Juniper Publishers | Journal of Polymer science (AJOP) is a Journal which publishes state-of-the-art overview articles by internationally recognized
Don't wanna be here? Send us removal request.
Text
Juniper Publishers wishes Happy Easter to you and your family members

0 notes
Text
Investigation of Extensional Flow Behavior of Polyethylene Melts through Birefringence by using Extrusion Cross-slot Die

Abstract
Flow parameters of polymers melt under steady state condition in shear are required to assess flow behaviour of the molten material in the die or downstream operations. Very often this is not sufficient to have a full understanding of the polymer processability, and additional information of flow response in extension is also needed. In this paper, the extensional properties of two molten polyethylene have been investigated by measuring stress response when a high extensional deformation is applied in a cross-slot die. Flow induced birefringence analysis and stress-optical rule are applied to determine rheological properties. Two polyethylene’s with similar rheology in shear but different molecular structure (HDPE and LLDPE) are analysed. Very interestingly, this approach appears capable of capturing differences in extensional flow that are not detectable using other conventional analytical methods.
Keywords: Flow Birefringence; Stress Optical Rule; Extensional Flow; Extensional Rheometry; Viscoelasticity
Introduction
Viscoelastic responses in shear and extensional regime of polymer melts are of paramount importance as they dominate most of the processing of polymers in the molten state [1]. Therefore, in industrial field, knowledge of rheology of polymers plays a central role in designing and understanding many processing operations: extrusion, blow molding, cast-blow extrusion, injection molding [2,3]. Specifically, extensional flow al high strain rate (ε ̇) is easily encountered in many industrial processes and there is increasing demand for generating more information about it [4]. It is usually more difficult to characterise properties of extensional flow than for simple shear. This kind of flow is commonly achieved with rheometers designed to uniaxially stretch the material. They typically include filament stretching [5] and dual wind-up stretching device [6]. However, true steady-state flow conditions are quite often difficult or impossible to reach because unlimited deformation is theoretically required [7], whereas elongational test is prone to sample inhomogeneity (due to localized necking) and rupture [8,9]. Gravity and non-isothermal condition on the sample, if the test is not carried out in climatic chamber or in thermostatic fluid, conspire to add limitation with these approaches. In many cases, when materials need to be tested at relative high temperature and broad strain rate close to effective industrial processing conditions, conventional lab-scale approaches in extensional flow with existing stretching rheometers are not capable to fully provide the required information. Low melt strength or sagging of molten materials promote premature deformation of the specimen, forcing to carrying out test at lower temperatures and it is not representative of real process conditions. This can limit the usage of conventional devices and methods. Therefore, there are relatively few data available for extensional flow [10] and especially for low viscosity molten polymers, measurements are very often overlooked and sometimes results are an over-simplification of real behavior.
For these reasons, full characterization of true steady state value for extensional stress response over a broad range strain rates is still an open topic in the field of polymer melts. In this scenario, cross-slot die, and rheo-optical approach appear to be a valid tool to get insight into rheology of complex flow and mimic and capture more closely extensional flow typically encountered in industrial operations [11,12]. Specifically, rheo-optical method offers potential to explore and evaluate the spatial-temporal evolution of the stress response of polymer melts [13]. It is an elegant and non-invasive way to generate extensional flow in controlled environment and confined geometries (the cross-slot die), it is well suited for polymer melts with poor melt strength, and/or non-homogeneous deformation at relatively high Henky strain or in case of not-achievement of a robust steady state [14]. Lastly, extensional flow can be sustained indefinitely in long run. This is possible through the connection of flow cell to a couple of extruders that provide a continuous feed of molten polymers up to relatively high flow rate. Thanks to the execution of the test in confined geometries, the method is insensitive to problems of melt yielding and high fluidity that, on the contrary, could make problematic the use of current rheometers operating in “not confined” environment. The specific geometry of the crossslot used in these experiments provides, for material transiting close to the stagnation point, high planar extensional flow that propagates along the entire plane of symmetry toward the outlet, which results in high level of extensional rate. Flow induced birefringence measurement (FIB) is used to evaluate interaction of the light with the polymer melt flow. Moreover the birefringence property is related to the stress distribution, polymer chains orientation and stretching with respect to stress directions.
Stresses in the melt stream were evaluated and quantified from flow-induced-birefringence (FIB) pattern along the centerline using the stress optical rule (SOR). It is well known that there exists, under a wide variety of conditions, a constant in the ratio of birefringence n and stress σ and is expressed as:
Where SOC is the stress optical coefficient for the polymers under analysis. It is given in unit of Pa-1. For the work presented in this paper a SOC of 1,80 x 10-9 Pa-1 is used for HDPE and LLDPE, which is in good agreement with the range given in literature for PE from 1,2 to 2,4 x10-9 Pa-1 [15,16]. Stress-optical rule has been found to remain linear in a wide stress range [17] and SOC weakly dependent on temperature [17,18]. The main goal of this paper is to investigate if melt flow induced stress birefringence can capture and well allow distinction of the behavior in extensional flow of the two polymers, otherwise not distinguishable with conventional rheological techniques. At the same time, we want to verify how sensitive the method is to capture differences in behaviour due to branching.
Materials and Experimental Setups
Materials
Materials used in this paper are a linear HDPE (Eraclene ML70U) and LLPDE (Clearflex CLD0) produced by Versalis, both with a very similar melt flow index. The first one is designed for extrusion application, whereas the second one is more suitable for injection moulding and film processes. They have very different degree of branching but despite this different molecular structure and application, still exhibit similar shear viscosity (Table 1).
Material Characterization in Shear Flow
The viscoelastic behaviour of two polyethylene samples has been evaluated in shear capillary experiment and by dynamic analysis. Results are reported in Table 1 and in Figure 1 (Master Curves). The grades have been selected because have very similar shear viscosity and master curve but very different degree of branching. It must be pointed out that both PE’s grades, neither elongational viscosity test nor fiber melt spinning measurements can be performed, due to the very low melt strength and sagging of the polymers at high temperature. Oscillating shear capillary rheometry (OSCAR) [19] has been also used to assess the complex rheology of LLDPE and of HDPE. The behaviour of the elastic modulus was measured at 190 °C for a shear cycle of period p=180s and amplitude . The elastic shear modulus reaches almost the same low shear rate value of 0.12 MPa for both PE but shows a plateau only for the HDPE up to 200 s-1 while for the LLDPE it keeps constantly decreasing within the same range of shear. It is also important to report that the shear viscosity modulus V(γ ̇) for both samples is perfectly superimposable.The elastic shear modulus behaviour can give some explanation to the industrial evidence on why the LLDPE is not suitable for extrusion production due to the inconsistency of its melt strength, also providing an explanation on why those polymers are not suitable for the same transformation process. Unfortunately, this approach requires a dedicated instrument with a very time-consuming experimental approach. In this work we have investigated the extensional viscosity at high ε ̇ rate of strain to fully access the rheological response of both material in many industrial processes.
Experimental Set Up
Equipment used for flow induced birefringence (from now on named as FIB) experiments is called “GEMINA”. It was designed and manufactured by Isotattica [20]. It basically consists of a couple of extruders and a patent pending cross-slot die including an optical bench for flow visualization.
Single screw extruders
To obtain a continuous flow of molten polymer to be delivered to the cross-slot die, the two independent-controlled extruders are coupled with the cross-slot apparatus. Each extruder is equipped with a 30 mm barrel/screw diameter and a L/D ratio of 25. Each of them is heated by three electrically powered zones on the barrel, a fourth one on the flange and a fifth one on die. It is powered by a 4 kW electric motor with a drive gear and electronic speed controller. Experiments are carried out at three different melt temperatures: 160, 190 and 220 °C, the extents of this range has been chosen in order to reproduce typical situations of use in machining processes. Pellets of polymers are feed in the hopper and molten polymer is transported along the extruder into the die. Setting temperatures from hopper to the die are selected in such way to get an effective temperature of the molten polymers as indicated above. Flow visualization experiments were performed after waiting for a certain time (15 min) for extruder parameters stabilization. Typical data collected for each run are as follows: flow rate, video and picture capturing for subsequent sequencing and processing via FIB. The range of flow rate used for experiments is from 20 cm3/min up to 290 cm3/min (i.e. from 16 s-1 to 225 s-1 in terms of apparent shear rates near inlet flow passageway).
Cross-slot die
A modular cross-slot die is fitted at the extruders exit from when the molten polymers flow. The cross-slot geometry provides four perpendicular, intersecting coplanar channels (width 4 mm, channel depth 8 mm), rounded at intersection point (Figure 2). The flow involves two opposed inlet streams which lies on the same axis meeting at a planar stagnation point and then exit orthogonally. It has a pair of stress-free transparent viewing windows (borosilicate glass) that allows a light beam to pass through the midsection of flow field and orthogonal analyzer before being captured using a digital video camera. The entire die is heated with electric heaters deeply inserted in the die, then connected with two independent temperature probe and controlled by the two zones 5B of the extruder panel control. The die is also equipped with 4 thread holes, in the passageway between adapters and cross die, for inserting melt pressure transducer or temperature probe (flush mounted). The two molten polymers are extruded with controlled flow rate and temperature in opposite directions towards the centre along the opposing collinear flow channels. By impinging these two fluids, flow induced stress birefringence is generated, allowing visualization and mapping of stress fringes, that correspond to a locus of constant value of principal stress differences PSD. Largestrain extensional flow deformations, preferred alignments and stagnation flow are generated along the inlet-outlet symmetry plane of the collinear channels and then analysed via flow-induced birefringence.
Optical bench
The flow birefringence measurements are performed using an optical bench as shown schematically in Figure 3. From top to the bottom, it consists of light source (white light), polarizer, two quarter wave plate (the cross-slot die is placed between them), analyser, extension tube with camera lens, and digital video camera. Firstly, the light beam passes the polarizer and quarter wave pate, enter through glass windows the melt stream which rotates its polarization state, then exit through the other glass window, quarter wave plate and analyser before reaching video camera. With this arrangement polarization of light is circular (circular polariscope in dark or bright field), isoclinic extinction bands (loci of points where principal stresses directions are constant) are not visible, leaving only stress-related isochromatic fringes (loci of points where the difference of principal normal stresses is constant). The optical parts are designed for fast assembly on a stand, are mechanically independent and moveable in all three dimensions for precise tuning of each component. Additionally, said stand, after competition of runs, can be moved away from die housing and extruders. With white light, coloured bands are observed, they are called “isochromatic fringes”. Firstly, fringe tracking and fringe order assignment are done. Considering that each isochromatic fringe with same colour carries the same light retardation and corresponds to a constant value of principal stress difference, the latter can be quantified through the stress optical rule.
The most important relation that allows us to calculate the PSD is given below:
Here N is the fringe order, l is the wavelength of light, d the channel depth. Combining stress optical rule (Equation 1) and Equation 2 it is possible to calculate the PSD by determining birefringence in molten flow on each isochromatic fringe.
Results and Discussion
Elongational flow/FIB
In the symmetry plane inlet-outlet along the centreline, at steady state regime, polymer flow experiences a constant extension rate ε̇. It can be estimated by the formula:
Where Vavg is the average velocity in the passageway and w the channel width. In this area flow approaches fully developed planar stretching flow. The steady state elongational viscosity is calculated from tensile stress (σstd is the PSD-principal stress difference between X-extensional and y-compressional axes, determined by FIB analysis) and the steady state strain rate ε̇ (Equation 4):
The evolution of the fringes as function of flow rate indicates a progressive increase of level of anisotropy between inlet and outlet channels (Figure 4). The isochromatic fringes of the birefringence are used to determine the principal stress difference in steadystate condition: each fringe carries a fixed stress contribution and through fringe sequencing and assignment of fringe order along the exit symmetry plane (fringe counting technique) is possible to determine PSD. An increase on concentration of isochromatic fringes is observed at high flow rate, mainly located along the exit symmetry plane where stretching flow is supposed to be fully developed. Quantitative representation of stress build-up versus extension rate is well illustrated in Figure 5. The graph corresponds to steady state conditions of fringe patterns. It is observed that the difference in stress between HDPE and LLDPE is maintained throughout the extended range of extension rate investigated. For both materials, the level of stress anisotropy between inlet and outlet channel flow increases as flowrates increase. The difference in stress concentration leads finally to a different evolution of extensional viscosity vs extension rate (Figure 6). The effect of difference in structure between HDPE and LLDPE is also well illustrated in Figure 7 & 8. The differences in fringe numbers sequencing made at same level of extensional rate are representative of the polymer type. LLDPE (short chain branching) shows the lower level on center-line stress fringes on comparison to HDPE (extensive branching), thus indicating for the former a lower stress concentration localized in the area of outlet symmetry plane.
As explained above, the configuration of the optical train used for the evaluations (circular polarization) is such as to examine isochromatic fringes. We have mentioned that with a different optical setup (linear polarization in dark field) is possible to also evaluate the isocline bands, loci of points where principal stresses directions are constant. With this configuration the initial field is dark, the principal stresses on melt produce a pattern of coloured fringes that vary with loading (isochromatic), on the same time some dark bands remain fixed with stress (isoclines); by rotating the polariser and analyser together, the coloured fringes do not vary (isochromatic) while the dark fringes change (clearly detectable isoclines). It has been made an exploratory survey and it has provided an interesting mapping of their distribution (Figure 9). Isoclinic fringes of HDPE are practically superimposable to those of LLDPE, an indicator that materials express a similar spatial orientation of stresses. There are some points which remain dark during simultaneous rotation, they are called singular points (S1 and S2) where both principal stresses are equal and hence σ1 = σ2 or σ1 – σ2 = 0. If σ1 = σ2 they represent fringe order 0 (black colour) in isochromatic evaluation. Isoclinic fringes allow, through a subsequent graphic elaboration, to determine the so-called isostatics which are the trajectories of the stresses. However, this issue is not the subject of this investigation, it will be addressed in future studies.
Summary and Conclusion
The cross-slot device coupled with two extruders allows measurement of steady-state planar extensional viscosity in a broad strain rate regime through stress birefringence. Specifically, the properties of two polyethylenes under extensional flow have been assessed by using this non-invasive rheo-optical approach. This analysis show how steady-state extensional flow can be evaluated through cross-slot measurement and how different branching in the molecular structure has an impact on stress in elongational flow. The PSD at different level of strain rate was captured using flow-induced birefringence. The PSD pattern developed from an initial near-Newtonian response (slow flow, quasi symmetric pattern between inlet and outlet) to gradually increasing level of asymmetry between inlet and outlet flow as the flow rate increases (stretching flow). It has been experimentally verified that these two materials show a difference in extensional viscosity, and the presence of long chain branches in the molecular morphology in HDPE affects the stress in extensional flow. On the other hand, LLDPE shows a lower extensional viscosity. The advantage of this method lies in the possibility to replicate the same temperature of industrial process and extensional optical device has confined geometry that allows the testing on molten polymers that, due their poor melt strength, sagging, localized necking at test temperature, cannot be otherwise tested in free-surface devices (not-confined geometries) like current commercial stretching rheometers.
Additionally, high strain steady-state limit can also be reached in long stable extrusion run. According to our findings, this approach is capable to detect and differentiate extensional rheology of different polymers that would not otherwise be captured with other techniques Cross slot die and rheo-optical analysis does have some limitations. At high stress values, fringes stratification become very high and the sequencing and counting may be more demanding. The PSD patterns for HDPE show a higher level of stress vs LLDPE at same strain rate, and it is observed that sequence of patterns of HDPE is always approximately two levels of fringe orders more forward than the LDPE compared at same level on central extension rate. The difference in growing number of fringes for HDPE versus LLDPE ultimately lead to a different extensional viscosity response. It should be remembered that these two materials have very similar shear viscosity and mater curve, despite significant different degree of branching is present. On the same time the differentiation observed in extensional viscosity is in good qualitative agreement with mechanical behaviour - elastic shear modulus under shear flow - performed via-OSCAR technique. Results obtained with these two techniques corroborate the explanation of the different evidence of extensional behaviour of these two polyethylenes. This also reflected in the industrial field: HDPE is more suitable for extrusion than LLDPE.
The design of the die inevitably leads to a compromise in relation to channel dimension and aspect ratio. Ideally twodimensional flow is desirable, even if an increase in branching enhance localized three-dimensional flow with stress pattern increasingly asymmetric. In conclusion, it has been shown that rheo-optical approach with flow induced birefringence analysis can be used for mapping and measuring properties of melts in extension along the plane of the outlet channels, or simultaneously shear properties in the inlet channels as well, depending on geometry of die, thus providing to be a very useful and intriguing tool to capture the smallest differences in flow behaviour and assess polymer performances. This methodology can become an indicator of rarely observed phenomenon that are not made manifest with conventional approaches and/or non-fully predict with simulation. It can be seen as a complementary approach to current extensional rheometers, towards which it strengthens the measuring range, provides kinematically steady extensional flow, exceeds their limits in maximum allowable testing temperature and applicable extension rate. The connection of the rheooptical die with extruders also guarantees a constant support of homogeneous molten material to the flow cell and above all a long and stable test duration. Lastly, this kind of lay out (cross-slot die and extruder coupling) makes this equipment quite innovative among the available equipment for industrial rheology. It can be used with the purpose of quality control, polymer and compounds design, R&D and optimization in tooling designing.
Future Works
Rheo-optic analysis with cross-slot die coupled with extruders is certainly a field of investigation still to be fully exploited. Further tests with polymers different from each other or belonging to the same monomeric identity but different molecular architecture are to be encouraged, making the most of the potential of the flow-induced birefringence. New potential field of study is certainly the evaluation of isoclines, in parallel to birefringence analysis of isochromatic. Isotropic points appearing in low fringe order zones are often either overlooked or entirely missed in conventional rheo-optical analysis. Vice versa, isoclines and especially their processing into isostatics, could provide complementary information to understand the stress trajectories in flowing materials. This will be the subject of a future work.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/
For more articles in Academic Journal of Polymer Science please click on: https://juniperpublishers.com/ajop/index.php
0 notes
Text
Manufacturing and Evaluation of High-Quality Composites using Out-of-Autoclave Prepregs
Abstract
Carbon fiber-reinforced thermoset polymers have become popular in a wide variety of applications such as primary aerospace structures, sporting goods and wind energy systems. Autoclave processing has been the preferred method for fabricating high performance composites. However, the need for low-cost, high-performance composites prompted researchers and industries to develop new techniques such as vacuum aided resin transfer molding (VARTM) and vacuum-bag-only cure out-of-autoclave (OOA). Manufacturing parts with less than 1% void content, on the other hand, remains a difficulty. In the present study, the OOA technique was used to create high-quality (less than 0.25 percent void content) carbon/epoxy composites. The phases in the processing that result in good quality are described. Physical, mechanical, and fatigue properties of the manufactured composites were evaluated.
Keywords: Fiber-reinforced; Polymers; Carbon; Composites; Vacuum
Introduction
In spite of numerous application possibilities, the usage of composites has been limited because of high costs. While the material costs sum to 8-10% of the total costs, manufacturing and processing costs contribute to the majority of the overall costs of the composites [1]. Cost savings of up to 75% have been achieved by using low-cost composite manufacturing techniques and by making integral parts [2]. A reduction in man-hours by 70-85% was also reported when implementing automated composite tape layers [3]. Hence, several studies have been devoted over the past few decades in developing non-autoclave manufacturing techniques that can significantly reduce the manufacturing costs of composites [4-10]. Bond et al. [11] presented a comparative summary of the physical, mechanical, and thermal performance of composites manufactured using different non-autoclave processes developed in the past few decades. In addition to huge capital and tool cost-savings, non-autoclave composite manufacturing processes offer several advantages such as scalability to large parts, and flexibility to manufacture hybrid, complex-shaped parts [5]. The out-of-autoclave (OOA) process is a vacuum-bag-only cure process that uses engineered prepregs that can be cured in regular ovens instead of an autoclave. Centea et al. [12] conducted a literature review on the processing of vacuum- bag-only prepreg and their effect on composite quality. They also presented the development and defining properties of vacuum bag only prepreg. The cost and environmental performance are also discussed in their study. The OOA process not only results in less energy consumption but the lower capital and tooling costs, fewer coefficient of thermal expansion issues, and the scalability to larger and integral parts made it a competitive alternative to the autoclave process. Developing low-cost advanced composites will allow to fully utilize the advantages of composites and to advance the usage of composites in several applications. And the improved performance of the composites is directly related to the fiber, resin, and especially void content. While void content less than 2% is typically desirable in aerospace composites, OOA has to produce composites with less than 1% to truly deliver advanced composite products that are comparable to autoclave composites.
Park et al. [13] utilized vacuum-bag-only to manufacture carbon/epoxy composites and investigated curing techniques for producing high-performance composites with low void content. They stated that improving the resin flow may allow for producing parts with minimal void content (1.3%). The composite laminates generated by their recommended technique showed a slight decrease in compressive strength compared to autoclave curing.
The compressive strength decreased by 6.5% for [0/90]₄ stacking sequence and 7.6% for [0/452/90]s stacking sequence. The inplane shear strength increased by 3% compared to laminates obtained by autoclave curing. In the present study, high-quality composites with a void content of less than 0.25% have been consistently manufactured employing the OOA manufacturing process. The manufacturing process utilized in accomplishing the high-quality composites is presented. Physical, mechanical, and fatigue tests have been conducted to evaluate the performance of the manufactured composites. Low-velocity impact tests were performed on the manufactured composite panels. Residual compressive strength of the impacted panels was evaluated. The effect of impact on the fatigue life of the composites was studied.
Materials
MTM45-1/CF2412 carbon prepregs obtained from Cytec Engineered Materials Inc., NJ, USA have been used for the present study. These prepregs contain 6K 5HS AS4C carbon fabric impregnated with MTM 45-1, a variable cure temperature, highperformance toughened epoxy resin.
Manufacturing
Flat composite panels have been manufactured using the OOA manufacturing process. The schematic of the bagging procedure employed for the OOA process is shown in Figure 1. The manufacturing procedure includes laying up the prepregs that were cut to the required dimensions and orientations onto an aluminum mold free from surface defects and already coated with Frekote release agent. Hand pressure and rollers were used to press the prepregs over the mold starting from one side of the prepreg and moving progressively towards the rest of the surface. This process is repeated for all the prepregs to remove entrapped air bubbles as well as folds or wrinkles. Thin glass strings, FEP release film, breather, and vacuum outlets were placed and sealed with a vacuum bag. A vacuum line was connected to the vacuum pump and checked for any leaks. A two-stage vacuum pump with a capacity of 5 L s-1and an ultimate vacuum of 0.013 Pa has been used to manufacture these panels. The set-up was maintained under vacuum for 12 hours. The lay-up is heated to 180oF and held for 4.5 hours. The temperature is then increased to 250oF and held for 4.5 hrs. The part is then cooled down to room temperature, demolded, and post-cured at 350oF for 2 hrs.
Characterization
Fiber volume fraction testing using sulphuric acid digestion method
Fiber volume fraction tests were conducted on the manufactured OOA composite panels using sulphuric acid digestion method. Four specimens each weighing from 0.50 to 2 gm. were cut from the panel. The edges of the specimens were polished thoroughly to facilitate accurate density measurements. The samples were dried in an oven for 1 hour at 120°C to remove any surface moisture, weighed, and tested for density. Table 1 shows the density, fiber volume fraction, and void content of the composite samples. The samples had an average fiber volume fraction of 53.99 %, and void content of 0.21 %. According to published studies, the amount of voids in a material has a direct impact on its mechanical properties [13-15]. For interlaminar shear strength, flexural strength, and flexural modulus, Ghiorse et al. reported reductions of 9.7%, 10.3%, and 5.3 percent per percent void, respectively [14]. Sergio et al. discovered that increasing void content had a significant negative impact on the fatigue life of composite constructions [15]. As a result, lowering the void percentage from 1% to around 0.25 percent should improve mechanical qualities.
Tensile Tests
Static tensile tests were performed on the OOA composites to evaluate the ultimate tensile strength required for fatigue testing. Samples of 2.286 mm (0.09 in.) thickness (6 layers) with 25.4 mm (1 in.) and 12.7 mm (0.5 in.) width either slipped or failed in the grips. Hence the thickness of the samples was decreased to 0.064 in (4 layers). While samples with 25.4 mm (1 in.) width failed in the grips without slipping, 12.7 mm (0.5 in.) wide samples failed in the middle. The test results obtained for 12.7 mm (0.5 in.) width and 1.626 mm (0.064 in.) thickness were reported below. Composites coupons were cut from the panels were manufactured using 4 layers of MTM45-1/CF2412 OOA prepregs. Tests were conducted on the coupons using Instron 4204 testing machine in accordance with ASTM D 3039. Samples were tested at a crosshead speed of 12.7 mm/min. (0.05 in./min). The ultimate tensile strength, modulus, and failure strain are tabulated in Table 2. The samples had an average tensile modulus, strength, and strain to failure of 824.79 MPa, 65.20 GPa, and 1.27% respectively. Hence the post-impact fatigue tension tests were performed at three stress levels – 50% Sult = 413.68 MPa, 65% Sult = 53.78 MPa and 75% Sult = 62.05 MPa (Sult -ultimate strength). When the samples fail at these levels during the fatigue tests, the stress level values were further dropped down.
Flexure Tests
Static flexure tests were performed on the OOA composites to evaluate the bending properties. Samples of 0.09 in thickness (6 layers) with 12.7 mm (0.5 in.) width manufactured from 6 layers of MTM45-1/CF2412 prepregs were used as test specimens. Tests were conducted on an Instron testing machine according to ASTM D790-03. A span to depth ratio of 40:1 was used to avoid failure by shear. Six specimens were tested at a crosshead speed of 6.096 mm/ min. (0.24 in./min.) The ultimate flexural strength, modulus, and strain to failure values are tabulated in Table 3.
Low-Velocity Impact Tests
Low-velocity impact tests have been performed on the composite panels manufactured using Out-of-Autoclave (OOA) process. A Dynatup Instron Model 9250 Impact Testing Machine with impulse control and a data system was used to carry out the low-velocity impact tests. Three different energy levels of 10J, 20J, and 25J were considered. The hemi-spherical impactor had a mass of 6.88 Kg and a diameter of 12.7 mm (0.5 in). The energytime history, load vs. displacement, and velocity-time history plots are shown in Figures 2 & 4 respectively. The impactor penetrated the samples at 30J of energy.
Compression-After-Impact (CAI) Tests
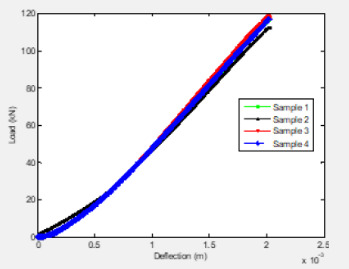
CAI tests have been conducted on MTM45-1/CF2412 composites manufactured using the OOA process. The tests were performed according to ASTM D7137. Four specimens of size 152.4 mm (6 in.) x 152.4 mm (6 in.) were first subjected to low-velocity impact tests and then machined to 152.4 mm (6 in.) x 101.6 mm (4 in.) for the CAI tests. Laminate construction consists of 12 fabric plies with a stacking sequence of [(+45/-45)/(0/90)]3S. Impact energy per unit thickness of 6672 J/m, an industry standard for evaluating thick, quasi-isotropic laminates was selected. Just clearly visible impact damage (VID) has been observed at 32J. The CAI test fixture is edge-loaded between the flat platens. Loads were applied at a cross-head speed of 1.27 mm/min. (0.05 in./ min). Compression load vs. deflection curves are shown in Figure 5. The ultimate compression-after-impact strength values of the specimens are tabulated in Table 4. The front view of the tested samples is shown in Figure 6.
Tension Fatigue Tests
Fatigue tests have been conducted on unimpacted OOA composites in an MTS 810 closed-loop servo-hydraulic testing system. Tests were performed on 12.7 mm (0.5 in.) wide x 254 mm (10 in.) long x 1.6256 mm (0.064 in.) thick MTM45-1/CF2412 samples. Fatigue behavior of the coupons at sinusoidal tension – tension loadings of 80%, 85%, 86%, 88%, and 90% of the ultimate strength (827.37 MPa) or ultimate tensile load of 15.57 kN (3500 lb) have been observed. A frequency of 2Hz and a load ratio of R = 0.1 (R = σmin/ σmax) have been used. Failure of samples in the grips was not observed with an increase in grip pressure to 5.75 MPa. The fatigue life of the coupons at different loadings is presented in Table 5. Figure 7 shows the S-N curve of an unimpacted sample under tension–tension (T-T) loading. Since the gap between the fatigue life at 88% and 90% stress levels is huge, more fatigue tests will be conducted at 89% of the ultimate load and other stress levels as needed. Figure 8 shows the failed specimens. Both fiber fracture and delaminations throughout the length of the specimen were observed. Post-impact compression fatigue tests are in progress.
Post-Impact Compression Fatigue Tests
CAI fatigue tests have being conducted on MTM45-1/CF2412 composites manufactured using the OOA process. The laminate construction consists of 8 fabric plies with a stacking sequence of [(+45/-45)/(0/90)]2S. Samples had a thickness of 3.35 mm (0.132 in). The 152.4 mm (6 in.) x 101.6 mm (4 in.) panels were subjected to the 15J of impact energy according to ASTM D7136. The CAI test fixture is edge-loaded between the flat platens as shown in Figure 9. Fatigue behavior of the coupons in sinusoidal compression–compression loadings of 60%, 65%, 70%, 75%, 80%, and 90% of the ultimate strength (224 MPa) or ultimate compressive load of 76.02 kN (17,090 lb) has been used. Initially, panels of 12 fabric plies have been constructed. The ultimate compressive failure load of the specimens was 115.65 kN (26,000 lb). Due to the load cell limits of the available test machines, panels with lower thickness have been chosen. A frequency of 2Hz and a load ratio of R = 10 (R = σmin/ σmax) have been used. The fatigue life of the composites at different load percentiles is given in Table 6. The fatigue curves are shown in Figures 10 & 11. The samples did not fail at 60% of loading even after 700,000 cycles and the tests were stopped.
Conclusion
A low-cost OOA vacuum-bag-only cure prepreg technology was successfully used to produce high-quality carbon fiber composites with void content less than 0.25 percent. The processing stages that lead to high-quality parts are shown. The fabricated panels were put through tensile, flexure, impact, compression-after-impact, and tensile fatigue tests. The effects of post-impact compression fatigue were studied. The results reveal that the OOA method is capable of producing parts with quality and performance comparable to those produced by the autoclave process at a fraction of the cost.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/
For more articles in Academic Journal of Polymer Science please click on: https://juniperpublishers.com/ajop/index.php
#polymer#rubber#juniper publishers in USA#copolymer#juniper publisher journals#juniper open access journals
0 notes
Text
Human-Centric Regulatory in Point-of-Care Manufacturing for 3D Printed PEEK Polymer Implants with Functionalized Implant Surface
Abstract
This article aims to define a regulatory approach for future medical technologies to be applied to the research, design, development, and manufacturing of smart medical devices. In the scope of this perspective: A human-centric regulatory approach and regulatory thinking method for 3D printed PEEK polymer implants.
Read more about this article: https://juniperpublishers.com/ajop/AJOP.MS.ID.555663.php
Read more Juniper Publishers Google Scholar articles: https://scholar.google.com/citations?view_op=view_citation&hl=en&user=WwdhOCwAAAAJ&citation_for_view=WwdhOCwAAAAJ:UebtZRa9Y70C
0 notes
Text
Which Sustainable Development Goals and Eco-challenges Matter Most to Niger’s Farmers and Herdsmen? A Best Worst Scaling Approach
Abstract
The sustainable Development Goals proposed by United Nations are increasingly becoming integrated in socioeconomic and environmental projects. Eco-challenges have also been widely studied and documented. Several researchers have well defined and documented what is sustainable development and how can be it achieved. However, fewer studies have investigated farmers and herdsmen’ preferences and awareness for United Nations Sustainable goals and eco-challenge. Based on Previous studies reported by United Nations Development Agency, seventeen sustainable development goals and nine eco-challenges were included in this study. In this study, the authors use the balanced incomplete block design to collect data from 136 respondents. For each question, respondents were asked to select their best and worst sustainable development goals, while they were asked to select their three best and their three worst eco-challenges. The conditional logit model and the count-based methods were used to model the sustainable development goals and eco-challenges respectively. Results show that gender equality, followed by industry; innovation and infrastructures, no poverty, climate action, reduced inequalities, clean water and sanitation, zero hunger and quality education are the most preferred sustainable development goals. Results also suggest that water, health and food are the most preferred eco-challenges. These findings may be useful to plan and implement sustainable development goals and eco-challenges and thereby stimulating economic growth and prosperity in the study.
Read more about this article: https://juniperpublishers.com/artoaj/ARTOAJ.MS.ID.556284.php Read more Juniper Publishers Google Scholar articles: https://scholar.google.com/citations?view_op=view_citation&hl=en&user=Zt1YgWcAAAAJ&citation_for_view=Zt1YgWcAAAAJ:-f6ydRqryjwC
0 notes
Text
Polymer Composite Materials from Current Status to Future Prospects
Abstract
A call for novel material, new ideas, applications, and techniques are still and will be challenging all the time. The increased development of science and modern technology allows one to use a high-throughput search for novel materials that could give positive feedback in different areas of life. With increasing demand for high performance materials, the focus of recent researches has been to produce products with enhanced properties at minimal changes in the equipment, process and cost of inputs. The addition of clay minerals or nano-metal oxides to the polymers is to improve the polymer properties, their wide and demand characteristics or desired potential applications by producing the polymer nanocomposites. Scanning and transmission electron microscopy have led to a deeper understanding of polymer nanocomposites, a better account surface topology, structure and morphology.
Read more about this article: https://juniperpublishers.com/ajop/AJOP.MS.ID.555626.php
Read more Juniper Publishers Google Scholar articles: https://scholar.google.com/citations?view_op=view_citation&hl=en&user=WwdhOCwAAAAJ&citation_for_view=WwdhOCwAAAAJ:ZeXyd9-uunAC
0 notes
Text
Recent Advances in the Synthesis and Analysis of Polyamide 6 and Products therefrom: From Polymerization Chemistry of εcaprolactam to Thermoplastic Resin Transfer Moulding (t-rtm)
Abstract
Significant research and developments are taking place in the field of synthetic polyamides (Nylons). One of the most investigated members of this family of polymers is poly (ε-caprolactam) (PCL, Polyamide 6, PA6, Nylon 6). Herein, a brief overview will be presented on the major recent advancements in the anionic ringopening polymerization (AROP) of ε-caprolactam (CL) by the initiating combinations of metal lactamates and carbamoylcaprolactams and by some other initiating systems, e.g. protected N-heterocyclic carbenes , NaHMDI and σ-borane-barium complexes. The in situ polymerization of CL in the presence of additives, such as flame retardants, reinforcing agents and fillers, is also discussed. Examples are presented on the determination of the molecular weight distributions (MWD) and average molecular weights of Polyamide 6 by GPC (SEC) measurements with 1,1,1-trifluoroethanol as eluent used routinely in our laboratories. Finally, a glimpse will be provided into the ongoing intensive research related to thermoplastic resin transfer molding (T-RTM) processes aiming at producing Polyamide 6 products, such as reinforced PA6 and composites, by rapid in situ polymerization of CL.
Read more about this article: https://juniperpublishers.com/ajop/AJOP.MS.ID.555629.php
Read more Juniper Publishers Google Scholar articles: https://scholar.google.com/citations?view_op=view_citation&hl=en&user=WwdhOCwAAAAJ&citation_for_view=WwdhOCwAAAAJ:qUcmZB5y_30C
0 notes
Text
Gellan Gum Immobilized Anticancer Drugs and Gold Nanoparticles in Nanomedicine
Abstract
This review is devoted to recent progress in the design of anticancer drug delivery systems with participation of unique polysaccharide gellan gum. At first a brief literature survey on conformational and phase behavior of gellan gum as a function of external stimuli, such as temperature, pH, salt addition etc. is presented. Then the immobilization protocol of anticancer drugs and gold nanoparticles within gellan-based hydrogel matrix is discussed. Release of anticancer drugs from gellan gel matrix to outer solution is considered. Cytotoxicity of gellan gum-immobilized gold nanoparticles together with their anticancer activity is summarized.
Read more about this article: https://juniperpublishers.com/ajop/AJOP.MS.ID.555588.php
Read more Juniper Publishers Google Scholar articles: https://scholar.google.com/citations?view_op=view_citation&hl=en&user=WwdhOCwAAAAJ&citation_for_view=WwdhOCwAAAAJ:RGFaLdJalmkC
0 notes
Text
Excited Triplet States of Organic Molecules and Reactive Free Radicals in Polymers

Abstract
The mini-review is devoted to the recent investigation the role of cage effect during photopolymerization. Benzophenone was selected as a photoinitiator of polymerization.
Introduction
Free-radical photopolymerization (UV-cure) of organic coatings is a very important process that has been used for more than half a century. Academic and industrial researchers study the basics of this complex process [1-4] In this mini review we will briefly consider the important role of low MW free radicals of photoinitiators (PIs) and the excited triplet states of PIs in photopolymerization. On the contrary, residual PIs in the cured coatings and other low MW photoreactive species in the cured coatings/polymers lead to a negative effect: photodecomposition of polymers outdoors, poor weatherability. We will comment on that as well.
Discussion
A primary chemical act of free radical photopolymerization is the generation of radical pairs (RP) in the triplet spin state. PI absorbs UV- or visible light; populates the excited singlet state of PI which in turn undergoes intersystem crossing into the triplet state. PIs often contain a carbonyl group in their structure. Reactive triplet state either undergoes dissociation into two radicals (Norrish 1 process) or H-abstraction from co-initiator or even from the coatings material or a polymer, both possessing C-H bonds. The first PIs are dubbed as Type 1 PIs, and the latter PIs as Type 2 PIs [4-6]. We will use a commercial PI benzophenone (BP) as an exemplar. Photochemistry of BP and reactions of corresponding free radicals BPH• have been extensively studied. Thus, the action of BP as a PI or as a residual PI in the cured coatings can be presented by eq. (1):
Here RH is a hydrogen donor. The RP formed in reaction (1) either decays in the polymer cage or radicals escape into the polymer bulk, as per (Scheme 1) shown below. The formation of an RP in the triplet state is a hurdle for a cage reaction. RP can react only in the singlet state with few exceptions. We include in our discussion low MW radicals which are formed at a high degree of photopolymerization (conversion of vinyl groups), say at . At high the media can be regarded as a polymer. A fraction of photogenerated radicals which decay inside a cage is called cage effect. Obviously, chemists are interested in the lowest possible in the case of photoinitiation of polymerization and in the highest possible in the polymers outdoors. (For fairness sake, we will mention that an aromatic radical BPH• does not initiate polymerization, but a counter radical R• does.) 3BP and R• are damaging species in the cured coatings or polymers that facilitate photodegradation. Photopolymerization usually does not lead to complete consumption of PI. On average 20% of the dissolved PI stays in the cured coatings. Being outdoors, the cured coating is subjected to destruction initiated in particular by the residual PI and possibly by carbonyl compounds formed during autoxidation. (Here we talk about processes which last years.) Thus, cage effect value and its dependence upon the degree of photopolymerization and properties of a polymer are very important for practice [7,8] It was demonstrated that the value of depends in particular upon the free volume of a polymer Vf and the glass transition temperature of a polymer Tg [3,9]. Cage effect dynamics (Scheme 1) since the inception of RP at t 0 till relatively long time upon completion (milliseconds) of cage reactions has been studied by ns flash photolysis with optical or ESR detection. This research was done mainly with PI=BP. 10 ns resolution of such instruments is okay. Cage reactions in the viscous media (polymers) lasts microseconds [1-3,9,10]. Application of moderate external magnetic field affects [1-3,7] and should find a practical application in the cure of coatings. An interesting type of coatings are coatings which can be photopolymerized without a PI [10,11].
Conclusion
It is not surprising, that commercial PIs [4-6] are molecules that have a high quantum yield of an excited triplet state and which dissociate from a triplet state with a formation of a triplet RP [10]. Triplet RP has a high probability of dissociation and initiation of polymerization even in a viscous media.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/
For more articles in Academic Journal of Polymer Science please click on: https://juniperpublishers.com/ajop/index.php
For more about Juniper Publishers Please click on: https://juniperpublishersblog.wordpress.com/
0 notes
Text
Recent Advances in the Synthesis and Analysis of Polyamide 6 and Products therefrom: From Polymerization Chemistry of εcaprolactam to Thermoplastic Resin Transfer Moulding (t-rtm)

Abstract
Significant research and developments are taking place in the field of synthetic polyamides (Nylons). One of the most investigated members of this family of polymers is poly (ε-caprolactam) (PCL, Polyamide 6, PA6, Nylon 6). Herein, a brief overview will be presented on the major recent advancements in the anionic ringopening polymerization (AROP) of ε-caprolactam (CL) by the initiating combinations of metal lactamates and carbamoylcaprolactams and by some other initiating systems, e.g. protected N-heterocyclic carbenes , NaHMDI and σ-borane-barium complexes. The in situ polymerization of CL in the presence of additives, such as flame retardants, reinforcing agents and fillers, is also discussed. Examples are presented on the determination of the molecular weight distributions (MWD) and average molecular weights of Polyamide 6 by GPC (SEC) measurements with 1,1,1-trifluoroethanol as eluent used routinely in our laboratories. Finally, a glimpse will be provided into the ongoing intensive research related to thermoplastic resin transfer molding (T-RTM) processes aiming at producing Polyamide 6 products, such as reinforced PA6 and composites, by rapid in situ polymerization of CL.
Keywords:Polyamide 6; Nylon 6; ε-Caprolactam; Anionic ROP; Initiator; Activator; PA 6 additives; GPC (SEC); Thermoplastic Resin Transfer Moulding (T-RTM)
Introduction
Synthetic polyamides (Nylons) are still among the most important macromolecular materials with a wide variety of useful properties and applications even after nine decades of their discovery [1]. Significant research and developments are taking place with these polymers worldwide. These include exploring new polymerization chemistries for their production, modification of their structure and properties, and finding new ways of processing, especially by thermoplastic resin transfer molding (T-RTM), for a large variety of composites and products. In this brief overview, we attempt to provide a glimpse into the recent major advancement with one of the members of the Nylon family, Nylon 6 (Polyamide 6, PA6).
Polymerization of ε-Caprolactam: Effect of Catalysts, Additives, Fillers and Reinforcing Materials
Beyond doubt, poly (ε-caprolactam) (i.e. Polyamide 6, PA6, Nylon 6) is one of the most widely investigated polymers due to its versatile properties and broad application possibilities. Polyamide 6 is mainly made by the anionic ring-opening polymerization (AROP) of ε-caprolactam (CL). The major current polymerization technologies are based on the AROP of CL by initiator-activator combinations [2-5]. The most widely used initiator-activator initiating systems include metal lactames (e.g. Na-, K-, Mg-lactames) in conjunction with carbamoylcaprolactams (CCL) such as hexamethylene-1,6-dicarbamoylcaprolactam. The major mechanistic steps of the polymerization of CL by such initiating systems are displayed in Scheme 1. One of the main advantages of this polymerization reaction is related to the fact that it can be carried out by bulk polymerization in the molten state of CL and the components of the initiating system, i.e. initiator and activator, to high yields without significant induction period at elevated temperatures. This solventless process with nearly quantitative monomer conversion without any side product can be considered as one of the most efficient green polymerization processes.
However, it is well-known that AROP is sensitive to moisture, which is able to inhibit the CL polymerization by reacting with either the components of the initiating systems or with the amide anion in the propagating chain [Scheme 1]. Interestingly, the effect of moisture on CL polymerization with industrial initiator and activator combinations has only recently been studied in a systematic manner by Wilhelm et al. [2,3]. They have found by polymerization kinetic investigations that the water present in the polymerization system of CL can be compensated by adding appropriate amounts of initiator and activator. Other systematic investigations on the effect of the condition of CL bulk polymerization with commercially used initiatoractivator mixtures are still lacking, although such studies are of paramount importance for the rapidly developing thermoplastic resin transfer molding (T-RTM) techniques, used for obtaining, in addition to other polymers, Polyamide 6 products as well. In one particular case, Kang and coworkers [4] have recently attempted to determine the optimal polymerization conditions for obtaining carbon fiber reinforced PA6 by TRTM with the use of lactamate-CCL initiating system by varying the injection rate of the components.
Investigations with other than lactamatecarbamoylcaprolactame initiating systmes have also been reported for AROP of CL. Buchmeiser et al. [6,7] carried out CL polymerization with latent, protected Nheterocyclic carbenes, and revealed the catalytic effect of such compounds as a function of the structure of the substituents. Sodium hydride (NaH) in combination with 4,4′-Methylenebis(phenyl isocyanate) (MDI) was used by Kim and coworkers [8] to initiate bulk (melt) CL polymerization to prepare Polyamide 6 composites with multiwalled carbon nanotubes. A complex of barium with σ-borane was successfully applied by Battacharjee et al. [9] to catalyze AROP of CL under mild conditions. Polymerization of CL in the presence of various additives is under intensive investigations nowadays. This can be considered as the major current trend in the field of the chemistry and processing of Polyamide 6, attempting mainly to reach short processing times to obtain advanced composite products, especially by TRTM. The following classes of additives have been recently investigated for the in-situ polymerization of CL by AROP: flame retardants [10-12], fillers, such as titania [13], zinc oxide [14], boron nitride [15] silica [16], montmorillonite [17] and multi-walled carbon nanotubes [8,18,19], and reinforcing agents, like glass fiber [20,21] and carbon fiber [4]. It has to be noted that in spite of its importance usually neither the CL monomer conversion nor the average molecular weights and the molecular weight distribution (MWD) are determined in such instances.
Characterization of Poly(ε-caprolactam) by Gel Permeation Chromatography
Although it is widely known that the MWD and the average molecular weights as well play critical role in the major properties of polymers for both processing and application purposes, it is quite surprising that MWD determinations of Polyamide 6 by GPC (called also size exclusion chromatography, SEC) is rather rare in the open literature. In the few recent instances, GPC of PA6 and CL copolymers has been carried out in solvents like hexafluoroisopropanol (HFIP) [22,23], DMF [24], THF (for low MW PA6) [19] and m-cresol (for CL copolymer) [25]. In the course of our recent investigations on CL polymerization, we have found that 1,1,1trifluorethanol (TFE), as an alternative to HFIP, can also be used effectively as mobile phase in GPC for the determination of the MWD and average MWs of PA6 with a broad range of molecular weights. As displayed in Figure 1, GPC can also be utilized to follow the progress of CL polymerization. In this Figure, the low MW peak, i.e. the peak at higher elution volumes, corresponds to the CL monomer. The gradual disappearance of the monomer peak and simultaneous increase of the polymer peak can definitely be applied to follow the polymerization process in addition to the determination of the MWD and the average MWs of the resulting Polyamide 6. These findings by us indicate that GPC with 1,1,1-trifluoroethanol as eluent can be routinely utilized for not only the determination of the MWD of Polyamide 6 products, but it can also be used to investigate the progress of the polymerization reaction of CL as well.
Polymerization of ε-Caprolactam by Thermoplastic Resin Transfer Moulding (T-RTM) for Obtaining Polyamide 6 and Its Composites
Intensive research and development is taking place worldwide with significant interest and activities in the field of T-RTM for producing the broadest possible range of products based on Polyamide 6 [2,4,21,26]. This is due to the fact that the melt (bulk) polymerization of CL, having low melt viscosity at elevated temperatures, can be carried out in situ in the presence of suitable initiator-activator combinations in a short time. Such a process provides economic and energy saving technological advantages over classical injection molding technologies which preferentially use preformed polymers. T-RTM processing of Polyamide 6 composites has been recently used with a variety of additives, such as reinforcing materials [4,18,19] and fillers [15-17]. However, investigations on the effect of the reaction conditions on the CL conversion and MWD of the resulting Polyamide 6 obtained under T-RTM or similar techniques are very rare. As mentioned earlier, Wendel et al. [2,3] explored the effect of water on the rate and outcome of CL polymerization in a T-RTM process. The optimization of the injection speed in T-RTM of CL by varying the sodium caprolactamate/CCL ratios in the presence of carbon fiber was recently attempted by Kang and coworkers [4]. However, systematic investigations on the effect of the major reaction parameters on the CL bulk (melt) polymerization with industrial reagents is still lacking. Considering this situation, our recent research efforts aim at revealing these correlations, which might be broadly utilized in T-RTM and other in situ bulk polymerization reactions with CL for finding optimal processing conditions [27].
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/
For more articles in Academic Journal of Polymer Science please click on: https://juniperpublishers.com/ajop/index.php
For more about Juniper Publishers Please click on: https://juniperpublishersblog.wordpress.com/
0 notes
Text
The Evaluation of Poly(imide) Siloxane to use in Biomedical and Radiotherapy Applications

Abstract
Poly(imide) siloxane encourages new research studies that offer innovative and comprehensive industrial areas, experimental approaches and their innovative products. Poly(imide) siloxane covers the studies in fundamental organic polymer chemistry and physical organic chemistry, noval researches on membranes and interfaces. The possible applications of the poly(imide) siloxane as shielding material include facilities such as biological shielding at nuclear medicine departments.
Keywords: Copolymers; Poly(imide) siloxane; Radiation
Introduction
Polymer based drug gene delivery systems with critical structural features at the nanometere scale have attracted immense interest. Besides, polymer applications to drug/gen delivery has offered unprecedented opportunities for the creation of a variety of polymeric structures. The drugs including nanocarriers can be derived from poly(imide) siloxane. The medical polymers such as flexible poly(imide) siloxane depending on anti-infective properties can be used in several medical applications [1-3]. Flexible poly(imide) siloxane with high mechanical performance (with the modification of hydrophobic and/or hydrophilic properties) depends on the formation of functional groups such as hydroxyl at the surface.
Results and Discussion
Polymeric materials can be used to manufacture balloons, particularly dilatation balloons. Measurable characteristics of balloons in general, and more specifically dilatation balloons, include distensibility (the percent radial expansion with increased pressure), elastic stress response (repeatability of obtaining the same diameter at the same pressure during repeated inflation-deflation cycles), flexibility, tensile strength and optical clarity [4,5].
Polymeric structures for drug/gene delivery will be described in this study. Tissue engineering requires to be coated with drug-loaded micelles in order to release drugs or growth factors to prevent infection or enhance tissue regeneration. Poly(imide) siloxane can support to produce polymeric micelle coatings which can control drug release from surfaces. The flexible poly(imide) siloxane having rubber properties can be effective in several areas (for example; special nerve can be regenerated to conduct nerve stimulation and to improve peripheral nerve regeneration in tubulation of neural system) [6-8]. Starting from this point, it can be said that flexible poly(imide) siloxane block copolymer can be accep as the suitable material to use in medical applications, nervous system repair and to minimize bacterial adhesion to the polymer. The researches on flexible poly(imide) siloxane are focused on the success of the production and application of biodegradable polymers, biocompatibility polymers with high mechanical performance and resistant against radiation. Poly(imide) siloxane can used to evaluate the equivalent tissue thickness (corresponding to half value layer thickness of the poly(imide) siloxane by using different synthesis and heat treatment conditions [9,10].
Conclusion
Poly(imide) siloxane is recognized as new generation polymer in polymer science and engineering. The application area of poly(imide) siloxane covers several fields of particular interest are biomedical applications, organic electronics and photonics, nanostructures, micro- and nano-fabrication, biological molecules (DNA, proteins, carbohydrates) applications, polymers for renewable energy and many other influences, potential industrial application areas such as food, textiles, adhesives, biodegradables, biorefining, pharmaceuticals, and oil recovery, exchange of research in the area of macromolecular substances.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/
For more articles in Academic Journal of Polymer Science please click on: https://juniperpublishers.com/ajop/index.php
For more about Juniper Publishers Please click on: https://juniperpublishersblog.wordpress.com/
0 notes
Text
The Explanation of Magnetic Metal Carbon Mesocomposites Synthesis Peculiarities by Means of Mesoscopics Notions

Abstract
Mechanism of mesoparticles modification reactions are considered with the application of such notions as charges quantization, phase coherence, interference and annihilation. On the base of theoretical Mesoscopics ideas the formation of covalent bonds because of the interference of negative charges quants in modification reactions is discussed. The hypothesis about possibility of annihilation at the interaction of positive and negative charges quants in redox processes is presented. The magnetic metal carbon mesoscopic composites synthesis (for example, initial metal carbon Mesocomposites) is realized by mechanochemical method at the grinding of metal oxides microscopic particles with polyvinyl alcohol macromolecules. Then in the result the Copper or Nickel Carbon mesocomposites which have the following atomic magnetic moments: for Copper – 1,3 μB, for Nickel – 1,8 μB are obtained. The investigations are carried out on the analysis examples of processes of Copper and Nickel Carbon mesoparticles modification by the compounds containing p, d elements. In the middle of its such substances as polyethylene polyamine, ammonium iodide, ammonium polyphosphate (APP), silica (SiO2), aluminum oxide, iron oxide, nickel oxide and copper oxide are used. It’s noted that the red ox processes are accompanied by the metal atomic magnetic moments growth, that is explained by the electron shift on high energetic levels because of the annihilation phenomenon. The hypothesis concerning to the passing of two phenomena (annihilation and interference) at redox processes is proposed.
Keywords: Macro Molecules; Nanoparticles; Suspension; Emulsions; Polymers; Toxicity
Abbreviations: NCPs: Cationic Polymers; DEX: Cationic Dextran; PLL: Poly-L-lysine; WHO: World Health Organization; PEG: Polyethylene Glycol; PDMAEMA: Poly(2-N,N-dimethylaminoethylmethacrylate)
Introduction
The process occurs at the charge’s quantization with the certain phase coherence and then with the chemical bond’s formation because of interference as well as in the red ox processes possible annihilation takes place [1,2]. If chemical reactions are realized without the changes of atoms oxidation states, then the negative charges quants quantization and the interference are carried out. However, the most reactions flow with the changes of elements oxidation states and then according to known schemes of reduction-oxidation processes it’s necessary to take into consideration of positive charge quants.At the interaction of positive charge quants with the negative charge quants the annihilation phenomenon with the electromagnetic radiation or/and the direct electromagnetic field is possible. Also, the interaction of positive charges with the formation of “dark hole” must not excluded (Figure 1). In this case the explosion with diffusion of many most quantity of energy into surroundings is possible. The phenomena of charge quantization, interference and annihilation are considered on the examples of metal carbon mesoparticles interactions with reagents containing p, d elements. At these investigations the basic method of researches is x-ray photoelectron spectroscopy.
Results and Discussion
The production of Metal Carbon mesoscopic composites is carried out with the using of mechanochemical interaction between microscopic particles of metal oxides and macromolecules of polymers at the active medium presence [3]. At the mesoscopic composites production, the sign variable loadings are applied. These loadings are appeared at the grinding with pressing. At the common grinding of Copper oxide particles with Polyvinyl alcohol (PVA) particles (or concentrated water solution) the metallic phase clusters falls between macromolecules of polyvinyl alcohol or, in other words, into reservoir (according to mesoscopic notions), in which banks are PVA macromolecules. The metal (example – Copper) within cluster has the positive charge. Therefore, the negative charge quants are directed to positive charged atom. In our case the negative charged quants from polyvinyl alcohol acetate and hydroxyl groups are transferred to copper positive charge quants. As a result, the annihilation with the electromagnetic direct field formation takes place. In this process the acetic acid and water are formed, and also the banks structures are changed: the poly acetylene and carbine fragments are appeared. There are unpaired electrons on joints of these fragments. The process of pair electron division and the shift of electrons on the high atomic levels for metal are explained by the annihilation origin. In this case the metal atomic magnetic moment growth is observed in the dependence on the electrons number which participates in red ox process.
The hypothesis about possibility of annihilation at the interaction of positive and negative charges quants in red ox processes is confirmed by the examples of processes of Copper and Nickel Carbon mesocomposites modification with application such substances as polyethylene polyamine, ammonium iodide, ammonium polyphosphate (APP), silica (SiO2), aluminum oxide, iron oxide, nickel oxide and copper oxide [3-5]. In the case, when polyethylene polyamine and ammonium iodide are applied, the connection reactions take place. At the interactions of polyethylene polyamine with mesoparticles the C=N bond formation is explained by the interference of negative charges quants. When the mesoparticles modification reactions with the using APP, SiO2, metal oxides are carried out, the redox processes are realized. In these cases, the modifiers reduction reactions take place. The structures of metal carbon mesoscopic composites with active carbon shells are defined by means of the complex of methods including x-ray photoelectron spectroscopy, transition electron microscopy with high permission, electron microdiffraction and EPR spectroscopy. In correspondent reactions the element reduction for reagents and Nickel or Copper atomic magnetic moments growth in mesoparticles take place. Below in (Table 1) the examples of metal atomic magnetic moments changes for mesoparticles modified by APPh or silica after the mechanochemical modification processes are given. The presence of unpaired electrons on mesoparticles carbon shells in above systems is determined by means of electron paramagnetic resonance (EPR) (Table 2).
Cu C NC – APPh (or SiO2) and Ni C NC – APPh (or SiO2).
The metal atomic magnetic moment growth proceeds owing to the redox processes with above chemical compounds. In papers [4,5] it’s shown that the reduction reactions of Phosphorus and Silicon from correspondent substances at the interaction on the interphase boundary with mesoparticles are realized.
The relations of mesoparticles to above oxides are changed from 1:1 to 1:0,2 depending on the qualitive spectra obtaining, for example, the relations of 1:1 and 1:0,5 for system “Ni/C NC – Al2O3” leads to full mask of mesoparticles. Therefore, the quantity of aluminum oxide is decreased to the relation 1:0,2. In accordance with Al3s spectra Aluminum is completely reduced during the modification process, and Nickel atomic magnetic moment is increased to 4,8μB (Table 3). In this case the reduction process is related to not only Aluminum oxide but also to Nickel oxide from metal cluster of mesoparticles (Ni C MC). Therefore, the reduction processes are stipulated by the electron transport from carbon shell of mesoparticles in direction to Al+3 and Ni+2 of atoms in correspondent oxides. Exceptional properties of modified Metal Carbon nanostructures with magnetic characteristics lead to the property’s improvement of nanostructured polymeric coatings [6-8]. For example, the introduction of 0,008% Cu C MC into the melamine-formaldehyde resins stimulates the polarization growth in two times (on the AFM data). Similar results can be received at the combination of phase coherency and interference of charges quants during the preparation process of modified polymeric materials. The decreasing of nanostructures activity is possible when the modification is carried out with the ultrasound processing or the violation of phase coherency takes place at the nanostructures quantities changes [9,10].
Conclusion
The present investigation has fundamental character. It’s based on the ideas concerning to the change of Metal Carbon mesoscopic composites reactivity. The investigations are dedicated the mechanochemical red ox processes in which the electron transport from mesoscopic composite cluster to carbon shell takes place. In this case the electron delocalization is found. For the first time the metal carbon mesoscopic composite modification by mechanochemical process with the using of active substances including also bioactive systems is possible. The activity of metal carbon mesoscopic composites is caused by the structure and composition of correspondent composites, which contain the delocalized electrons and double bonds on the surface of carbon shell. Thus, at the mechanic chemical reduction/oxidation synthesis the changes of element oxidation states as well as the increasing of metal atomic moment for cluster can be appeared. At the same time, the modifiers elements and functional groups are discovered in carbon shell of mesoscopic composites modified. The creation of reactive mesoscopic materials with regulated magnetic characteristics which can find the application as modifiers of materials properties, catalysts for different processes, effective inhibitors of corrosion, sorbents, stimulators of plant growth, is very topical. These facts open new era for further investigations and development of metal carbon mesoscopic composites application fields.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/
For more articles in Academic Journal of Polymer Science please click on: https://juniperpublishers.com/ajop/index.php
For more about Juniper Publishers Please click on: https://juniperpublishersblog.wordpress.com/
0 notes
Text
Fast Triggered Controllable Electrically Actuated Shape Memory Epoxy: Graphene oxide Nanocomposites

Abstract
Shape memory polymers (SMPs) are the new class of smart fascinating polymer materials with different potential applications. This research work aims to present systematic investigations of electrically actuated shape memory (EASM) properties at different electric voltage of epoxy polymer filled with 0.1 to 0.4 wt. % of Graphene (GO) to develop SMPs nanocomposites. EASM effect and surface morphology functional analysis of composites were observed after EASM characterization by Field Emission-Scanning Electron Microscopy (FE-SEM) and Fourier Transform Infrared Spectroscopy (FT-IR). 0.4 wt. % addition of GO showed highest shape recovery at 180 S, 140 S and 95 S for 40 V, 60 V and 80 V respectively. These results suggest that GO could be promising materials for electrically actuated shape memory applications.
Keywords: Graphene Oxide; Epoxy; Smart Polymer; Shape Memory; Polymer Nanocomposites
Introduction
Shape memory polymers (SMPs) are one of the promising smart materials, which can recover their original shape upon applying an external stimulus, such as heat, light and mechanical force etc [1-3]. Among these SMPs, polymer can recover an original shape in very short period of time from large deformation when subjected to an electrical current. In order to recover the shape of polymer, in case of electrically based SMPs, various types of electrically conductive fillers have been developed, so they can be actuated by passing an electric current. Carbon based nanomaterials such as, CNTs and Graphene have attracted much attention due to their novel properties of electrical conductivity [4-6]. So, development of electrical SMPs filled with carbon nanomaterials can be a way to new research. Most of the research studies found that electrically conductive composites have relatively poor mechanical and thermal properties. By contrast, a new class of thermoset SMPs shows better mechanical and thermal properties than thermoplastic SMPs, and it can be widely used as a functional or a structural material [7-10].These unique characteristics have led SMPs to be used in a myriad of fields, including automobile and aerospace engineering, medical treatment, and many other high-performance applications [9].
Many significant developments have been achieved for SMP composites for which shape recovery actuation can be carried out by electrically resistive heating with respect to maintain the mechanical and thermal properties. However, for the fabrication of electro active SMP composite, almost all previous works were focused on conductive fillers blended into pure polymers with the potential applications of SMPs, but some major limitations still exist and impose major challenges to their broad utilization. Some of the key limitations include low recovery due to low recovery strength, and low recovery speed due to the low thermal conductivity and inertness to electromagnetic stimuli [11]. Among the all conductive materials, GO has tremendous interest in scientific research due to its outstanding electrical responsive shape recovery property. It has two dimensional (2D) and conducting layered one atom-thick planar sheets of sp2-bonded honeycomb structure of carbon atoms. Graphene is a basic building block of graphitic materials having all dimensionalities and used to fabricate/prepare varieties of composite/blend films and polymer nanocomposites. It has extraordinary mechanical [12,13], thermal [14,15], high specific surface, excellent aqueous processability, amphiphilicity and surface functionalizability [7,16,17] and hence extensively investigated for applications in many technological fields [18-24].
It is expected that GO should be able to fulfil its roles as a fixed structure as well as a filler for reinforcement in epoxy-based polymer due to the potential covalent bonds. Larger specific surface area provides a strong connection between GO and polymer. On the other hand, the uniform distribution of GO on surfaces enables good dispersion of GO in the polymer. Even at low volume fractions, the vast interfacial areas created by welldispersed GO can affect the behavior of the surrounding polymer matrix and create a co-continuous network to fundamentally change the physical properties of the polymer matrix [25-27]. In this research article benefits to the electrical actuated shape memory properties of the developed conductive SMP composites were experimentally demonstrated. The improved conductivity also permitted electrical actuation of conductive SMP composites in a low electric power. Epoxy resin was selected as the matrix and GO was as one type of conductive filler for the investigated SMP composites due to their superb electrical, mechanical, and thermal properties; and expected to significantly improve the overall performance of the composite material.
Experimental
Ant
Commercially available Hand Layup Epoxy matrix and hardener, grade name Lapox L-12 and K-6 were procured from polymer division of Atul Limited, Mumbai, India. GO sheets with average 50 nm in thickness and up to the certain μm in length,were synthesized by modified Hummers method which has already been reported in our previous research works [28,29]. Solvents like acetone, ethanol and toluene for experimental and washing purpose were procured from Sigma Aldrich, Mumbai, India. To fabricate the nanocomposites, the epoxy/GO mixture was first prepared with different wt. % (0.1 to 0.4 wt. %) loadings. To lower the viscosity of the epoxy resin, and thus to obtain a good dispersion of modified GO within the matrix, the epoxy resin was diluted by acetone in a ratio of 100:30 and stirred simultaneously with heating up to 60 °C for 12 h for slow solvent evaporation. An additional 12 h of heating under vacuum conditions ensured the complete acetone removal. The hardener was added to the dispersions at a weight ratio of 100:6 and mixed for 5 min. The resulting mixtures were poured into a Teflon mold. In order to characterize and testing, the desired specified samples were cut from the sheets. Rectangular samples (100×10×3 mm3) were cut from the sheet of GO filled epoxy nanocomposites to investigate electrically actuated shape memory effect [30].
Electro-active shape memory testing
The rectangular samples (100×10×3 mm3) of the nanocomposites were heated to the certain temperature and held for 10 min. After getting certain temperature, the samples were bent into a “U” shape and then quickly dipped into ice water to maintain the “U” shape. Different voltages were applied on both ends of the different samples with crocodile pin and recorded the shape recovery process and recovery time.
Characterization
Topographical surface morphology of GO and GO filled epoxy nanocomposites were determined by using field emission scanning electron microscope (FE-SEM, S-4800 Hitachi, Tokyo, Japan) operated at an accelerating voltage of 30 kV). Functional groups of epoxy and GO filled epoxy were recorded on FT-IR spectrophotometer (FTIR-8000 Spectrophotometer, Shimadzu, Tokyo, Japan). The number of scans per sample was 25 and resolutions of the measurements were 4 cm-1. The recorded wave number range was 500-4500 cm-1. Thermal stability of epoxy and GO filled epoxy polymer was determined by a thermo gravimetric analyzer (TGA-50, Shimadzu, Tokyo, Japan). Approximately 10 mg of the sample was placed in a platinum pan for TGA analysis. The temperature range was kept from room temperature to 600 °C and a heating rate was kept 10 °C/min under nitrogen atmosphere to avoid thermo-oxidative degradation.
Results and Discussion
Electrically activated shape memory analysis
The samples were tested for direct current (DC), interpreted the obtained results, validated and complemented with the references of current research works on enhanced properties achieved with the use of GO. The results are illustrated in Figures 1-5 and Figures S1-S10. A comparable magnitude of improvement in shape memory properties with the different loading and different current are discussed. With the chosen wt. % of GO, the electrically conductive shape memory of the nanocomposite was rapidly improved by increasing the loading of GO and current (V) up to the 0.4 wt. % at 80 V respectively. Figures 1 and S1, S2 show the electroactive shape recovery behavior for the virgin epoxy polymer. The original shape of the sample was almost same; there was no change observed when an electric field of 40 V and above i.e. 60 and 80 V was applied. In the beginning at 0.1 wt. % (Figures 2 and S3, S4), a change was observed in shape memory of nanocomposites in 350 S at 40 V which further increased the shape memory properties in less time i.e. 210 S and 150 S for 60 V and 80 V respectively (Table 1). Figures 3-5 show the electro active shape recovery behavior for the epoxy containing 0.2, 0.3 and 0.4 wt. % GO. The original shape of the nanocomposites was almost completely recovered in 0.2, 0.3 and 0.4 wt. % for all the electric fields. From the figure 100(±2) % recovery were observed in 0.2- 0.4 wt. % loading of GO. The comparison of shape recovery was 0.2 to 0.4 wt. % loading with different electric fields and degree of shape recovery are shown in Figures S5-S10 and also mention in Tables 1-4. It is observed from the tables that the rate of shape recovery was strongly dependent on the magnitude of the applied voltage and the GO wt. % content in the polymer (at 0.4 wt. % loading shape recovery time was 95 S for 80V) [31]. But at lower loading (0.2 wt. %) from our previous research study [30] found that it provides better water thermal sensitive shape memory properties. The GO filled composites could be heated more rapidly to a higher temperature with higher voltage application (80 V) in comparison that of the lower voltage (40 and 60 V) because of their higher conductivity.
Morphological and functional analysis of composites
Morphological Study and functional analysis are rather important in order to explain and interpretate the reason behind electrically conductive shape memory properties. Cross sectional FE-SEM images of GO filled epoxy (0.1 to 0.4 wt. % loading) after electrically conductive shape memory testing at 80 V are shown in Figure 6. It shows different morphological changes at different wt. % loadings. At the initial stages of 0.1 and 0.2 wt. % loading (Figure 6 a and b), the GO sheets are properly embalmedand exfoliated with epoxy matrix. But at 0.3 and 0.4 wt. % loading (Figure 6 c and d), there is availability of higher amount of GO with distributed and aggregated nature towards the whole polymer matrix and it provides a higher and fast electrical conductivity. So, the electrical actuation in the case of 0.4 wt.% loading is higher than lower wt. % loadings (0.1 and 0.2. wt. %). But in our earlier research work of GO filled epoxy and their thermally water sensitive shape memory behavior, it was found that GO creates better stacking galleries in epoxy at lower loading (0.2 wt. %) and that have generate a specific and rapidly thermally water sensitive properties as compared to 0.3 and 0.4 wt. % loading [30].
In the case of functional analysis, FTIR was used to characterize and differentiate between composites with electrically conductive shape memory polymer composites. From the FTIR spectra (Figure 7), no much consistent difference was observed in GO filled epoxy at 0.1 to 0.4 wt. % loading; and suggested that only physical changes were found in composites. Figure 7(d) shows the strong and broad peak at 3360 cm-1 in 0.3 wt. % indicating the presence of surface O–H stretching due to the vibrations of the H-O-H groups of water. The other peaks correspond to oxygen functional groups, such as carboxyl (C=O) stretching of COOH groups (1725 cm-1), aromatic (C=C) stretching (1615 cm-1), epoxy (C–O) group stretching (1218 cm-1) and alkoxy (C–OH) group stretching vibrations (1043 cm-1) indicate the presence of GO.
Thermal properties of composites
Figure 8 shows TGA curves of the neat epoxy and its composites with 0.1 to 0.4 wt. % loading. As can be seen from the curves, all the samples begin to decompose at 370-390 °C after electrical active shape memory testing and degrade almost completely below 445 °C due to the pyrolysis of polymer mainchains. It should be noted that the thermal stabilization that is onset thermal degradation temperature (don) of the composites was observed to be increased with increase in GO loading (Figure 8 and Table 5). The higher don degradation temperature was recorded as 391 °C and offset thermal degradation temperature (doff) as 447 °C for 0.3 wt. % loadings (Table 5). In case of thermal properties of the GO filled epoxy, it is observed that don and doff temperature were increased with increase in weight loss before any actuation [18]. But after the electrical actuations, don and doff were observed to be increased with increase in GO loading with decrease in weight loss as compared to characterization of thermal properties before electrical actuation. This happened when electric current passes through the higher amount of GO, there is occurrence of electrical resistive heating in the GO. The resistive heating is transferred from the GO to the epoxy matrix resulting from the temperature difference [32].
Conclusion
This research work presented a new approach to significantly improve the electrical actuated epoxy shape memory of epoxy nanocomposites embedded with GO. With the varying amount of GO, the electrically actuated shape memory property increased with increase in both GO wt. % loading and electric voltage.The actual electrically actuated shape memory property at 0.4 wt. % loading is 180 S, 140 S and 95 S with 40 V, 60 V and 80 V as compared to 0.3 wt. % loading (280 S, 160 S and 110 S for 40 V, 60 V and 80 V respectively).The demonstrated experimental shape memory results showed that GO filled epoxy composites have potential electrical conductivity.Which will be beneficial for their applications in a shape recovery forces output.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/
For more articles in Academic Journal of Polymer Science please click on: https://juniperpublishers.com/ajop/index.php
For more about Juniper Publishers Please click on: https://juniperpublishersblog.wordpress.com/
0 notes
Text
Cationic Polymerization of Para-Methyl Styrene

Abstract
Cationic polymerization of p-methyl styrene by phenacyl triphenyl phosphonium, phenacyl triphenyl arsonium and p, p`-bis[(triphenylphosphonio) methyl benzophenone salts [1-3], and cation radical salt of p-substituted triphenylamine having different p-substilments was carried out photochemically and/or thermally. Cation radical amine salts were found to be more effective thermally in comparison to phosphonium or arsonium salts. The effects of initiator structure, counter ion, concentrations of salts and reaction conditions on the rate of polymerization in dichloromethane on the isolated polymer will be presented Figure 1-3.
Keywords: Phenacyl triphenylphosphonium salts; Phenacyl triphenylparsonium salts; tri p-substituted triphenyl amine salts; p-methyl styrene; photo polymarization; thermal polymarization
Introduction
Poly (p-methyl styrene), PMS has excellent potential for a broad range of applications in industrial products, for example, reinforced plastic composites achieve higher thermal stability when PMS substituted for styrene [1]. The reactivity of para-substituted styrene towards cationic polymerization depends on the type of substituent with the following order; OCH3> CH3 > H > Cl have been established. Phase transfer catalyzed chlorination of poly (p-methyl styrene) occurs by the action of commercial aqueous sodium hypochlorite solution to produce the chloromethylated polystyrene, which is a key intermediate in the preparation of anion exchange resins [2], and for lithographic evaluation [3-5].
phosphonium salts such as p, p`-bis [(triphenylphosphonio)methyl] benzophenone are reported to be useful photoinitiators for the cationic polymerization of epoxide and vinyl monomers [6]. Recently, we reported on the use of phenacyltriphenyl phosphonium and arsonium salts as photoinitiators for the cationic polymerization of cyclohexene oxide [7], and styrene [8] and p-methylstyrene [9].
This paper will further examine the photoinitiated polymerization of p-methyl styrene by onium salts [1-3] and thermally by cation radical salts [4-6] at room temperature, and in dichloromethane.
Experimental Procedures
Onium salts preparations and characterizations were performed as reported previously [7]. The bromide salts were converted to the required photo initiator by adding 0.1 mole of each salt in 150mL of water to a mole of the KPF6 or KPF6 dissolved in 50mL of water. Para-methyl styrene, and dichloromethane (Fluka) were dried over calcium hydride and distilled prior to their use. Acetone (Fluka) “AnalaR” grade was used as received.
Spectroscopic measurements
Ultraviolet spectra were obtained on a Cary-2300 spectrophotometer. Infrared spectra were recorded on a Nicolet 50xB FT-IR spectrophotometer. NMR spectra were taken in CDCl3, on a XL- 200 pulsed Fourier transform NMR spectrometer with tetramethyl silane as the internal standard.
Photopolymerization by onium salts (1-3)
Photoinitiated polymerization was carried out in a 15mm diameter Pyrex tube using a tight syringe for monomer addition. A homogeneous solution was formed. The reaction tubes were then closed with rubber septum, and irradiation was carried out using a merry-go-round photo reactor, Model RPR 100, obtained From the Southern New England Company. Inside the reactor’s barrel was a “merry-go-round” holder, which rotate continuously by a motor, and was surrounded by sixteen Honovia 450 watt, medium pressure mercury lamps placed at a distance of 5cm from the sample. The samples were placed in the holder, and were irradiated for the required period, using a light source of 350 nm wavelength. Poly (p-methylstyrene) was precipitated by addition of methanol, filtered, dried and weighed. From the polymer masses, the rate of polymerization was determined gravimetrically. The polymerization of p-methylstyrene by salts (1-3) is shown in Figure 4.
Thermal polymerization by cation radical salts (4-6)
Tris-p-substituted triphenylamines were fairly readily oxidized to form stable cation- radicals, and polymerization of cationically susceptible epoxide and vinyl monomers have been carried out by stable soluble, tris-(p-Bromotriphenyl) amine cation radical salts having stable counter anion such as SbF6 - [10,11], which are prepared as shown in Figure 3.
Solution of the isolated salts [4-6] was found to be stable in dichloromethane, for a period of several days under laboratory conditions. The relative stability of these salt solutions in dichloromethane and under normal laboratory conditions can be arranged as follows: salt 4 > salt 5 > salt 6 Figure 6.
Polymerization procedure
Selected amounts of monomer and initiator solution, in dichloromethane were placed into a Pyrex tube closed with rubber septum and flushed with nitrogen. The tube was placed on a holder and left immersed for the required period in a water bath held at 25oC. Rapid addition of initiator was achieved by syringe injection. The polymer was precipitated into methanol, filtered, dried, and weighed. Polymer yield percentage and rate of polymerization were determined gravimetrically [9]. The number averaged molecular weight (Mn) of the isolated polymer was measured in toluene on a “Hewlett Packard” (Model 501), and High-Speed Membrane Osmometer in toluene. Molecular weight distributions were determined using a “Waters Associates” (Model 200) GPC, fitted with differential refractometer detector. The system was examined at 25oC in THF with a solvent flow rate of 1.0mL/min and a polymer concentration of 1.0mg/mL. Molecular weights were calculated with reference to polystyrene standards.
Results and Discussion
For polymerization by onium salt (3) PMS obtained after 30minutes, the weight average molecular weight, MW was found to be 111, 287g per mole, and the number average 41, 680 g per mol, Mw/Mn = 2.67. In the case of phenacyltriphenyl phosphoniu and Arsonium salts, Bronsted acid is most likely to be the initiating species. Upon photolysis of these salts, the resonance stalilized ylide and protons are formed according to Figure 5. The suggested mechanism by which photopolymerization of p-methylstyrene initiated by the use of onium salts is given in figure below.
Figure 6 compares the efficiency of cation radical salts [4-6], under the same conditions. The reaction mixture contained 2.53M monomer and 3.3 x 10-4M in dichloromethane. The results in Figure 6 show that the efficiency of cation- radical salts (4-6) fall in the following sequences: salt 6 > salt 5 > salt 4. Photolysis of reaction solution containing the monomer and these cation radical salts in the concentration range reported, changed the reaction mixture color from blue to orange/red upon photolysis of salt 5 and salt 6, and low polymerization, while solution of salt 4 showed significant increase in the amount of polymer obtained. These results suggested that photolysis of salt 5 and 6 leads to splitting of the Br anion, which terminated the propagated chains at the early stages of the reaction. Interaction of the cation radical salt with the monomer is the key step in the polymerization process, as polymer chains would grow following thermal initiation by both the protonic acid (H+) produced and the amine ended carbonium ion. Glass transition temperature of PMS obtained by salt (6) after 6 minutes was 381.750K, the weight average for the same sample was 68,766g/mole, the number average was 27, 022g/mol, and the Mw/Mn = 2.54.
Conclusion
Triphenyl phosphonium, arsonium and cation radical salts were found to be effective thermal initiators for the cationic polymerization of p-methyl styrene. The polymerization was initiated thermally when the cation radical salts were utilized, and photochemically when phosphonium and arsonium salts were used.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/
For more articles in Academic Journal of Polymer Science please click on: https://juniperpublishers.com/ajop/index.php
For more about Juniper Publishers Please click on: https://juniperpublishersblog.wordpress.com/
2 notes
·
View notes
Text
Elution Dynamics of Adsorption: Comparing the Solutions of Direct and Inverse Problems

Abstract
Studies of the elution dynamics of adsorption have revealed a close correspondence between the analytical solutions to (i) a direct problem formulated using an equilibrium adsorption layer model and (ii) an inverse problem formulated using the method of moments.
Keywords: Elution Dynamics; Adsorption; Charcoal; chromatograph; Numerical Equations
Introduction
The effects of adsorption dynamics on the elution peak profile for an arbitrary adsorption isotherm were previously analyzed in [1] using numerical simulations based on a model that assumed a layer of equilibrium adsorption (LEA model) [2]. Assuming linear adsorption dynamics, [1] revealed that the elution curves simulated using the LEA model agreed well with experimental data obtained for a given integer unit of relative adsorbent bed length (n). The solution parameters to the inverse problem, formulated using the method of moments for integer numbers [3] and expressed in units of relative adsorbent bed length, was found to correspond to the retention and spreading parameters in the LEA model simulation studies. For example, the adsorption dynamics of ethyl chloride on activated charcoal (with bed lengths of 1 and 5 in n units) [4] or halogen hydrocarbons on activated charcoal (n = 1, 2, 3) [5] were found to agree. In parallel, the analysis of elution curves measured from non-unitary adsorbent beds (n = 0.78, 1.54, etc.) by the method of moments [5] was also found to agree with the results obtained from integer-length beds. Solutions to the discrete LEA model use equations that include a factorial term. Previously, solutions to the direct adsorption dynamics problems formulated using the LEA model [6] were shown to be applicable over the real numbers n if the factorial term in the elution curve equation was approximated by the gamma function. The purpose of this study was to compare the solutions of the direct and inverse problems associated with the adsorption dynamics, formulated in terms of the LEA model and method of moments, respectively.
Discussion
The elution curve in the LEA model can be described as [1]
where c(t) is the concentration on the adsorbent bed, equal to n, t is time, n=L/Le is the relative length of the adsorbent bed, L is the absolute length of the bed, Le is the effective kinetic constant of the LEA model, c0 is the maximal concentration determined by the amount of solute added, .)/(1ebuL=+Γ, u is the linear velocity of the mobile phase, and Γ is Henry’s adsorption constant. An equation similar to (1) has been applied in plate theory [7] and may be used to describe chromatographic processes under the approximation that n→∞, for concentration functions represented by a Gaussian curve. Equation (1) differs from most equations used in plate theory because n-1 is taken to be the exponent and the factorial, in place of n. The method of moments [3] was proposed as a method for solving the inverse chromatography problem, and it is used in many techniques, such as statistics. The use of this method in modeling adsorption dynamics proposes calculation of two statistical moments. A first equation is used to define the position of the center of gravity tc of the experimental curve,
where time tc defines the adsorption Henry’s constant.
A second central moment μ2 is defined by deviations and provides a measure of the spread in the experimental curve,
The mathematical equality along with equation (1) easily yields an expression for the moments in the LEA model,namely. These equations are described here for the first time and were verified by comparison with experimental data.
(Figure 1) plots the values of tc and μ2 , calculated as reported previously [5] from the experimental elution profiles corresponding to the adsorption of CF3Cl on activated charcoal beds of varying lengths. The curves shown in (Figure 1) were fit using a linear approximation to yield the equations:
The slopes of these numerical equations were given by b, and in all cases were found to be equal to b= 0.41.
Recently the same data were analyzed without points tc and μ2 at n=0 [8]. The result of approximation is not differed strongly: 2.46. 0.02( 2 0.998) c t= n + R =and μ2 = 6.04.n + 0.12(R2 = 0.993) and in all cases b= 0.41. These results demonstrated that the LEA model and the method of moments are homologous, and they describe and predict the linear adsorption dynamics, including for adsorbent beds of short length.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/
For more articles in Academic Journal of Polymer Science please click on: https://juniperpublishers.com/ajop/index.php
For more about Juniper Publishers Please click on: https://juniperpublishersblog.wordpress.com/
0 notes
Text
Happy Thanksgiving Day – Juniper Publishers

Wishing you hope, joy, peace, good health, favor, and love on this Thanksgiving Day!
1 note
·
View note
Text
Polymeric Based Materials via Palladium- Catalyzed Polycondensation

Abstract
The significant access to the structurally pure, high molar mass, N-containing polymers via palladium-catalyzed polycondensation of various aromatic diamines with various aromatic dibromo compounds is an interesting target. One of the major advantages is to apply inexpensive monomers; aromatic amines and aromatic bromides. Furthermore, many diamines are readily available or easy to synthesize giving fast access to numerous new functionalized polymers either by applying amino-functionalized or bromo-functionalized monomers to optimize and improve the polymer properties. With the increasing demand for different functionalized polymers, the focus of relevant studies has been to generate polymers with improved properties by palladium-catalyzed polycondensation. A series of poly(imino ether)s (PIEs), poly(imino ketone)s (PIKs), poly(imino azobenzene)s (PIAzos), poly(imino acridine)s (PIAcs), poly(imino fluorenone)s (PIFOs), and poly(imino carbazole)s (PICs) have been fabricated via palladium-catalyzed polycondensation of different dibromides with different aromatic diamines.
Keywords:Poly(imino ether)s; polycondensation; poly(imino ketone)s; poly(imino azobenzene)s; poly(imino acridine)s; poly(imino fluorenone)s; poly(imino carbazole)s.
Introduction
In the past few years, various categories of new materials such as copolymer/bentonite [1-5], copolymer/kaolinite [6-8], copolymer/pyrogenic silica [9,10], terpolymer/bentonite [11,12], and terpolymer/kaolinite [13,14] composites have been reported due to their distinctive chemistry and physical properties. Moreover, core-shell nanocomposites (CSNCs) based on TiO2 [15-17], Fe2O3 [18], and Al2O3 [19,20] nanoparticles have attracted enormous research interest. The Pd-catalyzed arylation amination reaction is a broadly useful method for the construction of a wide variety of aromatic polymers in a one-pot synthesis [21-24]. Consequently, the corresponding high-performance polymers containing varied functionality were constructed. The careful optimization of reaction conditions and reagents, supplemented by a sound mechanistic understanding of both organic and organometallic chemistry was a key to this approach.
Discussion
In continuation of our work, a series of poly(imino ether)s (PIEs), poly(imino ketone)s (PIKs), poly(imino azobenzene)s (PIAzos), poly(imino acridine)s (PIAcs), poly(imino fluorenone)s (PIFOs), and poly(imino carbazole)s (PICs) have been fabricated via palladium-catalyzed polycondensation of various dibromides with various aromatic diamines [21-24]. The catalytic system generated from tris(dibenzylideneacetone)dipalladium (0) [Pd2(dba)3] and 2,2´-bis(diphenylphosphino)-1,1´-binaphthyl (BINAP) has been applied. The chemical inertness, thermal stability, and low dielectric constant characteristics of the polymer materials make them attractive for advanced technology applications since aromatic polyelectrolytes containing azobenzene have photoresponsive properties [22]. The Pd-catalyzed system may be used to synthesize these polyelectrolytes in a one-pot reaction. It is anticipated that future studies might not only improve the scope and understanding of this process but will lead to new types of interesting polymers followed by their new applications by using Pd-catalyzed cross-coupling reactions. One can use cheaper monomers e.g. chloro- or bromo-functionalized aromatic ketones instead of the corresponding difluoro structures. Also using the nitro group leading to the generation of nitrite ions that become reactive and cause side reactions at elevated temperature as is the case for the preparation of poly(arylene ether)s is avoided. Furthermore, many diamines and dibromo compounds are readily available or easy to synthesize giving fast access to numerous new polymers with optimized properties such as thermal stability, mechanical behavior, or solubility.
Conclusion
An efficient approach aimed at producing a series of promising high-performance polymers were successfully achieved by palladium-catalyzed polycondensation. Soluble PIEs, PIKs, PIAzos, PIAcs, PIFOs, and PICs with high molecular weights have been obtained by Pd-catalyzed polycondensation of aromatic dibromides and aromatic diamines. Numerous PIEs, PIKs, PIAzos, PIAcs, PIFOs, and PICs have been fabricated by using different monomers. Pd/BINAP catalyst system is found to be an effective catalyst for the cross-coupling of aromatic diamines with the aromatic dibromides achieving high yields with high molecular weight polymers. Most of these polymers are characterized by high solubility in aprotic solvents and high thermal stability. The amorphous nature of these polymers was proven by X-ray diffraction and was also reflected in their good solubility. The resulting PIEs, PIKs, PIAzos, PIAcs, PIFOs, and PICs are likely to have interesting electronic and chemical properties.
All PIEs, PIKs, PIAzos, PIAcs, PIFOs, and PICs were fully characterized by Fourier transform infrared spectroscopy, elemental analyses, differential scanning calorimetry, thermogravimetric analyses, gel permeation chromatography, and X-ray diffraction. These polymers exhibit high thermal stability and high decomposition temperatures. A model compound for each case has been fabricated and characterized by standard spectroscopic methods to confirm the proposed structure and to assist in the structure proof of the polymer. Most of the objectives of these researches with good characterizations have been achieved. The Pd-catalyzed polycondensation technique familiarizes a valuable setup to produce new high-performance polymers obsessed with varied functionality.
For more Open Access Journals in Juniper Publishers please click on: https://juniperpublishers.com/
For more articles in Academic Journal of Polymer Science please click on: https://juniperpublishers.com/ajop/index.php
0 notes