Kerone are offered the complete heating solution like as heating, cooling & drying in Mumbai, India. http://www.kerone.com
Don't wanna be here? Send us removal request.
Text

Best wishes to all for the beautiful day of Eid Al-Adha ! May the almighty fill your life with bliss, peace, good health & prosperity. May he show the path to rise above the self and to serve humanity with compassion, love and the spirit of sacrifice. #Kerone #eidmubarak2023 #EidAlAdha
0 notes
Text

May the festival of Baisakhi bring you good fortune, success, and happiness in all your endeavours! Wishing you a joyous spring season. Happy Baisakhi.
0 notes
Text

May God gift you all the colours of life, colours of joy, colours of happiness, colours of friendship, colours of love and all other colours you want to paint your life in. Happy Holi. #holi #kerone #happyholi
0 notes
Text

On this special occasion, let us make a promise to our motherland that we will do all we can to enrich and preserve our heritage and our national ethos. Wishing you all a very Happy Republic Day 2023! #Kerone #repulicday #repulicday2023
0 notes
Text
Industrial Revolution in the Manufacturing Industry
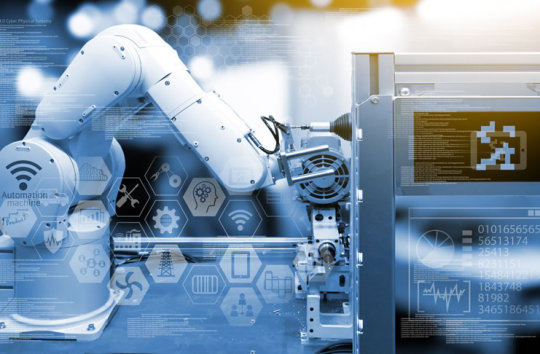
Textiles were the dominant industry of the industrial Revolution in terms of employment, worth of output and capital invested with. The textile industry was conjointly the primary to use modern production methods.
The Industrial Revolution began in great United Kingdom, and many of the technological innovations were of British origin. By the mid-18th century United Kingdom was the world’s leading business nation, controlling a world trading empire with colonies in North America and also the Caribbean, and with major military and political hegemony on the Indian subcontinent, notably with the proto-industrialised Mughal Bengal, through the activities of the east India Company. The development of trade and also the rise of business were among the main causes of the industrial Revolution.
The earliest recorded use of the term “Industrial Revolution” seems to have been in a letter from 6 July 1799 written by French envoy Louis-Guillaume Otto, saying that France had entered the race to industrialise. “The plan of a replacement social order supported major industrial change was clear in Southey and Owen, between 1811 and 1818, and was implicit as early as Blake within the early 1790s and words worth at the turn of the [19th] century.” The term industrial revolution applied to technological amendment was becoming a lot of common by the late decade, as in Jérôme-Adolphe Blanqui’s description in 1837 of la révolution industrielle.
Six factors facilitated industrialization: high levels of agricultural productivity to produce excess workforce and food; a pool of managerial and entrepreneurial skills; accessible ports, rivers, canals and roads to cheaply move raw materials and outputs; natural resources like coal, iron and waterfalls; political stability and a legal system that supported business; and financial capital available to invest.
The commencement of the industrial Revolution is closely connected to a small variety of innovations, starting within the second half of the eighteenth century. By the 1830s the following gains had been made in important technologies:
Textiles – mechanised cotton spinning powered by steam or water increased the output of a worker by a factor of around 500. The power loom increased the output of a worker by a factor of over 40. The cotton gin increased productivity of removing seed from cotton by a factor of 50. Large gains in productivity also occurred in spinning and weaving of wool and linen, but they were not as great as in cotton.
Steam power – the efficiency of steam engines increased so that they used between one-fifth and one-tenth as much fuel. The adaptation of stationary steam engines to rotary motion made them suitable for industrial uses. The high pressure engine had a high power to weight ratio, making it suitable for transportation. Steam power underwent a rapid expansion after 1800.
Iron making – the substitution of coke for charcoal greatly lowered the fuel cost of pig iron and wrought iron production. Using coke also allowed larger blast furnaces, resulting in economies of scale. The steam engine began being used to pump water and to power blast air in the mid-1750s, enabling a large increase in iron production by overcoming the limitation of water power. The cast iron blowing cylinder was first used in 1760. It was later improved by making it double acting, which allowed higher blast furnace temperatures. The puddling process produced a structural grade iron at a lower cost than the finery forge. The rolling mill was fifteen times faster than hammering wrought iron. Hot blast (1828) greatly increased fuel efficiency in iron production in the following decades.
Invention of machine tools – The first machine tools were invented. These included the screw cutting lathe, cylinder boring machine and the milling machine. Machine tools made the economical manufacture of precision metal parts possible, although it took several decades to develop effective techniques.
The Industrial revolution has been criticised for complete ecological collapse, inflicting mental illness, pollution and unnatural systems of organizing for humanity. Since the beginning of the industrial revolution individuals have criticised it by stating the industrial Revolution turned humanity and nature into slaves and destroying the world. It’s also been criticised by valuing profits and company growth over life and wellbeing, multiple movements have arose philosophically against the industrial revolution and include groups like the Amish and Primitivism.
We at KERONE have a team of experts to help you with your need in various products range from our wide experience.
0 notes
Text

Warm wishes on the occasion of Makar Sankranti! May this festive occasion bless you with abundance and prosperity in life. Happy Makar Sankranti 2023! #kerone #makarsankranti2023 #pongal #uttrayan #bhogifestival
0 notes
Text
Importance and applications of Industrial Minerals

Industrial resources (minerals) are geological materials that are mined for their industrial worth, that are not fuel (fuel minerals or mineral fuels) and aren’t sources of metals (metallic minerals) but are utilized in the industries based on their physical and/or chemical properties. they’re utilized in their natural state or after beneficiation either as raw materials or as additives in a very wide range of applications.
Industrial minerals could also be defined as minerals mined and processed (either from natural sources or synthetically processed) for the value of their non-metallurgical properties, that provides for their use in a particularly wide range of industrial and domestic applications. As a general rule, they’ll also be defined as being non-metallic, non-fuel minerals.
Obvious examples of naturally occurring industrial minerals include:
clays
sand
talc
limestone
gypsum
pumice
potash
Other examples of natural industrial minerals include minerals that also have a metallurgical as well as non-metallurgical value, such as:
bauxite (aluminium metal + bauxite used in cements, abrasives, refractories & alumina source for many applications)
chromite (chrome metal & ferrochrome alloy + foundry sand, chemicals, pigments)
rutile (titanium metal + white pigment for paints, paper, plastics)
zircon (zirconium metal + source of zirconia for ceramics, glass)
manganese (manganese metal + source of manganese dioxide for batteries, pigments)
stibnite (antimony metal + source of antimony trioxide used as flame retardant)
Quartz (silicon metal + source of silica in glass, ceramics, fillers).
There are also synthetic industrial minerals that are factory-made from natural minerals. Artificial minerals are usually processed as a result of the inferior characteristics and/or scarcity of their natural counterparts.
Quite frankly, without industrial minerals, an enormous range of everyday domestic and important industrial product would simply not exist. In a median 9-5 working day you’ll probably acquire contact with a minimum of 100 things that are factory-made from industrial minerals.
A useful example is a quick examination of your home kitchen to see just how important industrial minerals are to our everyday environment. Industrial minerals in your kitchen:
In essence, wherever there is demand for these industrial and domestic applications, i.e. a market, this will create a trading business specific to that market. The crucial point is that the pattern of industrial minerals trade is utterly dictated by the needs of the population and the performance of the economy, and then combined with mineral availability.
As an industrial minerals consultant once said: “Without a market, an industrial mineral deposit is merely a geological curiosity”. So, put simply, no market demand = no mineral development = no mineral trade.
Mineral consuming market existence and its performance directly affects demand, and therefore trade, for mineral raw materials
The route of a mineral from mine to market may involve more than one stage, i.e. its consumption in manufacturing an intermediate mineral or end product, which is then consumed in the manufacture of another end product, which is then sold to an end-use market.
Many industrial minerals can serve a range of markets, which also impacts the pattern of minerals trade in that a single mineral source can supply several different customers owing to market type, as well as market geography.
For example, bentonite sourced in Wyoming travels to domestic and overseas population centres owing to its widespread use as an absorbent in cat litter products. However, its equally important use as a major component in drilling fluids means that it is also freighted to centres of oil and gas drilling activity, eg. Gulf of Mexico.
Typical examples of industrial rocks and minerals are limestone, clays, sand, gravel, diatomite, kaolin, bentonite, silica, barite, gypsum, and talc. Some examples of applications for industrial minerals are construction, ceramics, paints, electronics, filtration, plastics, glass, detergents and paper.
In some cases, even organic materials (peat) and industrial products or by-products (cement, slag, silica fume) are categorised underneath industrial minerals, further as metallic compounds mainly used in non-metallic type (as AN example most titanium is used as AN oxide TiO2 instead of Ti metal).
The analysis of raw materials to see their suitability to be used as industrial minerals needs technical test-work, mineral processing trials and end-product analysis; free to transfer evaluation manuals are accessible for the following industrial minerals: limestone, flake graphite, diatomite, kaolin, clay and construction materials.
The best way to see who is involved in the industrial minerals business is to examine the mine to market supply chain.
All industrial minerals are mined (surface and underground) and so undergo processing to refine the crude mineral ore into a processed grade or series or grades for sale to the market. These are then transported from the source to a different plant for further process, or directly to the consuming markets.
We at KERONE have experience of 47+ years in helping the industries with their needs. We at KERONE have a team of experts to help you with your need for Industrial Minerals in various products range from our wide experience.
0 notes
Text

May this New Year be full of new dreams and new hopes for you. May you enter this year with smiles on your face and with the love of your dear ones Happy New Year..!!
kerone #HappyNewYear #newyear2023
0 notes
Text

Wishing you a merry Christmas and a happy new year filled with blessings, prosperity, and abundance. May you be surrounded by love and warmth during this special time. #kerone #merrychristmas #HappyNewYear #newyear2023
0 notes
Text
Different Methods of Metal Curing

Curing is a chemical process employed in polymer chemistry and process engineering that produces the toughening or hardening of a polymer material by cross-linking of polymer chains. Even if it is strongly associated with the production of thermosetting polymers, the term curing can be used for all the processes where starting from a liquid solution, a solid product is obtained.
During the curing process, single monomers and oligomers, mixed with or without a curing agent, react to form a tridimensional polymeric network.
In the initial part of the reaction branches molecules with numerous architectures are formed, and their molecular weight will increase in time with the extent of the reaction till the network size is up to the size of the system. The system has lost its solubility and its viscosity tends to infinite. The remaining molecules begin to be with the macroscopic network till they react with the network creating different crosslinks. The crosslink density will increase until the system reaches the end of the chemical reaction.
Curing can be initiated by heat, radiation, electron beams, or chemical additives. To quote from IUPAC: curing “might or might not require mixing with a chemical curing agent.
“Thus, two broad classes are
Curing induced by chemical additives (also called curing agents, hardeners).
Curing in the absence of additives. An intermediate case involves a mixture of resin and additives that requires external stimulus (light, heat, radiation) to induce curing.
The curing methodology depends on the resin and the application. Particular attention is paid to the shrinkage induced by the curing. Usually small values of shrinkage (2-3%) are desirable.
Curing induced by additives
Epoxy resins are typically cured by the use of additives, often called hardeners. Polyamines are often used. The amine group’s ring-open the epoxide rings.
In rubber, the curing is also induced by the addition of a cross linker. The resulting method is termed Sulfur vulcanization. Sulfur breaks down to form polysulfide cross-links (bridges) between sections of the polymer chains. The degree of crosslinking determines the rigidity and durability, similarly as different properties of the material.
Paints and varnishes usually contain oil drying agents; metal soaps that catalyse cross-linking of the unsaturated oils of which they’re largely comprised. As such, once paint is described as drying it’s infact hardening. Oxygen atoms serve the crosslinks, analogous to the role played by sulfur within the vulcanization of rubber.
Curing without additives
In the case of concrete, curing entails the formation of silicate crosslinks. The process is not induced by additives.
In several cases, the resin is provided as a solution or mixture with a thermally-activated catalyst, that induces crosslinking but solely upon heating. for example, some acrylate-based resins are formulated with dibenzoyl peroxide. Upon heating the mixture, the peroxide converts to a free radical, which adds to an acrylate, initiating crosslinking.
Some organic resins are cured with heat. As heat is applied, the viciousness of the resin drops before the onset of crosslinking, whereupon it will increase because the constituent oligomers interconnect. This method continues till a tridimensional network of oligomer chains is formed – this stage is termed gelation. In terms of processability of the resin this marks a very important stage: before gelation the system is relatively mobile, after it the quality is incredibly limited, the micro-structure of the resin and also the composite material is fixed and severe diffusion limitations to further cure are created. Thus, in order to achieve vitrification in the resin, it’s typically necessary to increase the process temperature after gelation.
When catalysts are activated by ultraviolet radiation, the process is called UV cure.
Different Monitoring Methods:
Rheological analysis
Thermal analysis
Dielectrometric analysis
Spectroscopic analysis
Ultrasonic analysis
We at KERONE have a team of experts to help you with your need for Curing in various products range from our wide experience.
0 notes
Text
Application and Popular Uses of Graphite

Graphite, archaically referred to as plumbago, is a crystalline type of the element carbon with its atoms organized in a very hexagonal structure. It happens naturally during this kind and is the most stable kind of carbon under standard conditions. Under high pressures and temperatures it converts to diamond. Graphite is utilized in pencils and lubricants. It’s a good conductor of heat and electricity. Its high conductivity makes it helpful in electronic product like electrodes, batteries, and solar panels.
The principal types of natural graphite, each occurring in different types of ore deposits, are
A crystalline small flake of graphite (or flake graphite) occurs as isolated, flat, plate-like particles with hexagonal edges if unbroken. When broken the edges can be irregular or angular;
Amorphous graphite: very fine flake graphite is sometimes called amorphous;
Lump graphite (or vein graphite) occurs in fissure veins or fractures and appears as massive platy intergrowths of fibrous or acicular crystalline aggregates, and is probably hydrothermal in origin.
Highly ordered pyrolytic graphite refers to graphite with an angular spread between the graphite sheets of less than 1°.
The name “graphite fiber” is sometimes used to refer to carbon fibres or carbon fiber-reinforced polymer.
Graphite occurs in metamorphic rocks as a result of the reduction of sedimentary carbon compounds during metamorphism. It also occurs in igneous rocks and in meteorites. Minerals associated with graphite include quartz, calcite, micas and tourmaline. The principal export sources of mined graphite are in order of tonnage: China, Mexico, Canada, Brazil, and Madagascar.
In meteorites, graphite occurs with troilite and silicate minerals. Small graphitic crystals in meteoritic iron are called cliftonite. Some microscopic grains have distinctive isotopic compositions, indicating that they were formed before the Solar system. They are one of about 12 known types of minerals that predate the Solar System and have also been detected in molecular clouds. These minerals were formed in the ejecta when supernovae exploded or low to intermediate-sized stars expelled their outer envelopes late in their lives. Graphite may be the second or third oldest mineral in the Universe.
Historically, graphite was called black lead or plumbago. Plumbago was commonly used in its massive mineral form. Both of these names arise from confusion with the similar-appearing lead ores, particularly galena. The Latin word for lead, plumbum, gave its name to the English term for this grey metallic-sheened mineral and even to the leadworts or plumbagos, plants with flowers that resemble this colour.
The term black lead usually refers to powdered or processed graphite, matte black in color.
Uses of natural graphite
Natural graphite is mostly used for refractories, batteries, steelmaking, expanded graphite, brake linings, foundry facings and lubricants.
Refractories
Batteries
Steelmaking
Brake linings
Foundry facings and lubricants
Pencils
Other uses
Natural graphite has found uses in zinc-carbon batteries, electric motor brushes, and various specialized applications. Graphite of various hardness or softness ends up in totally different qualities and tones when used as an artistic medium. Railroads would usually combine powdered graphite with waste oil or linseed oil to make a heat-resistant protective coating for the exposed portions of a steam locomotive’s boiler, like the smoke box or lower a part of the furnace.
A high-quality flake graphite product that closely resembles natural flake graphite may be made up of steelmaking kish. Kish may be a large-volume near-molten waste skimmed from the molten iron feed to a basic oxygen furnace, and consists of a mixture of graphite (precipitated out of the supersaturated iron), lime-rich slag, and a few iron. The iron is recycled on site, leaving a mixture of graphite and slag. The simplest recovery method uses hydraulic classification (which utilizes a flow of water to separate minerals by specific gravity: graphite is light-weight and settles nearly last) to urge a 70th graphite rough concentrate. Leaching this concentrate with hydrochloric acid gives a 95th graphite product with a flake size ranging from 10 meshes down.
We at KERONE have a team of experts to help you with your need for Graphite in various products range from our wide experience.
0 notes
Text

On this auspicious occasion, I wish the colour, bliss and beauty of Dussehra be with you and your loved ones throughout the year! Wishing you a very happy Dussehra 2022! #kerone #HappyDussehra
0 notes
Text

On the auspicious occasion of Navratri, we pray that the following days of this festive occasion infuse your life with cheers and smiles. Wishing a very Happy Navratri to you and your family. #kerone #HappyNavratri
0 notes
Text
May Lord Ganesh remove all your hurdles, worries, griefs and negativity. May your life be filled with love, prosperity, success and happiness. Happy Ganesh Chaturthi

0 notes
Text
PET Flakes Crystallizer and Dryer

PET is highly hygroscopic and absorbs moisture from the atmosphere. Tiny amounts of moisture can hydrolyse PET within the melt part, reducing molecular weight. PET should be dry just prior to processing, and amorphous PET needs crystallization prior to drying so that the particles don’t stick together as they’re going through glass transition.
Hydrolysis can occur due to moisture and this often can be seen as a reduction in the IV (Intrinsic Viscosity) of the product. PET is “semi-crystalline”. When the IV is reduced, the bottles are more brittle and tend to fail at the “gate” (injection point) during blowing and filling. It is very possible that due to the initial moisture level in the resin, and the amount removed during vacuum that a significant amount of moisture still remains as it is reaching its melt phase in the extruder.
In its “crystalline” state it has both crystalline and amorphous portions in its molecular structure. The crystalline portion develops where the molecules can align themselves in a very compact linear structure. In the non-crystalline regions the molecules are in a more random arrangement. By insuring that your crystallinity is high, prior to processing, the result will be a more uniform and higher quality product.
Another thing to consider is the number of times the PET has been processed. Each time the PET is processed there is a reduction in IV. Therefore, PET that has been used to make a bottle, recycled and used again, does not have the IV of the original bottle. Each time the bottle is recycled, the IV is further reduced. This is why a percentage of virgin resin is often added to increase the products properties.
Flake size is determined by the grinder. I have seen customers who think that ¼ inch is perfect and those who think ¾ is best. The smaller the flake the more fines will be produced and the better the material will flow in the drying hopper and vacuum chambers. Extremely fine grinds tend to lead to higher pressure drop in the hopper and can cause reduced air flow and uneven distribution. Large grinds can sometimes cause uneven hopper material flow.
60% regrind or more is common in extrusion. Most bottle applications are less than 10% for food and beverage and less than 30% otherwise. It depends on the source of the regrind and the end product. Blow molding is more difficult when you use material with different heat history and IV. Fines can also cause processing problems.
The disadvantage of using regrind versus virgin resin is that regrinds have a heat history and have a significantly lower IV (Intrinsic Viscosity). Lower IV in the finished part causes it to be more brittle/less flexible. The second disadvantage is that there tends to be more “yellowing” in regrind materials that can cause a color or haziness issue.
It is more important to remove the moisture than to heat it. However, it is very difficult to measure moisture on-line in a process so time and temperature is generally used to set the moisture level achieved. For instance, with PET processing, it is generally assumed that if there is 4-6 hours in the drying hopper at 325-350° F, the moisture will be reduced to less than 50 ppm.
After the PET has been formed into a sheet, there isn’t typically any additional pre-conditioning required before thermoforming. The sheet will undergo heat in the thermoforming and as long as it doesn’t approach the melt temperature the process just changes the shape of the already formed sheet and the stresses imparted to the sheet tend to give it strength.
In general, crystallizing master batch can have a lot of drying/crystallizing issues. Unless the quantity is so large that you can afford to purchase a crystallizer, buying crystallized master batch is probably preferred.
We at KERONE have a team of experts to help you with your need for PET flakes crystallizer and dryer in various products range from our wide experience.
0 notes
Text

May this Janmashtami bring happiness to your life and hatred will be far apart from your life. Enjoy the festival with love in your heart and good wishes for others.
Happy Janmashtami..!!
#Kerone #Janmashtami #krishnajanmashtami
0 notes
Text

On the auspicious occasion of Muharram, may the almighty bless everyone with lots of happiness & give strength to face all types of problems in their lives.
My Best Wishes on Muharram!
Kerone #Muharram #muharram2022
0 notes