Student radiographer working in a hospital and hoping to become an Advanced Practitioner in. Um. I haven't decided yet. This blog is for education & entertainment, but I am not an authority! I just love the subject.
Don't wanna be here? Send us removal request.
Text

thought too hard about MRI machines today and had this come to me in a vision
43K notes
·
View notes
Note
Hi! I've seen you talking in the tags about genAI's use for medical imaging - would you up for giving a more detailed explanation? Because I don't really understand how you can have a "predictive" quality to the scan without losing necessary accuracy
So, in MRI we use 'sequences' - i.e., we test the different magnetic properties of the tissues in your body, and how they react to stimuli. I've written a more in-depth explanation here.
There are typically multiple sequences per scan, which is why the scans take sooooooo very long. The basic sequences, like T1/T2, take a couple minutes each. The highly specialised sequences, like NEX-5 neuromelanin sensitive scanning for Parkinson's Disease, can take 16 minutes each (imagine trying to lie still for the duration of that whole scan... which might get up to 90 minutes.... as a patient with a motor disorder that causes involuntary movement 🙃).
Plus, we use 'contrast' - a sort of dye - to better describe pathology, and that contrast is, um. A toxic heavy metal. That can kill you.
(DON'T WORRY IF YOU'RE GOING FOR AN MR SCAN. It's very, very rare that this happens, and only if your renal function is through the floor. But like. Still not ideal!)
So, we want to reduce scan time and contrast use. Thankfully, there is a lot of information contained in a basic-bitch T1/T2/FLAIR sequence that we cannot see with human eyes. The human eye can only differentiate about 50 shades of grey (hahaha) but the subtle differentiations in magnetic resonance that comprise an MR image can number significantly higher.
(In CT, we use a similar theory to select different window widths so we only irradiate you once... but it hasn't been applied to MR yet for various complex reasons)
If you feed a paired 'generator' CNN and a 'discriminator' CNN (typical GAN structure) like, a thousand T1 images and their GBCA counterparts when you're looking at, say, cerebral blood volume in Alzheimer's, the GAN can learn to add the contrast synthetically with very high (90%+!) sensitivity for this disease!
The application of this technology is in its infancy. It's currently limited to specific pathologies. But it's coming along massively, and could hugely improve the lives of anyone who needs frequent MR scans!
18 notes
·
View notes
Text

staghorn calculi, baby!!
if you're in the throes of cosmic despair i cannot recommend museums enough. art or science or history it doesn't matter. oh we're all connected, all of us and everything, throughout all time and space, and no one, no one, no one is alone? awesome. that's what i thought i just wanted to make sure.
33K notes
·
View notes
Text
‘While bats can only sense the outer shapes and textures of their targets, dolphins can peer inside theirs. If a dolphin echolocates on you, it will perceive your lungs and your skeleton. It can likely sense shrapnel in war veterans and fetuses in pregnant women. It can pick out the air-filled swim bladders that allow fish, their main prey, to control their buoyancy.
It can almost certainly tell different species apart based on the shape of those air bladders. And it can tell if a fish has something weird inside it, like a metal hook. In Hawaii, false killer whales often pluck tuna off fishing lines, and “they’ll know where the hook is inside that fish,” Aude Pacini, who studies these animals, tells me. “They can ‘see’ things that you and I would never consider unless we had an X-ray machine or an MRI scanner.”
This penetrating perception is so unusual that scientists have barely begun to consider its implications. The beaked whales, for example, are odontocetes that look dolphin-esque on the outside—but on the inside, their skulls bear a strange assortment of crests, ridges, and bumps, many of which are only found in males.
Pavel Gol’din has suggested that these structures might be the equivalent of deer antlers—showy ornaments that are used to attract mates. Such ornaments would normally protrude from the body in a visible and conspicuous way, but that’s unnecessary for animals that are living medical scanners.’
-Ed Yong, An Immense World
#oh!!!#one has to ponder the implications....#we can already use sonography to bust clots in the brain and so forth#but obviously it's not exactly the prime modality for looking inside bony cavities like the skull
28K notes
·
View notes
Text
They trained an AI model on a widely used knee osteoarthritis dataset to see if it would be able to make nonsensical predictions - whether the patient ate refried beans, or drank beer. It did, in part by somehow figuring out where the x-ray was taken.
The authors point out that AI models base their predictions on sneaky shortcut effects all the time; they're just easier to identify when the conclusions (beer drinking) are clearly spurious.
Algorithmic shortcutting is tough to avoid. Sometimes it's based on something easy to identify - like rulers in images of skin cancer, or sicker patients getting their chest x-rays while lying down.
But as they found here, often it's a subtle mix of non-obvious correlations. They eliminated as many differences between x-ray machines at different sites as they could find, and the model could still tell where the x-ray was taken - and whether the patient drank beer.
AI models are not approaching the problem like a human scientist would - they'll latch onto all sorts of unintended information in an effort to make their predictions.
This is one reason AI models often end up amplifying the racism and gender discrimination in their training data.
When I wrote a book on AI in 2019, it focused on AIs making sneaky shortcuts.
Aside from the vintage generative text (Pumpkin Trash Break ice cream, anyone?), the algorithmic shortcutting is still completely recognizable today.
2K notes
·
View notes
Text
oh, very cool! I'm doing a bunch of research into CNN-generated synthetic MRI at the moment, which is super-cool, so this was dabbling in a new area of the field for me! That sounds incredibly interesting, and a very good example of how this tech can be used as a tool to help practitioners!
Been a while, crocodiles. Let's talk about cad.

or, y'know...

Yep, we're doing a whistle-stop tour of AI in medical diagnosis!
Much like programming, AI can be conceived of, in very simple terms, as...
a way of moving from inputs to a desired output.
See, this very funky little diagram from skillcrush.com.
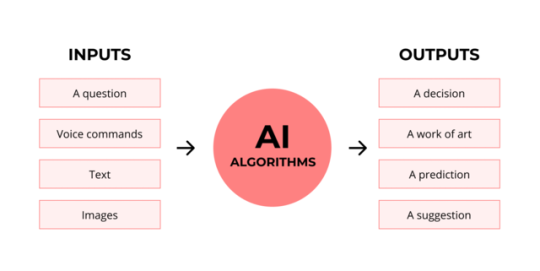
The input is what you put in. The output is what you get out.
This output will vary depending on the type of algorithm and the training that algorithm has undergone – you can put the same input into two different algorithms and get two entirely different sorts of answer.
Generative AI produces ‘new’ content, based on what it has learned from various inputs. We're talking AI Art, and Large Language Models like ChatGPT. This sort of AI is very useful in healthcare settings to, but that's a whole different post!
Analytical AI takes an input, such as a chest radiograph, subjects this input to a series of analyses, and deduces answers to specific questions about this input. For instance: is this chest radiograph normal or abnormal? And if abnormal, what is a likely pathology?
We'll be focusing on Analytical AI in this little lesson!
Other forms of Analytical AI that you might be familiar with are recommendation algorithms, which suggest items for you to buy based on your online activities, and facial recognition. In facial recognition, the input is an image of your face, and the output is the ability to tie that face to your identity. We’re not creating new content – we’re classifying and analysing the input we’ve been fed.
Many of these functions are obviously, um, problematique. But Computer-Aided Diagnosis is, potentially, a way to use this tool for good!
Right?
....Right?

Let's dig a bit deeper! AI is a massive umbrella term that contains many smaller umbrella terms, nested together like Russian dolls. So, we can use this model to envision how these different fields fit inside one another.

AI is the term for anything to do with creating and managing machines that perform tasks which would otherwise require human intelligence. This is what differentiates AI from regular computer programming.
Machine Learning is the development of statistical algorithms which are trained on data –but which can then extrapolate this training and generalise it to previously unseen data, typically for analytical purposes. The thing I want you to pay attention to here is the date of this reference. It’s very easy to think of AI as being a ‘new’ thing, but it has been around since the Fifties, and has been talked about for much longer. The massive boom in popularity that we’re seeing today is built on the backs of decades upon decades of research.
Artificial Neural Networks are loosely inspired by the structure of the human brain, where inputs are fed through one or more layers of ‘nodes’ which modify the original data until a desired output is achieved. More on this later!
Deep neural networks have two or more layers of nodes, increasing the complexity of what they can derive from an initial input. Convolutional neural networks are often also Deep. To become ‘convolutional’, a neural network must have strong connections between close nodes, influencing how the data is passed back and forth within the algorithm. We’ll dig more into this later, but basically, this makes CNNs very adapt at telling precisely where edges of a pattern are – they're far better at pattern recognition than our feeble fleshy eyes!
This is massively useful in Computer Aided Diagnosis, as it means CNNs can quickly and accurately trace bone cortices in musculoskeletal imaging, note abnormalities in lung markings in chest radiography, and isolate very early neoplastic changes in soft tissue for mammography and MRI.
Before I go on, I will point out that Neural Networks are NOT the only model used in Computer-Aided Diagnosis – but they ARE the most common, so we'll focus on them!

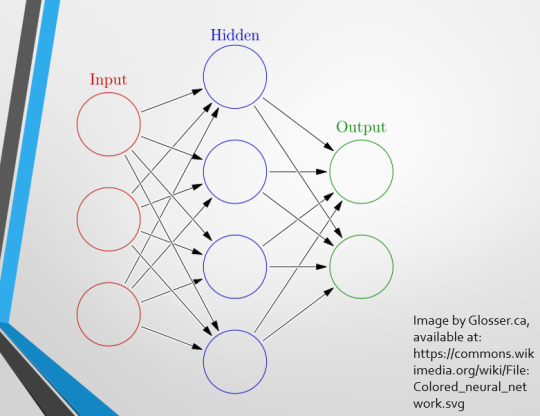
This diagram demonstrates the function of a simple Neural Network. An input is fed into one side. It is passed through a layer of ‘hidden’ modulating nodes, which in turn feed into the output. We describe the internal nodes in this algorithm as ‘hidden’ because we, outside of the algorithm, will only see the ‘input’ and the ‘output’ – which leads us onto a problem we’ll discuss later with regards to the transparency of AI in medicine.
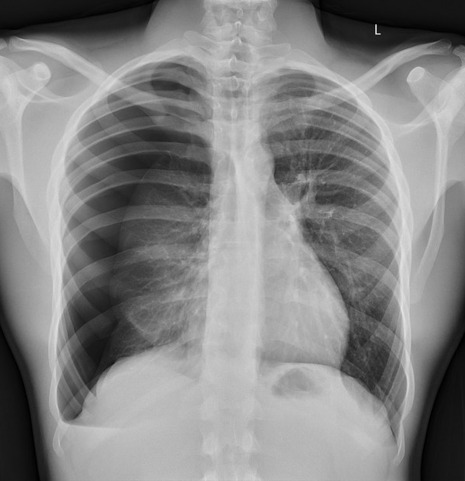
But for now, let’s focus on how this basic model works, with regards to Computer Aided Diagnosis. We'll start with a game of...
Spot The Pathology.
yeah, that's right. There's a WHACKING GREAT RIGHT-SIDED PNEUMOTHORAX (as outlined in red - images courtesy of radiopaedia, but edits mine)
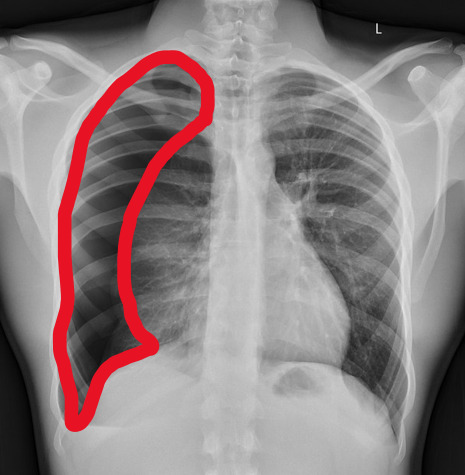
But my question to you is: how do we know that? What process are we going through to reach that conclusion?
Personally, I compared the lungs for symmetry, which led me to note a distinct line where the tissue in the right lung had collapsed on itself. I also noted the absence of normal lung markings beyond this line, where there should be tissue but there is instead air.
In simple terms.... the right lung is whiter in the midline, and black around the edges, with a clear distinction between these parts.
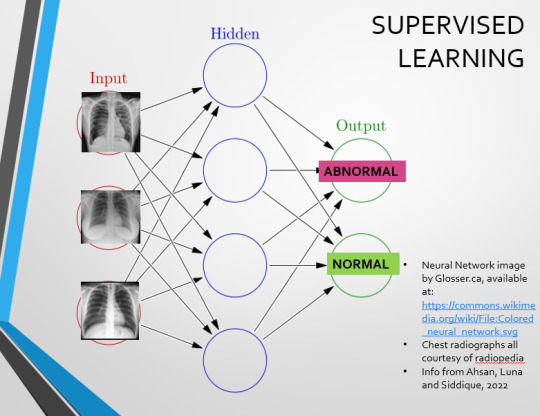
Let’s go back to our Neural Network. We’re at the training phase now.
So, we’re going to feed our algorithm! Homnomnom.
Let’s give it that image of a pneumothorax, alongside two normal chest radiographs (middle picture and bottom). The goal is to get the algorithm to accurately classify the chest radiographs we have inputted as either ‘normal’ or ‘abnormal’ depending on whether or not they demonstrate a pneumothorax.
There are two main ways we can teach this algorithm – supervised and unsupervised classification learning.
In supervised learning, we tell the neural network that the first picture is abnormal, and the second and third pictures are normal. Then we let it work out the difference, under our supervision, allowing us to steer it if it goes wrong.
Of course, if we only have three inputs, that isn’t enough for the algorithm to reach an accurate result.
You might be able to see – one of the normal chests has breasts, and another doesn't. If both ‘normal’ images had breasts, the algorithm could as easily determine that the lack of lung markings is what demonstrates a pneumothorax, as it could decide that actually, a pneumothorax is caused by not having breasts. Which, obviously, is untrue.
or is it?
....sadly I can personally confirm that having breasts does not prevent spontaneous pneumothorax, but that's another story lmao
This brings us to another big problem with AI in medicine –

If you are collecting your dataset from, say, a wealthy hospital in a suburban, majority white neighbourhood in America, then you will have those same demographics represented within that dataset. If we build a blind spot into the neural network, and it will discriminate based on that.
That’s an important thing to remember: the goal here is to create a generalisable tool for diagnosis. The algorithm will only ever be as generalisable as its dataset.
But there are plenty of huge free datasets online which have been specifically developed for training AI. What if we had hundreds of chest images, from a diverse population range, split between those which show pneumothoraxes, and those which don’t?
If we had a much larger dataset, the algorithm would be able to study the labelled ‘abnormal’ and ‘normal’ images, and come to far more accurate conclusions about what separates a pneumothorax from a normal chest in radiography. So, let’s pretend we’re the neural network, and pop in four characteristics that the algorithm might use to differentiate ‘normal’ from ‘abnormal’.
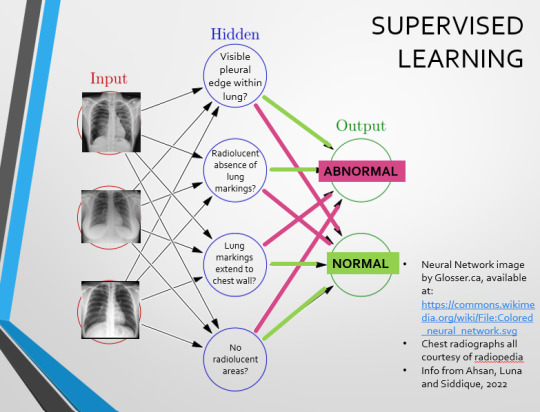
We can distinguish a pneumothorax by the appearance of a pleural edge where lung tissue has pulled away from the chest wall, and the radiolucent absence of peripheral lung markings around this area. So, let’s make those our first two nodes. Our last set of nodes are ‘do the lung markings extend to the chest wall?’ and ‘Are there no radiolucent areas?’
Now, red lines mean the answer is ‘no’ and green means the answer is ‘yes’. If the answer to the first two nodes is yes and the answer to the last two nodes is no, this is indicative of a pneumothorax – and vice versa.
Right. So, who can see the problem with this?
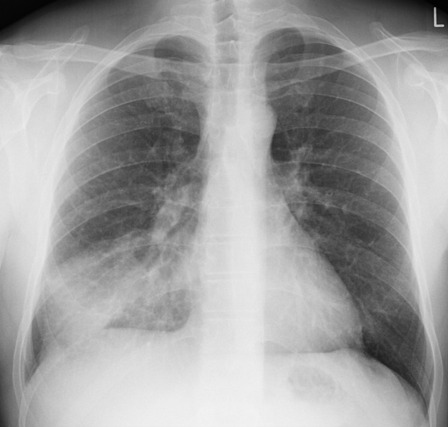
(image courtesy of radiopaedia)
This chest radiograph demonstrates alveolar patterns and air bronchograms within the right lung, indicative of a pneumonia. But if we fed it into our neural network...
The lung markings extend all the way to the chest wall. Therefore, this image might well be classified as ‘normal’ – a false negative.
Now we start to see why Neural Networks become deep and convolutional, and can get incredibly complex. In order to accurately differentiate a ‘normal’ from an ‘abnormal’ chest, you need a lot of nodes, and layers of nodes. This is also where unsupervised learning can come in.
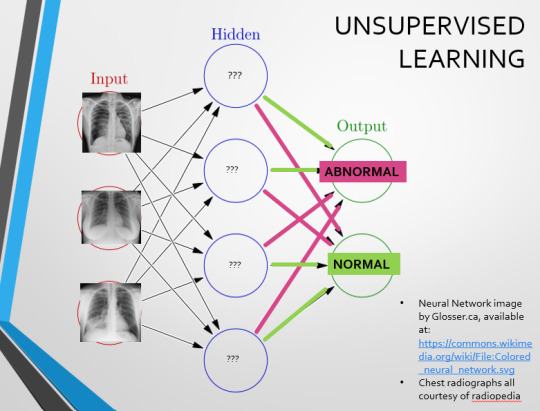
Originally, Supervised Learning was used on Analytical AI, and Unsupervised Learning was used on Generative AI, allowing for more creativity in picture generation, for instance. However, more and more, Unsupervised learning is being incorporated into Analytical areas like Computer-Aided Diagnosis!
Unsupervised Learning involves feeding a neural network a large databank and giving it no information about which of the input images are ‘normal’ or ‘abnormal’. This saves massively on money and time, as no one has to go through and label the images first. It is also surprisingly very effective. The algorithm is told only to sort and classify the images into distinct categories, grouping images together and coming up with its own parameters about what separates one image from another. This sort of learning allows an algorithm to teach itself to find very small deviations from its discovered definition of ‘normal’.
BUT this is not to say that CAD is without its issues.
Let's take a look at some of the ethical and practical considerations involved in implementing this technology within clinical practice!
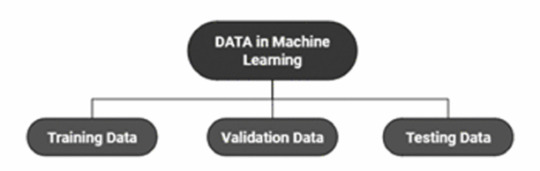
(Image from Agrawal et al., 2020)
Training Data does what it says on the tin – these are the initial images you feed your algorithm. What is key here is volume, variety - with especial attention paid to minimising bias – and veracity. The training data has to be ‘real’ – you cannot mislabel images or supply non-diagnostic images that obscure pathology, or your algorithm is useless.
Validation data evaluates the algorithm and improves on it. This involves tweaking the nodes within a neural network by altering the ‘weights’, or the intensity of the connection between various nodes. By altering these weights, a neural network can send an image that clearly fits our diagnostic criteria for a pneumothorax directly to the relevant output, whereas images that do not have these features must be put through another layer of nodes to rule out a different pathology.
Finally, testing data is the data that the finished algorithm will be tested on to prove its sensitivity and specificity, before any potential clinical use.
However, if algorithms require this much data to train, this introduces a lot of ethical questions.
Where does this data come from?
Is it ‘grey data’ (data of untraceable origin)? Is this good (protects anonymity) or bad (could have been acquired unethically)?
Could generative AI provide a workaround, in the form of producing synthetic radiographs? Or is it risky to train algorithms on simulated data when the algorithms will then be used on real people?
If we are solely using CAD to make diagnoses, who holds legal responsibility for a misdiagnosis that costs lives? Is it the company that created the algorithm or the hospital employing it?
And finally – is it worth sinking so much time, money, and literal energy into AI – especially given concerns about the environment – when public opinion on AI in healthcare is mixed at best? This is a serious topic – we’re talking diagnoses making the difference between life and death. Do you trust a machine more than you trust a doctor? According to Rojahn et al., 2023, there is a strong public dislike of computer-aided diagnosis.
So, it's fair to ask...
why are we wasting so much time and money on something that our service users don't actually want?
Then we get to the other biggie.

There are also a variety of concerns to do with the sensitivity and specificity of Computer-Aided Diagnosis.
We’ve talked a little already about bias, and how training sets can inadvertently ‘poison’ the algorithm, so to speak, introducing dangerous elements that mimic biases and problems in society.
But do we even want completely accurate computer-aided diagnosis?
The name is computer-aided diagnosis, not computer-led diagnosis. As noted by Rajahn et al, the general public STRONGLY prefer diagnosis to be made by human professionals, and their desires should arguably be taken into account – as well as the fact that CAD algorithms tend to be incredibly expensive and highly specialised. For instance, you cannot put MRI images depicting CNS lesions through a chest reporting algorithm and expect coherent results – whereas a radiologist can be trained to diagnose across two or more specialties.
For this reason, there is an argument that rather than focusing on sensitivity and specificity, we should just focus on producing highly sensitive algorithms that will pick up on any abnormality, and output some false positives, but will produce NO false negatives.
(Sensitivity = a test's ability to identify sick people with a disease)
(Specificity = a test's ability to identify that healthy people do not have this disease)
This means we are working towards developing algorithms that OVERESTIMATE rather than UNDERESTIMATE disease prevalence. This makes CAD a useful tool for triage rather than providing its own diagnoses – if a CAD algorithm weighted towards high sensitivity and low specificity does not pick up on any abnormalities, it’s highly unlikely that there are any.
Finally, we have to question whether CAD is even all that accurate to begin with. 10 years ago, according to Lehmen et al., CAD in mammography demonstrated negligible improvements to accuracy. In 1989, Sutton noted that accuracy was under 60%. Nowadays, however, AI has been proven to exceed the abilities of radiologists when detecting cancers (that’s from Guetari et al., 2023). This suggests that there is a common upwards trajectory, and AI might become a suitable alternative to traditional radiology one day. But, due to the many potential problems with this field, that day is unlikely to be soon...

That's all, folks! Have some references~
16 notes
·
View notes
Text
Been a while, crocodiles. Let's talk about cad.

or, y'know...

Yep, we're doing a whistle-stop tour of AI in medical diagnosis!
Much like programming, AI can be conceived of, in very simple terms, as...
a way of moving from inputs to a desired output.
See, this very funky little diagram from skillcrush.com.

The input is what you put in. The output is what you get out.
This output will vary depending on the type of algorithm and the training that algorithm has undergone – you can put the same input into two different algorithms and get two entirely different sorts of answer.
Generative AI produces ‘new’ content, based on what it has learned from various inputs. We're talking AI Art, and Large Language Models like ChatGPT. This sort of AI is very useful in healthcare settings to, but that's a whole different post!
Analytical AI takes an input, such as a chest radiograph, subjects this input to a series of analyses, and deduces answers to specific questions about this input. For instance: is this chest radiograph normal or abnormal? And if abnormal, what is a likely pathology?
We'll be focusing on Analytical AI in this little lesson!
Other forms of Analytical AI that you might be familiar with are recommendation algorithms, which suggest items for you to buy based on your online activities, and facial recognition. In facial recognition, the input is an image of your face, and the output is the ability to tie that face to your identity. We’re not creating new content – we’re classifying and analysing the input we’ve been fed.
Many of these functions are obviously, um, problematique. But Computer-Aided Diagnosis is, potentially, a way to use this tool for good!
Right?
....Right?

Let's dig a bit deeper! AI is a massive umbrella term that contains many smaller umbrella terms, nested together like Russian dolls. So, we can use this model to envision how these different fields fit inside one another.

AI is the term for anything to do with creating and managing machines that perform tasks which would otherwise require human intelligence. This is what differentiates AI from regular computer programming.
Machine Learning is the development of statistical algorithms which are trained on data –but which can then extrapolate this training and generalise it to previously unseen data, typically for analytical purposes. The thing I want you to pay attention to here is the date of this reference. It’s very easy to think of AI as being a ‘new’ thing, but it has been around since the Fifties, and has been talked about for much longer. The massive boom in popularity that we’re seeing today is built on the backs of decades upon decades of research.
Artificial Neural Networks are loosely inspired by the structure of the human brain, where inputs are fed through one or more layers of ‘nodes’ which modify the original data until a desired output is achieved. More on this later!
Deep neural networks have two or more layers of nodes, increasing the complexity of what they can derive from an initial input. Convolutional neural networks are often also Deep. To become ‘convolutional’, a neural network must have strong connections between close nodes, influencing how the data is passed back and forth within the algorithm. We’ll dig more into this later, but basically, this makes CNNs very adapt at telling precisely where edges of a pattern are – they're far better at pattern recognition than our feeble fleshy eyes!
This is massively useful in Computer Aided Diagnosis, as it means CNNs can quickly and accurately trace bone cortices in musculoskeletal imaging, note abnormalities in lung markings in chest radiography, and isolate very early neoplastic changes in soft tissue for mammography and MRI.
Before I go on, I will point out that Neural Networks are NOT the only model used in Computer-Aided Diagnosis – but they ARE the most common, so we'll focus on them!


This diagram demonstrates the function of a simple Neural Network. An input is fed into one side. It is passed through a layer of ‘hidden’ modulating nodes, which in turn feed into the output. We describe the internal nodes in this algorithm as ‘hidden’ because we, outside of the algorithm, will only see the ‘input’ and the ‘output’ – which leads us onto a problem we’ll discuss later with regards to the transparency of AI in medicine.
But for now, let’s focus on how this basic model works, with regards to Computer Aided Diagnosis. We'll start with a game of...
Spot The Pathology.

yeah, that's right. There's a WHACKING GREAT RIGHT-SIDED PNEUMOTHORAX (as outlined in red - images courtesy of radiopaedia, but edits mine)

But my question to you is: how do we know that? What process are we going through to reach that conclusion?
Personally, I compared the lungs for symmetry, which led me to note a distinct line where the tissue in the right lung had collapsed on itself. I also noted the absence of normal lung markings beyond this line, where there should be tissue but there is instead air.
In simple terms.... the right lung is whiter in the midline, and black around the edges, with a clear distinction between these parts.

Let’s go back to our Neural Network. We’re at the training phase now.
So, we’re going to feed our algorithm! Homnomnom.
Let’s give it that image of a pneumothorax, alongside two normal chest radiographs (middle picture and bottom). The goal is to get the algorithm to accurately classify the chest radiographs we have inputted as either ‘normal’ or ‘abnormal’ depending on whether or not they demonstrate a pneumothorax.
There are two main ways we can teach this algorithm – supervised and unsupervised classification learning.
In supervised learning, we tell the neural network that the first picture is abnormal, and the second and third pictures are normal. Then we let it work out the difference, under our supervision, allowing us to steer it if it goes wrong.
Of course, if we only have three inputs, that isn’t enough for the algorithm to reach an accurate result.
You might be able to see – one of the normal chests has breasts, and another doesn't. If both ‘normal’ images had breasts, the algorithm could as easily determine that the lack of lung markings is what demonstrates a pneumothorax, as it could decide that actually, a pneumothorax is caused by not having breasts. Which, obviously, is untrue.
or is it?
....sadly I can personally confirm that having breasts does not prevent spontaneous pneumothorax, but that's another story lmao
This brings us to another big problem with AI in medicine –

If you are collecting your dataset from, say, a wealthy hospital in a suburban, majority white neighbourhood in America, then you will have those same demographics represented within that dataset. If we build a blind spot into the neural network, and it will discriminate based on that.
That’s an important thing to remember: the goal here is to create a generalisable tool for diagnosis. The algorithm will only ever be as generalisable as its dataset.
But there are plenty of huge free datasets online which have been specifically developed for training AI. What if we had hundreds of chest images, from a diverse population range, split between those which show pneumothoraxes, and those which don’t?
If we had a much larger dataset, the algorithm would be able to study the labelled ‘abnormal’ and ‘normal’ images, and come to far more accurate conclusions about what separates a pneumothorax from a normal chest in radiography. So, let’s pretend we’re the neural network, and pop in four characteristics that the algorithm might use to differentiate ‘normal’ from ‘abnormal’.

We can distinguish a pneumothorax by the appearance of a pleural edge where lung tissue has pulled away from the chest wall, and the radiolucent absence of peripheral lung markings around this area. So, let’s make those our first two nodes. Our last set of nodes are ‘do the lung markings extend to the chest wall?’ and ‘Are there no radiolucent areas?’
Now, red lines mean the answer is ‘no’ and green means the answer is ‘yes’. If the answer to the first two nodes is yes and the answer to the last two nodes is no, this is indicative of a pneumothorax – and vice versa.
Right. So, who can see the problem with this?

(image courtesy of radiopaedia)
This chest radiograph demonstrates alveolar patterns and air bronchograms within the right lung, indicative of a pneumonia. But if we fed it into our neural network...
The lung markings extend all the way to the chest wall. Therefore, this image might well be classified as ‘normal’ – a false negative.
Now we start to see why Neural Networks become deep and convolutional, and can get incredibly complex. In order to accurately differentiate a ‘normal’ from an ‘abnormal’ chest, you need a lot of nodes, and layers of nodes. This is also where unsupervised learning can come in.

Originally, Supervised Learning was used on Analytical AI, and Unsupervised Learning was used on Generative AI, allowing for more creativity in picture generation, for instance. However, more and more, Unsupervised learning is being incorporated into Analytical areas like Computer-Aided Diagnosis!
Unsupervised Learning involves feeding a neural network a large databank and giving it no information about which of the input images are ‘normal’ or ‘abnormal’. This saves massively on money and time, as no one has to go through and label the images first. It is also surprisingly very effective. The algorithm is told only to sort and classify the images into distinct categories, grouping images together and coming up with its own parameters about what separates one image from another. This sort of learning allows an algorithm to teach itself to find very small deviations from its discovered definition of ‘normal’.
BUT this is not to say that CAD is without its issues.
Let's take a look at some of the ethical and practical considerations involved in implementing this technology within clinical practice!


(Image from Agrawal et al., 2020)
Training Data does what it says on the tin – these are the initial images you feed your algorithm. What is key here is volume, variety - with especial attention paid to minimising bias – and veracity. The training data has to be ‘real’ – you cannot mislabel images or supply non-diagnostic images that obscure pathology, or your algorithm is useless.
Validation data evaluates the algorithm and improves on it. This involves tweaking the nodes within a neural network by altering the ‘weights’, or the intensity of the connection between various nodes. By altering these weights, a neural network can send an image that clearly fits our diagnostic criteria for a pneumothorax directly to the relevant output, whereas images that do not have these features must be put through another layer of nodes to rule out a different pathology.
Finally, testing data is the data that the finished algorithm will be tested on to prove its sensitivity and specificity, before any potential clinical use.
However, if algorithms require this much data to train, this introduces a lot of ethical questions.
Where does this data come from?
Is it ‘grey data’ (data of untraceable origin)? Is this good (protects anonymity) or bad (could have been acquired unethically)?
Could generative AI provide a workaround, in the form of producing synthetic radiographs? Or is it risky to train CAD algorithms on simulated data when the algorithms will then be used on real people?
If we are solely using CAD to make diagnoses, who holds legal responsibility for a misdiagnosis that costs lives? Is it the company that created the algorithm or the hospital employing it?
And finally – is it worth sinking so much time, money, and literal energy into AI – especially given concerns about the environment – when public opinion on AI in healthcare is mixed at best? This is a serious topic – we’re talking diagnoses making the difference between life and death. Do you trust a machine more than you trust a doctor? According to Rojahn et al., 2023, there is a strong public dislike of computer-aided diagnosis.
So, it's fair to ask...
why are we wasting so much time and money on something that our service users don't actually want?
Then we get to the other biggie.

There are also a variety of concerns to do with the sensitivity and specificity of Computer-Aided Diagnosis.
We’ve talked a little already about bias, and how training sets can inadvertently ‘poison’ the algorithm, so to speak, introducing dangerous elements that mimic biases and problems in society.
But do we even want completely accurate computer-aided diagnosis?
The name is computer-aided diagnosis, not computer-led diagnosis. As noted by Rajahn et al, the general public STRONGLY prefer diagnosis to be made by human professionals, and their desires should arguably be taken into account – as well as the fact that CAD algorithms tend to be incredibly expensive and highly specialised. For instance, you cannot put MRI images depicting CNS lesions through a chest reporting algorithm and expect coherent results – whereas a radiologist can be trained to diagnose across two or more specialties.
For this reason, there is an argument that rather than focusing on sensitivity and specificity, we should just focus on producing highly sensitive algorithms that will pick up on any abnormality, and output some false positives, but will produce NO false negatives.
(Sensitivity = a test's ability to identify sick people with a disease)
(Specificity = a test's ability to identify that healthy people do not have this disease)
This means we are working towards developing algorithms that OVERESTIMATE rather than UNDERESTIMATE disease prevalence. This makes CAD a useful tool for triage rather than providing its own diagnoses – if a CAD algorithm weighted towards high sensitivity and low specificity does not pick up on any abnormalities, it’s highly unlikely that there are any.
Finally, we have to question whether CAD is even all that accurate to begin with. 10 years ago, according to Lehmen et al., CAD in mammography demonstrated negligible improvements to accuracy. In 1989, Sutton noted that accuracy was under 60%. Nowadays, however, AI has been proven to exceed the abilities of radiologists when detecting cancers (that’s from Guetari et al., 2023). This suggests that there is a common upwards trajectory, and AI might become a suitable alternative to traditional radiology one day. But, due to the many potential problems with this field, that day is unlikely to be soon...

That's all, folks! Have some references~
#medblr#artificial intelligence#radiography#radiology#diagnosis#medicine#studyblr#radioactiveradley#radley irradiates people#long post
16 notes
·
View notes
Text

do NOT ask
8 notes
·
View notes
Text

[I/D: (the first rule of radiation safety is to remember that any quantity of radioactive material is potentially dangerous, no matter how small. the second rule of radiation safety is to have fun and be yourself :heart emoji:)]
I had great fun in the PET-CT hub yesterday! Remind me to give you guys that lecture on Nuclear Medicine at some point~
16 notes
·
View notes
Text
oh. my. god. This is atrocious!
If you saw me agreeing with being annoyed about wasted helium in a fictional context and were like "I bet she has some more helium based anger in her life" good news LAPD fucked up a raid on a medical facility they thought was a pot farm and flat out ruined thousands of gallons of the stuff.
10K notes
·
View notes
Text
can confirm; definitely angels. if you want a little demystification, check out this post!
Does Gen Z even care about MRI machines?
854 notes
·
View notes
Text
they should let you get xrays and mris just cause. i wanna see what my skelinton looks like. i wanna see my organs and shit
#My inner rad: yes but radiation protections and money#Every other bone (ha) in my body: I WANNA SEE MY INTERIOR GOOS
43K notes
·
View notes
Photo
tragically, it's not actually that accurate... like, at all. but still a fun bit of historical trivia!

Throckmorton
#this is one of my most delightful (horrid) facts to get out at parties#we used to think that your peepee points to your pain!
592K notes
·
View notes
Text
*coyly holds dangerously radioactive material and wags finger like a girl in a makeup tutorial*
1K notes
·
View notes
Text
Me: yeah I've been eating nothing but buckshot for like six days is that gonna be a problem?
Nurse who runs the MRI machine: Duuude this is gonna be so cool
35K notes
·
View notes
Text

research project off to a great start

18 notes
·
View notes
