#Chemiluminescence Immunoassay (CLIA) Market trends
Text
Chemiluminescence Immunoassay (CLIA) Market Application, Growth 2024-2032

The Reports and Insights, a leading market research company, has recently releases report titled “Chemiluminescence Immunoassay (CLIA) Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2024-2032.” The study provides a detailed analysis of the industry, including the global Chemiluminescence Immunoassay (CLIA) Market Forecast share, size, trends, and growth. The report also includes competitor and regional analysis and highlights the latest advancements in the market.
Report Highlights:
How big is the Chemiluminescence Immunoassay (CLIA) Market?
As per Reports and Insights Analysis, the chemiluminescence immunoassay (CLIA) market is expected to grow at a CAGR of 7.8% during the forecast period of 2024 to 2032.
What are Chemiluminescence Immunoassay (CLIA)?
Chemiluminescence immunoassay (CLIA) is a precise diagnostic method used to detect and measure specific substances, such as proteins, hormones, or antibodies, in a sample. It employs chemiluminescent labels attached to antibodies or antigens that emit light when they bind to their target analytes. This emitted light is quantified using a luminometer, providing a measurement proportional to the concentration of the target substance. CLIA is renowned for its high sensitivity and specificity, making it a valuable tool in clinical diagnostics, research, and various applications in medical and biochemical analysis.
Request for a sample copy with detail analysis: https://www.reportsandinsights.com/sample-request/1671
What are the growth prospects and trends in the Chemiluminescence Immunoassay (CLIA) industry?
The chemiluminescence immunoassay (CLIA) market growth is driven by various factors and trends. The chemiluminescence immunoassay (CLIA) market is experiencing significant growth due to its widespread application in clinical diagnostics and research for accurate detection and quantification of biomarkers. This expansion is fueled by the increasing demand for precise diagnostic tools, advancements in assay technology, and the rising prevalence of chronic diseases. Ongoing innovations in CLIA technology, including the development of new reagents and platforms, are also driving market growth. Leading companies are heavily investing in research and development to improve assay performance and address the evolving needs of the healthcare and research sectors, thereby boosting the overall CLIA market. Hence, all these factors contribute to chemiluminescence immunoassay (CLIA) market growth.
What is included in market segmentation?
The report has segmented the market into the following categories:
By Product Type:
Analyzers
Reagents & Consumables
By Analyzer Type:
Benchtop Analyzers
Floor-standing Analyzers
By Application:
Infectious Diseases
Oncology
Cardiology
Autoimmune Diseases
Others
By End-Use:
Hospitals & Diagnostic Laboratories
Research & Academic Institutes
Pharmaceutical & Biotechnology Companies
Contract Research Organizations (CROs)
Segmentation By Region:
North America:
United States
Canada
Asia Pacific:
China
India
Japan
Australia & New Zealand
Association of Southeast Asian Nations (ASEAN)
Rest of Asia Pacific
Europe:
Germany
The U.K.
France
Spain
Italy
Russia
Poland
BENELUX (Belgium, the Netherlands, Luxembourg)
NORDIC (Norway, Sweden, Finland, Denmark)
Rest of Europ
Latin America:
Brazil
Mexico
Argentina
Rest of Latin America
The Middle East & Africa:
Saudi Arabia
United Arab Emirates
South Africa
Egypt
Israel
Rest of MEA (Middle East & Africa)
Who are the key players operating in the industry?
The report covers the major market players including:
Roche Diagnostics
Siemens Healthineers
Abbott Laboratories
Beckman Coulter (a subsidiary of Danaher Corporation)
Ortho Clinical Diagnostics
bioMérieux SA
DiaSorin S.p.A.
Sysmex Corporation
Thermo Fisher Scientific Inc.
Mindray Bio-Medical Electronics Co., Ltd.
Randox Laboratories Ltd.
Snibe Diagnostic
View Full Report: https://www.reportsandinsights.com/report/Chemiluminescence Immunoassay (CLIA)-market
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us:
Reports and Insights consistently mееt international benchmarks in the market research industry and maintain a kееn focus on providing only the highest quality of reports and analysis outlooks across markets, industries, domains, sectors, and verticals. We have bееn catering to varying market nееds and do not compromise on quality and research efforts in our objective to deliver only the very best to our clients globally.
Our offerings include comprehensive market intelligence in the form of research reports, production cost reports, feasibility studies, and consulting services. Our team, which includes experienced researchers and analysts from various industries, is dedicated to providing high-quality data and insights to our clientele, ranging from small and medium businesses to Fortune 1000 corporations.
Contact Us:
Reports and Insights Business Research Pvt. Ltd.
1820 Avenue M, Brooklyn, NY, 11230, United States
Contact No: +1-(347)-748-1518
Email: [email protected]
Website: https://www.reportsandinsights.com/
Follow us on LinkedIn: https://www.linkedin.com/company/report-and-insights/
Follow us on twitter: https://twitter.com/ReportsandInsi1
#Chemiluminescence Immunoassay (CLIA) Market share#Chemiluminescence Immunoassay (CLIA) Market size#Chemiluminescence Immunoassay (CLIA) Market trends
0 notes
Link
Chemiluminescence Immunoassay (CLIA) Analyzer Tube Market
0 notes
Text
The Carcinoembryonic Antigen Market is projected to grow from USD 1235 million in 2024 to an estimated USD 3522.944 million by 2032, with a compound annual growth rate (CAGR) of 14% from 2024 to 2032.The global Carcinoembryonic Antigen (CEA) market has been experiencing significant growth, driven by advancements in cancer diagnostics, increased prevalence of cancer worldwide, and the growing awareness of the role of biomarkers in early cancer detection. CEA is a glycoprotein involved in cell adhesion and is commonly used as a tumor marker in cancer diagnostics, particularly for colorectal cancer. This article provides an in-depth analysis of the CEA market, exploring key drivers, restraints, opportunities, and trends shaping its future trajectory.
Browse the full report at https://www.credenceresearch.com/report/carcinoembryonic-antigen-cea-market
Market Definition and Scope
Carcinoembryonic Antigen (CEA) is a biomarker that is frequently measured in blood samples to monitor cancer progression, especially in patients with colorectal, breast, pancreatic, and lung cancers. CEA levels can provide essential insights into cancer recurrence, response to therapy, and prognosis. It plays a crucial role in detecting cancer at an early stage, allowing for timely intervention and personalized treatment plans. The global CEA market encompasses various diagnostic tests, including CEA assays, kits, reagents, and instrumentation.
Key Drivers of the CEA Market
1. Rising Prevalence of Cancer: The increasing incidence of cancer globally is one of the primary factors driving the CEA market. According to the World Health Organization (WHO), cancer is the second leading cause of death worldwide, accounting for approximately 10 million deaths in 2020. Colorectal cancer, in particular, is among the most common cancers, and CEA is widely used in its diagnosis and management.
2. Advancements in Diagnostic Technologies: Technological advancements in diagnostic tools and techniques have improved the accuracy and efficiency of CEA testing. The introduction of highly sensitive immunoassays, such as enzyme-linked immunosorbent assays (ELISAs) and chemiluminescence immunoassays (CLIAs), has enhanced the ability to detect CEA levels in patients, contributing to better cancer management.
3. Growing Demand for Personalized Medicine: As the healthcare industry shifts towards personalized medicine, the demand for biomarkers like CEA is increasing. CEA testing helps in tailoring treatment strategies based on an individual’s tumor biology and response to therapy, improving treatment outcomes and reducing adverse effects.
4. Increasing Awareness and Government Initiatives: Governments and healthcare organizations worldwide are raising awareness about cancer prevention and early detection. In many countries, cancer screening programs that include CEA testing are being implemented to identify high-risk populations. This has further propelled the demand for CEA diagnostic tests.
Challenges and Restraints
1. Limited Specificity of CEA Testing: One of the primary limitations of CEA as a biomarker is its lack of specificity. Elevated CEA levels can also be observed in non-cancerous conditions such as smoking, liver disease, and inflammatory conditions. This can lead to false-positive results, complicating the diagnostic process. Consequently, CEA testing is often used in conjunction with other diagnostic tools to improve accuracy.
2. High Cost of Advanced Diagnostic Tools: The cost associated with advanced diagnostic technologies, including CEA assays, can be prohibitive for healthcare providers, especially in low- and middle-income countries. This limits the accessibility of CEA testing in certain regions, thereby restricting market growth.
3. Regulatory and Reimbursement Challenges: Regulatory approval processes and reimbursement policies for diagnostic tests vary significantly across regions. Stringent regulations and the absence of uniform reimbursement policies can hinder market growth, particularly for new entrants.
Opportunities in the CEA Market
1. Emerging Markets: Developing countries, particularly in Asia-Pacific, are witnessing a surge in healthcare infrastructure development and cancer awareness programs. These regions present significant opportunities for market expansion as healthcare providers invest in advanced diagnostic tools, including CEA testing.
2. Integration of Artificial Intelligence (AI): The integration of AI and machine learning in diagnostic tools offers promising opportunities for the CEA market. AI-powered algorithms can enhance the interpretation of CEA test results, leading to more accurate and timely diagnoses.
3. Collaboration Between Key Players: Collaborations between pharmaceutical companies, diagnostic laboratories, and research institutions can accelerate the development of innovative CEA tests and assays. Such partnerships can also help in overcoming challenges related to regulatory approvals and market penetration.
Market Segmentation
The CEA market can be segmented based on product type, application, and end-user. Product types include CEA assays, kits, reagents, and instruments. Applications of CEA testing are primarily in colorectal cancer, followed by breast, pancreatic, and lung cancers. Key end-users of CEA testing products are hospitals, diagnostic laboratories, and research institutions.
Competitive Landscape
Several key players dominate the CEA market, including Abbott Laboratories, F. Hoffmann-La Roche AG, Siemens Healthineers, and Thermo Fisher Scientific. These companies focus on product innovation, partnerships, and geographic expansion to maintain a competitive edge. For instance, Roche’s Elecsys CEA assay is a popular product in cancer diagnostics, offering high precision and reliability.
Key Player Analysis:
Abbott (U.S.)
Aviva Systems Biology Corporation
Boster Biological Technology (U.S.)
Cigna (U.S.)
Correlogic Systems, Inc. (Hong Kong)
Creative Diagnostics (U.S.)
Hoffmann-La Roche Ltd (Switzerland)
Genway Biotech, LLC. (U.S.)
Laboratory Corporation of America Holdings (U.S.)
Lee BioSolutions (U.S.)
Mayo Foundation for Medical Education and Research (MFMER) (U.S.)
Merck KGaA (Germany)
Omega Diagnostics Group PLC (U.K.)
Prospec-Tany Technogene Ltd (Israel)
Quest Diagnostics Incorporated (U.S.)
RayBiotech Life, Inc (U.S.)
Segmentation:
By Type,
Serum CEA
Tissue CEA.
By Gender,
Male
Female
By Product,
Kits,
Reagents,
Instruments
By Test,
Clinical testing
Research testing.
By End-Use,
Hospitals,
Diagnostic laboratories,
Research institutes.
By Region
North America
The U.S
Canada
Mexico
Europe
Germany
France
The U.K.
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/carcinoembryonic-antigen-cea-market
About Us:
Credence Research is committed to employee well-being and productivity. Following the COVID-19 pandemic, we have implemented a permanent work-from-home policy for all employees.
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes
Text
Blood Screening Market 2023- Business Planning Research and Resources, Revenue, and Forecasts 2030
Blood Screening Market Size & Trends
The global blood screening market size was valued at USD 2.76 billion in 2022 and is anticipated to grow at a compound annual growth rate (CAGR) of 11.7% from 2023 to 2030.
Blood screening is a process in which donated blood is screened for infectious diseases such as HBV, HCV, HIV1, and HIV2. The high growth of this market is attributed to rising blood donations, an increase in the incidence of infectious diseases, and government initiatives. According to World Health Organization (WHO), 118.54 million blood donations are collected yearly. In the U.S., 6.8 million individuals donate blood annually, and 13.6 million units of red blood cells and whole blood are collected annually.
Gather more insights about the market drivers, restrains and growth of the Blood Screening Market
The market is primarily driven by the rise in the rate of disorders such as HIV, diphtheria, measles and chronic diseases such as hemophilia, cancer, and other blood-related disorders. Blood-based diagnostics are used to diagnose a wide range of diseases, including infectious diseases, cancer, and cardiovascular diseases. Serology tests detect the presence of antibodies to a specific disease-causing organism. These tests diagnose various infectious diseases, including HIV, hepatitis B, and syphilis. Molecular tests detect the presence of DNA or RNA from a specific disease-causing organism. These tests are more sensitive than serology tests and can be used to diagnose diseases at an earlier stage. Biochemical tests measure the levels of certain substances in the blood. These tests can diagnose a wide range of diseases, including diabetes, kidney disease, and liver disease.
The COVID-19 pandemic had a significant impact on the market. Although respiratory droplets are the primary means of COVID-19 virus transmission, research has shown that viral RNA may be discovered in blood samples, supporting blood screening for COVID-19 identification, hence driving the market significantly.
Blood Screening Market Segmentation
Grand View Research has segmented the global blood screening market based on technology, product, and region:
Technology Outlook (Revenue, USD Million, 2018 - 2030)
Nucleic Acid Amplification Test (NAT)
ELISA
Chemiluminescence Immunoassay (CLIA) and Enzyme Immunoassay (EIA)
Next Generation Sequencing
Western Blotting
Product Outlook (Revenue, USD Million, 2018 - 2030)
Reagent
Instrument
Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
US
Canada
Europe
UK
Germany
France
Italy
Spain
Sweden
Norway
Denmark
Asia Pacific
Japan
China
India
Australia
Thailand
South Korea
Latin America
Brazil
Mexico
Argentina
Middle East and Africa
Saudi Arabia
South Africa
UAE
Kuwait
Browse through Grand View Research's Medical Devices Industry Research Reports.
The global embolic protection devices market size was valued at USD 612.9 million in 2023 and is projected to grow at a CAGR of 8.7% from 2024 to 2030.
The global covered stent market size was estimated at USD 1.13 billion in 2023 and is projected to grow at a CAGR of 4.1% from 2024 to 2030.
Key Companies & Market Share Insights
Product launches, approvals, strategic acquisitions, and innovations are just a few of the important business strategies used by market participants to maintain and grow their global reach.
For instance, in March 2023, Abbott received U.S Food and Drug Administration (FDA) clearance for a laboratory traumatic brain injury blood test, the first commercially available lab-based test for the assessment of mild traumatic brain injuries (TBIs), commonly referred to as concussions, which will be made widely available to hospitals across the U.S. This test, which is powered by Abbott’s Alinity i laboratory tool, will enable clinicians to evaluate individuals with mild traumatic brain injuries in a timely manner.
Furthermore, in May 2023, Siemens Healthcare introduced Atellica HEMA 570 and 580 next-generation hematology analyzers, which have user-friendly interfaces and can be connected to multiple analyzers to remove workflow barriers and provide high throughput time.
Key Blood Screening Companies:
Abbott
Danaher Corporation (Beckman Coulter)
Becton Dickinson and Company
Bio-Rad Laboratories, Inc.
Hoffman-La Roche Ltd.
Grifols, S.A.
Ortho-Clinical Diagnostics, Inc.
Siemens Healthcare GmbH
Thermo Fisher Scientific, Inc.
SOFINA s.a (Biomerieux)
Order a free sample PDF of the Blood Screening Market Intelligence Study, published by Grand View Research.
0 notes
Text
Chemiluminescence Immunoassay Market Sluggish Growth Rate Foreseen by 2024–2030
The Chemiluminescence Immunoassay Market was valued at USD 12.6 billion in 2023 and will surpass USD 20.2 billion by 2030; growing at a CAGR of 7.0% during 2024 - 2030. Chemiluminescence Immunoassay is a biochemical method used to detect and measure the concentration of various analytes in blood or other bodily fluids. It is widely used for diagnosing diseases by identifying specific antibodies, hormones, or proteins associated with various medical conditions. CLIA offers a highly sensitive, specific, and automated approach to diagnostics, making it a preferred choice in laboratories and hospitals.
The principle behind CLIA is based on the use of chemiluminescent molecules that emit light during the immunological reaction. The amount of emitted light correlates with the presence of the target analyte, allowing accurate quantification.
Key Market Players
Several key players are contributing to the competitive landscape of the global CLIA market. Some of the prominent companies include:
Siemens Healthineers
Abbott Laboratories
Roche Diagnostics
Danaher Corporation
BioMérieux SA
Thermo Fisher Scientific Inc.
Read More about Sample Report: https://intentmarketresearch.com/request-sample/chemiluminescence-immunoassay-market-3625.html
Key Trends Driving Growth in the CLIA Market
Rise in Chronic Diseases: The growing incidence of chronic diseases such as cardiovascular diseases, diabetes, and cancer has boosted the demand for reliable diagnostic tools like CLIA. Early detection and monitoring of these diseases are critical to improving patient outcomes, creating significant demand for CLIA testing solutions.
Technological Advancements in Diagnostics: Continuous innovations in immunoassay platforms, including the integration of automation and artificial intelligence, have enhanced the efficiency and accuracy of CLIA tests. Automation reduces manual errors, speeds up the testing process, and facilitates large-scale testing, which is crucial for diagnostic laboratories.
Growing Focus on Infectious Disease Detection: The COVID-19 pandemic underscored the importance of rapid and accurate diagnostic testing. CLIA played a pivotal role in detecting viral antibodies and monitoring immune responses. With the threat of emerging infectious diseases, there is an ongoing need for rapid, sensitive, and scalable diagnostic solutions.
Increasing Preference for Point-of-Care Testing: The shift towards decentralized healthcare has led to a rise in demand for point-of-care (POC) diagnostics, where CLIA-based POC tests are gaining traction. Portable CLIA analyzers enable quick and accurate testing at the patient’s bedside or in remote locations, reducing the need for hospital visits.
Expansion in Research and Development: Pharmaceutical and biotechnology companies are heavily investing in R&D to develop new CLIA-based tests for various applications, from oncology to infectious disease diagnostics. This has led to the expansion of the market with new products, addressing previously unmet diagnostic needs.
Challenges in the CLIA Market
Despite its robust growth, the CLIA market faces several challenges, including:
High Equipment Costs: The initial setup costs for CLIA analyzers and related instruments can be prohibitively high for smaller laboratories and clinics, limiting market penetration in low-resource settings.
Skilled Workforce Requirements: Proper execution of CLIA tests requires highly skilled personnel. A shortage of trained professionals in certain regions may slow down the widespread adoption of CLIA technology.
Regulatory Compliance: The regulatory landscape for diagnostic testing is complex, with various approval processes differing by region. Ensuring compliance with local and international regulations can pose challenges for market players.
Regional Market Insights
North America: Dominates the global CLIA market, thanks to its well-established healthcare infrastructure, the presence of major market players, and the growing prevalence of chronic diseases. The United States, in particular, accounts for a significant market share due to high healthcare expenditure and ongoing technological advancements.
Europe: The European CLIA market follows closely behind North America, driven by the increasing demand for diagnostic tools, especially in countries like Germany, France, and the UK.
Asia-Pacific: This region is anticipated to experience the fastest growth, fueled by the rising healthcare needs of a growing population, increased investment in healthcare infrastructure, and the expanding middle class in countries like China, India, and Japan. Additionally, improving awareness of early disease detection in these markets further boosts demand for CLIA-based diagnostics.
Latin America and Middle East & Africa: These regions are gradually adopting CLIA technology, driven by the need for advanced diagnostic solutions. However, the market growth in these areas is slower due to limited resources and the high costs associated with the technology.
These companies are continuously investing in R&D, strategic partnerships, and product launches to strengthen their positions in the market.
Ask for Customization Report: https://intentmarketresearch.com/ask-for-customization/chemiluminescence-immunoassay-market-3625.html
Future Outlook and Opportunities
The future of the Chemiluminescence Immunoassay market looks promising, with ample opportunities for growth. The increasing demand for precision medicine, advancements in molecular diagnostics, and the growing focus on preventive healthcare are expected to create significant expansion opportunities.
Personalized Diagnostics: As the healthcare industry moves towards personalized medicine, CLIA technology will play a vital role in developing tailored diagnostics that can cater to individual patient needs.
Emerging Applications: Beyond infectious disease and chronic condition diagnostics, CLIA holds potential in emerging fields such as pharmacogenomics, environmental testing, and food safety, offering diverse growth avenues.
Conclusion
The Chemiluminescence Immunoassay market is poised for continued growth, driven by the increasing demand for efficient diagnostic solutions, technological advancements, and rising awareness about early disease detection. Despite challenges related to costs and regulatory hurdles, the market presents immense opportunities for innovation, especially in personalized medicine and emerging diagnostic applications. As healthcare continues to evolve, CLIA will remain a critical tool in shaping the future of diagnostics.
#Chemiluminescence Immunoassay#Chemiluminescence Immunoassay Size#Chemiluminescence Immunoassay Demand#Chemiluminescence Immunoassay Growth
0 notes
Text
Forecasted at $13.75 Billion by 2028, the Chemiluminescence Immunoassay Market Is Poised for Considerable Growth in the Upcoming Years
Chemiluminescence Immunoassay Market Growth & Trends
The global chemiluminescence immunoassay market size is expected to reach USD 13.75 billion by 2028, according to a new report by Grand View Research, Inc. It is expected to expand at a CAGR of 7.9% from 2021 to 2028. The high prevalence of chronic diseases, several advantages of chemiluminescence immunoassay (CLIA) technique, and approval and…
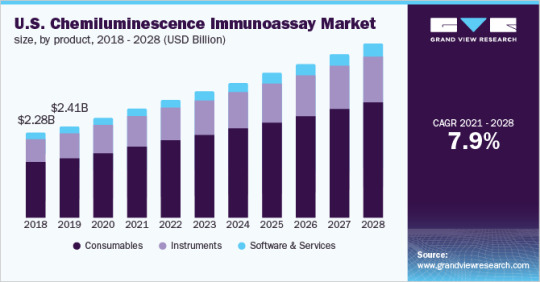
View On WordPress
0 notes
Text
Immunoassay Revolution: Understanding Market Size, Share, and Trends
The global immunoassay market is experiencing robust growth, driven by the increasing prevalence of chronic diseases, advancements in technology, and a growing emphasis on early disease detection. As of 2023, immunoassays, which encompass a diverse range of diagnostic techniques, play a pivotal role in the detection and quantification of various analytes, including proteins, hormones, and biomarkers. These assays find extensive application in clinical diagnostics, pharmaceutical research, and food testing, contributing to the overall improvement of healthcare and life sciences.
Over the projection period of 2022–2032, the global immunoassay market is expected to increase at a 6% compound annual growth rate (CAGR) and reach a market size of US$ 53 billion. With a 5.7% year-over-year growth rate, the market, estimated at US$ 28 billion in 2021, is expected to expand to US$ 29.6 billion in 2022.
Download a Sample Copy of This Report:
https://www.factmr.com/connectus/sample?flag=S&rep_id=4766?PJ
Competitive Landscape
The key players are taking on extensive initiatives to address the unmet needs of patients in the domain of immunoassays and stressing more on new product development, mergers and acquisitions, and regional expansion.
Molecular biology subsidiary BioFire Diagnostics, a subsidiary of BioMérieux SA, filed an application with the Food and Drug Administration in January 2020 for clearance of its BIOFIRE Blood Culture Identification 2 (BCID2) Panel. The BIOFIRE BCID2 Panel has an expanded list of antibiotic resistance genes, considerably more pathogens, and several revised targets compared to the previous BIOFIRE BCID Panel.
Siemens Healthineers announced in November 2020 that its SARS-CoV-2 IgG Antibody Test has been certified as a CE mark measurement of neutralizing antibodies. The test is an improved version of the COVID-19 antibody test it previously launched.
Maglumi HIV Ab/Ag Combi CLIA kit, Snibe Diagnostics’ fourth-generation chemiluminescence immunoassay that can detect HIV-1 and HIV-2 antibodies as well as the HIV-1 p24 antigen, obtained CE marking in April 2021, allowing it to be sold in the European Union and other regions accepting the designation.
Key Companies Profiled
DiaSorin S.p.A.
Sysmex Corporation
bioMerieux SA
Ortho Clinical Diagnostics
Thermo Fisher Scientific Inc.
Becton
Dickinson and Company
Merck KGaA.
Increased prevalence of infectious and chronic illnesses, such as COVID-19, is linked to market expansion. Further driving increase will be the ageing population, which is more prone to infectious and chronic illnesses.
Key Market Drivers:
Rising Disease Burden: The escalating burden of chronic diseases, such as cardiovascular disorders, cancer, and infectious diseases, is a primary driver for the immunoassay market. The need for accurate and timely diagnosis to initiate appropriate treatment regimens has spurred the demand for immunoassay-based diagnostic solutions.
Technological Advancements: Ongoing advancements in immunoassay technologies are enhancing the sensitivity, specificity, and speed of diagnostic tests. Automation, multiplexing, and the integration of novel detection platforms are improving the efficiency of immunoassays, making them invaluable tools in clinical laboratories and research settings.
Point-of-Care Testing (POCT): The growing trend towards decentralized testing and the demand for rapid diagnostic results have propelled the adoption of immunoassays in point-of-care settings. Portable immunoassay devices allow for quick on-site testing, enabling healthcare providers to make immediate clinical decisions and improve patient outcomes.
Challenges and Opportunities:
Complexity of Assay Development: The complexity associated with the development of immunoassays, including the selection of appropriate antibodies, assay optimization, and standardization, poses a challenge to market growth. However, overcoming these challenges presents opportunities for innovation and the creation of more sophisticated and reliable immunoassay platforms.
Competitive Landscape: The immunoassay market is highly competitive, with numerous players offering a wide array of products. To thrive in this landscape, companies are exploring niche markets, developing specialized assays, and engaging in strategic collaborations to expand their product portfolios and gain a competitive edge.
Current Industry Trends (2023):
Expansion of Biomarker Discovery: Immunoassays are integral in the discovery and validation of biomarkers for various diseases. The emphasis on precision medicine and personalized treatment approaches has fueled the expansion of biomarker research, driving the demand for immunoassay platforms capable of detecting specific biomolecules indicative of disease states.
Adoption of Multiplex Immunoassays: Multiplex immunoassays, allowing the simultaneous detection of multiple analytes in a single sample, are gaining prominence. These assays streamline the diagnostic process, conserve sample volumes, and provide a comprehensive understanding of the patient's health status. The adoption of multiplexing is particularly notable in cancer research and autoimmune disease diagnostics.
Integration of Artificial Intelligence (AI): The integration of artificial intelligence and machine learning in immunoassay data analysis is a growing trend. AI algorithms enhance the accuracy and speed of result interpretation, aiding healthcare professionals in making more informed decisions. This integration contributes to the evolving landscape of precision diagnostics.
Key Segments Covered in the Immunoassay Market Report
By Technology Type :
Chemiluminescence Immunoassay (CLIA)
Enzyme Linked Fluorescence Assay (ELFA)
Enzyme-linked Immunosorbent Assay (ELISA)
Radioimmunoassay (RIA)
Others
By Product :
Analyzer Immunoassay
Consumable Immunoassay
By Application :
Infectious Diseases
Orthopedics
Cardiology
Oncology
Endocrinology
Other Applications
By End-User :
Immunoassay for Blood Banks
Immunoassay for Hospitals & Diagnostic Laboratories
Immunoassay for Others (Pharmaceutical & Biotech Companies, Forensic Labs, Academic & Research Institutes, etc.)
By Region :
North America
Western Europe
Asia Pacific
Middle East & Africa
Eastern Europe
Latin America
Global Industry News (2023):
Rapid COVID-19 Diagnostics: The ongoing global response to the COVID-19 pandemic has highlighted the pivotal role of immunoassays in infectious disease diagnostics. Rapid immunoassay tests for detecting SARS-CoV-2 antigens and antibodies have been pivotal in mass testing efforts, emphasizing the adaptability and versatility of immunoassay technologies in addressing emerging health challenges.
Investments in Research and Development: Key players in the immunoassay market are actively investing in research and development to introduce novel assays with enhanced performance characteristics. This includes the development of ultrasensitive assays, novel biomarker panels, and innovative detection methodologies to meet the evolving needs of the healthcare and research communities.
Strategic Collaborations: Strategic collaborations and partnerships between diagnostic companies, pharmaceutical firms, and research institutions are prevalent in the immunoassay market. These collaborations aim to leverage collective expertise, access shared resources, and accelerate the development and commercialization of novel immunoassay-based diagnostics.
Focus on Sustainability: Sustainability is gaining traction in the immunoassay market, with a focus on developing eco-friendly assay components, reducing waste generation, and adopting green laboratory practices. This aligns with broader industry efforts to minimize environmental impact and promote sustainable healthcare solutions.
The immunoassay market continues to be a dynamic and vital component of the diagnostics and life sciences landscape. The ongoing convergence of technological advancements, the expansion of biomarker research, and the market's response to global health challenges underscore the adaptability and resilience of immunoassay technologies. As the industry navigates complexities and embraces opportunities for innovation, immunoassays are expected to play an increasingly integral role in shaping the future of diagnostics and healthcare.
0 notes
Text
Chemiluminescence Immunoassay Market Is Estimated To Witness High Growth Owing To Increasing Demand for Accurate and Rapid Diagnostic Solutions

The global Chemiluminescence Immunoassay Market is estimated to be valued at US$ 6.01 Bn in 2021 and is expected to exhibit a CAGR of 8.0% over the forecast period 2021-2028, as highlighted in a new report published by Coherent Market Insights.
A) Market Overview:
Chemiluminescence immunoassay (CLIA) is a widely used diagnostic technique that combines the principles of chemiluminescence and immunoassay to detect the presence of specific analytes in patient samples. This technique offers several advantages such as high sensitivity, specificity, and rapid results, making it a preferred choice in clinical laboratories and research settings. The increasing prevalence of infectious diseases, cancer, and chronic conditions, along with the need for accurate and rapid diagnostic solutions, is driving the demand for chemiluminescence immunoassay products.
B) Market Key Trends:
The key trend driving the growth of the chemiluminescence immunoassay market is the increasing adoption of automated platforms and systems. Automated chemiluminescence immunoassay systems offer several advantages such as reduced turnaround time, increased throughput, and improved accuracy compared to manual testing methods. These systems also enable simultaneous testing of multiple analytes, reducing the overall cost and time spent on sample processing. For example, Beckman Coulter Inc. offers the Access 2 Immunoassay System, which is a fully automated platform for chemiluminescence immunoassay testing.
C) PEST Analysis:
Political: The chemiluminescence immunoassay market is influenced by government regulations and policies related to the approval and reimbursement of diagnostic tests. Stringent regulatory requirements for assay validation and quality control drive manufacturers to adhere to strict standards.
Economic: The growing healthcare expenditure and increasing investments in research and development activities in the healthcare sector are driving the growth of the chemiluminescence immunoassay market. The market is also influenced by factors such as healthcare infrastructure, insurance coverage, and affordability of diagnostic tests.
D) Key Takeaways:
- The global Chemiluminescence Immunoassay Market is expected to witness high growth, exhibiting a CAGR of 8.0% over the forecast period. This growth is primarily driven by the increasing demand for accurate and rapid diagnostic solutions.
- North America is expected to dominate the chemiluminescence immunoassay market due to factors such as well-established healthcare infrastructure, high healthcare expenditure, and strong research and development activities in the region.
- Key players operating in the global chemiluminescence immunoassay market include DiaSorin S.p.A., Abbott Laboratories, Shenzhen Mindray Bio-Medical Electronics Co., Ltd., Siemens Healthineers, Beckman Coulter Inc., F. Hoffmann-La Roche AG, Inova Diagnostics, Inc., Maccura Biotechnology Co., Ltd., Tosoh Corporation, and Ortho Clinical Diagnostics.
In conclusion, the global chemiluminescence immunoassay market is poised for significant growth in the coming years. The increasing demand for accurate and rapid diagnostic solutions, coupled with advancements in technology, is driving the adoption of chemiluminescence immunoassay products. Automated platforms and systems are emerging as a key trend in the market, offering improved efficiency and accuracy in diagnostic testing. North America is expected to dominate the market, given its well-established healthcare infrastructure and strong research and development activities. Key players in the market are focused on innovations and collaborations to gain a competitive edge in this growing market.
#Chemiluminescence Immunoassay#Chemiluminescence Immunoassay Market#Chemiluminescence Immunoassay Market Size#Chemiluminescence Immunoassay Market Share#Clinical Diagnostic
0 notes
Text
Chemiluminescence Immunoassay Market : Value Chain, Stakeholder Analysis and Trends by 2032
The Chemiluminescence Immunoassay (CLIA) Market is experiencing rapid growth and is revolutionizing the field of diagnostic testing. Chemiluminescence immunoassay is a highly sensitive and specific technique that uses light emission to detect and quantify analytes of interest, such as biomarkers and antibodies, in patient samples. CLIA offers several advantages over other immunoassay methods, including enhanced sensitivity, wide dynamic range, and shorter assay times.
One key driver for the growth of the Chemiluminescence Immunoassay Market is the increasing demand for accurate and efficient diagnostic tools in clinical laboratories. CLIA provides a reliable and precise method for the detection and measurement of biomarkers associated with various diseases, such as infectious diseases, cancer, and hormonal disorders. The ability of CLIA to deliver quick and accurate results has made it a preferred choice for diagnostic laboratories worldwide.
Additionally, advancements in technology have led to the development of automated CLIA systems, which have streamlined workflow and improved efficiency in laboratory operations. These automated systems offer high-throughput testing capabilities, reducing the turnaround time for test results and increasing the overall productivity of diagnostic laboratories. The integration of robotics, advanced software, and data management systems has further enhanced the performance and usability of CLIA platforms.
Moreover, the increasing prevalence of chronic and infectious diseases, along with the rising geriatric population, has fueled the demand for diagnostic tests that can accurately and rapidly detect these conditions. CLIA meets these requirements by providing sensitive and specific measurements of disease biomarkers, aiding in early detection, disease monitoring, and treatment optimization.
For More Info@ https://www.persistencemarketresearch.com/market-research/chemiluminescence-immunoassay-market.asp
In conclusion, the Chemiluminescence Immunoassay Market is witnessing significant growth as it offers a highly sensitive and reliable diagnostic tool for a wide range of medical conditions. With technological advancements and the development of automated systems, CLIA has become a cornerstone in clinical laboratory testing. As the demand for accurate and efficient diagnostic methods continues to rise, the market is expected to expand further, driving innovation and improving patient care in the field of diagnostics.
0 notes
Text
0 notes
Text
Chemiluminescence Immunoassay (CLIA) Market Application, Growth 2024-2032

The Reports and Insights, a leading market research company, has recently releases report titled “Chemiluminescence Immunoassay (CLIA) Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2024-2032.” The study provides a detailed analysis of the industry, including the global Chemiluminescence Immunoassay (CLIA) Market share, size, trends, and growth forecasts. The report also includes competitor and regional analysis and highlights the latest advancements in the market.
Report Highlights:
How big is the Chemiluminescence Immunoassay (CLIA) Market?
As per Reports and Insights Analysis, the chemiluminescence immunoassay (CLIA) market is expected to grow at a CAGR of 7.8% during the forecast period of 2024 to 2032.
What are Chemiluminescence Immunoassay (CLIA)?
Chemiluminescence immunoassay (CLIA) is a precise diagnostic method used to detect and measure specific substances, such as proteins, hormones, or antibodies, in a sample. It employs chemiluminescent labels attached to antibodies or antigens that emit light when they bind to their target analytes. This emitted light is quantified using a luminometer, providing a measurement proportional to the concentration of the target substance. CLIA is renowned for its high sensitivity and specificity, making it a valuable tool in clinical diagnostics, research, and various applications in medical and biochemical analysis.
Request for a sample copy with detail analysis: https://www.reportsandinsights.com/sample-request/1671
What are the growth prospects and trends in the Chemiluminescence Immunoassay (CLIA) industry?
The chemiluminescence immunoassay (CLIA) market growth is driven by various factors and trends. The chemiluminescence immunoassay (CLIA) market is experiencing significant growth due to its widespread application in clinical diagnostics and research for accurate detection and quantification of biomarkers. This expansion is fueled by the increasing demand for precise diagnostic tools, advancements in assay technology, and the rising prevalence of chronic diseases. Ongoing innovations in CLIA technology, including the development of new reagents and platforms, are also driving market growth. Leading companies are heavily investing in research and development to improve assay performance and address the evolving needs of the healthcare and research sectors, thereby boosting the overall CLIA market. Hence, all these factors contribute to chemiluminescence immunoassay (CLIA) market growth.
What is included in market segmentation?
The report has segmented the market into the following categories:
By Product Type:
Analyzers
Reagents & Consumables
By Analyzer Type:
Benchtop Analyzers
Floor-standing Analyzers
By Application:
Infectious Diseases
Oncology
Cardiology
Autoimmune Diseases
Others
By End-Use:
Hospitals & Diagnostic Laboratories
Research & Academic Institutes
Pharmaceutical & Biotechnology Companies
Contract Research Organizations (CROs)
Segmentation By Region:
North America:
United States
Canada
Asia Pacific:
China
India
Japan
Australia & New Zealand
Association of Southeast Asian Nations (ASEAN)
Rest of Asia Pacific
Europe:
Germany
The U.K.
France
Spain
Italy
Russia
Poland
BENELUX (Belgium, the Netherlands, Luxembourg)
NORDIC (Norway, Sweden, Finland, Denmark)
Rest of Europ
Latin America:
Brazil
Mexico
Argentina
Rest of Latin America
The Middle East & Africa:
Saudi Arabia
United Arab Emirates
South Africa
Egypt
Israel
Rest of MEA (Middle East & Africa)
Who are the key players operating in the industry?
The report covers the major market players including:
Roche Diagnostics
Siemens Healthineers
Abbott Laboratories
Beckman Coulter (a subsidiary of Danaher Corporation)
Ortho Clinical Diagnostics
bioMérieux SA
DiaSorin S.p.A.
Sysmex Corporation
Thermo Fisher Scientific Inc.
Mindray Bio-Medical Electronics Co., Ltd.
Randox Laboratories Ltd.
Snibe Diagnostic
View Full Report: https://www.reportsandinsights.com/report/Chemiluminescence Immunoassay (CLIA)-market
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us:
Reports and Insights consistently mееt international benchmarks in the market research industry and maintain a kееn focus on providing only the highest quality of reports and analysis outlooks across markets, industries, domains, sectors, and verticals. We have bееn catering to varying market nееds and do not compromise on quality and research efforts in our objective to deliver only the very best to our clients globally.
Our offerings include comprehensive market intelligence in the form of research reports, production cost reports, feasibility studies, and consulting services. Our team, which includes experienced researchers and analysts from various industries, is dedicated to providing high-quality data and insights to our clientele, ranging from small and medium businesses to Fortune 1000 corporations.
Contact Us:
Reports and Insights Business Research Pvt. Ltd.
1820 Avenue M, Brooklyn, NY, 11230, United States
Contact No: +1-(347)-748-1518
Email: [email protected]
Website: https://www.reportsandinsights.com/
Follow us on LinkedIn: https://www.linkedin.com/company/report-and-insights/
Follow us on twitter: https://twitter.com/ReportsandInsi1
#Chemiluminescence Immunoassay (CLIA) Market share#Chemiluminescence Immunoassay (CLIA) Market size#Chemiluminescence Immunoassay (CLIA) Market trends
0 notes
Text
Therapeutic Drug Monitoring Market to Reflect Impressive Growth Rate by 2024
Therapeutic drug monitoring (TDM) is the clinical practice of determining the concentration of a specific drug in a patient’s bloodstream at different intervals. Therapeutic drug monitoring (TDM) helps to sustain a constant volume of drug in bloodstream required to show therapeutic effects without toxic effects, thereby optimizing individual dosage regimens. Therapeutic drug monitoring (TDM) is generally not deployed for majority of drugs, rather it is used primarily for monitoring drugs with narrow therapeutic window, medicines for which required concentrations are challenging to monitor, drugs with marked pharmacokinetic variability, and drugs known to cause adverse effects. TDM begins with the initiation of medication, and involves determining a preliminary dosage regimen suitable for the clinical state and patient characteristics as age, body mass index, weight, organ function, and associated drug therapy. TDM serves in assessing the efficiency and safety of a medication when administered into the individual patient.
Request a PDF Brochure - https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=39239
The therapeutic drug monitoring (TDM) market is anticipated to witness healthy growth during the forecast period, owing to steep rise in the demand for better healthcare for the growing urban population. Increased knowledge of pharmacogenetics and pharmacokinetics of drugs is inclining the clinicians towards therapeutic drug monitoring (TDM) of certain medications. Rapid increase in the prevalence of complex neurological diseases and cancer have fueled the demand for high end diagnostic methods such as genetic testing which in turn is anticipated to boost therapeutic drug monitoring (TDM) market. Moreover, increasing investments in research and development of novel drugs and vaccines by private and government bodies is also expected to propel the demand for the therapeutic drug monitoring (TDM) market in the near future. In addition, rise in the geriatric population, especially in developing geographies and development of innovative tools in medication management are further driving this market. However, low research and development (R&D) returns, high infrastructure cost, stringent regulatory framework, and lack of trained resources are some of the restraints of the therapeutic drug monitoring (TDM) market.
The therapeutic drug monitoring (TDM) market can be segmented on the basis of product type, technology, drug class, end user and region. The product type segment can be further segmented into number of equipment such as immunoassay analyzers, spectrophotometers, chromatograms, and consumables. Increasing preference for technologically advanced services, product innovation, and growing number of diseases is anticipated to fuel the therapeutic drug monitoring (TDM) market growth. On the basis of technology the market is segmented into immunoassays such as chemiluminescent assays (CLIA), enzyme-linked immunosorbent assay (ELISA), proteomics technologies, chromatography, etc. On the basis of drug class, the market can be segmented into antiepileptics, antibiotics, psychoactive drugs, antiarrhythmic drugs, bronchodilators, immunosuppressants, and others.
Request for Analysis of COVID19 Impact on Therapeutic Drug Monitoring Market - https://www.transparencymarketresearch.com/sample/sample.php?flag=covid19&rep_id=39239
Geographically, the therapeutic drug monitoring (TDM) market can be segmented into North America, Europe, Asia Pacific, Middle East and Africa and Latin America. North America is estimated to hold the largest share of the global therapeutic drug monitoring (TDM) market due to the presence of superior health care base, strong regional economics, and greater emphasis on prevention and wellness by government. This trend is also reflected in other countries. The therapeutic drug monitoring (TDM) market in Europe is expected to witness rapid growth, attributed to high consciousness about wellbeing in the region coupled with increasing spending on healthcare services. Countries such as China, Japan, and India in the Asia Pacific are poised to be the most promising markets for therapeutic drug monitoring (TDM) in the near future. Major factors for the positive outlook are extensive development of health care infrastructure, and growing emphasis on research and development in the health care sector.
Key players operating in the therapeutic drug monitoring market include Abbott Diagnostics, Danaher Corporation, F. Hoffmann-La Roche AG, Bio-Rad Laboratories Inc., Siemens Healthcare, Roche Diagnostics, Thermo Fisher Scientific, and Agilent Technologies.
The report offers a comprehensive evaluation of the market. It does so via in-depth qualitative insights, historical data, and verifiable projections about market size. The projections featured in the report have been derived using proven research methodologies and assumptions. By doing so, the research report serves as a repository of analysis and information for every facet of the market, including but not limited to: Regional markets, technology, types, and applications.
Buy now Therapeutic Drug Monitoring Market Report - https://www.transparencymarketresearch.com/checkout.php?rep_id=39239<ype=S
The report has been compiled through extensive primary research (through interviews, surveys, and observations of seasoned analysts) and secondary research (which entails reputable paid sources, trade journals, and industry body databases). The report also features a complete qualitative and quantitative assessment by analyzing data gathered from industry analysts and market participants across key points in the industry’s value chain.
A separate analysis of prevailing trends in the parent market, macro- and micro-economic indicators, and regulations and mandates is included under the purview of the study. By doing so, the report projects the attractiveness of each major segment over the forecast period.
More Trending Reports by Transparency Market Research:
https://www.prnewswire.com/news-releases/magnifying-prevalence-of-malnutrition-and-other-disorders-to-add-extra-stars-of-growth-global-medical-foods-market-to-reach-valuation-of-us-33-3-bn-by-2030--301123806.html
https://www.prnewswire.com/news-releases/technological-strides-in-ultrasound-devices-market-expand-scope-of-diagnosing-complex-diseases-market-to-clock-cagr-of-5-6-from-2018-to-2026-tmr-301129995.html
About Us
Transparency Market Research is a next-generation market intelligence provider, offering fact-based solutions to business leaders, consultants, and strategy professionals.
Our reports are single-point solutions for businesses to grow, evolve, and mature. Our real-time data collection methods along with ability to track more than one million high growth niche products are aligned with your aims. The detailed and proprietary statistical models used by our analysts offer insights for making right decision in the shortest span of time. For organizations that require specific but comprehensive information we offer customized solutions through ad-hoc reports. These requests are delivered with the perfect combination of right sense of fact-oriented problem solving methodologies and leveraging existing data repositories.
TMR believes that unison of solutions for clients-specific problems with right methodology of research is the key to help enterprises reach right decision.
Contact
Transparency Market Research
State Tower,
90 State Street,
Suite 700,
Albany NY - 12207
United States
USA - Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com
1 note
·
View note
Text
Cancer Diagnostics Global Market - Forecast to 2026
The understanding of the pathways of cancer development and their causes has led to the development of novel methodologies, and there is an emerging trend towards precision health in today’s world to enhance cancer diagnosis, treatment, prevention and monitoring by using innovative diagnostic technologies with broader range.
Over the past few years technical advancements has led to the progression in cancer screening and treatment. The rise in number of new cases necessitates for more improvised technologies in diagnosis and monitoring of cancer. Cancer diagnostics are the foundation for the successful cancer prevention, where it provides quantitative measurements, changes at the genetic level thereby finding the underlying mechanism of disease and enabling the oncologist to tailor care at individual level and facilitating the personalized medicine practice.
Due to the side effects of radiation and other image-based diagnosis of cancer, there is a huge opportunity for non-image based techniques such as in-vitro diagnostics comprising tests based on molecular biology and immunology. These tests provide rapid and precise information about cancer and have revolutionized cancer management around the world. Some of the advantages of non-image based diagnostics are, up to 70% of the healthcare decisions are influenced by molecular and immune-based tests further improving the quality of personalized care of the patient, potential improvement in the patient outcome and facilitate early detection of cancer with sensitive assays thereby reducing the cost of healthcare of an individual.
According to IQ4I analysis, the cancer diagnostics global market is expected to reach $10,627.4 million by 2026 growing at a high single digit CAGR from 2019 to 2026. The growth of cancer diagnostic market is driven by the increasing number of cancer associated with the lifestyle, dramatic changes in the demography, advances in the disease genomics, also adoption of nationwide cancer screening programs as a part of national cancer control plans by individual countries (almost 120 countries have included national plans for cervical and breast cancer screening programs respectively) and discovery of new drugs. Cancer diagnostic tests have provided a greater value and are likely to play an escalating role in the future market.
The commercialization of cancer diagnostics techniques depends on economical, clinical and logistic challenges. One among them is the reimbursement system with suitable coding, positive coverage, and assessment of the price of novel diagnostics. With the reimbursement policies, all patients can access all new diagnostic tests on sustained bases thereby reducing the financial pressure. Also, high investment by major players for the production and release of new diagnostics techniques to the market will have a major role in driving the cancer diagnostics market. The restraints of the cancer diagnostics will include lack of skilled professionals, high cost of advanced techniques, product recall due to manufacturing defects and quality issues, stringent and time consumption for getting approvals from the regulatory bodies, lack of reimbursement will also hamper the growth of cancer diagnostic market.
The cancer diagnostics global market is segmented based on technology, sample source, application and end-users. The technology market is further categorized into immunoassays, molecular-based assays and others. The immunoassays global market is further sub-segmented into
Access Free PDF With Graphs and Charts : https://www.sdki.jp/sample-request-116339
Immunohistochemistry (IHC), Enzyme Linked Immunosorbent Assay (ELISA) and others comprising of RIA (Radioimmunoassay), CLIA (Chemiluminescence), and FIA (Fluorescence immunoassays), among which, ELISA commanded the largest revenue in 2019, and IHC is expected to grow at a strong double digit CAGR from 2019 to 2026. The molecular-based global market is further sub-segmented into in-situ hybridization, PCR, microarray and sequencing, among which PCR commanded the largest revenue in 2019, the sequencing segment is expected to grow at a strong double digit CAGR from 2019 to 2026. The other technology segment is expected to grow at a mid single digit CAGR from 2019 to 2026.
Based on the source of the sample taken for performing the diagnostic test, the market is segmented into three categories, viz., blood, tissue and others. Among these segment, blood accounted for the largest revenue generation in 2019 and is expected to grow at a strong double digit CAGR from 2019 to 2026. The blood-based tests provide accurate results when compare to tissue and other source and its less painful for collection from the individual compare to tissue and other source such as cerebrospinal fluid, bone marrow aspirate sample. Also, blood as a sample source can be used for early diagnosis before the occurrence of symptoms.
The cancer diagnostics by the application are classified into cancer type and cancer care segment. The market based on cancer type is further segmented into lung, breast, colorectal, prostate, ovarian, liver and other cancers. The largest revenue was generated by colorectal cancer in 2019 and is expected to grow at a strong double digit CAGR from 2019 to 2026. The cancer care segment is further classified into diagnosis, early screening, companion diagnostics, prognosis and monitoring. Among which, diagnosis accounted for the largest revenue in 2019 whereas, companion diagnostics segment is expected to grow at a strong double digit CAGR from 2019 to 2026.
The cancer diagnostics end-user market is segmented into hospitals, clinical/centralized laboratories, and other end-users such as biopharma, academics and research. Clinical/centralized laboratories segment accounted for the largest revenue in 2019 owing to the access of advanced and novel technologies for the diagnosis and also the availability of skilled professionals with more capital investments as compared with hospitals. Hospitals segment is expected to grow at a high single digit CAGR from 2019 to 2026.
Geographical wise, North America region commanded the largest revenue in 2019, owing to the high demand for early detection, treatment and prevention of cancer with advanced technology due to larger outbreaks of cancer associated with the lifestyle. However, the Asia-Pacific region is expected to grow at a strong double digit CAGR from 2019 to 2026 owing to higher incidence of cancer cases compare to other regions and also increasing awareness programmes on the need for screening and prevention of cancer at early stages.
Request Sample For More Insights : https://www.sdki.jp/sample-request-116339
The cancer diagnostics global market is a competitive market and all the existing players in this market are involved in developing new and advanced techniques for diagnosis to maintain their market shares and also acquiring companies for product expansion and technological innovations.
Some of the key players in cancer diagnostics global market are Abbott Laboratories (U.S.), F.Hoffmann-LA Roche AG (Switzerland), Qiagen (Netherlands), Exact Sciences Corporation (U.S.), Agilent Technologies (U.S.), Danaher Corporation (U.S.), Hologic, Inc. (U.S.), Myriad Genetics, Inc. (U.S.), Siemens Healthineers (Germany), and Guardant Health (U.S.).
0 notes
Text
Automated Immunoassay Analysers Market 2021 Key Companies, Geographical Analysis, Research Development, and Forecast 2027
Automated Immunoassay Analysers Market Overview:
The rise is accompanied by a growing tendency toward lab automation, as well as an increase in the occurrence of infectious diseases. Due to incremental improvements to current items and expanded clinical applications, these products are gaining appeal. The Global Automated Immunoassay Analyzers Industry is being driven by current trends in automation and integration, which have resulted in the launch of unique products in the market. They are increasingly being used for drug discovery by a variety of pharmaceutical and biotechnology companies.
Automated Immunoassay Analysers Market Report Scope the latest industry report on the global Automated Immunoassay Analysers market assesses the opportunities and current market landscape, offering insights and updates on the corresponding segments for the forecasted period of 2021-2027. The report contains a complete analysis of major market dynamics as well as detailed information on the global Automated Immunoassay Analysers market's structure. This market research report provides unique insights into how the global Automated Immunoassay Analysers market is expected to grow from 2021 to 2027.
The primary goal of the Automated Immunoassay Analysers market research is to provide detailed information on market opportunities that are assisting in the transformation of global Automated Immunoassay Analysers enterprise. Report provide projected growth rates along with the compound annual growth rate (CAGR) for forecasted period to enable readers to better understand the monitoring and assessment of the global Automated Immunoassay Analysers market, as well as to discover lucrative opportunities in the market.
Request for free sample: https://www.maximizemarketresearch.com/request-sample/11000
Automated Immunoassay Analysers Market Scope:
Maximize Market Research, report provide overall market insights for manufacturers, suppliers, distributors, and investors in the global Automated Immunoassay Analysers market. The information and data offered in the report may be used by all stakeholders in the global Automated Immunoassay Analysers market, as well as industry professionals, researchers, journalists, and business researchers.
Maximize Market Research, report provides a unique research approach to conduct detailed research on the global Automated Immunoassay Analysers market and make conclusions on the market's future growth factors. Primary and secondary research methodologies are combined in the research approach to assure the authenticity and validity of the conclusions in this report.
The report discusses the Automated Immunoassay Analysers market's drivers, restraints, opportunities, and challenges. The research helps to identify the market growth drivers and determining how to utilize these factors as strengths. Restraints can assist readers in identifying traits that are restricting the Automated Immunoassay Analysers market, as well as reducing them before they become an issue. This will assist readers in comprehending the aspects that will influence your ability to capitalise on possibilities.
Automated Immunoassay Analysers Market Segmentation:
•Global Automated Immunoassay Analysers Market, By Technology
o Chemiluminescence Immunoassay (CLIA)
o Enzyme Linked Fluorescent Immunoassay (ELFA)
o Enzyme-linked Immunosorbent Assay (ELISA)
o Radioimmunoassay (RIA)
o Others (Biochip Assay, Immunofluorescent Assay, and Counting Assay)
• Global Automated Immunoassay Analysers Market, By Sales Model
o Reagent Rental / Lease
o Outright Sale
•Global Automated Immunoassay Analysers Market, By Applications
o Infectious diseases
o Endocrinology
o Drug Monitoring
o Cardiology
o Oncology
o Allergy Testing
o Others
• Global Automated Immunoassay Analysers Market, By End-User
o Hospitals
o Diagnostic Laboratories
o Blood Banks
o Others (Research Institutes, Pharmaceutical & Biotechnology Industry, Food & Beverages Industry)
Due to the consolidation trend among hospitals, which has resulted in high test volumes, and favourable health reforms to improve hospital treatment, hospitals accounted for the biggest revenue share. Furthermore, the growing number of corporate hospital chains is expected to enhance demand for Global Automated Immunoassay Analysers in the coming years. A growing number of hospitals is one of the other factors. Automated solutions allow hospitals to devote their professional resources to more vital tasks such as analysis and review of test data.
Get more Report Details: https://www.maximizemarketresearch.com/market-report/global-automated-immunoassay-analysers-market/11000/
Automated Immunoassay Analysers Market Key Players:
Siemens Healthineers
Abbott Laboratories
bioMérieux SA
Bio-Rad Laboratories, Inc
Pall Corporation
Beckman Coulter, Inc.
Sigma-Aldrich Corporation
Sartorius AG
Ortho Clinical Diagnostics
Radiometer APS
Sartorius
Danaher
Randox Laboratories
F. Hoffmann-La Roche Ltd.
DiaSorin S.p.A.
Agilent Technologies
SNIBE Diagnostics
Pfizer Inc.
Hospitech
CSL Behring
The competitive landscape shows the market share of major key competitors, as well as their key development plans and current financial performance over the previous five years. This information is anticipated to help businesses understand their competitors on a global level. Furthermore, the reports feature company profiles, product offers, critical financial data, country-level research, and a synthesis of demand and supply variables that influence market growth.
Automated Immunoassay Analysers Market Regional Analysis:
Geographically, Automated Immunoassay Analysers market report is segmented into several key regions are as follows,
Asia-Pacific (Vietnam, China, Malaysia, Japan, Philippines, Korea, Thailand, India, Indonesia, and Australia)
Europe (Turkey, Germany, Russia UK, Italy, France, etc.)
North America (the United States, Mexico, and Canada.)
South America (Brazil etc.)
The Middle East and Africa (GCC Countries and Egypt.)
Furthermore, the study covers market size, growth rate, import and export, as well as country-level analysis, integrating the demand and supply forces of the Automated Immunoassay Analysers market in these countries, which are impacting market growth.
COVID-19 Impact Analysis on Automated Immunoassay Analysers Market:
COVID-19's global influence on the Automated Immunoassay Analysers market was examined in this research. During this crisis, the report examines the Automated Immunoassay Analysers market's alternatives, demanding conditions, and difficult possibilities in detail. In terms of funding and market expansion, the paper briefly examines the COVID-19's merits and limitations. The study also contains a set of concepts that should aid readers in developing and planning company strategies.
The report considers consultations to overcome past disruptions and foresees potential ones in order to improve preparation. Businesses can use the frameworks to design their strategic alignments in order to recover from such disruptive trends. Maximize Market Research analysts can also assist readers in breaking down a complex circumstance and bringing resiliency to a situation that is uncertain.
About Us:
Maximize Market Research provides B2B and B2C research on 12000 high growth emerging opportunities technologies as well as threats to the companies across the Healthcare, Pharmaceuticals, Electronics Communications, Internet of Things, Food and Beverages, Aerospace and Defence and other manufacturing sectors.
Contact Us:
MAXIMIZE MARKET RESEARCH PVT. LTD.
3rd Floor, Navale IT Park Phase 2,
Pune Bangalore Highway,
Narhe, Pune, Maharashtra 411041, India.
Email: [email protected]
Phone No.: +91 20 6630 3320
Website: www.maximizemarketresearch.com
0 notes
Text
By the Year 2028, the Chemiluminescence Immunoassay Market Is Expected to Witness Robust Expansion, With a Projected Size of $13.75 Billion
Chemiluminescence Immunoassay Market Growth & Trends
The global chemiluminescence immunoassay market size is expected to reach USD 13.75 billion by 2028, according to a new report by Grand View Research, Inc. It is expected to expand at a CAGR of 7.9% from 2021 to 2028. The high prevalence of chronic diseases, several advantages of chemiluminescence immunoassay (CLIA) technique, and approval and…

View On WordPress
0 notes