#Chromatography Reagents
Explore tagged Tumblr posts
Text
#romatography Consumables#Analytical Reagents#Chromatography Reagents#Chromatography Buffers#Chromatography Reagents Market
0 notes
Text
This report studies the Chromatography Reagents market size (value and volume) by players, regions, product types and end industries, history data 2018-2022 and forecast data 2023-2030
0 notes
Text
Gas Chromatography Market: Upcoming Opportunities with SWOT Analysis By 2037
In 2024, the global gas chromatography (GC) market was estimated at USD 4.61 billion and is anticipated to exceed USD 11.66 billion by 2037, growing at a compound annual growth rate (CAGR) of more than 7.4% throughout the forecast period from 2025 to 2037.This robust growth is fueled by rising demand across a broad range of industries including pharmaceuticals, environmental monitoring, petrochemicals, and food safety testing. Increasing regulatory requirements and the need for highly accurate, real-time analysis are accelerating adoption rates globally.
Gas Chromatography Industry Demand
Gas chromatography is an advanced analytical method employed to separate, detect, and measure volatile and semi-volatile substances within complex mixtures.The method relies on the differential distribution of sample components between a stationary phase and a mobile gas phase, offering rapid and precise analytical results.
The demand for GC technology is primarily driven by its cost-effectiveness, operational simplicity, and long shelf life of consumables. Its widespread applicability in critical sectors—ranging from forensic science to food safety—adds to its increasing adoption. Environmental regulations and public health safety mandates continue to expand the scope and necessity of advanced GC systems.
Gas Chromatography Market: Growth Drivers & Key Restraint
Growth Drivers –
Technological Advancements: Modern gas chromatographs now feature enhanced sensitivity, compact designs, and automated functionalities. Integration with mass spectrometry and AI-powered data analytics is driving higher throughput and better accuracy, spurring market growth.
Outsourcing and CRO Trends: Pharmaceutical and biotech companies increasingly rely on Contract Research Organizations (CROs) for R&D and quality control. This outsourcing trend is amplifying the demand for GC systems in third-party labs worldwide.
Stringent Environmental and Safety Regulations: Rising governmental and international regulations around environmental safety, air pollution, and food quality have pushed industries to adopt more accurate and reliable GC technologies for routine testing and compliance.
Restraint –
High Initial Investment and Skilled Operation Needs: Although gas chromatography is cost-effective in the long term, the initial capital investment and requirement for trained professionals often hinder adoption in small- to mid-sized enterprises, particularly in emerging markets.
Request Sample@ https://www.researchnester.com/sample-request-4930
Gas Chromatography Market: Segment Analysis
Segment Analysis by Product Type –
Instruments: Core gas chromatographs are evolving with modular systems, automated injectors, and enhanced detectors.
Accessories & Consumables: Columns, injectors, detectors, vials, and septa fall under this category. High replacement frequency and compatibility with a range of analytical applications ensure consistent demand.
Reagents: Reagents, including carrier gases and derivatization agents, play a critical role in the accuracy and specificity of GC results. Growing application in pharmaceuticals and food safety testing is propelling this segment.
Segment Analysis by End‑User –
Pharmaceuticals & Biotechnology: Used extensively for drug development, quality control, and impurity profiling.
Academic & Research Institutes: Universities and scientific labs utilize GC systems for exploratory research, toxicology studies, and innovation in analytical chemistry.
Oil & Gas: GC is vital in hydrocarbon analysis, process optimization, and environmental monitoring. The sector’s push for refining efficiency and pollution control enhances GC adoption.
Gas Chromatography Market: Regional Insights
North America:
North America remains the dominant region, driven by a well-established pharmaceutical sector, advanced research infrastructure, and strict environmental standards. The U.S. leads in adoption due to consistent government funding and industrial R&D initiatives.
Europe:
Europe follows closely, with significant uptake in environmental testing, food safety, and petrochemical industries. EU regulations on emissions and contamination levels foster strong demand for reliable and high-performance GC systems.
Asia-Pacific (APAC):
APAC is expected to witness the fastest growth, attributed to rapid industrialization, increasing investment in healthcare infrastructure, and the expansion of local pharmaceutical manufacturing. China, India, and Japan are particularly active markets due to rising domestic production and export needs.
Top Players in the Gas Chromatography Market
Leading companies in the gas chromatography market include Waters Corporation, Shimadzu Corporation, Thermo Fisher Scientific, PerkinElmer, Merck KGaA, Phenomenex, Bio-Rad Laboratories, and Cytiva. These organizations are recognized as global providers of analytical instruments, life science tools, and laboratory technologies used extensively in research, diagnostics, quality assurance, and industrial analysis.
Access Detailed Report@ https://www.researchnester.com/reports/gas-chromatography-market/4930
Contact for more Info:
AJ Daniel
Email: [email protected]
U.S. Phone: +1 646 586 9123
U.K. Phone: +44 203 608 5919
0 notes
Text
Analytical and Scientific Instrumentation Market to see 6% CAGR from growing biotech by 2030
The analytical and scientific instrumentation market is growing at a CAGR of approximately 6% over the forecast period. The market expansion is driven by increasing investments in pharmaceutical research, environmental monitoring, and industrial quality control. However, high instrument costs and stringent regulatory compliance requirements hinder wider implementation, particularly in developing countries.
Analytical and scientific instruments are a broad category of devices and systems used to measure, analyze, and quantify physical, biological, and chemical properties. These instruments are crucial to fields including environmental science, biotechnology, medicine, and food safety because they enable precise data collection and validation. Scientists can use techniques like chromatography, spectroscopy, and electrophoresis to discover pollutants, assess the composition of materials, and produce new products. Their importance goes beyond making sure safety regulations are followed, increasing the effectiveness of R&D, and raising the calibre of scientific findings.
🔗 Want deeper insights? Download the sample report:https://meditechinsights.com/analytical-and-scientific-instrumentation-market/request-sample/
Rising Demand for Precision in Pharmaceutical and Biotech R&D
The analytical and scientific equipment market growth is driven by the rising demand for drug discovery & development, precision medicine, and quality control, and to meet these needs, laboratories and research institutes require more advanced and reliable tools. For example, techniques like mass spectrometry & chromatography are crucial in pharmacokinetics and biomarker investigations to make sure that drug formulations meet safety and efficacy standards. Additionally, the regulatory push for stricter validation and compliance has necessitated the use of more sophisticated analytical techniques. Furthermore, companies are investing heavily in high-throughput screening techniques in an attempt to expedite drug development, which is driving increased demand for advanced tools. Also, due to the growing demand for biosimilars and complex biologics, there is an increasing need for specialized analytical tools for their structural characterization and stability testing. This is trending in personalized medicine, where analytical and scientific instruments are significant for genetic and molecular profiling, further propelling the market growth.
Advancements in Microfluidics for High-Throughput Analysis
The adoption of microfluidics in analytical and scientific instruments has improved accuracy and efficiency in various applications. The key benefit of microfluidics is its capacity to combine several analytical processes, such as sample preparation, reaction, and detection, onto an automated platform, which reduces human error and boosts throughput. Microfluidics has reduced analysis times while increasing sensitivity and repeatability in clinical diagnostics, pharmaceutical research and development, and other medical fields. With the use of microchannels, this technology has made it possible to manipulate small fluid volumes, enabling quick chemical and biological tests with minimal use of reagents. Additionally, there is a growing adoption of microfluidic-based lab-on-a-chip devices in POC diagnostics as they provide cost-effective and compact real-time analysis options. All these developments have streamlined intricate processes, encouraging downsizing trends and creating highly accurate scientific and analytical tools.
Competitive Landscape Analysis
The global analytical and scientific instrumentation market is marked by the presence of established and emerging market players such as Thermo Fisher Scientific, Inc.; Shimadzu Corp.; Danaher Corp.; Agilent Technologies, Inc.; PerkinElmer, Inc.; Mettler Toledo; Bio-Rad Laboratories, Inc.; Illumina, Inc.; Eppendorf SE; F. Hoffmann-La Roche AG; Sartorius AG; Waters Corp.; and Avantor, Inc among others. Some of the key strategies adopted by market players include new product development, strategic partnerships and collaborations, and geographic expansion.
Gain a competitive edge-request a sample report now! https://meditechinsights.com/analytical-and-scientific-instrumentation-market/request-sample/
Global Analytical and Scientific Instrumentation Market Segmentation
This report by Medi-Tech Insights provides the size of the global analytical and scientific instrumentation market at the regional- and country-level from 2023 to 2030. The report further segments the market based on product type, technology and end-user.
Market Size & Forecast (2023-2030), By Product Type, USD Million
Instruments
Services
Software
Market Size & Forecast (2023-2030), By Technology, USD Million
Chromatography
Mass Spectrometry
Spectroscopy
Microscopy
PCR
Flow Cytometry
Others
Market Size & Forecast (2023-2030), By End-user, USD Million
Pharmaceutical & Biotechnology Companies
Environmental Testing Labs
Food & Beverage Industry
Academic & Research Institutes
Others
Market Size & Forecast (2023-2030), By Region, USD Million
North America
US
Canada
Europe
UK
Germany
France
Italy
Spain
Rest of Europe
Asia Pacific
China
India
Japan
Rest of Asia Pacific
Latin America
Middle East & Africa
About Medi-Tech Insights
Medi-Tech Insights is a healthcare-focused business research & insights firm. Our clients include Fortune 500 companies, blue-chip investors & hyper-growth start-ups. We have completed 100+ projects in Digital Health, Healthcare IT, Medical Technology, Medical Devices & Pharma Services in the areas of market assessments, due diligence, competitive intelligence, market sizing and forecasting, pricing analysis & go-to-market strategy. Our methodology includes rigorous secondary research combined with deep-dive interviews with industry-leading CXO, VPs, and key demand/supply side decision-makers.
Contact:
Ruta Halde Associate, Medi-Tech Insights +32 498 86 80 79 [email protected]
0 notes
Text
Biochemical Reagents Market experiencing steady growth driven by 2037
The Biochemical Reagents Market has witnessed significant momentum in recent years, with expanding applications across diagnostics, therapeutics, and life sciences research. In 2024, the market was valued at USD 34.5 billion, and it is projected to escalate to USD 98.1 billion by 2037 rising at CAGR of approximately 8.3% during the forecast period from 2025 to 2037. The market's robust trajectory is underpinned by rising healthcare expenditures, continuous innovation in molecular biology tools, and increasing biopharmaceutical research activity worldwide.
Biochemical Reagents Industry Demand
The Biochemical Reagents Market encompasses a wide array of chemical compounds and formulations used in biological research and diagnostic procedures. These reagents are essential for assays, molecular research, and drug development. The market spans multiple sectors, including genomics, proteomics, cell biology, microbiology, and diagnostic testing.
Key demand drivers include:
Cost-effectiveness and versatility: Many biochemical reagents are easy to use and offer a high return on investment, especially in routine lab settings.
Ease of administration: These reagents integrate seamlessly with existing laboratory workflows and automated platforms.
Extended shelf life: Stable storage conditions and long shelf life make them ideal for both high-volume testing facilities and research labs with budget constraints.
As the global emphasis on personalized medicine and high-throughput screening intensifies, the need for robust, scalable, and efficient reagents continues to grow.
Request Sample@ https://www.researchnester.com/sample-request-7699
Biochemical Reagents Market: Growth Drivers & Key Restraint
Key Growth Drivers
Rising Outsourcing to CROs and CDMOs Pharmaceutical companies are increasingly outsourcing R&D and production tasks to specialized Contract Research and Manufacturing Organizations, boosting the demand for quality biochemical reagents used in drug discovery, toxicity studies, and clinical trials.
Prevalence of Chronic and Infectious Diseases The persistent global burden of conditions like cancer, cardiovascular diseases, and emerging infectious diseases has intensified the demand for reagents used in diagnostic assays, biomarker analysis, and precision medicine initiatives.
Advancements in Molecular Biology Technologies Innovations such as CRISPR, next-generation sequencing (NGS), and real-time PCR have dramatically elevated the use of sophisticated reagents in genomics and proteomics, opening new frontiers in biomedical research and diagnostics.
Major Restraint
High Cost of Specialized Reagents and Equipment While standard reagents are relatively affordable, specialized or custom reagents often come with high costs. This poses a challenge for small-scale labs, especially in developing economies, and may restrict adoption across underfunded research institutions.
Biochemical Reagents Market: Segment Analysis
By End Use
Pharmaceutical and Biotechnology Companies: These organizations dominate the market owing to the increasing focus on biopharmaceutical development, monoclonal antibody production, and biologics research.
Academic and Research Institutes: A major consumer of reagents for experimental and teaching purposes, particularly in fields such as molecular biology, genetics, and microbiology.
Hospitals and Diagnostic Laboratories: Rising demand for rapid diagnostics and biomarker testing propels consistent usage of reagents in clinical settings.
Contract Research Organizations (CROs): As drug sponsors increasingly depend on CROs for trial management, reagent consumption rises in toxicology and pharmacokinetics.
Contract Development and Manufacturing Organizations (CDMOs): CDMOs rely on bulk reagents for formulation, process development, and scale-up, particularly in biosimilars and advanced therapies.
By Product Type
Chromatography Reagents: Essential in separation sciences, these reagents play a critical role in quality control and purity assessment of drug compounds.
Cell & Tissue Culture Reagents: These reagents enable the in vitro cultivation of cells, supporting research in cancer, stem cell therapy, and vaccine development.
PCR Reagent Kits: Widely used in molecular diagnostics and genetic analysis, especially following their prominent role during the COVID-19 pandemic.
Electrophoresis Reagents: Critical in DNA/RNA/protein separation processes, these reagents remain fundamental in academic and clinical labs.
Flow Cytometry Reagents: Increasing applications in immunophenotyping, cell sorting, and disease diagnostics continue to drive demand.
Others: Includes immunoassay reagents, buffers, dyes, and stains used in varied life science and analytical applications.
By Application
Drug Discovery & Development: Biochemical reagents support target validation, hit identification, and toxicity screening during early-phase R&D.
Genomics: Used extensively in gene expression studies, SNP analysis, and epigenetics, helping advance personalized medicine.
Proteomics: Facilitates protein identification and functional analysis, crucial in understanding disease mechanisms and therapeutic pathways.
Diagnostics: Core components in clinical chemistry and molecular diagnostics, enabling early detection and disease monitoring.
Biotechnology Research: Includes agricultural biotechnology, food testing, and environmental monitoring applications.
Others: Encompasses forensic science, veterinary research, and industrial biotechnology sectors.
Biochemical Reagents Market: Regional Insights
North America
North America remains the most mature market, supported by strong government funding for life sciences, a thriving pharmaceutical industry, and a well-established diagnostic infrastructure. The U.S. particularly sees high adoption due to technological readiness and robust biotech ecosystems.
Europe
Europe grow with countries like Germany, the UK, and France driving growth. Strong academic research output, supportive regulatory frameworks, and increasing collaborations between academia and industry act as key enablers.
Asia-Pacific (APAC)
APAC is expected to witness the fastest growth due to expanding biotech sectors in China, India, South Korea, and Japan. Rising healthcare investments, clinical trials, and government pharma incentives are driving regional demand.
Top Players in the Biochemical Reagents Market
Leading Competitive Landscape & Strategic Initiatives of the Biochemical Reagents Market include Thermo Fisher Scientific Inc., Merck KGaA, F. Hoffmann-La Roche AG, Takara Bio Inc., Bio-Rad Laboratories, Inc., Agilent Technologies, Inc., QIAGEN N.V., Danaher Corporation, Abbott Laboratories, Siemens Healthineers AG, Becton, Dickinson and Company, Promega Corporation, Waters Corporation, Fujirebio, and BioMérieux SA. These companies compete based on product innovation, portfolio breadth, global distribution networks, and strategic collaborations in biopharmaceutical and diagnostic segments.
Access Detailed Report@ https://www.researchnester.com/reports/biochemical-reagents-market/7699
Contact for more Info:
AJ Daniel
Email: [email protected]
U.S. Phone: +1 646 586 9123
0 notes
Text
Cas No: 768-66-1 Manufacturers
A Deep Dive into CAS No: 768-66-1 – Manufacturing and Global Landscape
In the expansive world of chemical compounds, every substance holds a unique role in industrial, pharmaceutical, or agricultural applications. One such compound is 2,2,6,6-Tetramethylpiperidine, commonly recognized by its CAS number: 768-66-1. This blog takes a deep dive into the manufacturing landscape of this compound, exploring its characteristics, production considerations, global market trends, and overall significance — all without mentioning specific company names.
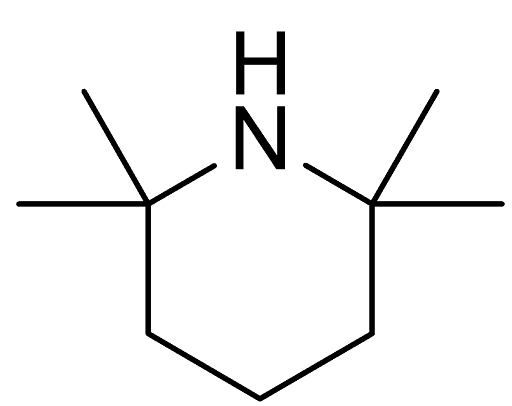
Understanding 2,2,6,6-Tetramethylpiperidine
The compound is air-sensitive and needs to be stored under inert conditions, typically using nitrogen or argon to avoid degradation or unwanted reactions.
One of the key reasons this compound is widely produced and used is due to its strong basicity and steric hindrance properties. These characteristics make it particularly useful in synthetic organic chemistry, especially in reactions requiring selective deprotonation or where a hindered base is essential.
Applications Driving Its Demand
The applications of 2,2,6,6-Tetramethylpiperidine span several industrial sectors:
Pharmaceutical Synthesis: It serves as a precursor or reagent in the synthesis of various active pharmaceutical ingredients (APIs), helping in the creation of complex molecules.
Polymer Industry: The compound is a key building block in the development of stabilizers used in polymers, particularly in light and heat stabilization processes.
Specialty Chemicals: TMP is used in the development of certain high-performance materials and specialty chemicals due to its unique structural features.
Organic Synthesis: Its strong base properties and steric hindrance make it ideal for deprotonation steps in lab-scale and industrial-scale synthesis alike.
Due to these varied uses, the compound is manufactured globally and supplied to research laboratories, pharmaceutical producers, and chemical processing plants.
The Manufacturing Process
Manufacturing 2,2,6,6-Tetramethylpiperidine requires a specialized setup due to the need for precision in chemical handling and environmental control. Generally, the production process involves the catalytic hydrogenation of precursors such as 2,2,6,6-tetramethylpyridine or related piperidine derivatives. The process must be carried out under controlled temperatures and pressures to maintain product purity and yield.
After synthesis, the compound undergoes several stages of purification, typically via distillation, to achieve high purity levels — often exceeding 98% or even 99% depending on the application. Quality control measures, such as gas chromatography (GC) and nuclear magnetic resonance (NMR) spectroscopy, are employed to ensure batch consistency and performance suitability.
Global Manufacturing Hubs
The production of 2,2,6,6-Tetramethylpiperidine is not limited to one specific country or region. It is part of a global supply chain supported by chemical manufacturers in:
Asia: Especially in regions with strong chemical infrastructure, where large-scale manufacturing plants produce bulk quantities of this compound.
Europe: Often focused on high-purity or specialty-grade TMP for use in pharmaceuticals and high-performance materials.
North America: Also engaged in manufacturing, with some facilities focusing on smaller, research-grade batches for laboratory use.
The geographical diversity ensures supply chain resilience and gives buyers the flexibility to source based on factors like purity, packaging, shipping logistics, and price.
Quality and Compliance
For manufacturers, maintaining high standards of purity is critical. The desired purity level of TMP typically ranges from 98% to above 99%, depending on the end-use. Manufacturers usually provide a certificate of analysis (CoA) with every batch to verify compliance with these specifications.
Apart from quality, regulatory compliance is another essential factor. Manufacturers often operate under Good Manufacturing Practices (GMP) or ISO-certified facilities, particularly when the end product is destined for pharmaceutical applications. They also adhere to REACH and other chemical safety regulations, ensuring the safe handling, storage, and transportation of the compound.
Market Trends and Future Outlook
As industries continue to evolve, the demand for 2,2,6,6-Tetramethylpiperidine remains steady. There's also increasing demand in the field of green chemistry and materials science, where sterically hindered amines play a crucial role.
With ongoing innovations in synthesis and purification, the cost-efficiency and environmental friendliness of the production process are expected to improve. This will likely make TMP even more accessible for newer applications in advanced chemistry.
Manufacturers are now offering TMP in various formats, such as drum packaging for industrial users and small-volume bottles for research institutions.
Conclusion
2,2,6,6-Tetramethylpiperidine (CAS No: 768-66-1) is a highly functional chemical that plays an indispensable role in multiple industries. Its manufacturing involves precise chemical engineering and quality assurance protocols to meet the needs of a broad customer base. With a well-established global network of producers, this compound remains a key reagent in modern chemical synthesis. Whether for pharmaceuticals, polymers, or specialty applications, TMP continues to be a compound of choice for chemists around the world.
URL: For more information, visit Vasista Pharma: Cas No: 768-66-1 Manufacturers
0 notes
Text
A Complete Guide to Buying Lab Analysis Equipment in Malaysia
Purchasing lab analysis equipment in Malaysia requires careful planning and a deep understanding of your laboratory’s specific needs. Whether you're setting up a new lab or upgrading existing equipment, making the right choice ensures accuracy, efficiency, and compliance with industry standards. This guide will walk you through everything you need to know about buying lab analysis equipment in Malaysia—from understanding types of equipment to selecting a trusted supplier.
1. Understand Your Lab's Requirements
Before diving into product catalogs, it’s essential to clearly define what your lab needs. Consider:
Type of analysis: Are you conducting chemical, biological, or physical analysis? Each type requires different equipment.
Volume of testing: High-throughput labs need automated systems, while small labs may manage with manual or semi-automated tools.
Compliance standards: Laboratories in Malaysia may need to adhere to ISO, GMP, GLP, or MOH regulations depending on their industry.
Create a checklist of required functions, technical specifications, and preferred brands or technologies.
2. Types of Lab Analysis Equipment
Here are some common categories of lab analysis equipment found in Malaysian labs:
a. Analytical Balances
Used for precise weight measurements, especially in pharmaceutical and chemical labs. Look for balances with high sensitivity and internal calibration features.
b. Spectrophotometers
Used in chemical and biological analysis to measure light absorbance. Available in UV-Vis, IR, and atomic absorption variants.
c. Chromatography Systems
Gas chromatography (GC) and high-performance liquid chromatography (HPLC) systems are essential for separating and analyzing complex mixtures.
d. Centrifuges
Used for separating substances based on density, common in clinical, molecular biology, and food testing labs.
e. pH Meters and Electrochemical Analyzers
Used to test acidity, ion concentration, and conductivity in water and other solutions.
f. Microscopes
From basic optical microscopes to high-end electron microscopes, these are fundamental in biology, medical, and materials research labs.
g. Water Purification Systems
Crucial for preparing reagent-grade water, especially in molecular and analytical labs.
h. Fume Hoods and Safety Cabinets
Provide protection against hazardous fumes and biological agents, ensuring lab safety and compliance.
3. Choose the Right Supplier
In Malaysia, numerous suppliers cater to lab analysis needs, offering both local and international brands. When evaluating a supplier:
Check reputation and reviews: Look for testimonials, online reviews, and case studies.
Evaluate technical support: Ensure they provide installation, calibration, maintenance, and training services.
Verify product authenticity: Choose authorized distributors of reputed brands like Thermo Fisher, Shimadzu, Agilent, or Sartorius.
After-sales service: Reliable customer support and readily available spare parts are critical for long-term equipment performance.
Popular suppliers in Malaysia include Chemopharm, Medigene, Crest Systems, and Fisher Scientific Malaysia.
4. Consider Budget and Financing Options
Lab analysis equipment can be a significant investment. While budget constraints are real, it’s important not to compromise on quality. Here are some tips:
Get multiple quotations: Compare prices and value-added services.
Ask about warranties: Ensure the equipment has a warranty and service agreement.
Explore leasing or financing options: Some suppliers offer installment plans or leasing for large equipment purchases.
Also, consider the total cost of ownership, which includes maintenance, calibration, consumables, and energy usage.
5. Ensure Regulatory Compliance
In Malaysia, labs operating in pharmaceuticals, food, water, and environmental testing must follow specific regulatory requirements:
National Pharmaceutical Regulatory Agency (NPRA) for pharmaceutical labs.
Department of Standards Malaysia for ISO certification and laboratory accreditation (e.g., ISO/IEC 17025).
Ministry of Health (MOH) for clinical and food labs.
Make sure the equipment you buy meets the standards required by these agencies to ensure compliance and avoid penalties.
6. Installation, Calibration, and Training
Once the equipment arrives, proper setup is vital:
Installation should be carried out by trained professionals to avoid damage.
Calibration must be done according to manufacturer guidelines and validated for accuracy.
Training your lab personnel is crucial to maximize equipment utility and ensure proper handling.
Ask your supplier whether these services are included in the purchase or come at an additional cost.
7. Maintenance and Support
Regular maintenance keeps your equipment in optimal condition and extends its lifespan. Many suppliers in Malaysia offer annual service contracts that include:
Preventive maintenance
Performance verification
Emergency repair services
Replacement parts
Document all maintenance activities to comply with auditing standards and ensure data integrity.
8. Future-Proofing Your Lab
Technology in laboratory analysis evolves rapidly. When selecting equipment, consider features like:
Modular design: Allows for upgrades without full replacement.
Software integration: Compatible with LIMS (Laboratory Information Management Systems).
Energy efficiency: Reduces operational costs.
Remote diagnostics: Allows for quicker troubleshooting and support.
Investing in scalable, upgradable systems ensures your lab stays competitive and adaptable.
Final Thoughts
Buying lab analysis equipment in Malaysia is a strategic investment that impacts the quality, safety, and efficiency of your operations. By understanding your specific needs, choosing reliable suppliers, and planning for future growth, you can ensure a successful setup that meets regulatory standards and delivers accurate results. Whether you're in pharmaceuticals, healthcare, environmental science, or food testing, the right equipment is the foundation of your lab’s success.
0 notes
Text
0 notes
Text
Acetaminophen Reagent Market Report: Trends, Opportunities, and Forecast 2025-2031
Acetaminophen Reagent Market, Global Outlook and Forecast 2025-2032
Global Acetaminophen Reagent Market is projected to grow from USD 590 million in 2023 to USD 824.67 million by 2030, at a CAGR of 4.9%, driven by increasing demand for accurate medical diagnostics and pharmaceutical quality control. This standardized reagent plays a critical role in pain management drug development and toxicology screening across healthcare systems worldwide.
Acetaminophen reagents are essential for drug formulation analysis, therapeutic drug monitoring (TDM), and overdose diagnostics. Their precision and reliability make them indispensable in clinical laboratories and pharmaceutical manufacturing, especially with the growing prevalence of chronic pain conditions requiring careful medication management. Regulatory emphasis on drug safety continues to drive innovation in reagent formulations.
Download FREE Sample Report: https://www.24chemicalresearch.com/download-sample/284829/global-regional-acetaminophen-reagent-forecast-supply-dem-analysis-competitive-market-2025-2032-829
Market Overview & Regional Analysis
North America currently leads the market with 26% global share, attributed to advanced healthcare infrastructure and strict drug safety regulations that mandate rigorous acetaminophen level monitoring. The region's established pharmaceutical R&D ecosystem and widespread adoption of clinical chemistry analyzers further bolster market growth.
Europe follows closely with sophisticated hospital networks utilizing acetaminophen reagent tests for therapeutic drug monitoring. Meanwhile, Asia-Pacific emerges as the fastest-growing region thanks to expanding healthcare access and strengthening pharmaceutical manufacturing capabilities, particularly in India and China. Latin America and Middle East markets show steady growth, though constrained by healthcare budget limitations in some countries.
Key Market Drivers and Opportunities
The market thrives on rising global acetaminophen consumption across prescription and OTC medications, necessitating precise formulation analysis. Clinical laboratories represent 58% of end-use demand as emergency departments enhance overdose testing protocols. Pharmaceutical QC applications account for 32% share, driven by FDA and EMA requirements for drug content verification.
Significant opportunities exist in developing multiplex assay platforms that combine acetaminophen testing with other drug class panels. Emerging point-of-care testing solutions for emergency medicine and the expansion of hospital-based toxicology services present additional growth avenues. Custom reagent development for specialized clinical research applications also shows promising potential.
Challenges & Restraints
Market growth faces headwinds from reagent price sensitivity in cost-conscious healthcare systems and competition from alternative testing methodologies including chromatography. Evolving regulatory standards for reagent validation require continuous manufacturer investment in documentation and quality systems.
Market Segmentation by Type
Antibody/Substrate Reagent
Enzyme Reagent
Acetaminophen Antiserum
Acetaminophen Fluorescein Tracer
Pretreatment Solution
Download FREE Sample Report: https://www.24chemicalresearch.com/download-sample/284829/global-regional-acetaminophen-reagent-forecast-supply-dem-analysis-competitive-market-2025-2032-829
Market Segmentation by Application
Hospitals
Diagnostics Laboratories
POC Testing
Forensic Laboratories
Others
Market Segmentation and Key Players
Biorbyt Ltd
Thermo Fisher Scientific
Siemens Healthineers AG
Abbott
Sekisui Diagnostics
Beckman Coulter
Randox Laboratories
American Screening Corporation
EKF Diagnostics
Bio-Rad Laboratories Inc
Henry Schein
Bio-Techne
Report Scope
This market analysis provides comprehensive coverage of the global Acetaminophen Reagent industry from 2024-2032, with detailed examination of:
Market size projections and growth trends
Technology adoption patterns across regions
Regulatory landscape impacts
Competitive environment mapping
Our research methodology combines primary interviews with laboratory directors, purchasing managers, and product specialists from:
200+ hospital and reference laboratories
Leading diagnostic manufacturers
Pharmaceutical QC departments
The report evaluates:
Reagent formulation innovations
Pricing trends across product categories
Supply chain dynamics
Emerging application areas
Get Full Report Here: https://www.24chemicalresearch.com/reports/284829/global-regional-acetaminophen-reagent-forecast-supply-dem-analysis-competitive-market-2025-2032-829
About 24chemicalresearch
Founded in 2015, 24chemicalresearch has rapidly established itself as a leader in chemical market intelligence, serving clients including over 30 Fortune 500 companies. We provide data-driven insights through rigorous research methodologies, addressing key industry factors such as government policy, emerging technologies, and competitive landscapes.
Plant-level capacity tracking
Real-time price monitoring
Techno-economic feasibility studies
With a dedicated team of researchers possessing over a decade of experience, we focus on delivering actionable, timely, and high-quality reports to help clients achieve their strategic goals. Our mission is to be the most trusted resource for market insights in the chemical and materials industries.
International: +1(332) 2424 294 | Asia: +91 9169162030
Website: https://www.24chemicalresearch.com/
Follow us on LinkedIn: https://www.linkedin.com/company/24chemicalresearch
0 notes
Text
Natural Paeonol vs. Synthetic Paeonol: A Complete Guide to Sources, Differences, and Applications
Paeonol is a natural compound with various pharmacological effects, including anti-inflammatory, analgesic, and antioxidant properties. It is widely used in pharmaceuticals, cosmetics, and health supplements. Paeonol can be extracted from plants like tree peony bark (Moutan Cortex) and Cynanchum paniculatum, or synthesized chemically. But what are the key differences between natural and synthetic paeonol? Which one is better for your needs? This article compares them in terms of sources, production methods, purity, cost, and applications.

Sources and Production Methods
(1) Natural Paeonol
Source: Extracted from medicinal plants like peony root bark (Moutan Cortex), Paeonia lactiflora, and Cynanchum paniculatum.
Extraction Process: Uses solvents (ethanol, water), followed by purification steps like column chromatography and crystallization.
Characteristics: Relies on plant cultivation, has a longer production cycle, but aligns with traditional medicine principles.
(2) Synthetic Paeonol
Source: Derived from petrochemical products (e.g., resorcinol, p-hydroxybenzoic acid) via organic synthesis.
Production Process: Chemical reactions (e.g., Friedel-Crafts acylation), followed by purification to obtain high-purity paeonol.
Characteristics: Shorter production time, suitable for large-scale industrial production, and more cost-effective.
✅ Key Difference: Natural paeonol depends on plant resources, while synthetic paeonol relies on chemical synthesis, offering higher efficiency.
Composition and Purity Comparison
AspectNatural PaeonolSynthetic PaeonolMain ComponentsPaeonol + co-extracted compounds (e.g., flavonoids, glycosides)High-purity paeonol (typically ≥98%)ImpuritiesMay contain tannins, pigmentsMay contain synthetic intermediates or catalyst residuesBioactivityMulti-component synergy, gentler effectsSingle compound, precise but may lack synergistic effects
✅ Key Difference: Natural paeonol may offer broader bioactivity, while synthetic paeonol is more standardized for pharmaceutical use.
Cost and Environmental Impact
Natural Paeonol:
Higher cost (due to plant cultivation and extraction inefficiency).
More eco-friendly (but solvent recovery must be managed).
Synthetic Paeonol:
Lower cost (industrial production is more efficient).
Environmental concerns (may involve toxic reagents and waste disposal).
✅ Key Difference: Synthetic paeonol is more economical, but natural paeonol aligns better with green consumer trends.
Applications and Market Acceptance
(1) Pharmaceuticals & Supplements
Natural Paeonol: Preferred in traditional medicine and natural health products.
Synthetic Paeonol: Used in standardized drugs (e.g., anti-inflammatory or cardiovascular medications).
(2) Cosmetics
Natural Paeonol: Used in high-end skincare (marketed as “plant-derived”).
Synthetic Paeonol: Common in mass-market products for anti-allergy and antioxidant benefits.
(3) Regulatory Standards
Natural extracts must comply with heavy metal and pesticide residue limits.
Synthetic versions must meet chemical purity (e.g., ICH guidelines) and solvent residue regulations.
How to Choose?
Choose Natural Paeonol if:
You need traditional medicine formulations, premium cosmetics, or “natural” branding.
Choose Synthetic Paeonol if:
You require high purity, low cost, and large-scale production for pharmaceuticals or cosmetics.
Conclusion
Both natural and synthetic paeonol have pros and cons, and the choice depends on cost, efficacy needs, and market positioning. Natural extracts are ideal for traditional medicine and high-end markets, while synthetic paeonol dominates in standardized drugs and mass-market products. In the future, bio-synthesized paeonol (e.g., microbial fermentation) may emerge as a sustainable alternative!
0 notes
Text
#romatography Consumables#Analytical Reagents#Chromatography Reagents#Chromatography Buffers#Chromatography Reagents Market
0 notes
Text
Biopharma lab Products
Let’s face it, the magic behind every vaccine, drug, or biologic begins in the lab. And what powers these labs? You guessed it — Biopharma lab products. These aren’t just test tubes and microscopes; they’re the foundation of modern medicine.

Email Us : [email protected]
What Are Biopharma Lab Products?
Biopharma lab products include all the specialized equipment, consumables, and kits used in pharmaceutical and biotech research. From high-end analyzers to DNA extraction kits, these tools help bring life-saving drugs from concept to reality.
Why They Matter in the Modern Pharmaceutical Landscape
The pharmaceutical world is evolving faster than ever. And without the right tools, even the most brilliant ideas can stall. Biopharma lab products ensure experiments are accurate, efficient, and compliant with international standards. In short, they’re the silent heroes of drug development.
Categories of Biopharma Lab Products
Let’s break it down — because not all lab tools are created equal.
Analytical Instruments
Tools like mass spectrometers and spectrophotometers that provide detailed insights into molecular structures and concentrations.
Cell Culture Equipment
Includes incubators, biosafety cabinets, and CO2 monitors — crucial for growing cells in controlled environments.
Chromatography Tools
Used to separate mixtures, identify compounds, and purify substances. Think HPLC and gas chromatography systems.
Protein Purification Systems
Essential for isolating proteins during biotherapeutic development, ensuring purity and efficacy.
Genomic and Proteomic Kits
These kits support DNA/RNA extraction, PCR amplification, protein assays, and other high-throughput bioanalytical tasks.
Must-Have Biopharma Lab Products for Your Lab
No lab is complete without a few power players. Here are the must-haves:
High-Performance Liquid Chromatography (HPLC)
If precision had a face, it’d look like HPLC. It separates, identifies, and quantifies compounds ��� ideal for drug formulation studies.
Bioreactors & Fermenters
Think of these as mini-factories that help grow bacteria, yeast, or mammalian cells in optimal conditions.
Cryopreservation Tools
From ultra-low temperature freezers to cryovials, these tools preserve biological samples for future use.
Filtration Units and Membrane Technology
Helps in separating particles and purifying solutions — crucial during formulation and packaging stages.
How to Choose the Right Biopharma Lab Products
Not sure what to buy? Here’s your checklist:
Factors to Consider
Quality and Certification
Look for products with ISO, CE, or FDA approval. Compliance matters — big time.
Compatibility with Existing Systems
Your new toy is useless if it doesn’t play nice with your current setup.
Vendor Support and Maintenance
Great tech means nothing without solid support. Opt for vendors who offer installation, training, and maintenance.
Top Brands in Biopharma Lab Products
When in doubt, trust the pioneers:
Thermo Fisher Scientific
Known for its high-quality instrumentation and analytical tools.
Merck Millipore
Leaders in filtration, reagents, and lab water purification systems.
Bio-Rad Laboratories
They’ve got it all — from life science kits to imaging tools.
Sartorius
Masters in precision scales, bioprocessing equipment, and lab filtration.
Innovations in Biopharma Lab Products
We’re not in the '90s anymore. Welcome to the era of smart labs!
Automation and AI Integration
From robotic arms to AI-powered data analytics, automation is speeding up everything.
Lab-on-a-Chip Technologies
Miniaturized devices doing complex analyses — faster and with fewer samples.
Green Lab Solutions
Sustainability is no longer optional. Eco-friendly reagents and energy-efficient devices are in.
Challenges in Biopharma Lab Equipment Selection
It's not always rainbows and butterflies…
Budget Constraints
Let’s be honest — quality comes at a cost. Planning and prioritizing is key.
Regulatory Compliance
From FDA to EMA, staying compliant is a hurdle but non-negotiable.
The Future of Biopharma Lab Products
Smart labs, AI-powered diagnostics, mobile labs — the future is flexible, efficient, and personalized. As precision medicine gains ground, expect lab products to become even more tech-driven, modular, and integrated with digital platforms.
FAQs
1. What are Biopharma lab products used for? Biopharma lab products are used for conducting research, analysis, testing, and manufacturing processes in pharmaceutical and biotech industries.
2. Are Biopharma lab products different from traditional lab tools? Yes, they are more specialized, regulated, and designed for high-precision tasks specific to biopharma applications.
3. How can I verify the quality of lab products? Look for certifications like ISO, FDA, or CE and always purchase from reputed manufacturers with proven track records.
4. Which equipment is essential for a startup biopharma lab? Basic necessities include HPLC systems, incubators, biosafety cabinets, centrifuges, and filtration systems.
5. What’s the future of lab technology in the biopharma sector? Expect AI integration, portable lab devices, eco-friendly equipment, and smarter automation in the coming years.
Conclusion
Biopharma lab products are the unsung heroes of modern science. They enable breakthroughs, ensure quality, and drive efficiency in a high-stakes industry. Whether you're setting up a new lab or scaling an existing one, investing in the right tools is a game-changer. With the right biopharma lab products, the future of medicine is not just possible — it's already happening.
Email Us : [email protected]
https://www.linkedin.com/company/foxxlifesciences
0 notes
Text
How Palbociclib API Is Manufactured: Process Overview and Quality Challenges

Palbociclib, a selective cyclin-dependent kinase (CDK) 4/6 inhibitor, has become a vital drug in the treatment of hormone receptor-positive, HER2-negative advanced breast cancer. Its widespread clinical adoption has driven global demand for high-quality Palbociclib Active Pharmaceutical Ingredient (API). For pharmaceutical companies and drug developers, understanding how Palbociclib API is manufactured—and the quality challenges involved—is essential for selecting reliable Palbociclib API manufacturers.
Overview of Palbociclib API Manufacturing
Palbociclib's molecular complexity and stringent purity requirements make its production a highly controlled, multi-step chemical synthesis process. Typically, the API is manufactured using a series of intermediate reactions that involve key raw materials, reagents, catalysts, and solvents under tightly regulated conditions.
1. Synthetic Route Design
The manufacturing of Palbociclib starts with designing a scalable and patent-compliant synthetic route. Most Palbociclib API manufacturers adopt routes that avoid patented intermediates or processes (depending on regional patent laws). The synthetic pathway generally involves:
Construction of the pyrido[2,3-d]pyrimidin-7-one core, which is the pharmacologically active part of the molecule.
Introduction of cyano and methylamino functional groups, crucial for its selective kinase inhibition.
Multiple purification steps like crystallization or chromatography to eliminate impurities and residual solvents.
The entire process must be robust enough to handle multi-kilogram or ton-scale batches without compromising purity or yield.
2. Process Optimization and Scalability
Once the route is finalized, the focus shifts to process optimization:
Yield improvement to reduce waste and cost.
Cycle time reduction for improved manufacturing efficiency.
Solvent recovery systems to minimize environmental impact and enhance sustainability.
Leading Palbociclib API manufacturers often use Process Analytical Technology (PAT) to monitor real-time reactions, helping ensure consistency and reducing deviations.
Key Quality Challenges in Palbociclib API Manufacturing
Despite being a well-established molecule, manufacturing Palbociclib API at commercial scale presents multiple challenges. Ensuring high-quality output demands strict control over every step of the process.
1. Impurity Control and Profiling
Due to its complex chemical structure, Palbociclib synthesis may yield structural isomers, degradation products, and process-related impurities. Regulatory bodies like the USFDA and EMA require detailed impurity profiling and setting tight specifications for:
Organic impurities (e.g., unreacted intermediates)
Inorganic impurities (e.g., residual catalysts)
Residual solvents (as per ICH Q3C guidelines)
Achieving impurity levels within limits is one of the biggest quality hurdles.
2. Polymorphism and Crystallinity
Palbociclib is known to exhibit polymorphism, which can influence its bioavailability, stability, and solubility. Manufacturers must consistently produce the correct polymorphic form (usually Form A or B) and ensure proper crystallization conditions are maintained across batches.
3. Batch-to-Batch Consistency
Uniformity in particle size distribution, color, and flow properties is essential to maintain downstream formulation compatibility. This requires:
Precise control over temperature, pH, and solvent ratios
Repeatable equipment performance (reactors, dryers, filtration systems)
Strong in-process control and end-product testing protocols
4. Regulatory Compliance
Palbociclib API is subject to stringent regulatory standards. Manufacturers must comply with:
Current Good Manufacturing Practices (cGMP)
Drug Master File (DMF) submissions
ICH guidelines for stability and validation
Only experienced and well-audited Palbociclib API manufacturers can consistently meet these benchmarks, which are essential for gaining approval in regulated markets like the US, EU, and Japan.
Choosing the Right Palbociclib API Manufacturer
Given the complexity of synthesis and regulatory demands, selecting a reliable manufacturer is crucial. Ideal Palbociclib API manufacturers should offer:
Validated production facilities with cGMP certification
Available DMFs (Type II or open part)
Strong R&D and process development capabilities
Proven record of on-time delivery and global supply chain support
Conclusion
Manufacturing Palbociclib API is a meticulous process involving multi-step synthesis, tight impurity control, and stringent regulatory adherence. Only qualified Palbociclib API manufacturers with robust systems and technological expertise can ensure consistent quality, scalability, and compliance. For pharmaceutical companies entering the oncology space, partnering with the right manufacturer can make all the difference in bringing safe and effective drugs to market efficiently.
0 notes
Text
Comprehensive Guide to Tomopal 50 ml Luer Lock Glass Syringe
Introduction
In industrial and laboratory settings, precision, durability, and safety are non-negotiable. The 50.0 ml Luer Lock Tomopal Glass Syringe is engineered to meet these exact demands. Manufactured in Japan and designed for high-performance fluid handling, this syringe is trusted in environments where accuracy and compatibility with advanced systems are critical.

Product Description
The Tomopal 50 ml glass syringe is part of a broad range of syringes spanning 1.0 ml to 500.0 ml, designed exclusively for industrial and laboratory use. This syringe is custom-fit, crafted from high-grade borosilicate glass, and renowned for its thermal and chemical resistance.
Key Specifications (Part #140-4050)
· ★ Volume: 50.0 ml ±1.5%
· ★ Graduation Increments: 2.0 ml
· ★ Material: Heat-resistant borosilicate glass
· ★ Autoclavable: Yes, up to 134°C
· ★ Sterility: Non-sterile (requires autoclaving before use)
· ★ Fitting Type: Chrome-plated brass Luer Lock tip
· ★ Piston Diameter (D1): 27.45 mm ± 0.20 mm
· ★ Barrel Outside Diameter (D2): 32.35 mm ± 0.55 mm
· ★ Barrel Collar Diameter (D3): 44.00 mm ± 0.75 mm
· ★ Piston Collar Diameter: 34.05 mm ± 0.65 mm
· ★ Length (L): 178.00 mm ± 0.50 mm
Features and Benefits
· ★ Reinforced Structure: Reinforcement at the luer lock tip and barrel base — the most common points of breakage — enhances long-term durability.
· ★ Leak-Proof Precision: The cylinder and plunger are individually ground and fitted for smooth, leak-proof operation that meets Federal Specification GG-S-921b.
· ★ User-Friendly Design: A flat, wide barrel rim offers easy fingertip grip and prevents rolling on lab benches.
· ★ Chemical and Thermal Resilience: Borosilicate glass construction ensures resistance to sudden temperature shifts and corrosive chemicals.
· ★ Permanent Graduations: Clearly fused 1.0 ml and 10.0 ml markings for long-lasting legibility.
· ★ Custom Fit Option: Syringes are uniquely numbered for matching barrel and piston sets, ensuring consistent performance and calibration.
Compatibility
Tomopal Glass Syringes are fully compatible with a wide range of industry-standard equipment, including:
· • SGE
· • Hamilton Company
· • Cadence Science
· • BD Glass Syringes
Common Applications
Laboratory Use
· ★ Titration: Enables exact volume control during acid-base titrations and complexometric reactions.
· ★ Chromatography: Precise sample injection into HPLC or GC systems.
· ★ Reagent Preparation: Used to accurately handle and transfer small fluid volumes.
Industrial Use
· ★ Pharmaceutical Manufacturing: Dispensing active pharmaceutical ingredients (APIs) during formulation.
· ★ Chemical Processing: Precise mixing and transferring of corrosive or volatile substances.
· ★ Quality Control: Trusted for accurate volumetric analysis in QA labs.
Maintenance and Care
· ★ Cleaning: Rinse thoroughly after each use with appropriate solvents or hot water. Avoid abrasive tools that can damage the inner surface.
· ★ Sterilization: Autoclave before reuse as per your SOP or lab guidelines.
· ★ Inspection: Regularly check for wear or cracks. Ensure plunger-barrel fit is smooth and secure.
Conclusion
The Tomopal 50 ml glass syringe delivers unmatched precision, structural integrity, and system compatibility for demanding laboratory and industrial applications. Its robust design and custom-fit manufacturing offer peace of mind for professionals who rely on exact fluid handling every day.
0 notes
Text
PFAS Testing Market Expected to Witness a Sustainable Growth over 2025 | Global Market Vision
The global PFAS (per- and polyfluoroalkyl substances) testing market is experiencing significant growth, driven by increasing environmental and health concerns associated with these persistent chemicals. Valued at approximately USD 429.2 million in 2024, the market is projected to reach USD 969.5 million by 2030, registering a compound annual growth rate (CAGR) of 14.5% during the forecast period.
This growth is fueled by stringent regulatory measures and heightened public awareness regarding PFAS contamination in water, soil, air, and food. Governments worldwide are implementing policies to limit PFAS exposure, leading to a surge in demand for testing services. Advanced analytical techniques, such as liquid chromatography-tandem mass spectrometry (LC-MS/MS) and gas chromatography-mass spectrometry (GC/MS), are commonly employed to detect various PFAS compounds, including PFOA, PFOS, PFNA, and PFHxS
Get Full PDF Sample Copy of Report (Including Full TOC, List of Tables & Figures, Chart) @ https://futuremarketconsulting.com/request-sample/53593
Key Market Players:
Merck KGaA
• Agilent Technologies
• LGC Limited
• Waters Corporation
• Biotage
• Accustandard Inc.
• Revvity Inc
• Thermo Fisher Scientific Inc
• Phenomenex
• MACHEREY-Nagel GMBH & Co. KG
• Shimadzu Corporation
• Others
By Consumables (Chromatography Columns, Sample Preparation Techniques, Solvents, Reagents, Reference Materials & Analytical Standards, Membrane & Syringe Filters, and Other)
By Technique (LC-MS-MS, GC/MS, Mass Spectrometry, NMR Spectroscopy, Combustion Chromatography, ELISA, and Other)
By Analyte (Electrochemical, Semiconductor, Solid State/MOS, Photo-ionization Detector (PID), Catalytic, Infrared (IR), and Others)
By Application (Wastewater
Drinking Water, Ground & Surface Water, Air, Food & Beverages, Soil, Serum/Blood, Cosmetics, and Others)
Key Target Audience:
• PFAS Testing manufacturers and other stakeholders
• Organizations, forums and alliances related to PFAS Testing distribution
• Government bodies such as regulating authorities and policy makers
• Market research organizations and consulting companies
The study is useful in providing answers to several critical questions that are important for industry stakeholders such as PFAS Testing manufacturers, customers and policy makers. The study would also help them to target the growing segments over the coming years, thereby aiding the stakeholders in taking investment decisions and facilitating their expansion.
The following are the major objectives of the study.
To define, describe, and forecast the global PFAS Testing market size on the basis of grade, application, type, and region
To provide detailed information regarding the significant factors influencing the growth of the market (drivers, restraints, opportunities, and industry-specific challenges)
To analyze the opportunities in the market for stakeholders and details of a competitive landscape for market leaders
To forecast the market size, in terms of value and volume, with respect to five main regions, namely, North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa
To strategically profile key players and comprehensively analyze their market shares and core competencies
To track and analyze competitive developments such as joint ventures, mergers & acquisitions, new product developments, and research & developments (R&D) in the PFAS Testing market
During this research study, major players operating in the PFAS Testing market in various regions have been identified, and their offerings, regional presence, and distribution channels have been analyzed through in-depth discussions. Top-down and bottom-up approaches have been used to determine the overall market size. Sizes of the other individual markets have been estimated using the percentage splits obtained through secondary sources such as Hoovers, Bloomberg BusinessWeek, and Factiva, along with primary respondents. The entire procedure includes the study of the annual and financial reports of the top market players and extensive interviews with industry experts such as CEOs, VPs, directors, and marketing executives for key insights (both qualitative and quantitative) pertaining to the market. The figure below shows the breakdown of the primaries on the basis of the company type, designation, and region considered during the research study.
Frequently asked questions
How much is the global PFAS Testing market worth?
What was the value of the PFAS Testing market in 2021?
At what CAGR is the PFAS Testing market projected to grow in the forecast period (2022-2028)?
What is the leading segment in the market?
What is the key factor driving the market?
Which are the leading players in the market?
Which country held the highest market share in the market?
Which factors are expected to drive the adoption of the product?
Buy Exclusive Report @: https://futuremarketconsulting.com/buy-now/53593
NOTE: Our analysts monitoring the situation across the globe explains that the market will generate remunerative prospects for producers post the COVID-19 crisis. The report aims to provide an additional illustration of the latest scenario, economic slowdown, and COVID-19 impact on the overall industry.
Related Repots:
0 notes