#Cleanroom Design And Build
Explore tagged Tumblr posts
Note
What are some of the coolest computer chips ever, in your opinion?
Hmm. There are a lot of chips, and a lot of different things you could call a Computer Chip. Here's a few that come to mind as "interesting" or "important", or, if I can figure out what that means, "cool".
If your favourite chip is not on here honestly it probably deserves to be and I either forgot or I classified it more under "general IC's" instead of "computer chips" (e.g. 555, LM, 4000, 7000 series chips, those last three each capable of filling a book on their own). The 6502 is not here because I do not know much about the 6502, I was neither an Apple nor a BBC Micro type of kid. I am also not 70 years old so as much as I love the DEC Alphas, I have never so much as breathed on one.
Disclaimer for writing this mostly out of my head and/or ass at one in the morning, do not use any of this as a source in an argument without checking.
Intel 3101
So I mean, obvious shout, the Intel 3101, a 64-bit chip from 1969, and Intel's first ever product. You may look at that, and go, "wow, 64-bit computing in 1969? That's really early" and I will laugh heartily and say no, that's not 64-bit computing, that is 64 bits of SRAM memory.
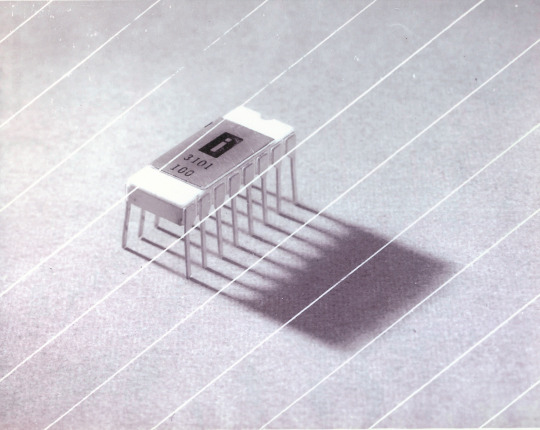

This one is cool because it's cute. Look at that. This thing was completely hand-designed by engineers drawing the shapes of transistor gates on sheets of overhead transparency and exposing pieces of crudely spun silicon to light in a """"cleanroom"""" that would cause most modern fab equipment to swoon like a delicate Victorian lady. Semiconductor manufacturing was maturing at this point but a fab still had more in common with a darkroom for film development than with the mega expensive building sized machines we use today.
As that link above notes, these things were really rough and tumble, and designs were being updated on the scale of weeks as Intel learned, well, how to make chips at an industrial scale. They weren't the first company to do this, in the 60's you could run a chip fab out of a sufficiently well sealed garage, but they were busy building the background that would lead to the next sixty years.
Lisp Chips
This is a family of utterly bullshit prototype processors that failed to be born in the whirlwind days of AI research in the 70's and 80's.
Lisps, a very old but exceedingly clever family of functional programming languages, were the language of choice for AI research at the time. Lisp compilers and interpreters had all sorts of tricks for compiling Lisp down to instructions, and also the hardware was frequently being built by the AI researchers themselves with explicit aims to run Lisp better.
The illogical conclusion of this was attempts to implement Lisp right in silicon, no translation layer.

Yeah, that is Sussman himself on this paper.
These never left labs, there have since been dozens of abortive attempts to make Lisp Chips happen because the idea is so extremely attractive to a certain kind of programmer, the most recent big one being a pile of weird designd aimed to run OpenGenera. I bet you there are no less than four members of r/lisp who have bought an Icestick FPGA in the past year with the explicit goal of writing their own Lisp Chip. It will fail, because this is a terrible idea, but damn if it isn't cool.
There were many more chips that bridged this gap, stuff designed by or for Symbolics (like the Ivory series of chips or the 3600) to go into their Lisp machines that exploited the up and coming fields of microcode optimization to improve Lisp performance, but sadly there are no known working true Lisp Chips in the wild.
Zilog Z80
Perhaps the most important chip that ever just kinda hung out. The Z80 was almost, almost the basis of The Future. The Z80 is bizzare. It is a software compatible clone of the Intel 8080, which is to say that it has the same instructions implemented in a completely different way.
This is, a strange choice, but it was the right one somehow because through the 80's and 90's practically every single piece of technology made in Japan contained at least one, maybe two Z80's even if there was no readily apparent reason why it should have one (or two). I will defer to Cathode Ray Dude here: What follows is a joke, but only barely

The Z80 is the basis of the MSX, the IBM PC of Japan, which was produced through a system of hardware and software licensing to third party manufacturers by Microsoft of Japan which was exactly as confusing as it sounds. The result is that the Z80, originally intended for embedded applications, ended up forming the basis of an entire alternate branch of the PC family tree.
It is important to note that the Z80 is boring. It is a normal-ass chip but it just so happens that it ended up being the focal point of like a dozen different industries all looking for a cheap, easy to program chip they could shove into Appliances.
Effectively everything that happened to the Intel 8080 happened to the Z80 and then some. Black market clones, reverse engineered Soviet compatibles, licensed second party manufacturers, hundreds of semi-compatible bastard half-sisters made by anyone with a fab, used in everything from toys to industrial machinery, still persisting to this day as an embedded processor that is probably powering something near you quietly and without much fuss. If you have one of those old TI-86 calculators, that's a Z80. Oh also a horrible hybrid Z80/8080 from Sharp powered the original Game Boy.
I was going to try and find a picture of a Z80 by just searching for it and look at this mess! There's so many of these things.

I mean the C/PM computers. The ZX Spectrum, I almost forgot that one! I can keep making this list go! So many bits of the Tech Explosion of the 80's and 90's are powered by the Z80. I was not joking when I said that you sometimes found more than one Z80 in a single computer because you might use one Z80 to run the computer and another Z80 to run a specialty peripheral like a video toaster or music synthesizer. Everyone imaginable has had their hand on the Z80 ball at some point in time or another. Z80 based devices probably launched several dozen hardware companies that persist to this day and I have no idea which ones because there were so goddamn many.
The Z80 eventually got super efficient due to process shrinks so it turns up in weird laptops and handhelds! Zilog and the Z80 persist to this day like some kind of crocodile beast, you can go to RS components and buy a brand new piece of Z80 silicon clocked at 20MHz. There's probably a couple in a car somewhere near you.
Pentium (P6 microarchitecture)
Yeah I am going to bring up the Hackers chip. The Pentium P6 series is currently remembered for being the chip that Acidburn geeks out over in Hackers (1995) instead of making out with her boyfriend, but it is actually noteworthy IMO for being one of the first mainstream chips to start pulling serious tricks on the system running it.

The P6 microarchitecture comes out swinging with like four or five tricks to get around the numerous problems with x86 and deploys them all at once. It has superscalar pipelining, it has a RISC microcode, it has branch prediction, it has a bunch of zany mathematical optimizations, none of these are new per se but this is the first time you're really seeing them all at once on a chip that was going into PC's.
Without these improvements it's possible Intel would have been beaten out by one of its competitors, maybe Power or SPARC or whatever you call the thing that runs on the Motorola 68k. Hell even MIPS could have beaten the ageing cancerous mistake that was x86. But by discovering the power of lying to the computer, Intel managed to speed up x86 by implementing it in a sensible instruction set in the background, allowing them to do all the same clever pipelining and optimization that was happening with RISC without having to give up their stranglehold on the desktop market. Without the P5 we live in a very, very different world from a computer hardware perspective.
From this falls many of the bizzare microcode execution bugs that plague modern computers, because when you're doing your optimization on the fly in chip with a second, smaller unix hidden inside your processor eventually you're not going to be cryptographically secure.
RISC is very clearly better for, most things. You can find papers stating this as far back as the 70's, when they start doing pipelining for the first time and are like "you know pipelining is a lot easier if you have a few small instructions instead of ten thousand massive ones.
x86 only persists to this day because Intel cemented their lead and they happened to use x86. True RISC cuts out the middleman of hyperoptimizing microcode on the chip, but if you can't do that because you've girlbossed too close to the sun as Intel had in the late 80's you have to do something.
The Future
This gets us to like the year 2000. I have more chips I find interesting or cool, although from here it's mostly microcontrollers in part because from here it gets pretty monotonous because Intel basically wins for a while. I might pick that up later. Also if this post gets any longer it'll be annoying to scroll past. Here is a sample from a post I have in my drafts since May:
I have some notes on the weirdo PowerPC stuff that shows up here it's mostly interesting because of where it goes, not what it is. A lot of it ends up in games consoles. Some of it goes into mainframes. There is some of it in space. Really got around, PowerPC did.
237 notes
·
View notes
Note
Hello dear google, I'm struggling to find a career I might enjoy in the future based on the things I already do and like, could I ask for some help?
In my school I'm working with q Environment control, like water treatment controlling trash in the nature, suffering to research some stuff I don't know how to translate and keep the water quality cool for consume. I basically deal with water quality, soil quality, air quality, etc. It's hard to describe in English ngl.
I was thinking on something around biology, chemistry and lab work but I'm kinda dumb for trying to research for things like this and why not try a help from a nerdo freak in the nearby Tumblr blog? Pls don't hate me, shrimp just is out of questions.
- 🦐
Dumb? You’re literally doing environmental systems management, probably with outdated resources and limited lab infrastructure— and you still care enough to “keep the water quality cool for consume.”
So let’s start there: you’re already doing work that takes real grit, patience, and actual scientific thinking.
You’re not dumb. You’re just swimming in real-world complexity without a guidebook. That’s not failure. That’s fieldwork.
Based on what you’re describing, you’re already neck-deep in applied environmental science. That could branch into…
Environmental chemistry (monitoring pollutants, analyzing contaminants, water/soil/air interaction) Public health & sanitation science (figuring out how environmental factors affect communities) Ecotoxicology (how chemicals move through ecosystems and mess things up) Water resources engineering (designing systems to treat and move water safely) Or even analytical lab tech work if you like the testing and measuring part more than the fieldwork.
If you like biology, chemistry, and lab work? You’re the kind of person who could build the systems and test them. You don’t have to choose between being out there in the dirt or in a cleanroom with a centrifuge. You could do both.
You want to help people. You want to understand the planet. You want your work to matter. That’s not aimless. That’s direction with rough coordinates.
Just keep going. Don’t wait until you “feel smart.” The smartest people I know are still googling half of what they do.
And don’t apologize for asking questions.
That’s how progress is ignited. 🗣️🗣️🗣️
10 notes
·
View notes
Text

SwRI adds new chamber for spacecraft-related EMC, EMI testing
Southwest Research Institute (SwRI) has added a semi-anechoic shielded enclosure for electromagnetic compatibility and interference (EMC/EMI) testing for spacecraft. The test chamber is the next step in SwRI’s plans to create a turnkey spacecraft integration and test center within its 74,000-square-foot Space System Spacecraft and Payload Processing Facility.
The 400-square-foot EMC/EMI Chamber is semi-anechoic, or free of echo, and shielded from electromagnetic interference. It supports performance of standard emissions and susceptibility testing with an upper frequency limit of 40 gigahertz (GHz) (Ka-band) and also provides the ability to perform spacecraft self-compatibility testing, which ensures that spacecraft subsystems and components work correctly and do not interfere with each other. The chamber will also evaluate radio frequency performance and compliance, important capabilities for wireless and telecommunications operations.
“This new chamber performs tests that indicate how a spacecraft will respond to a space environment. It incorporates significant automation, allowing us to test satellites and instruments more quickly and efficiently while maintaining appropriate cleanliness levels,” said Institute Engineer John Stone. “Locating the EMC/EMI chamber adjacent to other test facilities will also reduce the time lost and risk incurred while moving the test articles between buildings on the SwRI campus.”
The chamber is part of a 11,000-square-foot environmental testing facility within SwRI’s Space System Spacecraft and Payload Processing Facility, which also includes a high-decibel acoustic test chamber. The same building is home to 20,000 square feet of integration facilities, including two ISO 7 class 10,000 cleanrooms with 5-ton overhead bridge cranes and two ISO 8 class 100,000 high-bay cleanrooms outfitted with 7.5-ton bridge cranes.
In late September, the chamber performed EMC and EMI testing for four satellites that comprise the Polarimeter to UNify the Corona and Heliosphere (PUNCH) spacecraft. PUNCH, a SwRI-led NASA mission that will image how the Sun’s outer corona becomes the solar wind, is set to launch in February 2025.
“This chamber is an exciting addition, and we plan to continue to grow the capabilities of this facility as a site for comprehensive spacecraft integration and testing,” Stone said.
IMAGE: SwRI’s new semi-anechoic shield enclosure is designed to perform electromagnetic compatibility and interference and radio frequency testing for spacecraft. The wall and ceiling coverings absorb radio frequencies, allowing researchers and engineers to measure the performance of various electronic components. Credit Southwest Research Institute
3 notes
·
View notes
Text
GMP Training: Building a Foundation for Quality and Compliance
Introduction
Good Manufacturing Practices (GMP) are a set of guidelines that ensure products in regulated industries, such as pharmaceuticals, food, and cosmetics, are consistently produced and controlled according to quality standards. Central to the successful implementation of GMP is comprehensive training for employees, which equips them with the knowledge and skills to maintain compliance and uphold product safety. GMP training is not just a regulatory requirement but a critical investment in operational excellence, consumer trust, and business sustainability. As industries face increasing scrutiny from regulators and consumers, effective GMP training programs are essential for fostering a culture of quality. This article explores GMP training through four key subtopics: its purpose and importance, core components, delivery methods, and benefits for organizations, providing a detailed understanding of its role in regulated industries.
Purpose and Importance of GMP Training
The primary purpose of GMP training is to ensure that employees understand and adhere to GMP guidelines, which are established by regulatory bodies like the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the World Health Organization (WHO). These guidelines cover all aspects of production, from raw material handling to equipment maintenance and documentation, to prevent contamination, errors, and safety risks.
GMP training is crucial for several reasons. First, it ensures compliance with regulatory requirements, helping organizations avoid costly penalties, product recalls, or license suspensions. Second, it protects consumers by ensuring products are safe, effective, and of high quality. Third, it fosters a culture of accountability, where employees at all levels understand their role in maintaining standards. Without proper training, even well-designed GMP systems can fail due to human error or lack of awareness.
The importance of GMP training extends beyond compliance. It aligns organizations with global standards, enabling them to compete in international markets where GMP certification is often a prerequisite. Regular training also keeps employees updated on evolving regulations, technologies, and best practices, ensuring long-term operational success.
Core Components of GMP Training
Effective gmp training programs cover a range of topics tailored to the industry and specific roles within an organization. While content varies, the core components typically include:
GMP Principles and Regulations: Employees learn the fundamentals of GMP, including relevant regulations (e.g., FDA’s 21 CFR Part 211 for pharmaceuticals or WHO GMP guidelines). This includes understanding quality assurance, risk management, and regulatory expectations.
Standard Operating Procedures (SOPs): Training emphasizes the importance of following SOPs for processes like cleaning, testing, and documentation. Employees learn how to execute and document tasks consistently to ensure traceability.
Hygiene and Contamination Control: This covers personal hygiene, cleanroom protocols, and measures to prevent cross-contamination, particularly critical in pharmaceuticals and food production.
Documentation and Record-Keeping: Known as the “backbone” of GMP, proper documentation ensures compliance and traceability. Training teaches employees how to maintain accurate records, handle deviations, and prepare for audits.
Additional topics may include equipment maintenance, change control, and corrective and preventive actions (CAPA). Training is often customized to address specific risks, such as microbial contamination in food production or sterile manufacturing in pharmaceuticals. Role-specific modules ensure that operators, quality assurance staff, and management receive relevant instruction.
Delivery Methods for GMP Training
GMP training can be delivered through various methods, each offering unique advantages depending on the organization’s needs, size, and resources. Common delivery methods include:
In-Person Training: Conducted by internal experts or external consultants, in-person sessions allow for hands-on demonstrations, interactive discussions, and immediate feedback. This is ideal for complex processes or cleanroom training.
Online Training: E-learning platforms provide flexible, self-paced courses, often with quizzes and multimedia content. Online training is cost-effective and suitable for large or geographically dispersed teams, with platforms tracking completion and performance.
On-the-Job Training: Practical training occurs in the workplace, where employees apply GMP principles under supervision. This reinforces theoretical knowledge and builds confidence in real-world settings.
Blended Learning: Combining online modules with in-person or on-the-job sessions, blended learning offers flexibility while ensuring practical application. This approach is increasingly popular for balancing cost and effectiveness.
Training frequency varies, with initial onboarding sessions followed by annual refreshers or updates based on regulatory changes or audit findings. Organizations must also maintain records of training completion to demonstrate compliance during inspections. Choosing the right delivery method depends on factors like workforce size, regulatory requirements, and operational complexity.
Benefits of GMP Training
Investing in GMP training yields significant benefits for organizations, employees, and consumers. Key advantages include:
Regulatory Compliance: Well-trained employees reduce the risk of non-compliance, ensuring successful audits and adherence to regulations, which protects the organization from fines or shutdowns.
Improved Product Quality: Training minimizes errors, contamination, and defects, leading to consistent, high-quality products that meet customer and regulatory expectations.
Enhanced Employee Performance: GMP training empowers employees with clear guidelines and confidence, improving productivity and fostering a sense of responsibility for quality.
Market Competitiveness: GMP-trained organizations are better positioned to secure contracts with major clients, enter global markets, and build a reputation for reliability and safety.
Additionally, training reduces the likelihood of costly recalls or legal issues, saving resources in the long term. It also enhances employee morale by demonstrating the organization’s commitment to professional development and safety. For consumers, the ultimate benefit is access to safe, reliable products, reinforcing trust in the brand.
Conclusion
GMP training is a vital pillar of quality assurance in regulated industries, ensuring that organizations meet stringent standards while delivering safe, high-quality products. By equipping employees with the knowledge and skills to implement GMP principles, training fosters compliance, operational efficiency, and consumer trust. From understanding core regulations to mastering SOPs and contamination control, GMP training prepares workforces to navigate the complexities of modern manufacturing. With flexible delivery methods like online, in-person, and on-the-job training, organizations can tailor programs to their needs, reaping benefits like improved performance, market access, and regulatory success. As global demand for safe and reliable products grows, GMP training remains an essential investment for organizations committed to excellence and sustainability in the pharmaceutical, food, and cosmetic industries.
0 notes
Text
Hospital Engineering Supplies: Essential Infrastructure Solutions for Modern Healthcare Facilities

The foundation of a safe, efficient, and compliant medical facility begins with the right hospital engineering supplies. From HVAC systems to structural supports, medical gas systems, and custom metalwork, every component contributes to the overall performance, hygiene, and regulatory compliance of a healthcare environment.
Whether you're planning a new hospital build or upgrading an existing facility, sourcing quality healthcare engineering supplies is critical for long-term reliability and patient safety.
What Are Hospital Engineering Supplies?
Hospital engineering supplies include a wide range of products used to support the operational systems and physical infrastructure of medical buildings. These supplies are tailored to meet:
🧼 Hygiene and infection control standards
⚙️ Mechanical and structural performance
🏥 Medical-grade compliance (NFPA, ADA, ASHRAE)
💡 Long-term durability and energy efficiency
Examples include:
Medical gas outlets and piping systems
Stainless steel handrails and wall guards
Structural brackets and support systems
Electrical panels and wiring components
Cleanroom accessories and air handling systems
Waste containment and safety railings
Core Categories of Healthcare Engineering Supplies
🧯 Mechanical and Fire Protection Equipment
HVAC systems, ductwork, smoke dampers, and fire-rated doors are vital for air quality and life safety.
🧰 Custom Fabricated Metalwork
Custom stainless steel wall guards, utility supports, and cabinetry help meet specific layout needs and infection control standards.
💨 Medical Gas Engineering Supplies
From copper piping to zone valves and gas outlets, these systems are central to patient care in ICUs and ORs.
🚮 Waste Management Infrastructure
Includes industrial waste containers, biohazard bins, and laundry chutes built for hygiene and OSHA compliance.
🛠️ Structural and Utility Support Systems
Catwalks, ceiling grids, and mounting brackets provide strong, reliable support for lighting, equipment, and plumbing systems.
Why Quality Hospital Engineering Supplies Matter
Investing in top-quality hospital infrastructure supplies ensures:
✅ Regulatory compliance with healthcare codes
💪 Strength and durability for 24/7 hospital use
🧼 Sanitary surfaces that resist bacterial growth
🛡️ Protection from mechanical and environmental stress
💲 Lower maintenance and long-term cost savings
Cheap or poorly sourced materials can lead to system failures, safety hazards, or failed inspections.
Who Needs Hospital Engineering Supplies?
🏥 Hospital Facility Managers
🛠️ Healthcare Construction Contractors
🧱 Architects and Project Engineers
🧰 Biomedical Equipment Planners
🧬 Laboratory Designers and Builders
🏛️ Public Health Infrastructure Departments
Where to Source Reliable Medical Engineering Products
When sourcing engineering supplies for healthcare facilities, prioritize vendors who offer:
📦 In-stock availability and fast delivery
🧾 Product certifications and documentation
🛠️ Installation and technical support
🧰 Custom fabrication and sizing options
🔧 On-site field measuring or retrofitting services
Final Thoughts
Whether you’re outfitting a surgical suite or upgrading a hospital’s back-end infrastructure, hospital engineering supplies are the backbone of a safe and efficient facility. With the right components—from structural supports to medical gas systems—you can ensure your project meets the highest standards of safety, cleanliness, and performance.
Need help selecting or ordering hospital-grade engineering products? Contact a reliable supplier today to discuss your project and get a quote tailored to your facility’s specific needs.
0 notes
Text
Femoral Arterial Cannula Manufacturers in India – Why SurgiKart Leads the Market
In the highly specialized and quality-sensitive world of medical devices, finding reliable femoral arterial cannula manufacturers in India is crucial for healthcare providers who prioritize patient safety, clinical performance, and international compliance. Among the top-tier names in the Indian medical device landscape, SurgiKart India stands out as a global leader in manufacturing high-precision, CE-certified, and ISO 13485:2003 compliant femoral arterial cannulas and other critical care surgical devices.
With over two decades of industry expertise and a worldwide footprint, SurgiKart India has not only positioned itself as a trusted Indian brand but also emerged as a global exporter of medical excellence—delivering to over 52 countries with unmatched quality standards and competitive pricing.
Introduction to Femoral Arterial Cannulas
Femoral arterial cannulas are vital components in cardiac surgeries and extracorporeal circulation procedures. These cannulas are designed to ensure effective blood flow, minimal trauma, and enhanced safety during bypass surgeries or any procedure requiring femoral artery access.
Due to the complexity and risk associated with vascular access, only the most advanced and meticulously engineered femoral arterial cannulas are deemed suitable for use. This is why hospitals, surgical centers, and OEM buyers consistently seek femoral arterial cannula manufacturers in India who meet global benchmarks—and SurgiKart is leading that charge.
Why SurgiKart India is the Preferred Choice Among Femoral Arterial Cannula Manufacturers in India
World-Class Manufacturing Infrastructure
SurgiKart operates a state-of-the-art manufacturing facility in New Delhi, equipped with:
Controlled molding areas
ETO gas sterilization plant
Class 10,000 cleanroom environments
Cutting-edge testing and validation instruments
Every femoral arterial cannula produced is subjected to stringent quality checks, ensuring compliance with global safety and performance norms.
CE Certification and US FDA 510(k) Compliance
Unlike many other local players, SurgiKart’s commitment to international standards is reflected in its:
ISO 13485:2003 certified QMS
CE marking
Multiple products approved by the US FDA via the 510(k) process
This gives international buyers and domestic healthcare institutions full confidence in the safety, sterility, and functional excellence of SurgiKart’s arterial cannulas.
Customizable OEM and Contract Manufacturing Solutions
SurgiKart isn’t just a brand—it’s a reliable OEM partner. As a top femoral arterial cannula manufacturer in India, SurgiKart offers:
Private label manufacturing
Custom configurations
High-volume production
Packaging flexibility
This makes them the perfect partner for global brands looking to outsource their vascular product lines while maintaining top-tier standards.
In-House R&D and Engineering Excellence
Innovation is at the core of SurgiKart’s philosophy. Their engineering and R&D teams are constantly:
Improving flow dynamics in arterial cannulas
Enhancing tip design for smoother insertion
Reducing trauma during cannulation
Innovating flexible, kink-resistant tubing
Their femoral arterial cannulas are known for:
Optimal blood flow rate
Easy insertion
High resistance to collapse or leakage
Biocompatible materials
These design features make SurgiKart a preferred partner not just within India but for hospitals and buyers around the world.
Global Reach of SurgiKart India
While headquartered in India, SurgiKart’s influence spans:
North America
Europe
Asia-Pacific
Middle East
Africa
They work with over 300+ overseas distributors and actively engage in international medical expos and trade shows, building trust and expanding partnerships across continents.
For any global medical device buyer or hospital chain seeking femoral arterial cannula manufacturers in India, SurgiKart offers the ideal blend of quality, scalability, and price advantage.
Focus on Cardiac and Surgical Precision
SurgiKart’s range of femoral arterial cannulas is specially tailored for:
Cardiopulmonary bypass
Femoral artery cannulation during emergency surgeries
Aortic dissection procedures
ECMO setups (in adult and pediatric care)
Every design is carefully engineered with patient safety and surgeon ease-of-use as the top priorities.
Customer-Centric Mission and Vision
The mission of SurgiKart India is crystal clear: “To become the leading provider of high-quality, innovative medical devices globally, with a focus on customer satisfaction, competitive pricing, and uncompromising quality.”
Their commitment to:
Transparent business practices
Long-term OEM partnerships
Product innovation
End-user satisfaction …has made them a go-to name when it comes to femoral arterial cannula manufacturers in India.
Trusted Leadership and Skilled Team
Under the leadership of Mr. Sachin Chawla, who has 20+ years of experience in the medical device industry, the company has grown from a regional manufacturer to a global player.
SurgiKart employs over 300 trained professionals across departments including:
Production
QA and Sterilization
Regulatory Affairs
Domestic and International Marketing
Their agile workforce and efficient documentation systems ensure timely delivery, product consistency, and smooth communication across borders.
A Partner You Can Trust
Whether you’re a:
Hospital chain
Medical device distributor
International brand looking for OEM partners
Government healthcare agency
Startup in need of custom cardiac disposables
SurgiKart India is your best bet for high-grade, cost-effective femoral arterial cannulas and related cardiovascular surgical tools.
They don’t just offer a product—they offer end-to-end support, from prototyping and regulatory documentation to packaging and international shipping.
Product Highlights: SurgiKart’s Femoral Arterial Cannulas
Available in adult and pediatric variants
Radiopaque line for placement visualization
Ergonomic connector for secure attachment
Curved and straight tip options
Sterile, disposable, and ready-to-use
Available in various French sizes
Smooth tapered tip for easy insertion
DEHP-free and latex-free
CE-certified for international acceptance
SEO Meta Description
Looking for reliable femoral arterial cannula manufacturers in India? SurgiKart India delivers CE-certified, OEM-ready cannulas trusted in over 52 countries.
Final Thoughts: Choose SurgiKart India – Where Precision Meets Trust
In the rapidly evolving world of surgical innovation and patient care, settling for average is not an option. With SurgiKart India, you get a partner who combines world-class manufacturing, deep industry knowledge, international certifications, and an unshakable commitment to patient outcomes.
When searching for femoral arterial cannula manufacturers in India, remember: the right manufacturer doesn’t just provide a product—they provide trust, performance, and peace of mind.
Choose SurgiKart India. Where life-saving precision begins.
0 notes
Text
Organized Precision: High-Quality PCB Magazine Rack in India – Only at Ascomp Inc
When handling delicate printed circuit boards (PCBs), proper storage and handling are critical to prevent physical damage, static discharge, and contamination. In production lines, testing labs, and assembly units, using a reliable PCB magazine rack in India ensures safe transport, handling, and storage of PCBs without compromising quality or efficiency. At Ascomp Inc, we offer a wide range of robust and anti-static PCB racks, designed for industrial use and optimized for Indian manufacturing environments.
What is a PCB Magazine Rack?
A PCB magazine rack is a specialized storage unit designed to hold multiple PCBs vertically or horizontally. Made from heat-resistant and ESD-safe materials like conductive plastic or aluminum alloy, these racks protect PCBs during handling, transit, and storage. They are commonly used in:
SMT lines
Assembly and inspection workstations
Wave soldering and reflow zones
Testing and quality control areas
Each PCB magazine rack in India from Ascomp Inc is built to ensure mechanical stability, cleanroom compatibility, and anti-static protection.
Features of Ascomp Inc’s PCB Racks
✅ Adjustable slot width to accommodate different PCB sizes
✅ Stackable and lightweight design for space efficiency
✅ Durable, ESD-safe materials to prevent static damage
✅ Heat-resistant structure for high-temp production areas
✅ Models with rollers for easy mobility between lines
✅ Locking mechanisms to keep PCBs securely in place
Our racks are compatible with both manual handling and robotic automation systems used in advanced SMT and EMS setups.
Applications Across Industries
Ascomp Inc supplies PCB magazine racks to industries such as:
Electronics manufacturing and SMT assembly
Automotive electronics and EV units
Aerospace and defense electronics labs
Medical device and telecom PCB fabrication
R&D labs and prototyping facilities
Consumer electronics repair and refurbishment hubs
We help companies ensure that PCBs move through the production lifecycle with safety, efficiency, and traceability.
Customization Options
Different PCB sizes and handling requirements call for tailored solutions. That’s why Ascomp Inc offers:
Adjustable rack models for varying board thickness
Horizontal and vertical slot orientations
Customized branding and labeling
Options with antistatic wheels and handles
Cleanroom-grade builds upon request
We also provide full technical guidance to select the right rack model based on your product mix and workflow.
Why Choose Ascomp Inc?
🧰 Experienced supplier of ESD and assembly accessories
📦 PAN-India delivery and fast fulfillment for bulk orders
🛠️ Durable and industry-tested products
📞 Dedicated support for technical queries and custom needs
💼 Trusted by EMS, OEM, and R&D labs across India
We are committed to supporting India’s growing electronics ecosystem with the infrastructure it needs to scale safely and efficiently.
#PCBMagazineRackIndia#AscompInc#ESDStorageIndia#ElectronicsManufacturingTools#PCBHandlingIndia#SMTAssemblyTools#CleanroomStorage#AntiStaticRacks#PCBProductionIndia#IndustrialStorageSolutions
0 notes
Text
Wafer Carrier Tray Market: Integration with Smart Building Solutions by 2025-2032

MARKET INSIGHTS
The global Wafer Carrier Tray Market size was valued at US$ 567.4 million in 2024 and is projected to reach US$ 934.1 million by 2032, at a CAGR of 7.43% during the forecast period 2025-2032. This growth aligns with the expanding semiconductor industry, which itself is projected to grow from USD 579 billion in 2022 to USD 790 billion by 2029 at a 6% CAGR.
Wafer carrier trays are specialized containers designed to safely transport and store semiconductor wafers during manufacturing processes. These precision-engineered trays protect delicate silicon wafers from contamination and physical damage while ensuring proper handling through various fabrication stages. Common variants include 150mm, 200mm, and 300mm wafer trays, with the 300mm segment currently dominating due to industry adoption of larger wafer sizes for improved manufacturing efficiency.
The market growth is driven by several key factors, including increased semiconductor production capacity globally and stricter cleanliness requirements in fabrication facilities. While the semiconductor segment accounts for approximately 85% of wafer tray usage, the solar energy sector is emerging as a secondary growth driver. Recent developments include advanced polymer formulations that reduce particle generation and static charge accumulation, with key players like Entegris and Shin-Etsu Polymer leading innovation in materials science for wafer handling solutions.
MARKET DYNAMICS
MARKET DRIVERS
Expansion of Semiconductor Manufacturing to Fuel Demand for Wafer Carrier Trays
The global semiconductor industry, valued at $579 billion in 2022, is projected to reach $790 billion by 2029, growing at a 6% CAGR. This rapid expansion directly correlates with increased demand for wafer carrier trays as they are essential for handling delicate silicon wafers during manufacturing and transportation. With foundries expanding production capacity to meet the insatiable demand for chips across automotive, IoT, and consumer electronics sectors, semiconductor manufacturers are requiring more sophisticated wafer handling solutions. Modern wafer carrier trays are engineered to minimize particle contamination – a critical factor as feature sizes shrink below 5nm nodes where even microscopic contaminants can ruin chips worth thousands of dollars.
Transition to Larger Wafer Sizes Accelerating Market Growth
The industry’s ongoing migration from 200mm to 300mm wafer processing continues to drive replacement demand for carrier trays. While 300mm wafers now represent over 75% of global silicon area processed, many analog and power semiconductor manufacturers still operate 200mm fabs, creating a sustained dual-market demand. The transition allows for 2.25x more chips per wafer but requires complete requalification of handling equipment including carrier trays to ensure compatibility. This technological migration presents substantial revenue opportunities for tray manufacturers able to meet the exacting specifications for 300mm processing environments where thermal stability and cleanroom compatibility are paramount.
Additionally, the emerging adoption of 450mm wafer prototypes in R&D facilities suggests a future wave of upgrades, though full commercialization remains several years away due to the billion-dollar costs of fab conversions. Industry experts project that the global wafer carrier tray market will maintain steady growth through at least 2030 as multiple generation technologies remain in concurrent production.
MARKET RESTRAINTS
High Material Costs and Supply Chain Constraints Impacting Market Expansion
The wafer carrier tray market faces significant headwinds from volatile raw material pricing, particularly for specialized engineering plastics and advanced composites. High-purity polycarbonate and PEEK polymer prices have fluctuated between 15-25% annually since 2020, squeezing manufacturer margins. The sophisticated molding and machining equipment required for tray production represents a multi-million dollar investment, creating high barriers to entry. These capital-intensive requirements favor established players but limit market expansion and technological diversification.
Additional constraints include:
Supply Chain Fragility The global semiconductor equipment supply chain remains vulnerable to geopolitical tensions and logistics disruptions. With tray manufacturers dependent on specialty chemical suppliers concentrated in specific regions, any trade restrictions or transportation bottlenecks can cause production delays. This was evidenced during recent disruptions where lead times for critical tray components extended from 8 weeks to over 6 months.
Technical Certification Requirements Each new generation of wafer trays must undergo rigorous qualification testing with semiconductor OEMs – a process often requiring 12-18 months. This lengthy certification cycle slows the introduction of innovative materials and designs that could potentially reduce costs or improve performance.
MARKET OPPORTUNITIES
Emerging Photovoltaic Applications Opening New Growth Frontiers
While semiconductor applications dominate current demand, the solar energy sector presents substantial untapped potential for wafer carrier tray manufacturers. The photovoltaic industry processed over 300 million silicon wafers in 2023, with solar cell production capacity expanding at 25% annually in key markets. Unlike semiconductor-grade trays requiring nanometer-level cleanliness, solar wafer handling solutions can utilize more cost-effective materials while still meeting industry specifications. This allows manufacturers to extend their product portfolios with differentiated offerings for the renewable energy sector.
➤ Leading manufacturers are already adapting semiconductor-grade tray technologies to create solar-specific solutions that reduce wafer breakage during transport – a persistent challenge costing PV manufacturers approximately $150 million annually in yield losses.
Furthermore, the development of reusable tray systems for solar wafer handling presents a compelling value proposition, as the industry seeks to improve sustainability metrics. These systems can reduce single-use packaging waste by up to 80% while maintaining the necessary protection for delicate solar wafers during factory transfers.
MARKET CHALLENGES
Increasing Technical Demands Outpacing Material Science Advancements
As semiconductor fabrication pushes toward 2nm and smaller process nodes, wafer carrier trays must meet increasingly stringent requirements that challenge existing material capabilities. Modern trays must simultaneously provide exceptional dimensional stability across temperature fluctuations, extreme chemical resistance to cleaning solvents, and minimize particulate generation – properties that often have competing material requirements. This technical tightrope has led to situations where leading-edge chipmakers reject entire batches of trays due to sub-nanometer measurement deviations detected during qualification.
Other critical challenges include:
Industry Consolidation Pressures The wafer carrier tray market has seen accelerating consolidation, with the top five manufacturers now controlling over 60% of global capacity. This creates significant competitive barriers for mid-sized players attempting to invest in next-generation tray technologies, potentially slowing overall innovation in the sector.
AI-Driven Quality Expectations The adoption of AI-based wafer inspection systems in fabs has dramatically increased detection sensitivity for tray-related defects. Where human inspectors might have overlooked minor imperfections, machine vision systems flag them as critical failures – requiring tray manufacturers to implement near-zero-defect manufacturing processes that significantly increase production costs.
WAFER CARRIER TRAY MARKET TRENDS
Semiconductor Industry Expansion Driving Demand for Wafer Carrier Trays
The global wafer carrier tray market is experiencing significant growth due to the rapid expansion of the semiconductor industry. With the semiconductor market projected to grow from $579 billion in 2022 to $790 billion by 2029 at a 6% CAGR, demand for reliable wafer handling solutions has intensified. Wafer carrier trays play a critical role in protecting delicate semiconductor wafers during manufacturing, transportation, and storage. The increasing adoption of 300mm wafers, which now account for over 75% of all semiconductor wafers produced, has particularly boosted demand for larger capacity trays. Furthermore, the industry’s shift toward more complex 3D NAND and advanced logic nodes requires superior contamination control, positioning high-performance wafer carriers as essential components in fab operations.
Other Trends
Material Innovation for Advanced Nodes
Manufacturers are increasingly focusing on developing contamination-resistant materials for wafer carrier trays to meet the stringent requirements of sub-7nm semiconductor fabrication. Traditional polycarbonate trays are being supplemented with advanced polymer blends featuring static-dissipative properties and ultra-low particulate generation. The market for specialized material solutions is growing at approximately 9% annually, outpacing standard tray segments. This trend aligns with semiconductor makers’ need to minimize yield loss in advanced processes where even nanometer-scale contaminants can cause significant device failures. Recent developments in carbon fiber-reinforced composites are particularly promising for handling larger wafers while maintaining dimensional stability.
Renewable Energy Sector Adoption
The solar energy sector is emerging as a substantial consumer of wafer carrier trays, driven by massive investments in photovoltaic manufacturing capacity. While the semiconductor segment still dominates with 82% market share in wafer tray applications, solar wafer handling now accounts for nearly 15% of total demand and is growing at 11% CAGR. This growth correlates with solar panel manufacturers’ transition to larger 210mm wafers, requiring redesigned carrier solutions. Unlike semiconductor-grade trays that emphasize contamination control, solar applications prioritize durability and cost-efficiency, creating distinct product segments within the market. The increasing automation in solar panel production lines has further accelerated adoption of standardized carrier systems to enable seamless robotic handling.
COMPETITIVE LANDSCAPE
Key Industry Players
Manufacturers Focus on Product Innovation to Cater to Evolving Semiconductor Demand
The global wafer carrier tray market features a mix of established players and emerging competitors, primarily concentrated across Asia-Pacific and North America. Entegris currently leads the market, holding approximately 18-22% revenue share in 2024 due to its comprehensive portfolio of contamination-control solutions and strategic acquisitions like the purchase of CMC Materials in 2022. The company’s dominance stems from its ability to provide complete wafer handling ecosystems.
Shin-Etsu Polymer and Miraial Co., Ltd. collectively account for nearly 25-30% of the Asian market, benefiting from their proximity to semiconductor fabrication hubs in Japan, Taiwan, and South Korea. These companies are investing heavily in R&D to develop advanced polymer compounds that reduce particulate generation – a critical requirement for next-generation chip manufacturing.
The competitive intensity is increasing as mid-sized players like 3S Korea and Gudeng Precision expand their production capacities. 3S Korea recently announced a $30 million facility expansion to meet growing demand from Samsung Electronics and SK Hynix. These companies are gradually eroding the market share of larger players through competitive pricing and faster delivery times.
Meanwhile, European suppliers such as SPS-Europe are differentiating themselves through specialized designs for compound semiconductor applications, particularly in the power electronics segment. The industry is witnessing a surge in strategic partnerships, with Ferrotec recently collaborating with several Chinese foundries to localize production.
List of Key Wafer Carrier Tray Manufacturers Profiled
Entegris (U.S.)
Shin-Etsu Polymer (Japan)
Miraial Co., Ltd. (Japan)
3S Korea (South Korea)
Chuang King Enterprise (Taiwan)
EPAK Electronics (Germany)
Dainichi Shoji K.K. (Japan)
Gudeng Precision (Taiwan)
E-SUN (China)
SPS-Europe (Germany)
Peak International (U.S.)
Ferrotec (Japan)
Segment Analysis:
By Type
300 mm Wafer Tray Segment Leads Due to High Adoption in Advanced Semiconductor Manufacturing
The market is segmented based on type into:
150 mm Wafer Tray
200 mm Wafer Tray
300 mm Wafer Tray
Other specialized trays
By Application
Semiconductor Segment Dominates with Increasing Demand for Advanced Chips and IoT Devices
The market is segmented based on application into:
Semiconductor manufacturing
Solar energy panel production
Research and development
By Material
Plastic Composite Segment Gaining Traction Due to Lightweight and Cost Advantages
The market is segmented based on material into:
High-purity plastics
PP (Polypropylene)
PEEK (Polyether ether ketone)
Others
Advanced composites
Stainless steel
By End-Use Industry
Foundries Segment Leads with Expanding Semiconductor Production Capacity Worldwide
The market is segmented based on end-use industry into:
Semiconductor foundries
IDMs (Integrated Device Manufacturers)
Solar panel manufacturers
Research institutions
Regional Analysis: Wafer Carrier Tray Market
North America The North American wafer carrier tray market is driven by strong semiconductor manufacturing capabilities and significant R&D investments in advanced fabrication technologies. The U.S. holds the dominant position, accounting for approximately 38% of regional revenue, supported by major semiconductor hubs like Arizona and Texas. Stringent contamination control standards and adoption of Industry 4.0 practices in wafer handling are accelerating demand for high-precision 300mm trays. However, rising material costs and supply chain disruptions present challenges. The market benefits from government initiatives like the CHIPS Act, which allocates $52 billion to bolster domestic semiconductor production, creating indirect demand for wafer handling solutions.
Europe Europe’s wafer carrier tray market exhibits steady growth, characterized by specialization in automotive and industrial semiconductor applications. Germany leads regional consumption due to its robust MEMS (Micro-Electro-Mechanical Systems) manufacturing sector. The region shows strong preference for 200mm trays owing to mature fabrication facilities, though 300mm adoption is growing among foundries upgrading capacity. Environmental regulations regarding polymer materials influence product development, with manufacturers focusing on recyclable and cleanroom-compatible solutions. The European Chips Act aims to double the region’s semiconductor market share to 20% by 2030, indicating long-term potential for wafer handling equipment suppliers.
Asia-Pacific Asia-Pacific dominates the global wafer carrier tray market, contributing over 60% of worldwide demand. This reflects the region’s concentration of semiconductor fabrication plants, particularly in Taiwan, South Korea, and China. Rapid expansion of 300mm wafer fabs and government-backed semiconductor self-sufficiency initiatives in China are primary growth drivers. Japanese manufacturers lead in high-purity tray production, while Southeast Asia emerges as a cost-effective manufacturing hub. The region faces intense price competition, prompting suppliers to balance cost optimization with contamination prevention – a critical factor given tightening chip yield requirements. India’s nascent semiconductor ecosystem presents future growth opportunities as the country develops its first major fabs.
South America South America represents a niche market primarily serving the solar energy sector’s silicon wafer requirements. Brazil accounts for most regional demand, though volumes remain modest compared to other regions. The lack of advanced semiconductor manufacturing limits adoption of sophisticated carrier trays, with most demand concentrated on basic 150mm and 200mm solutions. Economic instability and limited local production capabilities result in heavy reliance on imports, affecting supply consistency. Nonetheless, growing renewable energy investments and potential nearshoring benefits as global supply chains diversify suggest gradual market development prospects.
Middle East & Africa The MEA wafer carrier tray market remains in early development stages, with activity concentrated in Israel’s specialized semiconductor sector and Gulf Cooperation Council (GCC) countries’ solar energy projects. Limited local fabrication capacity keeps demand minimal, though the region serves as a strategic logistics hub for tray shipments between Asia and Europe. Abu Dhabi’s G42 and Israel’s Tower Semiconductor represent key local demand sources. While not yet a significant market, MEA’s growing technology investment strategies and potential as an alternative manufacturing location warrant long-term monitoring by industry participants.
Report Scope
This market research report provides a comprehensive analysis of the global and regional Wafer Carrier Tray markets, covering the forecast period 2025–2032. It offers detailed insights into market dynamics, technological advancements, competitive landscape, and key trends shaping the industry.
Key focus areas of the report include:
Market Size & Forecast: Historical data and future projections for revenue, unit shipments, and market value across major regions and segments. The global Wafer Carrier Tray market was valued at USD million in 2024 and is projected to reach USD million by 2032.
Segmentation Analysis: Detailed breakdown by product type (150mm, 200mm, 300mm, and others), application (semiconductor and solar energy), and end-user industry to identify high-growth segments and investment opportunities.
Regional Outlook: Insights into market performance across North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa, including country-level analysis where relevant.
Competitive Landscape: Profiles of leading market participants including Pozzetta, Entegris, Shin-Etsu Polymer, and Miraial Co.,Ltd., including their product offerings, R&D focus, manufacturing capacity, and recent developments.
Technology Trends & Innovation: Assessment of emerging technologies in wafer handling, material science advancements, and evolving semiconductor fabrication standards.
Market Drivers & Restraints: Evaluation of factors driving market growth along with challenges such as supply chain constraints, regulatory issues, and market-entry barriers.
Stakeholder Analysis: Insights for component suppliers, OEMs, system integrators, investors, and policymakers regarding the evolving ecosystem and strategic opportunities.
Related Reports:https://semiconductorblogs21.blogspot.com/2025/06/laser-diode-cover-glass-market-valued.htmlhttps://semiconductorblogs21.blogspot.com/2025/06/q-switches-for-industrial-market-key.htmlhttps://semiconductorblogs21.blogspot.com/2025/06/ntc-smd-thermistor-market-emerging_19.htmlhttps://semiconductorblogs21.blogspot.com/2025/06/lightning-rod-for-building-market.htmlhttps://semiconductorblogs21.blogspot.com/2025/06/cpe-chip-market-analysis-cagr-of-121.htmlhttps://semiconductorblogs21.blogspot.com/2025/06/line-array-detector-market-key-players.htmlhttps://semiconductorblogs21.blogspot.com/2025/06/tape-heaters-market-industry-size-share.htmlhttps://semiconductorblogs21.blogspot.com/2025/06/wavelength-division-multiplexing-module.htmlhttps://semiconductorblogs21.blogspot.com/2025/06/electronic-spacer-market-report.htmlhttps://semiconductorblogs21.blogspot.com/2025/06/5g-iot-chip-market-technology-trends.htmlhttps://semiconductorblogs21.blogspot.com/2025/06/polarization-beam-combiner-market.htmlhttps://semiconductorblogs21.blogspot.com/2025/06/amorphous-selenium-detector-market-key.htmlhttps://semiconductorblogs21.blogspot.com/2025/06/output-mode-cleaners-market-industry.htmlhttps://semiconductorblogs21.blogspot.com/2025/06/digitally-controlled-attenuators-market.htmlhttps://semiconductorblogs21.blogspot.com/2025/06/thin-double-sided-fpc-market-key.html
0 notes
Text
Reliable Jumbo Bag Suppliers – Why Rishi FIBC Is a Trusted Name in Bulk Packaging

When it comes to transporting and storing large quantities of goods, choosing the right packaging solution is critical. This is especially true for industries like agriculture, food processing, chemicals, pharmaceuticals, and construction. One of the most effective and widely used solutions is the jumbo bag, also known as an FIBC (Flexible Intermediate Bulk Container). If you're looking for dependable jumbo bag suppliers, Rishi FIBC is a name that stands out with trust, quality, and global expertise.
What Are Jumbo Bags?
Jumbo bags are large, durable, and flexible containers made from woven polypropylene. They are designed to hold anywhere between 500 kg to 2000 kg of dry bulk material. These bags are widely used for transporting grains, seeds, chemicals, plastic granules, cement, and other bulk products safely and efficiently.
Why Rishi FIBC?
Rishi FIBC is one of India’s leading manufacturers and suppliers of jumbo bags. The company is known for its commitment to quality, innovation, and customer satisfaction. Here's what makes Rishi FIBC a reliable partner for your bulk packaging needs:
1. High-Quality Manufacturing Standards
Rishi FIBC operates advanced production facilities that are ISO and BRC certified. From extrusion and weaving to coating and stitching, every process is handled with care and precision. Their production units also feature cleanroom environments for food-grade and pharma-grade jumbo bags, ensuring product safety and hygiene.
2. Wide Range of Jumbo Bags
No two businesses have the same needs, and Rishi FIBC understands that. They offer a broad portfolio of jumbo bags including:
Standard Jumbo Bags
Baffle Bags
Conductive (Type C) Bags
UN Certified Bags
Food Grade Bags
Pharma Grade Bags
Ventilated Bags
Each bag is designed to meet specific industry requirements, with full customization options available in terms of size, filling/discharge mechanisms, lifting loops, and printing.
3. Global Reach, Local Understanding
Though based in India, Rishi FIBC supplies jumbo bags to customers across more than 40 countries. Their strong export capabilities are supported by efficient logistics and timely delivery, making them a preferred choice for international clients as well.
4. Customer-Focused Approach
Rishi FIBC doesn’t just supply bags – they provide solutions. Their expert team helps customers select the right type of bag, offers technical guidance, and ensures smooth after-sales support. This commitment to building long-term relationships is what sets them apart from others.
5. Sustainability and Innovation
As a responsible supplier, Rishi FIBC also focuses on eco-friendly production practices and recyclable materials. They are constantly innovating to deliver better, safer, and more sustainable packaging options.
If you are searching for a reliable jumbo bag supplier, Rishi FIBC brings you the perfect balance of quality, customization, service, and global credibility. With years of experience and a passion for delivering value, Rishi FIBC ensures that your bulk packaging needs are met with confidence and care.
Visit www.rishifibc.com to learn more or connect with their team for customized solutions.
0 notes
Text
medical devices manufacturing
Imagine a world where diagnosis, monitoring, and treatment of diseases were impossible without machines. That’s the world we would live in without medical devices manufacturing. This field is the backbone of modern healthcare, producing everything from surgical tools to smart implants.
With rising global demand, the industry has transformed significantly. One company making waves in this space is Foxxtechnologies, known for its cutting-edge manufacturing solutions tailored for the medical sector.

What is Medical Devices Manufacturing?
Simply put, it’s the process of designing, engineering, producing, and distributing devices that assist in medical treatment or diagnosis. These range from basic thermometers to complex robotic surgical systems.
Importance of the Medical Devices Industry
This industry ensures hospitals and clinics have the tools they need to save lives. It’s not just about machines — it’s about healthcare innovation, patient safety, and efficiency.
Understanding the Manufacturing Process
Research and Development (R&D)
Every great product starts with an idea. In medical devices manufacturing, R&D is where the magic begins.
Ideation and Concept Testing
Teams brainstorm, sketch, and simulate product ideas. Then, they test concepts through small trials and user feedback to determine viability.
Prototyping and Product Design
Before going to mass production, a prototype is created. This helps identify design flaws and gather early clinical feedback.
Role of CAD and 3D Modelling
Design engineers use advanced software to build detailed 3D models, helping predict performance and optimize the structure.
Materials Used in Medical Device Production
The choice of material can make or break a device.
Biocompatible Materials
Manufacturers use plastics, metals, and ceramics that are non-toxic and accepted by the human body. Titanium and medical-grade silicone are common choices.
Regulatory Standards and Certifications
You can’t just create a device and sell it — there are rules.
FDA, ISO, and CE Certifications
Medical devices must meet strict quality and safety regulations. These include FDA approvals in the U.S., CE marks in Europe, and ISO 13485 certification globally.
Key Technologies in Medical Devices Manufacturing
Automation and Robotics
Modern factories use robotics to improve precision, reduce errors, and accelerate production.
3D Printing in Medical Devices
3D printing is transforming the industry by allowing customized implants, faster prototyping, and reduced waste.
AI and IoT Integration
Smart medical devices connected through IoT can transmit real-time health data to doctors. AI helps in predictive maintenance and quality control.
Foxxtechnologies – Leading the Innovation
Overview of Foxxtechnologies
Foxxtechnologies is not your average manufacturer. They specialize in innovative, scalable, and high-quality medical device production services. With a solid reputation, they cater to both startups and large healthcare brands.
Unique Manufacturing Capabilities
Customization and Rapid Prototyping
Need a device tailored to your needs? Foxxtechnologies provides rapid prototyping, saving both time and cost in development.
Cleanroom Production Facilities
Sterility is crucial. Their ISO-class cleanrooms ensure that every product meets stringent hygiene standards.
Compliance and Quality Assurance
Foxxtechnologies doesn’t just build — they ensure every product is tested, validated, and certified according to international standards.
Trends Shaping the Future of Medical Device Manufacturing
Sustainability and Eco-Friendly Practices
As the world shifts towards greener practices, manufacturers like Foxxtechnologies are adopting recyclable materials and low-waste processes.
Smart Devices and Wearables
From fitness trackers to glucose monitors, wearable technology is booming and changing how we manage health.
Global Market Growth and Expansion
The global market for medical devices is expected to surpass $800 billion by 2030. Companies must scale fast — and smart.
Challenges in Medical Device Manufacturing
Navigating Regulations
Each country has its own rules. Global manufacturing means tackling multiple regulatory frameworks.
Ensuring Sterility and Biocompatibility
The challenge is to ensure every device is safe and performs flawlessly inside the human body.
High Costs of R&D and Manufacturing
Innovation isn’t cheap. It demands huge investments in technology, skilled labor, and compliance.
Why Choose Foxxtechnologies for Medical Device Manufacturing?
Industry Expertise and Experience
With years of hands-on experience, Foxxtechnologies knows what works and what doesn't in this highly sensitive sector.
Client-Centric Solutions
From idea to delivery, the team works closely with clients, offering end-to-end support.
Scalable and Efficient Processes
Whether it’s a batch of 100 or 10,000 units, Foxxtechnologies scales seamlessly without compromising quality.
Conclusion
Medical devices manufacturing is not just a process — it’s a commitment to healthcare, innovation, and patient safety. As technology evolves, companies like Foxxtechnologies are leading the charge by integrating cutting-edge tech, adhering to global standards, and providing client-focused solutions.
Whether you're a startup with a prototype idea or an established healthcare brand looking to expand production, Foxxtechnologies is your go-to partner in the realm of medical device manufacturing.
Email Us : [email protected]
0 notes
Text
Turnkey Pharmaceutical Projects A Complete Solution For Modern Pharma Facilities
The pharmaceutical industry is under constant pressure to deliver high-quality medicines efficiently, safely, and cost-effectively. From stringent regulatory standards to changing market demands, pharmaceutical companies must plan and build production facilities that meet global benchmarks without delay. This is where turnkey pharmaceutical projects provide unmatched value. By offering end-to-end solutions, turnkey providers help pharma companies set up, expand, or upgrade manufacturing plants with minimum hassle and maximum compliance. In this detailed guide, learn how turnkey pharmaceutical projects work, what benefits they offer, and why choosing an expert partner for pharma turnkey projects is crucial for business success.
What are Turnkey Pharmaceutical Projects?
In simple terms, a turnkey pharmaceutical project means a complete, ready-to-use facility delivered to the client. The term “turnkey” implies that the client only has to “turn the key” to start production — because every aspect, from design to validation, has been taken care of by the turnkey provider.
A typical turnkey pharmaceutical project covers:
Site master planning
Facility and utility design
Procurement and installation of equipment
Cleanroom design and construction
HVAC and environmental control systems
Process automation and control integration
Qualification and validation
Documentation and regulatory support
Training and handover
Such projects ensure that all systems work seamlessly together, meet international GMP standards, and can be scaled as business needs grow.
Why Pharma Companies Prefer Turnkey Solutions
Building a pharmaceutical plant involves multiple stakeholders — architects, engineers, equipment vendors, automation experts, validation teams, and regulatory authorities. Managing this complexity is time-consuming and risky.
Turnkey pharmaceutical projects solve this problem by providing a single point of responsibility. Here’s why more pharma companies now opt for turnkey execution:
1. Faster Project Completion: A turnkey provider manages the entire project timeline — from concept to commissioning. This reduces delays caused by coordinating multiple vendors.
2. Cost Control: With a fixed scope and clear deliverables, the client avoids unexpected expenses. Turnkey contracts provide better budget predictability.
3. Regulatory Compliance: Turnkey experts are well-versed in global GMP, WHO, USFDA, and EU standards. They design facilities and processes that pass inspections smoothly.
4. Quality Assurance: Each phase is executed by industry specialists, ensuring high-quality construction, validated equipment, and consistent product safety.
5. Flexibility and Scalability: Turnkey pharmaceutical projects are designed with future expansions in mind, so companies can increase production capacity with minimal disruption.
6. Single Point Accountability: Instead of managing multiple contracts, clients work with one partner who is accountable for quality, cost, and timely delivery.
Key Components of Pharma Turnkey Projects
A well-executed pharma turnkey project involves careful planning and flawless execution of several integrated parts:
Facility Layout:Efficient layouts optimize material flow, minimize contamination risks, and ensure compliance with cGMP standards.
Cleanroom Design and Construction: Cleanrooms are critical for sterile manufacturing. Turnkey providers build ISO-classified cleanrooms with proper HVAC, filtration, and airlocks.
Equipment Selection and Installation: From reactors to packaging lines, turnkey partners source, install, and qualify all equipment suited to the product type.
Utility Systems: Design and integration of water systems (WFI, PW), compressed air, steam, and waste management ensure uninterrupted operations.
Process Automation: Modern pharma plants use automation to monitor processes, reduce human error, and maintain data integrity. Turnkey projects deliver integrated automation solutions.
Validation and Documentation: Proper validation is key for regulatory approval. Turnkey firms perform Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ), along with full documentation.
Choosing the Right Partner for Turnkey Pharmaceutical Projects
Not all turnkey providers are equal. Choosing the right partner can make or break a project. Here’s what to look for:
Experience and Expertise: Check the provider’s track record in handling similar pharma turnkey projects, especially for your type of product (oral solids, injectables, biologics, etc.).
Regulatory Knowledge: A good turnkey partner must know the latest GMP guidelines of USFDA, MHRA, EUGMP, and other global bodies.
Customization: Every project is unique. The partner should offer customized solutions instead of one-size-fits-all layouts.
Integrated Team: Architects, process engineers, validation experts, and automation specialists should work as one team under the same roof.
After-Sales Support: Even after the handover, the provider should offer support for troubleshooting, maintenance, and future upgrades.
By partnering with a trusted turnkey specialist, pharma companies can focus on core operations while experts handle facility creation.
Future Trends in Turnkey Pharmaceutical Projects
As the industry evolves, so do turnkey solutions. Some emerging trends include:
Modular Construction: Pre-fabricated modules reduce onsite construction time and enable faster plant commissioning.
Sustainable Facilities: Turnkey providers design energy-efficient plants that reduce carbon footprints and operating costs.
Digital Twins: Virtual replicas of plants help test design changes and optimize operations before implementation.
AI and IoT Integration: Smart sensors and analytics improve process control, predictive maintenance, and data-driven decision-making.
By embracing these trends, pharma companies stay competitive and ready for future demands.
Conclusion
For pharmaceutical companies aiming to build world-class facilities, turnkey pharmaceutical projects are the smart choice. They deliver a fully functional, compliant, and scalable plant with minimal risk and maximum efficiency. Investing in expert pharma turnkey project providers ensures projects are delivered on time, within budget, and up to global standards. This not only helps companies bring medicines to market faster but also strengthens their reputation for quality and compliance. Whether you’re starting a new venture or expanding existing capacity, a trusted turnkey partner is your most valuable asset in navigating the complexities of the pharmaceutical manufacturing landscape.
#turnkey pharmaceutical project#pharma turnkey project#biotech management consulting#pharma consultancy#biopharmaceutical consulting#turnkey solutions#cleanroom construction#pharma turnkey projects#cleanroom design
0 notes
Text
When sourcing stainless steel pipes for plumbing, fabrication, or industrial projects, knowing the accurate weight per meter is essential for planning, pricing, and installation. For those working with Jindal Stainless SS 304 pipes, having a verified weight chart helps eliminate guesswork and ensures compliance with project load calculations.
In this guide, Udhhyog India, your trusted stainless steel pipe supplier, offers a free downloadable Jindal SS 304 Pipe Weight Chart PDF (2025) covering standard sizes, schedules, wall thicknesses, and pipe weights.
🔍 What Is SS 304 Pipe?
SS 304 pipe is a corrosion-resistant stainless steel pipe made of austenitic grade 304 alloy containing 18% chromium and 8% nickel. It is the most widely used grade for its excellent resistance to oxidation, high strength, and easy fabrication properties.
✅ Key Features:
Non-magnetic
Excellent corrosion resistance
Ideal for high-temperature and sanitary environments
Suitable for both domestic and industrial pipelines
Jindal Stainless Ltd. is India’s leading manufacturer of SS 304 pipes, and Udhhyog proudly supplies Jindal pipes across PAN India.
📏 Standard SS 304 Pipe Sizes Supplied by Udhhyog
NB (Inch)OD (mm)Common Thickness (mm)Schedule½”21.31.5 – 3.0SCH 10 – 801”33.42.0 – 3.5SCH 10 – 802”60.32.5 – 4.0SCH 10 – 803”88.93.0 – 5.0SCH 10 – 804”114.33.6 – 6.0SCH 10 – 806”168.34.0 – 7.1SCH 10 – 80
📎 Sizes from ½ inch to 12 inch and up to SCH 160 available on request.
��� How Is Pipe Weight Calculated?
Formula to calculate weight per meter:
📐 Weight (kg/m) = (OD – WT) × WT × 0.02466 × 1000
Where:
OD = Outer Diameter (mm)
WT = Wall Thickness (mm)
0.02466 = Density factor for SS 304 (g/mm³)
Udhhyog uses this formula to prepare verified weight charts for Jindal SS 304 pipes.
⚖️ Jindal SS 304 Pipe Weight Chart – Udhhyog 2025 Preview
NB SizeOD (mm)Wall Thickness (mm)Approx. Weight (kg/m)½”21.32.01.04¾”26.92.01.331”33.42.62.181½”48.33.03.862”60.33.25.443”88.93.69.724”114.34.013.636”168.34.822.84
📥 [Click here to download full Jindal SS 304 Pipe Weight Chart PDF – Udhhyog Verified]
🏗️ Why Is Pipe Weight Chart Important?
Understanding pipe weight is important for:
📐 Design load calculations in building projects
💸 Accurate cost estimation (per kg or per meter pricing)
🚚 Logistics and freight calculation
📦 Storage, handling, and installation planning
🛠 Applications of Jindal SS 304 Pipes
SectorApplicationsIndustrialProcess piping, chemical plants, steam linesConstructionStructural supports, railing systemsPlumbingHot/cold water pipelines, gas distributionFood & PharmaDairy and beverage transfer, cleanroom pipingInfrastructureUnderground water lines, borewell casings
🏭 Why Buy from Udhhyog?
✅ Feature🔍 AdvantageAuthorized Jindal Supplier100% verified stock with test certificatesPAN India DeliveryFast delivery from warehouses in Delhi, Mumbai, PuneReady Stock Available½” to 6” in SCH 10/40/80 pipesAccurate Weight BillingInvoices based on certified kg/m valuesPDF & Technical SupportFree downloads, assistance with sizing and standards
💰 SS 304 Pipe Price Per Kg & Meter – Udhhyog (Indicative)
Size (Inch)ThicknessPrice/kg (₹)Approx. ₹/Meter1”2.6 mm₹275 – ₹285₹600 – ₹6252”3.2 mm₹270 – ₹280₹1,450 – ₹1,5503”3.6 mm₹270 – ₹275₹2,620 – ₹2,7004”4.0 mm₹260 – ₹270₹3,550 – ₹3,700
📞 Bulk rates available. Contact Udhhyog for GST-included project quotes.
📜 What’s Included in the Downloadable PDF?
The free Jindal SS 304 Pipe Weight Chart PDF includes:
Nominal Bore (NB) in inch and mm
Outer diameter (OD)
Wall thickness in mm
Weight per meter (kg/m)
Schedule classification (SCH 5/10/40/80)
Udhhyog contact details for quotation and orders
🙋 Frequently Asked Questions (FAQ)
❓1. Where can I get an official Jindal SS 304 weight chart?
Answer: You can download it directly from Udhhyog’s website or request it via email. It includes verified weights for Jindal-manufactured SS 304 pipes.
❓2. Is the chart suitable for both seamless and welded pipes?
Answer: Yes. The chart provides values that apply to both ERW (welded) and seamless SS 304 pipes, depending on the thickness and OD.
❓3. Is there a difference between Jindal pipe and local SS pipe?
Answer: Yes. Jindal SS pipes offer better quality control, tighter dimensional tolerances, and certified chemistry. Udhhyog supplies only Jindal Verified Stock.
❓4. Can I get a price per meter based on this chart?
Answer: Yes. Pipe is typically priced per kg, but with Udhhyog’s chart, you can multiply weight × rate/kg to get price/meter.
❓5. Are these pipes suitable for drinking water lines?
Answer: Yes. SS 304 is food-grade compliant, making it safe for potable water, beverage processing, and RO system pipelines.
0 notes
Text
The Future of Construction Dust and Debris Containment Systems: Smarter, Safer, Greener
In the ever-evolving construction industry, safety, cleanliness, and sustainability are no longer optional—they are essential. At the heart of modern jobsite cleanliness is the construction dust and debris containment system, which is rapidly transforming thanks to new technologies and rising environmental standards.
As we step into the future, these systems are becoming smarter, safer, and greener, aligning with growing regulatory demands and customer expectations. Here's a look at how these systems are shaping the next generation of construction practices.
🧠 Smarter: Technology-Driven Containment Solutions
Gone are the days of relying solely on plastic sheets and duct tape. Today’s construction dust and debris containment systems are enhanced with smart technologies designed to improve performance and reduce manual oversight.
Innovations include:
IoT-connected air quality sensors that monitor particulate levels in real time.
HEPA-filtered negative air machines that automatically adjust airflow based on dust concentration.
Digital alerts sent to site managers when containment breaches or system failures occur.
Modular systems with RFID tracking, ensuring fast setup and optimised inventory use.
These smart systems allow for better control of contamination, especially in sensitive environments like hospitals, data centers, and pharmaceutical facilities.
🦺 Safer: Protecting Workers and Occupants
A well-designed construction dust and debris containment system does more than just block dust—it actively contributes to jobsite safety.
Key safety benefits include:
Improved indoor air quality, reducing the risk of respiratory illnesses among workers and building occupants.
Compliance with OSHA and EPA regulations, helping avoid costly fines.
Controlled containment zones, which reduce trip hazards and cross-contamination.
As health and safety standards become more stringent in 2025 and beyond, these systems will be essential in maintaining a compliant and secure workspace.
🌱 Greener: Eco-Conscious Materials and Sustainable Practices
Sustainability is a growing concern across all sectors, and construction is no exception. The future of dust and debris containment includes a strong shift toward eco-friendly materials and reusable systems.
Green advancements include:
Reusable modular wall systems made from recyclable composites or low-VOC materials.
Biodegradable containment wraps designed for short-term use without long-term waste.
Energy-efficient negative air machines that reduce carbon footprint while maintaining performance.
Zero-waste planning that integrates dust containment into broader sustainability strategies.
These improvements not only help projects qualify for certifications like LEED but also reflect a commitment to environmental responsibility.
🏗 Use Cases Leading the Charge
The most forward-thinking industries are already implementing next-gen dust containment systems:
🏥 Healthcare Facilities: Infection control during renovations.
🏢 Corporate Offices: Minimal disruption to daily operations.
🧪 Labs and Cleanrooms: Zero tolerance for air contaminants.
🏫 Schools and Universities: Safer environments for students during campus upgrades.
In each case, an advanced construction dust and debris containment system proves to be a cost-effective and risk-reducing investment.
🛠 Final Thoughts: Prepare for a Cleaner Tomorrow
As construction practices continue to evolve, adopting a smarter, safer, greener construction dust and debris containment system isn’t just a trend—it’s a necessity. By integrating advanced technology, prioritising safety, and committing to sustainability, contractors can future-proof their sites while meeting the expectations of clients, regulators, and the planet.
Whether you're managing a high-sensitivity renovation or a standard tenant improvement, the containment solution you choose today will define your success tomorrow.
0 notes
Text
Comparing Hastelloy C22 and Hastelloy C276 in Industrial Use

Comparing Hastelloy C22 and Hastelloy C276 in Industrial Use
This comprehensive comparison will explore the differences between Hastelloy C22 and C276, their chemical compositions, mechanical and physical properties, applications, and product forms including pipe fittings, round bars, and flanges. Book A Consultation In high-performance industrial environments, where resistance to corrosion, extreme temperature, and aggressive chemicals is critical—Hastelloy alloys are among the most trusted materials. Two of the most prominent grades in this category are Hastelloy C22 and Hastelloy C276. While both are part of the nickel-chromium-molybdenum alloy family, they are tailored for different use cases and chemical resistances. What Is Hastelloy? Hastelloy is a registered trademark for a family of corrosion-resistant metal alloys primarily made from nickel, with varying amounts of molybdenum, chromium, iron, and cobalt. These alloys are designed to withstand oxidizing and reducing environments, making them ideal for: Chemical Processing

Hastelloy is widely used in chemical plants due to its remarkable resistance to harsh substances like hydrochloric acid, sulfuric acid, and chlorine compounds. Equipment such as reactors, heat exchangers, and pressure vessels benefits from its durability, helping manufacturers maintain efficiency and safety even under continuous exposure to corrosive chemicals. Power Generation In the energy sector, Hastelloy components are exposed to high temperatures and pressures, especially in nuclear and fossil fuel plants. Its superior thermal stability and resistance to stress corrosion cracking make it ideal for turbines, boiler systems, and heat exchangers, helping ensure uninterrupted performance over extended lifespans. Marine Engineering

Marine environments pose a serious threat of corrosion due to constant contact with seawater and salty air. Hastelloy stands up to these challenges by offering excellent resistance to pitting and crevice corrosion. It's commonly used in offshore platforms, seawater piping, and ship components where long-term durability is essential. Pollution Control Environmental systems like flue gas scrubbers and waste treatment tanks depend on materials that can handle corrosive gases and acidic byproducts. Hastelloy’s resilience in such chemically aggressive environments makes it a go-to material for equipment that manages emissions and supports sustainability efforts in industrial operations. Pharmaceuticals Pharmaceutical manufacturing requires high standards of cleanliness and material integrity. Hastelloy is ideal for use in drug production equipment, cleanroom walls, and piping systems due to its non-reactive nature and ability to maintain purity. It helps ensure compliance with regulatory standards while preserving product quality and safety. Choosing between Hastelloy C22 and C276 depends on your specific operational needs: Choose Hastelloy C22 if: - You work in environments with oxidizing chemicals - You need enhanced corrosion protection in acidic media - You're building pollution control systems Choose Hastelloy C276 if: - Your system handles reducing chemicals like hydrochloric acid - You prioritize weldability and mechanical stability - You're sourcing material for legacy or standard designs Read the full article
0 notes
Text
Stainless Steel Corner Guards: Durable, Hygienic Wall Protection for High-Traffic Spaces

In healthcare, hospitality, education, and commercial environments, wall corners are frequent victims of impact damage. From hospital beds and carts to cleaning equipment and wheelchairs, these high-contact zones need strong protection. That’s where stainless steel corner guards make all the difference.
Engineered for durability, hygiene, and aesthetic appeal, stainless steel corner protectors prevent costly repairs and maintain a clean, professional look in any setting.
Why Choose Stainless Steel Corner Guards?
Stainless steel corner guards are a top choice for environments that require:
🛡️ High-impact resistance
🧼 Easy-to-clean, non-porous surfaces
🧲 Sleek, modern appearance
✅ Compliance with sanitation and safety regulations
🔩 Long-term durability without rust or corrosion
These benefits make them ideal for hospitals, restaurants, warehouses, airports, schools, and even luxury interiors.
Key Benefits of Stainless Steel Corner Guards
💪 Maximum Durability
Unlike plastic or vinyl alternatives, stainless steel resists dents, scratches, and corrosion—even under heavy use.
🧽 Easy Maintenance
Non-porous surfaces resist stains and bacteria, making them perfect for healthcare and food-service facilities where sanitation is critical.
🧯 Fire and Heat Resistance
Stainless steel maintains its structural integrity in high-heat environments and is non-combustible.
🛠️ Variety of Mounting Options
Available in adhesive-backed or screw-on formats for different surfaces and installation needs.
🎨 Sleek Design
Blends seamlessly with both modern and industrial interiors for a clean, professional finish.
Applications of Stainless Steel Corner Guards
🏥 Hospitals and Medical Facilities
🛒 Grocery Stores and Warehouses
🏢 Office Buildings and Commercial Complexes
🧼 Cleanrooms and Laboratories
🛏️ Hotels and Assisted Living Facilities
🍽️ Restaurants and Food Prep Areas
They’re commonly installed in hallways, kitchen corridors, waiting rooms, loading areas, and any high-traffic space where wall damage is likely.
Popular Types and Styles
90-Degree and 135-Degree Guards – Fit various corner angles
Standard and Custom Heights – Ranging from 4" up to full wall height
Brushed or Polished Finish – Choose the right look for your space
With or Without Wings – For added surface coverage
Perforated, Flush-Mount, or Beveled Edges – Customize based on use and aesthetics
What to Look for in a Supplier
✅ Custom size and finish options
🧰 Mounting hardware or adhesive included
🧾 Fast turnaround and bulk pricing
📜 Compliance with ADA, OSHA, or healthcare regulations
🚚 Reliable shipping and packaging to prevent transit damage
Final Thoughts
If you manage a facility where appearance and durability matter, stainless steel corner guards are a must-have. They provide superior protection against everyday wear and tear, reduce maintenance costs, and contribute to a cleaner, safer, more professional space.
Ready to upgrade your wall protection? Contact a trusted stainless steel corner guard supplier today for product options, custom sizing, and fast delivery.
0 notes
Text
Hoist Manufacturers: Powering Industrial Lifts with Precision and Safety
In the age of automation and speedy industrialization, hoists have emerged as an crucial component for lifting, lowering, and positioning heavy substances throughout multiple sectors. Whether it’s in factories, warehouses, strength flora, or construction sites, deciding on the proper hoist producers can significantly influence operational safety, efficiency, and lengthy-time period performance.
Hoists are now not just mechanical gadgets—they may be clever, engineered answers built to deal with intense workloads with utmost reliability. In this newsletter, we explore the position of present day hoist producers, what makes a terrific one, and how to pick out the first-class partner for your business desires.

What Do Hoist Manufacturers Offer?
Hoist manufacturers layout, engineer, and deliver numerous varieties of hoisting systems, custom designed to particular lifting requirements. These systems may be electric powered, guide, or pneumatic, and are engineered to provide safe and seamless vertical motion of masses in various environments.
Core services from leading hoist producers commonly encompass:
Custom layout and engineering
Electric wire rope and chain hoists
Manual hoists and lever blocks
Pneumatic hoists for risky regions
Overload safety and protection systems
Spare elements and accessories
Installation and commissioning
Annual renovation and servicing
From compact workshop fashions to heavy-obligation hoists for steel flora, cutting-edge producers build structures that are rugged, precise, and built for longevity.
Why Choosing the Right Hoist Manufacturer Matters
The performance of your lifting device heavily depends at the fine and design precision presented by the manufacturer. Here’s why choosing the right hoist manufacturer is vital:
1. Safety First
Certified manufacturers make certain that each hoist meets countrywide and international protection standards, decreasing the threat of mechanical failures and place of business accidents.
2. Tailored Engineering
Good hoist producers customise their systems in step with lifting top, load potential, frequency of use, and placement constraints—ensuring you get a solution that fits your workflow.
3. Higher Efficiency
Electric and automated hoists designed with the aid of pinnacle producers enhance handling velocity, lessen manual effort, and improve productiveness on the shop floor.
4. Longer Equipment Life
Well-manufactured hoists use superb materials, sealed bearings, and durable automobiles to make certain long lifestyles with minimal preservation.
5. Reliable Support
Top producers offer installation help, person education, spares, and AMC help, ensuring clean operations even after purchase.
Industries That Rely on Hoist Manufacturers
Hoists are imperative across a extensive style of sectors. Trusted hoist producers serve industries together with:
Automotive and Auto Components
Steel and Metal Processing
Oil & Gas and Chemical Industries
Textile and Food Processing
Power Generation and Substations
Construction and Infrastructure
Warehousing and Logistics
Each of those industries demands distinctive hoisting specifications—from cleanroom requirements in pharma to explosion-proof structures in oil refineries. This makes specialised manufacturing expertise crucial.
Popular Types of Hoists Offered
Modern hoist manufacturers provide a complete range of system to fulfill precise lifting desires. Some of the important thing types include:
1. Electric Wire Rope Hoists
Ideal for high-load, high-speed programs in factories and massive workshops. These hoists offer easy operation and advanced protection capabilities.
2. Electric Chain Hoists
Compact and dependable, suitable for medium-obligation use in manufacturing strains, assembly shops, and packaging devices.
3. Manual Chain Pulley Blocks
Economical and smooth to perform, perfect for low-frequency lifting duties or faraway activity web sites.
4. Pneumatic Hoists
Designed for use in explosive or high-temperature environments—common in oil and chemical industries.
5. Custom Built Hoists
Manufactured to meet non-wellknown lifting heights, unconventional mounting, or extreme load coping with requirements.
What to Look for in a Hoist Manufacturer
To make certain protection, reliability, and an excellent return on investment, examine a hoist manufacturer primarily based on the following:
Engineering Capability: Can they layout according to your custom specs?
Certifications: Look for ISO 9001, IS requirements, CE mark, and different enterprise compliance.
Material Quality: High-grade metal, automobiles, and gearboxes imply higher sturdiness.
Service Network: Are they able to imparting quick servicing and guide at your vicinity?
Experience: Years in enterprise, consumer list, and enterprise reputation all rely.
Safety Features: Does the hoist include brakes, restriction switches, and overload safety?

Final Thoughts
The right hoist can redefine how your facility handles substances. But a terrific hoist starts with a depended on producer—one that combines innovation, precision, and a deep knowledge of lifting dynamics. Whether you are putting in a brand new plant or upgrading your present structures, choosing the proper hoist producer is a strategic choice that impacts productiveness, protection, and fee-performance.
From fashionable chain hoists to custom-engineered wire rope structures, dependable producers make sure that your lifting answer is not simply purposeful—but destiny-prepared.
0 notes