#Clinical Trials Market Share
Text
Clinical Trials Market is expected to reach US$ 83.16 Billion by 2030
The Global Clinical Trials Market is projected to reach approximately US$ 83.16 Billion by 2030, as per Renub Research. Clinical trials, governed by precise protocols, aim to address specific patient care queries. These trials span five phases, each serving a distinct purpose, with defined criteria for participant selection and procedures, tests, medications, and doses. Rising drug development…

View On WordPress
#clinical trials market#clinical trials market by indication#clinical trials market by phase#clinical trials market report#clinical trials market share#clinical trials market size#global clinical trials market
0 notes
Text
The Rise of Clinical Trial Support Services: Trends and Market Insights
The clinical trial support services industry plays a critical role in the advancement of medical research and drug development. As the demand for innovative therapies continues to grow, the need for efficient, reliable, and comprehensive clinical trial support services is more important than ever. The Clinical Trials Support Services Market Size is projected to be valued at USD 26.10 billion in 2024 and is anticipated to grow to USD 37.5 billion by 2029, reflecting a compound annual growth rate (CAGR) of 7.52% throughout the forecast period (2024-2029).
Market Overview
The clinical trial support services market has been experiencing significant growth, driven by an increasing number of clinical trials and the rising complexity of drug development processes. With a projected market size valued in billions by 2024, this industry encompasses a range of services, including project management, regulatory affairs, site management, patient recruitment, and data management. The global focus on accelerating drug approval processes and improving patient outcomes is propelling the demand for these services.
Key Trends Influencing the Industry
Increased Focus on Patient-Centric Approaches
As clinical trials evolve, there is a growing emphasis on patient engagement and recruitment. Companies are adopting patient-centric strategies that prioritize the needs and experiences of participants. This includes using digital tools for better communication, streamlining enrollment processes, and ensuring that trials are designed with patient feedback in mind.
Adoption of Technology and Digital Solutions
The integration of technology is transforming clinical trial support services. Electronic data capture (EDC), electronic patient-reported outcomes (ePRO), and telemedicine are becoming standard practices. These technologies enhance data accuracy, improve patient monitoring, and streamline trial processes, making it easier to manage large-scale studies.
Regulatory Changes and Compliance Requirements
With evolving regulatory landscapes, particularly in regions like North America and Europe, clinical trial support services must adapt to new compliance requirements. This has led to increased demand for regulatory affairs experts who can navigate complex regulations and ensure that trials meet necessary standards.
Growth in Outsourcing
Pharmaceutical and biotechnology companies are increasingly outsourcing clinical trial support services to specialized providers. This trend allows sponsors to focus on core competencies while leveraging the expertise of service providers to enhance trial efficiency, reduce costs, and accelerate timelines.
Emphasis on Data Analytics
Data-driven decision-making is becoming essential in clinical trials. Companies are investing in advanced analytics to derive insights from trial data, improving operational efficiency and enhancing the likelihood of successful outcomes. This trend is leading to better patient selection, optimized trial designs, and improved regulatory submissions.
Expansion of Global Clinical Trials
As pharmaceutical companies seek to tap into diverse patient populations and expedite timelines, global clinical trials are on the rise. Clinical trial support services are adapting to accommodate the unique challenges of conducting studies across multiple countries, including managing logistics, regulatory approvals, and cultural considerations.
Challenges Facing the Industry
Despite the promising growth outlook, the clinical trial support services industry faces several challenges. These include rising operational costs, recruitment and retention of qualified staff, and navigating complex regulatory environments. Additionally, the ongoing impact of the COVID-19 pandemic has introduced uncertainties that require adaptability and resilience.
Future Outlook
The future of the clinical trial support services industry looks promising. As healthcare continues to advance and the demand for new therapies grows, the need for efficient clinical trial processes will remain critical. Companies that can leverage technology, prioritize patient engagement, and maintain compliance will be well-positioned to thrive in this dynamic landscape.
Conclusion
The clinical trial support services industry is integral to the success of clinical research and drug development. With increasing complexity and a growing emphasis on patient-centricity, the market is poised for substantial growth. By embracing technology, fostering collaboration, and navigating regulatory challenges, stakeholders can drive innovation and improve patient outcomes in the evolving landscape of clinical trials.
For a detailed overview and more insights, you can refer to the full market research report by Mordor Intelligence https://www.mordorintelligence.com/industry-reports/clinical-trial-support-services-market
#clinical trial support services market#clinical trial support services market size#clinical trial support services market share#clinical trial support services market trends#clinical trial support services market analysis
0 notes
Text

#Global Clinical Trials Market#Global Clinical Trials Market Size#Global Clinical Trials Market Share#Global Clinical Trials Market Report#Global Clinical Trials Market Trends
0 notes
Text
Virtual Clinical Trials Market Future: Trends, Challenges, and Opportunities

Virtual Clinical Trials Market Outlook, Scope & Overview:
Industry reports indicate that the global virtual clinical trials market was valued at USD 8.39 billion in 2023 and is projected to reach USD 13.17 billion by 2031, growing at a CAGR of 5.8% over the forecast period 2024-2031.
Technological Advancements to Drive Growth of Global Virtual Clinical Trials Market
The adoption of virtual clinical trial solutions will continue to influence global market revenues. The shift towards virtual and decentralized clinical trials is driven by the need for more flexible, efficient, and patient-centric trial designs that can enhance data collection and reduce operational costs.
As a product segment, remote patient monitoring and digital data collection solutions currently hold a significant share of the global virtual clinical trials market. This segment is anticipated to grow at a year-over-year rate of 5.8% in 2024 over 2023 and reach USD 13.17 billion in revenues by 2031. The increasing demand for real-time data access and the need to overcome geographical and logistical challenges in clinical trials are expected to drive market growth.
Virtual Clinical Trials Solutions – Market Dynamics
Drivers:
Virtual clinical trials are witnessing significant growth due to their ability to provide greater flexibility, enhance patient engagement, and improve the efficiency of trial processes. The advancements in digital health technologies, such as remote monitoring devices, telemedicine platforms, and electronic data capture systems, are key factors driving the adoption of virtual clinical trials. Additionally, the need for faster trial recruitment and the growing focus on patient-centric approaches are further propelling market growth.
Restraints:
Despite the growth potential, challenges such as data privacy concerns, regulatory hurdles, and the complexity of integrating virtual trial technologies with existing systems are hindering the widespread adoption of virtual clinical trials. Moreover, issues related to technology accessibility, patient engagement, and the need for robust cybersecurity measures pose additional challenges to market expansion.
Virtual Clinical Trials Solutions – Market Outlook
The proven benefits of virtual clinical trials in enhancing trial efficiency, improving patient participation, and reducing operational costs have contributed to the market's growth. Virtual clinical trials are expected to witness increased adoption across major markets, including North America, Europe, and Asia Pacific, driven by advancements in digital health technologies and the growing emphasis on decentralized trial models.
Global Virtual Clinical Trials Market
The rise in demand for virtual clinical trials in developed and emerging markets is expected to drive market growth over the forecast period. North America currently holds a significant market share in the global virtual clinical trials market, with the US being a key contributor to market revenues. Europe and Asia Pacific regions are also experiencing rapid adoption of virtual trial solutions, supported by favorable regulatory frameworks and increasing investments in digital healthcare infrastructure.
Key Players in the Virtual Clinical Trials Solutions Market
Leading companies in the virtual clinical trials solutions market include Medidata Solutions, Parexel International, Veeva Systems, and Oracle Corporation. These companies are at the forefront of developing and commercializing advanced virtual trial platforms and technologies for various clinical research applications, including remote monitoring, data management, and patient engagement.
In conclusion, the global virtual clinical trials market is poised for substantial growth over the forecast period, driven by technological advancements, increasing demand for decentralized trial models, and the expanding adoption of digital health solutions across diverse clinical research settings.
Other Trending Reports
Artificial Intelligence in Ultrasound Imaging Industry Growth
Growth Hormone Deficiency Industry Growth
Patient Registry Software Industry Growth
Contrast Media/Contrast Agent Industry Growth
#Virtual Clinical Trials Market#Virtual Clinical Trials Market Size#Virtual Clinical Trials Market Share#Virtual Clinical Trials Market Trends#Virtual Clinical Trials Market Growth#Virtual Clinical Trials Market Analysis#Virtual Clinical Trials Market Outlook
0 notes
Text
The Oncology Clinical Trials Market in 2023 is US$ 13.44 billion, and is expected to reach US$ 20.82 billion by 2031 at a CAGR of 5.60%.
#Oncology Clinical Trials Market#Oncology Clinical Trials Market Growth#Oncology Clinical Trials Market Share
0 notes
Text
Early Phase Clinical Trial Outsourcings Market Scope & Growth Projection till 2032
Early Phase Clinical Trial Outsourcings Market provides in-depth analysis of the market state of Early Phase Clinical Trial Outsourcings manufacturers, including best facts and figures, overview, definition, SWOT analysis, expert opinions, and the most current global developments. The research also calculates market size, price, revenue, cost structure, gross margin, sales, and market share, as well as forecasts and growth rates. The report assists in determining the revenue earned by the selling of this report and technology across different application areas.
Geographically, this report is segmented into several key regions, with sales, revenue, market share and growth Rate of Early Phase Clinical Trial Outsourcings in these regions till the forecast period
North America
Middle East and Africa
Asia-Pacific
South America
Europe
Key Attentions of Early Phase Clinical Trial Outsourcings Market Report:
The report offers a comprehensive and broad perspective on the global Early Phase Clinical Trial Outsourcings Market.
The market statistics represented in different Early Phase Clinical Trial Outsourcings segments offers complete industry picture.
Market growth drivers, challenges affecting the development of Early Phase Clinical Trial Outsourcings are analyzed in detail.
The report will help in the analysis of major competitive market scenario, market dynamics of Early Phase Clinical Trial Outsourcings.
Major stakeholders, key companies Early Phase Clinical Trial Outsourcings, investment feasibility and new market entrants study is offered.
Development scope of Early Phase Clinical Trial Outsourcings in each market segment is covered in this report. The macro and micro-economic factors affecting the Early Phase Clinical Trial Outsourcings Market
Advancement is elaborated in this report. The upstream and downstream components of Early Phase Clinical Trial Outsourcings and a comprehensive value chain are explained.
Browse More Details On This Report at @https://www.globalgrowthinsights.com/market-reports/early-phase-clinical-trial-outsourcings-market-101449
Global Growth Insights
Web: https://www.globalgrowthinsights.com
Our Other Reports:
Global Early Phase Clinical Trial Outsourcings Market Share
Smart Microwave Oven Market Forecast
Shot Peening Market Size
Spectrometer Market Growth Rate
Operating Room Management Market Analysis
Indoor Location-based Services (LBS) Market Share
Digital Refractometers Market Growth
Electric Trucks Market
Global CMP Pad Regulator Market Size
Global Advanced Metering Infrastructure (AMI) Market Growth
RFID Printer Market Forecast
Global Laser Communication Terminal Market Share
Concrete Waterproofing Admixture Market Growth Rate
Thermal Treatment Air Filtration Systems Market Size
5G Security Market Share
Single Lead ECG Equipment Market Analysis
Personal Cloud Market
Fiber Coupled Superluminescent Light Emitting Diodes (SLED) Market Growth
Global Digital Talent Acquisition Market Growth
Global Hydraulic Dock Leveler Market Size
Global Robotic Process Automation Market Share
Carpet Manufacturing Machines Market Forecast
EV Charging Cables Market Size
Lighters Market Growth Rate
Reduced Iron Powder Market Analysis
Medical Audiometers Market Share
Peptide CDMO Market Growth
Intelligent Flow Meter Market
Global Tax Management Software Market Size
Global Aqua Feed Market Growth
Refurbished Medical Devices Market Forecast
Global Civil Engineering Design Software Market Share
Invisible Orthodontics Market Growth Rate
Self-Tanning Products Market Size
Laser Communications Terminals (LCTs) Market Share
Flower Pots and Planters Market Analysis
Battery Energy Storage Systems (BESS) Market
Potassium Bicarbonate Market Growth
Optical Transceivers Market Size
Digital Twin Technology Market Share
#Early Phase Clinical Trial Outsourcings Market Size#Early Phase Clinical Trial Outsourcings Market Share#Early Phase Clinical Trial Outsourcings Market Trends#Early Phase Clinical Trial Outsourcings Market Industry#Early Phase Clinical Trial Outsourcings Market Growth
0 notes
Text
Global AI-Enabled Drug Discovery and Clinical Trials Market Report
In today's rapidly evolving world, the convergence of technology and healthcare is propelling remarkable advancements, none more promising than the integration of Artificial Intelligence (AI) in drug discovery and clinical trials
According to our market analysis, the global AI-enabled drug discovery and clinical trials market accounted for a market revenue of $250 million and is estimated to reach the mark of $4238.7 million by the end of 2030, with a whopping CAGR of 24.88% during 2018-2030. The market growth is expected to be pushed by several factors that include the rising drug development expenditure and rising number of synergistic activities.
Global AI- Enabled Drug Discovery Overview
The Global AI-Enabled Drug Discovery and Clinical Trials Market Report stands as a testament to the transformative power of AI in healthcare. Compiled with meticulous research and insightful analysis, this report offers a comprehensive overview of the landscape, shedding light on key trends, challenges, and opportunities shaping the future of pharmaceuticals and clinical research.
Regulatory bodies such as the U.S. Food and Drug Administration (FDA) have yielded guidelines and introduced several initiatives for encouraging the implementation of AI in the drug discovery and development process. For instance, the “Enrichment Strategies for Clinical Trial to Support Approval of Human Drugs and Biological Products” developed by the FDA in 2012 encourages technology usage for improving the quality of clinical trials.
AI in Drug Discovery: Pioneering Innovation
Traditionally, drug discovery has been a laborious and time-consuming process, often plagued by high costs and low success rates. However, with the advent of AI, the paradigm is shifting. AI-powered algorithms are revolutionizing the way researchers identify potential drug candidates, predict their efficacy, and optimize molecular structures. By harnessing the vast amounts of data available in the realms of genomics, proteomics, and chemical databases, AI algorithms can rapidly sift through information, uncovering novel insights and accelerating the drug discovery process.
The Global AI-Enabled Drug Discovery and Clinical Trials Market Report highlights the exponential growth of AI applications in drug discovery. From virtual screening and target identification to lead optimization and toxicity prediction, AI is driving efficiency and innovation across every stage of the drug development pipeline. Moreover, AI-enabled platforms facilitate collaboration between researchers, enabling data sharing and accelerating the pace of discovery.
Get a free sample @ Global AI Enabled Drug Discovery Market Report
Market Growth Drivers, Challenges,Opportunities
Growth Drivers-
Increasing Drug Development Expenditure
Facilitation of Polypharmacology
Growing Number of Synergistic Activities
Market Challenges
Lack of Regulations
Ethical Issues
Market Opportunities
Expansion of business in developing economies such as India and Brazil
Introduction of Solutions in different languages
Market Segmentation
By Application
By Therapeutic Applications
By End-User
By Region
Key Companies Profiled
Accutar Biotechnology Inc.,
AiCure, LLC,
Ardigen,
Atomwise, Inc.,
Benevolent AI,
Berg, LLC,
Exscientia Ltd.,
And many others.
Transforming Clinical Trials: Enhancing Efficiency and Efficacy
Clinical trials represent a crucial phase in the drug development process, providing essential data on safety, efficacy, and dosage.The Global AI-Enabled Drug Discovery and Clinical Trials Market Report delve into the myriad ways AI is transforming clinical trials. Machine learning algorithms can analyze patient data to identify suitable candidates for clinical trials, thereby optimizing patient recruitment and retention.
Visit our vertical page for better understanding @ Global AI-Enabled Drug Discovery Market Report
Challenges and Opportunities
Despite the immense promise of AI in drug discovery and clinical trials, significant challenges persist. Data privacy concerns, regulatory hurdles, and the need for robust validation of AI algorithms pose formidable obstacles to widespread adoption.
However, amidst these challenges lie unparalleled opportunities. The Global AI-Enabled Drug Discovery and Clinical Trials Market Report underscore the potential of AI to revolutionize healthcare, driving personalized medicine, and fostering the development of targeted therapies..
Conclusion
The Global AI-Enabled Drug Discovery and Clinical Trials Market Report paints a compelling picture of a future where AI serves as a catalyst for transformative change in healthcare. By harnessing the power of AI, researchers and pharmaceutical companies can accelerate the pace of drug discovery, optimize clinical trial processes, and deliver more effective treatments to patients worldwide. As we stand on the cusp of a new era in medicine, the convergence of AI and healthcare holds the promise of a brighter, healthier future for all.
#AI-Enabled Drug Discovery and Clinical Trials Market#AI-Enabled Drug Discovery and Clinical Trials Market Report#AI-Enabled Drug Discovery and Clinical Trials Market Industry#AI-Enabled Drug Discovery and Clinical Trials Market Trends#AI-Enabled Drug Discovery and Clinical Trials Market Size#AI-Enabled Drug Discovery and Clinical Trials Market Share
0 notes
Text
https://cynochat.com/read-blog/184199_clinical-trials-market-size-overview-share-and-forecast-2031.html
0 notes
Text
Clinical Trial Supplies Market Size, Share & Trends Analysis Report, 2030
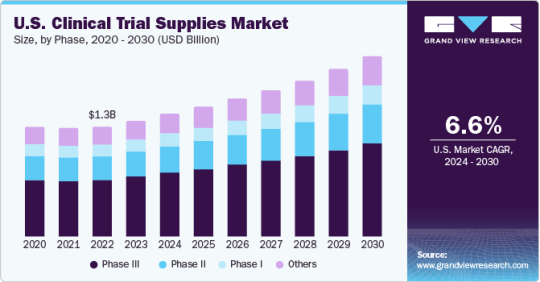
Clinical Trial Supplies Market Growth & Trends
The global clinical trial supplies market size was estimated at USD 3.97 billion in 2030 and is anticipated to grow at a compound annual growth rate (CAGR) of 6.5% from 2024 to 2030. Increasing volume of clinical trial studies coupled with the growing complexity in conduction of these trials are some of the major factors driving the market growth.
Globally, an increase in the prevalence of chronic diseases and the rapidly aging population are expected to drive the growth of R&D of biologics, which is expected to further propel the demand for efficient clinical supplies and contribute to the growth of the clinical trial supplies industry. Furthermore, an increase in the demand for orphan drugs and high investment in the R&D of rare diseases are also expected to contribute toward the development of biologic drugs. Thus, owing to these factors, this segment is likely to witness significant growth during the forecast period.
For instance, in 2022, Novartis invested around USD 10 billion in research and development. It also secured 23 approvals in the European Union, Japan, China, and the U.S. for new drugs and rare diseases. The company is also conducting 44 ongoing phase III programs in India with 17 clinical programs running in rare diseases such as atypical hemolytic uremic syndrome (aHUS), Immune thrombocytopenic purpura (ITP), spinal muscular atrophy (SMA), and Lupus Nephritis.
Direct-to-Patients (DTP) is an upcoming segment in the distribution of clinical trial supplies, which is expected to be the future model of distribution. DTP is one of the emerging models that involves delivering drugs to patients directly to create patient-centric trials. This would facilitate fewer visits to the site and reduce the burden on participants. The COVID-19 outbreak has led to the increased adoption of such a model, to continue clinical trial studies with minimum disruption. In addition, patient retention and a diverse pool of patients worldwide are some of the notable reasons that can be attributed to the high adoption of this model.
Request a free sample copy or view report summary: https://www.grandviewresearch.com/industry-analysis/clinical-trial-supplies-market
Clinical Trial Supplies Market Report Highlights
Based on the clinical phase, the market is anticipated to be dominated by the Phase III trial segment with a 52.7% revenue share in 2022. The presence of a large number of molecules currently under Phase III makes it the primary factor responsible for this deduction
Among services, the storage, and distribution segment is anticipated to witness the fastest growth at a CAGR of 6.8% during the forecast period. The rise in global biologics pipeline and temperature-sensitive drugs is expected to increase the complexities related to the logistics of clinical trial supplies
Biologicsare expected to witness the fastest growth at 6.7% CAGR during the forecast period owing to the increasing research in the field of genetics and biotechnology such as the development of nanoparticle-based drug delivery systems
In terms of therapeutic use, oncology dominated the market with a revenue share of 38.8% in 2022. According to the United Press International, hospitals in the U.S. are disposing of billions of cancer drug vials due to improper dosage, thereby indicating the need for appropriate supply management
Clinical Trial Supplies Market Segmentation
Grand View Research has segmented the global clinical trial supplies market based on clinical phase, product/service, end-use, therapeutic use, and region:
Clinical Trial Supplies Clinical Phase Outlook (Revenue, USD Billion, 2018 - 2030)
Phase I
Phase II
Phase III
Others
Clinical Trial Supplies Product/Service Outlook (Revenue, USD Billion, 2018 - 2030)
Manufacturing
Storage & distribution
Cold chain based
Non-cold chain based
Supply chain management
Clinical Trial Supplies End-Use Outlook (Revenue, USD Billion, 2018 - 2030)
Pharmaceuticals
Biologics
Medical device
Others
Clinical Trial Supplies Therapeutic Use Outlook (Revenue, USD Billion, 2018 - 2030)
Oncology
CNS
Cardiovascular
Infectious disease
Metabolic disorders
Others
Clinical Trial Supplies Regional Outlook (Revenue, USD Billion, 2018 - 2030)
North America
S.
Canada
Europe
K.
Germany
France
Italy
Spain
Denmark
Sweden
Norway
Asia Pacific
Japan
China
India
Australia
South Korea
Thailand
Latin America
Brazil
Mexico
Argentina
Middle East & Africa
South Africa
Saudi Arabia
UAE
Kuwait
List of Key Players in the Clinical Trial Supplies Market
Almac Group
Biocair
Catalent Inc.
KLIFO
Movianto
PCI Pharma Services
Sharp Services, LLC
Thermo Fischer Scientific Inc.
Marken
PAREXEL International Corporation
Browse Full Report: https://www.grandviewresearch.com/industry-analysis/clinical-trial-supplies-market
#Clinical Trial Supplies Market#Clinical Trial Supplies Market Size#Clinical Trial Supplies Market Share#Clinical Trial Supplies Market Trends
0 notes
Text
AI-based Clinical Trial Solutions For Patient Matching Market Segmented On The Basis Of Patient Matching Market Report Based On The Therapeutic Application, End-Use, Region And Forecast To 2030: Grand View Research Inc.
San Francisco, 11 Aug 2023: The Report AI-based Clinical Trial Solutions For Patient Matching Market Size, Share & Trends Analysis Report By Therapeutic Application, By End-use, By Region, And Segment Forecasts, 2022 – 2030
The global AI-based clinical trial solutions for patient matching market size is expected to reach USD 1.9 billion by 2030, registering a compound annual growth rate (CAGR)…

View On WordPress
#AI-based Clinical Trial Solutions For Patient Matching Industry#AI-based Clinical Trial Solutions For Patient Matching Market 2030#AI-based Clinical Trial Solutions For Patient Matching Market Revenue#AI-based Clinical Trial Solutions For Patient Matching Market Share#AI-based Clinical Trial Solutions For Patient Matching Market Size
0 notes
Text
Clinical Trials Market Witnessed A Decline In 2020 Owing To COVID-19 Pandemic
The global clinical trials market size is expected to reach USD 78.3 billion by 2030, registering a CAGR of 5.8% during the forecast period, according to a new report by Grand View Research, Inc. An increase in the volume and complexity of clinical trials has been witnessed lately, which plays an important role in the R&D of new drugs and products. The market witnessed a decline of 6% in 2020 owing to the COVID-19 pandemic. However, the market is projected to recover from 2021 onwards. In addition, clinical trials have become increasingly costly, adding to the overall cost of developing a drug.
The increasing need for developing new drugs for chronic diseases, such as cancer, respiratory disorders, diabetes, cardiovascular diseases, and others, is creating immense pressure on the healthcare industry. The COVID-19 pandemic and the increasing demand for developing a suitable treatment are driving the market. The high number of people affected by the disease further depicts an increasing need for therapeutics & vaccines. Currently, there are 288 therapeutics and 106 vaccines under development, out of which, nearly 7.0% of therapeutics are in Phase IV, 21.0% in Phase III, and 43.0% & 13.0% in Phase II & Phase I, respectively.
Gain deeper insights on the market and receive your free copy with TOC now @: Clinical Trials Market Report
The pandemic has resulted in the global disruption of traditional onsite clinical trials. Hence, regulatory bodies worldwide have undertaken various initiatives for fast-tracking clinical trials for the development of innovative solutions. One such instance is Solidarity, an international clinical trial launched by the WHO to find effective treatment against COVID-19. Although the pandemic has forced many medical device & drug developers to revise the approach to such crises, integrating best practices within clinical trial procedures & adapting to virtual trials, which can support the continuous development of therapeutics.
#Clinical Trials Market Size & Share#Global Clinical Trials Market#Clinical Trials Market Latest Trends#Clinical Trials Market Growth Forecast#COVID-19 Impacts On Clinical Trials Market#Clinical Trials Market Revenue Value
0 notes
Text
Clinical Trial Management System Market Trends: Future Growth and Opportunities

Clinical Trial Management System Market Outlook, Scope & Overview:
Industry reports indicate that the global clinical trial management system (CTMS) market was valued at USD 1.82 billion in 2023 and is projected to reach USD 5.49 billion by 2031, growing at a CAGR of 14.8% over the forecast period 2024-2031.
Technological Advancements to Drive Growth of Global Clinical Trial Management System Market
The adoption of advanced clinical trial management systems will continue to influence global market revenues. Healthcare providers and research organizations are increasingly turning to CTMS solutions due to their efficiency in managing clinical trials, ensuring compliance, and enhancing data accuracy.
As a product segment, cloud-based CTMS solutions currently hold a significant share of the global clinical trial management system market. This segment is anticipated to grow at a year-over-year rate of 14.8% in 2024 over 2023 and reach USD 3 billion in revenues by 2031. The increasing complexity of clinical trials and the need for streamlined management processes are driving the demand for advanced CTMS solutions.
Clinical Trial Management Systems – Market Dynamics
Drivers:
Clinical trial management systems are witnessing significant growth in the global market due to their ability to streamline clinical trial processes, improve data management, and ensure regulatory compliance. The use of advanced technologies in CTMS solutions has enhanced their capabilities, driving demand across the healthcare and research sectors. Additionally, the growing number of clinical trials and the increasing need for efficient management systems are key factors fueling the growth of the CTMS market.
Restraints:
Despite the growth potential, challenges such as high implementation costs, data security concerns, and the need for specialized training are hindering the widespread adoption of CTMS solutions. Moreover, regulatory challenges and the complexity of integrating CTMS with existing systems in healthcare organizations can impact market growth.
Clinical Trial Management Systems – Market Outlook
The effective outcomes observed from using CTMS solutions in managing clinical trials have contributed to the market's growth. CTMS solutions are projected to witness a steady increase in demand, particularly in developed regions where the number of clinical trials is higher and the need for efficient management systems is critical.
Global Clinical Trial Management System Market
The rise in demand for CTMS solutions in North America, Europe, and the Asia Pacific regions is expected to drive market growth over the forecast period. North America currently holds a significant market share in the global CTMS market, with the US being a key contributor to market revenues. Europe and Asia Pacific regions are also experiencing a surge in demand for CTMS solutions, fueled by the increasing number of clinical trials and advancements in CTMS technologies.
Key Players in the Clinical Trial Management System Market
Leading companies in the clinical trial management system market include Oracle Corporation, Medidata Solutions, PAREXEL International Corporation, BioClinica, and Veeva Systems. These companies offer a range of CTMS solutions, including cloud-based and on-premise systems, designed to enhance the efficiency and effectiveness of clinical trial management.
In conclusion, the global clinical trial management system market is poised for significant growth over the forecast period, driven by technological advancements, the increasing number of clinical trials, and the growing need for efficient management systems in the healthcare and research sectors.
Other Trending Reports
Antipsychotic Drugs Industry Trends
Computer Vision in Healthcare Industry Trends
Bioinformatics Industry Trends
Neuroscience Industry Trends
#Clinical Trial Management System Market#Clinical Trial Management System Market Size#Clinical Trial Management System Market Share#Clinical Trial Management System Market Trends#Clinical Trial Management System Market Growth#Clinical Trial Management System Market Analysis#Clinical Trial Management System Market Outlook
0 notes
Text
https://www.databridgemarketresearch.com/reports/global-artificial-intelligence-ai-based-clinical-trials-market
#Artificial-Intelligence-(AI)-based-Clinical-Trials-Market-by-Product#Artificial-Intelligence-(AI)-based-Clinical-Trials-Market-Global-Opportunity#Analysis-and-Industry-regional#Artificial-Intelligence-(AI)-based-Clinical-Trials-Market-Size-Share#Artificial-Intelligence-(AI)-based-Clinical-Trials-Market-Growth#Artificial-Intelligence-(AI)-based-Clinical-Trials-Market-Insights-Country-Share-Competitors-Research-Study
0 notes
Text
Clinical Trial Packaging Market Growth, Overview with Detailed Analysis 2022-2028
Clinical Trial Packaging Market Growth, Overview with Detailed Analysis 2022-2028
The Clinical Trial Packaging Market research report 2022-2030 provides an in-depth analysis of the changing trends, opportunities, and challenges influencing the growth over the next decade. The study includes a detailed summary of each market along with data related to demand, supply and distribution. The report examines Clinical Trial Packaging market growth strategies adopted by leading…
View On WordPress
#Clinical Trial Packaging#Clinical Trial Packaging forecast#Clinical Trial Packaging Industry#Clinical Trial Packaging Market#Clinical Trial Packaging price#Clinical Trial Packaging report#Clinical Trial Packaging research#Clinical Trial Packaging share#Clinical Trial Packaging trends#Covid-19 Impact Analysis
0 notes