#Global Scientific Data Management System Market
Explore tagged Tumblr posts
Text
Europe Laboratory Information System (LIS) Market Key Companies Profile, Supply, Demand and SWOT Analysis (2022-2028)
The Europe laboratory information system (LIS) market in is expected to grow from US$ 244.03 million in 2022 to US$ 431.39 million by 2028. It is estimated to grow at a CAGR of 10.0% from 2022 to 2028.
Europe Laboratory Information System (LIS) Market Players
The laboratory information system (LIS) market players are actively pursuing innovative solutions to strengthen their market presence amidst intense competition. For instance, in March 2021, Autoscribe Informatics unveiled an updated version of its Regulated Manufacturing LIMS. This configured solution, built upon the Matrix Gemini LIS solution, is designed for all manufacturing organizations, including highly regulated industries such as medical devices and pharmaceuticals. This web-enabled version aims to improve the user experience, offering both enhanced visual appeal and increased flexibility in user setup, dashboards, reports, and other components crucial for SoftBank users. The software provides a cohesive, integrated platform with a single user interface, along with configurable workflows, the ability to automate routine tasks, and robust data exchange tools. In a related move, in July 2021, LabVantage Solutions, Inc. launched a new edition of its flagship LabVantage LIMS. The LabVantage LIS 8.7 portal features systems for managing laboratory data security, while also providing self-service access to external customers.
Laboratory Information System (LIS) Market Overview
The Europe laboratory information system (LIS) market is geographically segmented to include Germany, the UK, France, Italy, Spain, and the Rest of Europe. Europe occupies a significant position in the global laboratory information management systems market and is expected to record robust growth throughout the forecast period. This growth is primarily driven by increasing R&D expenditure, the rapid expansion of pharmaceutical companies, and the rising adoption of information technology solutions for clinical decision support, all of which will fuel market expansion. Furthermore, the market for laboratory information systems is poised for growth due to ongoing advancements within the healthcare sector. Germany is the largest market for medical technology in the European region. According to Germany Trade & Invest in 2019, the German Medical Technology Industry generated €33.4 billion in sales (domestic and exports) and committed a substantial 9% of its annual turnover to R&D expenses.
Europe Laboratory Information System (LIS) Market Segmentation
The Europe laboratory information system (LIS) market is meticulously segmented by product, delivery mode, component, end user, and country.
Based on product, the market is bifurcated into standalone LIS and integrated LIS. The standalone LIS segment held the larger market share in 2022.
By delivery mode, the market is segmented into cloud-based delivery mode, web-based delivery mode, and on-premises delivery mode. The cloud-based delivery mode segment held the largest market share in 2022.
In terms of component, the market is segmented into software and services. The software segment held the larger market share in 2022.
Regarding end user, the market is segmented into hospital labs, independent labs, and physician office labs. The hospital labs segment commanded the largest market share in 2022.
Finally, by country, the market is segmented into Germany, France, Italy, Spain, the UK, and the Rest of Europe. Germany dominated the market share in 2022.
Key industry players in the laboratory information system (LIS) market in the region include AAC Infotray AG, CompuGroup Medical, Illumina, Inc., LabLynx, Inc., LabVantage Solutions, Inc., McKESSON CORPORATION, and Thermo Fisher Scientific Inc.
Download our Sample PDF Report
@ https://www.businessmarketinsights.com/sample/BMIRE00027734
About Us:
Business Market Insights is a market research platform that provides subscription service for industry and company reports. Our research team has extensive professional expertise in domains such as Electronics & Semiconductor; Aerospace & Defense; Automotive & Transportation; Energy & Power; Healthcare; Manufacturing & Construction; Food & Beverages; Chemicals & Materials; and Technology, Media, & Telecommunications
0 notes
Text
U.S. Cerebral Palsy Market Size, Share, Trends, Opportunities, Key Drivers and Growth Prospectus
Executive Summary U.S. Cerebral Palsy Market :
Data Bridge Market Research analyses the U.S. cerebral palsy market, which was USD 954.53 million in 2023, is expected to reach USD 1,727.89 million by 2031, at a CAGR of 7.70% during the forecast period 2024-2031.
U.S. Cerebral Palsy Market report is a comprehensive background analysis of the industry, which includes an assessment of the parental market. With the global market data provided in the report, it has become easy to achieve global perspective for the international business. This market report also contains market drivers and market restraints for industry that are derived from SWOT analysis, and also shows what all the recent developments, product launches, joint ventures, mergers and acquisitions by the several key players and brands that are driving the market are by systemic company profiles.
For producing this U.S. Cerebral Palsy Market report, data has been sourced from in-house databases, secondary and primary research performed by a team of industry experts. In this business report, complete and crystal clear outline of the market is penned down which is useful for many businesses. This market report can be explored in terms of breakdown of data by manufacturers, region, type and application, market status, market share, growth rate, future trends, market drivers, opportunities and challenges, emerging trends, risks and entry barriers, sales channels, and distributors. With this U.S. Cerebral Palsy Market report not only an unskilled individual but also a professional can easily extrapolate an entire market within a few seconds.
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive U.S. Cerebral Palsy Market report. Download Full Report: https://www.databridgemarketresearch.com/reports/us-cerebral-palsy-market
U.S. Cerebral Palsy Market Overview
**Segments**
- **By Type**: The U.S. cerebral palsy market can be segmented by type, including spastic cerebral palsy, dyskinetic cerebral palsy, ataxic cerebral palsy, and mixed cerebral palsy. Each type requires different treatment approaches and therapies based on the specific symptoms and severity of the condition.
- **By Treatment**: Treatment options for cerebral palsy in the U.S. market include medications, therapy (physical, occupational, speech), surgery, assistive devices, and alternative therapies. The availability and effectiveness of these treatments vary for each individual based on factors such as age, severity of symptoms, and overall health.
- **By End User**: The target end users in the U.S. cerebral palsy market comprise hospitals, clinics, rehabilitation centers, and home care settings. Each end user segment has distinct requirements in terms of equipment, expertise, and resources to provide comprehensive care and support for individuals with cerebral palsy.
**Market Players**
- **Medtronic**: A leading player in the U.S. cerebral palsy market, Medtronic offers a range of medical devices and therapies for managing spasticity and movement disorders associated with cerebral palsy. Their innovative solutions aim to improve quality of life and functional outcomes for patients.
- **Abbott Laboratories**: Abbott Laboratories is another key player that provides pharmaceutical products and nutritional supplements for individuals with cerebral palsy. Their products focus on addressing specific nutritional deficiencies and supporting overall health and wellness.
- **Johnson & Johnson**: Johnson & Johnson's portfolio includes orthopedic devices and surgical instruments that are used in the treatment of cerebral palsy, particularly for corrective procedures and rehabilitation interventions. The company's commitment to innovation drives advancements in patient care and outcomes.
- **Boston Scientific Corporation**: Boston Scientific Corporation specializes in neurostimulation technologies that can help manage pain and spasticity associated with cerebral palsy. These advanced therapies offer targeted relief and improved mobility for affected individuals.
The U.S. cerebral palsy market is characterized by a diverse range of segments and players, each contributing to the overall landscape of care and treatment options available to patients. As research and technology continue to advance, we can expect further innovations and improvements in managing this complex neurological condition.
The U.S. cerebral palsy market is witnessing significant growth driven by an increasing prevalence of the condition, advancements in medical technology, and a growing focus on enhancing the quality of life of individuals with cerebral palsy. One of the key trends shaping the market is the shift towards personalized treatment approaches tailored to the specific needs of patients based on factors such as age, type of cerebral palsy, and severity of symptoms. This trend is driving innovation among market players to develop targeted therapies and interventions that can deliver better outcomes for patients.
Moreover, the adoption of multidisciplinary care models involving collaboration between healthcare professionals such as neurologists, orthopedic surgeons, physical therapists, and speech therapists is gaining prominence in the U.S. cerebral palsy market. This holistic approach to care aims to address the complex needs of individuals with cerebral palsy and improve their overall well-being.
In addition, there is a growing emphasis on preventive strategies and early intervention to manage cerebral palsy more effectively. Early diagnosis and intervention have been shown to be crucial in minimizing the impact of the condition on an individual's development and long-term outcomes. This focus on early detection and intervention is driving investments in research and development to identify novel biomarkers, screening tools, and therapeutic approaches that can improve the prognosis for individuals with cerebral palsy.
Furthermore, the U.S. cerebral palsy market is witnessing a surge in technological advancements, particularly in the field of neurostimulation and assistive devices. Innovations such as implantable neurostimulators, robotic exoskeletons, and mobile apps for therapeutic monitoring are revolutionizing the way cerebral palsy is managed and treated. These technologies not only offer new treatment options but also empower patients to actively participate in their care and rehabilitation process.
On the regulatory front, the U.S. cerebral palsy market is subject to stringent oversight by agencies such as the FDA to ensure the safety and efficacy of treatments available to patients. Compliance with regulatory standards and guidelines is crucial for market players to bring new products to market and expand their offerings.
Overall, the U.S. cerebral palsy market is dynamic and evolving, driven by a combination of technological advancements, shifting treatment paradigms, and a growing focus on patient-centered care. As market players continue to innovate and collaborate with healthcare providers and researchers, we can expect to see further improvements in the diagnosis, management, and outcomes for individuals living with cerebral palsy in the United States.The U.S. cerebral palsy market is a multifaceted and dynamic space that is witnessing significant growth and innovation driven by various factors. One key trend shaping the market is the increasing prevalence of cerebral palsy, which is leading to a higher demand for advanced treatment options and care solutions. With advancements in medical technology, market players are focusing on developing personalized treatment approaches tailored to the specific needs of patients. This trend is driving innovation in therapies and interventions aimed at improving outcomes and quality of life for individuals with cerebral palsy.
Another important aspect shaping the market is the adoption of multidisciplinary care models involving collaboration between healthcare professionals from different specialties. This holistic approach to care is gaining prominence as it addresses the complex needs of individuals with cerebral palsy and aims to enhance their overall well-being. The emphasis on preventive strategies and early intervention is also a significant trend in the market, highlighting the importance of early diagnosis and timely interventions to minimize the impact of cerebral palsy on patients' development and long-term outcomes.
Technological advancements play a crucial role in the evolution of the U.S. cerebral palsy market, particularly in the development of neurostimulation technologies and assistive devices. Innovations such as implantable neurostimulators, robotic exoskeletons, and mobile apps for therapeutic monitoring are transforming the landscape of cerebral palsy management and treatment. These technologies not only offer new avenues for treatment but also empower patients to actively engage in their care and rehabilitation processes, enhancing their overall experience and outcomes.
Regulatory oversight by agencies like the FDA is another key aspect influencing the U.S. cerebral palsy market. Compliance with regulatory standards is essential for market players to introduce new products and expand their offerings in a safe and effective manner. Stricter regulations ensure that patients have access to high-quality and reliable treatment options, fostering trust and confidence in the market.
In conclusion, the U.S. cerebral palsy market is characterized by its complexity, innovation, and focus on improving patient outcomes. As market players continue to collaborate, innovate, and invest in research and development, we can expect further advancements in the diagnosis, management, and overall care of individuals living with cerebral palsy in the United States. The market's evolution is driven by a commitment to providing personalized, holistic, and technology-driven solutions that enhance the quality of life for individuals with cerebral palsy.
The U.S. Cerebral Palsy Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now: https://www.databridgemarketresearch.com/reports/us-cerebral-palsy-market/companies
DBMR Nucleus: Powering Insights, Strategy & Growth
DBMR Nucleus is a dynamic, AI-powered business intelligence platform designed to revolutionize the way organizations access and interpret market data. Developed by Data Bridge Market Research, Nucleus integrates cutting-edge analytics with intuitive dashboards to deliver real-time insights across industries. From tracking market trends and competitive landscapes to uncovering growth opportunities, the platform enables strategic decision-making backed by data-driven evidence. Whether you're a startup or an enterprise, DBMR Nucleus equips you with the tools to stay ahead of the curve and fuel long-term success.
Key Questions Answered in This Report: –
How has this U.S. Cerebral Palsy Marketperformed so far and how will it perform in the coming years?
Which are the key product types available in this U.S. Cerebral Palsy Market?
Which are the major application areas in theU.S. Cerebral Palsy Market?
What are the key distribution channels in the global U.S. Cerebral Palsy Market?
What are the key regions in this U.S. Cerebral Palsy Market?
What are the price trends?
What are the various stages in the value chain of this industry?
What are the key driving factors and challenges in the market?
Browse More Reports:
Europe Transradial Access Market Global Fruit and Herbal Tea Market Global Security Assertion Markup Language (SAML) Authentication Market Global Metal Fabrication Market Europe Soy Protein Concentrate Market Global Dental Intraoral Radiology Equipment Market Global Metal Packaging Market Global Copper Sulphate Market Global Breast Ultrasound Market North America Biostimulants Market Global Instant Noodles Market Asia-Pacific Radiopharmaceuticals Market Global Agrochemical Intermediates Market Global Flight Simulator Market Global Polyurethane (PU) Microspheres Market Global Biostimulants Market Global Virtual Pipeline Systems Market Global Stand-Up Paddleboard Market Asia-Pacific Bladder Disorders Market North America Augmented Reality (AR) and Mixed Reality (MR) Market Guatemala Specific Pollen Allergies Market Global Cardiovascular Digital Solutions Market Middle East and Africa Automotive Battery Thermal Management System Market Asia- Pacific Plasma Fractionation Market Global Ribonucleic Acid (RNA) Interference Market Global Canned Tuna Market Global Walnut Oil Market Global Digital Shipyard Market Global Guar Gum Market Global Shoe Rack Market Global Needle Free Blood Drawing Devices Market Global Free Range Eggs Market Global Acrylic Bathtub Market Global Vegan Footwear Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us: Data Bridge Market Research US: +1 614 591 3140 UK: +44 845 154 9652 APAC : +653 1251 975 Email:- [email protected]
Tag:- U.S. Cerebral Palsy, U.S. Cerebral Palsy Size, U.S. Cerebral Palsy Share, U.S. Cerebral Palsy Growth
0 notes
Text
Thymidine Kinase 2 Deficiency Treatment: Evolving Therapies and Emerging Research
Bridging the Gap in Thymidine Kinase 2 Deficiency Treatment
Thymidine Kinase 2 Deficiency is a rare mitochondrial disorder characterized by depletion of mitochondrial DNA, primarily affecting skeletal muscle function. This condition often results in progressive muscle weakness and respiratory complications. Despite the severity of its symptoms and the impact on patient lifespan, therapeutic options for Thymidine Kinase 2 Deficiency remain limited. Current treatment largely centers on managing symptoms rather than altering the course of the disease, underlining the pressing need for disease-modifying therapies in Thymidine Kinase 2 Deficiency treatment.
Learn more about the latest efforts in Thymidine Kinase 2 Deficiency treatment: https://www.delveinsight.com/blog/thymidine-kinase-2-deficiency-treatment-landscape?utm_source=blog&utm_medium=promotion&utm_campaign=akpr
Limited Therapeutic Development in Thymidine Kinase 2 Deficiency Treatment
The pipeline for developing therapies targeting Thymidine Kinase 2 Deficiency remains notably underpopulated. Although some investigational approaches, such as nucleoside replacement therapy and gene-based interventions, have demonstrated encouraging results in early-stage research, they have not yet progressed to regulatory approval. This reflects the broader challenge of advancing clinical development in ultra-rare disorders like Thymidine Kinase 2 Deficiency, where research is often constrained by the complexity of mitochondrial biology and limited clinical data.
Key Challenges in Advancing Thymidine Kinase 2 Deficiency Treatment
Multiple barriers continue to slow the progress of developing effective treatment for Thymidine Kinase 2 Deficiency. Chief among them are the rarity of the condition, insufficient longitudinal data, and the logistical hurdles of conducting adequately powered clinical trials. The variability in disease manifestation further complicates the establishment of reliable clinical endpoints. In addition, the limited commercial appeal of the Thymidine Kinase 2 Deficiency market discourages significant pharmaceutical investment, compounding the difficulty of bringing therapies to market.
The Road Ahead for Thymidine Kinase 2 Deficiency Treatment
Despite these hurdles, there is growing optimism surrounding the future of Thymidine Kinase 2 Deficiency treatment. Advancements in gene therapy techniques, enhanced diagnostic capabilities, and a rise in global rare disease advocacy are setting the stage for renewed progress. Strategic partnerships among academic institutions, biotechnology firms, and patient advocacy organizations will be instrumental in expanding research and driving innovation. As interest in mitochondrial diseases continues to rise, the prospect for meaningful therapeutic breakthroughs in Thymidine Kinase 2 Deficiency is becoming increasingly tangible.
Explore ongoing clinical trials and research collaborations in Thymidine Kinase 2 Deficiency: https://www.delveinsight.com/blog/thymidine-kinase-2-deficiency-treatment-landscape?utm_source=blog&utm_medium=promotion&utm_campaign=akpr
Conclusion
The development of effective Thymidine Kinase 2 Deficiency treatment remains a significant challenge. However, with continued scientific exploration and increased collaboration, the path forward holds promise. A renewed commitment to clinical research and patient advocacy can pave the way for transformative change, offering improved care and hope to those living with this debilitating condition.
Latest Reports Offered By DelveInsight:
nash market, which country has the largest pharmaceutical industry, amz001, current hc delivery system, ketone supplements, crispr's pipeline, bio chips, health rating app, alnylam pipeline, pharmaceutical company hub of the world, complement inhibitors markers, ad109 side effects, chronic illness symptom tracker app, spinal stimulator brands, varon oxygen concentrator, venoferrum, drugs for colitis, robotics and prosthetics, osteosarcoma vs ewing sarcoma, automated pharmacy systems, celiac disease infographic
Other Reports Offered By DelveInsight:
https://www.delveinsight.com/report-store/seborrhoeic-dermatitis-epidemiology-forecasthttps://www.delveinsight.com/report-store/von-hippel-lindau-disease-epidemiology-forecast
#tk2d treatment#tk2 deficiency treatment#tk2d disease treatment#treatment for thymidine kinase 2 deficiency#thymidine kinase 2 deficiency market#thymidine kinase 2 mitochondrial#thymidine kinase 2 deficiency#tk2d life expectancy#tk2 deficiency
0 notes
Text
Rethinking Clinical Research Methodologies in a Post-Pandemic World
The global pandemic didn’t just reshape economies, education, or work culture—it also brought an unprecedented transformation to the world of clinical research. Traditional methodologies, while once considered reliable and standard, were suddenly forced to evolve under pressure. This shift has triggered a significant wave of rethinking and restructuring in how clinical trials and healthcare research are designed, executed, and analyzed—especially in emerging markets like India.
The New Clinical Landscape
When COVID-19 struck, it highlighted both the strengths and weaknesses of existing research frameworks. The speed at which vaccines and treatments were developed was commendable, but it also showed how rigid and time-consuming conventional trial setups can be. The post-pandemic world now demands agility, innovation, and inclusivity in research methodologies.
One of the most promising transformations has been the adoption of decentralized and hybrid trial models. These rely heavily on digital tools, telemedicine, remote patient monitoring, and real-world data, allowing researchers to conduct studies without always requiring participants to visit centralized sites. It’s a game-changer that could make clinical research more accessible, especially in regions with limited infrastructure.
Why the Shift Was Inevitable
Clinical research has always been crucial for medical progress. But the pandemic revealed how fragile our global health systems are and emphasized the importance of clinical trials that are quicker, more efficient, and patient-centric.
Key shortcomings of pre-pandemic research models:
Time-consuming participant recruitment and monitoring
Limited geographic diversity in trial subjects
High dropout rates due to logistical challenges
Underrepresentation of minority groups
These flaws made it clear that the clinical research world needed to pivot fast—and smartly.
India’s Rising Role in Global Clinical Research
India is rapidly positioning itself as a global hub for healthcare innovation. The future of healthcare research in India looks particularly promising due to several factors:
A large and diverse population ideal for trials
Skilled workforce with strong medical and scientific backgrounds
Increasing government support and regulatory reforms
Growth of specialized training and education centers
Educational institutions, especially in cities like Pune, are stepping up to bridge the skill gap. For instance, enrolling in a clinical research course in Pune can now equip students with knowledge not just in traditional research protocols but also in modern trial techniques and digital tools.
Training the New Generation of Researchers
As the research ecosystem evolves, there’s an urgent need for well-trained professionals who understand modern methodologies. Institutes are therefore offering specialized programs like the CRC certification course, designed to train Clinical Research Coordinators on trial management, documentation, ethical considerations, and tech-enabled processes.
A reputable clinical research institute in Pune may now offer hands-on experience with tools used in decentralized trials, helping professionals stay relevant in this fast-changing landscape.
Modern Methodologies in Focus
Here are some of the key shifts in research methods we’re witnessing in the post-pandemic era:
Decentralized Clinical Trials (DCTs): Enabling remote participation and reducing geographical barriers.
Real-World Evidence (RWE): Using data from wearable devices, EHRs, and health apps to support clinical decisions.
AI and Big Data: Streamlining patient recruitment, predicting outcomes, and improving trial design.
Patient-centric Models: Prioritizing patient convenience, engagement, and feedback to improve adherence and outcomes.
Adaptive Trials: Allowing researchers to modify trial parameters in real time without compromising scientific integrity.
These techniques not only make research more effective but also faster and safer—an essential factor in times of health crises.
Reassessing the Purpose of Clinical Research
The purpose of clinical research has always been to evaluate new treatments, interventions, and medical approaches to ensure safety and efficacy. But in the post-pandemic world, that purpose expands to include:
Speed of innovation: We must develop treatments and vaccines faster.
Wider access: Trials must include more diverse populations across urban and rural areas.
Inclusion of tech: Digital tools are now integral, not optional.
Public trust: Transparent processes and ethical frameworks are more important than ever.
Looking Ahead: The Way Forward
As we reflect on the lessons from the pandemic, it’s clear that the future lies in adaptive, tech-powered, and globally inclusive research. India, with its pool of trained professionals and improving infrastructure, stands to benefit immensely. The rise in enrollment for clinical research course in Pune institutions is a testament to this momentum.
To summarize, here’s what the future of clinical research looks like:
Hybrid and remote trial models becoming the norm
Skilled workforce trained through CRC certification courses and modern syllabi
Investment in institutions like clinical research institute in Pune
AI, analytics, and real-time data playing a vital role in research efficiency
India’s emergence as a global research hub
Continued focus on the importance of clinical trials in public health strategy
In conclusion, the disruption caused by COVID-19 has turned out to be a catalyst for much-needed change. From digital transformation to ethical evolution, clinical research is being redefined in ways that promise to shape a more resilient and responsive healthcare future. For aspirants and professionals alike, now is the time to invest in knowledge, upgrade skills, and actively contribute to this evolving domain—because the future of healthcare research in India depends on how well we adapt today.
Let me know if you'd like this repurposed for a blog, LinkedIn post, or emailer.
0 notes
Text
Biomedical Refrigerator Market Insights: Technological Advancements and Regional Trends Reshaping Global Demand
1. Technological Advancements Driving Market Evolution 🚀
1.1 Smart Refrigeration: IoT & Remote Monitoring
Modern Biomedical Refrigerator Market increasingly feature IoT-based sensors, real‑time alerts, digital consoles, cloud connectivity, and remote management tools. These innovations ensure continuous temperature tracking, timely alerts for deviations, and automated data logging—vital for complying with GMP/GSP and reducing sample spoilage .

1.2 Focus on Energy Efficiency and Sustainability
There’s a clear shift toward greener labs: manufacturers are incorporating eco‑friendly refrigerants, advanced insulation, dual‑compressor systems, and frequency‑converting compressors that reduce power consumption and carbon footprint . Projects like Haier’s TwinCool ULT freezers and Helmer’s ENERGY��STAR®‑certified units highlight industry momentum .
1.3 Precision Cooling for Advanced Therapies
Next‑generation therapies—cell and gene treatments—demand ultra‑low (–80 °C to –196 °C) and cryogenic storage. Market adoption of shock freezers, specialized plasma freezers, and ULT units is driven by increased global approvals (e.g., 36 US gene therapies by March 2024) and rising deal volumes (~ USD 3.5 billion in 2023 investments) .
2. Segmentation & Application-Specific Insights
2.1 Product Types
Ultra‑Low Temperature (ULT) Freezers dominate with ~35 % share, essential for cryogenics and molecular biology .
Plasma Freezers are the fastest‑growing segment (~29 %) due to expanded plasma-based therapies and diagnostics .
Laboratory/Pharmacy/Medical Refrigerators lead in unit numbers (~40 %), primarily used by hospitals, pharmacies, and clinics .
2.2 End‑User Demand
Hospitals hold ~45 % volume share, relying on cold storage for medicine, blood, vaccines, and reagents .
Pharmacies account for ~28‑29 %, particularly for specialty drugs and immunotherapies .
Diagnostic Labs & Research Facilities are the fastest‑growing, driven by AI labs, genomics, diagnostics — demand is increasing for precision refrigeration and data logging .
3. Regional Trends & Market Shifts
3.1 North America: Established Market Leader
Constituting ~40 – 45 % of global revenue, North America—especially the U.S.—leads due to advanced healthcare infrastructure, high R&D investment, and strict regulatory oversight (FDA, CDC) . Key factors: high incidence of chronic disease, growing number of blood transfusions (~16 M components/year), and surge in cell/gene clinical trials .
3.2 Europe: Steady Growth with High Compliance
Europe accounts for ~30 % of the market and shows steady growth with Germany leading at ~7 % CAGR. The region’s emphasis on regulation (CE marking, EU directives) and biotech hubs (UK, France, Germany) supports demand .
3.3 Asia‑Pacific: Rapid Expansion
APAC is growing fastest (7–7.8 %+ CAGR) driven by:
Infrastructure growth in China, India, Japan
Government vaccine programs (India provides 65–70 % of WHO needs)
Increasing blood banking and diagnostic services .
Countries like India, China, and Australia rank among fastest growing individual markets .
3.4 Latin America & MEA: Emerging but Promising
Together accounting for ~10 % of market share, these regions show growing demand due to rising healthcare investments, immunization drives, blood banks, and telemedicine expansion .
4. Market Size & Forecast
Current Market Size: Estimates vary from USD 2.64 billion (2023 FMI) to USD 4.33 billion–USD 4.4 billion across other reports .
Growth Forecast: Expected CAGR from 5.4 % to 7.0 % through 2030–2034, reaching USD 6–7.2 billion .
Type‑Segment Outlook:
Blood bank and plasma freezers: 8.1 % CAGR, projected USD 2.6 billion by 2030 .
Laboratory fridges/freezers: ~6–6.3 % CAGR .
5. Competitive Landscape & Strategic Moves
5.1 Prominent Manufacturers
Top global players include Thermo Fisher Scientific, Haier Biomedical, Panasonic Healthcare, Eppendorf, Helmer Scientific, B Medical Systems, Arctiko, Follett, and PHC Holdings .
5.2 Strategic Initiatives
Energy‑Star Certification for Helmer’s ULT freezers (Feb 2024) .
Eco models: Haier’s TwinCool ULT (Oct 2023) and Thermo Fisher’s locally‑made TSV series in India for cost‑sensitive labs .
M&A & Expansions: Haier acquired Labtech UK (2016); B Medical opened India plant in Gujarat (2022) .
New Product Lines: Arctiko’s Flexaline series (Aug 2023); Haier’s HYC‑461GD/FD pharmacy fridge (Sep 2024) .
6. Challenges & Market Barriers
High Capital Costs: Advanced ULT & IoT units range from USD 2,000–15,000+, deterring smaller facilities .
Operating & Compliance Costs: Energy usage and maintenance calibrations add ongoing expenses. IoT adds security and regulatory complexity.
Regulatory Requirements: Manufacturers must meet diverse standards (FDA, CE, ENERGY STAR, GxP), increasing time-to-market and costs .
7. Future Outlook: Trends to Watch
Precision Medicine Growth: Personalized therapies will drive demand for advanced cryogenic & ULT storage systems.
IoT & AI Integration: Edge-AI for predictive maintenance, blockchain for data integrity, and cloud analytics are emerging priorities.
Sustainable Innovation: Alternative refrigerants (e.g., HFOs), magnetic and caloric cooling R&D may redefine future refrigeration tech .
Market Expansion: APAC, LATAM, and MEA offer high growth potential as healthcare infrastructure spreads.
Distributed Storage: Telemedicine, home healthcare, and point-of-care diagnostics will need compact, smart cold units.
✅ Conclusion
Technological advancements—IoT, smart dashboards, energy-efficient cooling—are synergizing with regional healthcare expansion, especially in North America and APAC, to elevate biomedical refrigerators from functional equipment to critical infrastructure. With market value poised to reach USD 6–7 billion by 2030 and ~6–7 % CAGR, success lies in blending innovation with affordability, sustainability, and compliance.
0 notes
Text
The laboratory analytical equipment market is at the heart of scientific innovation and critical decision-making across various industries. From pharmaceutical drug development and food safety to environmental monitoring and forensic investigations, these instruments enable accurate measurement, detection, and characterization of materials at the molecular level. As global industries embrace precision-driven operations, the reliance on sophisticated analytical tools is intensifying. The market is projected to grow steadily from 2025 to 2035, fueled by increased investments in R&D, evolving healthcare diagnostics, and a push toward automation and digital transformation in laboratories.
Market Overview
The laboratory analytical equipment market encompasses a broad array of instruments used to analyze chemical, biological, and physical properties of substances. These include chromatographs, spectrometers, microscopes, titrators, and thermal analyzers, among others. The market is witnessing robust growth, underpinned by expanding applications in clinical diagnostics, pharmaceuticals, biotechnology, environmental science, materials testing, and food quality control. Moreover, advancements in miniaturization, real-time analysis, and integrated software systems are transforming how labs conduct testing and generate data.
As laboratories modernize and automation becomes more accessible, there is a significant shift toward instruments that offer high throughput, improved accuracy, and reduced human error. This transformation is especially evident in the pharmaceutical and biotech sectors, where fast and precise results are essential to meet stringent regulatory requirements and competitive timelines.
Key Market Drivers
1. Rising Demand from the Healthcare and Pharmaceutical Sectors
The global focus on health and wellness, particularly in the aftermath of COVID-19, has intensified investments in clinical research and diagnostics. Laboratory analytical equipment plays a crucial role in drug development, biomarker analysis, genetic screening, and disease diagnostics. The rise of personalized medicine, which requires highly accurate molecular-level analysis, has further boosted demand for next-generation instruments.
2. Technological Advancements
Modern analytical instruments are being integrated with AI, machine learning, and cloud-based data management to facilitate smarter laboratories. These technologies enhance data interpretation, speed up workflows, and enable predictive analytics. Instruments such as automated mass spectrometers, high-resolution microscopes, and real-time PCR systems are examples of how technology is reshaping laboratory operations.
3. Regulatory and Quality Assurance Requirements
Industries such as pharmaceuticals, food and beverage, and chemicals are bound by strict quality assurance and compliance standards. Regulatory frameworks including GMP (Good Manufacturing Practices), ISO certifications, and FDA requirements demand precise and reproducible testing protocols. This has led to increased procurement of high-performance analytical tools to ensure product safety and regulatory compliance.
4. Growing Focus on Environmental Monitoring
With increasing global awareness around environmental pollution and climate change, demand for analytical tools in water, air, and soil testing has surged. Instruments like atomic absorption spectrometers and gas chromatographs are essential for detecting contaminants and ensuring adherence to environmental standards. Government agencies, academic institutions, and private organizations are expanding their monitoring efforts, contributing to market growth.
Market Segmentation
By Product Type:
Chromatography Systems: Widely used in pharmaceuticals and food testing for separating and identifying components of complex mixtures.
Spectroscopy Instruments: Include UV-Vis, NMR, and Mass Spectrometry; vital for quantitative and structural analysis.
Microscopes: Optical, electron, and atomic force microscopes enable visualization of micro and nano-scale structures.
Titrators and Electrochemical Analyzers: Used in chemical and petrochemical labs for quality control.
Thermal Analyzers: Employed in material science to study properties like melting point and heat capacity.
Others: Includes particle size analyzers, rheometers, and pH meters.
Among these, chromatography and spectroscopy dominate the market due to their extensive use in analytical laboratories and their ability to deliver comprehensive chemical profiles.
By End-user:
Pharmaceutical & Biotechnology Companies: The largest end-user segment, driven by the need for consistent product testing, formulation research, and regulatory validation.
Academic & Research Institutions: These entities drive innovation through basic and applied research across disciplines.
Environmental Testing Laboratories: Utilize analytical instruments for pollution monitoring, resource management, and sustainability studies.
Food & Beverage Industry: Instruments are employed to verify ingredient authenticity, detect adulteration, and comply with food safety regulations.
Chemical & Petrochemical Industry: Analytical tools are essential for monitoring process chemistry, ensuring product specifications, and improving production efficiency.
Clinical Diagnostics Laboratories: Use instruments for routine tests, infectious disease detection, and personalized diagnostics.
By Region:
North America: Leading the market due to strong R&D infrastructure, high healthcare expenditure, and early adoption of automation.
Europe: Driven by stringent quality regulations and a strong academic research base.
Asia Pacific: Fastest-growing region, supported by expanding pharmaceutical manufacturing, increased government investments in R&D, and growing academic initiatives in countries like China, India, and South Korea.
Latin America and MEA: Emerging regions showing growth due to improvements in healthcare access and environmental monitoring initiatives.
Emerging Trends
1. Automation and Smart Labs
Laboratories are increasingly embracing automation to improve efficiency and data accuracy. Robotic sample handlers, smart sensors, and automated titration systems are being integrated with software platforms to streamline workflows and reduce human error. Fully integrated labs capable of remote operation are becoming a reality.
2. Portable and Miniaturized Instruments
There is a growing demand for handheld or portable analytical devices in industries such as agriculture, environmental testing, and food safety. These instruments offer real-time results at the point of use, significantly speeding up decision-making processes.
3. Cloud Connectivity and Remote Monitoring
Instruments with cloud-based data storage and real-time monitoring features are revolutionizing laboratory data management. Scientists can now access, share, and analyze data remotely, improving collaboration and workflow continuity.
4. Green Laboratory Practices
Sustainability is gaining importance in laboratory operations. Equipment that reduces chemical usage, energy consumption, and waste generation is increasingly preferred. Manufacturers are also focusing on developing eco-friendly instruments and consumables.
Competitive Landscape
The market is moderately consolidated, with leading players focused on continuous innovation, global expansion, and strategic partnerships. Major players include:
Thermo Fisher Scientific Inc.
Agilent Technologies Inc.
Shimadzu Corporation
PerkinElmer Inc.
Waters Corporation
Bruker Corporation
Danaher Corporation (including Beckman Coulter and Sciex)
Metrohm AG
Horiba Ltd.
Hitachi High-Tech Corporation
These companies offer comprehensive product portfolios and frequently invest in R&D to improve instrument sensitivity, portability, and automation. Strategic acquisitions and collaborations are also common as companies seek to enter new markets or expand their technological capabilities.
Market Forecast and Outlook (2025–2035)
The global laboratory analytical equipment market is poised for strong, sustained growth over the next decade. As more industries integrate data-driven and evidence-based decision-making processes, the need for robust analytical infrastructure will only intensify. While developed markets will continue to invest in high-end instruments and digital integration, emerging economies will witness increased adoption due to improved funding and awareness.
By 2035, the market is expected to benefit from:
Widespread adoption of AI and big data tools in labs
Growing importance of regulatory compliance
Rapid technological innovation in sample preparation and real-time analysis
Expansion of testing applications in non-traditional fields such as nutraceuticals, cosmetics, and agriculture
Conclusion
The laboratory analytical equipment market is undergoing a profound evolution shaped by technological advancements, changing regulatory landscapes, and shifting scientific priorities. As laboratories worldwide seek faster, smarter, and greener solutions, the demand for next-generation analytical tools will continue to accelerate. The period between 2025 and 2035 will be pivotal in defining the market's future—marked by greater automation, decentralized testing, and cross-sector innovation.
0 notes
Text
Global Electrophysiological (EP) Recording Systems Market sees steady 7% CAGR with tech by 2030
The global electrophysiological recording systems market is projected to grow at a CAGR of 7% from 2025 to 2030, driven by increasing demand for advanced electrophysiology procedures in both cardiac and neuroscience fields. Growth is further supported by rapid technological advancements, increasing arrhythmia burden, rising neuromodulation research, and growing adoption of EP recording systems in emerging markets.
Electrophysiology recorders are critical devices used for capturing, analyzing, and storing the electrical signals of the heart and brain during diagnostic and interventional procedures. The market is expanding due to rising procedural volumes in cardiac electrophysiology labs and increased neuroscience research. Additionally, integration of advanced mapping systems, digital connectivity, and data analytics capabilities are making EP recorders indispensable in modern electrophysiology workflows.
Discover the more details-Download the PDF brochure: https://meditechinsights.com/electrophysiological-ep-recording-systems-market/request-sample/
Rising Demand for EP Procedures and Neuro Applications Driving Market Growth
The growing clinical need for real-time, high-fidelity signal acquisition in both cardiac and neuro applications has substantially boosted demand for EP recorders. In cardiac care, rising arrhythmia cases and expanding electrophysiology labs are fuelling growth. In parallel, increased adoption of EP recorders in neuroscience for brain mapping, neuromodulation, and functional research is creating new market opportunities. Increasing investments in academic and translational research, particularly in neural interface technologies and closed-loop neuromodulation systems, are further accelerating market demand. Neuroscience EP recorder applications are expected to see high single-digit growth over the next 5 years, driven by demand in research and functional neurosurgery.
Technological Advancements Driving Innovation in EP Recording Systems
Advances in electrophysiology recording technology are reshaping the market. Innovations include high-density signal acquisition, customizable recording software, advanced noise-filtering algorithms, and integrated data management tools for improved diagnostics and treatment planning. Additionally, cloud-based data sharing and interoperability with 3D cardiac mapping systems and neurostimulation devices are enhancing procedural efficiency and clinical decision-making. Artificial intelligence and machine learning tools are also starting to augment data interpretation in both cardiac and neuroscience applications, enabling precision-guided interventions and predictive outcomes.
Competitive Landscape Analysis
The electrophysiology recorder market is competitive, with established players like GE HealthCare, Boston Scientific, and Alpha Omega Engineering leading the space. These companies are focusing on expanding product capabilities, integrating advanced signal processing, and forming partnerships with academic institutions and EP labs. While GE HealthCare and Boston Scientific are major players in cardiac EP recording, Alpha Omega Engineering is one of the key players in neuroscience.
Unlock key findings! Fill out a quick inquiry to access a sample report https://meditechinsights.com/electrophysiological-ep-recording-systems-market/request-sample/
Market Segmentation
This report by Medi-Tech Insights provides the size of the global electrophysiological recording systems market at the regional- and country-level from 2023 to 2030. The report further segments the market based on product type, application, and end-user.
Market Size & Forecast (2023-2030), By Product Type, USD Billion
EP Recording Systems
Software
Others
Market Size & Forecast (2023-2030), By Application, USD Billion
Cardiac Electrophysiology
Neuroscience Electrophysiology
Others
Market Size & Forecast (2023-2030), By End-user, USD Billion
Hospitals
Ambulatory Surgery Centers
Research Institutes
Market Size & Forecast (2023-2030), By Region, USD Billion
North America
US
Canada
Europe
Germany
France
UK
Italy
Spain
Rest of Europe
Asia Pacific
China
India
Japan
Rest of Asia Pacific
Latin America
Middle East & Africa
About Medi-Tech Insights
Medi-Tech Insights is a healthcare-focused business research & insights firm. Our clients include Fortune 500 companies, blue-chip investors & hyper-growth start-ups. We have completed 100+ projects in Digital Health, Healthcare IT, Medical Technology, Medical Devices & Pharma Services in the areas of market assessments, due diligence, competitive intelligence, market sizing and forecasting, pricing analysis & go-to-market strategy. Our methodology includes rigorous secondary research combined with deep-dive interviews with industry-leading CXO, VPs, and key demand/supply side decision-makers.
Contact:
Ruta Halde Associate, Medi-Tech Insights +32 498 86 80 79 [email protected]
0 notes
Text
How ISO 17025 Certified Labs Ensure Accurate Testing of Semi Finished Leather?
In the global leather industry, quality assurance and regulatory compliance are vital. As manufacturers seek to export leather goods to markets with strict safety and environmental standards, the importance of accurate semi finished leather testing cannot be overstated. Whether it's detecting restricted substances or verifying material durability, reliable testing is critical at the semi finished stage—before costly final processing.
This is where ISO 17025 certified laboratories come in. These labs follow internationally recognized protocols to ensure the highest levels of precision, accuracy, and repeatability in testing. In this blog, we’ll explore what makes ISO 17025 certification crucial, how it applies to semi finished leather testing, and why working with certified labs is a smart move for manufacturers and exporters alike.
What Is Semi Finished Leather?
Semi finished leather refers to hides that have been tanned and partially processed but are not yet finished into final products like shoes, jackets, or upholstery. This stage often involves:
Chemical treatments (tanning agents, dyes, softeners)
Structural modifications (splitting, shaving)
Preparation for final finishing (embossing, coating)
Because chemical residues and physical properties are still being shaped, semi finished leather testing is essential to ensure safety, quality, and compliance before final use.
Why Accuracy in Leather Testing Matters
Testing semi finished leather ensures:
Compliance with global regulations (e.g., REACH, RoHS, CPSIA)
Detection of harmful substances (e.g., chromium VI, formaldehyde, azo dyes)
Assessment of physical durability (e.g., tensile strength, tear resistance)
Protection of brand integrity and prevention of recalls or rejections
Inaccurate testing can lead to:
False compliance (leading to penalties and legal issues)
Missed contaminants that pose health and environmental risks
Inconsistent quality affecting customer trust
That’s why ISO 17025 certified labs are the gold standard for semi finished leather testing.
What Is ISO 17025?
ISO/IEC 17025 is an international standard developed by ISO (International Organization for Standardization) and IEC (International Electrotechnical Commission). It outlines the general requirements for the competence of testing and calibration laboratories.
Key components of ISO 17025 include:
Rigorous quality management systems
Technically competent staff
Validated test methods
Accurate measurement traceability
Regular equipment calibration
Transparent documentation and reporting
When a laboratory is ISO 17025 certified, it means its operations, procedures, and results are reliable, reproducible, and globally recognized.
How ISO 17025 Labs Ensure Reliable Semi Finished Leather Testing
1. Use of Validated Testing Methods
ISO 17025 labs employ standardized methods such as:
ISO 17075 – Determination of chromium VI in leather
ISO 17234 – Azo dye testing
ISO 3071 – pH testing of aqueous leather extracts
EN 1811 – Heavy metal analysis in leather products
ISO 5402 – Flexing endurance testing
These globally accepted methods ensure consistency and comparability of results across borders.
2. Highly Trained Technicians and Analysts
Certified labs ensure staff are qualified and trained in:
Complex analytical procedures (e.g., ICP-OES, GC-MS)
Sample preparation techniques
Data interpretation and uncertainty calculation
This reduces the risk of human error and increases the reliability of test outcomes.
3. Calibrated and Maintained Equipment
From spectrometers to tensile testers, ISO 17025 labs regularly calibrate and maintain their instruments. This guarantees accurate measurements for:
Chromium VI detection
Formaldehyde levels
Tear strength and abrasion resistance
Calibrated equipment provides confidence that results are scientifically sound.
4. Traceability and Documentation
Each test result from an ISO 17025 lab is traceable to:
The specific equipment used
Calibration records
Operator credentials
Reference materials and standards
This level of documentation ensures transparency and legal defensibility—essential in the event of a dispute or audit.
5. Measurement Uncertainty Evaluation
ISO 17025 requires labs to quantify uncertainty in measurement. This scientific approach allows users to understand the reliability and accuracy of results and make informed decisions.
6. Continuous Improvement and Auditing
Certified labs undergo regular internal audits and external assessments by accreditation bodies. This ensures that testing remains up to date with evolving regulations and best practices.
Benefits of Choosing ISO 17025 Labs for Semi Finished Leather Testing
✅ Global Recognition
ISO 17025 certificates are recognized by regulatory bodies, brands, and customs authorities worldwide—making your export journey smoother.
✅ Faster Market Entry
Reliable test results help meet regulatory deadlines and reduce delays at border checkpoints.
✅ Reduced Risk of Product Recalls
Accurate testing at the semi finished stage helps detect issues early, reducing the likelihood of failed final inspections.
✅ Enhanced Customer Trust
Brands and B2B customers are more likely to partner with suppliers who provide certified, accurate test reports.
✅ Support for Sustainability Goals
Accurate testing helps monitor and reduce harmful chemicals, supporting your brand’s ESG and sustainability commitments.
Real-World Scenario: A Global Exporter's Advantage
A leather exporter supplying semi finished hides to luxury fashion brands in Europe partnered with an ISO 17025 accredited lab to test for chromium VI and azo dyes. The lab detected chromium VI levels slightly above REACH limits, allowing the exporter to adjust their tanning process before final shipment. This prevented product rejection, saved thousands of dollars in losses, and enhanced the supplier's reputation with global buyers.
How to Choose the Right ISO 17025 Lab
When selecting a testing partner, consider:
Scope of Accreditation: Ensure the lab is accredited specifically for leather testing.
Turnaround Time: Time-sensitive shipments require quick and reliable results.
Testing Portfolio: Labs should offer both chemical and physical testing of leather.
Reporting Clarity: Results should be clear, detailed, and easy to understand.
Location & Logistics: A conveniently located lab with global affiliations reduces shipping time and cost.
Conclusion
As global regulations tighten and consumer awareness grows, semi finished leather testing has become a cornerstone of quality assurance and legal compliance. ISO 17025 certified labs offer the accuracy, transparency, and credibility that manufacturers need to succeed in international markets.
By partnering with an ISO 17025 certified lab, leather producers not only ensure compliance but also demonstrate a commitment to quality, safety, and sustainability—key factors in building lasting customer relationships and brand value.
#semi finished leather testing#leather testing lab#leather testing#testing lab near me#testing lab in delhi
0 notes
Text
Latin America NGS Market Demand and Forecast Analysis
Meticulous Research®—a leading global market research company, published a research report titled, "Latin America NGS Market by Offering (Kits [Library Prep, QC, DNA Extraction], System) Type (Genome, Exome, Targeted) Application (Reproductive, Oncology, Infectious, Drug Discovery) Technology (SBS, Nanopore, Nanoball, SMRT Seq, DNB) - Forecast to 2032."
According to this latest publication from Meticulous Research®, the Latin America NGS market is expected to reach $765.2 million by 2032, at a CAGR of 13.9% from 2025 to 2032. The growth of the Latin America NGS market can be attributed to various factors, including the increasing initiatives by governments and organizations for genomic sequencing projects, advancements in sequencing procedures, decreasing costs of genome sequencing, a rise in cancer prevalence, and the expanding application of NGS in cancer treatment and research. However, the high costs of NGS systems and consumables and the availability of alternative technologies restrain the market's growth.
The rising adoption of bioinformatics and genomic data management solutions, as well as collaborations and partnerships to support next-generation sequencing, are expected to create market growth opportunities. However, the lack of skilled professionals and ethical and legal issues related to NGS-based diagnosis pose a significant challenge to the market's growth.
Key Players
The key players profiled in the Latin America NGS market report are Thermo Fisher Scientific Inc. (U.S.), Illumina, Inc. (U.S.), Qiagen N.V. (Netherlands), F. Hoffmann-La Roche Ltd (Switzerland), PerkinElmer, Inc. (U.S.), Agilent Technologies, Inc. (U.S.), Danaher Corporation (U.S.), Bio-Rad Laboratories, Inc. (U.S.), Oxford Nanopore Technologies Plc. (U.K.), and 10X Genomics, Inc. 2(U.S.).
Latin America NGS Market: Future Outlook
The Latin America NGS market is segmented into Offering (Consumables [Sample Preparation Consumables {DNA Extraction and Amplification, Library Preparation & Target Enrichment, Quality Control}, Other Consumables], NGS Platforms/Instruments, Software, Services), Sequencing Type (Targeted Genome Sequencing, Whole Genome Sequencing, Whole Exome Sequencing, Other Sequencing Types), Technology (Sequencing by Synthesis, Ion Semiconductor Sequencing, Single-molecule Real-time Sequencing (SMRT), Nanopore Sequencing, DNA Nanoball Sequencing), Applications (Clinical Applications [Reproductive Health, Oncology, Infectious Diseases, Other Clinical Applications], Research Applications [Drug Discovery, Agriculture & Animal Research, Other Research Applications), End User (Hospitals and Diagnostic Laboratories, Pharmaceutical & Biotechnology Companies, Academic Institutes & Research Centers, Other End Users), and Geography.
Among the offerings, in 2025, the consumables segment is expected to account for the largest share of the Latin America NGS market. The large market share of this segment is attributed to recurring demand for consumables, advancements in consumables, and the rising adoption of NGS technology for clinical and research purposes. NGS is used in clinical and research studies to characterize disease-causing genomes, sequence the potential pathogen, analyze epigenetic markers, and identify specific genes and their sequences. These applications of NGS are instrumental in developing targeted medicines, understanding food microbiology and microbiota analysis in the beverage industry, and other environmental studies.
Among the sequencing types, in 2025, the targeted genome sequencing segment is expected to account for the largest share of the Latin America NGS market. The large market share of this segment is attributed to its low sequencing costs compared to other sequencing technologies, increased sensitivity in variant calling, and advancements in targeted genome sequencing technologies. Advances in targeted genome sequencing have enhanced the diagnosis of monogenic intestinal and pediatric disorders. Beyond disease diagnosis, this technology facilitates the exploration of gene-drug associations, enabling researchers to design gene-specific drugs with high specificity.
Among the technologies, in 2025, the sequencing by synthesis segment is expected to account for the largest share of the Latin America NGS market. The large market share of this segment is attributed to the advantages offered by sequencing by synthesis over other technologies. These advantages include the elimination of homopolymer errors, cost-effectiveness for various throughput or scale requirements, robust performance and data quality, unbiased coverage across the genome, and high-quality alignment achieved through paired-end sequencing.
Among the applications, in 2025, the research applications segment is expected to account for the larger share of the Latin America NGS market. The large market share of this segment is attributed to the increasing prevalence of genetic disorders, a rise in the adoption of sequencing-based tests in laboratories, the growing demand for gene-based medicines, increased investments in drug research and development activities, and increasing research programs for personalized medicine.
Among the end users, in 2025, the pharmaceutical & biotechnology companies segment is expected to account for the largest share of the Latin America NGS market. The large market share of this segment is attributed to the increasing R&D spending by pharmaceutical and biotechnology companies and the rising incidence of chronic diseases. For instance, according to GLOBOCAN, in 2020, 1.5 million people were diagnosed with cancer in Latin America. This number is projected to reach 1.9 million by 2032. NGS is often utilized to identify genomic mutations and variants in cancer research. NGS allows scientists and medical professionals to examine a vast amount of DNA and RNA simultaneously, aiding in the detection of abnormalities that might be connected to the disease.
Geographic Review
This research report provides a comprehensive analysis of the market in Brazil, Mexico, Argentina, Chile, and the Rest of Latin America. In 2025, Brazil is expected to account for the largest share of the Latin America NGS market. Brazil's significant market share can be attributed to the increasing adoption of advanced technologies for research purposes, the expanding applications of NGS in drug discovery, growing research and development spending by pharmaceutical and biotechnology companies, and the rising incidence of chronic diseases. These factors collectively drive the adoption of NGS technologies among pharmaceutical and biotechnology companies in Brazil.
Download Sample Report Here @ https://www.meticulousresearch.com/download-sample-report/cp_id=5780
Key questions answered in the report:
Which are the high-growth market segments in terms of the offering, sequencing type, technology, application, end user, and country?
What was the historical market size for NGS in Latin America?
What are the market forecasts and estimates for the period 2025–2032?
What are the major drivers, restraints, challenges, opportunities, and trends in the Latin America NGS market?
Who are the major players in the Latin America NGS market?
What is the competitive landscape like, and who are the market leaders in the Latin America NGS market?
What are the recent developments in the Latin America NGS market?
What are the different strategies adopted by the major players in the Latin America NGS market?
What are the geographical trends and high-growth countries?
Contact Us: Meticulous Research® Email- [email protected] Contact Sales- +1-646-781-8004 Connect with us on LinkedIn- https://www.linkedin.com/company/meticulous-research
#NGS#NextGenerationSequencing#LatinAmericaHealthcare#Genomics#PersonalizedMedicine#Biotechnology#HealthcareInnovation#GeneticTesting#ClinicalDiagnostics#NGSMarket
0 notes
Text
Western Blotting Processors Market Witnesses Growth Due to Rising Demand in Proteomics Research Globally
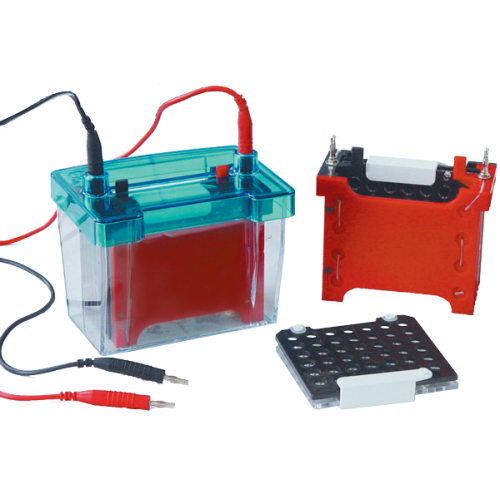
The western blotting processors market is witnessing substantial growth, driven by the increasing demand for automated laboratory systems, rising prevalence of chronic diseases, and continued advancements in proteomics research. As a widely utilized technique for protein detection and quantification, western blotting has evolved significantly with the incorporation of automated processors that enhance throughput, reproducibility, and data accuracy.
Market Overview
Western blotting, a cornerstone in molecular biology and clinical diagnostics, has traditionally been a labor-intensive process. However, the integration of automation through western blotting processors has significantly improved workflow efficiency in laboratories. These automated systems streamline the steps of protein transfer, blocking, antibody incubation, washing, and detection, minimizing human error and reducing the time required to generate results.
As of 2025, the global western blotting processors market is valued at over USD 350 million and is expected to exhibit a compound annual growth rate (CAGR) of approximately 6% over the next five years. North America currently dominates the market, followed closely by Europe and Asia Pacific, with the latter showing the fastest growth due to increasing investments in healthcare infrastructure and research activities in emerging economies like China and India.
Key Market Drivers
1. Growing Application in Disease Diagnosis and Drug Development
The growing incidence of diseases such as cancer, HIV, and neurodegenerative disorders has increased the demand for precise diagnostic tools. Western blotting is commonly employed in the detection of HIV proteins and biomarkers related to Alzheimer’s, Parkinson’s, and various cancers. Automated processors enable higher sample throughput, making them particularly valuable in clinical diagnostics and pharmaceutical R&D.
2. Rising Investments in Proteomics and Genomics Research
Government and private sector investments in proteomics research have surged over the past decade. Western blotting plays a critical role in validating gene expression studies and protein-protein interaction research. Automated systems offer scalability and accuracy, essential for high-volume laboratories involved in biomarker discovery, translational research, and personalized medicine.
3. Technological Advancements and Product Innovation
Manufacturers are focusing on the development of fully integrated systems with touch-screen interfaces, cloud-based data management, and advanced imaging capabilities. Innovations such as multiplex western blotting and chemiluminescent/fluorescent detection methods are enhancing the sensitivity and quantification capabilities of processors. These advancements are expanding the usability of western blotting systems across diverse applications.
Market Challenges
Despite its growth prospects, the western blotting processors market faces several challenges. High capital investment required for advanced systems remains a significant barrier for small and medium-sized laboratories, particularly in developing countries. Additionally, the presence of alternative technologies such as ELISA, mass spectrometry, and next-generation sequencing, which offer higher throughput and multiplexing capabilities, is posing competitive pressure on the western blotting space.
Another concern is the need for skilled personnel to operate and maintain these instruments effectively. While automation reduces manual intervention, knowledge of protocols, calibration, and troubleshooting remains essential, underscoring the importance of training and support services.
Competitive Landscape
The western blotting processors market is moderately consolidated, with a few key players holding substantial market shares. Leading companies include Bio-Rad Laboratories, Thermo Fisher Scientific, GE Healthcare (now Cytiva), Merck KGaA, and LI-COR Biosciences. These companies are actively engaged in strategic collaborations, product launches, and geographical expansion to strengthen their market positions.
For instance, Bio-Rad’s ChemiDoc MP Imaging System and Thermo Fisher’s iBind Flex Western Device have gained widespread adoption for their user-friendly designs and high performance. Meanwhile, emerging players are innovating with cost-effective solutions tailored for academic and research institutions, which are more price-sensitive markets.
Regional Insights
North America remains the largest market due to strong R&D spending, a well-established healthcare system, and a robust biopharmaceutical industry. Europe follows closely, supported by stringent regulatory standards and active research consortia. In Asia Pacific, rapid urbanization, expanding biotechnology sectors, and growing government initiatives in healthcare and life sciences research are accelerating market expansion.
Countries like China, Japan, and South Korea are investing in upgrading their laboratory infrastructure, thereby boosting the demand for automated western blotting processors. Moreover, academic-industry partnerships in the region are fostering innovation and encouraging the adoption of modern laboratory equipment.
Future Outlook
The future of the western blotting processors market looks promising with the convergence of automation, digital imaging, and bioinformatics. As laboratories continue to seek solutions that deliver faster and more reproducible results, the demand for high-throughput and user-friendly processors will increase. Environmental sustainability is also expected to play a role, with manufacturers focusing on energy-efficient systems and reduced reagent consumption.
In conclusion, while the western blotting processors market faces competition from emerging diagnostic technologies, it remains a critical tool in life sciences research and clinical diagnostics. Continuous innovation, affordability, and accessibility will be key factors shaping the market trajectory in the coming years.
0 notes
Text
Veterinary Antimicrobial Susceptibility Testing Market Size, Share, Key Drivers, Trends, Challenges and Competitive Analysis
Executive Summary Veterinary Antimicrobial Susceptibility Testing Market :
The global veterinary antimicrobial susceptibility testing market size was valued at USD 39.61 billion in 2023 and is projected to reach USD 59.60 billion by 2031, with a CAGR of 5.24% during the forecast period of 2024 to 2031.
This Veterinary Antimicrobial Susceptibility Testing Market report aids to establish correlative relationship between the product brand and consumers’ needs and preferences. This market research report is a comprehensive analysis on the study of industry. Market research covered in this report helps the management of a firm in planning by providing accurate and up- to-date information about the consumer’s demands, their changing tastes, attitudes, preferences, and buying intentions etc. Further, manufacturer can adjust production according to the conditions of demand which are analysed here. It also supports to secure economies in the distribution of products and find out the best way of approaching the potential. With the data covered in this Veterinary Antimicrobial Susceptibility Testing Market report, marketing of goods can be made efficient and economical which leads to elimination of all type of wastage.
This Veterinary Antimicrobial Susceptibility Testing Market report makes focus on the more important aspects of the market like what the market recent trends are. The market study provides details of drivers and restraints for the Veterinary Antimicrobial Susceptibility Testing Market with the help of SWOT analysis, along with the impact they have on the demand over the forecast period. It provides guidelines about planning of advertising and sales promotion efforts. Furthermore, the Veterinary Antimicrobial Susceptibility Testing Market report helps the manufacturer in finding out the effectiveness of the existing channels of distribution, advertising programmes or media, selling methods and the best way of distributing the goods to the eventual consumers.
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive Veterinary Antimicrobial Susceptibility Testing Market report. Download Full Report: https://www.databridgemarketresearch.com/reports/global-veterinary-antimicrobial-susceptibility-testing-market
Veterinary Antimicrobial Susceptibility Testing Market Overview
**Segments**
- **Product Type**: The veterinary antimicrobial susceptibility testing market can be segmented by product type into instruments, consumables, and software. Instruments include automated systems, semi-automated systems, and manual systems. Consumables consist of testing plates, reagents, and others. Software plays a crucial role in data analysis and interpretation.
- **Testing Method**: On the basis of testing method, the market can be categorized into disk diffusion, broth dilution, rapid automated methods, agar dilution, and others. Each method has its own advantages and limitations, impacting the choice of method based on the requirements of the testing scenario.
- **Animal Type**: Veterinary antimicrobial susceptibility testing is conducted on various animal types such as companion animals and livestock. The market can be segmented based on the type of animals being tested, as the testing requirements may vary significantly across different species.
**Market Players**
- **Thermo Fisher Scientific Inc.**: Thermo Fisher Scientific is a key player in the veterinary antimicrobial susceptibility testing market, offering a wide range of products such as instruments, consumables, and software for accurate and efficient testing.
- **Danaher Corporation**: Danaher Corporation provides innovative solutions for veterinary antimicrobial susceptibility testing, with a focus on advanced technologies and reliable results to support the healthcare needs of animals.
- **Merck & Co., Inc.**: Merck is a leading player in the veterinary antimicrobial susceptibility testing market, offering comprehensive solutions for testing various pathogens and determining their susceptibility to antimicrobial agents.
- **IDEXX Laboratories, Inc.**: IDEXX Laboratories is known for its high-quality testing solutions in the veterinary industry, including antimicrobial susceptibility testing products that ensure accurate and timely results for effective treatment decisions.
- **Bio-Rad Laboratories, Inc.**: Bio-Rad Laboratories offers a range of products for veterinary antimicrobial susceptibility testing, leveraging its expertise in diagnostics and research to provide reliable testing solutions for animal health.
The global veterinary antimicrobial susceptibility testing market is characterized by the presence of key players focusing on technological advancements and product innovations to cater to the evolving needs of the veterinary healthcare industry. Increasing awareness about antimicrobial resistance and the importance of proper testing methods in veterinary settings are driving the market growth. Factors such as the rise in pet ownership, growing demand for livestock products, and the implementation of stringent regulations promoting responsible antimicrobial use are also contributing to market expansion.
The global veterinary antimicrobial susceptibility testing market continues to witness significant growth driven by various factors shaping the industry landscape. One key trend impacting the market is the increasing focus on combatting antimicrobial resistance in animals, a critical concern for both animal health and public health. As awareness about the overuse of antibiotics and its implications on antimicrobial resistance grows, there is a heightened emphasis on implementing effective testing methods to ensure appropriate antimicrobial usage in veterinary settings.
Market players are continuously investing in research and development to introduce advanced technologies and innovative products that enhance the efficiency and accuracy of antimicrobial susceptibility testing. This focus on technological advancements is crucial in meeting the evolving needs of the veterinary healthcare industry, where precise and timely testing results are paramount for making informed treatment decisions.
Moreover, the market's segmentation based on product type, testing method, and animal type offers insights into the diverse applications of veterinary antimicrobial susceptibility testing. By offering a range of instruments, consumables, and software solutions tailored to specific testing scenarios and animal types, market players can address the varied requirements of veterinarians and researchers working across companion animals and livestock.
Collaborations and partnerships between key market players and academic institutions or research organizations play a pivotal role in fostering innovation and driving market growth. These strategic alliances enable knowledge sharing, access to specialized expertise, and the co-development of new testing solutions that can address emerging challenges in veterinary antimicrobial susceptibility testing.
Regulatory initiatives promoting responsible antimicrobial use in veterinary healthcare also contribute to market expansion by emphasizing the importance of accurate susceptibility testing and rational antibiotic prescribing practices. Compliance with regulatory guidelines not only ensures the effective management of antimicrobial resistance but also enhances the overall quality of care provided to animals.
In conclusion, the global veterinary antimicrobial susceptibility testing market presents a dynamic landscape characterized by technological advancements, regulatory developments, and a growing awareness of antimicrobial resistance issues. Market players are poised to capitalize on opportunities arising from these trends by offering innovative solutions that meet the changing demands of the veterinary healthcare sector. As the market continues to evolve, strategic investments in research and development, enhanced collaboration efforts, and a commitment to promoting responsible antimicrobial use will be key drivers of future growth and success in this critical segment of the veterinary industry.The global veterinary antimicrobial susceptibility testing market is witnessing significant growth propelled by various factors that are shaping the industry landscape. One of the key drivers of market expansion is the escalating concern surrounding antimicrobial resistance in animals, which is a critical issue impacting both animal health and public health. As awareness about the adverse effects of antibiotic overuse grows, there has been a notable emphasis on implementing effective testing methods to ensure judicious use of antimicrobials in veterinary practices. This heightened focus on combatting antimicrobial resistance has led to an increased demand for advanced testing solutions that can provide accurate and timely results to guide appropriate treatment decisions for animals.
Market players in the veterinary antimicrobial susceptibility testing sector are consistently investing in research and development endeavors to introduce cutting-edge technologies and innovative products that can enhance the efficiency and precision of testing procedures. By prioritizing technological advancements, these companies aim to meet the evolving needs of the veterinary healthcare industry, where the availability of precise and rapid testing outcomes is paramount for delivering optimal care to animals. Moreover, the market segmentation based on product type, testing method, and animal type offers valuable insights into the diverse applications of antimicrobial susceptibility testing in veterinary settings, enabling companies to tailor their offerings to meet specific requirements across companion animals and livestock.
Furthermore, strategic collaborations and partnerships between key market players and academic or research institutions are playing a crucial role in fostering innovation and propelling market growth. By engaging in such strategic alliances, companies can leverage specialized expertise, share knowledge, and co-create novel testing solutions that address emerging challenges in veterinary antimicrobial susceptibility testing. These collaborative efforts contribute to driving advancements in the field and enhancing the overall quality of testing practices in veterinary healthcare.
Additionally, regulatory initiatives aimed at promoting responsible antimicrobial use in veterinary settings are acting as catalysts for market expansion by underlining the significance of accurate susceptibility testing and appropriate antibiotic prescribing practices. Compliance with regulatory guidelines not only supports the effective management of antimicrobial resistance but also elevates the standard of care provided to animals. As the global veterinary antimicrobial susceptibility testing market continues to evolve, strategic investments in research and development, enhanced collaboration strategies, and a steadfast commitment to promoting responsible antimicrobial utilization will be pivotal in driving future growth and success in this critical segment of the veterinary industry.
The Veterinary Antimicrobial Susceptibility Testing Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now: https://www.databridgemarketresearch.com/reports/global-veterinary-antimicrobial-susceptibility-testing-market/companies
DBMR Nucleus: Powering Insights, Strategy & Growth
DBMR Nucleus is a dynamic, AI-powered business intelligence platform designed to revolutionize the way organizations access and interpret market data. Developed by Data Bridge Market Research, Nucleus integrates cutting-edge analytics with intuitive dashboards to deliver real-time insights across industries. From tracking market trends and competitive landscapes to uncovering growth opportunities, the platform enables strategic decision-making backed by data-driven evidence. Whether you're a startup or an enterprise, DBMR Nucleus equips you with the tools to stay ahead of the curve and fuel long-term success.
Key Benefits of the Report:
This study presents the analytical depiction of the global Veterinary Antimicrobial Susceptibility Testing Marketindustry along with the current trends and future estimations to determine the imminent investment pockets.
The report presents information related to key drivers, restraints, and opportunities along with detailed analysis of the global Veterinary Antimicrobial Susceptibility Testing Market share.
The current market is quantitatively analyzed from ��to highlight the Global Veterinary Antimicrobial Susceptibility Testing Market growth scenario.
Porter's five forces analysis illustrates the potency of buyers & suppliers in the market.
The report provides a detailed global Veterinary Antimicrobial Susceptibility Testing Market analysis based on competitive intensity and how the competition will take shape in coming years
Browse More Reports:
Global Organometallic Market Global Dome Security Market Global Rabies Prophylaxis Market Middle East and Africa Trauma Devices Market Global Neurological Biomarkers Market Global Automotive Rain Sensor Market Asia-Pacific Maintenance Repair and Operations (MRO) Market Global Automotive Battery Thermal Management System Market Global Subscription and Billing Management Market Global Osteosarcoma Drug Market Global Specialty PACS Market Global Gardening Pots Market Asia-Pacific Specialty Oilfield Chemicals Market North America Sweet Potato Powder Market Global Minimal Residual Disease Market Global Endometrial Resection Devices Market Europe Contrast Injector Market Global Discrete Diodes Market Global Composite Packaging Market Global Vascular Grafts and Peripheral Stents Market Global Colored Gemstones Market Global Web Analytics Market Global Tumor Necrosis Factor (TNF) Inhibitor Drugs Market Global Dental Adhesive Market California Biostimulants Market Global Thrombotic Thrombocytopenic Purpura (Moschcowitz Disease) Market Global Coagulation Testing Market North America Network Packet Broker Market Asia-Pacific Inherited Retinal Diseases Market Global Quicklime Market Global Carbon Fiber Tape Market Global Frozen Drinks Market Global User Experience (UX) Research Software Market Europe Augmented Reality (AR) and Mixed Reality (MR) Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us: Data Bridge Market Research US: +1 614 591 3140 UK: +44 845 154 9652 APAC : +653 1251 975 Email:- [email protected]
Tag:- Veterinary Antimicrobial Susceptibility Testing, Veterinary Antimicrobial Susceptibility Testing Size, Veterinary Antimicrobial Susceptibility Testing Share, Veterinary Antimicrobial Susceptibility Testing Growth
0 notes
Text
How UAE’s Accredited Laboratories Leverage Automation for Precision Testing? | +971 554747210
The UAE is rapidly advancing as a regional hub for scientific research, manufacturing, and quality assurance. At the heart of this progress are accredited laboratories in the UAE, which play a vital role in ensuring products and materials meet stringent standards. To stay ahead in a competitive global market, many of these labs are embracing automation technologies that revolutionize precision testing.
Automation is transforming how accredited laboratories operate, enabling faster, more accurate, and highly reliable test results. This blog explores how UAE’s accredited laboratories leverage automation for precision testing, the benefits of automation, and its impact on various industries.
The Growing Importance of Accredited Laboratories in the UAE
Laboratory accreditation, such as ISO/IEC 17025 certification granted by bodies like ENAS (Emirates National Accreditation System), guarantees that labs meet international quality and technical standards. These accredited labs are trusted to deliver reliable testing services essential for:
Regulatory compliance
Product certification
Quality assurance
Research and development
In sectors like oil and gas, pharmaceuticals, food safety, and manufacturing, precision testing is non-negotiable. Automation helps UAE’s accredited laboratories meet these high demands efficiently and consistently.
What Is Automation in Laboratory Testing?
Automation in laboratory testing involves using technology-driven systems, robotics, and software to perform test procedures with minimal human intervention. This includes:
Automated sample preparation and handling
Robotic liquid handling systems
Computer-controlled analytical instruments
Integrated data acquisition and management platforms
By reducing manual processes, automation minimizes human error, speeds up workflows, and enhances data accuracy.
How UAE’s Accredited Laboratories Use Automation for Precision Testing
1. Automated Sample Preparation
Sample preparation is often the most labor-intensive and error-prone part of testing. UAE labs use automated systems to:
Weigh and measure samples precisely
Perform dilution and mixing with exact proportions
Conduct sample digestion or extraction processes
Automation ensures uniformity across samples, which is critical for reproducible test results.
2. Robotic Liquid Handling
Accredited labs in the UAE implement robotic liquid handlers to transfer precise volumes of liquids during chemical analysis, molecular biology, and pharmaceutical testing. These robots offer:
High throughput processing
Reduced contamination risks
Consistent pipetting accuracy
This technology is vital for labs conducting food safety tests, water quality analysis, and drug potency assays.
3. Advanced Analytical Instruments
Automation extends to advanced instruments such as:
Chromatography systems (GC, HPLC) for separating chemical mixtures
Spectroscopy devices (UV-Vis, FTIR, Mass Spectrometry) for qualitative and quantitative analysis
Automated microscopes and imaging systems for material characterization
These instruments are often integrated with software that controls their operation, collects data, and processes results automatically.
4. Data Management and Reporting
Automated data management platforms collect, store, and analyze test data securely. Features include:
Real-time data monitoring
Automated calculation of results with statistical validation
Generation of standardized, customizable reports
Traceability and audit trails for compliance
Such platforms help UAE’s accredited laboratories maintain transparency and meet regulatory demands efficiently.
Benefits of Automation for UAE’s Accredited Laboratories
Enhanced Accuracy and Precision
Automation drastically reduces human error associated with manual handling and subjective interpretation. Precise control over sample volumes, instrument parameters, and data processing leads to more consistent and trustworthy results.
Increased Testing Throughput
Automated systems can process hundreds or thousands of samples simultaneously, dramatically increasing laboratory productivity. This is crucial in sectors like food testing or environmental monitoring where large sample volumes are routine.
Faster Turnaround Time
Automation shortens testing cycles, enabling faster delivery of results without compromising quality. This agility helps manufacturers and exporters meet tight deadlines and regulatory timelines.
Improved Safety
Handling hazardous chemicals and biological samples manually poses risks. Automated systems reduce operator exposure to dangerous substances, promoting safer laboratory environments.
Regulatory Compliance and Traceability
Automation supports compliance with international standards such as ISO/IEC 17025 by maintaining comprehensive records, reducing documentation errors, and facilitating external audits.
Impact of Automation on Key UAE Industries
Oil and Gas
Accredited labs use automated precision testing to analyze petroleum products, pipeline materials, and environmental samples. Rapid and accurate test results help companies comply with local and global standards, ensuring operational safety and efficiency.
Pharmaceuticals
Automation in pharmaceutical testing ensures drug quality, potency, and purity. Accredited labs in the UAE employ robotic systems for sample prep and automated instrumentation to meet stringent health authority requirements.
Food Safety
The UAE’s food import and manufacturing sectors depend heavily on accredited labs to test for contaminants, allergens, and nutritional content. Automation enables high-throughput screening of food samples, essential for consumer safety.
Manufacturing and Construction
Material testing labs use automated systems to assess the mechanical, chemical, and physical properties of metals, plastics, and composites. This ensures that products meet UAE’s regulatory and quality benchmarks.
Challenges and Considerations in Implementing Automation
Despite its advantages, automation implementation requires significant investment in equipment, staff training, and software integration. Accredited laboratories must:
Select compatible automated systems for their specific testing needs
Maintain rigorous calibration and validation of automated instruments
Ensure skilled personnel are trained to operate and troubleshoot automated workflows
UAE laboratories are increasingly partnering with global technology providers and investing in workforce development to overcome these challenges.
The Future of Automation in UAE’s Accredited Laboratories
With the UAE’s strategic focus on innovation and smart technologies, automation in accredited laboratories is poised for exponential growth. Emerging trends include:
Artificial Intelligence (AI) and Machine Learning: For predictive analytics and anomaly detection in test data
Internet of Things (IoT): Connected devices providing real-time monitoring of laboratory instruments
Cloud-based Data Solutions: Enhancing collaboration, storage, and remote access to lab results
Advanced Robotics: For fully autonomous lab workflows
These advancements will further improve the precision, efficiency, and scalability of testing services offered by accredited laboratories in the UAE.
Conclusion
Automation is revolutionizing the landscape of accredited laboratories in the UAE, especially in delivering precision testing critical to multiple industries. By integrating robotic systems, advanced instruments, and sophisticated data management platforms, UAE’s labs achieve unparalleled accuracy, faster throughput, and enhanced safety.
For businesses seeking reliable testing and certification, partnering with an ENAS-accredited, ISO/IEC 17025 certified laboratory that leverages automation is a smart move. It ensures compliance, quality, and operational excellence in today’s fast-paced market.
As the UAE continues to lead in technology adoption, the future of laboratory testing will undoubtedly be shaped by intelligent automation — empowering accredited labs to set new standards of precision and trust.
0 notes
Text
Ever Wondered What Happens in Clinical Research?
If you’ve ever paused and thought, “Ever wondered what happens in clinical research?” — you’re not alone. It’s a field that touches everyone’s life, yet remains largely behind the scenes. From life-saving vaccines to breakthrough cancer therapies, clinical research is the backbone of modern medicine.
But what does it really involve? What kind of training do you need? And what does the future hold for those who choose this path? Let’s explore the world of clinical research, its opportunities, and why it’s such an essential part of the healthcare ecosystem.
Understanding the Basics: What is Clinical Research?
At its core, clinical research involves the study of health and illness in people. It’s how we find new ways to detect, prevent, and treat diseases. The field is carefully structured into different phases, from early laboratory testing to trials involving volunteers and patients. These trials follow strict ethical and scientific standards.
For anyone curious about starting a career in this field, an introduction to clinical research is the first step. Many students and graduates opt for foundational courses that offer a comprehensive view of the industry, its terminology, and real-world applications.
Why Are Clinical Trials Important?
Clinical trials are more than just scientific experiments; they are vital tools for improving public health. Here’s why they matter:
Patient Safety: They ensure that new drugs or treatments are safe and effective before reaching the market.
Innovation: Trials foster innovation by supporting the development of new therapies and medical devices.
Regulatory Compliance: They help pharmaceutical companies meet legal and ethical obligations.
Personalized Medicine: Clinical studies are leading the way in customized treatments for individual patients.
The Role of Training: Why You Need It
India, and specifically Pune, has emerged as a hub for healthcare and pharmaceutical education. With its growing life sciences sector, many students are now enrolling in professional programs to gain relevant expertise. A clinical research course in Pune offers the right mix of theoretical knowledge and practical exposure.
These courses typically cover:
Basics of drug development
Clinical trial protocols
Ethics and regulatory guidelines
Role-specific responsibilities
Real-time case studies and simulations
Institutes like TechnoBridge Systems, recognized as a leading clinical research training institute in Pune, are playing a key role in nurturing the next generation of research professionals.
Becoming a Clinical Research Coordinator
If you’ve ever considered a specialized role in the industry, becoming a coordinator is a popular and respected choice. A clinical research coordinator training in Pune can prepare you for responsibilities such as:
Managing the day-to-day operations of a trial
Recruiting and interacting with participants
Ensuring protocol compliance
Collecting and maintaining data
Liaising with sponsors and regulatory authorities
Such training typically includes hands-on modules, internships, and certification — all designed to build job-ready skills.
Career Outlook: What’s the Future of Clinical Research Careers?
Thanks to a global push for better healthcare, the future of clinical research careers looks brighter than ever. Whether it's pharmaceutical companies, hospitals, CROs (Contract Research Organizations), or biotech firms, the demand for skilled professionals is steadily rising.
Here’s why the future is promising:
Global Expansion: Clinical trials are increasingly being outsourced to emerging markets like India, leading to more opportunities.
Technological Growth: AI, data analytics, and wearable health tech are revolutionizing the field.
Diverse Roles: From Clinical Data Management to Regulatory Affairs, a wide range of career paths are available.
Job Stability: The industry offers high job security due to its critical importance to healthcare.
Top Reasons to Choose a Clinical Research Course in Pune
Pune has gained a reputation for its educational infrastructure and industry tie-ups. Here’s what makes it a great choice:
Experienced Faculty: Courses are taught by professionals with real industry experience.
Placement Assistance: Many institutes have strong connections with pharmaceutical companies.
Affordable Education: Compared to metros like Mumbai or Delhi, Pune offers quality education at a lower cost.
Industry Exposure: With several biotech and pharma companies based in and around Pune, students often get real-world exposure during their training.
Conclusion
So the next time someone asks, “Ever wondered what happens in clinical research?”, you’ll know there’s more than meets the eye. It’s a deeply rewarding and impactful field that blends science, ethics, and innovation. Whether you’re a fresh graduate or a working professional looking to transition, enrolling in a reputed clinical research training institute in Pune can be your gateway to a successful career.
From understanding why clinical trials are important to exploring the future of clinical research careers, the journey is filled with knowledge, discovery, and opportunity. If you’re ready to be part of something that changes lives — this could be your calling.
0 notes
Text
Fish Counters Market Trends, Size, Forecast to 2024-2032

The Reports and Insights, a leading market research company, has recently releases report titled “Fish Counters Market: Global Industry Trends, Share, Size, Growth, Opportunity and Forecast 2024-2032.” The study provides a detailed analysis of the industry, including the global Fish Counters Market share, size, trends, and growth forecasts. The report also includes competitor and regional analysis and highlights the latest advancements in the market.
Report Highlights:
How big is the Fish Counters Market?
The global fish counters market size reached US$ 9.1 billion in 2023. Looking forward, Reports and Insights expects the market to reach US$ 15.6 billion in 2032, exhibiting a growth rate (CAGR) of 6.2% during 2024-2032
What are Fish Counters?
Fish counters are tools utilized to count fish as they move through a designated area, like a river, stream, or fish ladder. They play a critical role in fisheries management, research, and conservation by providing precise data on fish migration, population dynamics, and habitat utilization. These counters employ diverse technologies, such as infrared sensors, video cameras, and acoustic systems, to detect and tally fish without direct contact. This information is essential for scientists and policymakers to make well-informed decisions for the preservation and sustainable management of fish populations and their environments.
Request for a sample copy with detail analysis: https://www.reportsandinsights.com/sample-request/1949
What are the growth prospects and trends in the Fish Counters industry?
The fish counters market growth is driven by various factors. The fish counters market is steadily growing, fueled by increasing demand for precise and efficient fish counting solutions in fisheries and aquaculture. These systems play a vital role in monitoring fish populations, ensuring sustainable fishing practices, and enhancing production in aquaculture settings. Technological advancements, including the incorporation of artificial intelligence and machine learning, are further enhancing the accuracy and efficiency of fish counters. Additionally, there is a growing preference for portable and user-friendly fish counting devices, especially in smaller fisheries and research settings. Overall, the fish counters market is poised for continued expansion due to the growing emphasis on sustainable fishing and effective aquaculture management. Hence, all these factors contribute to fish counters market growth.
What is included in market segmentation?
The report has segmented the market into the following categories:
By Product Type:
Electronic Fish Counters
Mechanical Fish Counters
By Application:
Fisheries Management
Aquaculture
By End-Use:
Government Agencies and Research Institutes
Commercial Fisheries
Aquaculture Farms
Market Segmentation by Region:
North America
United States
Canada
Europe
Germany
United Kingdom
France
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
Rest of Asia Pacific
Latin America
Brazil
Mexico
Argentina
Middle East & Africa
Saudi Arabia
South Africa
United Arab Emirates
Israel
Who are the key players operating in the industry?
The report covers the major market players including:
VAKI Aquaculture Systems Ltd.
Precision Measurement Engineering, Inc.
Star-Oddi
AquaScan AS
Vidar Systemer AS
Marel hf.
Fishtek Marine
NOVIS S.A.
Pentair Aquatic Eco-Systems
Automated Aquatics
In-Situ Inc.
Fishtek Marine Ltd.
Otter Trawl (Oceantech)
OSMOSIA Scientific Instruments
AquaScan OY
View Full Report: https://www.reportsandinsights.com/report/Fish Counters-market
If you require any specific information that is not covered currently within the scope of the report, we will provide the same as a part of the customization.
About Us:
Reports and Insights consistently mееt international benchmarks in the market research industry and maintain a kееn focus on providing only the highest quality of reports and analysis outlooks across markets, industries, domains, sectors, and verticals. We have bееn catering to varying market nееds and do not compromise on quality and research efforts in our objective to deliver only the very best to our clients globally.
Our offerings include comprehensive market intelligence in the form of research reports, production cost reports, feasibility studies, and consulting services. Our team, which includes experienced researchers and analysts from various industries, is dedicated to providing high-quality data and insights to our clientele, ranging from small and medium businesses to Fortune 1000 corporations.
Contact Us:
Reports and Insights Business Research Pvt. Ltd. 1820 Avenue M, Brooklyn, NY, 11230, United States Contact No: +1-(347)-748-1518 Email: [email protected] Website: https://www.reportsandinsights.com/ Follow us on LinkedIn: https://www.linkedin.com/company/report-and-insights/ Follow us on twitter: https://twitter.com/ReportsandInsi1
0 notes