#Halofuginone
Explore tagged Tumblr posts
Text
Halofuginone Lactate CAS#: 82186-71-8
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product NameHalofuginone LactateIUPAC Name7-bromo-6-chloro-3--2-oxopropyl]quinazolin-4-one;2-hydroxypropanoic acidMolecular Structure
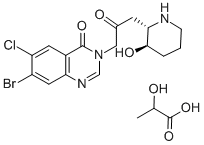
CAS Registry Number 82186-71-8MDL NumberMFCD00144429SynonymsSCHEMBL2562822; CHEMBL1162014; C-22970; 7-bromo-6-chloro-3-(3-((2S,3R)-3-hydroxypiperidin-2-yl)-2-oxopropyl)quinazolin-4(3H)-one 2-hydroxypropanoate; Halocur; Stenorol; TempostatinMolecular FormulaC19H23BrClN3O6Molecular Weight504.8InChIInChI=1S/C16H17BrClN3O3.C3H6O3/c17-11-6-13-10(5-12(11)18)16(24)21(8-20-13)7-9(22)4-14-15(23)2-1-3-19-14;1-2(4)3(5)6/h5-6,8,14-15,19,23H,1-4,7H2;2,4H,1H3,(H,5,6)/t14-,15+;/m0./s1InChI KeyGATQERNJKZPJNX-NUNOUFIPSA-NCanonical SMILESCC(C(=O)O)O.C1CC(C(NC1)CC(=O)CN2C=NC3=CC(=C(C=C3C2=O)Cl)Br)OIsomeric SMILESCC(C(=O)O)O.C1C(C(NC1)CC(=O)CN2C=NC3=CC(=C(C=C3C2=O)Cl)Br)O Patent InformationNo data available
Physical Data
AppearanceWhite or off-white powderMelting Point>70°C dec. Boiling Point595.8 °C at 760 mmHgFlash Point314.1 °CSolubilityDMSO : 9 mg/mL (21.70 mM; Need ultrasonic and warming)
Spectra
No data available
Route of Synthesis (ROS)
No data available
Safety and Hazards
Pictogram(s)





SignalDangerGHS Hazard StatementsH300: Fatal if swallowed H301: Toxic if swallowed H310: Fatal in contact with skin H315: Causes skin irritation H317: May cause an allergic skin reaction H318: Causes serious eye damage H330: Fatal if inhaled H361: Suspected of damaging fertility or the unborn child H372: Causes damage to organs through prolonged or repeated exposure H400: Very toxic to aquatic life H410: Very toxic to aquatic life with long lasting effects Information may vary between notifications depending on impurities, additives, and other factors. Precautionary Statement CodesP201, P202, P260, P261, P262, P264, P270, P271, P272, P273, P280, P281, P284, P301+P310, P302+P350, P302+P352, P304+P340, P305+P351+P338, P308+P313, P310, P314, P320, P321, P322, P330, P332+P313, P333+P313, P361, P362, P363, P391, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) For more detailed information, please visit ECHA C&L website
Other Data
TransportationEnvironmentally hazardous substance, solid, n.o.s. ; Class 9; Packaging Group: III; UN Number: 3077 Under the room temperature and away from lightHS Code294200StorageKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition. Recommended storage temperature: -20°C 3 years 4°C 2 years Shelf LifeMarket PriceUSD 2300/kg Use PatternHalofuginone Lactate CAS#: 82186-71-8 is used fvor the treatment of scleroderma, cancer, and restenosis.Halofuginone Lactate CAS#: 82186-71-8 is a low molecular weight quinazolinone alkaloid, and a potent inhibitor of collagen alpha1(I) and matrix metalloproteinase 2 (MMP-2) gene expression. Halofuginone also effectively suppresses tumor progression and metastasis in mice. Collgard Biopharmaceuticals is developing halofuginone for the treatment of scleroderma and received orphan drug designation from the U.S. Food and Drug Administration in March, 2000. Halofuginone (RU-19110) is a less-toxic form of Febrifugine, which is isolated from the plant Dichroa febrifuga. Halofuginone inhibits prolyl-tRNA synthetase in an ATP-dependent manner with a Ki of 18.3 nM. Halofuginone attenuates osteoarthritis (OA) by inhibition of TGF-β activity. Halofuginone is a potent inhibitor of collagen a1(I) and matrix metalloproteinase 2 (MMP-2) gene expression. Halofuginone also suppresses extracellular matrix deposition and cell proliferation. The profound antitumoral effect of halofuginone is attributed to its combined inhibition of the tumor stromal support, vascularization, invasiveness, and cell proliferation. Read the full article
0 notes
Text
Crocin attenuates lung inflammation and pulmonary vascular dysfunction in a rat model of bleomycin-induced pulmonary fibrosis.
PMID: Life Sci. 2019 Aug 26:116794. Epub 2019 Aug 26. PMID: 31465731 Abstract Title: Crocin attenuates lung inflammation and pulmonary vascular dysfunction in a rat model of bleomycin-induced pulmonary fibrosis. Abstract: Amongst the various forms of lung injury; pulmonary fibrosis remains the most intricate form with limited therapeutic options to both the patient and the physicians. Bleomycin (BLM) is a chemotherapeutic agent used for the treatment of various carcinomas; however, its therapeutic value is significantly limited by its associated pulmonary fibrosis. The current study highlights the prominent antioxidant, anti-inflammatory and anti-fibrotic effect of crocin against BLM-induced pulmonary fibrosis. Intratracheal BLM instillation induced significant biochemical, structural, functional and vascular pulmonary injury. BLM instillation increased oxidant load with quenching of antioxidant defenses together with increase inflammatory and fibrotic cytokines expression. Crocin significantly attenuated BLM-induced lung injury and its effect was comparable to the standard anti-fibrotic; halofuginone. The observed anti-inflammatory and anti-fibrotic and antioxidant impacts are thought to be embroiled in the therapeutic impacts of crocin. Down-regulation of TLR4, IL-10 expression is the major pathway involved in the observed anti-inflammatory effects and finally, down-regulation of tissue expression of TNF-α and TGF-β1 is the major pathways implicated in the observed anti-fibrotic activities and modulation of Nrf2 and HO-1 pathways is the main mechanism involved in the observed antioxidant effects.
read more
0 notes
Text
Antifibrotic Therapy Disrupts Stromal Barriers and Modulates the Immune Landscape in Pancreatic Ductal Adenocarcinoma
Pancreatic ductal adenocarcinoma (PDA) remains one of the deadliest forms of cancer, in part, because it is largely refractory to current therapies. The failure of most standard therapies in PDA, as well as promising immune therapies, may be largely ascribed to highly unique and protective stromal microenvironments that present significant biophysical barriers to effective drug delivery, that are immunosuppressive, and that can limit the distribution and function of antitumor immune cells. Here, we utilized stromal reengineering to disrupt these barriers and move the stroma toward normalization using a potent antifibrotic agent, halofuginone. In an autochthonous genetically engineered mouse model of PDA, halofuginone disrupted physical barriers to effective drug distribution by decreasing fibroblast activation and reducing key extracellular matrix elements that drive stromal resistance. Concomitantly, halofuginone treatment altered the immune landscape in PDA, with greater immune infiltrate into regions of low hylauronan, which resulted in increased number and distribution of both classically activated inflammatory macrophages and cytotoxic T cells. In concert with a direct effect on carcinoma cells, this led to widespread intratumoral necrosis and reduced tumor volume. These data point to the multifunctional and critical role of the stroma in tumor protection and survival and demonstrate how compromising tumor integrity to move toward a more normal physiologic state through stroma-targeting therapy will likely be an instrumental component in treating PDA.Significance:This work demonstrates how focused stromal re-engineering approaches to move toward normalization of the stroma disrupt physical barriers to effective drug delivery and promote antitumor immunity.See related commentary by Huang and Brekken, p. 328 http://bit.ly/2CpBVfa
0 notes
Text
Halofuginone hydrobromide CAS#: 64924-67-0
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product NameHalofuginone hydrobromideIUPAC Name7-bromo-6-chloro-3--2-oxopropyl]quinazolin-4-one;hydrobromide; trans-(±)-7-Bromo-6-chloro-3--4(3H)-quinazolinone monohydrobromideMolecular Structure
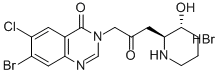
CAS Registry Number 64924-67-0EINECS Number241-422-7MDL NumberMFCD23843776Synonyms7-bromo-6-chlorofebrifugine Hbr; halofuginone; halofunginone; Stenorol; 7-bromo-6-chloro-3--2-oxopropyl]quinazolin-4-one HBr; Tempostatin; RU 19110; Molecular FormulaC16H18Br2ClN3O3 Molecular Weight495.60InChIInChI=1S/C16H17BrClN3O3.BrH/c17-11-6-13-10(5-12(11)18)16(24)21(8-20-13)7-9(22)4-14-15(23)2-1-3-19-14;/h5-6,8,14-15,19,23H,1-4,7H2;1HInChI KeySJUWEPZBTXEUMU-LDXVYITESA-NCanonical SMILESC1CC(C(NC1)CC(=O)CN2C=NC3=CC(=C(C=C3C2=O)Cl)Br)O.Br Patent InformationNo data available
Physical Data
AppearanceWhite or off-white powderMelting Point>217°C dec.Flash Point>70°C dec.SolubilityDMSO : 9 mg/mL (21.70 mM; Need ultrasonic and warming)
Spectra
Halofuginone hydrobromide CAS#: 64924-67-0 HNMR
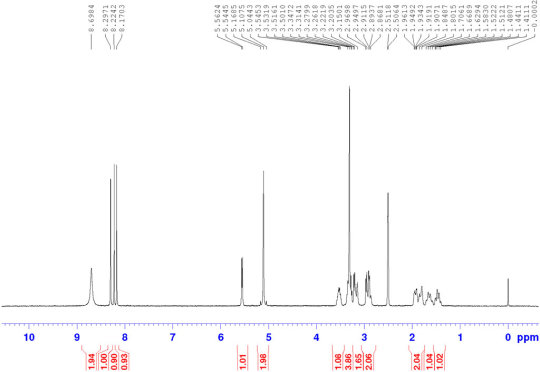
Route of Synthesis (ROS)
No data available
Safety and Hazards
Pictogram(s)

SignalWarningGHS Hazard StatementsH302 (92%): Harmful if swallowed Information may vary between notifications depending on impurities, additives, and other factors.Precautionary Statement CodesP264, P270, P301+P312, P330, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) For more detailed information, please visit ECHA C&L website Source: European Chemicals Agency (ECHA) License Note: Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. License URL: https://echa.europa.eu/web/guest/legal-notice Record Name: 7-bromo-6-chloro-3--2-oxopropyl]quinazolin-4-one;hydrobromide URL: https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/228694 Description: The information provided here is aggregated from the "Notified classification and labelling" from ECHA's C&L Inventory. Read more: https://echa.europa.eu/information-on-chemicals/cl-inventory-database
Other Data
TransportationNot dangerous goodsUnder the room temperature and away from lightHS Code294200StorageKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition. Shelf Life-20°C 3 years 4°C 2 yearsMarket PriceUSD 2300/kg Use PatternHalofuginone hydrobromide CAS#: 64924-67-0 is a specific collagen Type I inhibitor that antagonize or inhibit the development of new blood vessels, hence can prevent intimal hyperplasia at a vascular anastomosis. It is used in the treatment or prevention of coccidiosis in both humans and animals. Read the full article
0 notes
Text
Halofuginone CAS#: 55837-20-2
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product NameHalofuginoneIUPAC Name7-bromo-6-chloro-3--2-oxopropyl]quinazolin-4-oneMolecular Structure
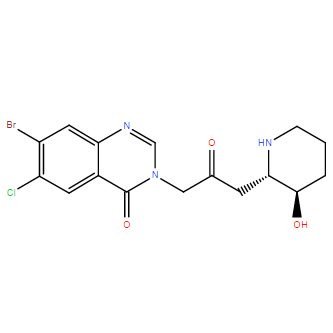
CAS Registry Number 55837-20-2Synonyms7-bromo-6-chlorofebrifugine; halofuginone; halofunginone; Stenorol; 7-bromo-6-chloro-3--2-oxopropyl]quinazolin-4-one; Tempostatin; RU 19110Molecular FormulaC16H17BrClN3O3 Molecular Weight414.686InChIInChI=1S/C16H17BrClN3O3/c17-11-6-13-10(5-12(11)18)16(24)21(8-20-13)7-9(22)4-14-15(23)2-1-3-19-14/h5-6,8,14-15,19,23H,1-4,7H2/t14-,15+/m0/s1InChI KeyLVASCWIMLIKXLA-LSDHHAIUSA-NCanonical SMILESC1CC(C(NC1)CC(=O)CN2C=NC3=CC(=C(C=C3C2=O)Cl)Br)OIsomeric SMILESC1C((NC1)CC(=O)CN2C=NC3=CC(=C(C=C3C2=O)Cl)Br)O Patent InformationPatent IDTitlePublication DateWO2007/120606METHODS FOR MODULATING FORMATION AND PROGRESSION OF CELLULITE2007EP1109559INHIBITION OF PATHOGENIC PROCESSES RELATED TO TISSUE TRAUMA2005US4665100Process for formulating a synthetic drug for use in animal feed, and resulting formulation1987
Physical Data
AppearanceWhite or off-white powderSolubilityDMSO : 9 mg/mL (21.70 mM; Need ultrasonic and warming)Boiling Point595.8 °C at 760 mmHgDensity1.73 g/cm3 Melting Point, °C 144 - 147160
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Frequency (NMR Spectroscopy), MHzChemical shifts1Hdimethylsulfoxide-d6 500Chemical shifts 13Cdimethylsulfoxide-d6125Chemical shifts13Cchloroform-d1125Chemical shifts 1Hchloroform-d1500 Description (IR Spectroscopy)Solvent (IR Spectroscopy)Bandsneat (no solvent, solid phase)
Route of Synthesis (ROS)

Route of Synthesis (ROS) of Halofuginone CAS 55837-20-2 ConditionsYieldWith potassium carbonate In water pH=10 Experimental Procedure The salt (-)-32HBr was basified topH 10 with 0.1Maq. K2CO3 and extracted with DCM (5 5 mL). Thecombined organic layers were washed with brine (25 mL), driedover MgSO4, filtered, and concentrated in vacuo (without heating) to afford (-)-3 (14 mg, 93percent) as a white solid. Mp 143e145 C;{lit.,9 Mp 160 C (decomp.)}; D20 5.4 (c 0.50, EtOH); {lit.,9D2010 (c 0.5, DMF)}; IR (neat): 3128, 2926, 2852, 1722, 1674,1607, 1448, 1382, 1309, 1223, 1106, 1075, 1043, 900 cm1; HRMS(ESI): calcd. for 414.0220, found 414.0212;1H NMR ((CD3)2SO, 500 MHz): d 1.16e1.42 (m, 2H), 1.52e1.61 (m,1H), 1.84e1.95 (m, 1H), 2.32e2.40 (m, 1H), 2.45 (dd, J 15.5, 9.0 Hz,1H), 2.64 (td, J 9.0, 4.0 Hz, 1H), 2.75e2.84 (m, 1H), 2.93e3.03 (m,2H), 4.76 (d, J 6.0 Hz, 1H), 4.94e5.06 (m, 2H), 8.16 (s, 1H), 8.22 (s,1H), 8.24 (s, 1H) ppm (OH not visible by 1H NMR spectroscopy); 13CNMR ((CD3)2SO, 125 MHz): d 26.3 (CH2), 34.7 (CH2), 44.1 (CH2), 46.0(CH2), 55.3 (CH2), 60.5 (CH), 71.2 (CH), 122.2 (C), 127.4 (CH), 128.8(C), 132.2 (C), 132.8 (CH), 147.8 (C), 150.2 (CH), 159.1 (C), 203.9 (C)ppm.93%
Safety and Hazards
Pictogram(s)





SignalDangerGHS Hazard StatementsH300: Fatal if swallowed H301: Toxic if swallowed H310: Fatal in contact with skin H315: Causes skin irritation H317: May cause an allergic skin reaction H318: Causes serious eye damage H330: Fatal if inhaled H361: Suspected of damaging fertility or the unborn child H372: Causes damage to organs through prolonged or repeated exposure H400: Very toxic to aquatic life H410: Very toxic to aquatic life with long lasting effects Information may vary between notifications depending on impurities, additives, and other factors. Precautionary Statement CodesP201, P202, P260, P261, P262, P264, P270, P271, P272, P273, P280, P281, P284, P301+P310, P302+P350, P302+P352, P304+P340, P305+P351+P338, P308+P313, P310, P314, P320, P321, P322, P330, P332+P313, P333+P313, P361, P362, P363, P391, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) For more detailed information, please visit ECHA C&L website
Other Data
TransportationNot dangerous goodsUnder the room temperature and away from lightHS Code294200StorageKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition. Recommended storage temperature: -20°C 3 years 4°C 2 yearsShelf Life1 yearMarket PriceUSD 2300/kg Use PatternHalofuginone CAS#: 55837-20-2 is usually used as a pharmaceuticalangioplastyatherectomyatherosclerosisbypass surgical proceduredrug-eluting stentHalofuginone CAS#: 55837-20-2 is a less-toxic form of Febrifugine, which is isolated from the plant Dichroa febrifuga. Halofuginone inhibits prolyl-tRNA synthetase in an ATP-dependent manner with a Ki of 18.3 nM. Halofuginone attenuates osteoarthritis (OA) by inhibition of TGF-β activity. Read the full article
0 notes
Text
Halofuginone CAS#: 55837-20-2
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product NameHalofuginoneIUPAC Name7-bromo-6-chloro-3--2-oxopropyl]quinazolin-4-oneMolecular Structure
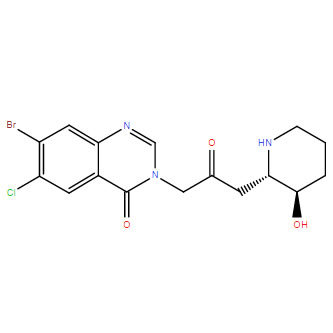
CAS Registry Number 55837-20-2Synonyms7-bromo-6-chlorofebrifugine; halofuginone; halofunginone; Stenorol; 7-bromo-6-chloro-3--2-oxopropyl]quinazolin-4-one; Tempostatin; RU 19110Molecular FormulaC16H17BrClN3O3 Molecular Weight414.686InChIInChI=1S/C16H17BrClN3O3/c17-11-6-13-10(5-12(11)18)16(24)21(8-20-13)7-9(22)4-14-15(23)2-1-3-19-14/h5-6,8,14-15,19,23H,1-4,7H2/t14-,15+/m0/s1InChI KeyLVASCWIMLIKXLA-LSDHHAIUSA-NCanonical SMILESC1CC(C(NC1)CC(=O)CN2C=NC3=CC(=C(C=C3C2=O)Cl)Br)OIsomeric SMILESC1C((NC1)CC(=O)CN2C=NC3=CC(=C(C=C3C2=O)Cl)Br)O Patent InformationPatent IDTitlePublication DateWO2007/120606METHODS FOR MODULATING FORMATION AND PROGRESSION OF CELLULITE2007EP1109559INHIBITION OF PATHOGENIC PROCESSES RELATED TO TISSUE TRAUMA2005US4665100Process for formulating a synthetic drug for use in animal feed, and resulting formulation1987
Physical Data
AppearanceWhite or off-white powderSolubilityDMSO : 9 mg/mL (21.70 mM; Need ultrasonic and warming)Boiling Point595.8 °C at 760 mmHgDensity1.73 g/cm3 Melting Point, °C 144 - 147160
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Frequency (NMR Spectroscopy), MHzChemical shifts1Hdimethylsulfoxide-d6 500Chemical shifts 13Cdimethylsulfoxide-d6125Chemical shifts13Cchloroform-d1125Chemical shifts 1Hchloroform-d1500 Description (IR Spectroscopy)Solvent (IR Spectroscopy)Bandsneat (no solvent, solid phase)
Route of Synthesis (ROS)

Route of Synthesis (ROS) of Halofuginone CAS 55837-20-2 ConditionsYieldWith potassium carbonate In water pH=10 Experimental Procedure The salt (-)-32HBr was basified topH 10 with 0.1Maq. K2CO3 and extracted with DCM (5 5 mL). Thecombined organic layers were washed with brine (25 mL), driedover MgSO4, filtered, and concentrated in vacuo (without heating) to afford (-)-3 (14 mg, 93percent) as a white solid. Mp 143e145 C;{lit.,9 Mp 160 C (decomp.)}; D20 5.4 (c 0.50, EtOH); {lit.,9D2010 (c 0.5, DMF)}; IR (neat): 3128, 2926, 2852, 1722, 1674,1607, 1448, 1382, 1309, 1223, 1106, 1075, 1043, 900 cm1; HRMS(ESI): calcd. for 414.0220, found 414.0212;1H NMR ((CD3)2SO, 500 MHz): d 1.16e1.42 (m, 2H), 1.52e1.61 (m,1H), 1.84e1.95 (m, 1H), 2.32e2.40 (m, 1H), 2.45 (dd, J 15.5, 9.0 Hz,1H), 2.64 (td, J 9.0, 4.0 Hz, 1H), 2.75e2.84 (m, 1H), 2.93e3.03 (m,2H), 4.76 (d, J 6.0 Hz, 1H), 4.94e5.06 (m, 2H), 8.16 (s, 1H), 8.22 (s,1H), 8.24 (s, 1H) ppm (OH not visible by 1H NMR spectroscopy); 13CNMR ((CD3)2SO, 125 MHz): d 26.3 (CH2), 34.7 (CH2), 44.1 (CH2), 46.0(CH2), 55.3 (CH2), 60.5 (CH), 71.2 (CH), 122.2 (C), 127.4 (CH), 128.8(C), 132.2 (C), 132.8 (CH), 147.8 (C), 150.2 (CH), 159.1 (C), 203.9 (C)ppm.93%
Safety and Hazards
Pictogram(s)





SignalDangerGHS Hazard StatementsH300: Fatal if swallowed H301: Toxic if swallowed H310: Fatal in contact with skin H315: Causes skin irritation H317: May cause an allergic skin reaction H318: Causes serious eye damage H330: Fatal if inhaled H361: Suspected of damaging fertility or the unborn child H372: Causes damage to organs through prolonged or repeated exposure H400: Very toxic to aquatic life H410: Very toxic to aquatic life with long lasting effects Information may vary between notifications depending on impurities, additives, and other factors. Precautionary Statement CodesP201, P202, P260, P261, P262, P264, P270, P271, P272, P273, P280, P281, P284, P301+P310, P302+P350, P302+P352, P304+P340, P305+P351+P338, P308+P313, P310, P314, P320, P321, P322, P330, P332+P313, P333+P313, P361, P362, P363, P391, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) For more detailed information, please visit ECHA C&L website
Other Data
TransportationNot dangerous goodsUnder the room temperature and away from lightHS Code294200StorageKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition. Recommended storage temperature: -20°C 3 years 4°C 2 yearsShelf Life1 yearMarket PriceUSD 2300/kg Use PatternHalofuginone CAS#: 55837-20-2 is usually used as a pharmaceuticalangioplastyatherectomyatherosclerosisbypass surgical proceduredrug-eluting stentHalofuginone CAS#: 55837-20-2 is a less-toxic form of Febrifugine, which is isolated from the plant Dichroa febrifuga. Halofuginone inhibits prolyl-tRNA synthetase in an ATP-dependent manner with a Ki of 18.3 nM. Halofuginone attenuates osteoarthritis (OA) by inhibition of TGF-β activity. Read the full article
0 notes
Text
Halofuginone hydrobromide CAS#: 64924-67-0
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product NameHalofuginone hydrobromideIUPAC Name7-bromo-6-chloro-3--2-oxopropyl]quinazolin-4-one;hydrobromide; trans-(±)-7-Bromo-6-chloro-3--4(3H)-quinazolinone monohydrobromideMolecular Structure
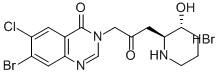
CAS Registry Number 64924-67-0EINECS Number241-422-7MDL NumberMFCD23843776Synonyms7-bromo-6-chlorofebrifugine Hbr; halofuginone; halofunginone; Stenorol; 7-bromo-6-chloro-3--2-oxopropyl]quinazolin-4-one HBr; Tempostatin; RU 19110; Molecular FormulaC16H18Br2ClN3O3 Molecular Weight495.60InChIInChI=1S/C16H17BrClN3O3.BrH/c17-11-6-13-10(5-12(11)18)16(24)21(8-20-13)7-9(22)4-14-15(23)2-1-3-19-14;/h5-6,8,14-15,19,23H,1-4,7H2;1HInChI KeySJUWEPZBTXEUMU-LDXVYITESA-NCanonical SMILESC1CC(C(NC1)CC(=O)CN2C=NC3=CC(=C(C=C3C2=O)Cl)Br)O.Br Patent InformationNo data available
Physical Data
AppearanceWhite or off-white powderMelting Point>217°C dec.Flash Point>70°C dec.SolubilityDMSO : 9 mg/mL (21.70 mM; Need ultrasonic and warming)
Spectra
Halofuginone hydrobromide CAS#: 64924-67-0 HNMR
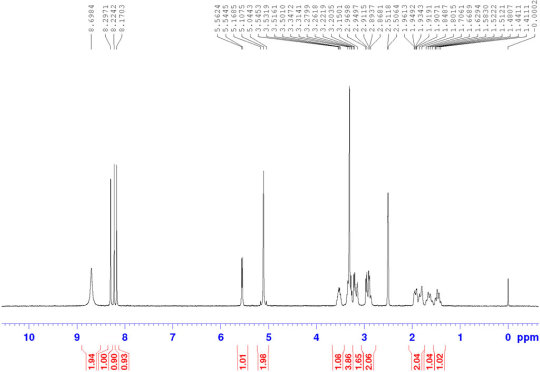
Route of Synthesis (ROS)
No data available
Safety and Hazards
Pictogram(s)

SignalWarningGHS Hazard StatementsH302 (92%): Harmful if swallowed Information may vary between notifications depending on impurities, additives, and other factors.Precautionary Statement CodesP264, P270, P301+P312, P330, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) For more detailed information, please visit ECHA C&L website Source: European Chemicals Agency (ECHA) License Note: Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page. License URL: https://echa.europa.eu/web/guest/legal-notice Record Name: 7-bromo-6-chloro-3--2-oxopropyl]quinazolin-4-one;hydrobromide URL: https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/228694 Description: The information provided here is aggregated from the "Notified classification and labelling" from ECHA's C&L Inventory. Read more: https://echa.europa.eu/information-on-chemicals/cl-inventory-database
Other Data
TransportationNot dangerous goodsUnder the room temperature and away from lightHS Code294200StorageKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition. Shelf Life-20°C 3 years 4°C 2 yearsMarket PriceUSD 2300/kg Use PatternHalofuginone hydrobromide CAS#: 64924-67-0 is a specific collagen Type I inhibitor that antagonize or inhibit the development of new blood vessels, hence can prevent intimal hyperplasia at a vascular anastomosis. It is used in the treatment or prevention of coccidiosis in both humans and animals. Read the full article
0 notes
Text
Halofuginone CAS#: 55837-20-2
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product NameHalofuginoneIUPAC Name7-bromo-6-chloro-3--2-oxopropyl]quinazolin-4-oneMolecular Structure

CAS Registry Number 55837-20-2Synonyms7-bromo-6-chlorofebrifugine; halofuginone; halofunginone; Stenorol; 7-bromo-6-chloro-3--2-oxopropyl]quinazolin-4-one; Tempostatin; RU 19110Molecular FormulaC16H17BrClN3O3 Molecular Weight414.686InChIInChI=1S/C16H17BrClN3O3/c17-11-6-13-10(5-12(11)18)16(24)21(8-20-13)7-9(22)4-14-15(23)2-1-3-19-14/h5-6,8,14-15,19,23H,1-4,7H2/t14-,15+/m0/s1InChI KeyLVASCWIMLIKXLA-LSDHHAIUSA-NCanonical SMILESC1CC(C(NC1)CC(=O)CN2C=NC3=CC(=C(C=C3C2=O)Cl)Br)OIsomeric SMILESC1C((NC1)CC(=O)CN2C=NC3=CC(=C(C=C3C2=O)Cl)Br)O Patent InformationPatent IDTitlePublication DateWO2007/120606METHODS FOR MODULATING FORMATION AND PROGRESSION OF CELLULITE2007EP1109559INHIBITION OF PATHOGENIC PROCESSES RELATED TO TISSUE TRAUMA2005US4665100Process for formulating a synthetic drug for use in animal feed, and resulting formulation1987
Physical Data
AppearanceWhite or off-white powderSolubilityDMSO : 9 mg/mL (21.70 mM; Need ultrasonic and warming)Boiling Point595.8 °C at 760 mmHgDensity1.73 g/cm3 Melting Point, °C 144 - 147160
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Frequency (NMR Spectroscopy), MHzChemical shifts1Hdimethylsulfoxide-d6 500Chemical shifts 13Cdimethylsulfoxide-d6125Chemical shifts13Cchloroform-d1125Chemical shifts 1Hchloroform-d1500 Description (IR Spectroscopy)Solvent (IR Spectroscopy)Bandsneat (no solvent, solid phase)
Route of Synthesis (ROS)

Route of Synthesis (ROS) of Halofuginone CAS 55837-20-2 ConditionsYieldWith potassium carbonate In water pH=10 Experimental Procedure The salt (-)-32HBr was basified topH 10 with 0.1Maq. K2CO3 and extracted with DCM (5 5 mL). Thecombined organic layers were washed with brine (25 mL), driedover MgSO4, filtered, and concentrated in vacuo (without heating) to afford (-)-3 (14 mg, 93percent) as a white solid. Mp 143e145 C;{lit.,9 Mp 160 C (decomp.)}; D20 5.4 (c 0.50, EtOH); {lit.,9D2010 (c 0.5, DMF)}; IR (neat): 3128, 2926, 2852, 1722, 1674,1607, 1448, 1382, 1309, 1223, 1106, 1075, 1043, 900 cm1; HRMS(ESI): calcd. for 414.0220, found 414.0212;1H NMR ((CD3)2SO, 500 MHz): d 1.16e1.42 (m, 2H), 1.52e1.61 (m,1H), 1.84e1.95 (m, 1H), 2.32e2.40 (m, 1H), 2.45 (dd, J 15.5, 9.0 Hz,1H), 2.64 (td, J 9.0, 4.0 Hz, 1H), 2.75e2.84 (m, 1H), 2.93e3.03 (m,2H), 4.76 (d, J 6.0 Hz, 1H), 4.94e5.06 (m, 2H), 8.16 (s, 1H), 8.22 (s,1H), 8.24 (s, 1H) ppm (OH not visible by 1H NMR spectroscopy); 13CNMR ((CD3)2SO, 125 MHz): d 26.3 (CH2), 34.7 (CH2), 44.1 (CH2), 46.0(CH2), 55.3 (CH2), 60.5 (CH), 71.2 (CH), 122.2 (C), 127.4 (CH), 128.8(C), 132.2 (C), 132.8 (CH), 147.8 (C), 150.2 (CH), 159.1 (C), 203.9 (C)ppm.93%
Safety and Hazards
Pictogram(s)





SignalDangerGHS Hazard StatementsH300: Fatal if swallowed H301: Toxic if swallowed H310: Fatal in contact with skin H315: Causes skin irritation H317: May cause an allergic skin reaction H318: Causes serious eye damage H330: Fatal if inhaled H361: Suspected of damaging fertility or the unborn child H372: Causes damage to organs through prolonged or repeated exposure H400: Very toxic to aquatic life H410: Very toxic to aquatic life with long lasting effects Information may vary between notifications depending on impurities, additives, and other factors. Precautionary Statement CodesP201, P202, P260, P261, P262, P264, P270, P271, P272, P273, P280, P281, P284, P301+P310, P302+P350, P302+P352, P304+P340, P305+P351+P338, P308+P313, P310, P314, P320, P321, P322, P330, P332+P313, P333+P313, P361, P362, P363, P391, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) For more detailed information, please visit ECHA C&L website
Other Data
TransportationNot dangerous goodsUnder the room temperature and away from lightHS Code294200StorageKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition. Recommended storage temperature: -20°C 3 years 4°C 2 yearsShelf Life1 yearMarket PriceUSD 2300/kg Use PatternHalofuginone CAS#: 55837-20-2 is usually used as a pharmaceuticalangioplastyatherectomyatherosclerosisbypass surgical proceduredrug-eluting stentHalofuginone CAS#: 55837-20-2 is a less-toxic form of Febrifugine, which is isolated from the plant Dichroa febrifuga. Halofuginone inhibits prolyl-tRNA synthetase in an ATP-dependent manner with a Ki of 18.3 nM. Halofuginone attenuates osteoarthritis (OA) by inhibition of TGF-β activity. Read the full article
0 notes
Text
Halofuginone CAS#: 55837-20-2
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product NameHalofuginoneIUPAC Name7-bromo-6-chloro-3--2-oxopropyl]quinazolin-4-oneMolecular Structure

CAS Registry Number 55837-20-2Synonyms7-bromo-6-chlorofebrifugine; halofuginone; halofunginone; Stenorol; 7-bromo-6-chloro-3--2-oxopropyl]quinazolin-4-one; Tempostatin; RU 19110Molecular FormulaC16H17BrClN3O3 Molecular Weight414.686InChIInChI=1S/C16H17BrClN3O3/c17-11-6-13-10(5-12(11)18)16(24)21(8-20-13)7-9(22)4-14-15(23)2-1-3-19-14/h5-6,8,14-15,19,23H,1-4,7H2/t14-,15+/m0/s1InChI KeyLVASCWIMLIKXLA-LSDHHAIUSA-NCanonical SMILESC1CC(C(NC1)CC(=O)CN2C=NC3=CC(=C(C=C3C2=O)Cl)Br)OIsomeric SMILESC1C((NC1)CC(=O)CN2C=NC3=CC(=C(C=C3C2=O)Cl)Br)O Patent InformationPatent IDTitlePublication DateWO2007/120606METHODS FOR MODULATING FORMATION AND PROGRESSION OF CELLULITE2007EP1109559INHIBITION OF PATHOGENIC PROCESSES RELATED TO TISSUE TRAUMA2005US4665100Process for formulating a synthetic drug for use in animal feed, and resulting formulation1987
Physical Data
AppearanceWhite or off-white powderSolubilityDMSO : 9 mg/mL (21.70 mM; Need ultrasonic and warming)Boiling Point595.8 °C at 760 mmHgDensity1.73 g/cm3 Melting Point, °C 144 - 147160
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Frequency (NMR Spectroscopy), MHzChemical shifts1Hdimethylsulfoxide-d6 500Chemical shifts 13Cdimethylsulfoxide-d6125Chemical shifts13Cchloroform-d1125Chemical shifts 1Hchloroform-d1500 Description (IR Spectroscopy)Solvent (IR Spectroscopy)Bandsneat (no solvent, solid phase)
Route of Synthesis (ROS)

Route of Synthesis (ROS) of Halofuginone CAS 55837-20-2 ConditionsYieldWith potassium carbonate In water pH=10 Experimental Procedure The salt (-)-32HBr was basified topH 10 with 0.1Maq. K2CO3 and extracted with DCM (5 5 mL). Thecombined organic layers were washed with brine (25 mL), driedover MgSO4, filtered, and concentrated in vacuo (without heating) to afford (-)-3 (14 mg, 93percent) as a white solid. Mp 143e145 C;{lit.,9 Mp 160 C (decomp.)}; D20 5.4 (c 0.50, EtOH); {lit.,9D2010 (c 0.5, DMF)}; IR (neat): 3128, 2926, 2852, 1722, 1674,1607, 1448, 1382, 1309, 1223, 1106, 1075, 1043, 900 cm1; HRMS(ESI): calcd. for 414.0220, found 414.0212;1H NMR ((CD3)2SO, 500 MHz): d 1.16e1.42 (m, 2H), 1.52e1.61 (m,1H), 1.84e1.95 (m, 1H), 2.32e2.40 (m, 1H), 2.45 (dd, J 15.5, 9.0 Hz,1H), 2.64 (td, J 9.0, 4.0 Hz, 1H), 2.75e2.84 (m, 1H), 2.93e3.03 (m,2H), 4.76 (d, J 6.0 Hz, 1H), 4.94e5.06 (m, 2H), 8.16 (s, 1H), 8.22 (s,1H), 8.24 (s, 1H) ppm (OH not visible by 1H NMR spectroscopy); 13CNMR ((CD3)2SO, 125 MHz): d 26.3 (CH2), 34.7 (CH2), 44.1 (CH2), 46.0(CH2), 55.3 (CH2), 60.5 (CH), 71.2 (CH), 122.2 (C), 127.4 (CH), 128.8(C), 132.2 (C), 132.8 (CH), 147.8 (C), 150.2 (CH), 159.1 (C), 203.9 (C)ppm.93% More: Inquiry all available synthetic routes with detailed experimental procedures
Safety and Hazards
Pictogram(s)





SignalDangerGHS Hazard StatementsH300: Fatal if swallowed H301: Toxic if swallowed H310: Fatal in contact with skin H315: Causes skin irritation H317: May cause an allergic skin reaction H318: Causes serious eye damage H330: Fatal if inhaled H361: Suspected of damaging fertility or the unborn child H372: Causes damage to organs through prolonged or repeated exposure H400: Very toxic to aquatic life H410: Very toxic to aquatic life with long lasting effects Information may vary between notifications depending on impurities, additives, and other factors. Precautionary Statement CodesP201, P202, P260, P261, P262, P264, P270, P271, P272, P273, P280, P281, P284, P301+P310, P302+P350, P302+P352, P304+P340, P305+P351+P338, P308+P313, P310, P314, P320, P321, P322, P330, P332+P313, P333+P313, P361, P362, P363, P391, P403+P233, P405, and P501 (The corresponding statement to each P-code can be found at the GHS Classification page.) For more detailed information, please visit ECHA C&L website
Other Data
TransportationNot dangerous goodsUnder the room temperature and away from lightHS Code294200StorageKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition. Recommended storage temperature: -20°C 3 years 4°C 2 yearsShelf Life1 yearMarket PriceUSD 2300/kg Use PatternHalofuginone CAS#: 55837-20-2 is usually used as a pharmaceuticalangioplastyatherectomyatherosclerosisbypass surgical proceduredrug-eluting stentHalofuginone CAS#: 55837-20-2 is a less-toxic form of Febrifugine, which is isolated from the plant Dichroa febrifuga. Halofuginone inhibits prolyl-tRNA synthetase in an ATP-dependent manner with a Ki of 18.3 nM. Halofuginone attenuates osteoarthritis (OA) by inhibition of TGF-β activity. Read the full article
0 notes