#Mifeprex
Text
Concerned about an Unplanned Pregnancy? Learn, empower yourself, and learn about online pills medication options
Learn useful facts to help you with your worries about an unintended pregnancy. Make wise judgments by arming yourself with knowledge about your options for buy pills online. Investigate your options and advice to confidently handle this difficult scenario. Take charge of your reproductive health by becoming informed about your options, getting assistance, and doing so. We give you the information you need to decide what is best for your particular set of circumstances. Your journey starts right here.
#UNPLANNED PREGNANCY#buy pills online#Mifepristone and Misoprostol Kit#MIFEPREX ABORTION PILL ONLINE
0 notes
Text

From AAPLOG on Facebook:
Alliance for Hippocratic Medicine v. U.S. Food and Drug Administration
"Description: By illegally approving chemical abortion drugs, the U.S. Food and Drug Administration failed to abide by its legal obligations to protect the health, safety, and welfare of girls and women. The FDA never studied the safety of the drugs under the labeled conditions of use, ignored the potential impacts of the hormone-blocking regimen on the developing bodies of adolescent girls, disregarded the substantial evidence that chemical abortion drugs cause more complications than surgical abortions, and eliminated necessary safeguards for pregnant girls and women who undergo this dangerous drug regimen."
On November 18, AAPLOG, alongside other plaintiffs, filed a lawsuit against the U.S. Food & Drug Administration (FDA) for illegally approving mifepristone (also known as “Mifeprex” and “RU-486”) and misoprostol for chemical abortion, as these dangerous drugs harm women and girls. The plaintiffs are represented by Alliance Defending Freedom, the law firm that worked with Mississippi in the US Supreme Court Case Dobbs v Jackson Women’s Health Center, the ruling for which overturned Roe v Wade. As the lawsuit explains, the FDA illegally approved mifepristone and has repeatedly removed the few safeguards governing it's use over the past two decades. The agency did not study the danger mifepristone posed to minors despite approving mifepristone for use by young girls. The FDA also has never required an ultrasound prior to a chemical abortion. An ultrasound is the best way to confirm the baby’s age and to rule out an ectopic pregnancy, which occurs in 1 in every 50 pregnancies. Without an ultrasound, the risks from a chemical abortion are significantly increased.
The FDA has not worked to correct, or even mitigate, the dangers intrinsic to chemical abortions. Instead, in 2016, the FDA dangerously expanded the approval of chemical abortion drugs from 7 weeks of pregnancy up to 10 weeks of pregnancy, changed the dosing regimen, reduced the number of in-person doctor visits from three to one, expanded who could prescribe and administer chemical abortion drugs beyond medical doctors, and eliminated the requirement for prescribers to report non-lethal complications from chemical abortion drugs. And in 2021, based on incomplete and unreliable data, the FDA removed the requirement that an abortionist physically meet with the woman and give her the chemical abortion drugs, thus allowing for chemical abortions by mail and telemedicine. This will only increase the danger to women, not to mention the children whose lives will be ended. As many as one out of five women who undergo a chemical abortion will suffer a complication. Women can face severe bleeding, life-threatening infections, and the inability to have future successful pregnancies—requiring emergency medical treatment, surgeries, blood transfusions, and hysterectomies. In addition, chemical abortion has a complication rate four times higher than surgical abortions.
AAPLOG has long fought against efforts by the FDA and pro-abortion advocates to prioritize abortion access over the health and safety of women and their preborn children. We are proud to be a part of this effort to hold the FDA accountable to its obligation to protect the health, safety, and welfare of women and girls. You can see the full press release about this lawsuit here. https://adfmedia.org/case/alliance-hippocratic-medicine-v-us-food-and-drug-administration .
60 notes
·
View notes
Text
Brenda Vise, 38 (USA 2001)

Brenda as Senior Class Secretary and Homecoming Queen in high school
It was 2001. A 38-year-old pharmaceutical representative named Brenda Colleen Vise took a pregnancy test on November 5 and it showed that she was pregnant. What the pregnancy test couldn’t tell her was that her pregnancy was ectopic.
Brenda found an advertisement for Volunteer Medical Clinic in the yellow pages. She had no idea that the deceptively named facility was actually an abortion facility that was not even licensed as a clinic. In fact, VMC had been administratively dissolved by the Tennessee Secretary of State for failure to comply with applicable law since September 17, 1999.
Staff members at VMC did another pregnancy test and then an ultrasound. Although Brenda was estimated to be 6 weeks pregnant, the ultrasound did not show a baby in the uterus. This is an obvious indicator of an ectopic pregnancy, which is a medical emergency and is a contraindication to the administration of the abortion pill.
Despite the glaring indicators of a serious condition, the VMC staff told Brenda that her ultrasound was blank because the baby was “too small to be seen.” At 6 weeks pregnant, a fetus in the uterus would have been easy to see with a competent ultrasound examination.
Brenda was given the RU-486 abortion pill and sent home with a dose of Cytotec to take by herself later. The FDA has never approved Cytotec for use in pregnant women and in fact has specifically warned against using it for an abortion. In August of the year before Brenda was killed, the manufacturer of Cytotec specifically issued a letter to healthcare providers that Cytotec was contraindicated in women who are pregnant and that Cytotec was not approved for the induction of labor or abortion, and in fact should not be used in an abortion.
After she got home, Brenda called VMC about her alarming symptoms. In Brenda’s repeated calls to VMC, she was told that her symptoms were “normal and routine.”
Brenda took the Cytotec as instructed roughly 48 hours after her initial dose of Mifeprex and continued to suffer from pain and nausea. She called VMC and told them she had a sub-normal body temperature, that she was pale and that she had significant pelvic pain. Instead of telling her to go to the hospital, VMC said that all of these were normal symptoms.
Brenda called again on September 10 in an even worse condition. She was told that her symptoms were “to be expected,” and was told to travel to VMC in Knoxville for a check-up instead of immediately getting care locally. She was specifically told not to go to a local hospital because according to VMC, “no hospital in Chattanooga would have knowledge about the drugs that had been administered.”
Brenda’s boyfriend called an ambulance, which rushed Brenda to a Chattanooga hospital. Sure enough, her ectopic pregnancy ruptured because of VMC’s incompetence. The rupture led to a massive infection and collapse of her vital systems. On September 12, 2001, Brenda was completely unresponsive and in a coma. A doctor declared her condition “terminal with no reasonable medical prospect of recovery.” She died later that day.
Even if Brenda’s baby had no chance of surviving, there was no reason that Brenda had to die too. VMC killed her with their extreme incompetence and refusal to follow medical guidelines. The state also should have better enforced the shutdown of the abortion facility that was still pretending to be a medical clinic.

48 notes
·
View notes
Text

The present is pregnant with the future?
I just saw a box of Mifeprex in the present’s mailbox.
2 notes
·
View notes
Link
Griswold v Connecticut (1965) married women and contraception
https://constitutioncenter.org/the-constitution/supreme-court-case-library/griswold-v-connecticut
United States Postal Service (1970)
https://www.govinfo.gov/content/pkg/STATUTE-84/pdf/STATUTE-84-Pg719.pdf
Eisenstadt v. Baird (1972) single women and contraception
https://tile.loc.gov/storage-services/service/ll/usrep/usrep405/usrep405438/usrep405438.pdf
Roe v Wade oral argument (1971)
https://www.supremecourt.gov/pdfs/transcripts/1971/70-18_12-13-1971.pdf
Roe v Wade oral argument (1972)
https://www.supremecourt.gov/pdfs/transcripts/1972/70-18_10-11-1972.pdf
Sarah Weddington (2021) Roe v Wade lawyer
https://www.npr.org/2021/12/26/1068168254/sarah-weddington-the-lawyer-who-at-26-successfully-argued-roe-v-wade-has-died
Supreme Court Justice Alito (2022)
https://www.politico.com/news/2022/05/02/read-justice-alito-initial-abortion-opinion-overturn-roe-v-wade-pdf-00029504
Supreme Court overturns Roe v Wade (2022)
https://d3i6fh83elv35t.cloudfront.net/static/2022/06/19-1392_6j37-2.pdf
#8 march#international#women#day#contraception#sarah weddington#griswold#fda#connecticut#massachusetts#usa#texas#baird#mifepristone#bluechew
2 notes
·
View notes
Text

What are the side effects of emergency contraceptive pill
An emergency contraceptive pill, or the morning-after pill, is a method of contraception used during an emergency. It prevents unintended pregnancy, and the side effects of emergency contraceptive pills are not usually severe. Women who have had unprotected sex or whose birth control method has failed can use emergency contraception to avoid getting pregnant. The morning-after pill should be used as a backup method of birth control and not as the primary method. These pills work by delaying or stopping ovulation, which will prevent fertilisation and hence there will be no pregnancy.
Levonorgestrel is an ‘over-the-counter’ pill which means that it is available without a prescription in India. Further, it’s very important for everyone to know that the emergency contraceptive pill cannot stop a pregnancy that has already happened.
Remember that mifepristone (Mifeprex), generally known as RU-486 or the abortion pill, is not the same as the morning-after pill. The abortion pill terminates a confirmed pregnancy, where the fertilised egg has attached itself to the lining of the uterus and has begun to develop.1
When to take the emergency contraceptive pill?
Emergency contraceptive pills are not the same as regular birth control pills or other forms of routine birth control. You should take it only when all other forms of birth control have failed or were ineffective. Use of emergency contraceptive pills may be appropriate in the following circumstances:
If you had sex without using any form of birth control.
Failure of the birth control method (a broken condom, forgotten birth control pill or missed dose of birth control shot).
If you have experienced non-consensual sex.2
The emergency contraceptive pill can cause a one-week delay in your cycle. Take a pregnancy test if your period doesn’t arrive three to four weeks after taking the emergency contraceptive pill.
How does the emergency contraceptive pill work?
The emergency contraceptive pill primarily functions by delaying ovulation. Ovulation is a natural aspect of the reproductive cycle. The release of an egg from your ovaries during this stage allows sperm to fertilise it and start the process of embryonic development. If you do not ovulate, there will be no egg to be fertilised by the sperm. Hence, you will not get pregnant.3
What are the side effects of taking the emergency contraceptive pill?
If you are planning to take the emergency contraceptive pill, you may feel glad to know that most of the side effects of these pills are temporary and go away in a couple of days. The side effects of these pills are similar to those of oral contraceptive pills. Call your doctor if the side effects of emergency contraceptive pills last for a prolonged period.4
The following are common minor side effects of both types of emergency contraceptive pills:
Nausea
Diarrhea
Fatigue
Headache
Stomach cramps
Sore or tender breasts
Decreased sexual drive 5
Later, you may also have other symptoms like:
Spotting — You may have some spotting within the next week. Also, your next period can be lighter or heavier than usual. It’s common, and you don’t have to worry about it. Call your doctor if you’re concerned or if the bleeding seems very heavy.
Irregular menstrual cycle — Due to the emergency contraceptive pill, your period may arrive a little early or late, which is normal. However, you should take a pregnancy test if you do not get your period within 3–4 weeks of taking the pill.
How effective is the emergency contraceptive pill?
If taken within 72 hours of having unprotected sex, emergency contraceptive pills can reduce the risk of pregnancy by up to 87% when taken as directed.7 The emergency contraceptive pills work for up to 3 days or 72 hours after having unprotected sex. But, the sooner you take the emergency contraceptive pill, the more likely it is to prevent pregnancy. Despite the name “morning-after pill,” you don’t need to wait until the next day to take these pills.
Are they any risks associated with emergency contraceptive pills?
While the emergency contraceptive pill is a viable option for avoiding pregnancy during unprotected sex, it is not advised for regular use because it is not as effective as other forms of contraception. Also, the emergency contraceptive pill does not protect against sexually transmitted infections (STIs).
Not everyone is advised to use emergency contraceptive pills. Avoid taking the emergency contraceptive pill if:
You have an allergy to one or more emergency contraceptive pill ingredients.
You’re taking a medicine that can make the emergency contraceptive pill less effective.
There are some indications that the emergency contraceptive pill won’t work as well to prevent pregnancy in obese or overweight people as it does for women who aren’t obese.
Additionally, before using ulipristal, make sure you are not pregnant. The effects of ulipristal on a developing baby are unknown. Ulipristal is not advisable if you are breastfeeding a baby.
What are the signs that the emergency contraceptive pill hasn’t worked?
If you take the emergency contraceptive pill right after having unprotected sex, it can be very effective. The longer you wait to use the emergency contraceptive pill, the less effective it is. A missed period is one of the primary indicators that an emergency contraceptive pill hasn’t worked. Take a pregnancy test if your period arrives more than three to four weeks later than expected.
The emergency contraceptive pill won’t interfere with your fertility or make it more difficult for you to conceive in the future.8
Consult a doctor:
In most cases, you don’t need to contact your doctor after taking the emergency contraceptive pill. However, get in contact if you experience significant lower abdomen pain three to five weeks after using the emergency contraceptive pill, bleeding, or spotting that lasts longer than a week. These could signify a miscarriage or the fertilised egg implanting outside of the uterus, typically in a fallopian tube (ectopic pregnancy).
It’s also crucial to keep in mind that taking the emergency contraceptive pill does not protect you from sexually transmitted infections (STIs). Contact your doctor if you have any doubts about the possibility of being exposed to an STI.
0 notes
Text
Supreme Court rejects challenge to FDAs approval of mifepristone : NPR
The U.S. Supreme Court on Thursday tossed out a challenge to the FDA’s rules for prescribing and dispensing abortion pills.
Erin Hooley/Chicago Tribune/Getty Images
hide caption
toggle caption
Erin Hooley/Chicago Tribune/Getty Images
The U.S. Supreme Court on Thursday tossed out a challenge to the FDA’s rules for prescribing and dispensing abortion pills.
Erin Hooley/Chicago Tribune/Getty Images
The U.S. Supreme Court on Thursday tossed out a challenge to the FDA’s rules for prescribing and dispensing abortion pills. By a unanimous vote, the court said the anti-abortion doctors who brought the challenge had failed to show they had been harmed, as they do not prescribe the medication, and thus, essentially, had no skin in the game.
The court said that the challengers, a group called the Alliance for Hippocratic Medicine, had no right to be in court at all since neither the organization nor its members could show they had suffered any concrete injury.
The court’s action amounted to a legal off-ramp, leaving the FDA rules in place, without directly addressing the regulations themselves.
The court’s decision also avoided, at least for now, a challenge to the entire structure of the FDA’s regulatory power to approve drugs and continually evaluate their safety—a system that for decades has been widely viewed as the gold standard for both safety and innovation.
Since the court reversed Roe v. Wade and the right to abortion in 2022, pills have become the most popular abortion method in the U.S. More than half the women who choose to terminate a pregnancy use a combination of pills approved by the FDA, including mifepristone, manufactured by Danco Laboratories and marketed as Mifeprex.
The pill regimen was first approved 24 years ago, and over the past seven years, the agency has approved changes in the dosing regimen and eliminated some restrictions that it found to be unnecessary. For instance, the pills can now be prescribed during the first 10 weeks of pregnancy, instead of the original seven weeks, and prescriptions can be filled by mail or at pharmacies, instead of at a doctor’s office. The result, according to Danco Labs, is that there have been fewer complications than when the drug was initially approved for just seven weeks in 2000.
Thursday’s Supreme Court decision reversed a ruling by the Fifth Circuit Court of Appeals, widely viewed as the most conservative federal appeals court in the country.
Siding with the FDA in the case were virtually all the major medical associations in the country, as well as almost all the pharmaceutical and bio-tech companies, big and small, that are regulated by the agency, making this the rare case in which a government regulator and the industry it regulates were on the same side. Dr. Jeremy Levin, the CEO of Ovid Therapeutics, one of the many pharmaceutical companies that sided with the FDA, earlier this year called the case “a dagger at the heart of the entire industry.”
For now, though, the prospect of dismantling the regulatory powers of the FDA has been averted. But the direct challenge to abortion pills and their accessibility has not been resolved, and could be revived in a different case.
Source link
via
The Novum Times
0 notes
Text
Beyond the 'abortion pill': Real-life experiences of individuals taking mifepristone (NPR)
For a while, it was known as RU-486. It's called Mifeprex or mifepristone – but many know it as "the abortion pill." It is one of two drugs – along with misoprostol – that areused in at least 63% of abortions in the U.S. now. And it is the subject of a Supreme Court case that could make it illegal or much harder to access.
As attorneys gather in Washington Tuesday to argue overwhether this medication should be removed from the market all over the country, NPR is reporting again some of what readers and listeners told us last year about theirexperiences using mifepristone.
The stories illustrate how mifepristone is indeed an "abortion pill" — but it also plays other important roles in people's lives.
Read more
0 notes
Text
“Tina Roe” (USA 2017)
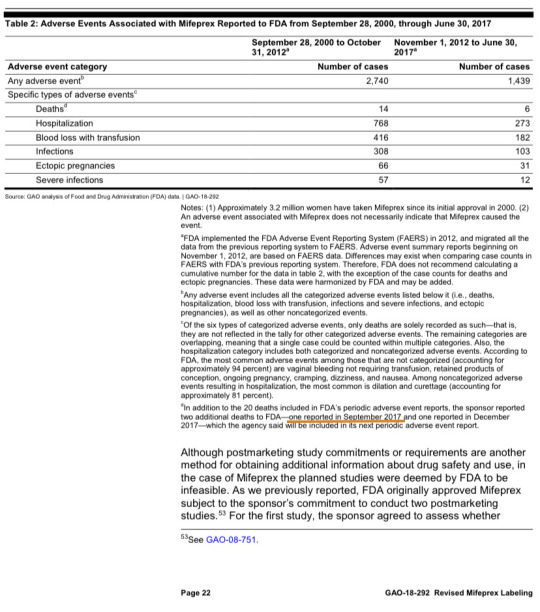
Recently a decision was made overturning the approval of RU-486 in the United States. While this will undoubtedly save many lives, it came too late for many victims of the abortion pill.
“Tina Roe” was one of many deaths from the legal use of the dangerous RU-486 pill, which is so dangerous it was only approved in the first place with a REMS requirement, a rare restriction reserved for extremely dangerous drugs. Her death was mentioned in a report by the US Government Accountability Office. In the span of a few months, a sponsor detected and reported multiple additional deaths to the FDA that the agency missed entirely— Tina’s death in September 2017 and another one reported in December 2017 (Toni Roe).
The report was made to evaluate labeling changes to the kill pill that would loosen safety standards. The report mentioned concerns that:
• “FDA may only be aware of a fraction of adverse events associated with Mifeprex. There are anecdotal examples of adverse events, such as severe bleeding, that may not be reported as such or that may be interpreted by emergency health care providers as a natural miscarriage. Underreporting may get worse under the revised Mifeprex label, which eliminates the follow-up visit and does not require prescribers to report nonfatal adverse events.”
• “Concerns have also been raised about FDA's oversight of the drug since approval, including the agency's response to deaths in U.S. women who had taken the drug.”
• “FDA may not have reliable data on the number of women who have used Mifeprex, which would affect the denominator for tracking adverse events. With an unclear denominator, FDA may not have an accurate measure of adverse event rates associated with Mifeprex.”
• “Even with additional dispensings beginning in 2012, FDA officials said there were still insufficient data captured to enable a robust safety assessment.”
It is worth mentioning that due to a voluntary reporting system for abortion deaths, we have no idea how many more of these cases there are. Even after reports of Tina and Toni’s deaths and thousands of dangerous events, safety requirements for the lethal pills were still loosened. The decision to remove some of the safety standards was catastrophic and was eventually overturned, but many died in the meantime.
(Please note that the date given is only the date that the FDA found out about her death. She may have died sooner, possibly even years before, but the faulty reporting system sometimes registers deaths far after they actually happen.)
#unsafe yet legal#pro life#death from legal abortion#tw abortion#unidentified victim#victims of roe#abortion#abortion pills kill#abortion bans save lives#tw ab*rtion#tw murder#abortion pill#the abortion pill kills
10 notes
·
View notes
Text
Buy Mifepristone Online
Mifeprex (mifepristone) is used, together with another medication called misoprostol, to end an early pregnancy. FDA first approved Mifeprex in 2000. In 2016, the agency approved a supplemental application for Mifeprex based on data and information submitted by the drug manufacturer. After reviewing the supplemental application, the agency determined that Mifeprex is safe and effective when used to terminate a pregnancy in accordance with the revised labeling. In 2019, FDA approved a generic version of Mifeprex, Mifepristone Tablets, 200 mg.
Usual Adult Dose for Abortion
Brand MIFEPREX:
-Day One: 200 mg mifepristone (MIFEPREX) orally as a single dose
-Day Two or Three: 800 mcg misoprostol buccally 24 to 48 hours after the first dose of mifepristone (Two 200 mcg misoprostol tablets should be placed in each cheek pouch [the area between the cheek and gums] for 30 minutes and then swallow any remnants with water or another liquid).
Brand Name : Mtp Kit
Packaging Size : Box
Packaging Type : Strips
Composition : Mifepristone & Misoprostol
Usage/Application : Mtp Kit helps terminate a pregnancy
Form : solid
Tracking Number Available
Delivery takes 24 hours
Maximum 48 ours within USA & Canada
International delivery takes 3 to 4 working days world wide
0 notes
Text
How to Buy Mifeprex Pills Online for Abortion

Online retailers of mifeprex tablets provide a secure and reliable method of having a medical abortion. Learn where to buy mifeprex abortion pill online and choose a pregnancy termination method that is right for you. Learn about the procedure, safety precautions, and factors to take into account for a private and confidential abortion experience.
0 notes
Text
Did you know there is a safe, private option for an at-home abortion?

It's called a medication abortion, or the "abortion pill." Whatsapp +60113597039
Women on Web helps to create access to safe medical abortion services.
A medical abortion requires two medicines (mifepristone and misoprostol) that will be delivered to you. A medical abortion has a success rate of more than 97% and can be done safely at home as long you have good information and access to emergency medical care should you experience any complications.
A medical abortion causes the non-surgical termination of an early pregnancy up until the 9th week. The safest, most effective type of medical abortion requires a combination of Mifepristone, (also known as, RU486, RU, Mifeprex, the abortion pill or mifegyne) and Misoprostol (also known as Cytotec, Arthrotec, Oxaprost, Cyprostol, Cyprostoll or Misotrol) to provoke the spontaneous expulsion of the pregnancy from the uterus.
Please note that our online abortion service can assist you if you have an unwanted pregnancy, you are less than 10 weeks pregnant and struggle to access safe abortion.
#CYTOPilGugu#CYTOTEC#200MCG#Telemedicine#pilgugur#ubatgugur#pilgugurcytotec#cytolog#pilgugur2023#ubatgugurkandungan2023#ubatgugurkandunganselamat#ubatgugurberkesan#pilgugurkl#abortionpillselangor#abortionpillmalaysia#mefiges#ubatgugurborong#mefikit#Mifepriston#abortionkit
0 notes
Text
The Abortion Pill’s Secret Money Men – Mother Jones
#private equity#abortion is healthcare#abortion is a human right#abortion rights#maybe this is why we shouldn't rely on private industry for public health hmmmmmm?#this is why we can't have nice things
0 notes
Text
“Toni Roe” (USA 2017)
“Toni Roe” died from the legal use of the RU-486 abortion pill, also known as mifepristone. Her death was reported by the US Government Accountability Office when they wrote a report to assess the newly weakened regulations on RU-486.
Toni’s death was reported sometime in December 2017. The FDA had no idea about her case until GAO found out and included notes in their report. She was one of multiple cases discovered while the report was being compiled that had not been known by the FDA.

The report was made to evaluate labeling changes to the kill pill that would eliminate safety standards. The report mentioned concerns that:
• “FDA may only be aware of a fraction of adverse events associated with Mifeprex. There are anecdotal examples of adverse events, such as severe bleeding, that may not be reported as such or that may be interpreted by emergency health care providers as a natural miscarriage. Underreporting may get worse under the revised Mifeprex label, which eliminates the follow-up visit and does not require prescribers to report nonfatal adverse events.”
• “Concerns have also been raised about FDA's oversight of the drug since approval, including the agency's response to deaths in U.S. women who had taken the drug.”
• “FDA may not have reliable data on the number of women who have used Mifeprex, which would affect the denominator for tracking adverse events. With an unclear denominator, FDA may not have an accurate measure of adverse event rates associated with Mifeprex.”
• “Even with additional dispensings beginning in 2012, FDA officials said there were still insufficient data captured to enable a robust safety assessment.”
(see Page 22)
(Please note that the date given is only the date that the FDA found out about her death. She may have died sooner, possibly even years before, but the faulty reporting system sometimes registers deaths far after they actually happen.)
#tw abortion#abortion#pro life#pro choice#tw murder#abortion debate#tw ab*rtion#tw death#death from legal abortion#unsafe yet legal#abortion pill#chemical abortion#the abortion pill kills
4 notes
·
View notes