#Pharmacovigilance Course
Text
The Pharmacovigilance Course equips you with essential knowledge of drug safety, adverse drug reactions, and regulatory compliance. Ideal for professionals in the pharmaceutical industry, this course offers practical skills in monitoring and managing drug risks, ensuring patient safety, and enhancing your career in pharmacovigilance and drug safety.
https://gaads.in/course/pharmacovigilance-course
#Pharmacovigilance Course#pharmacovigilance courses in delhi#pharmacovigilance training in india#pharmacovigilance course in india#bollywood
1 note
·
View note
Text

Post Graduate Diploma/Executive Diploma in Pharmacovigilance
The Post Graduate Diploma/Executive Diploma in Pharmacovigilance has been structured by experts from the industry themselves and thus comprehensive coverage and understanding of the industry and its functional areas is promised. The goal of the Post Graduate Diploma/Executive Diploma Programme is to familiarize the participant with the updated theoretical and practical aspects of Pharmacovigilance.
#Diploma in Pharmacovigilance#pharmacovigilance course#clinical Research#pharmacovigilance#Post Graduate Diploma in Pharmacovigilance#online Pharmacovigilance
0 notes
Text
Investigating Clinical Data Management and Pharmacovigilance Courses: A Way to Drug Greatness

Presentation
In the powerful scene of drugs, guaranteeing the wellbeing and adequacy of medications is principal. Clinical Data Management (CDM) and pharmacovigilance course (PV) assume essential parts in this space. As the interest for talented experts here develops, specific courses have arisen to address industry issues. How about we dive into what these courses involve and why they are fundamental for those trying to succeed in drugs.
Figuring out Clinical Data Management (CDM)
1. What is CDM?
CDM includes the assortment, mix, and management of data from clinical preliminaries. It guarantees that data gathered during clinical exploration is precise, solid, and genuinely sound. Experts in CDM administer the whole data lifecycle, from assortment to examination.
2. For what reason is CDM Significant?
Precise data is significant for administrative entries and dynamic by drug organizations. CDM guarantees consistence with administrative guidelines and improves the believability of clinical preliminary outcomes. It additionally works with proficient joint effort between different partners engaged with drug advancement.
Investigating Pharmacovigilance (PV)
1. What is PV?
Pharmacovigilance is the science and exercises connected with the recognition, appraisal, understanding, and avoidance of unfavorable impacts or some other medication related issues. It includes observing the wellbeing of promoted sedates and evaluating the dangers and advantages related with their utilization.
2. For what reason is PV Significant?
PV adds to general wellbeing by recognizing and limiting dangers related with drug items. Opportune discovery and revealing of unfriendly medication responses (ADRs) are fundamental for guaranteeing patient wellbeing and keeping up with public confidence in the medical services framework. Moreover, PV assumes a urgent part in consistence with administrative prerequisites.
The Meaning of Specific Courses
1. Extensive Educational program
Courses in CDM and PV give top to bottom information on applicable guidelines, systems, and apparatuses utilized in clinical examination and medication security. Understudies gain viable abilities through active preparation in data management programming and unfavorable occasion announcing frameworks.
2. Industry-Significant Abilities
These courses are intended to meet the developing necessities of the drug business. Graduates are outfitted with the abilities expected to explore complex administrative systems, lead clinical preliminaries proficiently, and guarantee the wellbeing of drug items all through their lifecycle.
3. Vocation Open doors
Experts with aptitude in CDM and PV are sought after across drug organizations, contract research associations (CROs), administrative offices, and medical services foundations. Vocation open doors incorporate jobs, for example, clinical data director, pharmacovigilance researcher, drug wellbeing partner, and administrative undertakings trained professional.
End
In a time of quick headways in drugs, the significance of clinical data management and pharmacovigilance couldn't possibly be more significant. Particular courses in these fields furnish hopeful experts with the information and abilities expected to succeed in the business. By guaranteeing the trustworthiness of clinical data and the security of drug items, CDM and PV experts assume a crucial part in propelling general wellbeing and development in medication.
0 notes
Text
Unlocking a Career in Drug Safety: Embark on a Pharmacovigilance Course
In the intricate world of pharmaceuticals, where medications hold the power to heal and sustain, the significance of pharmacovigilance cannot be overstated. Pharmacovigilance, the vigilant monitoring of drug safety, stands as a cornerstone of patient well-being, safeguarding individuals from adverse drug reactions (ADRs). Embarking on a pharmacovigilance course offers a gateway to a fulfilling career in this dynamic and essential field.
Delving into the Realm of Pharmacovigilance
A pharmacovigilance course immerses individuals in the intricacies of drug safety, equipping them with the knowledge and skills to navigate the multifaceted aspects of this field. Through comprehensive coursework, participants gain insights into:
The fundamental principles of pharmacovigilance
The regulatory framework governing drug safety
Methods for detecting and assessing ADRs
Strategies for risk management and signal detection
Effective communication of pharmacovigilance findings
The Whiteboard Academy: A Beacon of Pharmacovigilance Education
The Whiteboard Academy distinguishes itself as a leading provider of pharmacovigilance courses, offering a range of programs tailored to diverse career aspirations. Their curriculum, meticulously designed by industry experts, ensures that participants gain a thorough understanding of pharmacovigilance principles and practices. The Whiteboard Academy's pharmacovigilance courses are renowned for:
In-depth coverage of pharmacovigilance concepts
Hands-on training in ADR detection and management
Exposure to real-world case studies and simulations
Guidance on navigating regulatory requirements
Untapping Career Opportunities in Pharmacovigilance
Completion of a pharmacovigilance course opens doors to a plethora of rewarding career opportunities in the healthcare and pharmaceutical sectors. With the growing emphasis on patient safety, pharmacovigilance professionals are increasingly sought after by:
Pharmaceutical companies
Clinical research organizations (CROs)
Regulatory agencies
Healthcare institutions
Investing in a Pharmacovigilance Course: A Catalyst for Personal and Professional Growth
Enrolling in a pharmacovigilance course represents an investment in personal and professional growth. By acquiring expertise in this critical field, individuals can:
Contribute to the improvement of patient safety
Gain a competitive edge in the job market
Pursue a career in a rapidly growing industry
Make a meaningful impact on public health
Conclusion
Venturing into the realm of pharmacovigilance through a comprehensive course empowers individuals to become champions of patient safety. The Whiteboard Academy's commitment to providing exceptional pharmacovigilance education equips participants with the knowledge, skills, and confidence to excel in this dynamic field. Embrace the opportunity to safeguard patient well-being and embark on a fulfilling career in pharmacovigilance.
0 notes
Text
Why Should You Consider a Career in Medical Coding?
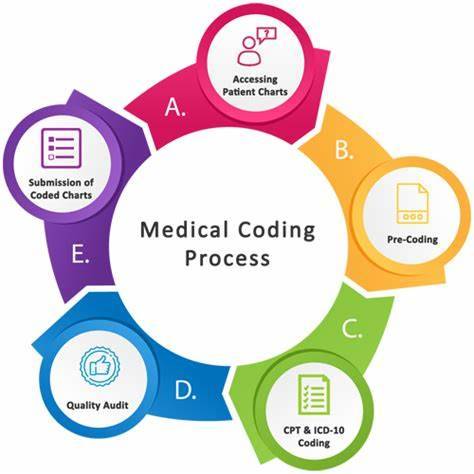
Introduction
Are you looking for a rewarding career in the healthcare industry that doesn't require years of medical school? If so, medical coding might be the perfect choice for you. In this blog, we'll explore the exciting world of medical coding, its importance in clinical research, and why you should seriously consider it as a career option. Let's dive in!
What is Medical Coding?
Medical coding is like the language of healthcare. It involves transforming medical information such as diagnoses, procedures, and treatments into universal codes. These codes are used for various purposes, including billing, insurance claims, and clinical research. Essentially, medical coders are responsible for ensuring that the healthcare system runs smoothly by accurately documenting patient records.
Why Choose a Career in Medical Coding?
1. In-Demand Career: The healthcare industry is constantly growing, and with it, the demand for skilled medical coders. Hospitals, clinics, insurance companies, and research institutions are always in need of qualified professionals to handle their coding needs.
2. Short Training Period: Unlike many other healthcare careers that require years of education, you can become a medical coder relatively quickly. Numerous institutes offer courses and training programs in medical coding that can be completed in a matter of months.
3. Diverse Opportunities: Medical coding isn't limited to just one type of job. You can find opportunities in various settings, including hospitals, private practices, pharmaceutical companies, and research organizations. If you want to explore related fields, you can also transition into areas like pharmacovigilance, drug regulatory affairs, or clinical data management.
4. Stability and Job Security: The healthcare industry is known for its stability, and medical coding is no exception. As long as there are healthcare services, there will be a need for medical coders. This translates to job security and peace of mind in your career.
5. Work-Life Balance: Many medical coding jobs offer excellent work-life balance. You'll typically work regular hours in a comfortable office setting, allowing you to maintain a healthy work-life balance.
6. Good Earning Potential: While salaries can vary based on location and experience, medical coders generally earn a competitive wage. With experience and additional certifications, you can increase your earning potential even further.
7. Contributing to Healthcare: By ensuring accurate coding, you help maintain the integrity of patient records, improve patient care, and support clinical research, pharmacovigilance, drug regulatory affairs, and clinical data management.
The Role of Medical Coding in Clinical Research
Now, let's delve into the connection between medical coding and clinical research. Clinical research plays a vital role in advancing healthcare treatments and therapies. It involves testing new drugs, medical devices, and treatment protocols to ensure their safety and effectiveness. Medical coding is an essential part of this process for several reasons:
1. Data Accuracy: Accurate coding ensures that the data collected during clinical trials is reliable. Researchers rely on this data to make informed decisions about the safety and efficacy of new treatments.
2. Regulatory Compliance: Regulatory agencies, such as the FDA, require precise documentation of clinical trial data. Medical coding helps maintain compliance with these regulations, which is critical for getting new drugs and treatments approved.
3. Patient Safety: Proper coding helps identify any adverse events or side effects experienced by patients during clinical trials. This information is crucial for patient safety and determining the risks and benefits of a new treatment.
4. Data Analysis: Medical coding simplifies the process of data analysis by categorizing information into standardized codes. This makes it easier for researchers to identify trends and draw conclusions from the data.
How to Start Your Career in Medical Coding?
1. Take a course: Look for reputable institutes or online courses that offer medical coding training. These courses cover topics like anatomy, medical terminology, and coding systems such as ICD-10 and CPT.
2. Get Certified: While certification isn’t always required, it can significantly boost your job prospects. Consider obtaining certifications like Certified Professional Coder (CPC) or Certified Coding Specialist (CCS) through recognized organizations.
3. Gain Experience: Entry-level positions may require some on-the-job experience. Look for internships or entry-level coding jobs to build your skills and resume.
4. Stay Updated: Medical coding guidelines and regulations can change, so it’s essential to stay current. Attend workshops, and seminars, and continue your education to remain competitive in the field.
5. Network: Join professional organizations such as the American Health Information Management Association (AHIMA) or the American Academy of Professional Coders (AAPC) to connect with other professionals in the industry.
Conclusion
In conclusion, a career in medical coding offers stability, good earning potential, and diverse opportunities within the healthcare industry. Moreover, it plays a crucial role in clinical research, contributing to the development of new and better treatments for various medical conditions. If you're interested in healthcare, have an eye for detail, and enjoy working in a structured environment, medical coding could be the perfect career choice for you. Consider enrolling in a reputable training program or course to kickstart your journey into this rewarding field. Your future as a medical coder awaits!
#medical coding institute#medical coding training#medical coding course#Clinical research training#Clinical Research Institute#Clinical research course#Pharmacovigilance course#Pharmacovigilance training institute#Pharmacovigilance jobs#Clinical data management course#Clinical data management training institute#Clinical research management
0 notes
Text
The Whiteboard offers a comprehensive pharmacovigilance course

The Whiteboard, a leading provider of education and training solutions, offers a comprehensive pharmacovigilance course designed for healthcare professionals, pharmacists, and regulatory affairs personnel. The course covers the fundamentals of pharmacovigilance, including the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems. The curriculum also emphasizes the legal and regulatory aspects of pharmacovigilance and provides a practical approach to implementing and maintaining pharmacovigilance systems. Participants will gain a thorough understanding of the importance of pharmacovigilance in ensuring the safety and efficacy of medicines and will be equipped with the necessary knowledge and skills to effectively manage and report adverse drug reactions. The course is delivered by experienced professionals in the field, using a range of interactive learning methods, including case studies, group discussions, and practical exercises. Upon completion of the course, participants will receive a certificate of attendance, demonstrating their commitment to pharmacovigilance and their dedication to patient safety.
For more details:- https://thewhiteboard.co.in/pv-pro.php
0 notes
Text
DATA VISUALIZATION IN CLINICAL TRIALS
"Data plays a key function in organizing, monitoring, and analyzing findings for a clinical study, similar to the foundation of a new house. Data provides new insights, helps evaluate hazards, and helps choose the best course of action for a study.
Introduction
In clinical trials, adverse events are often reported by simply counting the number of people who experienced each event. Reporting only frequency leaves out other data aspects that are crucial for stakeholders, such as severity, seriousness, rate (recurrence), timing, and groups of connected Adverse events. Data visualization is the process of displaying data in such a way that it can be easily understood. It helps to identify patterns and trends and make decisions based on this information. A good data visualization tool will allow you to quickly summarize your data and make it easy for others to understand what it means.
In the last ten years, there has been an increase of 183% in data per clinical trial because of breakthroughs in science and technology. The amount and variety of such data have grown well beyond what a straightforward spreadsheet can handle. The volume and variety of data generated by clinical trials will increase as they adopt decentralized methods. The main obstacles for sponsors of all sizes are importing and evaluating data from wearables, imaging systems, apps, and central labs. The importance of curating and delivering the data to stakeholders is growing along with the difficulty and time commitment. To complete these responsibilities, a platform that enables research teams to access all data in one location is now essential.
What is Data visualization?
Data visualization is the graphical representation of information and data using visual elements like charts, graphs, maps, and other visuals. It uses a variety of techniques, such as graphs and charts, pie charts and bar graphs, line graphs (to compare two sets), maps, timelines (to show repeated observations), histograms (to find outliers), box plots, etc.,
Comparison of traditional frequency tables and data visualization
Data visualization is more effective than frequency tables because it allows you to compare data more intuitively. A traditional frequency table shows how many times each option was selected but does not allow you to see any other information about the response (e.g., mean, or median). This can be time-consuming if multiple options are being compared or if many different metrics are being displayed on one page with no space between them
Power of Data Visualization
Traditional methods just don't allow for efficient use and administration of that data when the volume of data increases and decentralized trials become more prevalent. You may easily integrate, organize, and analyze clinical data using visualization tools to boost operational effectiveness and drive clinical trial success. In addition to the obvious benefit of being able to identify outliers and trends, data visualization also helps with identifying clusters, correlations, and relationships. These are all ways that you can use your data to inform decisions about a trial.
Benefits of data visualization
More informed decisions
Faster analysis
Improved organizational efficiency
Visualizations can better support investigators to assimilate large volumes of data and enable improved informal between-arm comparisons compared to tables
Increased competitive advantage
Improved customer experience
Visualization can show data quality issues, support robust temporal searches, or even discover cohorts of patients meeting selection criteria for clinical studies that depend on huge warehouses of patient data.
The availability of more internet information and personal sensors has begun to raise patient awareness of and ownership over their health. This is a significant departure from the paternalistic approach to healthcare in which patients trust their doctors with their health during annual checkups or in the event of an injury or illness.
Provide insights into the relationships between variables and help you identify potential flaws in your study design.
Conclusion
Data visualization is a powerful way to improve the quality of Clinical trial data. The use of tables, dot plots, and volcano plots can encourage differing interpretations. This can be achieved by providing interactive tools for data exploration and analysis, as well as visual displays that are easy to interpret and understand. Care in the construction of visual displays needs to be taken as there can be potential to overemphasize treatment effects in some circumstances.
https://www.clinosol.com/
#Clinical Research Training institute#certificate course in clinical research#clinical data management course#medical writing course#pharmacovigilance course
0 notes
Text

Clini India, one of the top Clinical Research institutes in Bangalore, Hyderabad, Pune and Mumbai, Our Institute offers courses in Clinical Research, Clinical Data Management, Pharmacovigilance, Medical Writing and Clinical SAS. We are SAS Accredited and ISO Certified Clinical Research Institute. Learn From online or offline, Flexible Classes, training with placement Assistance.
#clinical research course#pharmacovigilance course#medical writing course#clinical data management course#clinical SAS course
1 note
·
View note
Text
Regulatory Affairs Course

Discover our comprehensive Drug Regulatory Affairs courses designed to equip you with the knowledge and skills needed for a successful career in pharmaceutical regulatory compliance. Gain expertise in drug approval processes, regulations, and submissions. Enroll now for a rewarding educational journey with our online courses. Get more Information at: https://www.companysconnects.com or contact us at 9691633901 for more info.
#pharmacovigilance courses#mes training#clinical research certified professional course#drug regulatory affairs certification
0 notes
Text
#pharmacovigilance online courses#online certificate courses in pharmacovigilance#icri online learning
0 notes
Text
Navigating the Future of Drug Safety: A Comprehensive Guide to Pharmacovigilance Courses

Introduction
In the dynamic field of healthcare, ensuring the safety and efficacy of pharmaceutical products is of paramount importance. Pharmacovigilance, the science of monitoring and assessing the safety of drugs, plays a crucial role in safeguarding public health. This blog explores the significance of pharmacovigilance courses, shedding light on the key components that contribute to a successful career in drug safety
Understanding Pharmacovigilance
Definition and Scope
Pharmacovigilance is the science and activities related to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems. A pharmacovigilance course equips individuals with the knowledge and skills necessary to contribute to the safe use of medications.
Regulatory Landscape
A comprehensive pharmacovigilance course covers the regulatory frameworks governing drug safety at both national and international levels. Understanding these regulations is essential for professionals working in the pharmaceutical industry to ensure compliance with safety standards.
Role in Public Health
Emphasizing the broader impact of pharmacovigilance courses highlight its role in protecting public health. Professionals trained in pharmacovigilance contribute to the identification and mitigation of potential risks associated with drug use.
Key Components of Pharmacovigilance Courses
Drug Safety Surveillance
Courses delve into the methodologies and tools used for monitoring and surveillance of adverse drug reactions. This includes the analysis of real-world data, clinical trials, and post-marketing surveillance.
Signal Detection and Management
Participants learn how to identify signals that may indicate potential safety concerns. Additionally, courses cover the management of identified signals, including risk assessment and communication strategies.
Pharmacovigilance Systems and Databases
Understanding the infrastructure supporting pharmacovigilance is essential. Courses explore the design and implementation of pharmacovigilance systems, as well as the utilization of databases for efficient data collection and analysis.
Risk Management and Communication
An integral part of pharmacovigilance education is learning how to develop and implement risk management plans. Communication strategies for disseminating safety information to healthcare professionals and the public are also emphasized.
Ethics and Compliance
Ethics in pharmacovigilance is a critical aspect covered in courses. Professionals must adhere to ethical standards while reporting and managing safety data. Compliance with regulations and guidelines is essential to maintaining the integrity of drug safety practices.
Career Opportunities in Pharmacovigilance
Pharmacovigilance Officer
Monitoring and reporting adverse drug reactions, pharmacovigilance officers play a crucial role in ensuring drug safety compliance.
Drug Safety Specialist
Specialists focus on analyzing safety data, conducting risk assessments, and implementing risk management plans.
Regulatory Affairs Manager
Professionals in regulatory affairs ensure that pharmaceutical products comply with safety regulations and manage interactions with regulatory authorities.
Conclusion
Enrolling in a pharmacovigilance course is a strategic step towards a fulfilling and impactful career in drug safety. As the pharmaceutical industry continues to evolve, the demand for skilled pharmacovigilance professionals remains high. With the right education and training, individuals can contribute to a safer and more secure healthcare landscape, making a positive impact on public health globally
#Pharmacovigilance course#clinical Research#Medical Writing programmes#Clinical DataManagement Course#online Pharmacovigilance course
0 notes
Text
Navigating the Landscape of Pharmacovigilance: Online Courses and Clinical Data Management
Introduction
In the dynamic realm of pharmaceuticals, the significance of pharmacovigilance courses online and proficient clinical data management cannot be overstated. As the healthcare industry continues to evolve, the demand for skilled professionals in pharmacovigilance and clinical research data management escalates. In this article, we delve into the pivotal role of these disciplines, explore the avenues of online education, and shed light on the importance of adeptly managing clinical data.
The Foundation of Pharmacovigilance
Pharmacovigilance, often dubbed as the watchdog of the pharmaceutical world, encompasses the systematic detection, assessment, understanding, and prevention of adverse effects or any other drug-related problems. In the wake of increasing complexities in drug development and stringent regulatory standards, pharmacovigilance plays a central role in ensuring drug safety and efficacy throughout its lifecycle.
Unveiling Online Pharmacovigilance Courses
With the surge in demand for skilled pharmacovigilance professionals, the accessibility and flexibility of online courses have become paramount. Online pharmacovigilance courses offer a comprehensive curriculum tailored to meet industry standards and regulatory requirements. These courses provide aspiring professionals with a thorough understanding of pharmacovigilance principles, regulatory frameworks, risk management strategies, and signal detection methodologies.
The Crux of Clinical Research Data Management
Clinical data management serves as the backbone of clinical research, encompassing the collection, organization, validation, and analysis of data obtained from clinical trials. In an era driven by data-centric decision-making, efficient clinical data management is indispensable for ensuring the integrity, accuracy, and reliability of clinical trial data.
Navigating the Landscape of Clinical Data Management
In today's evolving healthcare landscape, the significance of adept clinical data management cannot be overstated. As the volume and complexity of clinical trial data continue to escalate, there is a growing demand for skilled professionals proficient in clinical data management. Online courses in clinical data management offer a structured curriculum covering essential aspects such as database design, data cleaning, quality control, and regulatory compliance.
The Intersection: Integrating Pharmacovigilance and Clinical Data Management
The convergence of pharmacovigilance and clinical data management heralds a new era of synergy and collaboration within the pharmaceutical industry. By seamlessly integrating pharmacovigilance principles into clinical data management processes, organizations can enhance their ability to detect, assess, and mitigate potential risks associated with investigational drugs.
Conclusion
In conclusion, the fields of pharmacovigilance and clinical data management stand as pillars of safety and efficacy in the pharmaceutical landscape. With the advent of online education, aspiring professionals now have unprecedented access to specialized courses that equip them with the knowledge and skills necessary to excel in these domains. As the healthcare industry continues to evolve, the demand for proficient pharmacovigilance and clinical data management professionals will only intensify, underscoring the importance of investing in education and training in these critical areas.
0 notes
Text
Aggregate Reporting in Pharmacovigilance Courses
Enhance your expertise in drug safety with our comprehensive aggregate reporting in pharmacovigilance courses. Learn the crucial skills needed to analyze and report adverse events, ensuring patient safety and regulatory compliance. Perfect for professionals looking to advance their careers in pharmacovigilance and drug safety, our courses offer in-depth training on aggregate data management, signal detection, and risk assessment. Enroll now to stay ahead in the rapidly evolving pharmaceutical industry
0 notes
Text
Balancing Benefit and Risk in Clinical Research
Introduction
The fields of medicine and healthcare are rapidly developing. Companies and research institutes play a vital role in advancing medical knowledge through clinical research. Clinical research surrounds various aspects, including medical coding, pharmacovigilance, drug regulatory affairs, and clinical data management. These fields are essential in ensuring the safety and effectiveness of new medical treatments. However, conducting clinical research comes with its own set of challenges, particularly when it comes to balancing the benefits and risks involved.

The Role of Clinical Research
Clinical research is the backbone of medical progress. It involves the systematic study of new drugs, medical devices, treatments, and procedures to determine their safety and efficacy. Companies and research institutes conduct clinical trials to gather data and evidence before these medical interventions are approved for widespread use.
Key Areas of Clinical Research
1. Medical Coding: Medical coding is like the language of healthcare. It involves translating medical records, diagnoses, and procedures into standardized codes. Accurate coding is crucial for proper billing and maintaining patient records.
2. Pharmacovigilance: This field focuses on monitoring the safety of drugs and vaccines post-approval. It helps identify and prevent adverse effects and ensures that patients receive safe medications.
3. Drug Regulatory Affairs: Drug regulatory affairs professionals work with regulatory agencies to ensure that new drugs meet safety and efficacy standards before they reach the market. They help companies navigate complex regulations.
4. Clinical Data Management: Managing clinical trial data is essential for maintaining the integrity of research. Data managers organize and validate information collected during trials.
Balancing Benefit and Risk
While clinical research is definitely important for medical progress, it also involves risks. Here are some ways in which companies and research institutes can strike a balance:
1. Ethical Considerations: Ethical guidelines and standards are the foundation of clinical research. Researchers must prioritize the well-being of participants and ensure that their rights and privacy are protected.
2. Informed Consent: Participants must provide informed consent before participating in a clinical trial. They should be fully aware of the potential risks and benefits, enabling them to make an informed decision.
3. Safety Monitoring: Continuous monitoring of participants' safety is essential. Any adverse events should be immediately reported and addressed.
4. Transparency: Transparency in reporting research findings is crucial. This includes disclosing both positive and negative results, helping to avoid biased information.
5. Regulatory Compliance: Companies and institutes must adhere to regulatory requirements in their respective fields, ensuring that the research meets high standards of safety and quality.
The Importance of Training
To ensure that clinical research is conducted responsibly, professionals in the field require acceptable training. Courses and training programs are available for medical coding, pharmacovigilance, drug regulatory affairs, and clinical data management. Proper training equips individuals with the knowledge and skills needed to conduct research while minimizing risks.
Job Placement in Clinical Research
For those interested in pursuing a career in clinical research, the job placement aspect is essential. Companies and institutes often offer placement opportunities for trained professionals, ensuring that they can apply their skills in real-world settings.
Conclusion
Balancing benefit and risk in clinical research is a complex but essential endeavor. Companies and research institutes play a critical role in advancing medical knowledge, but they must do so responsibly. Ethical considerations, informed consent, safety monitoring, transparency, and regulatory compliance are key factors in achieving this balance. Moreover, individuals interested in clinical research can benefit from training and job placement opportunities, enabling them to contribute to the field while ensuring the safety and well-being of patients. In this way, we can continue to make significant strides in healthcare while upholding the highest standards of ethics and safety.
#Medical billing and coding course#Pharmacovigilance jobs#Pharmacovigilance course#Pharmacovigilance training institute#Clinical data management course#Clinical data management training institute#Clinical research management#Clinical research training#Clinical Research Institute#Clinical research course#medical coding course#medical coding institute#medical coding training
0 notes
Text
pharmacovigilance courses - Pharma Connections
Unlock your potential in the pharmaceutical industry with our comprehensive Pharmacovigilance Training Course in bangalore. Designed to equip you with essential skills, our course focuses on drug safety monitoring practices, ensuring you are well-prepared for a successful career in pharmacovigilance.
0 notes
Text
Clinical data management course | Clinosol Research
Clinosol offers the best clinical research job-oriented training programs and collaborates with Life sciences companies to prepare students into professionals.

#certificate course in clinical research#clinical courses#clinical data management course#clinical research course#medical writing course#pharmacovigilance course#healthcare
0 notes