#Platform (Lateral Flow Assays
Explore tagged Tumblr posts
Text
United States point of care diagnostics market size is projected to exhibit a growth rate (CAGR) of 6.90% during 2024-2032. Numerous advancements in portable and handheld diagnostic devices have enhanced the convenience and user-friendliness of testing, which is primarily driving the market growth.
#United States Point of Care Diagnostics Market Report by Product Type (Blood-Glucose Monitoring Kit#Cardio-Metabolic Monitoring Kit#Pregnancy and Fertility Testing Kit#Infectious Disease Testing Kit#Cholesterol Test Strip#Hematology Testing Kit#and Others)#Platform (Lateral Flow Assays#Dipsticks#Microfluidics#Molecular Diagnostics#Immunoassays)#Prescription Mode (Prescription-Based Testing#OTC Testing)#End User (Professional Diagnostic Centers#Home Care#Research Laboratories#and Region 2024-2032
0 notes
Text
Beyond Vaccines: How Advanced Biologics are Revolutionizing Animal Disease Prevention and Treatment
Why are Veterinary Biologics Indispensable for Global Animal Health and Food Security?
Veterinary biologics are a critical class of products derived from living organisms that are used to prevent, treat, or diagnose animal diseases. This includes vaccines, diagnostic kits, immunomodulators, antiserums, and antibodies. Their indispensable role stems from their ability to bolster animal immunity, control infectious disease outbreaks, and improve overall animal welfare and productivity. The global veterinary biologics market is exhibiting strong growth, estimated at $13.65 billion in 2025 and projected to reach $18.38 billion by 2029, with a robust Compound Annual Growth Rate (CAGR) of 7.7%.
This significant growth is primarily driven by the surging prevalence of animal diseases worldwide, ranging from common infections in companion animals to highly contagious outbreaks in livestock that can devastate agricultural economies and pose zoonotic risks to humans (e.g., avian influenza, rabies). Biologics are the frontline defense against these threats, offering targeted and effective solutions. Furthermore, the increasing global demand for animal protein (meat, dairy, eggs) necessitates healthy and productive livestock, making disease prevention and control via biologics crucial for food security and sustainable agricultural practices. Rising pet ownership trends and a growing emphasis on companion animal health also contribute significantly to market expansion, as owners seek advanced preventive and therapeutic options for their beloved animals.
What Cutting-Edge Advancements are Driving Innovation in Veterinary Biologics?
The veterinary biologics market is a hub of biotechnological innovation, leading to more effective, targeted, and safer products for animals.
A primary driver of innovation is the rapid adoption of recombinant DNA technology and vector-based vaccines. Unlike traditional vaccines that use inactivated or live-attenuated pathogens, recombinant vaccines utilize genetic engineering to produce specific antigens that elicit an immune response without exposing the animal to the whole pathogen. This results in safer vaccines with fewer side effects and greater stability. Similarly, mRNA vaccine platforms, which proved revolutionary in human medicine, are increasingly being explored and developed for veterinary applications, promising rapid development, high efficacy, and flexible manufacturing. These technologies enable the creation of highly targeted and more potent vaccines against complex or emerging animal diseases.
The development of monoclonal antibodies (mAbs) represents another significant leap forward. mAbs offer highly specific and targeted therapeutic options for various animal conditions, including inflammatory diseases, certain cancers, and infectious diseases. For example, mAbs are being developed for canine atopic dermatitis, providing relief from chronic itching. These biologics can neutralize specific disease-causing agents or modulate immune responses with precision, offering an alternative to broad-spectrum pharmaceuticals and often with fewer side effects.
Advances in diagnostic kits are also transforming the market. Innovations include rapid diagnostic tests (e.g., lateral flow assays, pen-side tests) that provide quick and accurate detection of pathogens in the field, enabling faster containment of outbreaks. PCR-based diagnostic kits offer high sensitivity and specificity for early and definitive pathogen identification. These diagnostic tools are crucial for guiding the appropriate use of biologics and preventing disease spread.
The increasing focus on precision livestock farming and customized, targeted therapies is influencing biologic development. Data analytics and genomic insights are being used to develop vaccines and immunomodulators tailored to specific animal populations, breeds, or even individual animals, maximizing efficacy and minimizing waste. This shift moves away from a "one-size-fits-all" approach to more personalized animal health management.
Finally, the concept of "One Health" is significantly influencing the market. Recognizing the interconnectedness of human, animal, and environmental health, research and development in veterinary biologics are increasingly focusing on zoonotic diseases (diseases transferable between animals and humans), leading to biologics that benefit both animal and public health. This holistic approach drives the development of novel vaccines and diagnostic tools for diseases like rabies, avian influenza, and African swine fever.
What are the Key Drivers and Future Outlook for the Veterinary Biologics Market?
The veterinary biologics market is set for sustained expansion, propelled by strong underlying demand and continuous innovation, though it also faces specific challenges.
Key growth drivers include:
Rising Incidence of Animal Diseases: As mentioned, outbreaks of diseases like avian influenza, African swine fever, and new variants of common infections continually drive the need for new and updated biologics.
Increasing Pet Ownership and Humanization of Pets: Owners are more willing to invest in advanced preventive care, including vaccines and specialized immunotherapies, for their companion animals.
Growing Demand for Animal Protein: The global population's increasing consumption of meat, dairy, and eggs necessitates robust animal health management to ensure food safety and supply.
Technological Advancements in Biotechnology: Ongoing R&D in genomics, proteomics, and gene editing continues to unlock new targets and more effective biologic candidates.
Government Initiatives and Regulatory Support: Programs aimed at disease surveillance, control, and eradication, coupled with supportive regulatory pathways for faster market entry, accelerate market growth.
Looking ahead, the future of the veterinary biologics market holds several promising trends:
Emerging Infectious Diseases and Climate Change: The unpredictability of new pathogens and changing disease patterns due to climate change will necessitate rapid development of novel biologics.
Personalized and Targeted Therapies: Increased adoption of biologics tailored to specific animal genetics or individual disease profiles, moving beyond broad-spectrum treatments.
Eco-friendly and Sustainable Biologics: A growing emphasis on sustainable manufacturing processes and biologics that minimize environmental impact.
Integration with Digital Health Records and Precision Livestock Farming: Seamless data flow from biologics administration and animal health monitoring into digital platforms for better disease management and traceability.
Focus on Oral Biologics and Novel Delivery Methods: Exploring alternatives to injectables for easier administration and improved animal welfare.
Strengthening of the "One Health" Approach: Increased collaboration between human and animal health sectors to combat zoonotic diseases, driving investment in relevant veterinary biologics.
Challenges include the complex and often lengthy regulatory approval processes for new biologics, the high cost of R&D, and the potential for resistance or reduced efficacy against rapidly evolving pathogens. However, the critical role of veterinary biologics in safeguarding animal populations, ensuring food security, and protecting public health against zoonotic threats guarantees its continued growth and innovation as a vital sector of the animal healthcare industry.
Contact:
Market Research Future®
99 Hudson Street,5Th Floor
New York, New York 10013
United States of America
Phone:
+1 628 258 0071(US)
+44 2035 002 764(UK)
Email: [email protected]
Website: https://www.marketresearchfuture.com
0 notes
Text
Europe Point-Of-Care-Testing Market Size, Share, Trends, Growth Opportunities and Competitive Outlook
Executive Summary Europe Point-Of-Care-Testing (POCT) Market :
Europe Point-Of-Care Testing (POCT) Market size was valued at USD 21.63 billion 2024 and is projected to reach USD 10.65 billion by 2032, with a CAGR of 9.3% during the forecast period of 2025 to 2032.
Europe Point-Of-Care-Testing (POCT) Market business market research report help you stay up-to-date about the whole market and also give holistic view of the market. Market research analysis provides the insights which help to have a more precise understanding of the market landscape, issues that may impinge on the industry in the future, and how to position specific brands in the best way. With this report one can focus on the data and realities of the industry which keeps them on the right path. The insights covered in this Europe Point-Of-Care-Testing (POCT) Market report will guide for an actionable ideas, better decision-making and better business strategies.
With Europe Point-Of-Care-Testing (POCT) Market international market research report it becomes easy to do estimations about the investment in an emerging market, expansion of market share or success of a new product. Market research analysis makes the professional reputation better in the field, builds more credibility in the work and helps other participants to have more assurance and trust in your conclusions. This market report guides all sizes of businesses by providing informed decisions on the different aspects of business. Europe Point-Of-Care-Testing (POCT) Market report has been formulated by understanding the significance of sound facts and figures required for any research.
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive Europe Point-Of-Care-Testing (POCT) Market report. Download Full Report: https://www.databridgemarketresearch.com/reports/europe-poct-market
Europe Point-Of-Care-Testing (POCT) Market Overview
**Segments**
- Based on product, the Europe Point-Of-Care-Testing (POCT) market can be segmented into glucose monitoring kits, infectious disease testing kits, cardiometabolic monitoring kits, coagulation monitoring kits, pregnancy and fertility testing kits, tumor/cancer markers, urinalysis testing kits, cholesterol test strips, hematology testing kits, drug-of-abuse testing kits, fecal occult testing kits, and others. - On the basis of mode of prescription, the market can be categorized into prescription-based testing and OTC testing. - Depending on platform, the POCT market in Europe consists of lateral flow assays, dipsticks, microfluidics, molecular diagnostics, immunoassays, and others. - By end-user, the market is classified into hospitals, home care settings, clinics, diagnostic laboratories, and others.
**Market Players**
- Some of the key players operating in the Europe POCT market include Abbott, F. Hoffmann-La Roche Ltd, Siemens Healthcare GmbH, BD, bioMérieux SA, Johnson & Johnson Services, Inc., PTS Diagnostics, Werfen, QIAGEN, Sekisui Diagnostics, and Trividia Health, Inc.
The Europe Point-Of-Care-Testing (POCT) market is a dynamic and rapidly evolving sector that offers a wide range of opportunities for growth and innovation. One of the key trends shaping the market is the increasing demand for convenient and rapid diagnostic solutions, particularly in settings such as hospitals, clinics, and home care environments. The shift towards personalized medicine and the need for quicker and more accurate test results are driving the adoption of POCT devices across Europe.
One of the significant drivers of the Europe POCT market is the rising prevalence of chronic diseases such as diabetes, cardiovascular disorders, and infectious diseases. POCT devices play a crucial role in the early detection and monitoring of these conditions, leading to better patient outcomes and reduced healthcare costs. The focus on preventive healthcare and the emphasis on early diagnosis are also fueling the demand for POCT solutions in the region.
The competitive landscape of the Europe POCT market is characterized by the presence of a diverse range of players, including multinational corporations, medium-sized enterprises, and start-ups. Companies such as Abbott, F. Hoffmann-La Roche Ltd, Siemens Healthcare GmbH, and BD are among the key players driving innovation and technological advancements in the market. These companies are investing in research and development activities to launch new products and enhance their existing portfolio to cater to the evolving needs of healthcare providers and patients.
Regulatory factors also play a significant role in shaping the Europe POCT market. Stringent regulations governing the approval and commercialization of POCT devices are influencing the market dynamics and competition among players. Companies that can navigate the regulatory landscape effectively and ensure compliance with standards and guidelines are better positioned to succeed in the market.
The increasing adoption of digital health solutions and telemedicine platforms is creating new opportunities for POCT market players to expand their reach and offer integrated healthcare solutions. The convergence of healthcare technology, data analytics, and artificial intelligence is paving the way for innovative POCT devices that can deliver real-time insights and personalized recommendations to users.
Overall, the Europe POCT market holds immense potential for growth and innovation, driven by factors such as the increasing prevalence of chronic diseases, the emphasis on preventive healthcare, technological advancements, and regulatory developments. Market players that can differentiate their products, forge strategic partnerships, and stay ahead of the curve in terms of innovation are likely to experience sustained growth and success in the dynamic and competitive landscape of the Europe POCT market.The Europe Point-Of-Care-Testing (POCT) market is experiencing significant growth and evolution driven by various factors such as the demand for convenient and rapid diagnostic solutions, increasing prevalence of chronic diseases, and the shift towards personalized medicine. The market segmentation based on product diversity reflects the wide array of applications for POCT devices, ranging from glucose monitoring and infectious disease testing to pregnancy and fertility testing. This variety indicates the versatility and adaptability of POCT solutions to cater to different healthcare needs and diagnostic requirements.
The mode of prescription segmentation further showcases the different channels through which POCT devices are accessed, whether through prescription-based testing or over-the-counter availability. This distinction highlights the accessibility and flexibility of POCT devices, catering to both healthcare professionals and consumers seeking quick and reliable diagnostic results. The market players in the Europe POCT market consist of industry giants such as Abbott, Roche, and Siemens, along with other key players driving innovation and technological advancements in the sector.
The competitive landscape within the Europe POCT market underscores the importance of research and development activities in driving product innovation and differentiation. Companies investing in cutting-edge technologies and regulatory compliance are better positioned to capitalize on the growing demand for POCT solutions in the region. The integration of digital health solutions and telemedicine platforms further amplifies the opportunities for market expansion and the delivery of integrated healthcare services using POCT devices.
Regulatory factors play a crucial role in shaping the market dynamics and influencing competition among players. Compliance with stringent regulations and standards is essential for market players to navigate the regulatory landscape effectively and ensure the commercial success of their POCT devices. The convergence of healthcare technology, data analytics, and artificial intelligence presents new avenues for developing innovative POCT devices that provide real-time insights and personalized recommendations to users.
In conclusion, the Europe POCT market offers immense potential for growth and innovation, driven by the increasing prevalence of chronic diseases, technological advancements, regulatory developments, and the emphasis on preventive healthcare. Market players that focus on product differentiation, strategic partnerships, and continuous innovation are likely to thrive in the competitive landscape of the Europe POCT market. The evolving market trends and consumer demands present opportunities for companies to enhance their product offerings and create value-added solutions that address the evolving healthcare needs across Europe.
The Europe Point-Of-Care-Testing (POCT) Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now: https://www.databridgemarketresearch.com/reports/europe-poct-market/companies
DBMR Nucleus: Powering Insights, Strategy & Growth
DBMR Nucleus is a dynamic, AI-powered business intelligence platform designed to revolutionize the way organizations access and interpret market data. Developed by Data Bridge Market Research, Nucleus integrates cutting-edge analytics with intuitive dashboards to deliver real-time insights across industries. From tracking market trends and competitive landscapes to uncovering growth opportunities, the platform enables strategic decision-making backed by data-driven evidence. Whether you're a startup or an enterprise, DBMR Nucleus equips you with the tools to stay ahead of the curve and fuel long-term success.
Key Pointers Covered in the Europe Point-Of-Care-Testing (POCT) Market Industry Trends and Forecast
Europe Point-Of-Care-Testing (POCT) Market Size
Europe Point-Of-Care-Testing (POCT) Market New Sales Volumes
Europe Point-Of-Care-Testing (POCT) Market Replacement Sales Volumes
Europe Point-Of-Care-Testing (POCT) Market By Brands
Europe Point-Of-Care-Testing (POCT) Market Procedure Volumes
Europe Point-Of-Care-Testing (POCT) Market Product Price Analysis
Europe Point-Of-Care-Testing (POCT) Market Regulatory Framework and Changes
Europe Point-Of-Care-Testing (POCT) Market Shares in Different Regions
Recent Developments for Market Competitors
Europe Point-Of-Care-Testing (POCT) Market Upcoming Applications
Europe Point-Of-Care-Testing (POCT) Market Innovators Study
Browse More Reports:
Global Digital Holographic Display Market Global Animal Feed Acidifier Market Global Polyvinyl Butyral Market Global Lyme Disease Drug Market Asia-Pacific Reverse Logistics Market Middle East and Africa Remote Patient Monitoring and Care Market Global Hepatitis C Diagnosis and Treatment Market Global Sleep Tech Devices Market Global Paperboard Beverage Packaging Market Global Single-use Bioprocessing Systems Market Global Nanobots Market Global Oxidative Stress Assay Instruments Market Global Plastic Baby Food Packaging Market North America Conversational AI Market Global In-Memory Computing Market North America Reverse Logistics Market Global Imaging Infrared Light Emitting Diode (LED) Market Global Inherited Retinal Diseases Market Global Broadband Internet Access Services Market Global Plant Based Food Packaging Market Global Rare Earth Elements Market Global Automotive Natural Gas Vehicle Market North America Automotive DC-DC Converters Market Europe Non-stick Cookware Market Global Connected Care Market Global Triple A Syndrome Treatment Market North America Fluoroscopy - C Arms Market Global Video Conferencing Systems Market Global Automotive DC-DC Converters Market Global High Performance Lubricant Market North America Clinical Chemistry Analyser Market Global Performance Chemicals Market Global Vehicle Diagnostics Market Global Meatless Flavor Additives Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us: Data Bridge Market Research US: +1 614 591 3140 UK: +44 845 154 9652 APAC : +653 1251 975 Email:- [email protected]
0 notes
Text
Histamine Test Kit Market 2025
The Histamine Test Kit market refers to the market for tools and devices that allow the detection and measurement of histamine levels in various substances such as food, biological samples, and drinks. Histamine, a naturally occurring compound in the human body, plays a crucial role in immune response and digestion. However, it can also cause adverse reactions when consumed in high amounts, such as food allergies or intolerances. Histamine test kits are used in different sectors, including food safety, clinical research, and health diagnostics, to ensure that histamine levels remain within safe limits.
get free sample of this report at https://www.intelmarketresearch.com/download-free-sample/553/histamine-test-kit-market
These kits offer a quick, efficient way to measure histamine concentrations in products, preventing potential health risks like foodborne illnesses. Some of the primary tools used for histamine testing include ELISA (Enzyme-Linked Immunosorbent Assay) and Lateral Flow Immunoassays (LFIAs). Histamine test kits are predominantly used in the food and beverage industry, as well as in biological research and health diagnostics. Companies specializing in these kits provide accurate and reliable solutions for various end users, ensuring that they meet regulatory standards for histamine detection.
Market Size
The global Histamine Test Kit market was valued at US$ 154.59 million in 2024 and is projected to reach US$ 242.08 million by 2031, growing at a CAGR of 6.54% during the forecast period from 2025 to 2031. This growth is driven by an increasing need for food safety standards, advances in testing technologies, and rising consumer awareness about food allergies and intolerances.
The demand for histamine testing is steadily rising, particularly in regions such as North America and Europe, where food safety regulations are stringent, and the public is becoming more cautious about consuming food with high histamine content. Moreover, as histamine-related allergies and intolerances gain more recognition, testing solutions are becoming critical for both consumers and manufacturers. Additionally, the growth of the food and beverage industry, particularly the growing trend of fermented foods, directly impacts the market's expansion.
Market Dynamics (Drivers, Restraints, Opportunities, and Challenges)
Drivers
Increasing Food Safety Awareness: As foodborne illnesses caused by high histamine levels become more recognized, there is a rising demand for reliable testing kits. Regulatory frameworks in many countries require food producers to test and verify histamine levels in products like fish and fermented foods.
Growing Incidence of Histamine Intolerance: With the increasing awareness of food allergies, more consumers are seeking ways to avoid histamine-rich foods. Histamine test kits help manufacturers and consumers ensure products are safe for people with histamine sensitivities.
Technological Advancements: Innovations in testing methods, such as rapid ELISA and LFIA technologies, are making it easier to detect histamine levels quickly and accurately, thus boosting the adoption of histamine test kits in food production and biological research.
Restraints
High Cost of Advanced Test Kits: High-performance histamine test kits, especially those used for in-depth biological research, can be costly. This may limit the accessibility of these products for smaller companies or research facilities, particularly in emerging economies.
Lack of Standardization: Despite advancements in testing technologies, there is still a lack of universally accepted standards for histamine testing, which could affect market growth. Without a global consensus, there may be issues with accuracy and reliability across different testing platforms.
Opportunities
Expansion in Emerging Markets: As global awareness about food safety increases, regions such as Asia-Pacific and Latin America offer significant opportunities for histamine test kit manufacturers to expand. The growing middle class in these regions is leading to increased demand for safe and quality food products.
Rising Demand for Natural and Fermented Foods: The popularity of natural, fermented, and minimally processed foods has resulted in an increased demand for histamine testing to ensure that these foods are safe for consumption.
Challenges
Limited Awareness in Certain Sectors: While the food and beverage industry widely uses histamine test kits, other sectors like biological research and health diagnostics still have limited awareness. Widespread adoption requires more educational efforts and industry collaboration.
Regulatory Barriers: The regulatory environment around food safety testing can be complex and varies by region, creating challenges for companies operating internationally. Additionally, stringent compliance with local regulations can increase operational costs.
Regional Analysis
North America
The North American market for histamine test kits is estimated to grow from US$ 62.42 million in 2024 to US$ 88.93 million by 2031, at a CAGR of 4.94% during the forecast period. The growth is fueled by robust food safety regulations in the United States and Canada, particularly for seafood, dairy, and processed food industries. The awareness about histamine intolerance and food allergies is also pushing the adoption of these test kits.
Europe
Europe is expected to witness significant growth in the histamine test kit market, with its market size increasing from US$ 43.52 million in 2024 to US$ 71.69 million by 2031, at a CAGR of 7.26%. European countries have stringent food safety regulations, which encourage food manufacturers to adopt histamine testing. Countries like Germany, France, and the UK are particularly important players in this market.
Asia-Pacific
The Asia-Pacific region is experiencing rapid market growth, driven by increasing consumer demand for safe and high-quality food products, particularly in countries like China, India, and Japan. As the food and beverage industry in this region expands, the need for histamine testing solutions grows. Moreover, rising health awareness and the growing trend of food intolerance among consumers also contribute to the market expansion.
Competitor Analysis (in brief)
The global histamine test kit market is competitive, with several key players holding significant market shares. Leading companies include PerkinElmer, Eurofins Scientific, Neogen Corporation, Thermo Fisher Scientific, R-Biopharm, and Kikkoman Biochemifa Company. These companies are focused on innovation, improving test accuracy, and expanding their product portfolios to maintain their competitive advantage. In 2024, the top three players accounted for approximately 38.89% of the market revenue.
Global Histamine Test Kit: Market Segmentation Analysis
This report provides a deep insight into the global Histamine Test Kit market, covering all its essential aspects. This ranges from a macro overview of the market to micro details of the market size, competitive landscape, development trend, niche market, key market drivers and challenges, SWOT analysis, value chain analysis, etc.
The analysis helps the reader to shape the competition within the industries and strategies for the competitive environment to enhance the potential profit. Furthermore, it provides a simple framework for evaluating and assessing the position of the business organization. The report structure also focuses on the competitive landscape of the Global Histamine Test Kit. This report introduces in detail the market share, market performance, product situation, operation situation, etc., of the main players, which helps the readers in the industry to identify the main competitors and deeply understand the competition pattern of the market.
In a word, this report is a must-read for industry players, investors, researchers, consultants, business strategists, and all those who have any kind of stake or are planning to foray into the Histamine Test Kit in any manner.
Market Segmentation (by Application)
Food and Drink
Biological Research
Other
Market Segmentation (by Type)
ELISA
LFIAs
Other
Key Company
PerkinElmer
Eurofins Scientific
Neogen Corporation
Thermo Fisher Scientific
Kikkoman Biochemifa Company
Romer Labs
Hygiena
R-Biopharm
Merck Millipore
Revvity
Elabscience
Creative Diagnostics
Geographic Segmentation
North America
United States
Canada
Mexico
Asia-Pacific
China
Japan
South Korea
India
Australia
Southeast Asia
Europe
Germany
France
U.K.
Italy
Russia
Rest of Europe
Latin America
Brazil
Argentina
Middle East & Africa
GCC Countries
Turkey
South Africa
FAQ Section :
▶What is the current market size of the Histamine Test Kit market?
The Histamine Test Kit market is valued at US$ 154.59 million in 2024, with projections reaching US$ 242.08 million by 2031, growing at a CAGR of 6.54%.
▶Which are the key companies operating in the Histamine Test Kit market?
Key companies in the Histamine Test Kit market include PerkinElmer, Eurofins Scientific, Neogen Corporation, Thermo Fisher Scientific, and R-Biopharm.
▶What are the key growth drivers in the Histamine Test Kit market?
The primary growth drivers include increasing food safety awareness, growing incidence of histamine intolerance, and technological advancements in testing methods.
▶Which regions dominate the Histamine Test Kit market?
The North American and European markets dominate, with Asia-Pacific also showing rapid growth due to expanding food safety needs.
▶What are the emerging trends in the Histamine Test Kit market?
Emerging trends include advancements in rapid testing technologies and the increasing popularity of fermented foods, which require histamine testing to ensure safety for consumers
0 notes
Text
Microfluidic Devices: Shaping the Future of Lab-on-a-Chip Technology
The global microfluidic devices market is witnessing sustained growth as technological innovations, rising healthcare demands, and the shift toward miniaturized, portable diagnostic solutions continue to drive adoption. Microfluidics—the manipulation of fluids at the sub-millimeter scale—is rapidly transforming the landscape of healthcare, biotechnology, and pharmaceuticals, with applications ranging from lab-on-a-chip devices to high-throughput drug screening platforms.
Industry stakeholders across diagnostics, life sciences, and drug delivery sectors are increasingly investing in microfluidic solutions to enhance sensitivity, reduce reagent consumption, and streamline complex biological workflows. As microfluidic technology evolves, it is becoming a core enabler of next-generation medical diagnostics and personalized therapies.

Technological Advancements Accelerate Adoption
One of the central growth engines of the microfluidic devices market is the rapid advancement in microfabrication techniques. Innovations in soft lithography, 3D printing, and injection molding have enabled the design and production of microfluidic devices with improved precision, scalability, and cost efficiency. These advancements have lowered the entry barrier for new players and facilitated the production of complex, integrated systems for diverse biomedical applications.
Moreover, integration with electronics, sensors, and wireless communication modules is making microfluidic systems more intelligent and versatile. The convergence of microfluidics with fields such as nanotechnology, artificial intelligence, and biosensing has opened up new possibilities in disease detection, drug testing, and therapeutic monitoring.
Rising Demand for Point-of-Care and Home-Based Testing
The post-pandemic shift in diagnostic practices has heightened interest in decentralized and rapid testing. Microfluidic devices are at the forefront of this movement due to their capability to perform accurate and fast assays using minimal sample volumes. Point-of-care testing (POCT) applications—such as infectious disease screening, glucose monitoring, and cardiovascular risk assessment—are increasingly relying on microfluidic platforms for efficiency and portability.
Home-based diagnostic tools powered by microfluidics are gaining prominence, particularly in chronic disease management and preventive healthcare. The scalability and ease of integration into user-friendly formats such as lateral flow assays and wearable sensors are bolstering the adoption of microfluidic technology among both clinicians and consumers.
Expansion in Life Sciences and Drug Development
Beyond diagnostics, microfluidic devices are playing a pivotal role in revolutionizing the drug discovery and development process. Their ability to simulate in vivo environments on a microscale makes them ideal for organ-on-chip, cell culture, and high-throughput screening applications. Pharmaceutical companies are increasingly turning to microfluidic platforms to reduce lead times and improve the accuracy of preclinical testing.
Lab-on-a-chip systems, in particular, are enabling researchers to conduct complex biological and chemical analyses on a single, compact platform. This reduces reagent consumption and speeds up data generation, supporting faster decision-making in research and development pipelines.
Additionally, microfluidic devices are contributing to the growing field of precision medicine by facilitating real-time monitoring and enabling personalized therapeutic interventions based on individual biological responses.
Material Innovation and Manufacturing Trends
The choice of materials in microfluidic device fabrication has a significant impact on functionality, cost, and scalability. While traditional materials like glass and silicon have offered excellent performance in early-stage development, polymers such as polydimethylsiloxane (PDMS), polymethyl methacrylate (PMMA), and cyclic olefin copolymers (COC) are gaining popularity due to their cost-effectiveness, ease of prototyping, and biocompatibility.
The shift toward disposable and low-cost microfluidic devices is influencing material innovation. Manufacturers are focusing on developing hybrid devices that combine the benefits of different materials to optimize performance and manufacturability. Automated production techniques and roll-to-roll manufacturing are being explored to meet the increasing demand and facilitate large-scale deployment.
Regulatory Landscape and Standardization Efforts
The expanding applications of microfluidic devices in clinical and research settings necessitate stringent regulatory oversight and robust quality control. Regulatory authorities across key regions are increasingly developing frameworks for evaluating the safety, effectiveness, and consistency of microfluidic-based diagnostic and therapeutic devices.
Standardization of microfluidic components and interfaces is emerging as a critical focus area, especially in the context of interoperability and modularity. Industry bodies and consortiums are actively working toward establishing guidelines that can streamline regulatory approvals and facilitate wider market adoption.
Efforts toward harmonizing international standards are also helping manufacturers and developers navigate complex global regulatory environments more efficiently.
Competitive Landscape and Strategic Collaborations
The microfluidic devices market is characterized by a mix of established technology providers, innovative startups, and academic spin-offs. Key players are actively pursuing strategic collaborations, joint ventures, and acquisitions to expand their technological capabilities and market reach.
Partnerships between diagnostic companies and microfluidics developers are accelerating the commercialization of integrated platforms for disease detection and monitoring. Additionally, academic-industry collaborations are playing a crucial role in advancing research and translating innovations into market-ready solutions.
Intellectual property rights, including patents on design, materials, and fabrication techniques, remain a key area of competition, as companies strive to secure a technological edge in this rapidly evolving market.
Regional Insights and Emerging Markets
North America continues to lead the microfluidic devices market, supported by a strong ecosystem of medical device companies, advanced research institutions, and proactive regulatory frameworks. The region benefits from robust funding for biomedical innovation and a high adoption rate of point-of-care and personalized diagnostic technologies.
Europe follows closely, with increasing investments in healthcare infrastructure, research collaborations, and government-led initiatives promoting innovation in life sciences. Countries such as Germany, the UK, and France are notable hubs for microfluidic research and development.
Asia-Pacific is emerging as a significant growth region, driven by the expansion of healthcare access, rising demand for affordable diagnostics, and government incentives for medical technology innovation. Countries like China, India, South Korea, and Japan are witnessing increased activity in microfluidic research and commercialization, with a focus on addressing regional healthcare challenges.
Latin America and the Middle East & Africa are also gradually entering the market landscape, aided by improving healthcare infrastructure and growing awareness of point-of-care testing benefits.
Challenges and Outlook
Despite the promising outlook, the microfluidic devices market faces several challenges, including issues related to device standardization, reproducibility, and mass manufacturing. Ensuring biocompatibility and robustness across diverse applications remains a technical hurdle, especially in resource-limited settings.
Another concern is the integration of microfluidic systems with existing diagnostic workflows and electronic health records, which requires interoperability and data standardization. Addressing these challenges will be essential for broader adoption and sustained market penetration.
Looking ahead, the market is poised for continued expansion, driven by technological convergence, expanding application areas, and growing healthcare needs. The future will likely see microfluidic devices becoming more integrated, multifunctional, and patient-centric, enabling a new era of connected, data-driven healthcare.
Conclusion
Microfluidic devices are transforming the healthcare and life sciences sectors by enabling rapid, precise, and cost-effective biological analysis. As innovation continues to bridge the gap between complex lab-based protocols and real-world clinical needs, microfluidics is well-positioned to play a central role in the future of diagnostics, therapeutics, and personalized medicine. Stakeholders across the value chain—from device manufacturers to healthcare providers—are expected to benefit from the expanding capabilities and applications of this versatile technology.
0 notes
Text

Point of Care Testing: Market Size, Trend, Outlook by Products (Glucose Monitoring, Cardio-Metabolic, Infectious Disease Testers, etc.) Platforms (Immunoassay, Lateral Flow Assay, Microfluidics, Dipsticks, etc.) End Users, Regions, Major Players – Global Forecast to 2030
0 notes
Text
Self-Testing Market to Soar: $8.2B in 2023 to $18.5B by 2033 with 8.4% CAGR
Self-Testing Market is revolutionizing the way individuals manage their health, offering convenient, private, and accessible diagnostic solutions without requiring professional medical assistance. From home testing kits for diabetes and pregnancy to advanced genetic and infectious disease tests, this market reflects the growing demand for personalized healthcare.
To Request Sample Report: https://www.globalinsightservices.com/request-sample/?id=GIS26806 &utm_source=SnehaPatil&utm_medium=Article
Key Market Drivers 🌟
Technological Advancements: Innovations in molecular diagnostics, biosensors, and smartphone-connected devices enhance accuracy and ease of use.
Increased Health Awareness: Rising focus on preventive care and early diagnosis drives adoption.
Demand for Privacy: Home-based testing provides a discreet and comfortable alternative for individuals.
Market Highlights
Leading Segments:
Glucose Monitoring Kits: Capture 45% market share, driven by the growing prevalence of diabetes and the need for continuous health monitoring.
Pregnancy & Fertility Test Kits: Account for 30% market share, reflecting the rising importance of reproductive health management.
Infectious Disease Test Kits: Hold 25% market share, propelled by post-pandemic health consciousness and demand for rapid diagnostics.
Regional Insights:
North America: Leads the market with a proactive approach to personal health management and advanced healthcare infrastructure.
Europe: Benefits from strong government support and widespread adoption of self-testing kits.
Asia-Pacific: Exhibits rapid growth due to increased healthcare spending, a growing middle class, and rising awareness of self-diagnosis.
Market Segmentation
By Type: Rapid Tests, Laboratory Tests, Point-of-Care Tests, Home Tests, Self-Collection Kits, Digital Tests.
By Product: Blood Glucose Monitors, Pregnancy Tests, COVID-19 Tests, STD Tests, Allergy Tests, Genetic Tests, Cholesterol Tests.
By Services: Consultation, Diagnostic, Monitoring, Data Analysis Services.
By Technology: Lateral Flow Assays, Immunoassays, Molecular Diagnostics, Biosensors, Microfluidics.
By Application: Diabetes Management, Infectious Disease Detection, Cardiovascular Health, Fertility & Pregnancy, Genetic Screening, Cancer Screening.
By Device: Handheld Devices, Wearable Devices, Smartphone-Connected Devices.
By End User: Individuals, Healthcare Providers, Diagnostic Laboratories, Research Institutes.
By Functionality: Single-use, Reusable, Connected, Standalone.
By Mode: Online Platforms, Retail Stores, Pharmacies.
Market Outlook 🚀
2023 Volume: Approximately 300 million units, projected to grow as innovations in self-testing continue to improve accessibility and affordability.
Key Players: Roche Diagnostics, Abbott Laboratories, Everlywell, and LetsGetChecked are leading the market by expanding their product portfolios and integrating AI-driven analysis for accurate diagnostics.
#SelfTesting #HealthTech #GlucoseMonitoring #PregnancyTests #InfectiousDiseaseTests #PersonalizedHealthcare #RapidDiagnostics #MolecularDiagnostics #HealthInnovation #PreventiveCare #ConnectedHealth #HomeDiagnostics #WearableTech #DigitalHealth #FutureOfHealthcare
0 notes
Text
Point of Care Diagnostics Market worth $77.8 billion by 2028
The global point-of-care diagnostics market, valued at $49.7 billion in 2023, is projected to reach $77.8 billion by 2028, growing at a CAGR of 9.4%. Key drivers include the rising prevalence of infectious diseases like influenza, HIV, and tuberculosis, supportive government policies, and the shift towards healthcare decentralization. Challenges include pricing pressures due to reimbursement cuts and stringent regulatory approvals. Significant growth opportunities exist in emerging markets like China, India, Brazil, and Mexico. In 2022, glucose monitoring products held a significant market share, with lateral flow assays leading by platform. Major players include Abbott Laboratories, F. Hoffman-La Roche Ltd., BD, Danaher Corporation, and Siemens Healthineers. Recent developments feature FDA approvals for new diagnostic panels by bioMérieux and BD, and a partnership between Thermo Fisher Scientific and Project HOPE to expand HIV testing in Sub-Saharan Africa.

Download an Illustrative overview:
Browse in-depth TOC on "Point of Care Diagnostics Market"
191 - Tables
51 - Figures
322 – Pages
The Lateral Flow Assays segment, by platform, is expected to register the largest market share of the global point of care diagnostics industry in 2022.
Based on platform, the point-of-care diagnostics market is segmented into lateral flow assays, immunoassays, microfluidics, dipsticks, and molecular diagnostics. The lateral flow assays segment dominated this market with a share of in 2022.Lateral flow assays are simple in usage, relatively inexpensive, making it more accessible for patients and healthcare provider, LFA technology does not require trained person to operate, allowing them to be used in various healthcare settings, from clinics and pharmacies to remote arears, promoting healthcare decentralization. All these factors are contributing towards the dominance of lateral flow technology in point of care diagnostics market.
The OTC testing product segment, by mode of purchase, to dominate the global point of care diagnostics industry in 2022.
On the basis of mode of purchase OTC testing products segment accounted the largest market share in 2022 attributed to the increasing adoption of OTC products owing to its ease of usage and convenience. The presence of diverse range of OTC point of care devices for diseases screening, personal health monitoring and various other conditions further contribute to the rising demand for OTC PoC testing devices among consumers.
North America to account for the largest share of point of care diagnostics industry during the forecast period.
North America is the expected to be the largest regional market for point of care diagnostics during the forecast period. The presence of a well-established healthcare system, rapid adoption of advanced point of care testing products, increase in the availability of medical reimbursement, favourable government support for the novel product development, and higher user awareness about the presence of point of care testing products are anticipated to support the market growth in the region
Request Sample Pages:
Point of Care Diagnostics Market Dynamics:
Drivers:
Rising incidence of infectious diseases
Increasing prevalence of target conditions
Favorable government initiatives for POC testing
Rising number of CLIA-waived POC tests
Restraints:
Pricing pressure on POC manufacturers
Stringent regulatory approval process for product commercialization
Opportunities:
High growth potential of emerging markets
Decentralization of healthcare
Innovative product development
Challenge:
Inadequate standardization with centralized lab methods
Limited awareness in emerging markets
Premium pricing of novel platforms
Key Market Players of Point of Care Diagnostics Industry:
As of 2022, the point of care diagnostics market was dominated by Abbott Laboratories (US), F. Hoffmann-La Roche Ltd. (Switzerland), Siemens Healthineers (Germany), Danaher Corporation (US), and Becton, Dickinson and Company (US)
Point of Care Diagnostics Market - Key Benefits of Buying the Report:
The report will enable established firms as well as entrants/smaller firms to gauge the pulse of the market, which, in turn, would help them to garner a larger market share. Firms purchasing the report could use one or a combination of the below-mentioned strategies for strengthening their market presence.
This report provides insights on the following pointers:
Analysis of Key divers (rising prevalence of infectious and chronic disease, growing adoption of self-testing kits, helathacre decentralization, rising number of CLIA-wavier Poc Tests), restraints (rising pricing pressure on PoC manufactures, stringent regulatory approval procedures for new PoC devices), Opportunities (emerging markets, emerging technologies, such s microfluidics) , Challenge (Training & education in low resource countries)
Market Penetration: Comprehensive information on the product portfolios offered by the top players in the point-of-care diagnostics market
Product Development/Innovation: Detailed insights on the upcoming trends, R&D activities, and product launches in the point-of-care diagnostics market
Market Development: Comprehensive information on lucrative emerging regions
Market Diversification: Exhaustive information about new products, growing geographies, and recent developments in the point-of-care diagnostics market
Competitive Assessment: In-depth assessment of market segments, growth strategies, revenue analysis, and products of the leading market players.
Related Links:
1 note
·
View note
Text
The Rising Pace of the Diagnostic Testing of STDs Market Driven by Increasing Disease Prevalence Owing to Changing Sexual Behaviors

Diagnostic testing of STDs involves screening and diagnostic testing procedures that allow for the detection of sexually transmitted infections in asymptomatic individuals and confirmation of STDs in symptomatic patients. Common STDs that require diagnostic testing include Chlamydia, Gonorrhea, HIV/AIDS, Syphilis, Hepatitis, Herpes, HPV and Trichomoniasis. Effective testing helps identify infections early and allow timely treatment, reduce transmission and curb rising STD cases. The global diagnostic testing of STDs market is estimated to be valued at US$ 10.4 Bn in 2024 and is expected to exhibit a CAGR of 9.6% over the forecast period 2023 to 2030. Key Takeaways Key players operating in the diagnostic testing of STDs market are Vela Diagnostics USA Inc., Roche Holdings AG, Alere, Inc., Becton Dickinson & Company, bioMerieux, Danaher Corporation (Beckman Coulter), Hologic, Inc., binx health, Chembio Diagnostics, Pinpoint Science Inc., and bioLytical Laboratories. The major players are focusing on launching novel diagnostic products and services. For instance, in 2023, Vela Diagnostics launched an automated real-time PCR system for rapid and sensitive STD detection. The global diagnostic testing market is driven by the growing prevalence of STDs owing to rising high-risk sexual behaviors worldwide. According to WHO, every day more than 1 million STDs are acquired globally. This massive disease burden generates significant demand for effective diagnostic solutions for screening, detection, treatment monitoring and epidemiological surveillance. Rapid technological advancements are expanding diagnostic capabilities. Traditionally dependent on cultures and nucleic acid amplification tests (NAATs), the market is witnessing the adoption of novel platform technologies like CRISPR, microfluidics, and lateral flow assays for faster, integrated, multiplex and point-of-care testing. Industry players are developing smartphone-enabled testing devices and integrated diagnostic platforms to decentralize testing and improve access. Market Trends Growing preference for non-invasive diagnostic techniques: The trend towards non/minimally invasive testing methods is gaining traction. Technologies enabling self-sampling, sample stabilization and non-invasive molecular detection methods will improve patient compliance and uptake of routine screening programs. Increased automation and multiplexing of tests: STDs often require simultaneous detection of multiple pathogens. Industry is developing high-throughput, automated platforms for multiplex detection of STD panels from a single sample to streamline testing and reduce turnaround times. Market Opportunities Rising demand in low and middle-income countries: Most STDs disproportionately affect the developing world due to underdeveloped healthcare infrastructure and lack of awareness. Partnerships with governments, donor organizations can boost diagnostic accessibility in these regions. Integration of digital technologies: Leveraging telehealth, AI, blockchain and digital health records present opportunities to decentralize testing, strengthen surveillance and improve real-time clinical decision making in STD management. Impact of COVID-19 on Diagnostic Testing of STDs Market COVID-19 has significantly impacted the growth of the diagnostic testing of STDs market. During the initial lockdown phase, when people were confined to their homes, testing rates dropped dramatically as screening and testing centers were temporarily closed. Physical distancing norms also reduced the number of sexual partners, thereby lowering the risk of infections. This reduced the demand for STD diagnostic testing. However, as lockdowns eased, testing rates started increasing again.
#Diagnostic Testing of STDs Market Growth#Diagnostic Testing of STDs Market Trend#Diagnostic Testing of STDs Market Share
0 notes
Text
What are the primary components of a pathogen detection kit?
The primary components of a pathogen detection kit typically include sampling tools (such as swabs or collection tubes), reagents (including buffers, enzymes, and primers), and detection platforms (such as PCR machines or lateral flow assays). These kits may also contain positive and negative controls to validate test results. Additionally, they often come with instructions for sample collection and processing, as well as interpretation of results. Some advanced kits may incorporate specialized equipment or technologies for automated processing and analysis. Overall, these components work together to efficiently and accurately detect the presence of pathogens in various samples.
0 notes
Text
Data Bridge Market Research analyzes that the global point of care diagnostics market which was USD 40,280.13 million in 2023, is expected to reach USD 71,424.23 million by 2031, and is expected to undergo a CAGR of 9.1% during the forecast period of 2024 to 2031. “Lateral flow assays” dominate the platform segment of the market due to rising demand for point of care diagnostics. In addition to the insights on market scenarios such as market value, growth rate, segmentation, geographical coverage, and major players, the market reports curated by the Data Bridge Market Research also include depth expert analysis, patient epidemiology, pipeline analysis, pricing analysis, and regulatory framework.
1 note
·
View note
Text
Point-Of-Care-Testing Market Size, Share, Growth, Trends, Demand and Opportunity Analysis
Executive Summary Point-Of-Care-Testing (POCT) Market :
Point-Of-Care-Testing (POCT) Market report is offered to the business with a complete overview of the market, covering various aspects such as product definition, market segmentation based on various parameters, and the customary vendor landscape. All statistical and numerical information given in the report is symbolized with the help of graphs and charts which facilitates the understanding of facts and figures. All the data and information collected for research and analysis is denoted in the form of graphs, charts or tables for the sensible understanding of users. The Point-Of-Care-Testing (POCT) Market report defines CAGR value fluctuation during the forecast period of 2019 - 2025 for the market.
This Point-Of-Care-Testing (POCT) Market report is composed of myriad of factors that have an influence on the market and include industry insight and critical success factors (CSFs), market segmentation and value chain analysis, industry dynamics, market drivers, market restraints, key opportunities, technology and application outlook, country-level and regional analysis, competitive landscape, company market share analysis and key company profiles. This global Point-Of-Care-Testing (POCT) Market business report is very reliable as all the data and the information regarding the industry is collected via genuine sources such as websites, journals, annual reports of the companies, and magazines.
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive Point-Of-Care-Testing (POCT) Market report. Download Full Report: https://www.databridgemarketresearch.com/reports/point-care-testing-poct-market
Point-Of-Care-Testing (POCT) Market Overview
**Segments**
- **By Product (Blood Glucose Testing Kits, Cardiometabolic Monitoring Kits, Infectious Disease Testing Kits, Cholesterol Testing Kits, Pregnancy and Fertility Testing Kits, Tumor/Cancer Markers, Urinalysis Testing Kits, Others)** - **By Platform (Lateral Flow Assays, Dipsticks, Microfluidics, Molecular Diagnostics, Immunoassays, Agglutination Assays)** - **By Mode of Purchase (Prescription-Based Testing, OTC Testing)** - **By End-User (Hospitals, Home Care Settings, Clinics, Diagnostic Laboratories, Assisted Living Healthcare Facilities, Others)**
**Market Players**
- **Abbott Laboratories** - **F. Hoffmann-La Roche Ltd** - **Siemens Healthineers AG** - **Danaher** - **BD** - **Johnson & Johnson Services, Inc.** - **Bio-Rad Laboratories, Inc.** - **Thermo Fisher Scientific Inc.** - **Sysmex Corporation** - **bioMérieux SA**
Key market players in the global Point-Of-Care-Testing (POCT) market include Abbott Laboratories, F. Hoffmann-La Roche Ltd, Siemens Healthineers AG, Danaher, BD, Johnson & Johnson Services, Inc., Bio-Rad Laboratories, Inc., Thermo Fisher Scientific Inc., Sysmex Corporation, and bioMérieux SA. These companies are actively involved in product development, strategic collaborations, acquisitions, and regional expansion to enhance their market presence and cater to the growing demand for POCT products worldwide.
The global Point-Of-Care-Testing (POCT) market is witnessing significant growth driven by factors such as the increasing prevalence of various chronic and infectious diseases, the rising demand for rapid and accurate diagnostic tests, and the shift towards personalized healthcare. One of the key trends shaping the POCT market is the development of technologically advanced testing kits and platforms that offer quick results and require minimal expertise to operate. Companies like Abbott Laboratories, Roche, and Siemens Healthineers are at the forefront of innovation in this space, constantly introducing new products and improving existing ones to meet the evolving needs of healthcare providers and patients.
Another important factor contributing to the growth of the POCT market is the expanding adoption of decentralized diagnostic testing, especially in settings such as home care, clinics, and assisted living healthcare facilities. Point-of-care testing offers several advantages over traditional laboratory testing, including faster turnaround times, reduced costs, and improved patient outcomes. As a result, healthcare providers are increasingly integrating POCT into their diagnostic protocols to streamline care delivery and enhance treatment decisions.
Moreover, the COVID-19 pandemic has further accelerated the demand for POCT solutions, particularly for rapid antigen and antibody testing to detect the virus quickly and efficiently. This unprecedented global health crisis has underscored the importance of accessible and timely diagnostic testing, driving investments in POCT infrastructure and technology development. As a result, market players are focusing on expanding their product portfolios to include COVID-19 testing kits and platforms, consolidating their position in the market and contributing to overall market growth.
In terms of competition, the global POCT market is highly competitive, with key players leveraging strategies such as partnerships, acquisitions, and product launches to gain a competitive edge. Companies are also investing in research and development activities to innovate and introduce new technologies that offer higher sensitivity, specificity, and accuracy in diagnostic testing. Market consolidation through mergers and acquisitions is another prevalent trend, with larger companies acquiring smaller players to expand their geographic presence and market share.
Looking ahead, the global Point-Of-Care-Testing market is poised for robust growth, driven by ongoing technological advancements, the increasing prevalence of chronic diseases, and the growing emphasis on personalized medicine. As healthcare systems worldwide continue to prioritize rapid and accurate diagnostic testing, the demand for POCT solutions is expected to further surge, offering significant opportunities for market players to capitalize on this evolving landscape and shape the future of healthcare delivery.The Point-Of-Care-Testing (POCT) market is witnessing dynamic growth propelled by the increasing prevalence of chronic and infectious diseases worldwide. Key market players such as Abbott Laboratories, Roche, and Siemens Healthineers are driving innovation in POCT by developing advanced testing kits and platforms that offer rapid and accurate diagnostic results. This technological evolution is crucial in meeting the demand for efficient diagnostic solutions that require minimal expertise to operate, catering to the evolving needs of healthcare providers and patients.
The adoption of decentralized diagnostic testing is expanding across various healthcare settings, including home care, clinics, and assisted living facilities, due to the advantages offered by POCT such as quicker turnaround times, cost-effectiveness, and improved patient outcomes. Healthcare providers are incorporating POCT into their diagnostic protocols to streamline care delivery and make informed treatment decisions, contributing to the market growth.
The COVID-19 pandemic has further accelerated the demand for POCT solutions, particularly for rapid antigen and antibody testing to detect the virus swiftly. This global health crisis has highlighted the significance of accessible and timely diagnostic testing, leading to increased investments in POCT infrastructure and technology development. Market players are diversifying their product portfolios to include COVID-19 testing solutions, positioning themselves strongly in the market and driving overall growth.
Competition in the global POCT market is intense, with companies employing strategies like partnerships, acquisitions, and product launches to gain a competitive edge. Research and development activities are pivotal as companies strive to introduce innovative technologies that offer higher diagnostic accuracy. Market consolidation through mergers and acquisitions remains prevalent, with larger firms acquiring smaller players to enhance their market presence and expand their offerings.
Looking ahead, the POCT market is poised for substantial growth driven by ongoing technological advancements, the rising burden of chronic diseases, and the shift towards personalized medicine. As healthcare systems prioritize rapid and accurate diagnostic testing, the demand for POCT solutions is expected to surge further, presenting significant opportunities for market players to leverage this evolving landscape and shape the future of healthcare delivery.
The Point-Of-Care-Testing (POCT) Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now: https://www.databridgemarketresearch.com/reports/point-care-testing-poct-market/companies
DBMR Nucleus: Powering Insights, Strategy & Growth
DBMR Nucleus is a dynamic, AI-powered business intelligence platform designed to revolutionize the way organizations access and interpret market data. Developed by Data Bridge Market Research, Nucleus integrates cutting-edge analytics with intuitive dashboards to deliver real-time insights across industries. From tracking market trends and competitive landscapes to uncovering growth opportunities, the platform enables strategic decision-making backed by data-driven evidence. Whether you're a startup or an enterprise, DBMR Nucleus equips you with the tools to stay ahead of the curve and fuel long-term success.
Key Pointers Covered in the Point-Of-Care-Testing (POCT) Market Industry Trends and Forecast
Point-Of-Care-Testing (POCT) Market Size
Point-Of-Care-Testing (POCT) Market New Sales Volumes
Point-Of-Care-Testing (POCT) Market Replacement Sales Volumes
Point-Of-Care-Testing (POCT) Market By Brands
Point-Of-Care-Testing (POCT) Market Procedure Volumes
Point-Of-Care-Testing (POCT) Market Product Price Analysis
Point-Of-Care-Testing (POCT) Market Regulatory Framework and Changes
Point-Of-Care-Testing (POCT) Market Shares in Different Regions
Recent Developments for Market Competitors
Point-Of-Care-Testing (POCT) Market Upcoming Applications
Point-Of-Care-Testing (POCT) Market Innovators Study
Browse More Reports:
Global Phase Transfer Catalyst Market Global Pharmacy Inventory Management Software Solutions and Cabinets Market Global Pet Beds Market Global Peptide and Anticoagulant Drugs Market Global People Counting System Market Global Parasitology Identification Market Global Paraquat Market Global Parallel Robots Market Global Pandan Tea Market Global Palmoplantar Pustulosis Market Global Pain Relief Therapy Market Global OxoAlcohol Market Global Over the Top Content Market Global Oscillating Positive Expiratory Pressure (OPEP) Market Global Organo-modified Bentonite Market Global Organic Ruminant Feed Market Global Organic Beverages Market Global Optical Switches Market Global Optical Instrument and Lens Market Global Optical Character Recognition Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us: Data Bridge Market Research US: +1 614 591 3140 UK: +44 845 154 9652 APAC : +653 1251 975 Email:- [email protected]
Tag
Point-Of-Care-Testing (POCT) Market Size, Point-Of-Care-Testing (POCT) Market Share, Point-Of-Care-Testing (POCT) Market Trend, Point-Of-Care-Testing (POCT) Market Analysis, Point-Of-Care-Testing (POCT) Market Report, Point-Of-Care-Testing (POCT) Market Growth, Latest Developments in Point-Of-Care-Testing (POCT) Market, Point-Of-Care-Testing (POCT) Market Industry Analysis, Point-Of-Care-Testing (POCT) Market Key Player, Point-Of-Care-Testing (POCT) Market Demand Analysis
0 notes
Quote
Although low-dose computed tomography screening improves lung cancer survival in at-risk groups, inequality remains in lung cancer diagnosis due to limited access to and high costs of medical imaging infrastructure. We designed a needleless and imaging-free platform, termed PATROL (point-of-care aerosolizable nanosensors with tumor-responsive oligonucleotide barcodes), to reduce resource disparities for early detection of lung cancer. PATROL formulates a set of DNA-barcoded, activity-based nanosensors (ABNs) into an inhalable format. Lung cancer–associated proteases selectively cleave the ABNs, releasing synthetic DNA reporters that are eventually excreted via the urine. The urinary signatures of barcoded nanosensors are quantified within 20 min at room temperature using a multiplexable paper-based lateral flow assay. PATROL detects early-stage tumors in an autochthonous lung adenocarcinoma mouse model with high sensitivity and specificity. Tailoring the library of ABNs may enable not only the modular PATROL platform to lower the resource threshold for lung cancer early detection tools but also the rapid detection of chronic pulmonary disorders and infections.
Inhalable point-of-care urinary diagnostic platform | Science Advances
0 notes
Text
Biosensors, Vol. 13, Pages 988: Detection of Reverse Transcriptase LAMP-Amplified Nucleic Acid from Oropharyngeal Viral Swab Samples Using Biotinylated DNA Probes through a Lateral Flow Assay
This study focuses on three key aspects: (a) crude throat swab samples in a viral transport medium (VTM) as templates for RT-LAMP reactions; (b) a biotinylated DNA probe with enhanced specificity for LFA readouts; and (c) a digital semi-quantification of LFA readouts. Throat swab samples from SARS-CoV-2 positive and negative patients were used in their crude (no cleaning or pre-treatment) forms for the RT-LAMP reaction. The samples were heat-inactivated but not treated for any kind of nucleic acid extraction or purification. The RT-LAMP (20 min processing time) product was read out by an LFA approach using two labels: FITC and biotin. FITC was enzymatically incorporated into the RT-LAMP amplicon with the LF-LAMP primer, and biotin was introduced using biotinylated DNA probes, specifically for the amplicon region after RT-LAMP amplification. This assay setup with biotinylated DNA probe-based LFA readouts of the RT-LAMP amplicon was 98.11% sensitive and 96.15% specific. The LFA result was further analysed by a smartphone-based IVD device, wherein the T-line intensity was recorded. The LFA T-line intensity was then correlated with the qRT-PCR Ct value of the positive swab samples. A digital semi-quantification of RT-LAMP-LFA was reported with a correlation coefficient of R2 = 0.702. The overall RT-LAMP-LFA assay time was recorded to be 35 min with a LoD of three #RNA copies/µL (Ct-33). With these three advancements, the nucleic acid testing-point of care technique (NAT-POCT) is exemplified as a versatile biosensor platform with great potential and applicability for the detection of pathogens without the need for sample storage, transportation, or pre-processing. https://www.mdpi.com/2079-6374/13/11/988?utm_source=dlvr.it&utm_medium=tumblr
0 notes
Text
Point of Care Testing: Market Size, Trend, Outlook by Products (Glucose Monitoring, Cardio-Metabolic, Infectious Disease Testers, etc.) Platforms (Immunoassay, Lateral Flow Assay, Microfluidics, Dipsticks, etc.) End Users, Regions, Major Players – Global Forecast to 2030
0 notes
Text
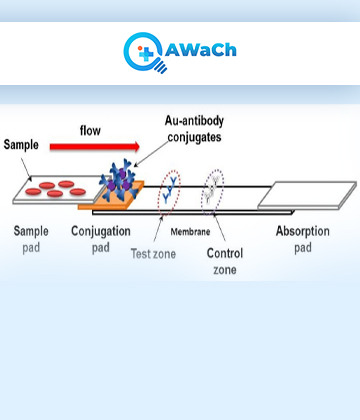
Lateral Flow Assay
A lateral flow assay (LFA), also known as a lateral flow test or rapid diagnostic tests (RDTs), is a simple and rapid diagnostic tool used to detect the presence or absence of a specific substance or analyte in a sample.
Lateral flow assay offer several advantages, including rapid results (usually within minutes), simplicity of use (no complex equipment or specialized training required), and suitability for point-of-care testing. However, it's important to note that LFAs may have limitations in terms of sensitivity and specificity compared to more complex laboratory-based diagnostic tests. We at QAWaCh Bio have developed Quantitative LFA-based testing that uses a combination of a LFA test card and a smartphone application to give results within 10-15 minutes at the bed-side of the patient. Our quantitative LFA platform, called Q-Plat, is made ultra-sensitive by incorporating indigenously made detection molecules in our lab called Q-probe, that greatly enhances the sensitivity of all our assays.
0 notes