#Polymerase Chain Reaction (PCR) Market Research
Text
Latin America Molecular Diagnostics Market: Analyzing Growth Drivers and Challenges
Meticulous Research®, a leading market research firm, has published a pivotal report titled, “Latin America Molecular Diagnostics Market by Offering (Reagents, Kits, Systems, Software, Services), Test Type (Lab, PoC), Technology (PCR, ISH, INAAT, Sequencing, Microarray), Application (HIV, Influenza, HPV, Oncology), End User - Forecast to 2031.”
According to this comprehensive report, the Latin America molecular diagnostics market is expected to reach an impressive valuation of $2.50 billion by 2031, exhibiting a robust compound annual growth rate (CAGR) of 6.3% from 2024 to 2031. This growth trajectory is largely fueled by several key factors: the rising geriatric population, the escalating prevalence of both communicable and non-communicable diseases, significant advancements in molecular diagnostics technologies, and increased healthcare expenditures.
Download Sample of Report @ https://www.meticulousresearch.com/download-sample-report/cp_id=5759?utm_source=article&utm_medium=social&utm_campaign=product&utm_content=24-09-2024
Factors Driving Market Growth
Aging Population: The demographic shift towards an aging population in Latin America has led to a heightened demand for effective diagnostic solutions. As people age, the risk of developing chronic diseases such as cancer and cardiovascular disorders increases, driving the need for advanced diagnostic tools.
Prevalence of Diseases: The region is witnessing an increase in both communicable diseases, such as HIV and influenza, and non-communicable diseases, including various cancers. This surge necessitates the adoption of precise and rapid diagnostic methods, propelling the molecular diagnostics market.
Technological Advancements: Continuous innovations in molecular diagnostic technologies, including Polymerase Chain Reaction (PCR), Next-Generation Sequencing (NGS), and microarrays, enhance the accuracy and speed of disease detection. These advancements are crucial for timely diagnosis and treatment, thereby increasing the uptake of molecular diagnostic tests.
Healthcare Expenditure: Rising healthcare expenditures in Latin America signify a growing commitment to improving healthcare systems. Governments and private entities are investing more in healthcare infrastructure, which includes the adoption of advanced diagnostic technologies.
Emerging Opportunities: The increasing focus on companion diagnostics and the rising trend of direct-to-consumer (DTC) testing further open up avenues for growth in the market. Emerging economies in Latin America are gradually embracing these trends, enhancing access to molecular diagnostics.
Market Challenges
While the market presents numerous opportunities, it also faces challenges that could impede growth:
Shortage of Skilled Professionals: A significant challenge is the shortage of trained professionals in molecular diagnostics. The complexity of the technologies involved necessitates a workforce with specialized skills, which is currently lacking in many parts of the region.
Regulatory Hurdles: The regulatory environment surrounding molecular diagnostics can be cumbersome. Variations in regulations across countries can lead to delays in product approval and market entry, which can be a significant barrier for companies looking to expand in this sector.
Cost Constraints: The high cost associated with molecular diagnostic tests can restrict their accessibility, particularly in low- and middle-income countries. Cost-effective solutions and increased funding for diagnostics are essential to overcome this barrier.
Segmentation Analysis
The Latin America molecular diagnostics market is segmented into various categories:
Offering:
Kits & Reagents: This segment is anticipated to dominate the market in 2024, primarily due to the wide range of diagnostic reagents available and the growing awareness regarding the importance of early disease diagnosis.
Instruments: Instruments used in molecular diagnostics, such as PCR machines and sequencers, also play a vital role in the market, offering precision and reliability in testing.
Test Type:
Laboratory Tests: The laboratory test segment is expected to capture the largest market share in 2024. The abundance of laboratory tests available in healthcare facilities and their reliability make them the preferred choice for diagnostics.
Point-of-Care (POC) Tests: POC testing is gaining traction due to its ability to provide rapid results. This segment is likely to witness significant growth, especially in emergency care and rural settings.
Technology:
Polymerase Chain Reaction (PCR): This technology is projected to lead the market due to its versatility and efficiency in detecting various pathogens and genetic disorders. PCR's ability to provide accurate results rapidly is invaluable in clinical settings.
Next-Generation Sequencing (NGS): NGS is also emerging as a powerful tool for molecular diagnostics, particularly in oncology and genetic testing. Its capability to analyze multiple genes simultaneously is driving its adoption.
Application:
Infectious Diseases: This application segment is expected to dominate the market in 2024. The increasing incidence of infectious diseases, coupled with the funding for developing new diagnostic tools, is enhancing the market's growth.
Oncology: The demand for early cancer detection through molecular diagnostics is also increasing, with significant investments being made in developing targeted therapies and companion diagnostics.
End User:
Hospitals & Clinics: This segment is expected to account for the largest market share in 2024 due to the increasing number of hospitalizations requiring molecular diagnostics. The proliferation of hospitals and clinics in emerging economies, such as Brazil, Chile, and Colombia, is a driving factor.
Diagnostic Laboratories: These facilities are critical in the molecular diagnostics landscape, providing specialized services and driving technological advancements.
Key Players in the Market
Several key players are shaping the landscape of the Latin America molecular diagnostics market, including:
Bio-Manguinhos (Brazil)
F. Hoffmann-La Roche Ltd. (Switzerland)
Thermo Fisher Scientific Inc. (U.S.)
Hologic, Inc. (U.S.)
Illumina, Inc. (U.S.)
OmicronLab (Mexico)
QIAGEN N.V. (Netherlands)
Danaher Corporation (U.S.)
Abbott Laboratories (U.S.)
Agilent Technologies, Inc. (U.S.)
These organizations are actively engaged in research and development, aiming to introduce innovative products and solutions that cater to the evolving needs of healthcare providers.
Future Outlook
The future of the Latin America molecular diagnostics market appears promising. The integration of advanced technologies, coupled with a focus on patient-centric care, is likely to drive growth. As healthcare systems continue to evolve and adapt, the demand for molecular diagnostics will increase, paving the way for more innovative solutions.
Stakeholders in the market must navigate challenges such as regulatory barriers and workforce shortages. Strategic partnerships, investments in training programs, and collaborations with local governments can help mitigate these challenges.
Moreover, as consumer awareness grows regarding the importance of early detection and personalized medicine, the acceptance and utilization of molecular diagnostics are expected to rise. The increasing role of telehealth and remote monitoring can also enhance access to molecular diagnostic tests, particularly in underserved areas.
Conclusion
In conclusion, the Latin America molecular diagnostics market is poised for substantial growth driven by various factors, including technological advancements, increasing disease prevalence, and rising healthcare expenditures. While challenges remain, the opportunities for innovation and expansion are significant. With a focus on overcoming obstacles and embracing new trends, the future of molecular diagnostics in Latin America looks bright.
Contact Us:
Meticulous Research®
Email- [email protected]
Contact Sales- +1-646-781-8004
Connect with us on LinkedIn- https://www.linkedin.com/company/meticulous-research
#Latin America Molecular Diagnostics Market#Molecular Diagnostics#Molecular Test#Molecular Laboratory#PCR#Molecular Testing#MDx
0 notes
Text
Automated Liquid Handling Technologies Market 2024-2032 Analysed by Business Growth, Development Factors, Applications, and Future Prospects
The global automated liquid handling technologies market is poised for substantial expansion, with the market valued at USD 2.11 billion in 2023. It is projected to grow at a compound annual growth rate (CAGR) of 11.18% during the forecast period of 2024 to 2032, reaching a market size of USD 5.24 billion by 2032. This surge is driven by the increasing demand for accuracy, efficiency, and automation in laboratory processes across various industries, particularly in pharmaceuticals, biotechnology, and clinical research.
Automated liquid handling (ALH) systems are advanced robotic tools designed to perform precise liquid transfers in laboratories, significantly reducing human error and increasing throughput. These technologies are instrumental in streamlining workflows for applications such as drug discovery, genomic research, clinical diagnostics, and high-throughput screening.
Key Market Drivers
Increased Focus on Laboratory Efficiency and Accuracy: As research processes become more complex and data-driven, there is a growing need for laboratories to improve efficiency while maintaining high accuracy. Automated liquid handling systems enable researchers to perform complex liquid handling tasks faster and more accurately than manual methods, thus reducing variability and improving reproducibility in experiments. This, in turn, accelerates scientific discoveries and product development timelines, making automation an attractive investment for laboratories worldwide.
Rising Demand in Pharmaceutical and Biotechnology Sectors: The pharmaceutical and biotechnology industries are experiencing a surge in demand for automated liquid handling systems due to their role in accelerating drug discovery, compound screening, and genomic studies. These industries rely on high-throughput automation to manage large sample volumes, particularly in the early stages of drug development. Automated systems allow pharmaceutical companies to process large datasets quickly, leading to faster drug candidate identification and reducing time to market.
Growth in Genomics and Proteomics Research: The expanding field of genomics and proteomics is another significant growth driver for the automated liquid handling market. As researchers explore the human genome and proteome, automated systems are becoming essential for handling complex workflows involving high sample volumes. Automated liquid handlers are used in next-generation sequencing (NGS), polymerase chain reaction (PCR), and other molecular biology techniques, helping labs to achieve higher throughput and accuracy.
Advancements in AI and Robotics: The integration of artificial intelligence (AI) and advanced robotics into automated liquid handling systems is further propelling market growth. AI-enhanced systems offer real-time monitoring, optimization of protocols, and predictive maintenance, which significantly enhance productivity and minimize downtime. The incorporation of machine learning algorithms allows these systems to adapt to complex workflows, making them indispensable in research-intensive sectors.
Access Free Sample Report: https://www.snsinsider.com/sample-request/4485
Challenges and Opportunities
While the automated liquid handling technologies market holds immense growth potential, certain challenges may impede its expansion. The high initial cost of automated systems can be a barrier for small and mid-sized laboratories. Additionally, the need for specialized training to operate and maintain these systems may limit their adoption in less experienced laboratories.
However, these challenges are being addressed as market players increasingly offer more cost-effective and user-friendly solutions. Many companies are investing in modular systems that can be tailored to specific needs, reducing upfront costs and allowing for gradual upgrades. Furthermore, the integration of cloud-based systems and remote monitoring tools enables laboratories to enhance their capabilities without significant infrastructure investments.
Regional Insights
North America currently dominates the automated liquid handling technologies market, largely due to the presence of leading pharmaceutical and biotechnology companies, as well as advanced research infrastructure. The region benefits from substantial R&D investments and early adoption of innovative laboratory automation technologies. Europe follows closely, driven by robust healthcare and life sciences sectors.
The Asia-Pacific region is expected to witness the highest growth during the forecast period, as countries like China, India, and Japan invest heavily in healthcare infrastructure, biopharmaceutical research, and laboratory automation. The increasing prevalence of diseases, growing demand for personalized medicine, and rising investments in biotechnology are major factors driving market growth in this region.
Future Outlook
As the demand for precision, reproducibility, and efficiency in laboratory workflows continues to grow, the automated liquid handling technologies market is set to witness significant advancements. From drug discovery to clinical diagnostics, the need for high-throughput, automated solutions is accelerating across industries. With a projected CAGR of 11.18% from 2024 to 2032, the market will see ongoing innovation, including AI-driven automation, modular designs, and increased integration with laboratory information management systems (LIMS).
In conclusion, the global automated liquid handling technologies market is positioned for rapid expansion, with its size expected to grow from USD 2.11 billion in 2023 to USD 5.24 billion by 2032. The continued evolution of laboratory automation technologies will shape the future of scientific research, diagnostics, and drug development, driving efficiencies and innovation across the life sciences sector.
Other Trending Reports
Pancreatic Cancer Treatment Market Size
gRNA Market Size
Patient Portal Market Size
Continuous Glucose Monitoring Market Size
0 notes
Text
Blood Group Typing Market Analysis: New Opportunities in the Healthcare Sector

The Blood Group Typing Market is expected to grow at a rapid pace over the coming years. In 2023, the market was valued at USD 1.9 billion, and by 2030, it's projected to surpass USD 3.3 billion, growing at a compound annual growth rate (CAGR) of 8.2%. This surge in market value highlights the increasing demand for blood typing in various healthcare sectors.
What is Blood Group Typing?
Blood group typing is the process of determining an individual's blood group, typically classified into A, B, AB, or O types. This crucial medical procedure ensures compatibility for blood transfusions, organ transplants, and managing pregnancies where blood group incompatibility may lead to complications. It also plays an important role in research, diagnostics, and therapeutics.
Why is the Blood Group Typing Market Growing?
Several factors contribute to the rapid growth of the blood group typing market:
Rising Demand for Blood Transfusions: The growing number of surgeries and trauma cases has driven the need for safe and compatible blood transfusions.
Advancements in Technology: With the advent of advanced testing technologies, blood typing has become quicker and more accurate.
Increasing Prevalence of Chronic Diseases: As chronic diseases like cancer and cardiovascular conditions become more common, the need for blood transfusions, organ transplants, and diagnostic tests rises.
Government Initiatives: Government health agencies across the globe are launching campaigns to improve healthcare infrastructure, which includes better access to blood banks and safe transfusion practices.
Access Full Report @ https://intentmarketresearch.com/latest-reports/blood-group-typing-market-3123.html
Key Segments of the Blood Group Typing Market
1. Based on Products
Consumables: Reagents, anti-sera, and red blood cells that are essential for blood typing procedures.
Instruments: Machines used in hospitals and laboratories for blood typing tests.
Services: Outsourcing services for hospitals and laboratories to perform blood group testing.
2. Based on Test Type
Antibody Screening: Detects antibodies in the blood that may cause immune reactions.
HLA Typing: Human leukocyte antigen typing is often used in organ transplantation to ensure compatibility.
ABO Blood Group Typing: The most common test, determining whether a person has blood type A, B, AB, or O.
Crossmatching: Ensures compatibility between donor and recipient blood before a transfusion.
3. Based on Technique
PCR-Based Testing: Polymerase chain reaction (PCR) testing is becoming popular due to its accuracy in detecting rare blood types.
Microarray Testing: A cutting-edge technique that offers detailed insights into blood group antigens.
Gel Agglutination: A traditional method where blood cells clump together to reveal their type.
4. Based on End-User
Hospitals and Clinics: The largest users of blood typing services, especially in emergency care, surgeries, and transfusions.
Blood Banks: Critical for maintaining safe blood supplies for hospitals and clinics.
Diagnostic Laboratories: Play a key role in providing accurate blood typing for various medical needs.
Research Institutes: Blood typing is essential in biomedical research and the development of new medical treatments.
Geographical Insights into the Blood Group Typing Market
North America
North America dominates the global blood group typing market, thanks to its advanced healthcare infrastructure and high demand for blood transfusions. The U.S. leads the region with significant investments in healthcare research and technological advancements.
Europe
Europe holds the second-largest market share, driven by increased demand for organ transplants and blood donations. Countries like Germany, the UK, and France are key players in the region.
Asia-Pacific
Asia-Pacific is the fastest-growing market, with a rapidly improving healthcare infrastructure, especially in countries like China, India, and Japan. Increased government initiatives and rising awareness of safe blood transfusion practices are fueling growth in this region.
Latin America and the Middle East
These regions are experiencing moderate growth, with improving healthcare systems and increased focus on safe transfusion practices. Brazil, Saudi Arabia, and the UAE are emerging as key markets.
Download Sample Report @ https://intentmarketresearch.com/request-sample/blood-group-typing-market-3123.html
Technological Advancements in Blood Group Typing
Technological innovations have dramatically improved the accuracy and speed of blood group typing. Some of the most noteworthy advancements include:
Automation: Automated blood typing machines reduce human error and streamline the testing process.
DNA-Based Testing: Genetic testing methods like PCR and next-generation sequencing (NGS) have made it easier to identify rare blood types.
Artificial Intelligence: AI-driven platforms can now analyze blood typing data more efficiently, predicting rare blood types and crossmatching results with greater precision.
Challenges in the Blood Group Typing Market
Despite its rapid growth, the blood group typing market faces several challenges:
High Cost of Advanced Technologies: While innovative testing methods improve accuracy, they also raise costs, limiting access in some regions.
Limited Awareness in Low-Income Countries: In developing countries, there is still a lack of awareness regarding the importance of safe blood transfusions.
Regulatory Hurdles: Compliance with government regulations varies by region, sometimes slowing the approval process for new technologies.
Key Players in the Blood Group Typing Market
Several companies are leading the charge in the global blood group typing market:
Bio-Rad Laboratories: Specializing in diagnostics and testing solutions.
Grifols: A global leader in plasma-derived medicines and transfusion diagnostics.
Ortho Clinical Diagnostics: A top player in immunohematology and blood typing products.
Immucor: Focuses on improving transfusion and transplantation diagnostics.
Future Outlook of the Blood Group Typing Market
The future of the blood group typing market looks promising, with continued growth expected through 2030. Some key trends include:
Increased Investment in R&D: Research into advanced blood typing methods will likely continue, focusing on automation, accuracy, and cost reduction.
Emerging Markets: Countries with developing healthcare systems are investing in better blood typing technologies, creating new growth opportunities.
Personalized Medicine: The rise of personalized treatments will likely drive demand for more precise blood typing methods to ensure compatibility in therapies like immunotherapy and gene editing.
Conclusion
The global blood group typing market is on a path of exponential growth, driven by technological advancements, rising demand for blood transfusions, and government initiatives. With the market projected to surpass USD 3.3 billion by 2030, the future looks bright for companies and healthcare providers in this field. As the industry continues to innovate, the accuracy and accessibility of blood group typing will only improve, saving more lives and ensuring safer medical procedures.
FAQs
What is the CAGR of the blood group typing market from 2024 to 2030?
The market is expected to grow at a CAGR of 8.2% during this period.
What is the importance of blood group typing?
Blood group typing ensures compatibility for blood transfusions, organ transplants, and other medical procedures, reducing the risk of complications.
Which regions are leading the blood group typing market?
North America holds the largest market share, followed by Europe and the rapidly growing Asia-Pacific region.
What are the major technologies used in blood group typing?
Key technologies include PCR-based testing, microarray testing, and gel agglutination methods.
What are the major challenges facing the blood group typing market?
Challenges include the high cost of advanced technologies, limited awareness in developing regions, and regulatory hurdles.
About Us
Intent Market Research (IMR) is dedicated to delivering distinctive market insights, focusing on the sustainable and inclusive growth of our clients. We provide in-depth market research reports and consulting services, empowering businesses to make informed, data-driven decisions.
Our market intelligence reports are grounded in factual and relevant insights across various industries, including chemicals & materials, healthcare, food & beverage, automotive & transportation, energy & power, packaging, industrial equipment, building & construction, aerospace & defense, and semiconductor & electronics, among others.
We adopt a highly collaborative approach, partnering closely with clients to drive transformative changes that benefit all stakeholders. With a strong commitment to innovation, we aim to help businesses expand, build sustainable advantages, and create meaningful, positive impacts.
Contact Us
US: +1 463-583-2713
0 notes
Text
Point of Care Diagnostics: Revolutionizing Healthcare with Real-Time Testing
The Advent of Quick and Accurate Medical Testing
Point of Care Diagnostics have emerged as a groundbreaking development in the medical field by enabling accurate testing to be done quickly and conveniently. Traditional diagnostic methods usually require samples to be sent to a centralized laboratory for analysis, which can delay vital treatment decisions by several days. However, point-of-care tests provide results within minutes using portable devices, bringing testing closer to the patient. This revolutionary approach is transforming healthcare delivery.
Rapid Testing for Better Patient Outcomes
By facilitating timely diagnosis, point-of-care testing leads to better patient outcomes. Speedy detection of conditions like infections or chronic diseases allows doctors to prescribe appropriate treatment without delay. For example, point-of-care tests are commonly used in emergency rooms to quickly identify heart attacks, strokes or life-threatening infections. Getting fast diagnostic results is crucial for such medical emergencies as it ensures patients receive the right therapy as soon as possible. The timely administration of antibiotics, anti-clotting medications or other critical treatments improves survival rates and recovery.
Patient Comfort and Convenience
Besides clinical benefits, Point of Care Diagnostics enhance patient comfort and convenience. People no longer have to wait anxiously for days to learn about their health while potentially worsening conditions go untreated. With devices that analyze samples on-site, patients get actionable results during the same clinical visit when treatment decisions are made. This spares them follow-up trips to the doctor or lab and unnecessary stress. Home testing using self-administered point-of-care kits even allows monitoring health remotely while maintaining independence. Finger-prick blood samples or urine specimens are all that's needed, eliminating difficulties obtaining specimens.
More Efficient Use of Resources
Speedy diagnostic testing optimizes use of limited healthcare resources. Quick turnaround times avoid unnecessary reliance on expensive treatments initiated just to address uncertainty in diagnoses. Point-of-care devices reduce laboratory workloads too by decentralizing testing. Moreover, decentralized testing is vital for resource-constrained settings like rural areas, refugee camps or developing countries where access to centralized labs is limited. Portable devices overcome infrastructure barriers and enable basic medical services even in remote areas. This promotes healthcare equity globally.
A Proliferation of Diagnostic Platforms
Rapid technological progress has enabled the development of varied point-of-care testing systems. Examples include paper microfluidic devices, electrochemical sensors, molecular diagnostics platforms and portable ultrasound machines integrated with imaging analysis software. Immunology-based tests detecting proteins or antibodies through lateral flow or microarray methods are commonly used for conditions like infections and cardiac markers. Molecular diagnostic platforms employ techniques like polymerase chain reaction (PCR) for swift nucleic acid amplification and analysis of viruses or genetic markers. Newer technologies like CRISPR gene editing also hold promise as a basis for point-of-care genetic testing. With ongoing research, the types of conditions examinable at the point of care continue expanding in scope and complexity.
Get more insights on Point Of Care Diagnostics
Also read related article on Gastroesophageal Reflux Disease Treatment Devices Market
For Deeper Insights, Find the Report in the Language that You want
French
German
Italian
Russian
Japanese
Chinese
Korean
Portuguese
About Author:
Money Singh is a seasoned content writer with over four years of experience in the market research sector. Her expertise spans various industries, including food and beverages, biotechnology, chemical and materials, defense and aerospace, consumer goods, etc. (https://www.linkedin.com/in/money-singh-590844163)

#Point Of Care Diagnostics#Poc Testing#Rapid Diagnostics#Bedside Testing#Portable Diagnostics#PointOfCare Testing#Poc Devices#NearPatient Testing#Decentralized Diagnostics
0 notes
Text
Digital PCR Market — Forecast(2024–2030)
Digital PCR Market Overview
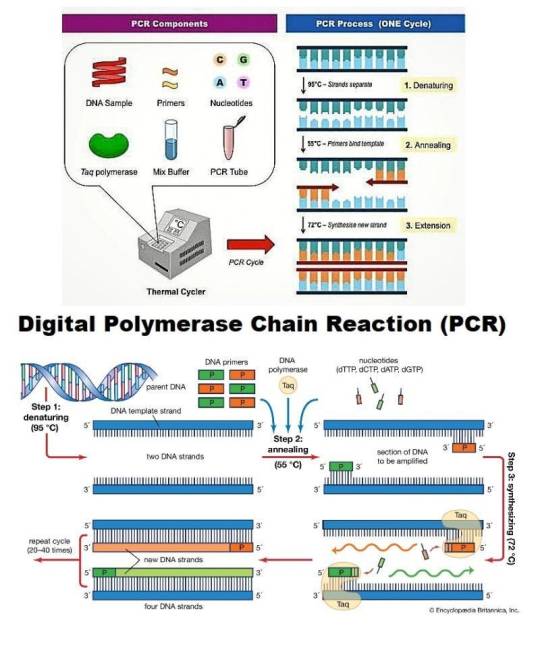
Request Sample
Report Coverage
The report: “Digital PCR Market Forecast (2024–2030)”, by Industry ARC, covers an in-depth analysis of the following segments of the Digital PCR Market.
By Product: Consumables & Reagents and Software & Services
By Technology Type: Droplet Digital PCR, Chip Based Digital PCR, and Beaming Digital PCR
By Indication: Infectious Disease, Oncology, Genetic Disorders, and Others
By Application: Research, Clinical Diagnostics, Forensics, and Others
By Geography: North America (U.S., Canada, Mexico), Europe (Germany, United Kingdom (U.K.), France, Italy, Spain, Russia, and Rest of Europe), Asia Pacific (China, Japan India, South Korea, Australia, and New Zealand, and Rest of Asia Pacific), South America (Brazil, Argentina, and Rest of South America), and Rest of the World (Middle East, and Africa).
Inquiry Before Buying
Key Takeaways
North America dominated the Digital PCR Market in 2020 owing to the increasing demand for rapid diagnostic tests and high diagnosis rates for infectious disease. The Digital PCR Market scope for different regions will be provided in the final report.
Technological advancements in digital PCR and growing adoption of digital PCR over real time PCR are likely to aid the market growth of the Digital PCR Market report.
Detailed analysis of the Strength, Weakness, and Opportunities of the prominent players operating in the market will be provided in the Digital PCR Market report.
High cost of digital PCR devices and reimbursement issues along with the technical limitations of PCR is poised to create hurdles for the Digital PCR Market.
Digital PCR Market Segment Analysis — By Technology Type
Droplet Digital PCR held the largest share in the Digital PCR Market in 2020 and is estimated to grow at a CAGR of 9.7% during the forecast period 2024–2030. This is attributed to the technological advances along with various new product launches. Droplet digital PCR is based on water oil emulsion droplet technology and for the amplification of the template molecules in each individual droplet. It also uses workflows and reagents for most standard probe based assays. Droplet digital PCR also measure the copy number variation by partitioning a PCR reaction into nanoliter droplets. The cross-contamination drawback of droplet digital PCR is increasing the demand of chip based digital PCR. Droplet digital PCR are estimated to register the highest CAGR over the period 2024–2030.
Schedule a Call
Digital PCR Market Segment Analysis — By Indication
Infectious disease held the largest share in the Digital PCR Market in 2020 and is estimated to grow at a CAGR 8.6% during the forecast period 2021–2026. This is attributed to the advantages of the droplet digital PCR of infectious diseases such as bacterial, viral, and parasitic indications. Digital PCR provides more accurate, sensitive, and reproductive detection of pathogens according to the National Center for Biotechnology and it is better than real time polymerase chain reaction that are used for clinical diagnostics. The demand of oncology is increasing owing to the growing prevalence of the condition and introduction of new product launches. Oncology are estimated to register the highest CAGR over the period 2024–2030.
Digital PCR Market Segment Analysis — By Geography
North America dominated the Digital PCR Market with a major share of 37.6% in 2020. This is attributed to the high prevalence & diagnosis rates for infectious disease and high awareness among patient population towards new diagnostic options. Availability of digital PCR devices, rising incidences of various types of cancer and metabolic diseases requiring advanced diagnosis and therapeutics is also increasing the growth of the market in this region.
However, Asia Pacific is estimated to grow at a higher CAGR during the forecast period 2024–2030 owing to the growing patient awareness regarding advanced digital polymerase chain reaction devices. Developing Healthcare infrastructure is also increasing the growth of the market in this region.
Buy Now
Digital PCR Market Drivers
Technological Advancements in Digital PCR
Technological advancements in digital PCR is increasing the growth of the Digital PCR Market. This is attributed to the growing demand for innovative devices and increasing research & development activities. Digital PCR is used to quantify and amplify nuclei acid. The introduction of various technologically advanced devices such as droplet digital PCR, chip based, and beam digital PCR is offering great benefits to the market. Thus, increasing the growth of the Digital PCR Market during the forecast period 2024–2030.
Growing Adoption of Digital PCR over Realtime PCR
Growing adoption of digital PCR over Realtime PCR is increasing the growth of the Digital PCR Market. This is attributed to the fact that digital PCR helps to deliver a compete measure to target nucleic acid molecules that is achieved from real time PCR. DNA quantification allows for reproducibility, precision, and sensitive that enables the researches to quantify smaller differences and measure minor variants very precisely. Thus, increasing the growth of the Digital PCR Market during the forecast period 2024–2030.
Digital PCR Market Challenges
High Cost of Digital PCR Devices and Reimbursement Issues Along with the Technical Limitations of PCR
Some of the factors that are set to impede the growth of the Digital PCR Market are high cost of digital PCR devices and reimbursement issues along with the technical limitations of PCR. The adoption of digital polymerase chain reaction techniques is limited owing to the lack of awareness about the digital PCR and the use of its advanced types.
Digital PCR Market Landscape
Product launches, mergers and acquisitions, joint ventures, and R&D activities are key strategies adopted by players in the Digital PCR Market. In 2020, the Digital PCR Market share is consolidated by the top ten players present in the market. Digital PCR Market, top 10 companies are Thermo Fisher Scientific Inc., BioMerieux SA, Stilla Technologies, Merck KgaA, Combinati Inc., and Bio-Rad Laboratories among others.
For more Lifesciences and Healthcare Market reports, please click here
0 notes
Text
Companion Diagnostics Market - Forecast(2024 - 2030)
Companion Diagnostic Market Overview:
The Harvard University, while addressing the risk associated with reactions of new drugs prescription, has stated some overwhelming facts. According to their findings, even properly prescribed drugs causes about 1.9 million hospitalizations a year and about 128,000 people die from drugs prescribed to them.[1] Such epidemic condition is being tailgated by the need of safe and effective and specific use of the drug. Owing to such demands, companion diagnostic drug market is poised for exponential growth. Companion diagnostics is an in-vitro diagnostic tool that assists physicians in optimizing treatment decisions for their patients and is crucial for myriad of cancer and other therapies. Riding on the back of economic burden of global healthcare and with abundant potential to restrict the liability, companion diagnostic market size is estimated to be $2,950 million as of 2018.
Inquiry Before Buying
Companion Diagnostic Market Outlook:
Companion diagnostic (CDx) is a diagnostic test used as an associate to a therapeutic drug to regulate its applicability to an individual person. It involves multiple monitoring methods including immunohistochemistry (IHC), polymerase chain Reaction (PCR), in-situ hybridization (ISH), real-time PCR (RT-PCR), and gene sequencing. The companion diagnostics uses technologies such as molecular biology technique, drug and diagnostic technology, and oncology therapy for the treatment of colorectal cancer, breast cancer, and other chronic diseases.
An acute analysis of the region-wise companion diagnostic market share concluded with reckoning North America as the most lucrative market for CDx. This region with cutting-edge healthcare technology in the United States and Canada generates 41% of the global companion diagnostic market demand for alarming need for cancer diagnosis and treatment. According to the American Cancer Society, prostate cancer is the most common cancer among males (19%), followed by lung (14%) and colorectal (9%) cancers and among females, breast (30%), lung (12%), and colorectal (8%) cancers are the most common. Increasing instances of cancer along with neurological disorders, infectious diseases, Hepatitis A is augmenting the North American companion diagnostic market.
Companion diagnostic market with abundant potential as an effective tool for personalized medicine has found a tremendous scope of application in pharmaceuticals, laboratories, research institutes and hospitals. Hospital as an end-user industry is the leading segment progressing with an application CAGR of 12.5% going through 2025. Hospitalized patients account for a total of 2.74 million serious adverse drug reactions. Each CDx test is specifically designed to be corresponding with an exact drug. Such tests can also save significant hospital expenditure by targeting specific patients with the most effective therapy.
Request Sample
Companion Diagnostic Market Trends and Growth Drivers:
· The necessity for personalized therapeutics for the cumulative geriatrics population and the increasing figure of diagnostics centers in both the developed and developing economies is predominant to determine profits in the global companion diagnostics market.
· FDA had issued "Guidance for Industry: In Vitro Companion Diagnostic Devices," to assist syndicates recognize the necessity for companion diagnostics at an initial stage in the drug development procedure and to strategize for co-development of the drug and companion diagnostic test.
On July 15, 2016, FDA introduced the draft regulation, "Principles for Co-development of an In Vitro Companion Diagnostic Device with a Therapeutic Product." This guidance text is envisioned to be a practical guide to support therapeutic product promoters and IVD sponsors in evolving a therapeutic product and an associated IVD companion diagnostic. The scientific progressions in the in-situ hybridization and automated silver-enhanced in-situ hybridization (SISH) for the monitoring of genes is trending in the global companion diagnostics market.
Schedule a Call
Companion Diagnostic Market Players Perspective:
Some of the key players influencing the global market are:- Abbott Laboratories, Agilent Technologies, biomerieux, Bio-Genex Laboratories, Danaher Corporation, GE Healthcare, Myriad Genetics, Inc., QIAGEN N.V., R-Biopharm AG, and Roche Diagnostics.
In April 2017, Abbott acquired Alere for a new price of about $5.3 billion. Alere is the global leader in point of care diagnostics focused on the areas of infectious disease, molecular, cardiometabolic and toxicology. The collective business will offer the biggest point of care menu of infectious disease, molecular, cardiometabolic and toxicology testing. Abbott's platforms will be expanded to comprise benchtop and rapid strip tests.
Buy Now
Companion Diagnostic Market Research Scope:
The base year of the study is 2018, with forecast done up to 2025. The study presents a thorough analysis of the competitive landscape, taking into account the market shares of the leading companies. It also provides information on unit shipments. These provide the key market participants with the necessary business intelligence and help them understand the future of the companion diagnostic market. The assessment includes the forecast, an overview of the competitive structure, the market shares of the competitors, as well as the market trends, market demands, market drivers, market challenges, and product analysis. The market drivers and restraints have been assessed to fathom their impact over the forecast period. This report further identifies the key opportunities for growth while also detailing the key challenges and possible threats. The key areas of focus include the various diagnostics in companion diagnostic market, and their specific advantages.
#companion diagnostics market#companion diagnostics market size#companion diagnostics market share#companion diagnostics market forecast#companion diagnostics market report#drugs#chronic diseases#treatment#">
0 notes
Text
Forensic Technologies Market: Innovations and Trends Shaping the Future
Forensic Technologies Market Overview: A lot of factors, such as geographic growth, segmentation, and market size by value and volume, are taken into account in the SkyQuest Technology Group research to provide a full and accurate analysis of the global Forensic Technologies market. This outstanding research study was created specifically to provide the most latest data on significant aspects of the global Forensic Technologies Industry. Numerous market estimates are provided in the analysis, including those for market size, output, revenue, consumption, CAGR, gross margin, price, and other critical factors. The best primary and secondary research methods and tools on the Forensic Technologies market were used to build it. Numerous research studies are included in it, including ones on pricing analysis, production and consumption analysis, company profile, and manufacturing cost analysis.
The competitive environment is a crucial element that every key factor needs to be aware of. The study explains the market's competitive landscape so that readers may gauge the degree of both domestic and global rivalry. Additionally, market researchers have provided summaries of each significant firm in the global Forensic Technologies industry, taking into consideration crucial elements including operational areas, production, and product portfolio. When analyzing the organizations in the study, significant factors including business size, market share, market growth, revenue, production volume, and profitability are also taken into account. The study report uses both qualitative and quantitative data to offer a thorough view of the market. It examines and forecasts the global market in a number of critical industries. The research provides a thorough overview of the industry by segmenting the Forensic Technologies market into groups based on application, end-user, and location. A thorough research of each market segment was conducted, taking into consideration current and upcoming market trends.
Global Forensic Technologies Market size was valued at USD 14.78 billion in 2022 and is poised to grow from USD 16.61 billion in 2023 to USD 41.92 billion by 2031, growing at a CAGR of 12.28% in the forecast period (2024-2031).
Chance to get a free sample @ https://www.skyquestt.com/sample-request/forensic-technologies-market
Detailed Segmentation and Classification of the report (Market Size and Forecast - 2031, Y-o-Y growth rate, and CAGR):
The Forensic Technologies Market can be segmented based on several factors, including product type, application, end-user, and distribution channel. Understanding these segments is crucial for companies looking to target specific markets and tailor their offerings to meet consumer needs.
Product
Digital & Computer Forensics, Ballistic Forensics, DNA Testing, Biometrics, Others
Application
Pharmacogenetics, Biodefense & Bio-Surveillance, Judicial, Law Enforcement, Others
Service
Laboratory Forensics (DNA Testing, Drug Testing, Biometrics, Others), Forensic Consulting
Technique
Polymerase Chain Reaction (PCR), Capillary Electrophoresis, Next-Generation Sequencing, Rapid DNA Analysis, Automated Liquid Handling Technology, Microarrays, Others
Location
Laboratory Forensic Technology, Portable Forensic Technology
Get your Customized report @ https://www.skyquestt.com/speak-with-analyst/forensic-technologies-market
Following are the players analyzed in the report:
GE Healthcare
Thermo Fisher Scientific Inc.
Agilent Technologies Inc.
LGC Limited
Danaher Corporation
Eurofins Scientific SE
Forensic Technology WAI Inc.
NMS Labs Inc.
SCIEX
Bruker Corporation
Bode Technology
Morpho (Safran)
IDEMIA Group
Qiagen N.V.
NicheVision Forensics Co.
Foster + Freeman Ltd.
FLIR Systems Inc.
Sirchie
Firearm and Toolmark Examination Unit
Forensic Pathology Services
Motives for purchasing this report-
- A full understanding of customer experiences, upcoming trends, and growth drivers may be obtained by market category analysis.
-Forensic Technologies Market participants will be able to quickly decide on their course of action in order to achieve a competitive advantage thanks to the essential information provided in this area.
The factors affecting the sales prospect are carefully examined by SkyQuest Technology Group across several important categories.
- Analysing market categories can provide detailed insights into consumer experiences, upcoming trends, and growth-promoting factors. A thorough analysis of market manufacturing trends is a crucial component of the study.
-These observations offer crucial information on the ways in which market participants are reacting to the most recent developments that are oversaturating the market.
-An in-depth analysis of the numerous organic
Buy your full Market Report now: https://www.skyquestt.com/buy-now/forensic-technologies-market
FAQs:
1. What are the main vendors' points of strength and weakness?
2. What are the primary business plans of the leading important players for the near future?
3. What will the market size and growth rate be for Forensic Technologies in the upcoming year?
4. Which prevailing global trends are affecting the Forensic Technologies market shares of the leading regions? What effect does Covid19 have on the Industry right now?
1 note
·
View note
Text
Preimplantation Genetic Testing Market Future Outlook: Analyzing Size, Share, and Growth Patterns
The global preimplantation genetic testing market size is expected to reach USD 1.54 billion by 2030, growing at 10.3% CAGR from 2024 to 2030, as per the new report by Grand View Research, Inc. Recent technological advancements in testing have changed the current practice of prenatal screening and early detection of chromosomal abnormalities in an embryo. Moreover, the introduction of novel technologies such as NGS, PCR, and FISH is expected to drive market growth over the forecast period.

Growing awareness about genetic diseases and novel techniques for the detection of chromosomal abnormalities and embryo screening for successful IVF holds growth prospects for this vertical over the forecast period. Key market players and service providers such as COOPER SURGICAL, INC. are running awareness campaigns to promote an increased understanding of the diseases and screening of these diseases at the early stages. Such favorable initiatives are expected to increase the demand for PDT in IVF procedures globally.
The increasing adoption of in vitro fertilization (IVF) technique consequently upsurges the utilization of preimplantation genetic testing (PGT). These techniques are advantageous in allowing the development of a healthy fetus and preventing the risk of selective pregnancy termination. Hence, PGT acts as an addendum to assisted reproductive technology.
Moreover, the increased risk of genetic disease in newborns and complications associated with IVF procedures push healthcare providers to recommend PGT. For instance, according to the CDC, in the U.S., about 6,000 babies are born with Down syndrome each year. It is the most common chromosomal condition diagnosed in newborns in the country. Moreover, the high risk associated with aged pregnancy further increases the adoption of PGT worldwide.
Key companies are involved in developing novel methods and solutions to carry out preimplantation genetic diagnosis and screening. To maintain their significant share in the revenue in the coming years, these participants are undertaking strategic initiatives. The strategic undertakings include regional expansion, collaborative development, and new product developments.
For instance, in October 2022, Ovation Fertility and Genomic Prediction partnered to expand genetic testing services by using the LifeView PGT platform for PFT. Moreover, in April 2022, Clevergene received PCPNDT for prenatal genetic testing. The company aimed to expand its services in preimplantation genetic screening and non-invasive prenatal testing.
For More Details or Sample Copy please visit link @: Preimplantation Genetic Testing Market Report
Preimplantation Genetic Testing Market Report Highlights
The rising incidence of genetic diseases has fueled the demand for preimplantation genetic testing (PGT). With increasing prevalence of genetic disorders, PGT has become crucial in IVF procedures.
The Polymerase Chain Reaction (PCR) segment dominated the preimplantation genetic testing market and contributed 39.8% to the market share in 2023.
The preimplantation genetic diagnosis segment dominated the preimplantation genetic testing market and contributed 77.0% of the market share in 2023.
Europe preimplantation genetic testing market dominated the global market in 2023, capturing the largest revenue share at 40.74%.
Gain deeper insights on the market and receive your free copy with TOC now @: Preimplantation Genetic Testing Market Report
Grand View Research has segmented the global Preimplantation Genetic Testing market on the basis of procedure, product, technology, application, end use, and region.
#PreimplantationGeneticTesting#PGT#GeneticScreening#IVF#ReproductiveHealth#GeneticTestingMarket#FertilityTreatment#EmbryoTesting#ReproductiveMedicine#HealthcareInnovation#PersonalizedMedicine#Genomics#FamilyPlanning#MedicalDiagnostics#HealthcareMarket
0 notes
Text
Polymerase Chain Reaction Market Surge: Future of Genetic Testing and Diagnostics

The Polymerase Chain Reaction (PCR) market is experiencing significant growth, driven by advancements in technology and increasing demand across various sectors. According to a recent report by SkyQuest Technology, the global PCR market is poised for substantial expansion, reflecting its crucial role in medical diagnostics, research, and biotechnology.
PCR, a technique developed in the 1980s, has revolutionized the field of molecular biology by allowing scientists to amplify specific DNA sequences. This process is essential for various applications, including disease diagnosis, genetic research, and forensic analysis. The report highlights that the PCR market has been growing steadily, with expectations of continued expansion in the coming years. The Polymerase Chain Reaction (PCR) Market size was valued at USD 24.17 billion in 2022 and is poised to grow from USD 24.75 billion in 2023 to USD 29.92 billion by 2031, growing at a CAGR of 2.4% during the forecast period (2024-2031).
Get Your Free Sample Report Here - https://www.skyquestt.com/sample-request/polymerase-chain-reaction-market
Key Drivers of Growth
Several factors are contributing to the growth of the PCR market:
1. Technological Advancements: Innovations such as real-time PCR and digital PCR are enhancing the accuracy and efficiency of genetic testing. These advancements are broadening the scope of PCR applications, from personalized medicine to infectious disease detection.
2. Increased Demand in Diagnostics: The global health crisis underscored the importance of rapid and reliable diagnostic tools. PCR has become a cornerstone in the detection of pathogens, including the SARS-CoV-2 virus, which has led to a surge in demand for PCR-based testing.
3. Expansion of Research and Development: The rise in research activities in genomics and proteomics is driving the need for advanced PCR technologies. Academic and research institutions are investing in PCR systems to support their studies and innovations.
4. Growing Biotechnology Sector: The biotech industry’s expansion is another significant driver. Companies are increasingly adopting PCR for drug discovery, development, and quality control, further propelling market growth.
Top Player’s Company Profile - Thermo Fisher Scientific, Inc., Roche Holdings AG, Bio-Rad Laboratories, Inc., QIAGEN N.V., Agilent Technologies, Inc., F. Hoffmann-La Roche Ltd., Becton, Dickinson and Company, Danaher Corporation, Promega Corporation, Merck KGaA, Fluidigm Corporation, Eppendorf AG, Takara Bio Inc., Abbott Laboratories, BioMérieux SA, PerkinElmer, Inc., BioFire Diagnostics, LLC, Biosearch Technologies, Inc., GenMark Diagnostics, Inc., Enzo Biochem, Inc., Illumina, Inc., New England Biolabs, Inc., Quantabio, LGC Limited, Bioer Technology Co., Ltd
Market Segmentation
The report provides a detailed analysis of the PCR market, segmented by product type, application, and region:
- Product Type: The market is categorized into instruments, reagents, and software. Instruments, including PCR machines and thermal cyclers, are the largest segment due to their essential role in the PCR process.
- Application: PCR is utilized in various fields such as clinical diagnostics, research, and forensic applications. Clinical diagnostics, particularly in infectious disease detection, holds the largest market share.
- Region: North America leads the market due to the presence of advanced healthcare infrastructure and high research funding. However, the Asia-Pacific region is expected to witness the fastest growth, driven by increasing healthcare investments and rising awareness.
Want to customize this report? Get Your Free Customize Report - https://www.skyquestt.com/speak-with-analyst/polymerase-chain-reaction-market
Recent Developments
In February 2023: Qiagen announced the release of its new QIAsymphony Dx Real-Time PCR System, that is designed to be greater person-pleasant and efficient than preceding PCR systems.
In March 2023: Roche Diagnostics launched its new cobas SARS-CoV-2 PCR check, which is a fast and accurate test for the detection of COVID-19.
In April 2023: Illumina announced the release of its new MiSeq 5Dx sequencing system, which is able to sequence up to 300 genomes consistent with day.
In May 2023: Thermo Fisher Scientific launched its new Applied Biosystems TaqMan Profiler Plus SARS-CoV-2 Assay, that's a quantitative PCR assay for the detection of SARS-CoV-2.
In June 2023: Agilent Technologies announced the release of its new 2100 Bioanalyzer System, which is a high-throughput DNA evaluation system. The PCR market is set for robust growth, underpinned by technological advancements and increasing demand across various applications. As the technology continues to evolve, it promises to deliver more precise and efficient solutions, further solidifying its role in medical diagnostics and research. For stakeholders in the PCR market, staying abreast of technological developments and market trends will be crucial in navigating the evolving landscape and leveraging opportunities for growth.
#PCRMarket#PolymeraseChainReactionMarket#PCRIndustry#PCRTrends#PCRGrowth#PCRTechnology#PCRDiagnostics#PCRApplications#PCRInnovation#PCRAdvancements#MolecularDiagnosticsMarket#GeneticTestingMarket#BiotechMarket#PCRResearch#PCRSolutions#DiagnosticMarket#PCRDevelopments#PCRInsights#PCRTrends2024#PCRExpansion
0 notes
Text
Molecular Diagnostics Market to Reach USD 37 Billion by 2031, Growing at 11.4% CAGR | SkyQuest Technology
SkyQuest projects that the Global Molecular Diagnostics Market will attain a value of USD 37.00 Billion by 2031, with a CAGR of 11.4% during the forecast period (2024-2031). The global molecular diagnostics market is a continuously expanding and evolving sector that includes a diverse set of technologies, techniques, and applications for detecting, evaluating, and monitoring diseases at the molecular level. It entails the utilization of nucleic acid-based tests, like polymerase chain reaction (PCR), next-generation sequencing (NGS), and microarrays, to detect genetic variations, mutations, and biomarkers related to a variety of diseases, such as infectious diseases, cancer, and genetic disorders. The market has been impacted by a number of factors, including the rising prevalence of chronic and infectious diseases, increasing demand for personalized medication, advances in genomes and proteomics research, and a requirement for efficient and precise diagnostic tools. The rise of targeted medicines and the move to precision medicine have increased demand for molecular diagnostics, which give critical information for medical care and monitoring.
Download a detailed overview:
https://www.skyquestt.com/sample-request/molecular-diagnostics-market
Browse in-depth TOC on the "Global Molecular Diagnostics Market"
Pages - 157
Tables - 61
Figures – 75
Rising Adoption of PCR is Driving the Molecular Diagnostics Market Growth
PCR is widely employed in CROs, research institutes, and hospitals, which boosts molecular diagnostics market growth rates. The increasing usage of high-throughput PCR technology for detecting viral and genetic illnesses is expected to drive market expansion. For example, Seegene introduced the Allplex SARS-CoV-2 Fast PCR Assay in 2022. The test has a 60-minute turnaround time, making it suited for mass testing. As a result, this component promotes market growth.
High Demand for Personalized Medicine is Boosting the Molecular Diagnostics Market
The increasing demand for personalized medication is a significant driver of the global molecular diagnostics market. Personalized medicine is the practice of personalizing medical therapies and treatments to individual patients depending on genetics, molecular traits, and other relevant criteria. Molecular diagnostics is essential for providing personalized therapy by delivering accurate and targeted information about a patient's ailment, prognosis, and treatment response. The growing understanding of personalized medicine's advantages for enhancing patient outcomes and managing healthcare resources has fuelled demand for molecular diagnostics, resulting in market growth.
Well-Established Healthcare Structure is Driving the Molecular Diagnostics Market of North America
North America is the leading region in the global molecular diagnostics market. This is due to factors like a well-established healthcare infrastructure, robust R&D skills, excellent laboratory facilities, and attractive reimbursement rules. The region also has an extensive use of modern diagnostic technologies, such as molecular diagnostics, which is fuelled by the presence of important market participants, the rising frequency of chronic diseases, and an emphasis on unique therapy. The Asia Pacific region is experiencing the most rapid expansion in the global molecular diagnostics market. It is due to rapid expansion like rising healthcare spending, increased knowledge of the benefits of early disease detection, improved access to advanced diagnostic tools, and increased investment in hospital infrastructure.
Molecular Diagnostics Market Insights
Drivers:
Increasing demand for personalized medicine that focuses on tailoring medical treatment is driving the molecular diagnostics market
Rise in elderly population that increases diseases like neurological disorders, diabetes, and obesity
High usage of PCR to identify infectious diseases
Restraints:
Strict regulatory framework can restrict the market growth as inaccurate results due to faulty diagnostic kits can cause issue
Regional and healthcare system reimbursement rules and coverage for molecular diagnostic tests can hinder market penetration and adoption
Molecular diagnostics involve advanced technologies making them expensive
Prominent Market Players of Global Molecular Diagnostics Market
Danaher (US)
F. Hoffmann-La Roche Ltd. (Switzerland)
Hologic, Inc. (US)
Abbott Laboratories (US)
Illumina, Inc. (US)
Thermo Fisher Scientific Inc. (US)
bioMérieux SA (France)
QIAGEN (Netherlands)
Agilent Technologies Inc. (US)
Becton Dickinson And Company (US)
Grifols S.A. (Spain)
Key Questions Answered in the Global Molecular Diagnostics Report
What are the primary drivers of the molecular diagnostics market?
Which are the top companies in the molecular diagnostics market?
Which product segment is leading the global molecular diagnostics market?
What are the challenges restricting the growth of the molecular diagnostics market?
This report provides the following insights:
Analysis of key drivers (Increasing demand for personalized medicine, rise in elderly population, and high usage of PCR), restraints (Different regional and healthcare system reimbursement and high expense of technologies used in molecular diagnostics), opportunities (Increasing adoption of next generation sequencing technologies and rising prevalence of chronic and infectious diseases), and challenges (strict regulatory framework) influencing the growth of molecular diagnostics market
Market Penetration: Comprehensive information on the product offered by the top players in the molecular diagnostics market
Product Development/Innovation: Detailed insights on the upcoming trends, R&D activities, and product launches in the molecular diagnostics market
Market Development: Comprehensive information on emerging regions
Market Diversification: Exhaustive information about new products, growing geographies, and recent developments in the market
Competitive Assessment: In-depth assessment of market segments, growth strategies, revenue analysis, and products of the leading market players.
About Us:
SkyQuest is an IP focused Research and Investment Bank and Accelerator of Technology and assets. We provide access to technologies, markets and finance across sectors viz. Life Sciences, CleanTech, AgriTech, NanoTech and Information & Communication Technology.
We work closely with innovators, inventors, innovation seekers, entrepreneurs, companies and investors alike in leveraging external sources of R&D. Moreover, we help them in optimizing the economic potential of their intellectual assets. Our experiences with innovation management and commercialization have expanded our reach across North America, Europe, ASEAN and Asia Pacific.
Contact:
Mr. Jagraj Singh
SkyQuest Technology
1 Apache Way,
Westford,
Massachusetts 01886
USA (+1) 351-333-4748
Email: [email protected]
Visit Our Website: https://www.skyquestt.com/
#Molecular Diagnostics Market Size#Molecular Diagnostics Market Share#Molecular Diagnostics Market Growth#Molecular Diagnostics Market Trends#Molecular Diagnostics Market News#Molecular Diagnostics Market Forecast#Molecular Diagnostics Market Analysis
0 notes
Text

Molecular Detection
PCR (polymerase chain reaction) plastic consumables are a group of laboratory supplies that are used in the PCR process for molecular detection. These consumables include items such as PCR tubes, PCR plates, PCR strip tubes, and PCR caps. They are typically made of polypropylene or polyethylene, and are designed to be heat-resistant, autoclavable, and chemically resistant to the reagents used in PCR. Some of these consumables come with attached or detachable caps that can be used to seal the tubes or plates, or to hold barcode labels for sample identification.
The imported medical grade high transparent polypropylene certified by USP is used as the raw material, and the surface is smooth to ensure high efficiency and uniformity between samples. Satisfy accurate and efficient PCR reaction, applied to genetic testing, disease
diagnosis, drug research and development and other fields.
Features
*No DNase, RNase, no pyrogen
*Provide a variety of specifications for single tube, 8-strip tube, and 12-strip tube to meet various experimental scenarios
*Ultra-thin tube wall, high heat conduction efficiency
*Excellent airtightness, low evaporation rate, and smooth opening
* Adapt to most commonly used brands of instruments on the market, such as thermo fisher, Bio-Rad, Eppendorf, Applied Biosystems, Roche, etc.
* Tube number identification, easy to distinguish and identify the samples in the tube
*Multi-color options: the white version is suitable for cold light and low-fluorescence signal detection; the transparent version has no impurities to reduce the influence of scattered light and improve the accuracy of the experiment. Different colors are available for different detection systems.
0 notes
Text
Latin America Molecular Diagnostics Market Overview: Challenges and Growth Opportunities
According to a recent report by Meticulous Research®, the Latin America molecular diagnostics market is forecasted to reach $2.50 billion by 2031, expanding at a compound annual growth rate (CAGR) of 6.3% from 2024 to 2031. The market growth is propelled by several key factors, including the increasing global geriatric population, rising prevalence of communicable and noncommunicable diseases, rapid advancements in molecular diagnostics technology, and a surge in healthcare spending across the region.
The rising demand for molecular diagnostics in Latin America is further supported by opportunities such as the expanding scope in emerging economies, increased focus on companion diagnostics, and the growing popularity of direct-to-consumer (DTC) testing. Despite these growth drivers, challenges like the shortage of skilled professionals, unfavorable regulatory frameworks, and the high costs of molecular diagnostic tests could restrain the market’s growth.
Download Sample of Report @ https://www.meticulousresearch.com/download-sample-report/cp_id=5759
Market Segmentation and Key Insights
The Latin America molecular diagnostics market is segmented based on offerings, test types, technologies, applications, and end users.
By Offering: In 2024, the kits and reagents segment is expected to dominate the market. This is attributed to the widespread availability of diagnostic reagents and consumables, disease-specific test kits and assays, and increasing awareness about the importance of early disease diagnosis.
By Test Type: The laboratory tests segment is projected to account for the largest share in 2024. Laboratory tests remain the preferred choice for hospitals, diagnostic laboratories, and academic institutions, driven by their wide availability and the significant developments in diagnostic testing within clinical settings.
By Technology: The polymerase chain reaction (PCR) segment is expected to lead the market in 2024. PCR's dominant position is due to its versatility in multi-drug resistance testing, widespread use in clinical and research laboratories, and applications in areas such as DNA fingerprinting, bacterial and viral detection (particularly HIV/AIDS), and genetic disorder diagnosis.
By Application: The infectious diseases segment is anticipated to hold the largest share of the market in 2024. The increasing prevalence of infectious diseases, rising funding for the development of innovative diagnostic tools, and the heightened focus on diagnostic technologies during the COVID-19 pandemic are driving the demand for molecular diagnostics in infectious disease management.
By End User: Hospitals and clinics are expected to dominate the market by end user in 2024. The growing number of hospitalizations due to a variety of diseases requiring molecular diagnosis, coupled with the proliferation of healthcare facilities in countries such as Brazil, Mexico, Chile, and Colombia, is contributing to the expansion of molecular diagnostic product utilization in this segment.
Key Industry Players
The competitive landscape of the Latin America molecular diagnostics market is shaped by several prominent global and regional players. Leading companies in the market include:
Bio-Manguinhos (Brazil)
F. Hoffmann-La Roche Ltd. (Switzerland)
Thermo Fisher Scientific Inc. (U.S.)
Hologic, Inc. (U.S.)
Illumina, Inc. (U.S.)
OmicronLab (Mexico)
QIAGEN N.V. (Netherlands)
Danaher Corporation (U.S.)
Abbott Laboratories (U.S.)
Agilent Technologies, Inc. (U.S.)
These companies are at the forefront of innovation and technological advancements in molecular diagnostics, driving market growth through product development, partnerships, and strategic expansions.
Opportunities and Challenges
The Latin America molecular diagnostics market is ripe with opportunities, particularly in emerging economies where there is increasing demand for advanced diagnostic tools. The rising focus on companion diagnostics and the expanding DTC testing market offer significant growth prospects. However, challenges such as the high cost of molecular diagnostic tests and regulatory hurdles may hinder market expansion.
Despite these challenges, the region’s healthcare landscape is undergoing rapid transformation, with increasing healthcare investments and advancements in diagnostic technologies expected to fuel further growth.
Read Full Report @ https://www.meticulousresearch.com/product/latin-america-molecular-diagnostics-market-5759
Contact Us:
Meticulous Research®
Email- [email protected]
Contact Sales- +1-646-781-8004
Connect with us on LinkedIn- https://www.linkedin.com/company/meticulous-research
#Latin America Molecular Diagnostics Market#Molecular Diagnostics#Molecular Test#Molecular Laboratory#PCR#Molecular Testing#MDx
0 notes
Text
Nucleic Acid Isolation and Purification Market Size, Key Vendors, Growth Rate, Drivers, and Volume & Forecast Report to 2024-2032
The global nucleic acid isolation and purification market is poised for significant expansion, with its market size estimated to grow from USD 6.60 billion in 2023 to an impressive USD 16.38 billion by 2032. This growth reflects a compound annual growth rate (CAGR) of 11.18% during the forecast period from 2024 to 2032, driven by advancements in molecular biology research, diagnostics, and biotechnological innovations.
Nucleic acid isolation and purification are critical steps in molecular biology and genetic research, enabling the extraction and purification of DNA or RNA from various biological samples. These processes are essential for a wide range of applications, including diagnostics, drug discovery, genomic studies, and the development of personalized medicine.
Key Market Drivers
Advancements in Genomic Research and Precision Medicine: The growing emphasis on genomics, personalized medicine, and gene-based therapies is a significant driver of the nucleic acid isolation and purification market. As scientists explore the human genome for disease markers and potential treatments, the demand for efficient and reliable nucleic acid extraction tools has surged. These processes play a crucial role in understanding genetic diseases, identifying biomarkers, and developing targeted therapies.
Expanding Use in Diagnostics and Infectious Disease Research: The nucleic acid isolation and purification market saw a notable boost during the COVID-19 pandemic, with its application in viral detection and monitoring through PCR (polymerase chain reaction) and other molecular diagnostic techniques. As the world continues to focus on infectious disease control, the need for accurate diagnostic tests, including those for emerging pathogens, remains critical. Nucleic acid isolation is the foundation for such molecular diagnostics, driving growth in this market segment.
Growing Demand in Biotechnology and Pharmaceutical Industries: In addition to diagnostics, biotechnology and pharmaceutical companies are leveraging nucleic acid isolation technologies for drug development and bioproduction. With increased funding for research into biologics, gene therapies, and vaccines, the pharmaceutical sector’s reliance on these processes is expected to continue growing. This demand is particularly significant for companies focused on RNA-based therapies, mRNA vaccines, and CRISPR gene-editing technologies.
Technological Innovations in Isolation and Purification Techniques: Continuous advancements in nucleic acid extraction technologies are transforming the market. Automated systems, high-throughput solutions, and faster, more efficient isolation kits are being developed to meet the needs of researchers and clinicians. These innovations not only reduce manual labor and errors but also improve reproducibility and sample integrity, making nucleic acid isolation and purification more accessible across various fields of study.
Access Free Sample Report: https://www.snsinsider.com/sample-request/4489
Challenges and Opportunities
The nucleic acid isolation and purification market faces several challenges, including the high cost of automated systems and consumables, particularly for smaller research labs and healthcare institutions. However, the benefits of increased efficiency, accuracy, and scalability are driving wider adoption, while ongoing research into cost-effective solutions presents opportunities for market expansion.
Moreover, the rise of next-generation sequencing (NGS) and its application in cancer research, genetic testing, and rare disease studies presents a growing opportunity for the market. As sequencing technologies advance and become more affordable, the demand for high-quality nucleic acid extraction methods will intensify.
Regional Insights
North America currently holds the largest share of the nucleic acid isolation and purification market, driven by the region’s strong focus on research and development, particularly in the areas of genomics, drug discovery, and molecular diagnostics. The United States, with its leading research institutions and biopharmaceutical companies, is a key contributor to this market growth.
Europe follows closely, with increasing investments in biotechnology and genomics research. The Asia-Pacific region is expected to witness the highest growth rate during the forecast period, driven by rising healthcare infrastructure, growing biotech industries, and increased research funding in countries such as China, India, and Japan.
Future Outlook
As genomic research, molecular diagnostics, and precision medicine continue to advance, the nucleic acid isolation and purification market is expected to see sustained growth. The increasing focus on personalized healthcare, gene-based treatments, and the use of nucleic acid technologies in various therapeutic applications will drive market expansion in the coming years. Furthermore, the growing prevalence of cancer, infectious diseases, and genetic disorders will fuel demand for nucleic acid extraction technologies for research and clinical applications.
In conclusion, the nucleic acid isolation and purification market is set for robust growth, with its value projected to rise from USD 6.60 billion in 2023 to USD 16.38 billion by 2032, at a CAGR of 11.18%. The increasing application of nucleic acid technologies in diagnostics, drug discovery, and genomics will continue to drive market dynamics, making this a vital area of growth within the life sciences and healthcare sectors.
Other Trending Reports
Sharps Containers Market Size
Gene Therapy Market Size
Orthopedic Devices Market Size
Real World Evidence/RWE Solutions Market Size
0 notes
Text
The Evolution of the COVID-19 Test Devices Market: From Early Pandemic to Present Day
The COVID-19 pandemic triggered a rapid and unprecedented surge in demand for diagnostic testing devices. As the pandemic unfolded, the market for COVID-19 tests underwent a remarkable transformation, characterized by rapid innovation, technological advancements, and increased global production capacity.

Buy the Full Report for More Insights on the COVID-19 Tests Market Forecast, Download a Free Sample Report
Early Pandemic: A Rush for Testing Devices
Initial Shortages: The early stages of the pandemic were marked by severe shortages of testing devices, particularly in regions with high infection rates. This scarcity hindered efforts to contain the virus and understand its spread.
Rapid Innovation: In response to the urgent need, manufacturers, researchers, and governments worldwide accelerated the development of new testing technologies, including RT-PCR, antigen tests, and rapid point-of-care (POC) tests.
Scaling Up Production: Global efforts were made to scale up the production of testing devices, leveraging existing manufacturing capabilities and establishing new facilities.
Advancements in Testing Technologies
RT-PCR Tests: Reverse-transcriptase polymerase chain reaction (RT-PCR) tests remained the gold standard for COVID-19 diagnosis, offering high sensitivity and specificity. However, their complexity and turnaround time limited their widespread use.
Antigen Tests: Rapid antigen tests, while less sensitive than RT-PCR, provided faster results and were more suitable for mass testing and screening purposes.
Point-of-Care Tests: POC tests, often lateral flow immunoassays, offered rapid results at the point of care but faced challenges in terms of sensitivity and specificity.
Saliva-Based Tests: The development of saliva-based testing simplified sample collection and made testing more accessible to the general public.
Market Expansion and Competition
Increased Production Capacity: Global manufacturers expanded their production capacities to meet the surging demand for COVID-19 tests.
New Market Entrants: Many new companies entered the market, driven by the lucrative opportunities presented by the pandemic.
Intense Competition: The market became highly competitive, with manufacturers vying for market share through price reductions, technological advancements, and improved turnaround times.
Challenges and Future Trends
Variant Challenges: The emergence of new COVID-19 variants, such as Omicron, posed challenges for testing devices, requiring adaptations to maintain accuracy.
Long-Term Demand: As the pandemic evolves, the long-term demand for COVID-19 tests remains uncertain, potentially impacting market dynamics.
Dual-Use Technologies: Some testing technologies, particularly those developed for COVID-19, have the potential to be repurposed for other infectious diseases, creating new market opportunities.
Home Testing: The increasing availability of at-home COVID-19 tests is changing the landscape of testing, empowering individuals to take control of their health.
The COVID-19 pandemic has significantly transformed the testing devices market, driving rapid innovation, increased production capacity, and a surge in demand. As the pandemic evolves, the market is likely to continue adapting to new challenges and opportunities, shaping the future of infectious disease testing.
0 notes
Text
Restriction Endonucleases Market Size, Share, Emerging Factors, Trends, Segmentation and Forecast to 2030

Market research “Restriction Endonucleases Market Size, Share, and Growth by 2031” enriched with data tables, pie charts, figures, and graphs spread through chapters reveal actionable insights. At present, the Restriction Endonucleases market is expanding at a lucrative CAGR. Through this assessment, The Insight Partners attempts to predict future trends, market values, growth factors, and related statistics. The report incorporates a broad range of strategies such as acquisition, collaborations, and investigation are embraced by market players to stay ahead in the competitive Restriction Endonucleases market space.
This market research is enriched with key statistics and facts allowing manufacturers to devise further business strategies. The market report also offers the company landscape and corresponding details of major market participants. The data contains company profiles, yearly turnover, product launches, income sources, and acquisitions.
Restriction Endonucleases market share has grown at a lucrative rate in recent years. Various factors that determine Restriction Endonucleases market growth is examined in this report, including opportunities, barriers, challenges, trends, and drivers. Authentic market determinants encourages innovation. This section addresses the distribution of firm activity and the factors that influence development. A comprehensive range of market-specific data is available, allowing investors to conduct an early assessment of the Restriction Endonucleases market's capabilities.
The main aim of the Restriction Endonucleases market report is to present an unbiased evaluation of the market based on industry growth potential, recent developments, trends, and growth opportunities. A detailed report is structured in a way such that users will find it easy to navigate and understand. We have ensured the optimal use of visual representations wherever necessary. This has increased pictorial presentation and the advantage of easy interpretation of industrial facts.
Scope of Restriction Endonucleases Market Research Report
Restriction Endonucleases Market size and forecast at global, regional, and country- level for all the key market segments covered under the scope
Market dynamics such as drivers, restraints, and key opportunities
Key future trends
Detailed PEST and SWOT analysis
Global and regional market analysis covering key market trends, key players, regulations, and recent market developments
Industry landscape and competition analysis covering market concentration, heat map analysis, key players, recent developments
Detailed company profiles
Unlock these Perks by Choosing this Report
Detailed historical data analysis, and market size forecast
Key market drivers influencing market growth
Emerging trends and opportunities in Restriction Endonucleases market
Analysis of major players to understand their strategies
Examination of regional dynamics
Report Attributes
Details
Segmental Coverage
Type
Type I
Type II
Type III
Type IV
Others
Application
Polymerase Chain Reaction (PCR)
Restriction Fragment Length Polymorphism (RFLP)
Epigenetics
Restriction Digestion
Sequencing
Cloning
End User
Hospitals
Clinics
Academic Research Institutes
Pharmaceutical and Biotechnology Companies
Diagnostic Centers
Geography
North America
Europe
Asia Pacific
and South and Central America
Regional and Country Coverage
North America (US, Canada, Mexico)
Europe (UK, Germany, France, Russia, Italy, Rest of Europe)
Asia Pacific (China, India, Japan, Australia, Rest of APAC)
South / South & Central America (Brazil, Argentina, Rest of South/South & Central America)
Middle East & Africa (South Africa, Saudi Arabia, UAE, Rest of MEA)
Market Leaders and Key Company Profiles
New England Biolabs
ThermoFisherScientific
Takara Bio Inc
Illumina
Agilent Technologies, Inc
F. Hoffmann-La Roche Ltd
NIPPON GENE CO., LTD
Promega Corporation
Merck KGaA
Jena Bioscience GmbH
Other key companies
Hope Your Search Ends Here! As We Offer Customization
The Insight Partners recognize that each business is unique and will encounter distinct challenges and opportunities. That’s why we offer tailor-made reports that are crafted by business requirements. From competitive analysis to consumer behavior insights offered under this research can address all key concerns.
The following are some customizations our clients frequently ask for:
The Restriction Endonucleases market report can be customized based on specific regions/countries as per the intention of the business
The report production was facilitated as per the need and following the expected time frame
Insights and chapters tailored as per your requirements.
Depending on the preferences we may also accommodate changes in the current scope.
About Us:
The Insight Partners is a one-stop industry research provider of actionable intelligence. We help our clients in getting solutions to their research requirements through our syndicated and consulting research services. We specialize in industries such as Semiconductor and Electronics, Aerospace and Defense, Automotive and Transportation, Biotechnology, Healthcare IT, Manufacturing and Construction, Medical Devices, Technology, Media and Telecommunications, Chemicals and Materials.
Contact Us: www.theinsightpartners.com
0 notes
Text
Digital PCR Market - Forecast(2024 - 2030)
Digital PCR Market Overview
Digital PCR Market size is forecast to reach $8.2 billion by 2026, growing at a CAGR of 9.3% during the forecast period 2021-2026. Digital Polymerase chain reaction is used for detection of nucleic acid that provides a reproducible and sensitive way to measure the amount of RNA present. It depicts high potential in clinical diagnostics, and forensics along with the in-vitro amplification of any DNA sample. It is also used for detection of viruses in cerebrospinal fluid of patients that are having neurological disease. The main advantage of digital PCR is that it has high tolerance to inhibitors, play an important role in targeted next-generation sequencing, and it also measures the cancer genes. It has various application in paternity testing and DNA cloning. Rise in prevalence of infectious disease and surge in awareness & acceptance of personalized medicines are the major factors driving the growth of the market. Advancement in technique and growing market penetration is set to further enhance the overall market development of the Digital PCR Market for the period 2021-2026.
Report Coverage
The report: “Digital PCR Market Forecast (2021-2026)”, by Industry ARC, covers an in-depth analysis of the following segments of the Digital PCR Market.
By Product: Consumables & Reagents and Software & Services
By Technology Type: Droplet Digital PCR, Chip Based Digital PCR, and Beaming Digital PCR
By Indication: Infectious Disease, Oncology, Genetic Disorders, and Others
By Application: Research, Clinical Diagnostics, Forensics, and Others
By Geography: North America (U.S., Canada, Mexico), Europe (Germany, United Kingdom (U.K.), France, Italy, Spain, Russia, and Rest of Europe), Asia Pacific (China, Japan India, South Korea, Australia, and New Zealand, and Rest of Asia Pacific), South America (Brazil, Argentina, and Rest of South America), and Rest of the World (Middle East, and Africa)
Request Sample
Key Takeaways
North America dominated the Digital PCR Market in 2020 owing to the increasing demand for rapid diagnostic tests and high diagnosis rates for infectious disease. The Digital PCR Market scope for different regions will be provided in the final report.
Technological advancements in digital PCR and growing adoption of digital PCR over real time PCR are likely to aid the market growth of the Digital PCR Market report.
Detailed analysis of the Strength, Weakness, and Opportunities of the prominent players operating in the market will be provided in the Digital PCR Market report.
High cost of digital PCR devices and reimbursement issues along with the technical limitations of PCR is poised to create hurdles for the Digital PCR Market.
Digital PCR Market Segment Analysis – By Technology Type
Droplet Digital PCR held the largest share in the Digital PCR Market in 2020 and is estimated to grow at a CAGR of 9.7% during the forecast period 2021-2026. This is attributed to the technological advances along with various new product launches. Droplet digital PCR is based on water oil emulsion droplet technology and for the amplification of the template molecules in each individual droplet. It also uses workflows and reagents for most standard probe based assays. Droplet digital PCR also measure the copy number variation by partitioning a PCR reaction into nanoliter droplets. The cross-contamination drawback of droplet digital PCR is increasing the demand of chip based digital PCR. Droplet digital PCR are estimated to register the highest CAGR over the period 2021-2026.
Inquiry Before Buying
Digital PCR Market Segment Analysis – By Indication
Infectious disease held the largest share in the Digital PCR Market in 2020 and is estimated to grow at a CAGR 8.6% during the forecast period 2021-2026. This is attributed to the advantages of the droplet digital PCR of infectious diseases such as bacterial, viral, and parasitic indications. Digital PCR provides more accurate, sensitive, and reproductive detection of pathogens according to the National Center for Biotechnology and it is better than real time polymerase chain reaction that are used for clinical diagnostics. The demand of oncology is increasing owing to the growing prevalence of the condition and introduction of new product launches. Oncology are estimated to register the highest CAGR over the period 2021-2026.
Digital PCR Market Segment Analysis – By Geography
North America dominated the Digital PCR Market with a major share of 37.6% in 2020. This is attributed to the high prevalence & diagnosis rates for infectious disease and high awareness among patient population towards new diagnostic options. Availability of digital PCR devices, rising incidences of various types of cancer and metabolic diseases requiring advanced diagnosis and therapeutics is also increasing the growth of the market in this region.
However, Asia Pacific is estimated to grow at a higher CAGR during the forecast period 2021-2026 owing to the growing patient awareness regarding advanced digital polymerase chain reaction devices. Developing Healthcare infrastructure is also increasing the growth of the market in this region.
Schedule a Call
Digital PCR Market Drivers
Technological Advancements in Digital PCR
Technological advancements in digital PCR is increasing the growth of the Digital PCR Market. This is attributed to the growing demand for innovative devices and increasing research & development activities. Digital PCR is used to quantify and amplify nuclei acid. The introduction of various technologically advanced devices such as droplet digital PCR, chip based, and beam digital PCR is offering great benefits to the market. Thus, increasing the growth of the Digital PCR Market during the forecast period 2021-2026.
Growing Adoption of Digital PCR over Realtime PCR
Growing adoption of digital PCR over Realtime PCR is increasing the growth of the Digital PCR Market. This is attributed to the fact that digital PCR helps to deliver a compete measure to target nucleic acid molecules that is achieved from real time PCR. DNA quantification allows for reproducibility, precision, and sensitive that enables the researches to quantify smaller differences and measure minor variants very precisely. Thus, increasing the growth of the Digital PCR Market during the forecast period 2021-2026.
Digital PCR Market Challenges
High Cost of Digital PCR Devices and Reimbursement Issues Along with the Technical Limitations of PCR
Some of the factors that are set to impede the growth of the Digital PCR Market are high cost of digital PCR devices and reimbursement issues along with the technical limitations of PCR. The adoption of digital polymerase chain reaction techniques is limited owing to the lack of awareness about the digital PCR and the use of its advanced types.
Buy Now
Digital PCR Market Landscape
Product launches, mergers and acquisitions, joint ventures, and R&D activities are key strategies adopted by players in the Digital PCR Market. In 2020, the Digital PCR Market share is consolidated by the top ten players present in the market. Digital PCR Market, top 10 companies are Thermo Fisher Scientific Inc., BioMerieux SA, Stilla Technologies, Merck KgaA, Combinati Inc., and Bio-Rad Laboratories among others.
Acquisitions/Product Launches
In 2020, BioMerieux SA received approval for Sars COV-2 gene tests that includes saliva specimens.
#Digital PCR Market#Digital PCR Market Size#Genetic Disorders#Digital PCR Industry#Oncology#Digital PCR Market Share#cerebrospinal fluid#Digital PCR top 10 companies#Digital PCR Market Report#Digital PCR Industry Outlook
0 notes