#Single-use Bioprocessing Material
Explore tagged Tumblr posts
Text
#Single-use Bioprocessing Material Market#Single-use Bioprocessing Material#Single-use Bioprocessing
0 notes
Text
Growth Dynamics and Future Roadmap of the Continuous Bioprocessing Industry
Continuous Bioprocessing Market
The global continuous bioprocessing market was valued at USD 349.3 million in 2024 and is projected to reach USD 911.4 million by 2030, expanding at a compound annual growth rate (CAGR) of 18.63% from 2025 to 2030. This rapid growth trajectory is primarily fueled by the increasing demand for cost-effective, scalable, and efficient biopharmaceutical manufacturing solutions, especially in the production of monoclonal antibodies, vaccines, and cell & gene therapies. The adoption of process intensification strategies, underpinned by automation, real-time monitoring technologies, and single-use systems, is significantly improving overall productivity while simultaneously reducing operational expenses.
Furthermore, global regulatory authorities, including the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), are actively promoting the implementation of continuous manufacturing methods. These regulatory bodies highlight the approach’s advantages in achieving superior process control, enhanced product consistency, and reduced production variability, making it a favorable alternative to traditional batch manufacturing.
The industry outlook remains highly optimistic, with market expansion being propelled by a combination of factors such as the accelerated adoption of cutting-edge biomanufacturing technologies, a rising global demand for biologic therapies, and ongoing investments in automation and process optimization across the biopharmaceutical sector. Continuous bioprocessing is rapidly becoming a preferred strategy among pharmaceutical and biotech companies due to its ability to deliver higher yields, minimize costs, and improve quality consistency. Moreover, the increasing global burden of chronic and complex diseases, including cancer, autoimmune disorders, and infectious diseases, is driving the need for faster, more cost-efficient production of biologic drugs—further amplifying the market’s momentum.
A pivotal growth driver lies in the technological advancements shaping bioprocessing equipment and systems. Innovations in real-time process analytics, single-use bioreactors, perfusion technologies, and predictive analytics have dramatically enhanced the practicality and reliability of continuous bioprocessing. Many biopharma firms are now investing heavily in digital biomanufacturing platforms, leveraging artificial intelligence (AI) and machine learning (ML) to fine-tune operations, elevate production yields, and uphold stringent quality standards. Regulatory support from agencies like the FDA and EMA—through published guidelines and streamlined approval pathways—is also easing the transition for companies moving from traditional batch methods to continuous operations.
The market is further propelled by the global shift toward cost-efficient and environmentally sustainable manufacturing. Traditional batch processing methods often involve large-scale facilities, higher capital investment, and excessive material waste. In contrast, continuous bioprocessing reduces energy and resource consumption, minimizes waste generation, and increases operational efficiency, making it an attractive solution for companies pursuing sustainable and agile manufacturing practices. The increasing trend of decentralized production and the need for flexible manufacturing infrastructure, especially in response to evolving global healthcare needs and pandemic preparedness initiatives, are accelerating the adoption of continuous bioprocessing technologies worldwide.
Key Market Trends & Insights
Regional Insights: In 2024, North America emerged as the leading revenue-generating region in the global market. This dominance is attributed to substantial investments in biopharmaceutical R&D, the presence of highly advanced manufacturing facilities, and favorable regulatory frameworks. Prominent industry leaders such as Thermo Fisher Scientific, Cytiva (a Danaher company), and Sartorius are channeling significant investments into next-generation bioprocessing technologies to support the production of biologics, biosimilars, and cell & gene therapies.
Country-Specific Insight: India is projected to register the highest CAGR in the market from 2025 to 2030, driven by its expanding biopharmaceutical sector, growing clinical research activity, and supportive government initiatives.
Segment Insights – Product Type: The consumables and reagents segment accounted for USD 214.6 million in revenue in 2024, fueled by the growing adoption of single-use technologies (SUTs), innovations in cell culture media, and the rising need for high-purity reagents to support uninterrupted and contamination-free bioproduction processes.
Segment Insights – Application: Monoclonal antibodies (mAbs) held the largest market share, contributing 98% of total revenue in 2024. The segment's dominance is linked to the surging global demand for biologic therapies, particularly those used in treating oncological, autoimmune, and infectious diseases.
Segment Insights – End-use: The pharmaceutical and biotechnology companies segment represented the largest end-user category, capturing a 43% revenue share in 2024. This is due to the increasing pressure on biopharma firms to produce high-yield, cost-effective, and scalable biologics, especially monoclonal antibodies, biosimilars, cell & gene therapies, and next-gen vaccines. As a result, many companies are transitioning from traditional batch processing models to continuous manufacturing platforms to improve efficiency, scalability, and product throughput.
Order a free sample PDF of the Continuous Bioprocessing Market Intelligence Study, published by Grand View Research.
Market Size & Forecast
2024 Market Size: USD 349.3 Million
2030 Projected Market Size: USD 911.4 Million
CAGR (2025-2030): 18.63%
North America: Largest market in 2024
Key Players
Danaher
Sartorius AG
Thermo Fisher Scientific Inc.
WuXi Biologics
Ginkgo Bioworks
Merck KGaA
GE Healthcare
Repligen Corporation
Asahi Kasei Bioprocess America, Inc.
Browse Horizon Databook on Global Continuous Bioprocessing Market Size & Outlook
Conclusion
The global continuous bioprocessing market is undergoing a significant transformation, driven by the urgent need for more efficient, scalable, and cost-effective biomanufacturing solutions. With a robust CAGR of 18.63% forecasted between 2025 and 2030, the market is poised for remarkable growth, reflecting a broader industry shift toward innovation, automation, and sustainability. Continuous bioprocessing offers substantial advantages over traditional batch methods—including enhanced product consistency, reduced operational costs, and improved process efficiency—making it increasingly attractive to pharmaceutical and biotechnology companies worldwide.
As chronic diseases and demand for biologics continue to rise, coupled with advancements in single-use technologies, real-time analytics, and AI-driven process control, continuous manufacturing is set to become the new standard in biologic drug production. Supportive regulatory frameworks from agencies such as the FDA and EMA further facilitate this transition, encouraging adoption through guidance and faster approvals.
Looking ahead, regions like North America will continue to lead the market due to their advanced infrastructure and high investment levels, while emerging markets such as India will offer strong growth opportunities. Key segments like monoclonal antibodies and consumables & reagents will remain central to revenue generation. In an era that demands flexible, rapid-response manufacturing—especially in light of global health emergencies—continuous bioprocessing stands out as a pivotal solution shaping the future of biologics production.
0 notes
Text
Sustainable Bioprocessing Gains Ground with Recyclable Single-Use Systems
The Single-use Bioprocessing Market is entering a transformative growth phase, fueled by the rapid expansion of biopharmaceutical manufacturing, rising demand for flexible production systems, and heightened focus on contamination control. Valued at USD 7.62 billion in 2021, the market is projected to reach USD 25.40 billion by 2031, growing at an impressive CAGR of 18.93% during the forecast period (2024–2031).

Unlock exclusive insights with our detailed sample report :
This market is evolving rapidly with the increasing adoption of single-use bioprocessing systems such as bioreactors, fermenters, mixers, filtration units, bags, and containers. These disposable systems, primarily made of sterilizable plastic components, support critical processes like upstream expression, purification, storage, and separation of biopharmaceutical products. As global biomanufacturing shifts toward agile, cost-effective, and contamination-free solutions, single-use technologies are being embraced by both established pharma giants and emerging biotech firms—especially in alignment with trends seen in the United States and Asia, where scalable, single-use systems are driving next-gen biologics production.
Key Market Drivers
1. Rise in Biologics and Personalized Medicine: The growth of monoclonal antibodies, gene therapy, and personalized medicine has created a strong need for scalable, contamination-free, and faster bioprocessing methods. Single-use systems (SUS) offer agility and ease of implementation, making them ideal for such advanced therapies.
2. Cost Efficiency and Operational Flexibility: Unlike traditional stainless-steel systems, SUS eliminate the need for cleaning and sterilization, significantly reducing downtime and water/chemical usage. This makes them highly attractive for small-to-mid-sized biotech firms and contract manufacturing organizations (CMOs).
3. Pandemic Preparedness and Vaccine Development: COVID-19 and subsequent global health threats highlighted the urgent need for rapid-response manufacturing capabilities. Single-use systems enabled fast-tracked vaccine production and played a pivotal role in scaling mRNA technologies.
4. Regulatory and Environmental Incentives: The U.S. FDA and EMA support the adoption of single-use bioreactors and modular facilities, facilitating market expansion. Meanwhile, vendors are innovating biodegradable materials to tackle concerns over plastic waste and sustainability.
Speak to Our Senior Analyst and Get Customization in the report as per your requirements:
Market Segmentation Snapshot
By Product Type: The market is segmented into bioreactors, mixers, bags, tubes & connectors, sampling systems, and others. Bioreactors and mixing systems account for the largest share due to their central role in upstream processing.
By Workflow: Upstream processing dominates the market share, given the high usage of SUS in cell culture and fermentation.
By End-User: Biopharmaceutical companies hold the majority share, followed by CMOs and academic research institutes.
Regional Insights
United States: The U.S. remains the largest and most mature market, fueled by robust biopharma R&D, government funding, and large-scale manufacturing investments. In 2024, the U.S. government announced a new initiative to strengthen domestic biologics production, allocating over USD 2 billion toward advanced manufacturing platforms—predominantly single-use facilities. Leading companies like Thermo Fisher Scientific, Danaher Corporation, and Sartorius Stedim Biotech are expanding their manufacturing capacities across North America.
Japan: Japan’s biopharma industry is rapidly integrating single-use systems as part of its strategic vision for regenerative medicine and mRNA vaccine production. In 2024, the Japanese Ministry of Health, Labour and Welfare (MHLW) introduced incentives for companies transitioning from conventional systems to disposable solutions. Furthermore, leading Japanese firms such as Asahi Kasei and Nipro Corporation are increasing R&D spending on sustainable single-use materials to enhance product lifecycle management.
Europe and Asia-Pacific: Germany, the UK, and Switzerland continue to be innovation hubs, while emerging economies in the Asia-Pacific region, such as China, South Korea, and India, are rapidly adopting SUS to scale biologics and biosimilars production.
Latest Industry Trends
AI Integration in Bioprocess Monitoring: Vendors are now embedding AI and data analytics into SUS platforms to allow predictive maintenance, batch tracking, and process optimization.
Modular Biomanufacturing Units: Companies are investing in mobile, modular units using SUS to cater to outbreak hotspots and rural regions, enhancing supply chain agility.
Green Bioprocessing Innovations: Environmental concerns are driving innovations in recyclable polymers and closed-loop systems to minimize single-use plastic waste.
M&A Activity on the Rise: The market is witnessing increased mergers and acquisitions. For example, in Q1 2025, Repligen Corporation announced the acquisition of a European tubing and bagging system manufacturer to strengthen its product portfolio.
Buy the exclusive full report here:
Competitive Landscape
The market is highly competitive and fragmented, with major players focusing on product innovation, capacity expansion, and strategic partnerships. Notable players include:
Thermo Fisher Scientific Inc.
Sartorius AG
Danaher Corporation (Cytiva and Pall)
Merck KGaA
Eppendorf AG
Parker Hannifin Corp.
Avantor, Inc.
Corning Incorporated
PBS Biotech, Inc.
Saint-Gobain Performance Plastics
These companies are investing in next-generation single-use assemblies, automation, and flexible manufacturing to meet evolving industry demands.
Growth Opportunities
CMO & CDMO Expansion: As pharmaceutical outsourcing grows, CMOs are increasingly deploying SUS to reduce turnaround time and manage multiple client processes efficiently.
Biosimilar Production: The patent cliff for blockbuster biologics has opened lucrative opportunities for biosimilars, where SUS offers a cost-effective pathway to scale.
Emerging Markets Penetration: Expansion in Latin America, Southeast Asia, and Africa presents a significant untapped opportunity, supported by international funding agencies.
Regenerative Medicine and Cell Therapy: As cell therapy and tissue engineering progress, single-use bioreactors and closed systems will be pivotal in clinical and commercial scale-up.
Stay informed with the latest industry insights-start your subscription now:
Conclusion
The global single-use bioprocessing market is on the cusp of a revolution, catalyzed by innovation in biologics, operational efficiency, and a growing preference for flexible, scalable manufacturing. The United States and Japan stand at the forefront of this transformation, while global demand signals a sustained, long-term market boom. As regulatory and environmental concerns are addressed through innovation, single-use technologies are poised to become the new standard in biopharmaceutical manufacturing.
#single-use bioprocessing market#single-use bioprocessing market size#single-use bioprocessing market growth#single-use bioprocessing market share#single-use bioprocessing market analysis
1 note
·
View note
Text
Viral Vector Industry Accelerates with Cancer Therapy Demand
Viral Vectors are Essential Components in gene therapy, cell-based treatments, and vaccine development. They act as carriers to deliver genetic material into cells. As the demand for targeted therapies increases, so does the need for high-quality, scalable viral vector production. The market’s growth is fueled by advancements in bioprocessing, a surge in gene therapy clinical trials, and rising funding from both public and private sectors.

To Get Sample Report: https://www.datamintelligence.com/download-sample/viral-vector-manufacturing-market
Market Drivers and Growth Opportunities
1. Gene Therapy Pipeline Expansion Gene therapy is one of the primary catalysts for market growth. Hundreds of clinical trials are underway globally, particularly for cancer and rare diseases, significantly increasing demand for viral vectors.
2. Oncology Segment Growth Cancer therapies using viral vectors are surging. The oncology application alone is expected to grow from USD 1.33 billion in 2024 to approximately USD 3.47 billion by 2029, making it a dominant segment.
3. Strong Government and Regulatory Support In the United States, federal funding and streamlined regulatory pathways support biotechnology innovation. Japan is also pushing for faster approvals and investment in domestic biomanufacturing.
4. Outsourced Manufacturing (CDMOs) Contract development and manufacturing organizations are essential players. Companies like Charles River and others are expanding GMP-compliant facilities to meet increasing demand.
5. Innovation in Bioprocessing Technologies Advancements in bioreactor design, vector purification systems, and multi-omics platforms have improved yield and reduced manufacturing time, making production more scalable.
6. Rise in Rare Disease Research The growing number of rare diseases requiring gene therapy has made personalized viral vector production a lucrative market segment.
Regional Insights
North America North America leads the market with a significant share, valued at USD 0.8 billion in 2024. The region benefits from a concentration of biotech firms, robust funding, and growing adoption of advanced manufacturing technologies.
Japan Japan’s viral vector manufacturing market was valued at USD 210 million in 2023 and is expected to reach approximately USD 782 million by 2030, growing at 20.6% CAGR. Investments in domestic capabilities and government support are key drivers.
Asia-Pacific The Asia-Pacific region is witnessing rapid growth with rising investments in gene therapy, especially in China and India. The region’s market is expected to reach USD 13.5 billion by 2032, reflecting strong momentum in both R&D and production infrastructure.
Europe European countries are investing in regional biomanufacturing hubs, supported by initiatives like Horizon Europe. Strategic partnerships between pharma companies and CDMOs are strengthening the market presence across Germany, France, and the UK.
Industry Trends
AAV Vectors Dominate Clinical Use Adeno-associated virus vectors remain the most popular choice due to safety and efficiency, especially in neurology and ophthalmology.
Adoption of Lentiviral Vectors for CAR-T Lentiviral vectors are increasingly used in CAR-T cell therapies for blood cancers, opening new avenues for market expansion.
Omics-based Optimization Genomic, transcriptomic, and proteomic technologies are being used to optimize vector development and improve yields.
Advanced Bioreactors and Automation Automated, single-use bioreactors are gaining popularity for their efficiency, scalability, and reduced contamination risk.
Strategic Collaborations and M&A Companies are forming alliances to expand capacity, develop proprietary platforms, and ensure supply chain resilience.
Challenges
Complex Regulatory Landscape Strict compliance requirements and evolving global standards can delay product approval and increase production costs.
High Manufacturing Costs Upfront capital investment and technical expertise needed for viral vector production remain significant barriers for new entrants.
Supply Bottlenecks Rapid demand growth is outpacing current supply capacities, especially in regions with limited infrastructure.
Strategic Opportunities
Scale-Up Through CDMOs Partnering with experienced CDMOs can enable faster scaling of production for emerging gene therapies.
Region-Specific Expansion Focus on expanding manufacturing capacity in APAC and Japan to meet local demand and regulatory needs.
Diversification of Vector Types Investing in a portfolio of AAV, adenoviral, and lentiviral vectors allows companies to serve multiple therapeutic areas.
R&D in Vector Engineering Genetic modification of vectors to enhance safety, targeting, and expression is a high-potential R&D area.
Conclusion and Future Outlook
The viral vector manufacturing market is poised for strong global growth, projected to exceed USD 5 billion by 2030. With increasing demand from oncology and gene therapy sectors, advancements in production technology, and rising investment from governments and private players, the market outlook remains highly favorable. Companies that invest in innovation, scalable infrastructure, and strategic partnerships will be well-positioned to lead the next generation of advanced therapeutics.
0 notes
Text
Bioprocess Bags Market Growth, Analysis and Future Outlook 2034

Bioprocess bags are specialized, single-use containers used in the biopharmaceutical and biotechnology industries for handling, storing, mixing, and transporting liquids and biological materials during the manufacturing process. Made from multi-layered polymer films, these bags are designed to maintain sterility and ensure chemical compatibility with a wide range of media, including cell cultures, buffers, and reagents. They serve as flexible, scalable alternatives to traditional stainless-steel vessels, reducing the risk of cross-contamination and eliminating the need for cleaning and sterilization. During different phases of production, such as fermentation, purification, and formulation, bioprocess bags are utilized. Their adaptability, cost-effectiveness, and ease of integration into closed-system manufacturing have made them essential components in modern bioprocessing, particularly in vaccine and biologics production.
According to SPER market research, ‘Bioprocess Bags Market Growth, Size, Trends Analysis - By Type, By Workflow, By End User - Regional Outlook, Competitive Strategies and Segment Forecast to 2034’ state that the Global Bioprocess Bags Market is estimated to reach USD 19.76 billion by 2034 with a CAGR of 16.61%.
Drivers:
The market for bioprocess bags worldwide is expanding significantly due to a number of important factors. Foremost is the increasing demand for biopharmaceuticals, including monoclonal antibodies, vaccines, and gene therapies, which necessitate efficient and scalable manufacturing processes. Bioprocess bags, integral to single-use systems, offer advantages such as reduced contamination risk, lower cleaning and validation costs, and increased flexibility in bioprocessing operations. The rise in personalized medicine and cell and gene therapies further amplifies this demand, as these treatments often require custom manufacturing processes that leverage bioprocess bags for efficiency and safety. Additionally, the growing emphasis on sustainable manufacturing practices positions bioprocess bags as a favorable alternative to traditional stainless-steel systems, due to their lower environmental footprint. All of these elements work together to support the bioprocess bags market's strong growth on a worldwide scale.
Request a Free Sample Report: https://www.sperresearch.com/report-store/bioprocess-bags-market.aspx?sample=1
Restraints:
There are many obstacles that could prevent the worldwide bioprocess bags market from expanding. A major concern is the environmental impact of disposing non-biodegradable plastic materials used in these bags, drawing increasing regulatory attention. Additionally, risks of leachables and extractables from plastic into biopharmaceutical products require stringent validation, raising production complexity and cost. High raw material and sterilization costs can limit affordability, particularly for small- and medium-sized manufacturers. Supply chain disruptions also pose a challenge, affecting the timely availability of these essential components. These factors collectively restrain the broader adoption and scalability of bioprocess bags despite their advantages in biomanufacturing.
The United States holds a dominant position in the global bioprocess bags market, primarily due to its robust biopharmaceutical industry, advanced healthcare infrastructure, and significant investments in research and development. Some significant market players are Thermo Fisher Scientific Inc, Sartorius AG, Danaher Corporation, Merck KGaA, Saint-Gobain, Corning Incorporated, Entegris and others.
For More Information, refer to below link: –
Bioprocess Bags Market Growth
Related Reports:
Disposable Blood Bags Market Size
Japan Disposable Toilet Bags Market Size
Follow Us –
LinkedIn | Instagram | Facebook | Twitter
Contact Us:
Sara Lopes, Business Consultant — USA
SPER Market Research
+1–347–460–2899
#Bioprocess Bags Market#Bioprocess Containers Market#Bioprocess Containers and Fluid Transfer Solutions Market#Bioprocess Bags Market Growth#Bioprocess Bags Market Trends#Bioprocess Bags Market Share#Bioprocess Bags Market Size#Bioprocess Bags Market Revenue#Bioprocess Bags Market Demand#Bioprocess Bags Market Analysis#Bioprocess Bags Market Segmentation#Bioprocess Bags Market Future Outlook#Bioprocess Bags Market Report#Bioprocess Bags Market Challenges#Bioprocess Bags Market Competition
0 notes
Text
New Chapter in Life Sciences Innovation
The life sciences industry is evolving faster than ever, driven by cutting-edge research, precision medicine, and breakthrough technologies in biopharma, diagnostics, and laboratory solutions. At the heart of this transformation stands Foxx Life Sciences, a trusted partner in delivering innovative and customizable laboratory products for researchers, scientists, and healthcare providers around the world.
Why This New Chapter Matters
As global health challenges become increasingly complex, the demand for smarter, safer, and more sustainable laboratory solutions has never been higher. Companies like Foxx Life Sciences are leading the New Chapter in Life Sciences Innovation equipment, single-use systems, and fluid management solutions can better serve the scientific community.

From supporting vaccine development to enhancing environmental testing capabilities, the innovations shaping this new chapter in life sciences are focused on efficiency, scalability, and environmental responsibility.
Foxx Life Sciences: At the Forefront of Change
Known for its comprehensive range of bioprocess, filtration, and laboratory safety products, Foxx Life Sciences continues to pioneer new product designs that meet the evolving needs of modern laboratories. Their commitment to quality, customization, and global accessibility positions them as a vital contributor in this exciting era of life sciences innovation.
Whether it's through cutting-edge single-use systems, eco-friendly laboratory consumables, or advanced fluid management technologies, Foxx Life Sciences is empowering researchers to push the boundaries of what's possible.
Embracing Emerging Trends in Life Sciences
As we step into this new era, several transformative trends are shaping the future of life sciences:
Single-Use Technology (SUT): The rapid adoption of single-use systems in bioprocessing is improving operational flexibility and reducing contamination risks. Foxx Life Sciences has been instrumental in developing reliable, customizable single-use solutions for biotech and pharmaceutical manufacturing.
Sustainable Laboratory Practices: Environmental responsibility is now a priority. Companies like Foxx Life Sciences are addressing this by offering eco-friendly lab consumables and fluid management products that minimize waste without compromising performance.
Smart Laboratory Infrastructure: From connected lab equipment to advanced filtration systems, modern laboratories are becoming more intelligent and automated. Foxx Life Sciences is contributing to this shift with innovative designs that integrate seamlessly into next-gen research facilities.
Global Collaboration & Supply Chain Solutions: In a world where timely access to high-quality lab materials is crucial, Foxx Life Sciences ensures global distribution capabilities, helping scientists and healthcare professionals around the world meet urgent research demands efficiently.
A Global Vision for a Healthier Tomorrow
What sets Foxx Life Sciences apart is not just their product innovation, but their global vision. By forging strategic partnerships and maintaining manufacturing and distribution hubs in key markets, the company supports life science breakthroughs across continents — from North America and Europe to Asia and beyond.
Their flexible, client-focused approach ensures tailored solutions for every need, whether it’s scaling up vaccine production or outfitting university labs with state-of-the-art equipment.
Final Thoughts: The Road Ahead
As the life sciences sector continues to navigate new frontiers, collaboration and innovation will remain its most valuable assets. Foxx Life Sciences exemplifies what it means to be a forward-thinking partner in this journey, providing the tools, technologies, and expertise needed to tackle today’s biggest health challenges and unlock tomorrow’s greatest discoveries.
Contact us: [email protected]
https://www.facebook.com/foxxlifescienceshttps://www.pinterest.com/foxxlifesciences/
0 notes
Text
Single-use Bioprocessing Market: Market Trends and Market Growth 2024-2032

The Single-use Bioprocessing Market Size was valued at USD 26.8 Billion in 2023 and is expected to reach USD 100.9 Billion by 2032, growing at a CAGR of 15.9% over the forecast period 2024-2032. This growth is driven by the increasing demand for biopharmaceuticals, advancements in single-use technology, and a shift toward cost-effective and flexible manufacturing solutions.
Get Free Sample Report @ https://www.snsinsider.com/sample-request/3661
Market Segmentation
By Product:
Simple & Peripheral Elements: Tubing, filters, connectors, transfer systems, bags, sampling systems, probes, sensors (pH, oxygen, pressure, temperature, conductivity, flow), and others.
Apparatus & Plants: Bioreactors (up to 1000L, 1000L-2000L, above 2000L), mixing, storage, filling systems, filtration systems, chromatography systems, pumps, and others.
Work Equipment: Cell culture systems, syringes, and others.
By Workflow:
Upstream bioprocessing
Fermentation
Downstream bioprocessing
By End-use:
Biopharmaceutical Manufacturers: CMOs & CROs, in-house manufacturers.
Academic & Clinical Research Institutes.
Regional Analysis
North America: Held the largest market share in 2023, driven by a strong biopharmaceutical sector, significant investments in research, and government support for biomanufacturing.
Asia-Pacific: Expected to witness the fastest growth due to rising healthcare investments, an expanding biopharmaceutical industry, and increased adoption of single-use technologies in manufacturing. Countries like China and India are playing a crucial role in market expansion.
Key Players
Service Providers / Manufacturers:
Thermo Fisher Scientific (HyPerforma Single-Use Bioreactors, BioProcess Containers)
Sartorius AG (Biostat STR Bioreactors, Flexsafe Bags)
Merck KGaA (Ultimus Film, Mobius Single-Use Systems)
Danaher Corporation (Xcellerex Bioreactors, Pall Allegro Systems)
GE Healthcare Life Sciences (Xcellerex XDR Bioreactors, WAVE Bioreactor Systems)
Eppendorf AG (BioBLU Single-Use Vessels, CelliGen BLU Bioreactors)
Corning Incorporated (HYPERStack Cell Culture Vessels, CellCube Modules)
Avantor, Inc. (J.T.Baker BPCs, Single-Use Mixer Systems)
Cellexus International (CellMaker Plus, CellMaker Regular)
Lonza Group AG (MODA-ES Bioprocess Software, Nunc Cell Factory Systems)
Key Market Trends
Growing demand for biologics, including vaccines and monoclonal antibodies.
Continuous technological advancements improving efficiency and scalability.
Increasing government support for biopharmaceutical manufacturing infrastructure.
Cost-effectiveness and flexibility of single-use systems compared to traditional stainless-steel setups.
Challenges include concerns over material leachables and extractables, as well as the need for standardization across single-use designs.
Future Scope
The single-use bioprocessing market is set for transformative growth, driven by the rising adoption of biologics and cell & gene therapies. Hybrid systems combining single-use and stainless-steel technologies are emerging to address scalability challenges. Moreover, the expansion into emerging markets presents substantial opportunities, as the demand for flexible and cost-efficient biomanufacturing solutions continues to rise.
Conclusion
The single-use bioprocessing market is undergoing rapid evolution, fueled by technological advancements and increasing global demand for biologics. While challenges related to standardization and material concerns remain, ongoing innovations and strategic investments will further strengthen the role of single-use technologies in the future of biopharmaceutical manufacturing.
Contact Us: Jagney Dave - Vice President of Client Engagement Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK)
Other Related Reports:
Fertility Services Market
Medical Power Supply Market
Post Traumatic Stress Disorder Treatment Market
MRI Guided Neurosurgical Ablation Market
#Single-use Bioprocessing Market#Single-use Bioprocessing Market Share#Single-use Bioprocessing Market Size#Single-use Bioprocessing Market Trends
0 notes
Text
Fermentation Vessels and Bioreactors: Driving Innovation in Bioprocessing
In the fields of pharmaceuticals, biotechnology, and food production, fermentation vessels and bioreactors play a crucial role in ensuring the growth of microorganisms, cells, and biologically active compounds under controlled conditions. These specialized systems are indispensable in producing vaccines, antibiotics, enzymes, and various biopharmaceutical products, making them the backbone of industrial bioprocessing.
Importance of Fermentation Vessels and Bioreactors
Fermentation vessels and bioreactors provide the ideal environment for cultivating microorganisms and cells essential for producing bio-based products. These vessels offer precise control over key parameters such as temperature, pH, oxygen levels, and agitation, ensuring optimal growth conditions and maximizing product yield.
The ability to maintain a sterile environment is paramount, as contamination can compromise entire production batches. Modern fermentation vessels and bioreactors are designed to minimize risks while enhancing productivity, making them indispensable for industries seeking consistency, scalability, and quality in their processes.
Compliance with Regulatory Standards
In pharmaceutical and biotech applications, adhering to strict regulatory standards is non-negotiable. Compliance with Good Manufacturing Practice (GMP) guidelines, the U.S. Food and Drug Administration (FDA), and other global regulatory bodies is essential to ensure product safety, quality, and efficacy.
Fermentation vessels and bioreactors must meet stringent standards for material selection, surface finish, and sterilization protocols. Regular validation and calibration processes are conducted to guarantee that every step of production aligns with regulatory requirements. Companies investing in compliant systems safeguard not only their products but also their reputations.
Innovation in Manufacturing Processes
Technological advancements have transformed fermentation vessels and bioreactors into highly sophisticated systems capable of automating complex bioprocesses. Innovations such as real-time monitoring, data-driven process control, and advanced sensors enable operators to fine-tune parameters, ensuring consistent performance and minimizing human error.
Additionally, the integration of single-use bioreactors has revolutionized the industry by reducing cross-contamination risks, accelerating production timelines, and lowering operational costs. These innovations empower manufacturers to remain agile in an increasingly competitive market.
Enhancing Productivity with Advanced Technologies
Modern fermentation vessels and bioreactors incorporate advanced technologies to enhance productivity and efficiency. Automation systems optimize processes, reducing downtime and ensuring uniform product quality. Real-time data collection and analysis enable predictive maintenance, minimizing disruptions and extending equipment lifespan.
Moreover, scalable designs allow for seamless transition from laboratory-scale to commercial production, accommodating the growing demands of pharmaceutical and biotech industries. By leveraging these technologies, companies can streamline their operations while maintaining the highest quality standards.
Ensuring Product Safety
Product safety is at the heart of every bioprocess. Fermentation vessels and bioreactors are meticulously designed to maintain sterile conditions throughout production. Features such as clean-in-place (CIP) and sterilize-in-place (SIP) systems ensure thorough cleaning and sterilization between production runs, preventing cross-contamination.
Material selection is equally important, with stainless steel and other non-reactive materials used to ensure product purity. Additionally, advanced filtration and monitoring systems help detect and eliminate potential contaminants, safeguarding both products and consumers.
Fermentation vessels and bioreactors are indispensable for the pharmaceutical, biotech, and food industries, ensuring consistent product quality, regulatory compliance, and enhanced productivity. Advanced technologies and rigorous manufacturing standards have made these systems more reliable and efficient than ever before.
Swjal Process Pvt. Ltd. stands at the forefront of delivering cutting-edge fermentation vessels and bioreactors. As a leading manufacturer in India, Swjal Process Pvt. Ltd. combines innovation, compliance, and expertise to provide robust solutions that empower industries to achieve excellence in bioprocessing.
#industrial vessles#mixing tanks#vessle with agiators#Fermentation vessels and bioreactors#swjal process
0 notes
Text
The Growing Importance of the Medical Filtration Market: A Comprehensive Overview

In today’s fast-paced world, the healthcare industry is constantly evolving, and one of the most critical yet often overlooked aspects is medical filtration. From ensuring the purity of pharmaceuticals to maintaining sterile environments in hospitals, medical filtration plays a pivotal role in safeguarding public health. In this blog, we’ll dive deep into the medical filtration market, exploring its significance, trends, and future prospects.
What is Medical Filtration?
Medical filtration refers to the process of removing contaminants, impurities, and particles from liquids and gases used in medical applications. This process is essential in various healthcare settings, including hospitals, laboratories, and pharmaceutical manufacturing units. The primary goal is to ensure the safety, efficacy, and quality of medical products and environments.
Medical filtration systems are used in a wide range of applications, such as:
Pharmaceutical Manufacturing: Ensuring the purity of drugs and vaccines.
Hospital Settings: Filtering air and water to maintain sterile environments.
Laboratories: Purifying samples and reagents for accurate testing.
Biotechnology: Supporting research and development processes.
Why is the Medical Filtration Market Booming?
The medical filtration market has witnessed significant growth in recent years, and this trend is expected to continue. Here are some key factors driving this expansion:
Rising Demand for Advanced Healthcare SolutionsWith the increasing prevalence of chronic diseases and the growing aging population, there is a heightened demand for effective healthcare solutions. Medical filtration ensures the safety and efficacy of treatments, making it indispensable in modern healthcare.
Stringent Regulatory StandardsGovernments and regulatory bodies worldwide have implemented strict guidelines to ensure the quality and safety of medical products. Compliance with these standards has fueled the adoption of advanced filtration technologies.
Technological AdvancementsInnovations in filtration materials and techniques, such as nanofiber membranes and ultrafiltration, have enhanced the efficiency and reliability of medical filtration systems.
Increased Focus on Infection ControlThe COVID-19 pandemic highlighted the importance of infection control in healthcare settings. Medical filtration systems, particularly air filtration, have become critical in preventing the spread of infectious diseases.
Growth of the Pharmaceutical and Biotechnology IndustriesThe expansion of these industries, driven by the development of new drugs and therapies, has created a surge in demand for high-quality filtration systems.
Key Trends Shaping the Medical Filtration Market
Adoption of Single-Use Filtration SystemsSingle-use filtration systems are gaining popularity due to their convenience, cost-effectiveness, and reduced risk of contamination. These systems are particularly useful in pharmaceutical manufacturing and bioprocessing.
Focus on SustainabilityAs environmental concerns grow, there is a shift toward eco-friendly filtration solutions. Manufacturers are developing reusable and biodegradable filtration products to minimize waste.
Integration of Smart TechnologiesThe integration of IoT and AI in filtration systems is revolutionizing the market. Smart filtration systems can monitor performance in real-time, predict maintenance needs, and optimize efficiency.
Expansion in Emerging MarketsDeveloping countries are investing heavily in healthcare infrastructure, creating new opportunities for the medical filtration market. Increased awareness about healthcare quality is also driving demand in these regions.
Challenges in the Medical Filtration Market
While the market is thriving, it is not without its challenges:
High Costs: Advanced filtration technologies can be expensive, posing a barrier for small-scale healthcare providers.
Complex Regulatory Landscape: Navigating the stringent and often varying regulations across different regions can be challenging for manufacturers.
Technical Limitations: Certain applications, such as filtering nanoparticles, require further technological advancements.
Future Outlook of the Medical Filtration Market
The future of the medical filtration market looks promising, with continued growth expected in the coming years. The global medical filtration market is projected to reach USD 9.35 billion by 2030 from USD 6.29 billion in 2024, at a CAGR of 6.8%.
Key factors contributing to this growth include:
Increasing investments in healthcare infrastructure.
Rising demand for personalized medicine and biologics.
Growing awareness about the importance of clean and safe medical environments.
How to Choose the Right Medical Filtration Solution
If you’re in the healthcare or pharmaceutical industry, selecting the right filtration system is crucial. Here are some tips to help you make an informed decision:
Identify Your Needs: Determine the specific application and requirements of your filtration system.
Evaluate Quality and Efficiency: Look for systems that meet industry standards and offer high filtration efficiency.
Consider Maintenance and Costs: Opt for solutions that are cost-effective and easy to maintain.
Check for Certifications: Ensure the product complies with relevant regulatory standards.
Conclusion
The medical filtration market is a cornerstone of modern healthcare, ensuring the safety and efficacy of medical products and environments. With technological advancements, increasing demand for healthcare solutions, and a focus on sustainability, the market is poised for significant growth. Whether you’re a healthcare provider, pharmaceutical manufacturer, or researcher, understanding the importance of medical filtration can help you make better decisions and contribute to a healthier world.
By staying informed about the latest trends and innovations in the medical filtration market, you can stay ahead of the curve and ensure the highest standards of quality and safety in your operations.
#Medical Filtration Market#Healthcare Filtration#Pharmaceutical Filtration#Filtration Trends#Infection Control
0 notes
Text
Bioprocess containers Market Industry Forecast, 2024–2030
Bioprocess Containers Market Overview

Request Sample :
A major trend is the growing demand for single-use bioprocess containers. Single-use bioprocess containers eliminate cleaning requirements between batches and reduce the risk of batch failure due to cross-contamination. Additionally, single-use systems are also cost effective. Respondents identified lower-cost single use devices as the most important area of interest for new products and technologies in 2023. Another notable trend is the rise in upstream bioprocess products driven by increasing demand for biopharmaceuticals, including biologics and gene therapies. As the industry focuses on enhancing cell culture, fermentation, and early-stage production efficiencies, there is a heightened need for advanced upstream bioprocess products to support these developments. According to a February 2024 article in BioProcess International, the top areas in upstream production were cell-culture media, bioreactors, and information technology (IT), which encompasses automation, software, and networking. Interest in IT solutions among respondents grew from 21.1% in 2022 to 29% in 2023.
COVID-19 / Ukraine Crisis — Impact Analysis:
The COVID-19 pandemic significantly accelerated the growth of the bioprocess containers market, driven by the urgent demand for vaccine production and biologics manufacturing. As pharmaceutical companies ramped up production, flexible and scalable solutions like single-use bioprocess containers became critical for handling cell cultures, reagents, and drug formulations. Additionally, disruptions in global supply chains and a heightened focus on healthcare infrastructure underscored the importance of efficient bioprocessing technologies. Post-pandemic, the market continues to grow, with increased interest in biologics, gene therapies, and personalized medicine fueling demand for flexible manufacturing solutions.
The Ukraine-Russia war has disrupted global supply chains, impacting the bioprocess containers market. The conflict has led to shortages of raw materials, increased transportation costs, and delays in production for biopharma manufacturing. Europe, a key region for bioprocessing, has been particularly affected by rising energy prices and supply chain instability. Companies have been forced to seek alternative suppliers and adjust logistics strategies to mitigate these disruptions. Despite the challenges, the growing demand for biologics and vaccines continues to drive the market, with manufacturers prioritizing resilient, flexible bioprocess solutions to ensure production continuity.
Key Takeaways 2D Bioprocess Containers are the Largest Segment
2D bioprocess containers dominate the bioprocess containers markets due to their cost-effectiveness and operational efficiency. Offering benefits such as easier storage, reduced contamination risk, and simplified handling, 2D containers meet the industry’s growing demand for scalable and flexible solutions. Additionally, their structure minimizes contamination risks and allows for easier handling, streamlining workflows and reducing labor-intensive procedures. As the biopharmaceutical industry grows, there is an increasing demand for scalable and flexible solutions to meet diverse production needs. 2D containers cater to these requirements by enabling single-use options that simplify transitions between processes, making them ideal for adaptable production setups.
Inquiry Before Buying:
Bioprocess Containers Market Segment Analysis — By Application
Cell culture is a major application of bioprocess containers (BPCs), playing a crucial role in the biomanufacturing industry. BPCs are extensively used for growing and maintaining mammalian, microbial, and insect cells, offering flexibility and scalability in production. They are designed with features like ports for sampling and reagent addition, and integrated sensors for monitoring environmental conditions. The increasing demand for biologics, including therapeutic proteins and vaccines, has driven the widespread adoption of BPCs in cell culture, making it a key focus in the development and manufacturing of biopharmaceutical products. According to Merck 2024, Merck invested $24.84 million to expand cell culture media production in Kansas, USA.
North America Dominates the Market
North America leads the global bioprocess container (BPC) market due to its advanced biopharmaceutical industry and substantial investments in research and development. The United States and Canada are at the forefront, driving significant growth with a strong focus on innovative single-use technologies and sophisticated manufacturing processes. The region’s robust infrastructure, presence of major biopharma companies, and commitment to cutting-edge advancements make North America a key player in the BPC market, particularly in applications such as cell culture, media preparation, and downstream processing. The United States dominates the pharmaceutical industry, home to some of the world’s largest companies like Pfizer, Johnson & Johnson, Merck & Co. and AbbVie. In July 2024, Despite the expected decrease in COVID-related revenue, Pfizer’s revenue rose 3% operationally to $28.2 billion. In December 2023, Pfizer finalized the $43 billion acquisition of Seagen (a biotech company), doubling its oncology pipeline. Such developments are a major catalyst for growth in the bioprocess container market, as it leads to a significant increase in the production of biopharmaceuticals, particularly in the oncology space.
Bioprocess Containers Market Drivers
Single Use Systems
The cost-effectiveness of single-use systems is a major driver of the bioprocess containers market. Single-use bioprocess containers significantly reduce operational costs compared to traditional stainless-steel systems by eliminating the need for time-consuming cleaning, sterilization, and maintenance. This leads to lower capital and operational expenses, making single-use systems an attractive option for biopharmaceutical manufacturers looking to streamline their processes and improve efficiency. As a result, the adoption of single-use bioprocess containers is increasing, driven by their economic advantages and the growing demand for flexible and cost-efficient manufacturing solutions. BioPlan’s 19th Annual Report and Survey of Biopharmaceutical Manufacturing indicates a substantial rise in the use of commercial-scale single-use (SUS) bioreactors, which grew from 32.5% in 2019 to 43% in 2022. This 32% increase is primarily due to the heightened demand for flexibility and rapid deployment in bioproduction during the COVID-19 pandemic.
Schedule A Call :
Rising Demand for Biopharmaceuticals
The rising demand for biopharmaceuticals is a key driver of the bioprocess containers market, particularly due to the increasing prevalence of chronic diseases such as cancer, diabetes, and autoimmune disorders among the aging population. As the global population of individuals aged 60 and above continues to grow, with significant increases in regions like North America, Europe, and parts of Asia, the need for advanced biologic treatments, including monoclonal antibodies, vaccines, and cell therapies, is intensifying. Bioprocess containers play a critical role in the production, storage, and transportation of these biopharmaceutical products, making them essential to meeting the growing demand. This surge in biopharmaceutical production is, in turn, driving the expansion of the bioprocess containers market. According to IDF projections, the number of adults living with diabetes will increase by 46% to 783 million by 2045, representing one in eight adults. The American Cancer Society (ACS) estimates that by 2050, the number of cancer cases is expected to rise to 35 million.
Bioprocess Containers Market Challenges
Regulatory Requirements
Regulatory requirements in the bioprocess container market pose a significant challenge due to the need to comply with stringent standards across various regions and applications. Manufacturers must navigate complex regulations, such as those from the FDA, ISO, and USP, which require rigorous validation and documentation. Staying up-to-date with evolving guidelines and ensuring that containers meet these standards is crucial for maintaining product safety and efficacy, but it adds complexity and cost to the manufacturing process. According to Cytiva, single-use bioprocess technologies are advanced but lack specific regulations, creating uncertainty about their suitability for therapeutic production and leaving bio manufacturers concerned about their appropriateness.
Buy Now :
Bioprocess Containers Market Key Players
Product/Service launches, approvals, patents and events, acquisitions, partnerships and collaborations are key strategies adopted by players in the Bioprocess Containers Market. The top 10 players in the Bioprocess Containers Market are
Lonza Group AG
Corning Incorporated
Entegris, Inc.
Parker Hannifin Corporation
Meissner Filtration Products, Inc.
Merck KGaA
Avantor Inc.
Sartorius AG
Thermo Fisher Scientific Inc.
Cytiva,
For more Lifesciences and Healthcare Market reports, please click here
#Bioprocessing 🌱#PharmaceuticalManufacturing 💊#SingleUseTechnology 🧪#BiotechIndustry 🧬#BioprocessContainers 🏭#LabTechnology 🔬#BiopharmaInnovation 🚀#HealthcareManufacturing 🏥
0 notes
Text
Biopharma Process Equipment Market Size, Trends, and Growth Forecast 2025–2032
Global Biopharma Process Equipment Market: Analysis and Forecast (2023-2031)
Introduction
The global Biopharma Process Equipment Market is undergoing remarkable growth, fueled by technological advancements and the increasing demand for biopharmaceuticals and personalized medicine. In 2023, the market was valued at USD 11,825.4 million and is expected to grow significantly, reaching USD 33,199.3 million by 2031, registering a compound annual growth rate (CAGR) of 13.9%.
The growing prevalence of chronic diseases such as cancer, diabetes, and autoimmune disorders has heightened the need for biopharmaceutical production, thereby increasing demand for advanced biopharma process equipment. Additionally, government investments in vaccine production, especially in response to global health crises, have further accelerated market expansion. The rising trend of single-use bioprocessing equipment, coupled with automation and digitalization in biopharma manufacturing, is reshaping the industry, making it more efficient and cost-effective.
This evolving market presents significant opportunities for innovation, particularly in bioreactors, chromatography systems, and single-use technologies. As pharmaceutical and biotechnology companies strive to improve drug production efficiency and product quality, demand for high-precision biopharma process equipment is expected to rise.
Get free sample copy @ https://www.statsandresearch.com/request-sample/40578-global-biopharma-process-equipment-market-
Market Dynamics
Market Drivers
Increasing Demand for Biopharmaceuticals The rising global burden of chronic diseases, along with increasing adoption of targeted therapies and personalized medicine, is fueling the demand for biopharmaceuticals. This, in turn, is driving the need for high-quality biopharma processing equipment to facilitate efficient drug production.
Technological Advancements in Bioprocessing Innovations such as single-use bioprocessing systems, AI-driven automation, and real-time monitoring technologies have revolutionized the biopharma equipment market. These advancements enhance operational efficiency, reduce contamination risks, and improve overall bioproduction quality.
Rising Investments in Vaccine Development Governments and private organizations are investing heavily in vaccine production due to the growing need for preventive healthcare solutions. This surge in vaccine production requires advanced filtration, storage, and bioreactor technologies, contributing to market expansion.
Shift Toward Single-Use Bioprocessing Equipment Single-use bioprocessing equipment is gaining popularity due to its reduced contamination risk, lower operational costs, and faster production cycles. This trend is expected to continue, especially as biopharmaceutical companies seek efficient and scalable solutions.
Growing Adoption of Automation and Digitalization Automation and AI-driven bioprocessing solutions are helping manufacturers improve accuracy, minimize human errors, and optimize production processes. The transition toward fully automated systems is expected to drive further growth in the biopharma process equipment market.
Market Challenges
High Costs of Advanced Bioprocessing Equipment The initial investment required for biopharma process equipment is high, particularly for fully automated and AI-integrated systems. This cost barrier may limit adoption among small and medium-sized enterprises (SMEs) in the biopharma industry.
Environmental Concerns Related to Single-Use Systems While single-use bioprocessing equipment offers numerous benefits, it also leads to increased plastic waste, raising concerns about environmental sustainability. The industry is exploring biodegradable and recyclable materials to address this issue.
Complex Regulatory Landscape The biopharma industry is subject to strict regulatory requirements set by agencies like the FDA, EMA, and WHO. Compliance with these quality and safety standards adds complexity to the biopharma process equipment market.
Risk of Contamination in Bioprocessing Biopharmaceutical manufacturing involves handling living cells and microbes, making contamination control a critical challenge. Companies are investing in advanced filtration and sterilization technologies to mitigate contamination risks.
Get full report @ https://www.statsandresearch.com/report/40578-global-biopharma-process-equipment-market-/
Market Opportunities
Expansion of Cell and Gene Therapy Applications The rising adoption of cell and gene therapy is driving demand for high-precision biopharma process equipment. These therapies require specialized bioreactors, chromatography systems, and filtration technologies, creating new growth opportunities.
Increased Focus on Predictive Medicine and AI Integration AI and machine learning are transforming biopharma manufacturing by enabling predictive analytics, process optimization, and real-time monitoring. The integration of AI-driven solutions presents a major opportunity for market expansion.
Growing Demand for Small-Scale Bioprocessing Equipment The emergence of biotech startups and research institutions has led to increased demand for small-scale biopharma equipment. This segment is expected to grow significantly due to its cost-effectiveness and flexibility for research applications.
Segmental Analysis
By Equipment Type:
Bioreactors – Dominates the market (35.9% share in 2023), expected to grow at a CAGR of 14.1% due to its critical role in bioprocessing.
Chromatography Systems – Second largest segment (23.5% market share), projected to reach USD 7,896.8 million by 2031.
Filtration Systems, Storage Tanks, and Others – Essential for purification and storage in biopharma manufacturing.
By Technology:
Single-Use Technology – Leads the market (63.2% share in 2023), expected to maintain dominance due to reduced contamination risks and faster processing times.
Reusable Technology – Expected to reach USD 11,000 million by 2031, catering to companies preferring long-term cost efficiency.
By Automation Level:
Semi-Automated Systems – Market leader in 2023 (37.9% share), projected to grow at a CAGR of 13.6%.
Fully Automated Systems – Gaining traction due to operational efficiency, expected to grow at a CAGR of 14.8%.
By Capacity:
Mid-Scale (2,000 L – 10,000 L) – Dominates the market (37.9% share), preferred due to versatility in production applications.
Small-Scale (500 L – 2,000 L) – Expected to grow rapidly (CAGR of 14.4%), driven by biotech startups and research institutions.
By Application:
Drug Development & Production – Largest segment (38.2% market share), driven by rising pharmaceutical demand.
Cell and Gene Therapy – Fastest-growing segment (CAGR of 14.8%), fueled by advancements in regenerative medicine.
By End-User:
Pharmaceutical Companies – Market leader (40.8% share in 2023), expected to reach USD 13,263.7 million by 2031.
Biotechnology Companies – Fastest-growing segment (CAGR of 14.5%), driven by biologics and biosimilars development.
By Region:
Asia-Pacific (APAC) – Fastest-growing region (CAGR of 14.8%), expected to reach USD 9,186.2 million by 2031.
North America – Well-established market, projected to reach USD 11,251.2 million by 2031.
Middle East & Africa (MEA) – Growing segment (CAGR of 14.5%), driven by healthcare investments.
Competitive Landscape
Key Players:
Cytiva
ThermoFisher Scientific
Eppendorf
Sartorius
PerkinElmer
Shimadzu Corporation
Recent Developments:
January 2021: Shimadzu Corporation launched iMScope QT, an advanced imaging mass spectrometry instrument.
December 2023: PerkinElmer acquired Covaris, expanding its life sciences and diagnostics portfolio.
Get enquiry before buying @ https://www.statsandresearch.com/enquire-before/40578-global-biopharma-process-equipment-market-
0 notes
Text
Bioprocess Bags Market: Innovations Driving Growth in Biopharmaceutical Production- UnivDatos
According to a new report by UnivDatos Market Insights, the Bioprocess Bags Market was valued at approximately USD 3.85 Billion in 2023 and is expected to grow at a substantial CAGR of around 17.5% during the forecast period (2024-2032) owing to the increased demand for biologics and biosimilars. The growth of the bioprocess bags market reflects the global trend towards the usage of single-use technologies in biopharmaceutical production. These industries avail themselves by minimized lead time, decreased probability of contamination, and improved production. The bioprocess bags market expansion is fueled by the increasing need for biologics, biosimilars, and vaccines. In October 2022, Austrian solution provider Single Use Support GmbH expanded its product portfolio with new single-use bioprocess containers under the brand name IRIS. The pioneering company leverages its know-how to provide reliable process solutions in the field of biopharmaceutical fluid management, continuing to pursue its vendor-agnostic approach.
Request To Download Sample of This Strategic Report - https://univdatos.com/get-a-free-sample-form-php/?product_id=68909&utm_source=LinkSJ&utm_medium=Snehal&utm_campaign=Snehal&utm_id=snehal
Segments that transform the industry
· Based on product type, the market is segmented into single-use bioprocessing bags and multi-use bioprocessing bags. Single-use bioprocessing bags held a significant share of the market in 2023 owing to enhanced growth by facilitating rapid production scaling, the need to clean them less often, and possessing lower risk of cross-contamination. Adding to this, companies use these bags in bioprocessing due to their adaptability to bioprocesses such as media preparation, mixing, and product storage. Their adaptability to automated bioreactors and closed systems makes them fundamental to the current biomanufacturing industry. For instance, on March 28, 2022 — ILC Dover LP (“ILC Dover” or the “Company”), specializing in innovative single-use solutions for biotherapeutics and pharmaceutical processing, announced its launch of liquid single-use bioprocessing bags, representing the first of many new products for handling and supply of sterile liquids for the biotherapeutics market. This expansion is a continuation of ILC Dover’s solution set across the entire biotherapeutic and pharmaceutical manufacturing workflow, from powder containment and handling, through sterile liquid handling and pre-filled liquid and powder bags.
According to the report, the impact of Bioprocess Bags has been identified to be high for the Asia-Pacific area.
For More Informative Information, Please Visit Us- https://univdatos.com/get-a-free-sample-form-php/?product_id=68909&utm_source=LinkSJ&utm_medium=Snehal&utm_campaign=Snehal&utm_id=snehal
Some of how this impact has been felt include:
Asia-Pacific is expected to grow with a significant CAGR during the forecast period (2024-2032). This is due to the booming biotechnology industry and rapidly growing consumption of biopharma products in the region’s emerging giants such as China and India. It is one of the most important bioprocessing locations because government policies support the production of local goods along with cheaper manufacturing expenses. Increased spending on single-use technologies and increasing outsourcing of CMC services through more CMOs/CROs are critical drivers. There are several reasons companies opt for bioprocess bags compliance with international quality standards and the need to scale up biosimilars and vaccine production. The region’s emphasis on new technologies and export-oriented biomanufacturing also drives adoption even more.
On June 1, 2021, Avantor, Inc. (NYSE: AVTR), a leading global provider of mission-critical products and services to customers in the life sciences, advanced technologies and applied materials industries, announced that it has acquired RIM Bio, a leading China-based manufacturer of single-use bioprocess bags and assemblies for biopharmaceutical manufacturing applications.
0 notes
Text
Revolutionizing Bioprocessing: Insights into the Single-use Assemblies Market
The global single-use assemblies market size is expected to reach USD 34.64 billion by 2030, registering a CAGR of 16.53% from 2024 to 2030, according to a new report by Grand View Research, Inc. Life science companies are eager to avoid the cost and time of cleaning needed with stainless steel. The augmented expansion of the international biotech industries has offered a growth environment, in which novel, disposable technologies are becoming more significant. As pharmaceutical companies, distributors, CDMOs, and manufacturers converge around the benefits of single-use assemblies, the industry will grow at a faster pace during the forecast period. From therapies for rare diseases to cancer research, the impact of single-use technology in accelerating cutting-edge research into these conditions is set to grow, thereby propelling industry growth.
In addition, the growing biologics market will further offer lucrative opportunities during the study period. Eradicating the risk of contamination is the major challenge faced by biopharmaceutical manufacturers, which currently involves high-level monitoring of critical manufacturing solutions. Single-use assemblies support manufacturers in overcoming this difficulty by eliminating or reducing the necessity for sterilization between the batches, thereby filtering the operational capability. SUTs are considered one of the important areas of growth among biomanufacturing companies, as several biopharmaceutical companies are venturing into disposable assembly offerings for the production of all sorts of biopharmaceuticals.
Moreover, SUTs are now being heavily adopted for the clinical manufacturing of biopharmaceuticals and have become more conventional within commercial manufacturing facilities. SUTs permit biologics manufacturers to reduce the facilities' footprint by almost 20% due to the need for utilities, which generate steam, water, and clean-in-place solutions. Furthermore, as per the research by BioProcess International, the engineers estimated that the capital expenditure for the single-use facility is 25-45% less than for a facility established on stainless-steel equipment. Similarly, they also estimated that single-use facilities need half the energy and water during operations and can be built in 18 months. Whereas the stainless-steel facility takes three years. Owing to such advantages, the adoption of single-use assemblies is increasing, thereby driving industry growth.
In recent years, CMOs have been integrating single-use assemblies into most or all their bioprocess. CMOs broadly use single-use assemblies for quicker processing and process changeover time. The quick turnaround time and flexibility between process runs and various client projects allowed by single-use assemblies can enhance the CMO efficiency, which, in turn, aids in the reduction of the overall costs. In addition, the growing number of CMO facilities are essentially fully single-use. CMOs adoption of single-use technology can save on campaign and facility costs, which helps reduce the operating costs and capital investments. Furthermore, single-use assemblies in CMOs decrease the complexity and reduces lead times in upstream processes.
They also enhance the quality of products by preventing cross-contamination in downstream processes, especially for processes handling potent or toxic materials, such as antibody-drug conjugates and viral vectors. Moreover, stringent regulatory requirements in the manufacturing systems also endorse the adoption of single-use assemblies in CMOs.COVID-19 has augmented an already rapidly rising demand for single-use assemblies as the SUTs allow for the speedy production and development of therapies and vaccines and improve accuracy and time. For instance, Aramus single-use bag assembly developed by Entegris, Inc. was used for the storage of COVID-19 vaccines as the vaccines need extremely low temperatures to preserve. With low leachable and extractable and an extensive operating-temperature variety, the patented single-use bags were ideal for cold chain storage and collection.
In addition, key manufacturers are also accelerating investment projects or increasing their capital expenditures. For instance, Pall Corp., invested USD 114 million to upsurge its single-use output. These investments comprise additional manufacturing capacity at 6 current manufacturing facilities in the United States and Europe. Moreover, MilliporeSigma, Thermo Fisher, and Cytiva have also augmented growth plans to support the industry’s backlog. For instance, in March 2021 Merck announced the addition of a single-use assembly production unit at its Life Science Center in Molsheim, France. The investment of USD 26.25 million will accelerate the European expansion plans of the company for single-use products critical to manufacturing vaccines and lifesaving therapies. On the other hand, regulatory concerns due to leachable and extractable might hamper the growth of disposable assemblies in the forecast period.
Leachable and extractable are set of undesired impurities in the product stream, these are compounds that can move into the product from manufacturing systems, packaging containers, or other product-contact systems. Extractable is extracted from the contact materials under extreme conditions, such as treatment with a harsh solvent or elevated temperature conditions. Leachable compounds, on the other hand, are those that are leached into the final product from contact materials during normal or real-time storage conditions. North America dominated the global industry in 2023 due to the growth in the biotechnology and pharmaceutical industries, advancement in SUT products, and increasing incidence of diseases, such as cancer coupled with increasing investments and funding in drug discovery research. Asia Pacific is expected to grow at a considerable CAGR over the forecast years owing to the strategic activities by key players coupled with funding by the government for biopharmaceutical R&D.
Single-use Assemblies Market Report Highlights
The filtration assemblies segment led the industry in 2023. Increased regulatory prospects and the need to diminish the risk of contamination have promoted the use of filtration assemblies for bulk and final fill operations
The bag assemblies segment is expected to grow at a lucrative CAGR over the forecast period. Advantages, such as no need for validation or cleaning, lower shipping cost due to less weight, and low maintenance cost & capital investment drive the segment growth
The customized solutions segment captured the highest revenue share in 2023. Several companies offer customized solutions to fast-track pharmaceutical manufacturing and drug development
The pharmaceutical & biotechnological companies end-user segment led the global industry in 2023. The growth of the current manufacturing facilities for biopharmaceuticals drives the demand for single-use assemblies
The CROs & CMOs segment is expected to grow at the fastest CAGR over the forecast period. In recent years, CMOs have been integrating single-use technology into most or all their bioprocess
The quick turnaround time and flexibility between process runs and various client projects allowed by single-use equipment can enhance the CMO efficiency, which aids in the reduction of the overall costs
Single-use Assemblies Market Segmentation
Grand View Research has segmented the global single-use assemblies market report based on product, application, solution, end-use, and region:
Single-use Assemblies Product Outlook (Revenue, USD Million, 2018 - 2030)
Bag Assemblies
2D bag assemblies
3D bag assemblies
Filtration Assemblies
Bottle Assemblies
Tubing Assemblies
Other Products
Single-use Assemblies Application Outlook (Revenue, USD Million, 2018 - 2030)
Filtration
Cell Culture & Mixing
Storage
Sampling
Fill-finish Applications
Other Applications
Single-use Assemblies Solution Outlook (Revenue, USD Million, 2018 - 2030)
Customized Solutions
Standard Solutions
Single-use Assemblies End-use Outlook (Revenue, USD Million, 2018 - 2030)
Biopharmaceutical & Pharmaceutical Companies
CROs & CMOs
Academic & Research Institutes
Single-use Assemblies Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
US
Canada
Europe
UK
Germany
France
Italy
Spain
Denmark
Sweden
Norway
Asia Pacific
Japan
China
India
South Korea
Australia
Thailand
Latin America
Brazil
Mexico
Argentina
Middle East and Africa
South Africa
Saudi Arabia
UAE
Kuwait
Order a free sample PDF of the Single-use Assemblies Market Intelligence Study, published by Grand View Research.
0 notes
Text
Tangential Flow Filtration Market: Growth, Trends, and Industry Insights from 2024-2031
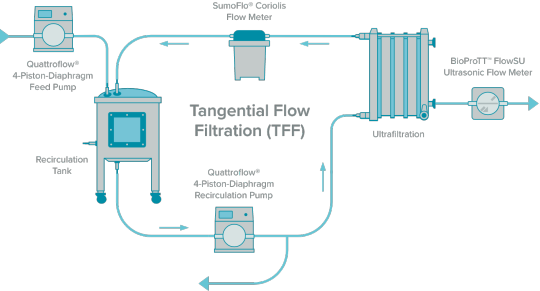
The global tangential flow filtration (TFF) market is witnessing significant growth, driven by rising demand for advanced filtration technologies across biopharmaceutical, food, and industrial applications. According to SkyQuest Technology, the tangential flow filtration market is projected to achieve a valuation of USD 5.61 billion by 2031, growing at an impressive CAGR of 11.53% from 2024 to 2031. This growth reflects the increasing adoption of TFF for efficient purification, separation, and concentration processes.
Market Dynamics: Key Drivers Accelerating Growth
Growing Biopharmaceutical Industry The booming biopharma sector, driven by the increasing production of monoclonal antibodies, vaccines, and recombinant proteins, is a major contributor to the demand for tangential flow filtration systems. TFF ensures high-efficiency separation, making it indispensable in drug development and production.
Advancements in Membrane Technology Technological advancements in membrane materials and filtration devices are improving the efficacy and scalability of tangential flow filtration. This is attracting widespread adoption across research and manufacturing settings.
Rising Demand for Purification Techniques Industries such as food & beverage, chemical, and water treatment are increasingly turning to TFF systems for applications like protein purification, cell harvesting, and wastewater management.
High Demand for Single-Use Systems Single-use tangential flow filtration systems are gaining popularity for their convenience, cost-efficiency, and ability to reduce contamination risks in bioprocessing.
Request a Sample Report - https://www.skyquestt.com/sample-request/tangential-flow-filtration-market
Market Segmentation: Understanding the Key Categories
The tangential flow filtration market is categorized based on product, technology, application, and end-user:
By Product
Single-Use Systems: Highly preferred for biopharmaceutical manufacturing due to lower maintenance costs.
Reusable Systems: Ideal for large-scale production and industrial applications.
Membranes & Accessories: Includes membranes, cassettes, and cartridges that optimize filtration performance.
By Technology
Microfiltration: Widely used for cell harvesting and protein separation.
Ultrafiltration: Popular for separating macromolecules and purification of antibodies and vaccines.
By Application
Bioprocessing: The largest application segment, primarily in monoclonal antibody and protein production.
Viral Vector Manufacturing: Increasing adoption in gene therapy and vaccine production.
Food & Beverage: Used for clarifying and purifying liquids in dairy, beverages, and food production.
By End-User
Biopharmaceutical Companies: Dominating the market due to increasing drug production.
Research Laboratories: Growing adoption for laboratory-scale purification processes.
Contract Manufacturing Organizations (CMOs): Increasing reliance on advanced filtration systems for outsourced production.
Speak to an Analyst - https://www.skyquestt.com/speak-with-analyst/tangential-flow-filtration-market
Regional Insights: TFF Market Trends Across Key Regions
North America North America remains a dominant region, led by the United States, where significant investments in biopharma R&D and manufacturing are driving the adoption of TFF systems.
Europe Europe’s market growth is fueled by increasing demand for high-purity biopharmaceuticals and government initiatives supporting advanced filtration technologies. Countries like Germany and France are major contributors.
Asia-Pacific The Asia-Pacific region is the fastest-growing market, with countries like China, India, and Japan emerging as biopharma hubs. Increased outsourcing of drug manufacturing and rising investments in life sciences are driving regional growth.
Rest of the World Latin America and the Middle East & Africa are witnessing gradual growth due to expanding healthcare infrastructure and the adoption of innovative bioprocessing technologies.
Make a Purchase Inquiry - https://www.skyquestt.com/buy-now/tangential-flow-filtration-market
Top Players Shaping the Tangential Flow Filtration Market
The tangential flow filtration market is led by several key players who are continuously innovating and expanding their product offerings to meet growing demand:
Merck KGaA
Danaher Corporation
Sartorius AG
Repligen Corporation
Parker Hannifin Corporation
Koch Membrane Systems, Inc.
GE Healthcare
Pall Corporation
Novasep
MilliporeSigma
Alfa Laval AB
Spectrum Laboratories, Inc.
These companies are focusing on enhancing product efficiency and introducing single-use filtration systems to address evolving industry needs.
Key Trends Transforming the Tangential Flow Filtration Industry
Shift Towards Single-Use Technologies Single-use TFF systems are gaining traction in bioprocessing due to their flexibility, cost savings, and contamination-free operation.
Rising Adoption in Viral Vector Manufacturing The rise of gene therapies and vaccines has increased demand for TFF systems capable of handling viral vectors and complex molecules.
Technological Innovations in Membrane Filtration Advancements in membrane materials and designs are improving the filtration efficiency and scalability of TFF systems.
Expansion of Bioprocessing Facilities The rapid growth of the biopharmaceutical industry is leading to the establishment of advanced manufacturing facilities, further driving the adoption of TFF systems.
The Future of Tangential Flow Filtration
The tangential flow filtration market is on a growth trajectory, driven by rising demand for efficient purification systems in biopharmaceuticals, food, and industrial applications. With advancements in membrane technology, increased adoption of single-use systems, and expanding bioprocessing capabilities, the market is poised for sustained expansion in the coming years.
As companies focus on innovation and sustainability, the TFF market will play a critical role in supporting the growing needs of industries worldwide.
#Tangential Flow Filtration Market#Tangential Flow Filtration Market Size#Tangential Flow Filtration Market Share#Tangential Flow Filtration Market Trends#Tangential Flow Filtration Market Growth#Tangential Flow Filtration Market Outlook#Tangential Flow Filtration Market Key Players#Tangential Flow Filtration Market Overview#Tangential Flow Filtration Market Competitor#Tangential Flow Filtration Market Insights#Tangential Flow Filtration Market Forecast#Tangential Flow Filtration Market Analysis#Tangential Flow Filtration Market Statistics#Tangential Flow Filtration Market Data#Tangential Flow Filtration Market PDF#Tangential Flow Filtration Market Excel#Tangential Flow Filtration Market Strategy#Tangential Flow Filtration Market Innovations
0 notes
Text
Bioprocess Bags Market Size, Share, Demand, Revenue, Trends, Manufacturers, Challenges and Future Opportunities: SPER Market Research

Bioprocess bags are special containers that provide a sterile space for cultivating cells or microorganisms. They are used in biopharmaceutical and biotechnology fields to produce biological products. The main types are 2D bioprocess bags, which are flat when empty, and 3D bags. These bags serve various workflows, such as upstream and downstream processes, come in different sizes, and support applications like buffer storage, cell culture, and product holding. They are utilized by pharmaceutical and biotechnology companies, along with academic labs.
According to SPER market research, ‘Global Bioprocess Bags Market Size- By Type, By Workflow, By End User - Regional Outlook, Competitive Strategies and Segment Forecast to 2034’ state that the Global Bioprocess Bags Market is predicted to reach 19.76 billion by 2034 with a CAGR of 16.61%.
Drivers: Bioprocess bags are made from flexible plastic and are designed for the biopharmaceutical manufacturing process. They come in various sizes to meet manufacturer needs. The market for these bags is projected to grow due to increasing demand for biopharmaceuticals, driven by an aging population, chronic diseases, and a preference for personalized medicine.
Additionally, the rise in outsourcing by Contract Manufacturing Organizations (CMOs) and Contract Research Organizations (CROs) is expected to boost the need for bioprocess bags. These organizations provide services to pharmaceutical and biotech companies, leading to greater demand as more firms outsource bioprocessing tasks like production, storage, and transportation of biologics.
Request a Free Sample Report: https://www.sperresearch.com/report-store/bioprocess-bags-market.aspx?sample=1
Restraints: The rising cost of raw materials Bioprocess bags must be made from high-quality materials and sterilized properly. However, these materials are far more expensive than typical plastics, and the sterilization process is costly. Furthermore, variables such as disease outbreaks, border disputes, and other issues may disrupt supply chains, raising raw material prices even further. As a result, the market for bioprocess bags is expected to slow down.
North America bioprocess bags market dominated the global market with a share in 2024. The growing demand for biopharmaceuticals is expected to boost the bioprocess bags market in the region. Factors contributing to this growth include the rising use of single-use bioprocessing technologies and a focus on lowering costs tied to advanced therapies. Moreover, the presence of major biopharmaceutical companies and contract manufacturing organizations (CMOs) in North America increases the need for high-quality bioprocess bags. Some significant market players are Thermo Fisher Scientific Inc, Sartorius AG, Danaher Corporation, Merck KGaA, Saint-Gobain, Corning Incorporated, and others.
For More Information, refer to below link: –
Bioprocess Bags Market
Related Reports:
Tendonitis Treatment Market Size- By Treatment, By Condition - Regional Outlook, Competitive Strategies and Segment Forecast to 2034
Dental Simulator Market Growth, Size, Trends Analysis - By Component, By Application, By End Use - Regional Outlook, Competitive Strategies and Segment Forecast to 2034
Follow Us –
LinkedIn | Instagram | Facebook | Twitter
Contact Us:
Sara Lopes, Business Consultant — USA
SPER Market Research
+1–347–460–2899
#Bioprocess Bags Market#Bioprocess Containers Market#Bioprocess Containers and Fluid Transfer Solutions Market#Bioprocess Bags Market Growth#Bioprocess Bags Market Trends#Bioprocess Bags Market Share#Bioprocess Bags Market Size
0 notes
Text
GMP Cell Therapy Consumables Market is valued at approximately USD 24.04 million and is projected to grow at a compound annual growth rate (CAGR) of 27.90% over the forecast period, reaching around USD 172.15 million by 2032.The Good Manufacturing Practice (GMP) cell therapy consumables market is evolving as a crucial segment within the biotechnology and pharmaceutical industries. As the demand for innovative therapies rises, particularly in the field of regenerative medicine and immune-oncology, the need for specialized consumables that adhere to stringent quality and safety standards has surged. This article explores the dynamics, key trends, and future outlook of the GMP cell therapy consumables market.
Browse the full report https://www.credenceresearch.com/report/gmp-cell-therapy-consumables-market
Understanding GMP Cell Therapy Consumables
GMP cell therapy consumables refer to the materials and reagents used during the production of cell-based therapies, manufactured under rigorous GMP guidelines. These consumables include cell culture media, reagents, growth factors, cytokines, cryopreservation solutions, and single-use bioprocessing systems. Adherence to GMP standards ensures the safety, efficacy, and quality of the final therapeutic products, essential for regulatory approval and patient safety.
Market Drivers and Trends
1. Rising Demand for Cell-Based Therapies Cell therapy, encompassing autologous and allogeneic approaches, has emerged as a promising frontier in treating chronic diseases, genetic disorders, and cancers. The success of therapies like CAR-T cells has fueled investments in cell therapy research and commercialization. Consequently, the demand for high-quality consumables to support these therapies has grown exponentially.
2. Stringent Regulatory Landscape Global regulatory bodies, including the FDA and EMA, emphasize compliance with GMP guidelines during the development and manufacturing of cell therapies. Companies must source GMP-compliant consumables to meet these regulatory requirements, driving demand in the market.
3. Technological Advancements in Bioprocessing The development of innovative single-use systems and automation technologies has revolutionized cell therapy manufacturing. These advancements enhance scalability, reduce contamination risks, and improve process efficiency. The adoption of these technologies has increased the reliance on GMP-certified consumables.
4. Expanding Biopharmaceutical Manufacturing With the growing pipeline of cell and gene therapies, biopharmaceutical companies are expanding their manufacturing capacities. This expansion necessitates a steady supply of consumables to ensure uninterrupted production, further propelling market growth.
Key Players and Competitive Landscape
The GMP cell therapy consumables market is characterized by the presence of established players and emerging companies. Key players such as Thermo Fisher Scientific, Sartorius AG, Merck KGaA, and Lonza Group dominate the market. These companies invest heavily in R&D to develop innovative and GMP-compliant products.
Startups and smaller firms are also entering the market, offering niche solutions and fostering competition. Strategic collaborations, mergers, and acquisitions have become common strategies to gain a competitive edge.
Challenges in the Market
1. High Production Costs Manufacturing GMP-compliant consumables involves stringent quality control measures and certification processes, leading to high production costs. These expenses are often passed on to end-users, making cost management a critical challenge.
2. Supply Chain Complexities The global supply chain for GMP cell therapy consumables is highly intricate, involving multiple stakeholders and geographies. Disruptions in supply chains, as observed during the COVID-19 pandemic, can impact market growth.
3. Regulatory Hurdles While regulations are necessary to ensure quality, navigating complex regulatory landscapes across different countries can be a time-consuming and costly process for manufacturers.
Future Outlook
The GMP cell therapy consumables market is poised for significant growth in the coming years. According to industry reports, the market is expected to grow at a compound annual growth rate (CAGR) of over 15% between 2023 and 2030. This growth will be fueled by increasing adoption of cell-based therapies, advancements in bioprocessing technologies, and supportive government initiatives.
Key Player Analysis:
Thermo Fisher Scientific Inc.
Lonza Group
Merck KGaA
Corning Inc.
Sartorius AG
Danaher Corporation
Miltenyi Biotec
Stemcell Technologies Inc.
GE Healthcare
Takara Bio Inc.
Segmentation:
Based on Product Type:
Cell Culture Media
Cell Separation Tools
Bioreactors
Cryopreservation Products
Other Consumables
Based on Technology:
Manual Systems
Automated Systems
Based on End-User:
Biopharmaceutical Companies
Research Institutions
Contract Manufacturing Organizations (CMOs)
Based on Region:
North America
United States
Canada
Europe
Germany
United Kingdom
France
Italy
Spain
Asia-Pacific
China
India
Japan
Australia
South Korea
Latin America
Brazil
Mexico
Argentina
Middle East and Africa
South Africa
UAE
Saudi Arabia
Browse the full report https://www.credenceresearch.com/report/gmp-cell-therapy-consumables-market
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes