#Transthyretin Amyloidosis Treatment Industry
Text
Transthyretin Amyloidosis Treatment Market Segment Analysis By Type, Therapy, Disease Type, Distribution Channel, Region And Forecast Till 2030: Grand View Research Inc.
San Francisco, 24 Aug 2023: The Report Transthyretin Amyloidosis Treatment Market Size, Share & Trends Analysis Report By Type (ATTR-PN, ATTR-CM), By Therapy, By Disease Type, By Distribution Channel, By Region, And Segment Forecasts, 2022 – 2030
The global transthyretin amyloidosis treatment market size is expected to reach USD 9.17 billion by 2030, according to a new report by Grand View…

View On WordPress
#Transthyretin Amyloidosis Treatment Industry#Transthyretin Amyloidosis Treatment Market#Transthyretin Amyloidosis Treatment Market 2030#Transthyretin Amyloidosis Treatment Market Share#Transthyretin Amyloidosis Treatment Market Size
0 notes
Text
From Symptom Management to Precision Therapeutics: The Evolution of Transthyretin Amyloidosis Treatment Market
Introduction:
Transthyretin Amyloidosis (ATTR) is a rare, progressive, and potentially life-threatening disease characterized by the accumulation of abnormal deposits of misfolded transthyretin protein in various organs. As research and understanding of this condition advance, so does the Transthyretin Amyloidosis Treatment Market. The market is witnessing significant growth, driven by innovative therapies, increased awareness, and a growing patient population.
Current Treatment Landscape:
Traditionally, the treatment options for ATTR have been limited, with therapies primarily focused on symptom management and supportive care. Liver transplantation, which involves replacing the patient's liver with a healthy donor liver to reduce the production of abnormal transthyretin protein, has been a standard approach. However, this procedure is not without challenges, including the scarcity of suitable donors and the associated risks.
Recent Developments:
In recent years, the landscape of Transthyretin Amyloidosis treatment has undergone a transformative shift with the emergence of novel therapeutic approaches. One notable advancement is the development of gene-silencing drugs that target the production of abnormal transthyretin protein at the genetic level. RNA-targeted therapies, such as patisiran and inotersen, have shown promising results in clinical trials, offering new hope for patients with ATTR.
Furthermore, small molecule drugs, such as tafamidis, have demonstrated efficacy in stabilizing transthyretin proteins, preventing their misfolding and subsequent deposition. These breakthroughs mark a paradigm shift in the approach to treating ATTR, moving beyond symptom management to directly address the underlying causes of the disease.
Market Dynamics:
The growing prevalence of ATTR, coupled with an aging population, has led to an increased demand for effective and targeted treatments. This rising patient pool, along with the expanding understanding of the disease's genetic basis, has attracted significant investments in research and development within the Transthyretin Amyloidosis Treatment Market.
Additionally, collaborations between pharmaceutical companies, academic institutions, and research organizations have played a crucial role in accelerating drug discovery and development. These partnerships leverage collective expertise, resources, and technologies to expedite the translation of scientific advancements into viable therapeutic options.
Challenges and Opportunities:
Despite the notable progress in the field, challenges persist, including the high cost of innovative therapies, limited accessibility, and the need for accurate and timely diagnosis. Overcoming these hurdles requires a comprehensive approach involving healthcare providers, policymakers, and the pharmaceutical industry to ensure equitable access to cutting-edge treatments.
The Transthyretin Amyloidosis Treatment Market presents lucrative opportunities for pharmaceutical companies to invest in research and development, with the potential for breakthrough therapies to enter the market. Moreover, fostering patient awareness and education is crucial to facilitate early diagnosis and intervention, enhancing the effectiveness of available treatments.
Conclusion:
The Transthyretin Amyloidosis Treatment Market is undergoing a transformative phase, with unprecedented advancements in therapeutic approaches. From traditional symptom management to innovative gene-silencing and protein-stabilizing therapies, the landscape is evolving rapidly. As research continues to unravel the complexities of ATTR, the market holds promise for improved patient outcomes, offering hope to individuals affected by this rare and challenging condition. The collaboration between stakeholders, continued investment in research, and enhanced patient access to innovative therapies will be pivotal in shaping the future of Transthyretin Amyloidosis treatment.
0 notes
Text
Symphony of Hope: Exploring Breakthrough Treatments for Rare Genetic Disorders

Introduction:
Within the intricate tapestry of human genetics, there exists a realm of rare genetic disorders that captivates researchers and challenges medical advancements. These conditions, affecting only a small fraction of the population, often pose complex treatment puzzles. However, with the constant evolution of innovative strategies, a glimmer of hope shines through for patients and their support networks. In this blog post, we embark on a journey to explore novel treatment strategies that are reshaping the landscape of rare genetic disorders. Additionally, we delve into the upcoming Orphan Drugs and Rare Diseases Conference, a gathering in London on the 9th and 10th of October 2023, where knowledge and collaboration will intertwine to advance our understanding and improve outcomes in this field.
Embracing Novel Treatment Strategies for Rare Genetic Disorders:
Gene Therapy: Like a symphony conductor guiding an orchestra, gene therapy orchestrates the introduction of healthy genes into a patient's cells, rectifying the underlying genetic abnormalities causing the disorder. In diseases such as spinal muscular atrophy (SMA) and Leber congenital amaurosis (LCA), this harmonious approach has showcased remarkable results, illuminating the path towards effective treatment.
Precision Medicine: The art of precision medicine lies in decoding the individual's genetic makeup and the intricacies of the disease. By meticulously analyzing a patient's genetic profile, medical professionals can tailor treatments to their unique genetic blueprint, unlocking targeted therapies that hold promise for heightened efficacy and reduced side effects.
Pharmacological Chaperones: Within the realm of rare genetic disorders lie proteins that are misfolded or rendered unstable. Pharmacological chaperones, akin to skilled sculptors, wield their expertise in stabilizing these proteins, restoring their natural form and function. Diseases such as Gaucher disease and Fabry disease have witnessed the transformative potential of this sculpting approach.
RNA-Based Therapies: The symphony of life dances to the rhythm of RNA-based therapies, where harmony is restored by modulating gene expression and correcting abnormal protein production. By wielding the power of antisense oligonucleotides and RNA interference, conditions like Duchenne muscular dystrophy and hereditary transthyretin amyloidosis are graced with newfound hope, as these therapies address the underlying genetic defects.
Stem Cell Transplantation: A beacon of regenerative medicine, stem cell transplantation breathes life into the realm of rare genetic disorders. By replacing damaged or malfunctioning cells with healthy counterparts, derived either from the patient's own body or a compatible donor, this technique bestows renewed vitality. Rare conditions such as sickle cell disease and thalassemia stand witness to the transformative power of this regenerative symphony.
Unveiling the Orphan Drugs and Rare Diseases Conference:
Mark your calendars for the 9th and 10th of October 2023 as the Orphan Drugs and Rare Diseases Conference descends upon London. This symposium of knowledge and collaboration stands as a testament to our collective dedication to unravelling the enigma of rare diseases. The conference serves as a nexus, attracting researchers, clinicians, pharmaceutical companies, regulatory authorities, and patient advocacy groups, igniting a vibrant tapestry of insights and advancements.
25% DISCOUNT IS WAITING – USE COUPON CODE: ORDC25
REGISTER NOW!
Attending the Conference Has Many Advantages, like:
Networking Opportunities: The conference provides a fertile ground for forging connections with esteemed experts, researchers, and industry professionals dedicated to rare diseases. These valuable connections can sprout collaborations, partnerships, and access to cutting-edge research and resources.
Exchange of Wisdom: Through captivating presentations, thought-provoking panel discussions, and engaging workshops, the conference channels a reservoir of knowledge. Attendees will immerse themselves in the latest advancements, emerging therapies, and regulatory updates, nurturing their intellectual acumen in this ever-evolving field.
Empowering Patient Advocacy: At the heart of this symphony of innovation lie the voices of patients and their families. The conference places significant emphasis on involving patient advocacy groups and encourages active patient participation. This inclusive approach empowers patients to share their experiences, challenges, and perspectives, ultimately shaping research priorities, clinical trial design, and access to innovative therapies.
Illuminating Regulatory Landscapes: As the crescendo of progress reverberates, the conference serves as a platform to illuminate the latest regulatory developments and policies surrounding orphan drugs and rare diseases. Participants gain a deeper understanding of the regulatory landscape, a vital cornerstone for drug development, approval, and access.
A Tapestry of Collaboration: The conference weaves a tapestry of collaboration, fostering interdisciplinary connections among stakeholders in rare disease research, including academia, industry, and patient organizations. By cultivating this harmonious collaboration, the conference aims to expedite the development of innovative therapies, ultimately symphonizing improved outcomes for patients.
Conclusion
Within the realm of rare genetic disorders, novel treatment strategies shine as beacons of hope, igniting a symphony of possibilities for patients and their support networks. The Orphan Drugs and Rare Diseases Conference in London, scheduled for the 9th and 10th of October 2023, serves as a rallying point for this symphony of innovation. Attending this conference provides an opportunity to embrace the transformative power of knowledge, collaboration, and patient advocacy, ultimately nurturing the evolution of rare disease treatments into a symphony of success.
AVAIL OF THE 25% DISCOUNT BEFOREHAND!
USE COUPON CODE – ORD25
0 notes
Link
0 notes
Photo

Transthyretin Amyloidosis Treatment Market Slated US$ 1,077 Mn by 2028| Prothena Corporation, Pfizer, Ionis Pharmaceuticals, Valeant, Celgene, Takeda, Johnson and Johnson, AstraZeneca, GlaxoSmithKline
Transthyretin Amyloidosis Treatment Market expecting for US$ 1,077.7 million, anticipated to register a CAGR of +58% by the term plan of 2021-28.
TTR amyloidosis is a specific type of amyloidosis that is very rare. TTR stands for transthyretin, a protein that is primarily made in the liver. TTR amyloidosis occurs when this protein “misfolds” or changes its shape in an abnormal way, and forms into fibrous clumps.
TTR amyloidosis is a systemic disease in which amyloid deposits can be visualized in most tissues such as skin, fat pad, rectal mu- cosa, gastric mucosa, nerve tissue or, myocardium. Tissue biopsy should be performed, ideally of an affected organ.
Liver transplantation removes the source of mutated TTR molecules and prolongs survival, with a 20-year survival of 55%.
On average, people with familial ATTR amyloidosis live for 7 to 12 years after they get their diagnosis, according to the Genetic and Rare Diseases Information Center. A study published in the journal Circulation found that people with wild-type ATTR amyloidosis live an average of about 4 years after diagnosis.
Request for a sample report here @ https://www.reportconsultant.com/request_sample.php?id=80362
Major Players Covered in this Report:
Prothena Corporation plc., Pfizer Inc., Ionis Pharmaceuticals Inc., Valeant Pharmaceuticals International Inc., Celgene Corporation, Takeda Pharmaceutical Company Ltd., Johnson and Johnson Pvt. Ltd., GlaxoSmithKline plc, Alnylam Pharmaceuticals Inc., SOM Innovation Biotech S.L., AstraZeneca plc.
Report Consultant announced latest research on growth factors and development of Global Transthyretin Amyloidosis Treatment Market. A detailed study accumulated to offer latest insights about acute features of the Transthyretin Amyloidosis Treatment market. The report contains different market predictions related to market size, revenue, production, CAGR, Consumption, gross margin, price, and other substantial factors. While emphasizing the key driving and restraining forces for this market, the report also offers a complete study of the future trends and developments of the market. It also examines the role of the leading market players involved in the industry including their corporate overview, financial summary and SWOT analysis.
Transthyretin Amyloidosis Treatment Market Study assures you to advise higher than your competition. With Structured tables and figures examining the Transthyretin Amyloidosis Treatment, the research document provides you a leading product, submarkets, revenue size and forecast to 2028.
The study report offers a comprehensive analysis of Transthyretin Amyloidosis Treatment market size across the globe as regional and country level market size analysis, CAGR estimation of market growth during the forecast period, revenue, key drivers, competitive background and sales analysis of the payers. Along with that, the report explains the major challenges and risks to face in the forecast period.
Market segmentation by drug:
Inostersen
Partisiran
Tafamidis
Others
Market segmentation by Indication:
Wild Type ATTR Amyloidosis
Hereditary ATTR Amyloidosis
Market segmentation by Distribution Channel:
Retail Pharmacy
Hospital Pharmacy
Online Pharmacy
Market segmentation by regions:
North America
Europe
Asia-Pacific
Middle East and Africa
Rest of the world
The research report of the Transthyretin Amyloidosis Treatment market offers broad analysis about the industry on the basis of different key segments. Moreover, the research report presents a comprehensive analysis about the opportunities, new products, and technological innovations in the market for the players.
Additionally, the research report on Transthyretin Amyloidosis Treatment market provides an in depth analysis about market status, market size, revenue share, industry development trends, products’ advantages and disadvantages of the enterprise, enterprise competition pattern, industrial policy and regional industrial layout characteristics. Thus the study report offers a comprehensive analysis of market size across the globe as regional and country level market size analysis, estimation of market growth during the forecast period.
Get upto 40% Discount available on this Report @ https://www.reportconsultant.com/ask_for_discount.php?id=80362
This study also covers company profiling, specifications and product picture, sales, market share and contact information of various regional, international and local vendors of Global Transthyretin Amyloidosis Treatment Market. The market opposition is frequently developing greater with the rise in scientific innovation and M&A activities in the industry. Additionally, many local and regional vendors are offering specific application products for varied end-users. The new merchant applicants in the market are finding it hard to compete with the international vendors based on reliability, quality and modernism in technology.
Detailed TOC of Transthyretin Amyloidosis Treatment Market Research Report-
– Transthyretin Amyloidosis Treatment Introduction and Market Overview
– Transthyretin Amyloidosis Treatment Market, by Application
– Transthyretin Amyloidosis Treatment Industry Chain Analysis
– Transthyretin Amyloidosis Treatment Market, by Type
– Industry Manufacture, Consumption, Export, Import by Regions
– Industry Value ($) by Region
– Transthyretin Amyloidosis Treatment Market Status and SWOT Analysis by Regions
– Major Region of Transthyretin Amyloidosis Treatment Market
i) Global Transthyretin Amyloidosis Treatment Sales
ii) Global Transthyretin Amyloidosis Treatment Revenue & market share
– Major Companies List
– Conclusion
0 notes
Text
Europe Genetic Testing Market Restraints, Key Driving Factors, Top Competitors, Growing Demand| Abbott Laboratories, Illumina Inc., Eurofins Scientific, Qiagen, Elitech Group
Europe Genetic Testing Market
Europe Genetic Testing market was valued at US$ 2,472.0 Mn in 2020 and it is projected to reach at US$ 4,262.4 Mn by 2027, at a CAGR of 8.5% during the forecast period.
Major factors accounting for this growth include the increase in incidences of genetic, infectious & chronic diseases, reduction in prices of genetic sequencing, rising adoption of precision medicine diagnostic techniques. Genetic testing is defined as medical test which detect changes in proteins, genes or chromosomes. The test results are able to confirm a speculated underlying genetic condition or aid in determining an individual’s chance of developing/ passing on a genetic disorder.
The growing focus by governments of several countries, to regulate& create awareness regarding genetic tests, has successfully resulted in the faster adoption of these tests worldwide. The rise in Research & Development funding, with the strong market presence by major players in the market, has created a strong entry barrier for new entrants. The increasing innovation in product design, improvement in quality, and strong distribution partnerships are key factors to retain a competitive edge in the market.
Get Request free sample copy @ https://qualiketresearch.com/request-sample/Europe-Genetic-Testing-Market/request-sample
Rising demands for newborn and pre-natal screening to detect congenital abnormalities, growing application of bio banking services that involve DNA testing as a crucial step for cord blood banking are further attributive for progress in this sector. Growing Physicians’ interest towards genetic testing in order to determine patient’s health at various stages of disease development is another factor anticipated to fuel demand in the coming years.
Factors such as increasing implementation of personalized medicine applications to treat chronic disorders enhancing the need for HLA typing & DNA identification for therapy are expected to further add to the growing demand for DNA diagnostics. Moreover, hi-tech advancements which result into commercialization of next generation diagnostic & testing solutions making use of genetic analysis are anticipated to positively impact on Europe genetic testing market growth.
The COVID-19 pandemic has left a prominent impact on the growth of the genetic testing market over the crisis. As per the research article published in Genetics in Medicine (2020), all the clinical genetic testing was restricted & telemedicine-based consultations and counseling were adopted across the globe. According to this study, 112 prenatal & 156 clinical genetics/cancer patients were evaluated in a span of one month at the Columbia University Irving Medical Center (CUIMC).
Most of the genetic testing services are adopted virtualization, coupled with home testing kit or home sample collection, to combat the transmission of the SARS-CoV2 virus. Moreover, as per the research article published in the Journal of Medical Genetics (2020), there was hindrance reported in accessing genetic testing blood draws, as a result of that the genetic testing was prominently impacted, wherein a reduction in genetic testing from 97.7% to 74.1% was observed during this period. Hence, COVID-19 is anticipated to have a direct & indirect impact on the genetic testing market over the ongoing crisis period.
Based on Technology, the Europe genetic testing market is categorized intoCytogenetics Testing, Biochemical Testing and Molecular Testing.
Cytogenetic testing segment is the second highest revenue generating technology in Europe genetic testing market. The growth is attributed due to rise in healthcare expenditure that has resulted in the increasing use of cytogenetic products in clinical and research laboratories, academic research institutes, and pharmaceutical and biotechnology companies. Also, cytogenetic is used in the development of targeted cancer treatment, personal medicine, and others. Currently, a wide range of techniques such as comparative genomic hybridization, fluorescence, karyotyping, and others are employed for the screening of genetic abnormalities and cancers. These techniques employ the use of cytogenetic products including media, kits, reagents, and others. This factor is expected to drive the Cytogenetic testing segment over the forecast period.
Europe Genetic Testing Market Share Analysis by Type
Based on type segment, the Europe genetic testing market is categorized into Carrier Testing, Diagnostic Testing, Newborn Screening, Predictive & Presymptomatic Testing, Prenatal Testing and Others.
Diagnostic Testing has accounted for the highest market share in 2020. This is due to rising incidence of genetic and other chronic disorders. As per the information published on World Health Organization (WHO), the increase in prevalence of inherited diseases like sickle cell anemia, cystic fibrosis and hemophilia will contribute the overall market size for genetic tests. Additionally, early disease diagnosis further allows patients to undergo therapeutic treatment at an early stage that minimizes the severity of disease leading to reduced mortality rate and hence proving beneficial for the segment expansion.
Based on Disease segment, the Europe genetic testing market is categorized into Alzheimer's Disease, Cancer, Cystic Fibrosis, Sickle Cell Anemia, Duchenne Muscular Dystrophy, Thalassemia, Huntington's Disease and Others.
Cancer disease has accounted for the highest market share in 2020. Genetic testing is very helpful to estimate the chances of developing cancer throughout an individual’s lifetime by examining for specific changes in chromosomes and genes, proteins. Cancer, globally, is the 2nd leading reason of death, and approximately70% of deaths from cancer occurs in low- and middle-income countries which raises the demand for genetic testing. As, significant enhancements can be made in the lives of cancer persons by diagnosing at an initial stage & avoiding postponements in care resulting in reduced morbidity, less expensive treatment & greater probability of surviving. This factor creases the demand for genetic testing for cancer.
Germany, The U.K., Italy and France are top country in Europe genetic testing market. These countries collectively accounted for more than 50% of market share in 2020. The presence of developed healthcare infrastructure, strong government initiatives and proactive funding for genetic testing research, and high prevalence of chronic diseases are the major drivers for industrial growth in these countries.
Other European countries is expected to create significant opportunity in genetic testing due to rising research incentives provided by the government in developing economies of this region that enable R&D and commercialization of technologically advanced products. Moreover, higher availability of target population owing to the incidence of chronic diseases in this region is also expected to supplement growth in the coming years.
Recent Developments:
In March 2020, Abbott had launched molecular point-of-care test to detect novel coronavirus in short time period, 5-15 minutes. The ID NOW platform is small, lightweight & portable (the size of a small toaster), and uses molecular technology, which is valued by clinicians and the scientific community for its high degree of accuracy. ID NOW is already the most widely available molecular point-of-care testing. The company has already shipped 150,000 PCR tests to its existing customers, for use on its m2000 Real-time in vitro diagnostic system.
In March 2021, Qiagen had unveiled its Cloud-based platform, QIAsphere, that helps laboratories & QIAstat-Dx users remotely monitor tests & instrument status around the clock. QIAstat-Dx connectivity with the platform will enable QIAGEN technical service to check customers instrument health in real time, simultaneously offering them a quick response to reduced systems downtime. QIAsphere connectivity is made possible by connecting a small connectivity hub, QIAsphere Base (Qbase), to QIAstat-Dx, as well as other platforms, in less time through LAN or Wi-Fi network of hospitals, retaining sensitive data of patients within the hospital network.
In December 2020, CENTOGENE and Alnylam Pharmaceuticals has launched a New Clinical Program Aimed at Revolutionizing the Diagnosis of Hereditary Transthyretin-Related Amyloidosis (“ATTRv”).
Market Segmentation
The Europe Genetic Testing Market is segmented into type such as Carrier Testing, Diagnostic Testing, Newborn Screening, Predictive & Presymptomatic Testing, Prenatal Testing, and Others. On the basis of Disease, market is segmented into Alzheimer's Disease, Cancer , Cystic Fibrosis, Sickle Cell Anemia, Duchenne Muscular Dystrophy, Thalassemia , Huntington's Disease, and Others. Further, market is segmented into technology such as Cytogenetic Testing, Biochemical Testing, and Molecular Testing.
Also, the Europe Genetic Testing Market is segmented into various countries such as Germany, UK, France, Turkey, Switzerland, Norway, Sweden, Spain, Italy, Denmark, Finland, Iceland, Poland, Luxembourg, Netherlands, and Belgium.
Market Key Players
Various key players are listed in this report such as Abbott Laboratories, Illumina Inc., Eurofins Scientific, Qiagen, Elitech Group, Thermo Fischer Scientific, Centogene AG, Blueprint Genetics, 23andMe Inc., F. Hoffmann-La Roche Ltd, etc.
Market Taxonomy
By Type
Carrier Testing
Diagnostic Testing
Newborn Screening
Predictive & Presymptomatic Testing
Prenatal Testing
Others
By Disease
Alzheimer's Disease
Cancer
Cystic Fibrosis
Sickle Cell Anemia
Duchenne Muscular Dystrophy
Thalassemia
Huntington's Disease
Others
By Technology
Cytogenetic Testing
Biochemical Testing
Molecular Testing
By Country
Germany
UK
France
Turkey
Switzerland
Norway
Sweden
Spain
Italy
Denmark
Finland
Iceland
Poland
Luxembourg
Netherlands
Belgium
Browse full Report @ https://qualiketresearch.com/reports-details/Europe-Genetic-Testing-Market
About Us
QualiKet Research is a leading Market Research and Competitive Intelligence partner helping leaders across the world to develop robust strategy and stay ahead for evolution by providing actionable insights about ever changing market scenario, competition and customers. QualiKet Research is dedicated to enhancing the ability of faster decision making by providing timely and scalable intelligence. We use different intelligence tools to come up with evidence that showcases the threats and opportunities which help our clients outperform their competition.
#Europe Genetic Testing Market#Europe Genetic Testing Market size#Europe Genetic Testing Market share#Europe Genetic Testing Market worth#Europe Genetic Testing Market rate
0 notes
Text
Europe Genetic Testing Market Top 5 Competitors, Regional Trend, Application, Marketing Strategy, Outlook Analysis and Forecast

Europe Genetic Testing market was valued at US$ 2,472.0 Mn in 2020 and it is projected to reach at US$ 4,262.4 Mn by 2027, at a CAGR of 8.5% during the forecast period. Major factors accounting for this growth include the increase in incidences of genetic, infectious & chronic diseases, reduction in prices of genetic sequencing, rising adoption of precision medicine diagnostic techniques. Genetic testing is defined as medical test which detect changes in proteins, genes or chromosomes. The test results are able to confirm a speculated underlying genetic condition or aid in determining an individual’s chance of developing/ passing on a genetic disorder.
Market Drivers: Increasing focus of government for genetic testing awareness
The growing focus by governments of several countries, to regulate& create awareness regarding genetic tests, has successfully resulted in the faster adoption of these tests worldwide. The rise in Research & Development funding, with the strong market presence by major players in the market, has created a strong entry barrier for new entrants. The increasing innovation in product design, improvement in quality, and strong distribution partnerships are key factors to retain a competitive edge in the market.
Get Sample Copy of this Report @ https://qualiketresearch.com/request-sample/Europe-Genetic-Testing-Market/request-sample
Rising demands for newborn and pre-natal screening to detect congenital abnormalities, growing application of bio banking services that involve DNA testing as a crucial step for cord blood banking are further attributive for progress in this sector. Growing Physicians’ interest towards genetic testing in order to determine patient’s health at various stages of disease development is another factor anticipated to fuel demand in the coming years.
Factors such as increasing implementation of personalized medicine applications to treat chronic disorders enhancing the need for HLA typing & DNA identification for therapy are expected to further add to the growing demand for DNA diagnostics. Moreover, hi-tech advancements which result into commercialization of next generation diagnostic & testing solutions making use of genetic analysis are anticipated to positively impact on Europe genetic testing market growth.
Market Restraints: COVID-19 has negative impact on Europe genetic testing market
The COVID-19 pandemic has left a prominent impact on the growth of the genetic testing market over the crisis. As per the research article published in Genetics in Medicine (2020), all the clinical genetic testing was restricted & telemedicine-based consultations and counseling were adopted across the globe. According to this study, 112 prenatal & 156 clinical genetics/cancer patients were evaluated in a span of one month at the Columbia University Irving Medical Center (CUIMC).
Most of the genetic testing services are adopted virtualization, coupled with home testing kit or home sample collection, to combat the transmission of the SARS-CoV2 virus. Moreover, as per the research article published in the Journal of Medical Genetics (2020), there was hindrance reported in accessing genetic testing blood draws, as a result of that the genetic testing was prominently impacted, wherein a reduction in genetic testing from 97.7% to 74.1% was observed during this period. Hence, COVID-19 is anticipated to have a direct & indirect impact on the genetic testing market over the ongoing crisis period.
Europe Genetic Testing Market Share Analysis by Technology
Based on Technology, the Europe genetic testing market is categorized intoCytogenetics Testing, Biochemical Testing and Molecular Testing.
Cytogenetic testing segment is the second highest revenue generating technology in Europe genetic testing market. The growth is attributed due to rise in healthcare expenditure that has resulted in the increasing use of cytogenetic products in clinical and research laboratories, academic research institutes, and pharmaceutical and biotechnology companies. Also, cytogenetic is used in the development of targeted cancer treatment, personal medicine, and others. Currently, a wide range of techniques such as comparative genomic hybridization, fluorescence, karyotyping, and others are employed for the screening of genetic abnormalities and cancers. These techniques employ the use of cytogenetic products including media, kits, reagents, and others. This factor is expected to drive the Cytogenetic testing segment over the forecast period.
Europe Genetic Testing Market Share Analysis by Type
Based on type segment, the Europe genetic testing market is categorized into Carrier Testing, Diagnostic Testing, Newborn Screening, Predictive & Presymptomatic Testing, Prenatal Testing and Others.
Diagnostic Testing has accounted for the highest market share in 2020. This is due to rising incidence of genetic and other chronic disorders. As per the information published on World Health Organization (WHO), the increase in prevalence of inherited diseases like sickle cell anemia, cystic fibrosis and hemophilia will contribute the overall market size for genetic tests. Additionally, early disease diagnosis further allows patients to undergo therapeutic treatment at an early stage that minimizes the severity of disease leading to reduced mortality rate and hence proving beneficial for the segment expansion.
Europe Genetic Testing Market Share Analysis by Disease
Based on Disease segment, the Europe genetic testing market is categorized into Alzheimer's Disease, Cancer, Cystic Fibrosis, Sickle Cell Anemia, Duchenne Muscular Dystrophy, Thalassemia, Huntington's Disease and Others.
Cancer disease has accounted for the highest market share in 2020. Genetic testing is very helpful to estimate the chances of developing cancer throughout an individual’s lifetime by examining for specific changes in chromosomes and genes, proteins. Cancer, globally, is the 2nd leading reason of death, and approximately70% of deaths from cancer occurs in low- and middle-income countries which raises the demand for genetic testing. As, significant enhancements can be made in the lives of cancer persons by diagnosing at an initial stage & avoiding postponements in care resulting in reduced morbidity, less expensive treatment & greater probability of surviving. This factor creases the demand for genetic testing for cancer.
Europe Genetic Testing Market Share Analysis by Country
Germany, The U.K., Italy and France are top country in Europe genetic testing market. These countries collectively accounted for more than 50% of market share in 2020. The presence of developed healthcare infrastructure, strong government initiatives and proactive funding for genetic testing research, and high prevalence of chronic diseases are the major drivers for industrial growth in these countries.
Other European countries is expected to create significant opportunity in genetic testing due to rising research incentives provided by the government in developing economies of this region that enable R&D and commercialization of technologically advanced products. Moreover, higher availability of target population owing to the incidence of chronic diseases in this region is also expected to supplement growth in the coming years.
Recent Developments:
In March 2020, Abbott had launched molecular point-of-care test to detect novel coronavirus in short time period, 5-15 minutes. The ID NOW platform is small, lightweight & portable (the size of a small toaster), and uses molecular technology, which is valued by clinicians and the scientific community for its high degree of accuracy. ID NOW is already the most widely available molecular point-of-care testing. The company has already shipped 150,000 PCR tests to its existing customers, for use on its m2000 Real-time in vitro diagnostic system.
In March 2021, Qiagen had unveiled its Cloud-based platform, QIAsphere, that helps laboratories & QIAstat-Dx users remotely monitor tests & instrument status around the clock. QIAstat-Dx connectivity with the platform will enable QIAGEN technical service to check customers instrument health in real time, simultaneously offering them a quick response to reduced systems downtime. QIAsphere connectivity is made possible by connecting a small connectivity hub, QIAsphere Base (Qbase), to QIAstat-Dx, as well as other platforms, in less time through LAN or Wi-Fi network of hospitals, retaining sensitive data of patients within the hospital network.
In December 2020, CENTOGENE and Alnylam Pharmaceuticals has launched a New Clinical Program Aimed at Revolutionizing the Diagnosis of Hereditary Transthyretin-Related Amyloidosis (“ATTRv”).
Market Segmentation
The Europe Genetic Testing Market is segmented into type such as Carrier Testing, Diagnostic Testing, Newborn Screening, Predictive & Presymptomatic Testing, Prenatal Testing, and Others. On the basis of Disease, market is segmented into Alzheimer's Disease, Cancer , Cystic Fibrosis, Sickle Cell Anemia, Duchenne Muscular Dystrophy, Thalassemia , Huntington's Disease, and Others. Further, market is segmented into technology such as Cytogenetic Testing, Biochemical Testing, and Molecular Testing.
Also, the Europe Genetic Testing Market is segmented into various countries such as Germany, UK, France, Turkey, Switzerland, Norway, Sweden, Spain, Italy, Denmark, Finland, Iceland, Poland, Luxembourg, Netherlands, and Belgium.
Market Key Players
Various key players are listed in this report such as Abbott Laboratories, Illumina Inc., Eurofins Scientific, Qiagen, Elitech Group, Thermo Fischer Scientific, Centogene AG, Blueprint Genetics, 23andMe Inc., F. Hoffmann-La Roche Ltd, etc.
Market Taxonomy
By Type
Carrier Testing
Diagnostic Testing
Newborn Screening
Predictive & Presymptomatic Testing
Prenatal Testing
Others
By Disease
Alzheimer's Disease
Cancer
Cystic Fibrosis
Sickle Cell Anemia
Duchenne Muscular Dystrophy
Thalassemia
Huntington's Disease
Others
By Technology
Cytogenetic Testing
Biochemical Testing
Molecular Testing
By Country
Germany
UK
France
Turkey
Switzerland
Norway
Sweden
Spain
Italy
Denmark
Finland
Iceland
Poland
Luxembourg
Netherlands
Belgium
Browse Full Research Report @ https://qualiketresearch.com/reports-details/Europe-Genetic-Testing-Market
About Us
QualiKet Research is a leading Market Research and Competitive Intelligence partner helping leaders across the world to develop robust strategy and stay ahead for evolution by providing actionable insights about ever changing market scenario, competition and customers. QualiKet Research is dedicated to enhancing the ability of faster decision making by providing timely and scalable intelligence. We use different intelligence tools to come up with evidence that showcases the threats and opportunities which helps our clients outperform their competition.
#Europe Genetic Testing Market size#Europe Genetic Testing Market Share#Europe Genetic Testing Market Trend#Europe Genetic Testing Mark
0 notes
Text
Cardiomyopathy Medication Market 2021: Gross Margin Analysis, Global Overview, Emerging Trends, Leading Growth Drivers, Future Estimation
Cardiomyopathy Medication Market Overview
The global cardiomyopathy medication market size is foreseen to reach USD 620 Million by 2025 at a CAGR of 4.60% during the gauge time of 2019 to 2025. Cardiomyopathy is an infection that causes the thickening and growth of the heart muscles and strange blood stream. Patients experiencing cardiomyopathy feature manifestations, for example, wooziness, tiredness, and expansion to legs, lower legs, and feet.
Widened adoption of cardiomyopathy medication is a cardiovascular issue brought about by development of heart. The strong dividers of the heart become frail because of expansion and consequently heart can't siphon blood productively. The turmoil causes impact on liver, lungs and other significant organs of the body consistently. It is the clinical condition which causes augmentation of at first left vertical of the heart, which is considered as principle siphoning chamber. Altogether, if may spread to right chamber over the time. cardiomyopathy medication additionally prompts visit blood clumps, sporadic pulses and valve issues, which may influence any individual regardless of age and sex, although the likelihood proportion of male over female is 3:1.
Different medicines accessible for cardiomyopathy medication centers in progress of blood stream which is it fundamental cause. The treatment of the cardiomyopathy medication should be possible with two procedures one is by oral medication and other is implantable gadgets. The kinds of cardiomyopathy medication drugs accessible are beta blockers, diuretics, digitalis, angiotensin 2 receptor blocker, warfarin, diuretics and angiotensin-changing over chemical inhibitors. In the second classification of treatment, implantable gadgets, heart siphons and cardioverter-defibrillators are unmistakably utilized.
The primary driver of cardiomyopathy medication are diseases in cardiovascular muscles, hereditary qualities or birth deformities, medication or liquor misuse or certain presentation to lethal substances, for example, mercury, lead or cobalt. In a portion of the cases cardiomyopathy medication may likewise be caused as symptom of disease medication.
Cardiomyopathy Medication Market Competitive Analysis:
Some of the major companies in the global cardiomyopathy medication market include Pfizer Inc., Array Biopharma, Inc., PhaseBio Pharmaceuticals, Inc., Sanofi-Aventis US LLC, AstraZeneca, F. Hoffmann-La Roche Ltd, Capricor Therapeutics., Merck & Co., Inc., MyoKardia, Janssen Products, LP, Ionis Pharmaceuticals, Inc., Becton And Dickson & Co., Medtronic, Biomerieux, and Teva Pharmaceutical Industries Ltd.
Cardiomyopathy Medication Market Segmentation:
The cardiomyopathy medication market is segmented on type, treatment, and end user.
Based on type, the market has been segmented into hypertrophic cardiomyopathy, dilated cardiomyopathy, restrictive cardiomyopathy, unclassified cardiomyopathy, and arrhythmogenic right ventricular dysplasia.
By treatment, the global cardiomyopathy medication market has been categorized as antiarrhythmics, anticoagulants, cardiac glycosides, antihypertensives, and diuretics.
The end user segments of the market are hospitals and clinics, homecare, and others. Of these, the hospitals and clinics segment are expected to dominate the market during the review period.
Cardiomyopathy Medication Market Regional Analysis:
Regionally, the global cardiomyopathy medication market is segmented into the Americas, Asia-Pacific, Europe, and Middle East & Africa. Of these, the market in the Americas is relied upon to overwhelm the global cardiomyopathy medication market because of the expanding per capita human services consumption and nearness of an enormous number of social insurance organizations in the district. The market in Asia-Pacific is anticipated to enroll the most elevated development rate during the estimate time frame inferable from the expanding attention to heart maladies and expanding government activities for human services changes.
Get Premium Research Report, Inclusive of COVID-19 Impact Analysis, Find more information @ https://www.marketresearchfuture.com/reports/cardiomyopathy-medication-market-8256
Cardiomyopathy Medication Industry News:
In 2019, the U.S. Nourishment and Drug Administration affirmed Vyndaqel (tafamidis meglumine) and Vyndamax (tafamidis) containers for the treatment of the coronary illness (cardiomyopathy) brought about by transthyretin interceded amyloidosis (ATTR-CM) in grown-ups.
In 2019, Researchers at the University of Arizona College of Medicine - Phoenix have appeared without precedent for preclinical investigations that Aliskiren, a medication that restrains the compound that manages pulse, can defer the movement of congestive cardiovascular breakdown and stretch endurance rates.
#Cardiomyopathy Medication Market#Cardiomyopathy Medication Market Size#Cardiomyopathy Medication Market Share#Cardiomyopathy Medication Market Growth#Cardiomyopathy Medication Market Analysis
0 notes
Text
CNS SPECIFIC ANTISENSE OLIGONUCLEOTIDE MARKET ANALYSIS
Antisense oligonucleotides are synthetic strings of nucleic acid, which reduce expression of messenger RNA (m-RNA). The function performed by interference with pre m- RNA splicing are restoration of protein and modification of protein. Antisense oligonucleotide is a new approach to treat several neurodegenerative disorders to prevent disease onset or stop disease development.
Market Dynamics
Increasing prevalence of neurodegenerative disease is a major factor driving CNS specific antisense oligonucleotide market growth. According to fact sheet from Alzheimer Association, an estimated around 5.7 million Americans were diagnosed with Alzheimer's dementia in 2018. Moreover, rich product pipeline for the treatment of CNS disorders and rare diseases associated with it is expected to boost the market growth. For instance, in December 2018, Biogen and Ionis Pharmaceuticals product IONIS-SOD1Rx (BIIB067) cleared Phase 1 clinical trial and is expected to be launched by 2020.
Key features of the study:
This report provides in-depth analysis of the CNS specific antisense oligonucleotide market and provides market size (US$ million) and Compound Annual Growth Rate (CAGR %) for the forecast period (2018 – 2026), considering 2017, as the base year
It elucidates potential revenue opportunities across different segments and explains attractive investment proposition matrices for this market
This study also provides key insights about market drivers, restraints, opportunities, new product launches or approval, market trends, regional outlook, and competitive strategy adopted by key players
It profiles key players in the global CNS specific antisense oligonucleotide market based on the following parameters – company overview, financial performance, product portfolio, market presence, distribution strategies, key developments, strategies, and future plans
Key companies covered as a part of this study include, Alnylam Pharmaceuticals Inc., Sarepta Therapeutics Inc., Biogen Inc., Ionis Pharmaceuticals Inc., Wave Life Sciences Ltd., Stroke Therapeutic Inc., Dynacure, ProQR Therapeutics N.V., Q-STATE BIOSCIENCES, INC.
Insights from this report would allow marketers and the management authorities of the companies to make informed decisions regarding their future product launches, technology up-gradation, market expansion, and marketing tactics
The global CNS specific antisense oligonucleotide market report caters to various stakeholders in this industry including investors, suppliers, product manufacturers, distributors, new entrants, and financial analysts
Stakeholders would have ease in decision-making through various strategy matrices used in analyzing the CNS specific antisense oligonucleotide market
Detailed Segmentation:
Patisiran
Nusinersen
Inotersen
IONIS-HTT Rx (RG6042)
Hereditary transthyretin amyloidosis (hATTR)/ Polyneuropathy
Spinal Muscular Atrophy
Huntington’s Disease
Hospital Pharmacy
Retail Pharmacy
Online Pharmacy
Patisiran
Nusinersen
Inotersen
IONIS-HTT Rx (RG6042)
Hereditary transthyretin amyloidosis (hATTR)/ Polyneuropathy
Spinal Muscular Atrophy
Huntington’s Disease
Hospital Pharmacy
Retail Pharmacy
Online Pharmacy
U.S.
Canada
Patisiran
Nusinersen
Inotersen
IONIS-HTT Rx (RG6042)
Hereditary transthyretin amyloidosis (hATTR)/ Polyneuropathy
Spinal Muscular Atrophy
Huntington’s Disease
Hospital Pharmacy
Retail Pharmacy
Online Pharmacy
Brazil
Mexico
Argentina
Rest of Latin America
Patisiran
Nusinersen
Inotersen
IONIS-HTT Rx (RG6042)
Hereditary transthyretin amyloidosis (hATTR)/ Polyneuropathy
Spinal Muscular Atrophy
Huntington’s Disease
Hospital Pharmacy
Retail Pharmacy
Online Pharmacy
Germany
U.K.
France
Italy
Spain
Russia
Rest of Europe
Patisiran
Nusinersen
Inotersen
IONIS-HTT Rx (RG6042)
Hereditary transthyretin amyloidosis (hATTR)/ Polyneuropathy
Spinal Muscular Atrophy
Huntington’s Disease
Hospital Pharmacy
Retail Pharmacy
Online Pharmacy
China
India
Japan
Australia
South Korea
ASEAN
Rest of Asia Pacific
Patisiran
Nusinersen
Inotersen
IONIS-HTT Rx (RG6042)
Hereditary transthyretin amyloidosis (hATTR)/ Polyneuropathy
Spinal Muscular Atrophy
Huntington’s Disease
Hospital Pharmacy
Retail Pharmacy
Online Pharmacy
GCC
Israel
Rest of Middle East
Patisiran
Nusinersen
Inotersen
IONIS-HTT Rx (RG6042)
Hereditary transthyretin amyloidosis (hATTR)/ Polyneuropathy
Spinal Muscular Atrophy
Huntington’s Disease
Hospital Pharmacy
Retail Pharmacy
Online Pharmacy
South Africa
Central Africa
North Africa
Company Overview
Product Portfolio
Financial Performance
Key Highlights
Market Strategies
Request the sample copy of here:
https://www.coherentmarketinsights.com/insight/request-sample/2477
Download the PDF Brochure here:
https://www.coherentmarketinsights.com/insight/request-pdf/2477
Buy now the market research report here:
https://www.coherentmarketinsights.com/insight/buy-now/2477
About Us:
Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions.
What we provide:
Customized Market Research Services
Industry Analysis Services
Business Consulting Services
Market Intelligence Services
Long term Engagement Model
Country Specific Analysis
Explore CMI Services here
Contact Us:
Mr. Shah
Coherent Market Insights Pvt. Ltd.
Address: 1001 4th ave, #3200 Seattle, WA 98154, U.S.
Phone: +1-206-701-6702
Email: [email protected]
Source:
https://www.coherentmarketinsights.com/market-insight/cns-specific-antisense-oligonucleotide-market-2477
0 notes
Text
Transthyretin (TTR) Amyloidosis Market | by Disease Type, Treatment, Gender, End User, and Region
Industry Research Report – Transthyretin (TTR) Amyloidosis Market forecast till 2023, is latest research report published by MRFR. The Global TTR Amyloidosis Market is segmented on the basis of treatment, gender, end-user, disease type, and Region.
Transthyretin (TTR) Amyloidosis Market Overview
The Transthyretin (TTR) amyloidosis market is likely to register a robust growth over the forecast period. Transthyretin (TTR) amyloidosis is a health condition characterized by anomalous deposits of a protein named amyloid (amyloidosis) in a person's tissues and organs. It usually affects autonomic neuropathy or peripheral neuropathic system and cardiac system. Amyloidosis symptoms are often ambiguous having similarity to symptoms caused by other conditions. The symptoms vary based on the location and type of the amyloid deposits. Primarily, three major types of transthyretin amyloidosis have been identified. The neuropathic type of transthyretin amyloidosis affects the autonomic and peripheral nervous systems, resulting in trouble controlling bodily functions and peripheral neuropathy. The leptomeningeal type of transthyretin amyloidosis mostly affects the central nervous system. The cardiac type of Transthyretin (TTR) amyloidosis puts patients at the risk of orthostatic hypertension, cardiomegaly, and arrhythmia.
Get a FREE Sample with Complete TOC By considering the COVID-19 impact on market @ https://www.marketresearchfuture.com/sample_request/6155
The surging African American population, increasing number of ATTR therapeutic drug launches, increasing average income of individuals, and growing healthcare awareness among people serves as key factors for driving the Transthyretin (TTR) amyloidosis market growth.
However, progress of the market can be restricted by strict governing policies, incorrect diagnosis of TTR ailments, restraints on clinical trials, and high cost of TTR drugs. Unavailability of advanced diagnostic methods in the middle-income nations and lack of knowledge about Transthyretin amyloidosis acts as major challenges to the market.
Transthyretin (TTR) Amyloidosis Market Segmentation
The worldwide Transthyretin (TTR) amyloidosis market has been segmented based on treatment, gender, end-user, and disease type. Based on treatment, the market has been segmented into organ transplantation, RNAi therapy, small molecules treatment, and others. Small molecules treatment has been sub-segmented as diflunisal and tafamidis. Based on gender, the market has been segmented into male and female. Based on end-user, the market has been segmented into ambulatory surgical centers, hospitals and clinics, and others. Based on disease type, the market has been segmented into Transthyretin (TTR) familial amyloid polyneuropathy, Transthyretin (TTR) familial amyloid cardiomyopathy, and others.
Transthyretin (TTR) Amyloidosis Market Regional Analysis
The Americas hold the largest market share for Transthyretin (TTR) amyloidosis. The growth is owing to the rising occurrence of amyloidosis among adults and older population and increasing elderly population. For instance, a report published by ASCO Journal in 2016 stated that almost 4,000 people suffered from amyloidosis each year in the United States. Additionally, increasing government funding, technologically advanced diagnostic equipment, and surging research initiatives in advanced medical treatment options are likely to drive the market. The existence of leading pharmaceutical brands such as Merck and Pfizer also boost the market in this region.
Europe represents the second largest share in the global Transthyretin (TTR) amyloidosis market. The Europe market has been anticipated to grow remarkably over the forecast period. The growth is owing to soaring healthcare expenditure, availability of progressive treatment facilities, amplifying demand for improved healthcare infrastructure collectively with government initiatives for healthcare reform. In the U.K., almost 60 new cases of Transthyretin (TTR) amyloidosis are reported every year. Furthermore, increased affordability coupled with well-established medical device manufacturers accelerate the market to a great extent. However, increasing incidences of wrong diagnosis and the inefficiency of treatment procedures are likely to restrict the growth in the Europe market.
Asia Pacific market has been fast expanding owing to rising aged population. Moreover, increasing patient pool, government initiatives to enhance the healthcare facility, availability of favorable insurance reimbursement policies, and a rapid adoption of advanced technology are likely to stimulate the Transthyretin (TTR) amyloidosis market.
The Middle East and Africa market has been anticipated to register least growth owing to limited access to treatment facilities and lack of health awareness. In the Middle East, Saudi Arabia and the United Arab Emirates are the largest markets. The market growth has been attributed to the easy accessibility of specialty care centers and growth of healthcare industry. However, the market is expected to witness steady development due to the genetical predisposition of Africans for transthyretin amyloidosis and administrative initiatives to excel the quality of healthcare in the region.
Transthyretin (TTR) Amyloidosis Market Key Players
The global Transthyretin (TTR) amyloidosis market has been dominated by key players such as Pfizer, Alnylam Pharmaceuticals, Ionis Pharmaceuticals Inc., Arcturus Therapeutics, Corino Therapeutics Inc, and Proclara Bioscience.
Obtain Premium Research Report Details, Considering the impact of COVID-19 @ https://www.marketresearchfuture.com/reports/transthyretin-amyloidosis-market-6155
About Market Research Future:
At Market Research Future (MRFR), we enable our customers to unravel the complexity of various industries through our Cooked Research Report (CRR), Half-Cooked Research Reports (HCRR), Raw Research Reports (3R), Continuous-Feed Research (CFR), and Market Research & Consulting Services.
MRFR team have supreme objective to provide the optimum quality market research and intelligence services to our clients. Our market research studies by Components, Application, Logistics and market players for global, regional, and country level market segments, enable our clients to see more, know more, and do more, which help to answer all their most important questions.
In order to stay updated with technology and work process of the industry, MRFR often plans & conducts meet with the industry experts and industrial visits for its research analyst members.
Contact:
Market Research Future
+1 646 845 9312
Email: [email protected]
NOTE: Our team of researchers are studying Covid-19 and its impact on various industry verticals and wherever required we will be considering covid-19 footprints for a better analysis of markets and industries. Cordially get in touch for more details.
#transthyretin amyloidosis market#TTR amyloidosis market#transthyretin amyloidosis market size#transthyretin amyloidosis market share#TTR amyloidosis market CAGR#ATTR#TTR familial amyloid polyneuropathy#TTR familial amyloid cardiomyopathy
0 notes
Text
By 2026, CNS Specific Antisense Oligonucleotide Market To Surpass US$ 6,843.0 Million - Coherent Market Insights
CNS Specific Antisense Oligonucleotide Market To exhibit a CAGR of 24.9% over the forecast period (2018 – 2026).
Request for Sample PDF Copy:
https://www.coherentmarketinsights.com/insight/request-pdf/2477
Description:
Antisense oligonucleotide plays a major role in modern healthcare, as it acts on the messenger RNA level before the protein is formed to inhibit expression of certain diseases-causing genes.
The global CNS specific antisense oligonucleotide market size was valued at US$ 883.7 million in 2017, and is expected to exhibit a CAGR of 24.9% over the forecast period (2018 – 2026).
Figure 1. Global CNS Specific Antisense Oligonucleotide Market Value (US$ Mn), by Region, 2017
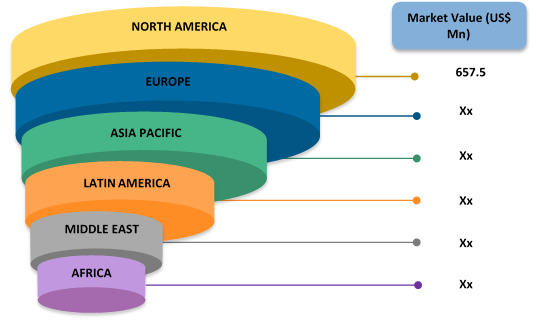
CNS Specific Antisense Oligonucleotide | Coherent Market Insights
Source: Coherent Market Insights Analysis (2019)
Increasing prevalence of cancer is expected to propel growth of the CNS specific antisense oligonucleotide market
CNS specific antisense oligonucleotide is used for treatment of neurodegenerative disorders and associated rare diseases. According to February 2017, report of National Center for Biotechnology Information (NCBI), an estimated the prevalence of Cerebral palsy ranges from 1.5 to more than 4 per 1,000 of children from age 10 to 14 years. The birth prevalence of Cerebral palsy is around 2 per 1,000 live births globally.
Furthermore, launch of new products for rare disease associated with CNS disorders is propelling growth of the CNS specific antisense oligonucleotide market over the forecast period. For instance, in October 2018, Ionis Pharmaceuticals, Inc. and Akcea Therapeutics, Inc. received the U.S. Food and Drug Administration (FDA) approval for its novel drug TEGSEDITM (inotersen) indicated for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults. In the same year, company received approval from Canadian Health authority for the novel drug TEGSEDITM (inotersen).
However, high cost of treatment and risk of toxicity is expected to hinder the market growth. According to the Journal Molecular Therapy, in May 2017, Biogen priced Spinraza (nusinersen injection) at US$ 750,000 for the first year’s treatment (US$125,000 per injection) and US$350,000 per year.
Figure 2. Global CNS Specific Antisense Oligonucleotide Market Share (%), by Drug, 2018 and 2026
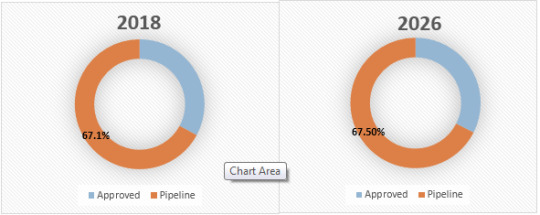
CNS Specific Antisense Oligonucleotide | Coherent Market Insights
Source: Coherent Market Insights Analysis (2019)
Government support for development and launch of antisense drugs in North America is expected to bolster the market growth
North America holds the dominant position in the global CNS specific antisense oligonucleotide market, owing to strong government support for antisense drugs. For instance, in March 2018, GeneTx Biotherapeutics LLC’s, GTX-101 received the U.S. Food and Drug Administration’s (FDA) orphan-drug designation for GTX-101 for the treatment of Angelman syndrome. It is a rare neurogenetic disorder that affects around one in 15,000 people, according to the FDA statement.
Increasing prevalence of Alzheimer disease in the region is also expected to boost the market growth. For instance, in August 2012, National Center for Biotechnology Information (NCBI) stated Alzheimer disease as the most frequent cause of dementia in Western societies and estimated around 5.5 million cases in the U.S. and the prevalence worldwide is estimated to be as high as 24 million.
Moreover, rich product pipeline for treatment of neurodegenerative disorders is expected to drive the market growth. For instance, in October 2017, Ionis Pharmaceuticals, Inc., initiated a Phase 1/2 clinical study of IONIS-MAPTRx in patients with mild Alzheimer's disease. Biogen funded US$ 10 million milestone payment to Ionis Pharmaceuticals, Inc.
Moreover, key players in the players are focused on receiving the U.S. Food and Drug Administration (FDA) approval for novel products to reduce the burden of CNS associated disorders. For instance, in September 2016, Sarepta Therapeutics received the U.S. Food and Drug Administration (FDA) approval for Exondys 51(eteplirsen) used for treatment of rare genetic disorder Duchenne muscular dystrophy.
Key Players
Major players operating in the global CNS specific antisense oligonucleotide market include Alnylam Pharmaceuticals Inc., Sarepta Therapeutics Inc., Biogen Inc., Ionis Pharmaceuticals Inc., Wave Life Sciences Ltd., Stroke Therapeutic Inc., Dynacure, ProQR Therapeutics N.V., and Q-STATE BIOSCIENCES, INC.
About Us:
Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions. We are headquartered in India, having office at global financial capital in the U.S. Our client base includes players from across all business verticals in over 150 countries worldwide. We do offer wide range of services such as Industry analysis, Consulting services, Market Intelligence, Customized research services and much more. We have expertise in many fields such as healthcare, chemicals and materials, Automation, semiconductors, electronics, energy, food and beverage, packaging and many more. Visit our website to know more.
Buy Report Here:
https://www.coherentmarketinsights.com/insight/buy-now/2477
Contact Us:
Mr. Shah
Coherent Market Insights
1001 4th Ave.
#3200
Seattle, WA 98154
Tel: +1-206-701-6702
Email: [email protected]
0 notes
Text
CNS Specific Antisense Oligonucleotide Market Development and Trends Forecasts Report 2018-2026

Antisense oligonucleotides are synthetic strings of nucleic acid, which reduce expression of messenger RNA (m-RNA). The function performed by interference with pre m- RNA splicing are restoration of protein and modification of protein. Antisense oligonucleotide is a new approach to treat several neurodegenerative disorders to prevent disease onset or stop disease development.
For In depth Information Get Sample Copy of this Report at: https://www.coherentmarketinsights.com/insight/request-sample/2477
Market Dynamics
Increasing prevalence of neurodegenerative disease is a major factor driving CNS specific antisense oligonucleotide market growth. According to fact sheet from Alzheimer Association, an estimated around 5.7 million Americans were diagnosed with Alzheimer's dementia in 2018. Moreover, rich product pipeline for the treatment of CNS disorders and rare diseases associated with it is expected to boost the market growth. For instance, in December 2018, Biogen and Ionis Pharmaceuticals product IONIS-SOD1Rx (BIIB067) cleared Phase 1 clinical trial and is expected to be launched by 2020.
Increasing prevalence of cancer is expected to propel growth of the CNS specific antisense oligonucleotide market
CNS specific antisense oligonucleotide is used for treatment of neurodegenerative disorders and associated rare diseases. According to February 2017, report of National Center for Biotechnology Information (NCBI), an estimated the prevalence of Cerebral palsy ranges from 1.5 to more than 4 per 1,000 of children from age 10 to 14 years. The birth prevalence of Cerebral palsy is around 2 per 1,000 live births globally.
Furthermore, launch of new products for rare disease associated with CNS disorders is propelling growth of the CNS specific antisense oligonucleotide market over the forecast period. For instance, in October 2018, Ionis Pharmaceuticals, Inc. and Akcea Therapeutics, Inc. received the U.S. Food and Drug Administration (FDA) approval for its novel drug TEGSEDITM (inotersen) indicated for the treatment of the polyneuropathy of hereditary transthyretin-mediated amyloidosis in adults. In the same year, company received approval from Canadian Health authority for the novel drug TEGSEDITM (inotersen).
However, high cost of treatment and risk of toxicity is expected to hinder the market growth. According to the Journal Molecular Therapy, in May 2017, Biogen priced Spinraza (nusinersen injection) at US$ 750,000 for the first year’s treatment (US$125,000 per injection) and US$350,000 per year.
Key Players
Major players operating in the global CNS specific antisense oligonucleotide market include Alnylam Pharmaceuticals Inc., Sarepta Therapeutics Inc., Biogen Inc., Ionis Pharmaceuticals Inc., Wave Life Sciences Ltd., Stroke Therapeutic Inc., Dynacure, ProQR Therapeutics N.V., and Q-STATE BIOSCIENCES, INC.
Browse Research Report At: https://www.coherentmarketinsights.com/market-insight/cns-specific-antisense-oligonucleotide-market-2477
Detailed Segmentation:
Global CNS Specific Antisense Oligonucleotide Market, By Drug:
Approved
Patisiran
Nusinersen
Inotersen
Pipeline
IONIS-HTT Rx (RG6042)
Global CNS Specific Antisense Oligonucleotide Market, By Indication:
Hereditary transthyretin amyloidosis (hATTR)/ Polyneuropathy
Spinal Muscular Atrophy
Huntington’s Disease
Global CNS Specific Antisense Oligonucleotide Market, By Distribution Channel:
Hospital Pharmacy
Retail Pharmacy
Online Pharmacy
Global CNS Specific Antisense Oligonucleotide Market, By Geography:
North America
Europe
Asia Pacific
Latin America
Middle East
Africa
Key questions answered in the report:
1. What will the market growth rate of market in 2026
2. What are the key factors driving the global market
3. Who are the key manufacturers in market space?
4. What are the market opportunities, market risk and market overview of the market?
5. What are sales, revenue, and price analysis by types and applications of market?
6. What are sales, revenue, and price analysis by regions of industry?
Request For Customization of Research Report @ https://www.coherentmarketinsights.com/insight/request-customization/2477
About Coherent Market Insights
Coherent Market Insights is a prominent market research and consulting firm offering action-ready syndicated research reports, custom market analysis, consulting services, and competitive analysis through various recommendations related to emerging market trends, technologies, and potential absolute dollar opportunity.
Contact Us
Mr. Shah
Coherent Market Insights
1001 4th Ave, #3200
Seattle, WA 98154
Tel: +1-206-701-6702
Email: [email protected]
0 notes
Text
Transthyretin Amyloidosis Treatment Market - Global Industry Insights, and Opportunity Analysis, 2025
Transthyretin amyloidosis is a rare disease caused due to abnormal build-up of amyloidosis in the tissues of the body. The deposits of transthyretin amyloids cause illness due to damage in structure and function of the organs and moreover, they can affect any part of the body where deposits of transthyretin amyloids are present. The deposits mainly consists of abnormal, insoluble protein fibers called amyloid fibrils that are soluble in healthy people but when the protein is misfolded it becomes sticky and form a clump together, which is insoluble in nature and accumulate in the tissue.
Furthermore, there are around 30 different types of protein known to form amyloid, which result in different clinical conditions as different amyloidosis tend to affect different body organs. Transthyretin amyloidosis is progressive and rare fatal disease that destroys the nerve cells that governs the various functions of body. There are 2 types of transthyretin amyloidosis, namely, , transthyretin familial amyloid polyneuropathy and transthyretin familial amyloid cardiomyopathy. Among this, in transthyretin familial amyloid polyneuropathy nerves that control senses, movements, and involuntary bodily functions are affected. Whereas, in transthyretin familial amyloid cardiomyopathy the damage is caused to heart muscles and also impedes the heart’s ability to pump blood throughout the whole body.
Request a sample copy of this report: http://bit.ly/2vVfz0F
0 notes
Text
Transthyretin Amyloidosis - Global Drug Forecast and Market Analysis to 2029 published on
https://www.sandlerresearch.org/transthyretin-amyloidosis-global-drug-forecast-and-market-analysis-to-2029.html
Transthyretin Amyloidosis - Global Drug Forecast and Market Analysis to 2029
Transthyretin Amyloidosis – Global Drug Forecast and Market Analysis to 2029
Summary
Amyloidosis is a protein confirmation disorder that results in the build-up of insoluble amyloid proteins in tissues. This build-up can be fatal as it leads to disruption of organ systems and causes oxidative stress within the body. There are various forms of amyloidosis, each associated with the protein involved and the underlying cause of protein mis-folding. Amyloid aggregates affect different organs, most commonly the heart, kidney and liver whilst also affecting the peripheral, autonomic and gastro-intestinal (GI) systems. Most amyloidosis diagnoses are made later in life, with the average patient aged 65 at diagnosis.
Transthyretin Amyloidosis (ATTR) is caused by the transthyretin (TTR) protein and occurs in two forms, either hereditary or wild type. Majority of organ involvement in ATTR is found in the heart and nerves are also affected. Liver transplant is a plausible treatment option for patients with ATTR-m as the liver is the cause of the mutated TTR protein, therefore removing the source of the issue seems to work well. However, a process described as seeding’ generally means that post-transplantation, the amyloid deposits eventually return due to the amyloid template left by pre-transplant mis-folding.
There are a number of unmet needs in the ATTR market, but drug treatment innovation is on the rise and diagnosis rates are expected to dramatically increase.
Key Highlights
– The main driver of the ATTR market expansion will be the approval and launch of the RNA interference therapeutics both ATTR polyneuropathy and ATTR cardiomyopathy. The approval of other pipeline agents of varying mechanism will also drive growth due to the current scarcity of the ATTR market.
– Another prominent contributor to sales growth the increase in diagnosed prevalence across the 7MM. This increase will be caused by improved understanding and awareness of the disease alongside campaigns by drug developers to increase genetic testing.
– The major global barrier for the ATTR market will be the annual cost of therapy for new pipeline drugs and the lack of options for advanced disease states, the stage at which majority of patients are diagnosed.
– The key market opportunities lie in addressing unmet needs through the development of efficacious therapies for clearing already deposited amyloid and increasing the number of therapies approved for ATTR cardiomyopathy.
The report will enable you to –
– Develop and design your in-licensing and out-licensing strategies through a review of pipeline products and technologies, and by identifying the companies with the most robust pipeline. Additionally a list of acquisition targets included in the pipeline product company list.
– Develop business strategies by understanding the trends shaping and driving the global ATTR therapeutics market.
– Drive revenues by understanding the key trends, innovative products and technologies, market segments, and companies likely to impact the global ATTR therapeutics market in future.
– Formulate effective sales and marketing strategies by understanding the competitive landscape and by analysing the performance of various competitors.
– Identify emerging players with potentially strong product portfolios and create effective counter-strategies to gain a competitive advantage.
– Track drug sales in the global ATTR therapeutics market from 2019-2029.
– Organize your sales and marketing efforts by identifying the market categories and segments that present maximum opportunities for consolidations, investments and strategic partnerships.
Scope
– Overview of ATTR, including epidemiology, etiology, pathophysiology, symptoms, diagnosis, and treatment guidelines.
– Annualized ATTR therapeutics market revenue, annual cost of therapy and treatment usage pattern data from 2019 and forecast for ten years to 2029.
– Key topics covered include strategic competitor assessment, market characterization, unmet needs, clinical trial mapping and implications for the ATTR therapeutics market.
– Pipeline analysis: comprehensive data split across different phases, emerging novel trends under development, and detailed analysis of late-stage pipeline drugs.
– Analysis of the current and future market competition in the global ATTR market. Insightful review of the key industry drivers, restraints and challenges. Each trend is independently researched to provide qualitative analysis of its implications.
Reasons to Buy
– A number of new therapies of varying mechanisms will enter the ATTR market over the forecast period. What strengths do these drugs bring to the market?
– What are the main unmet needs in this market? Will the drugs under development fulfil the unmet needs of the ATTR market?
– The current late-stage ATTR pipeline consists of a number of novel mechanisms such as the RNA interference therapies, Alnylam’s Onpattro, vutrisiran, and AKCEA’s Tegsedi and AKCEA-TTR-LRx, and Eidos’ TTR stabilizer, AG10. Will the late-stage drugs make a significant impact on the ATTR market?
– Which of these drugs will have the highest peak sales at the highest CAGR, and why?
– The ATTR patient population is currently very small, and the disease is said to hold a falsely perceived rarity. However, the diagnosed patient population is expected to dramatically increase over the forecast period. Why will this occur?
– How will epidemiological changes impact the growth of the future market?
0 notes
Text
Karl Friedrich Schinkel (Prussian, 1781–1841), Christian Peter Wilhelm Beuth (Allegory of Prussia's industrial renewal), The Snowman Raymond Briggs, German Christmas Songs, Zoonotic Virus Linked to Severe Encephalitis in Southern Germany, The Sydney J. Freedberg Lecture on Italian Art: Andrea Mantegna’s Stones, Caves, and Clouds National Gallery of Art, Florence : l'art ancestral de la parfumerie, Oculoleptomeningeal Amyloidosis associated with transthyretin Leu12Pro in an African patient
Karl Friedrich Schinkel (Prussian, 1781–1841), Christian Peter Wilhelm Beuth (Allegory of Prussia's industrial renewal), The Snowman Raymond Briggs, German Christmas Songs, Zoonotic Virus Linked to Severe Encephalitis in Southern Germany, The Sydney J. Freedberg Lecture on Italian Art: Andrea Mantegna’s Stones, Caves, and Clouds National Gallery of Art, Florence : l'art ancestral de la parfumerie, Oculoleptomeningeal Amyloidosis associated with transthyretin Leu12Pro in an African patient
https://blog.naver.com/artnouveau19/221819224285
Karl Friedrich Schinkel (Prussian, 1781–1841), Christian Peter Wilhelm Beuth (Allegory of Prussia's industrial renewal)
https://en.wikipedia.org/wiki/Karl_Friedrich_Schinkel#/media/File:Karl_Friedrich_Schinkel_Allegorie_auf_Beuth,_den_Pegasus_reitend,_1837.tif
Karl Friedrich Schinkel (13 March 1781 – 9 October 1841) was a Prussian architect, city planner, and painter who also designed furniture and stage sets. Schinkel was one of the most prominent architects of Germany and designed both neoclassical and neogothic buildings.[1] His most famous buildings are found in and around Berlin.
https://en.wikipedia.org/wiki/Karl_Friedrich_Schinkel
The Snowman is an animated television film based on Raymond Briggs' 1978 picture book The Snowman. It was directed by Dianne Jackson for the British public service Channel 4. It was first shown on 26 December 1982, and was an immediate success. It was nominated for the Academy Award for Best Animated Short Film and won a BAFTA TV Award.
The story is told through pictures, action and music, scored by Howard Blake, and is wordless, with the exception of the central song "Walking in the Air". The orchestral score was performed in the film by the Sinfonia of London and the song was performed by Peter Auty, a St Paul's Cathedral choirboy.[1]
The special ranks #71 on the 100 Greatest British Television Programmes, a list drawn up by the British Film Institute in 2000, based on a vote by industry professionals.[2] It was voted #4 in UKTV Gold's Greatest TV Christmas Moments. It came third in Channel 4's poll of 100 Greatest Christmas Moments in 2004. Its broadcast, usually on Christmas Eve, has become an annual festive event.
https://en.wikipedia.org/wiki/The_Snowman
The Snowman (1982) HD
https://youtu.be/5A3THighARU
German Christmas Songs
https://youtu.be/zE-xk_pUK54
JAMA
@JAMA_current
·
Feb 19
A recent study suggested that Borna disease virus 1 (BoDV-1), carried by wild shrews in Germany, Austria, Switzerland, and Liechtenstein, could be a more common cause of severe or fatal encephalitis among people in endemic areas than previously realized
https://twitter.com/JAMA_current/status/1229963817599324161
Global Health
February 18, 2020
Zoonotic Virus Linked to Severe Encephalitis in Southern Germany
Bridget M. Kuehn, MSJ
JAMA. 2020;323(7):599. doi:10.1001/jama.2020.0832
A recent study suggested that Borna disease virus 1 (BoDV-1), which is carried by wild shrews in Germany, Austria, Switzerland, and Liechtenstein, could be a more common cause of severe or fatal encephalitis among people in endemic areas than previously realized.
https://jamanetwork.com/journals/jama/fullarticle/2761100?guestAccessKey=accdc527-1b98-4b47-b5bc-cbc82345eea3&utm_source=twitter&utm_medium=social_jama&utm_term=3140055014&utm_campaign=article_alert&linkId=82591036
The Sydney J. Freedberg Lecture on Italian Art: Andrea Mantegna’s Stones, Caves, and Clouds
National Gallery of Art | Audio
https://podcasts.apple.com/kr/podcast/national-gallery-of-art-audio/id262840395?i=1000462428377
Gabriele Finaldi, director, National Gallery, London In his lecture, presented on December 8, 2019, Gabriele Finaldi of the National Gallery, London, discusses Mantegna's particular universe as constructed in stone: carved, cut, polished, and sometimes invented. In his compelling imaginarium, the ancient world is a severe construct of marble, alabaster, and porphyry. He juxtaposes sculpted stone with flesh, creating potent dualities of ancient and modern, eternal and transient, dead and alive. In the skies of his paintings, clouds take on mysterious forms, sometimes rocklike, that want to insinuate themselves into his narratives. This lecture explores how the realms of nature, art, and antiquity are fused into the unique vision of Mantegna's Renaissance world.
Karl Friedrich Schinkel (Prussian, 1781–1841), Christian Peter Wilhelm Beuth (Allegory of Prussia's industrial renewal) and Florence : l'art ancestral de la parfumerie
https://blog.naver.com/artnouveau19/221815875809
Florence : l'art ancestral de la parfumerie
https://www.francetvinfo.fr/culture/patrimoine/histoire/florence-l-art-ancestral-de-la-parfumerie_3831393.html#xtor=CS2-765-[twitter]-
J Neurol. 2015; 262(1): 228–234.
Published online 2014 Dec 9. doi: 10.1007/s00415-014-7594-2
PMCID: PMC4289971
PMID: 25488473
Oculoleptomeningeal Amyloidosis associated with transthyretin Leu12Pro in an African patient
P. McColgan,
S. Viegas, S. Gandhi, K. Bull, R. Tudor, F. Sheikh, J. Pinney, M. Fontana, D. Rowczenio, J. D. Gillmore, J. A. Gilbertson, C. J. Whelan, S. Shah, Z. Jaunmuktane, J. L. Holton, J. M. Schott, D. J. Werring, P. N. Hawkins, and M. M. Reilly
Abstract
Oculoleptomeningeal amyloidosis is a rare manifestation of hereditary transthyretin (TTR) amyloidosis. Here, we present the first case of leptomeningeal amyloidosis associated with the TTR variant Leu12Pro mutation in an African patient. A 43-year-old right-handed Nigerian man was referred to our centre with rapidly progressive neurological decline. He presented initially with weight loss, confusion, fatigue, and urinary and erectile dysfunction. He then suffered recurrent episodes of slurred speech with right-sided weakness. He went on to develop hearing difficulties and painless paraesthesia. Neurological examination revealed horizontal gaze-evoked nystagmus, brisk jaw jerk, increased tone, brisk reflexes throughout and bilateral heel-shin ataxia. Magnetic resonance imaging showed extensive leptomeningeal enhancement. Cerebrospinal fluid analysis showed a raised protein of 6.4 g/dl. Nerve conduction studies showed an axonal neuropathy. Echocardiography was characteristic of cardiac amyloid. TTR gene sequencing showed that he was heterozygous for the leucine 12 proline mutation. Meningeal and brain biopsy confirmed widespread amyloid angiopathy. TTR amyloidosis is a rare cause of leptomeningeal enhancement, but should be considered if there is evidence of peripheral or autonomic neuropathy with cardiac or ocular involvement. The relationship between different TTR mutations and clinical phenotype, disease course, and response to treatment remains unclear.
Keywords: Genetics, Amyloid, Transthyretin, Leu12Pro
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4289971/
0 notes
Text
Transthyretin Amyloidosis Treatment Market Growing At High Cagr By 2025 According To New Research Report
Transthyretin amyloidosis is a rare progressive fatal disease that destroys the nerve cells controlling various bodily functions. The symptoms of the disease are similar to those of many other diseases. Moreover, patients suffering with the condition die ten years after the onset of symptoms. Therefore, timely symptom identification, diagnosis, and treatment is important.
View Report: https://www.transparencymarketresearch.com/transthyretin-amyloidosis-treatment-market.html
Transthyretin amyloidosis is caused due to the misfolding of the transthyretin (TTR) protein, which is a secondary transporter of thyroxine and retinol-binding protein. The misfolding causes protein depositions known as amyloids to form within tissues and organs. There are three types of transthyretin amyloidosis. In familial amyloid polyneuropathy (TTR-FAP) amyloids lead to damage of the nerves that control the senses, movement, and involuntary bodily functions. The second type is transthyretin amyloidosis cardiomyopathy (TTR-CM) that leads to cardiomyopathy. It causes damage to the heart’s muscles, inhibiting the ability to pump blood throughout the body. The third type is senile systemic amyloidosis (SSA), which is more prevalent in men than women.
According to the Amyloidosis Foundation, there are roughly 126 different genetic variations in ATTR, with up to 53 types of genetic variations in non-transthyretin hereditary amyloidosis diseases. According to reports published on transthyretin amyloidosis, it has been estimated that nearly 10,000, or 1.1 per 100,000 individuals in the world are living with TTR-FAP. The age group of patients suffering from the disorder is between 30 and 40 years of age. It has also been observed that TTR-CM tends to affect older males aged 65 years and above. Familial amyloid polyneuropathy (TTR-FAP) leads to 100 different types of mutations in the transthyretin gene, which leads to protein misfolding. There is only a 50% chance of transferring the mutation to the next generation from an affected parent. On the other hand, the mutation that leads to familial amyloid cardiomyopathy is generally found in individuals of African origin. Amyloidosis related to age primarily affects Caucasian men who are aged 65 years and above.
Request a Brochure of Transthyretin Amyloidosis Treatment Market Report @ https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=60495
The diagnosis of transthyretin amyloidosis is carried out through tissue biopsy, genetic testing, and imaging studies of the heart. Currently, no efficient drugs for transthyretin amyloidosis are available in the market. In order to efficiently diagnose transthyretin amyloidosis, a complete study of the family should be carried out through neurological assessment tests, which include small-fiber assessment, electrocardiogram, and laboratory tests.
The global transthyretin amyloidosis treatment market is likely to be driven by an increase in the prevalence of disease, rise in the population with African origin, increase in awareness, improvement in diagnostic procedures, improving health care services, rapid economic growth in developing countries, and rise in research and development activity. On the other hand, major restraining factors for the transthyretin amyloidosis treatment market are less awareness in developing economies, miss-diagnosis of the disease, high cost associated with disease diagnosis and treatment, limited clinical trials, and lack of efficient medication.
The global transthyretin amyloidosis treatment market is segmented into treatment type, end user, and region. The global treatment type segment of transthyretin amyloidosis treatment market is divided into surgical procedure, and medication. The end user segment in divided into hospitals, specialized clinics, academic & research institutes.
In terms of region, the global transthyretin amyloidosis treatment market can be segmented into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. North America leads the transthyretin amyloidosis treatment market due of an increase in the African American population, rise in patient pool, increase in the number of diagnosis procedures, presence of leading manufacturers, and rapid drug development. The market in North America is followed by the market in Europe and Asia Pacific. According to the Amyloidosis Foundation, in the U.S., nearly 4,500 new cases of transthyretin amyloidosis are diagnosed every year. Asia Pacific is anticipated to provide lucrative opportunities to the transthyretin amyloidosis treatment market during the forecast period. Increase in demand for supportive care therapeutics because of an increase in health care expenditure in the region, rise in medical tourism, expansion in the pharmaceutical industry, and high rate of acceptance of new products are expected to drive the global transthyretin amyloidosis treatment market.
Major players operating in the global transthyretin amyloidosis market include Merck & Co., Pfizer Inc., AstraZeneca plc. , Prothena Corporation plc. , Ionis Pharmaceuticals, Inc., Bellus Health Inc., Alnylam Pharmaceuticals, Inc., and other prominent players.
0 notes