#defibrillator devices and equipment market trends
Text
Electrophysiology Market: Transforming Cardiac Care with Advanced Diagnostics
The Electrophysiology market is witnessing significant growth as cardiovascular diseases (CVDs) continue to be a leading cause of mortality worldwide. With advancements in diagnostic technology, electrophysiology is revolutionizing cardiac care by enabling precise diagnosis and treatment of heart rhythm disorders. This article explores the key market trends, segmentation, growth drivers, and leading companies shaping the electrophysiology industry.
Market Overview
According to SkyQuest’s Electrophysiology Market report, the market is currently valued at USD 8.20 billion in 2023, with a projected CAGR of 13%. The increasing prevalence of heart diseases, growing adoption of minimally invasive procedures, and advancements in electrophysiology devices are propelling the market forward.
Request Your Free Sample: - https://www.skyquestt.com/sample-request/electrophysiology-market
Market Segmentation
By Product Type:
Electrophysiology Ablation Catheters: Key devices for treating arrhythmias through minimally invasive procedures.
Electrophysiology Diagnostic Catheters: Widely used in diagnosing electrical activity in the heart.
Electrophysiology Lab Devices: Includes mapping systems and recording devices for advanced diagnostics.
Pacemakers and Defibrillators: Crucial for regulating heart rhythms in patients with severe arrhythmias.
Others: Includes specialized tools and equipment for electrophysiological procedures.
By Indication:
Atrial Fibrillation: One of the most common cardiac arrhythmias, where electrophysiology plays a significant role in treatment.
Atrioventricular Nodal Reentrant Tachycardia (AVNRT): A fast heart rhythm disorder often treated with ablation.
Wolff-Parkinson-White Syndrome (WPW): A rare condition where electrophysiology diagnostics are essential for management.
Other Arrhythmias: Includes ventricular tachycardia and flutter, both addressed through electrophysiology treatments.
By End-User:
Hospitals: Major centers for electrophysiology procedures and treatments.
Ambulatory Surgical Centers: Increasingly adopting electrophysiology for outpatient treatments.
Cardiac Centers: Specialized in diagnosing and treating heart rhythm disorders.
Others: Includes research institutions and academic centers focused on cardiac care.
Key Growth Drivers
Rising Incidence of Cardiovascular Diseases: The global rise in heart diseases is fueling demand for advanced electrophysiology diagnostics and treatments.
Technological Advancements: Innovations in catheter ablation, 3D mapping systems, and minimally invasive procedures are enhancing the market's growth potential.
Growing Preference for Minimally Invasive Surgeries: Electrophysiology procedures are less invasive, leading to faster recovery times, which is driving their adoption.
Increased Healthcare Spending: Governments and healthcare providers are investing heavily in cardiac care, boosting the demand for electrophysiology solutions.
Read More at: - https://www.skyquestt.com/report/electrophysiology-market
Leading Companies in the Market
SkyQuest’s report highlights key players dominating the Electrophysiology Market, including:
Johnson & Johnson
Abbott Laboratories
Medtronic PLC
Boston Scientific Corporation
Siemens Healthineers AG
MicroPort Scientific Corporation
Biotronik SE & Co. KG
GE Healthcare
Koninklijke Philips N.V.
Biosense Webster, Inc.
Take Action Now: Secure Your Report Today - https://www.skyquestt.com/buy-now/electrophysiology-market
Challenges and Opportunities
High costs associated with electrophysiology procedures and devices pose a challenge, especially in developing regions. However, the ongoing research and development efforts to create cost-effective and advanced devices offer vast opportunities for market growth. The increasing availability of mobile healthcare services and remote diagnostics also opens new avenues for expansion.
Future Outlook
The Electrophysiology Market is poised for continued growth, driven by technological advancements and the increasing burden of cardiovascular diseases globally. Companies that focus on developing innovative and cost-effective solutions are well-positioned to capitalize on the growing demand for electrophysiology procedures.
As the need for effective cardiac care intensifies, the electrophysiology market is at the forefront of diagnostic and treatment innovations. Healthcare providers and decision-makers must stay updated with the latest trends and technologies to ensure optimal patient outcomes. For a detailed analysis and strategic insights, consult SkyQuest's comprehensive Electrophysiology Market report.
0 notes
Text
The Emergency Medical Services Market is projected to grow from USD 22755 million in 2024 to an estimated USD 36542.63 million by 2032, with a compound annual growth rate (CAGR) of 6.1%from 2024 to 2032.The Emergency Medical Services (EMS) market has become an essential component of healthcare systems worldwide, playing a pivotal role in providing rapid medical care to those in critical situations. This market encompasses a wide range of services, including ambulance transportation, emergency room care, pre-hospital care, and medical emergency response teams. With the rising incidence of accidents, cardiovascular diseases, and other medical emergencies, the EMS market has experienced significant growth. This article delves into the key factors driving the EMS market, current trends, challenges, and future prospects.
Browse the full report at https://www.credenceresearch.com/report/emergency-medical-services-market
Market Overview
The EMS market is a rapidly expanding sector, fueled by increasing urbanization, rising incidences of chronic diseases, and a growing aging population. EMS provides life-saving care during emergencies, ranging from trauma incidents and cardiac arrests to strokes and severe allergic reactions. The market includes ambulance services, air medical services, emergency medical dispatchers, and specialized equipment such as defibrillators, stretchers, and life-support devices.
The market is divided into several segments based on service type (ground, air, and water ambulances), application (trauma, cardiac care, respiratory care, etc.), and end-user (hospitals, clinics, home care settings, etc.). North America leads the market due to its advanced healthcare infrastructure, followed by Europe and Asia-Pacific, which are experiencing rapid market growth driven by increasing healthcare expenditures and government initiatives.
Key Market Drivers
1. Rising Incidence of Medical Emergencies: The growing number of road accidents, natural disasters, and medical emergencies, such as heart attacks and strokes, is a major driver of the EMS market. According to the World Health Organization (WHO), road traffic accidents alone cause over 1.3 million deaths annually, highlighting the need for efficient EMS services.
2. Technological Advancements: Innovations such as GPS-enabled ambulances, telemedicine integration, and mobile healthcare apps have enhanced the efficiency of EMS. The advent of advanced life-support systems, automated external defibrillators (AEDs), and portable ventilators has significantly improved patient outcomes during emergency care.
3. Government Initiatives and Funding: Governments worldwide are investing heavily in improving EMS infrastructure. For instance, in the United States, the Emergency Medical Services for Children (EMSC) program funds initiatives aimed at enhancing pediatric emergency care. Similarly, emerging economies like India and China are upgrading their EMS systems to meet the growing demand for emergency medical care.
4. Growing Geriatric Population: The aging global population is contributing to the increased demand for EMS services. Older adults are more susceptible to emergencies like falls, cardiac issues, and respiratory complications, necessitating prompt medical intervention.
Market Challenges
Despite the growing demand, the EMS market faces several challenges. One of the main obstacles is the shortage of trained EMS professionals. Many regions, especially in developing countries, lack adequately trained paramedics and emergency medical technicians (EMTs), which can compromise the quality of care provided. Additionally, high operational costs, including ambulance maintenance, fuel, and medical equipment, pose financial challenges to service providers.
Regulatory compliance is another critical challenge. EMS providers must adhere to strict guidelines and regulations that vary by region, often complicating operations. Inconsistent response times and inadequate coverage in rural or underserved areas further hinder the market's growth.
Future Outlook
The future of the EMS market looks promising, with advancements in artificial intelligence, data analytics, and remote monitoring technologies poised to revolutionize emergency care. AI-powered predictive analytics can help EMS providers optimize response times by identifying high-risk patients and allocating resources more efficiently. The integration of wearable devices that monitor vital signs in real time could enable faster diagnosis and treatment during emergencies.
Moreover, the expansion of air ambulance services is expected to drive market growth, especially in remote or inaccessible regions. The increasing use of drones for delivering medical supplies, such as blood or medications, during emergencies is another emerging trend that holds significant potential.
Key Player Analysis:
Asahi Kasei Corporation
B. Braun Melsungen AG (Germany)
Baxter
BD (U.S.)
Boston Scientific Corporation
Cardinal Health (U.S.)
ConvaTec Inc
Danaher
Diagmed Healthcare
GENERAL ELECTRIC COMPANY (U.S.)
Johnson & Johnson Services Inc.
Koninklijke Philips N.V
Medtronic (Ireland)
Merit Medical System
Smith & Nephew (U.S.)
Stryker (U.S.)
Terumo Corporation
Segmentation:
By Type:
Ground ambulances
Air ambulances
And marine ambulances.
By Application:
Trauma care
Cardiac care
Respiratory care
Others
By End Use:
Hospitals
Private clinics
Emergency medical service providers.
Based on the Region:
North America
US
Canada
Mexico
Europe
Germany
France
UK
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/emergency-medical-services-market
About Us:
Credence Research is committed to employee well-being and productivity. Following the COVID-19 pandemic, we have implemented a permanent work-from-home policy for all employees.
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes
Text
Refurbished Medical Equipment Market Growth and Size for Strategic Decision-Making
The global refurbished medical equipment market size is expected to reach USD 45.81 Billion in 2032 and register a steady revenue CAGR of 11.8% during the forecast period, according to latest analysis by Emergen Research. Increasing preference for eco-friendly products is a major factor driving market revenue growth of. Benefits of used medical imaging equipment for environment must be emphasized.
The circular economy is supported by refurbishment, a sort of reuse and waste reduction that raises useful lifespan of medical imaging equipment from 5-7 to 10-14 years or even longer. Resources and energy required to produce new equipment are saved by refurbishment. It is critical from an environmental standpoint to maximize service life of imaging scanners due to energy and materials used throughout the production, safety testing, and regulatory compliance processes. For instance, improving Magnetic Resonance Imaging (MRI) scanners and extending their service life maximizes the return on initial investment of energy and materials.
Request a Sample Report with Table of Contents and Figures to click Here: https://www.emergenresearch.com/request-sample/1748
Competitive Terrain:
The section on the competitive landscape offers valuable and actionable insights related to the business sphere of the Refurbished Medical Equipment market, covering extensive profiling of the key market players. The report offers information about market share, product portfolio, pricing analysis, and strategic alliances such as mergers and acquisitions, joint ventures, collaborations, partnerships, product launches and brand promotions, among others. The report also discusses the initiatives taken by the key companies to combat the impact of the COVID-19 pandemic.
The leading market contenders listed in the report are:
GE HealthCare, Koninklijke Philips N.V., Siemens Healthcare GmbH, Block Imaging, Inc., Soma Tech INTL, Avante Health Solutions, Hilditch Group, EverX Pty. Ltd., Integrity Medical Systems, Inc., and Radiology Oncology Systems
Click to access the Report Study, Read key highlights of the Report and Look at Projected Trends: https://www.emergenresearch.com/industry-report/refurbished-medical-equipment-market
Emergen Research has segmented the global Refurbished Medical Equipment market on the basis of type, application, end-use, and region:
Segments Covered in this report are:
Product Outlook (Revenue, USD Billion; 2019–2032)
Medical Imaging Equipment
Computerized Tomography (CT) Scanners
Magnetic Resonance Imaging (MRI) Machines
X-Ray Machines
Nuclear Imaging Systems
Ultrasound Systems
Other Medical Imaging Equipment
Operating Room & Surgical Equipment
Anesthesia Machines
Electrosurgical Units
Operating Room Tables & Lights
Surgical Displays
Other Operating Room & Surgical Equipment
Patient Monitors
Multi-Parameter Monitors
Fetal Monitors
Electrocardiography Devices
Pulse Oximeters
Mobile Cardiac Telemetry Devices
Non-Invasive Blood Pressure Monitors
Other Patient Monitors
Cardiology Equipment
Defibrillators
Heart-Lung Machines
Other Cardiology Equipment
Urology Equipment
Dialysis Machines
Lithotripsy Devices
Neurology Equipment
Electromyograph Machines (EMG) Machines
Electroencephalography (EEG) Machines
Intensive Care Equipment
Ventilators
Infant Incubators & Warmers
Intravenous Therapy Systems
Endoscopy Equipment
Other Medical Equipment
Application Outlook (Revenue, USD Billion; 2019–2032)
Diagnostic Applications
Cardiology
Urology
Neurology
Orthopedics
Physiology
Emergency Medicine
Oncology
Obstetrics/Gynecology
Other Diagnostic Applications
Therapeutic Applications
Cardiology
Urology
Neurology
Orthopedics
Physiology
Emergency Medicine
Oncology
Obstetrics/Gynecology
Other Therapeutic Applications
End-Use Outlook (Revenue, USD Billion; 2019–2032)
Hospitals
Diagnostic Imaging Centers
Ambulatory Care Centers
Other End-Use
The various regions analyzed in the report include:
North America (U.S., Canada)
Europe (U.K., Italy, Germany, France, Rest of EU)
Asia Pacific (India, Japan, China, South Korea, Australia, Rest of APAC)
Latin America (Chile, Brazil, Argentina, Rest of Latin America)
Middle East & Africa (Saudi Arabia, U.A.E., South Africa, Rest of MEA)
Key Objectives of the Report:
Analysis and estimation of the Refurbished Medical Equipment Market size and share for the projected period of 2022-2030
Extensive analysis of the key players of the market by SWOT analysis and Porter’s Five Forces analysis to impart a clear understanding of the competitive landscape
Study of current and emerging trends, restraints, drivers, opportunities, challenges, growth prospects, and risks of the global Refurbished Medical Equipment Market
Analysis of the growth prospects for the stakeholders and investors through the study of the promising segments
Strategic recommendations to the established players and new entrants to capitalize on the emerging growth opportunities
Request Customization as per your specific requirement@ https://www.emergenresearch.com/request-for-customization/1748
0 notes
Text
Laminate Wood Flooring Market Size, Share, Growth, Trends [2032]
Laminate Wood Flooring Market provides in-depth analysis of the market state of Laminate Wood Flooring manufacturers, including best facts and figures, overview, definition, SWOT analysis, expert opinions, and the most current global developments. The research also calculates market size, price, revenue, cost structure, gross margin, sales, and market share, as well as forecasts and growth rates. The report assists in determining the revenue earned by the selling of this report and technology across different application areas.
Geographically, this report is segmented into several key regions, with sales, revenue, market share and growth Rate of Laminate Wood Flooring in these regions till the forecast period
North America
Middle East and Africa
Asia-Pacific
South America
Europe
Key Attentions of Laminate Wood Flooring Market Report:
The report offers a comprehensive and broad perspective on the global Laminate Wood Flooring Market.
The market statistics represented in different Laminate Wood Flooring segments offers complete industry picture.
Market growth drivers, challenges affecting the development of Laminate Wood Flooring are analyzed in detail.
The report will help in the analysis of major competitive market scenario, market dynamics of Laminate Wood Flooring.
Major stakeholders, key companies Laminate Wood Flooring, investment feasibility and new market entrants study is offered.
Development scope of Laminate Wood Flooring in each market segment is covered in this report. The macro and micro-economic factors affecting the Laminate Wood Flooring Market
Advancement is elaborated in this report. The upstream and downstream components of Laminate Wood Flooring and a comprehensive value chain are explained.
Browse More Details On This Report at @https://www.globalgrowthinsights.com/market-reports/laminate-wood-flooring-market-100005
Global Growth Insights
Web: https://www.globalgrowthinsights.com
Our Other Reports:
Global Hydrogenated Bisphenol A MarketMarket Size
Global Blood Clot Retrieval Devices MarketMarket Growth
Global Swimming Pool Treatment Chemicals MarketMarket Size
Global Membrane Pressure Vessel MarketMarket Growth
Global Defibrillator MarketMarket Size
Global Semiconductor Production Equipment MarketMarket Growth
Global Shale Gas Processing Equipment MarketMarket Size
Global Digital Experience Platform MarketMarket Growth
Global RFID Smart Cabinets MarketMarket Size
Global Desktop CNC Machines MarketMarket Growth
Global CMP Pad Regulator MarketMarket Size
Global Unmanned Surface Vessels (Usv) MarketMarket Growth
Global Fresh Avocado MarketMarket Size
Global Heat Pump Water Heater MarketMarket Growth
Global Motor For Robots MarketMarket Size
Global Personalized Hydration Solutions MarketMarket Growth
Global Silver Powders And Flakes MarketMarket Size
Global Depaneling Machine MarketMarket Growth
Global Pv Solar Energy Charge Controller MarketMarket Size
Global Fleece Knitting Yarn MarketMarket Growth
Global Super Capacitor MarketMarket Size
Global Bird Detection System MarketMarket Growth
Global Predictive Genetic Testing and Consumer Genomics MarketMarket Size
Global Recreation Management Software MarketMarket Growth
Global Analog Cheese MarketMarket Size
Global Artificial Intelligence Software MarketMarket Growth
Global EV Charging Cables MarketMarket Size
Global Remote Sensing Services MarketMarket Growth
Global Education Technology (EdTech) MarketMarket Size
Global Transcatheter Heart Valve Replacement and Repair MarketMarket Growth
Global Cervical Total Disc Replacement Device MarketMarket Size
Global Biomass Boiler MarketMarket Growth
Global Door Closers MarketMarket Size
Global Silicone Elastomers For Medical Applications MarketMarket Growth
Global Energy Recovery Ventilation System MarketMarket Size
Pegaspargase Drugs MarketMarket Analysis
0 notes
Text

𝑬𝒙𝒄𝒊𝒕𝒆𝒅 𝒕𝒐 𝒔𝒉𝒂𝒓𝒆 𝒊𝒏𝒔𝒊𝒈𝒉𝒕𝒔 𝒐𝒏 𝒕𝒉𝒆 𝒅𝒚𝒏𝒂𝒎𝒊𝒄 𝒍𝒂𝒏𝒅𝒔𝒄𝒂𝒑𝒆 𝒐𝒇 𝒕𝒉𝒆 𝑫𝒆𝒇𝒊𝒃𝒓𝒊𝒍𝒍𝒂𝒕𝒐𝒓𝒔 𝑴𝒂𝒓𝒌𝒆𝒕!
𝑺𝒆𝒄𝒖𝒓𝒆 𝒂 𝑭𝑹𝑬𝑬 𝑺𝒂𝒎𝒑𝒍𝒆: https://www.nextmsc.com/defibrillators-market/request-sample?utm_source=sanyukta-16-April-24&utm_medium=sanyukta-tumblr&utm_campaign=sanyukta-defibrillators-market
As advancements in healthcare technology continue to surge, the demand for reliable and efficient defibrillators is reaching new heights. From hospitals to public spaces, these life-saving devices play a crucial role in combating cardiac emergencies.
Key trends driving the defibrillator market include:
1️. 𝑻𝒆𝒄𝒉𝒏𝒐𝒍𝒐𝒈𝒊𝒄𝒂𝒍 𝑰𝒏𝒏𝒐𝒗𝒂𝒕𝒊𝒐𝒏𝒔: With the rise of AI and IoT, defibrillator manufacturers are introducing smarter, more intuitive devices that enhance accuracy and response time.
2️. 𝑰𝒏𝒄𝒓𝒆𝒂𝒔𝒆𝒅 𝑨𝒘𝒂𝒓𝒆𝒏𝒆𝒔𝒔: Growing awareness about the importance of early defibrillation in saving lives has spurred adoption across various sectors, including schools, gyms, and workplaces.
3️. 𝑹𝒆𝒈𝒖𝒍𝒂𝒕𝒐𝒓𝒚 𝑺𝒕𝒂𝒏𝒅𝒂𝒓𝒅𝒔: Stricter regulations and guidelines regarding cardiac care equipment are influencing product development and market expansion.
4️. 𝑮𝒍𝒐𝒃𝒂𝒍 𝑹𝒆𝒂𝒄𝒉: The defibrillator market is witnessing significant growth worldwide, fueled by rising healthcare expenditure and initiatives to improve emergency medical services.
5. 𝑲𝒆𝒚 𝑴𝒂𝒓𝒌𝒆𝒕 𝑷𝒍𝒂𝒚𝒆𝒓𝒔: Lucrative growth opportunities make the defibrillators market extremely competitive. Some of the major players in the market are Asahi Kasei Corporation, Stryker Corporation, Biotronic SE and Co. KG, Boston Scientific Corporation, Philips healthcare, LivaNova PLC, Medtronic PLC, Jude Medical Inc., Cardiac Science Corporation, Nihon Konden Corporation.
Exciting opportunities lie ahead for companies involved in the defibrillator sector, from innovators shaping the future of cardiac care to healthcare providers seeking to enhance their emergency response capabilities.
Let's continue to collaborate and innovate to ensure that life-saving technology remains accessible and effective for all!
#defibrillators#health care innovation#cardia ccare#medical technology#emergency response#market research#market trends#business insights
0 notes
Text
10 Essential FAQs About the Medical Devices Market: A Must-Read for Investors and Innovators
Q1. What is the size of the global medical devices market?
Ans. The global medical devices market is estimated to be worth over USD 568 billion in 2023 and is expected to reach USD 772.3 billion by 2028, growing at a CAGR of around 5.2%.
Q2. What are the major product segments in the medical devices market?
Ans. The largest segments include:
Diagnostics: Imaging equipment (MRI, CT scanners, etc.), laboratory instruments, and in vitro diagnostics (IVDs).
Therapeutics: Cardiac devices (pacemakers, defibrillators), orthopedic implants (joints, spinal devices), and surgical instruments.
Other: Dental equipment, ophthalmic devices, rehabilitation equipment, and home healthcare devices.

Q3. Which regions are leading the medical devices market?
Ans. North America: Holds the largest share, driven by advanced healthcare infrastructure and high disposable incomes.
Europe: Strong market with well-established regulatory frameworks and aging population.
Asia Pacific: Fastest-growing region due to population growth, rising healthcare spending, and improving medical facilities.
Q4. What are the driving factors for medical devices market growth?
Ans. Aging population: Increased demand for healthcare services and devices for chronic conditions.
Technological advancements: New generations of devices with improved functionality and minimally invasive procedures.
Rising healthcare spending: Increasing affordability and government initiatives in some regions.
Q5. What are the major medical devices market challenges?
Ans. Regulatory hurdles: Stringent regulatory requirements and lengthy approval processes for new devices.
Cybersecurity threats: Increasing vulnerabilities of connected medical devices to hacking and data breaches.
Cost containment pressures: Healthcare systems striving for cost-efficiency, impacting device manufacturers.
Q6. What are the latest trends in the medical devices market?
Ans. Artificial intelligence (AI): Integrating AI into diagnostics, surgical robots, and personalized medicine.
Telemedicine: Remote monitoring and healthcare delivery through connected devices.
3D printing: Personalization of medical devices and development of complex implants.
Wearable devices: Growing adoption for fitness tracking, chronic disease management, and remote monitoring.
Q7. Who are the major players in the medical devices market?
Ans. Here are some Medical Device Market Players
Johnson & Johnson
Medtronic
Siemens Healthineers
Abbott Laboratories
Stryker
Q8. How is the COVID-19 pandemic impacting the medical devices market?
Ans. Increased demand for ventilators and other critical care devices.
Disruptions in supply chains and manufacturing due to lockdowns and travel restrictions.
Shift towards telemedicine and virtual consultations.
Q9. What are the ethical considerations in the medical devices market?
Ans. Access to devices for patients in developing countries.
Data privacy and security concerns with connected devices.
Ensuring equitable distribution of new technologies and treatments.
Q10. What does the future hold for the medical devices market?
Ans. The market is expected to continue growing with advancements in technology, personalized medicine, and increasing demand in emerging economies. However, navigating regulatory challenges, cost pressures, and ethical considerations will be crucial for sustainable growth.
0 notes
Text
Global Emergency Medical Equipment Market Is Estimated To Witness High Growth Owing To Advancements in Technology

The global Emergency Medical Equipment market is estimated to be valued at US$ 23.82 billion in 2022 and is expected to exhibit a CAGR of 6.3% over the forecast period 2022-2030, as highlighted in a new report published by Coherent Market Insights.
Market Overview:
Emergency medical equipment includes devices and tools used by healthcare professionals to provide immediate medical assistance during emergencies. These equipment are crucial in saving lives and treating patients in critical conditions. The market for emergency medical equipment is driven by factors such as the increasing incidence of accidents and injuries, coupled with the need for immediate medical care. Moreover, the advancements in technology have led to the development of innovative and efficient equipment that are improving patient outcomes.
Market Key Trends:
One key trend in the global Emergency Medical Equipment market is the adoption of advanced technologies in medical devices. Healthcare providers are increasingly investing in innovative equipment that can provide accurate and timely diagnosis and treatment. For instance, the use of portable ultrasound devices has become common in emergency situations as they enable quick and accurate assessment of internal injuries. Similarly, the integration of artificial intelligence (AI) algorithms in emergency equipment is improving decision-making and enhancing patient care.
PEST Analysis:
- Political: The government plays a crucial role in regulating the production, distribution, and usage of emergency medical equipment. It sets standards and guidelines to ensure the safety and efficacy of these devices.
- Economic: The emergency medical equipment market is influenced by economic factors such as healthcare expenditure, insurance coverage, and reimbursement policies. The affordability and accessibility of these equipment are important considerations for market growth.
- Social: The increasing awareness about the importance of emergency medical care and the rising demand for prompt medical assistance are major social factors driving market growth.
- Technological: Advances in technology have revolutionized emergency medical equipment. Devices such as automated defibrillators, portable ultrasound, and telemedicine solutions are improving the delivery of emergency medical care.
Key Takeaways:
1: The Global Emergency Medical Equipment Market Size is expected to witness high growth, exhibiting a CAGR of 6.3% over the forecast period. This growth can be attributed to increasing accidents and injuries, which highlight the need for immediate medical care. The advancements in technology have led to the development of efficient and innovative equipment that are enhancing patient outcomes.
2: The Asia Pacific region is anticipated to be the fastest-growing and dominating region in the global Emergency Medical Equipment market. The growing population, increasing healthcare expenditure, and rising awareness about the importance of emergency medical care are driving market growth in this region.
3: Key players operating in the global Emergency Medical Equipment market include 3M, Abbott, Asahi Kasei Corporation, B. Braun Medical, BD, Cardinal Health, GE Healthcare, Henry Schein, Johnson & Johnson, Philips Healthcare, Smith & Nephew, and Stryker Corporation.
In conclusion, the global Emergency Medical Equipment market is on a growth trajectory fueled by advancements in technology and the increasing demand for immediate medical care. The adoption of advanced technologies and the integration of AI algorithms in emergency equipment are key trends shaping the market. However, government regulations and economic factors play a crucial role in shaping the market landscape. Market players should focus on innovation and ensuring affordability and accessibility of emergency medical equipment to capitalize on the growing market opportunities.
#Emergency Medical Equipment Market#Emergency Medical Equipment Market Growth#Emergency Medical Equipment Market forecast#Emergency Medical Equipment Market analysis#Emergency Medical Equipment Market values#Emergency Medical Equipment#Medical Equipment#Coherent Market Insights
0 notes
Text
Medical Device Software Development: From Software Design to Launch
Picture this: the global population is aging, quicker than you can say ‘baby boomers’, and their demand for medical products is shooting up like fireworks on New Year’s Eve.
According to the United Nations, by the time 2050 rolls around, we’ll have 1.5 billion people aged 65 and older, a giant leap from 793 million in 2019. Now, translate that into potential users, potential customers, who will be clamoring for the latest in medical instrument technology. We’re talking about a market on the cusp of exploding, like a star going supernova!
You want figures? We’ve got them. Statista foresees that the medical device software market will surge from its current worth of $570 billion to a whopping $719 billion by 2028. It’s like witnessing a small town growing into a megacity overnight.
From diagnostic tools to therapeutic devices, the future of healthtech is practically being written in code.
We’re helped to build and test software for dozens of digital health projects. Now, we offer you to delve into the intricacies of medtech software development, navigating the maze of regulatory landscape and many other challenges that developers face in this brave new world.
So, ready to decode the future of safe and effective medical devices? Let’s dive in!
Custom Medical Device Software: Types and Application
What’s the secret ingredient that brings a medical gadget to life? It’s the medical device software (MDS) – an essential component that orchestrates the functionality of these gadgets whether they’re seamlessly integrated or operating independently.
Take, for instance, a wearable fitness tracker on your wrist, quietly monitoring your health metrics (like heart rate) and counting steps as you go about your day. This everyday device is empowered by complex software designed to deliver critical data right at your fingertips.
On a larger scale, there’s radiology imaging software, enhancing X-Ray machines’ ability to produce detailed images, or ventilator software, adjusting the air flow delivered to patient rooms in hospitals. The breadth and depth of applications are truly extraordinary.
Let’s turn our attention to exploring the diverse types of clinical device software.
Key MDS Types In the Current Market
In a nutshell, there are two primary categories:
1. Embedded Medical Systems and Software
This software is integrated directly into the med. device and controls its core functionalities. Examples of devices that use embedded software include pulse oximeters, electronic defibrillators, and various types of medical imaging equipment.
2. Software as a Medical Device (SaMD)
Unlike embedded software, SaMD operates independently of any physical device. It is used for medical data visualization, processing, management, and specific technical diagnostics. One advantage of SaMD is that it can be more easily updated compared to traditional medical devices.
Grown Factors Every Medical Device Software Development Company Should Consider
The expansion in this sector hinges primarily on two driving forces: our escalating reliance on software within healthcare devices and the burgeoning rise of telemedicine and remote patient monitoring. These dynamics pave the way for a broad spectrum of stakeholders, ranging from manufacturers of medical tools to hospitals, NGOs, and healthtech companies, enabling them to enhance care delivery and pioneer innovative solutions.
On top of that, new technologies like AI, ML, and IoT are creating fresh opportunities. AI and ML are paving the way for smarter patient monitoring, while IoT is connecting medical instruments like never before. To stay ahead, companies need to keep up with trends, regulatory changes, and tech advances.
Healthtech device software is changing the game for doctors and patients alike. While the growing market value and potential revenue are a big draw, they’re not the only reasons companies are investing in custom medical instrument software development.
Top-5 Reasons Why Companies Need Software for Medical Devices
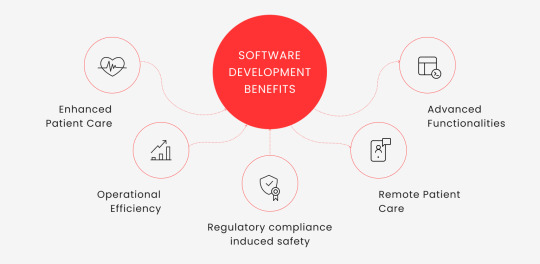
Putting it simply, medtech software development is vital for healthcare organizations, offering numerous benefits:
Enhanced Patient Care. Custom healthtech device software enables real-time patient monitoring, accurate diagnosis, and effective treatment plans, improving patient outcomes.
Operational Efficiency. Medical soft automates administrative tasks, reduces paperwork, and streamlines workflows, freeing up staff time for patient care.
Regulatory compliance induced safety. Software for a medical product must comply with stringent regulations. Failing to do so comes with massive fines. Custom software can be designed to meet these specific requirements, ensuring devices are safe for patient use and don’t cause financial trouble.
Remote Patient Care. Medical soft facilitates remote patient care, enabling data-driven decision-making and providing a platform for telemedicine services.
Advanced Functionalities. With technologies like AI and ML, healthtech software can support functionalities like predictive analysis and personalized patient care. This is particularly important considering the existing drag of the legacy business.
Medical app and desktop software development is a necessity for healthcare organizations, not a luxury. It significantly enhances patient care and operational efficiency, making it a worthwhile investment.
However, developing medtech software requires specialized skills and knowledge of healthcare regulations. Many companies partner with experienced software development services to leverage their expertise and ensure project success. However, before going into detail about compliance, let’s look at the type of medical gadgets one can upgrade with medical gadget software development services.
How Software Solutions Make Medical Devices Better
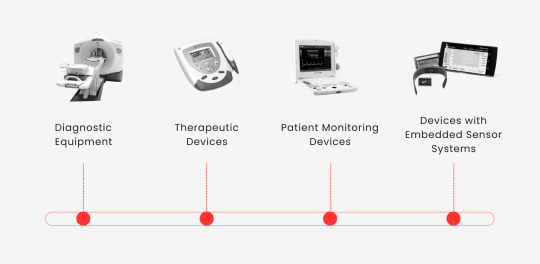
In the rapidly evolving healthcare landscape, software solutions play a crucial role in augmenting the capabilities of medical devices. This might be as simple as improving the readability of data from a heart monitor or as complex as enabling remote diagnostics. Here’s how these advancements contribute to enhancing medical devices:
Diagnostic Equipment. MRI, CT scanners, and X-ray machines rely on software for data processing, image creation, abnormality identification, and diagnostic reporting.
Therapeutic Devices. Software in devices like insulin pumps and pacemakers ensures accurate medication or electrical stimulation delivery.
Patient Monitoring Devices. Heart rate, blood pressure, and glucose monitor software collects and analyzes patient data, providing real-time feedback and alerting healthcare providers to significant changes.
Devices with Embedded Sensor Systems. Wearable fitness trackers and smartwatches use software to analyze real-time health data, provide insights, and offer personalized health and fitness recommendations.
Hospitals and clinics use these medical tools for diagnosis, treatment, patient monitoring, baby care devices management and personal health tracking. In such a context, developing software for these devices is a chance to kill two birds with one store — make healthcare services more effective, and build new revenue streams. Nevertheless, only a few companies dare to enter the realm of healthcare software development in general. The reason for that is two-fold—compliance and regulation.
Regulatory Requirements for SAMD Development and Beyond
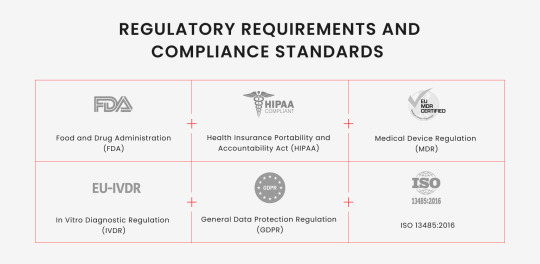
Navigating the regulatory landscape is a critical aspect of healthtech device software development. With various international and regional standards in place, understanding these regulations is essential to ensure medical tools’’ safety, effectiveness, and quality. The regulatory environment for the phenomenon is intricate and governed by various international and regional standards.
In the United States, the Food and Drug Administration (FDA) oversees the development and distribution of medical instruments, including software. The FDA’s regulations aim to ensure the safety and effectiveness of these devices, focusing on risk management and quality assurance. In addition, there is the Health Insurance Portability and Accountability Act (HIPAA). HIPAA is a crucial aspect of medtech software development in the United States, as it sets the standard for protecting sensitive patient data. Any healthcare-related software must ensure that all necessary physical, network, and process security measures are followed.
The Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR) regulate medical gadget software in the European Union. These regulations, effective in May 2021, emphasize clinical evaluation and post-market surveillance, requiring manufacturers to monitor and report their devices’ performance continuously. Regarding data protection, adhering to General Data Protection Regulation (GDPR) is vital.
In the global landscape, ISO 13485:2016 is a globally recognized standard for quality management systems in the medical instrument industry. It supports healthtech device software creators in designing quality management systems that establish and maintain their processes’ effectiveness. This standard ensures the consistent design, development, production, installation, and delivery of healthtech devices that are safe for their intended purpose.
Regulations and standards environment for new software in the healthcare industry is complex but necessary to ensure patient safety and device effectiveness. Adherence to these regulations, from the FDA guidelines to the ISO standards, is a legal obligation and a commitment to quality and safety in healthcare. As we move forward to the dev process, keeping compliance in mind is a must.
Understanding the Impact of IEC 62304 on Healthtech Software Development
In the realm of digital health software development, one international standard has taken precedence as a guiding force: the International Electrotechnical Commission’s IEC 62304. This standard lays out the lifecycle requirements for medtech device software, providing a comprehensive framework that ensures safety and performance.
Key facets of IEC 62304 include:
Risk Management. The standard insists on the adoption of risk management process compliant with ISO 14971, which ensures all possible risks are identified, evaluated, and mitigated effectively.
Software Development Lifecycle (SDLC). IEC 62304 mandates a well-structured SDLC, which includes planning, development, testing, maintenance, and eventual retirement of the software.
Software Classification. It introduces a classification scheme (Classes A, B, C) based on the potential risk to patients — the higher the risk, the stricter the development requirements.
Configuration Management. A robust configuration management system is necessary, allowing for the traceability of all software versions and modifications.
Problem Resolution. The standard requires a systematic process for identifying, documenting, and resolving all software-related problems.
Software Maintenance: It prescribes the need for ongoing maintenance to address software issues, continuously improve functionality, and manage updates.
IEC 62304, by covering every aspect of medtech software development, from inception to sunset, offers a clear and solid foundation for developers. It ensures patient safety and product efficacy while providing a roadmap for consistent, high-quality software development.
In conclusion, compliance with IEC 62304 is not just a regulatory requirement, but a blueprint for success. It streamlines processes, mitigates risks, and instills confidence among users, ultimately elevating the software reliability and credibility in a dynamic and demanding healthcare landscape.
The Ins and Out of the Medical Device Software Development Process
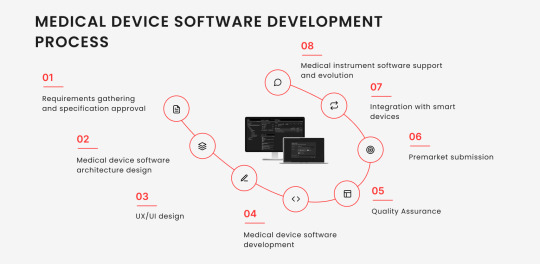
Developing software for medical instruments is a meticulous process involving several critical steps. From identifying a market need to ensuring the software’s safety and effectiveness, each stage of the software development life cycle (SDLC) plays a crucial role in successfully deploying healthtech device software. At this point, let’s take a closer look at these steps.
Step #1. Requirements gathering and specification approval
The initial phase of the process involves:
Identifying the software concept.
Prioritizing software requirements.
Creating a detailed specification of the medical instrument software.
This phase also includes risk analysis and creating a risk management plan, following the ISO 14971 standard in medical tool manufacturing.
Any oversights or missteps at this juncture can lead to significant issues further down the road. Moreover, this stage serves as the platform to identify potential software-related hazards, assess the associated risks, and evaluate their potential impact.
Step #2. Medical device software architecture design
Next, software developers design a reliable and scalable healthcare device software architecture. This architecture allows for adding new modules or device types with minimal rework. It ensures system configurability, clear module interfaces, and good encapsulation of every module.
The architecture design phase is pivotal in shaping the software’s performance, scalability, and ease of maintenance and updating. This stage also serves as a platform where engineers of clinical device software examine its interoperability with other systems and devices, aiming for a seamless and secure exchange of data.
Step #3. UX/UI design
Designing software for medical devices is an intricate task that hinges greatly on user experience. Our aim is to create a user interface that is not only sleek but also accessible for everyone, no matter their age or level of tech expertise.
Consider, for example, a heart rate monitor app on a smartwatch. The interface needs to be easy for a tech-savvy young adult to navigate, but also simple enough for an elderly person who may not be as comfortable with technology. This delicate balance is one of the world’s most vital challenges in medical device software design.
The interaction between the user and the software significantly impacts user satisfaction. So, we don’t just build software; we design software that helps people. It has to be intuitive and user-friendly, serving healthcare professionals and patients alike.
In short, the focus is on creating a software design for medical devices that enhances healthcare delivery and user satisfaction, while being easily navigable by all users. It’s all about making sure technology is an aid, not a hindrance, to care.
In one of my healthcare projects, we worked on a medical device that pricked your arm and drew blood. We didn’t want our users feeling uneasy or scared about it. So, we prioritized their experience and expectations from designing the app’s interface stage to drafting the packaging. We wanted them to trust us and our device. And that kind of trust only comes when you genuinely empathize with your end users. By empathizing, you create devices and interfaces that cover practical functionality and a human touch.
Yehor Sokhan, Head of Design at QArea
Step #4. Medical device software development
The software is developed with cross-platform compatibility during this phase to cater to multiple operating systems. It is integrated seamlessly with healthcare software (EHR, ADT) via HL7 v.3 or FHIR. The development methodology can be either Waterfall (full-fledged version delivered in one iteration) or Iterative (Agile, Scrum) with MVP delivery and updates every 2-4 weeks.
This step is all about coding the software according to the specifications and design documents created in the previous steps. It’s also the moment where the software’s ability to interact with wearable and non-wearable smart devices is considered, ensuring regular communication with the IoMT system for patient monitoring.
Step #5. Quality Assurance
During software testing and quality assurance, OWASP’s Secure Software Development Life Cycle (S-SDLC) practices are applied. These involve comprehensive multi-level QA. In addition, the step includes continuous testing, software validation/verification, and regular code reviews.
Testing strategies validate that the software meets its intended use purpose and can operate safely under normal and abnormal conditions. This step is crucial to ensure the software is defect-free and performs as expected. It’s also the stage where the software’s cybersecurity measures are considered, including data encryption, secure user authentication, and regular security audits.
Step #6. Premarket submission
To meet the requirements of the FDA and the Council of the European Union and ensure software safety, development services are provided according to ISO 13485, IEC 62304, and IEC 82304-1. During this stage, experts prepare detailed documentation for FDA 510 (k) premarket notification, CE marking, HIPAA compliance audits, etc. This step is critical to ensure the software meets all regulatory requirements and is safe for use.
Step #7. Integration with smart devices
At this stage, the software for the medical instrument is enabled to ensure smooth interaction with the devices. Stable communication with the IoMT system is ensured for remote patient monitoring, and comprehensive analytics of patient-generated health data collected by devices is provided. This step ensures the software can effectively communicate with other devices and systems.
Step #8. Medical instrument software support and evolution
Finally, if required, support is provided for the healthtech device software. It manages the software’s performance and security. Routine software administration tasks are mainly performed, and the software is helped to evolve further. This step is crucial to ensure the software is always up-to-date.
The stages above give you an idea of custom medical tool software development. The rule of thumb dictates—the better you handle each step, the more cost-efficient the process will be. Yet, even for clinical device software developers with years of experience, some crucial challenges remain to consider during the entire dev and design process.
Medical device software engineering challenges: From regulation to device application
The development of healthtech device software involves navigating a complex landscape of challenges. Developers must address many factors, from regulatory compliance to cybersecurity, to ensure successful software deployment and operation.
Challenge 1: Regulatory compliance
Again and again, regulatory compliance is crucial to remember within the dev and design process. The price of non-compliance can go as high as $1.5 million. Medical device software must adhere to FDA guidelines, HIPAA, and EU MDR regulations.
Developers must stay updated with these regulations and design the software to meet these standards. Outsourcing to experienced partners can be a viable strategy to navigate these complex regulatory waters.
Challenge 2: Interoperability
Medtech devices frequently have to communicate and exchange data with a variety of other systems and devices. This interaction is vital for the consolidation and comprehension of the data received from multiple sources, aiding in accurate diagnosis and effective treatment plans.
Let’s take the example of a heart rate monitor and a glucose level tracker in a hospital setting. These devices need to share information with the hospital’s main system so doctors can get a complete view of a patient’s health status. To enable this seamless exchange of information, developers adopt standard communication protocols and data formats, such as HL7 or FHIR.
In addition, the integration of these protocols is not a one-time event but must be maintained throughout the software lifecycle. As technology evolves and upgrades, these standards may also be updated. Your development team needs to ensure that the software remains compatible with these changes, maintaining the smooth flow of data exchange.
Challenge 3: Cybersecurity
Healthtech devices handle sensitive data, making them attractive targets for cybercriminals. A recent report by IBM indicates the average cost of a healthcare data breach surpasses $10 million. To avoid data breaches, MDS developers must implement robust security measures, including Advanced Encryption Standard (AES), SSH File Transfer Protocols (SFTP), encryption tools like BitLocker/FileVault, two-factor (2FA) or multi-factor (MFA) user authentication, single sign-on (SSO), and regular security audits including vulnerability assessment, penetration testing, and code reviews.
Challenge 4: Usability
The usability of SAMD and software for medical procedures and monitoring plays a crucial role in their effectiveness. It’s essential that these tools are user-friendly for everyone involved — from healthcare professionals to patients.
Let’s think about a future medical app designed to help patients track their symptoms. If the app is too complex, it may discourage users or lead to inaccurate data input. That’s why developers need to adopt user-centered design principles.
When we design and build healthtech soft, it’s not just about technical functionality. The software must be intuitive, ensuring users can operate it efficiently and effectively without added stress.
Challenge 5: Software validation
Rigorous testing and validation processes should be in place to verify the software’s functionality and performance. This includes coordinating, designing, and conducting trials and research studies for product validation.
To develop and deploy medical device software successfully, several hurdles must be overcome. For those developing this type of software, smooth sailing is possible through continuous learning about new regulatory changes, making certain that the software can interact seamlessly with other systems, implementing strong cybersecurity defenses, prioritizing user-friendly design, and thoroughly validating the software. It’s essentially about remaining adaptable, secure, user-oriented, and thorough in all stages of the software creation process.
The future of medical device app development
The medical device software development services now have a potential to thrive is an ever-evolving landscape. Technological advancements and the need for improved healthcare solutions also drive it. The following points highlight some of the key trends shaping this field:
Artificial Intelligence (AI) and Machine Learning (ML). These technologies are revolutionizing healthtech device software capabilities by enhancing diagnostics, predictive analytics, and personalized medicine. They analyze vast data sets to identify patterns and make predictions, improving patient outcomes. For example, AI can analyze imaging data for early disease detection.
Internet of Medical Things (IoMT). The IoMT, a connected infrastructure of medical tools, software applications, and health systems, allows devices to communicate and exchange data, improving efficiency and patient care. The trend of increasing device connectivity is expected to continue.
Telemedicine and Remote Patient Monitoring. The COVID-19 pandemic has accelerated the need for distance patient monitoring and telemedicine. Medical device software is being developed to facilitate remote consultations, monitor patient health data, and provide real-time feedback to healthcare providers.
Personalized Medicine. Leveraging AI and data analytics, healthtech device software is being developed to provide personalized treatment plans based on a patient’s unique characteristics. This approach can improve treatment outcomes and patient satisfaction.
Blockchain Technology. Blockchain can provide a secure and transparent platform for patient data exchange. It can maintain patient privacy, ensure data integrity, and facilitate interoperability between healthcare systems.
These trends highlight the dynamic nature of medical device software development. Outsourcing to experienced medical software development services can be a strategic move to leverage the opportunities presented by these trends.
Wrapping up
Faced with rigid regulations and constantly shifting trends, the challenges of development of medical device software are hefty but, with the right approach, entirely surmountable.
Agile development plays a significant role in tackling these challenges, allowing for flexibility and quick adaptation to changing circumstances or requirements. Mobile development, too, is an exciting frontier that brings healthcare tools right into the hands of those who need them, making care more accessible and efficient.
It’s in this environment that the innovative medical software truly shines — helping healthcare providers deliver superior patient care, improving clinical outcomes, and streamlining workflows.
The importance of high-quality medical tool software continues to grow exponentially. This makes it an ideal time to explore and leverage the immense potential that software design for healthtech devices holds.
However, navigating these waters and turning challenges into opportunities requires not just the right tools and strategies, but also the right team. A professional development partner, experienced in the nuances of healthcare software, can guide you through the intricate labyrinth of regulations, lifecycle management, and more, ensuring your product is not just compliant, but a step ahead.
The journey may be intricate, but the results — improved healthcare outcomes through powerful, intuitive software — are undoubtedly worth it.
0 notes
Text
North American Arrhythmia Management System (AMS) Market Analysis Demand, Statistics, Top Manufacturers, Revenue by Reports and Insights 2030
The latest market report published by Credence Research, Inc. “Global North American Arrhythmia Management System (AMS) Market: Growth, Future Prospects, and Competitive Analysis, 2016 – 2028. The North American Arrhythmia Management System (AMS) market has been gradually growing in recent years and is expected to grow at a 6.60% CAGR between 2023 and 2030. The market was valued at USD 3.7 billion in 2022 and is expected to expand to USD 5.78 billion by 2030.
North American Arrhythmia Management System (AMS) market. Our in-depth analysis focuses on key trends, market segmentation, major players, growth drivers, challenges, and future opportunities. As leaders in the field of arrhythmia management, we aim to provide valuable insights for industry stakeholders, healthcare professionals, and investors looking to understand and capitalize on this flourishing market.
North American Arrhythmia Management System (AMS) Market Top Report Findings shed light on the current state of arrhythmia management in this region. This comprehensive study provides an extensive analysis of key factors affecting market growth, including technological advancements, regulatory frameworks, and competitive landscape. The report highlights that the North American AMS market is witnessing a steady expansion due to increasing prevalence of cardiac disorders and rising awareness regarding early diagnosis and treatment options. It also reveals that advanced technologies such as implantable cardioverter-defibrillators (ICDs) are gaining significant traction among healthcare professionals for their effectiveness in managing arrhythmias.
Key Segments of the North American AMS Market
Test Equipment Segment
The AMS market is segmented by test equipment, with the Electrocardiogram (ECG) leading the pack. ECG remains a critical tool for diagnosing arrhythmias, providing valuable insights into the patient's heart rhythm and guiding appropriate treatment plans.
Site of Origin Atrial Segment
When it comes to the site of origin atrial, Sinus Bradycardia emerges as the leading segment. This condition is characterized by a slower-than-normal heart rate and requires effective monitoring and management.
Type Segment
Among different types of arrhythmias, Supraventricular Tachycardias show the highest Compound Annual Growth Rate (CAGR) during the forecast period. Supraventricular tachycardias refer to a group of arrhythmias originating above the ventricles, demanding accurate detection and timely interventions.
Country Segment
The United States is the driving force behind the growth of the North American Arrhythmia Management System (AMS) industry. Canada is the second largest country in the market, while Mexico is expected to be the fastest-growing country in this sector
Browse 220 pages report North American Arrhythmia Management System (AMS) Market By Test Equipment (Electrocardiogram (ECG), Holter monitor) By Site of Origin Atrial (Sinus bradycardia, Premature atrial contractions (PACs), Wandering atrial pacemaker, Atrial tachycardia, Multifocal atrial tachycardia, Supraventricular tachycardia (SVT), Atrial flutter, Atrial fibrillation)- Growth, Future Prospects & Competitive Analysis, 2016 – 2030)- https://www.credenceresearch.com/report/north-american-arrhythmia-management-system-ams-market
Scarcity of Qualified Healthcare Workers
Effectively utilizing AMS technology requires skilled healthcare professionals, including cardiologists, electrophysiologists, and technicians. However, certain areas face a shortage of skilled professionals, hindering the uptake and implementation of AMS technology. Limited training opportunities and the specialized nature of arrhythmia therapy can exacerbate this challenge.
Limited Reimbursement Coverage
Although reimbursement policies may incentivize AMS adoption, limited coverage for specific devices and services can hinder market growth. Insurance companies and government healthcare initiatives may not fully cover the cost of all AMS devices and procedures. This limited reimbursement coverage may create cost constraints for patients and healthcare providers, influencing the adoption of advanced AMS systems.
Focus on Patient Education and Engagement
Educating patients about arrhythmias, self-monitoring techniques, and the importance of adhering to treatment plans can improve patient engagement and self-care. AMS providers can develop instructional tools, mobile applications, or interactive platforms to educate patients and promote active involvement in managing their condition.
Advancements in Wearable Devices and Sensors
Wearable devices such as smartwatches, ECG monitors, or patches enable continuous monitoring of heart rhythms throughout the day, facilitating real-time surveillance and analysis. This constant monitoring aids in the early detection of anomalies and arrhythmias, providing valuable insights for healthcare practitioners. Long-term data collection through wearable devices allows for identifying patterns, triggers, and evaluating the effectiveness of treatment strategies, enhancing arrhythmia management practices.
Competitive Landscape
The North American Arrhythmia Management System (AMS) market is highly competitive, with several leading players vying for market share. Some notable competitors in the market include:
Applied Cardiac Systems
AliveCor
Biotronik
Biotricity
GE Healthcare
iRhythm Technologies
Koninklijke Philips N.V.
Medtronic plc.
Nihon Kohden Corporation
St. Jude Medical (Abbott Laboratories)
Spacelabs Healthcare (OSI Systems Inc.)
Welch Allyn (Hillrom Services Inc.)
These key players focus on product innovation, expanding their market reach, and maintaining competitive pricing to stay ahead of the competition.
Future Outlook
The North American Arrhythmia Management System (AMS) market holds immense promise, driven by the rising demand for remote patient monitoring and telemedicine solutions. Key growth factors, such as the increasing prevalence of arrhythmia and the growing senior population, continue to propel the market forward. To remain competitive and successful, key businesses in the sector must prioritize product innovation, expand market reach, and maintain a customer-centric approach.
Why to Buy This Report-
The report provides a qualitative as well as quantitative analysis of the global North American Arrhythmia Management System (AMS) Market by segments, current trends, drivers, restraints, opportunities, challenges, and market dynamics with the historical period from 2016-2020, the base year- 2021, and the projection period 2022-2028.
The report includes information on the competitive landscape, such as how the market's top competitors operate at the global, regional, and country levels.
Major nations in each region with their import/export statistics
The global North American Arrhythmia Management System (AMS) Market report also includes the analysis of the market at a global, regional, and country-level along with key market trends, major players analysis, market growth strategies, and key application areas.
Browse Full Report: https://www.credenceresearch.com/report/north-american-arrhythmia-management-system-ams-market
Visit: https://www.credenceresearch.com/
Related Report: https://www.credenceresearch.com/report/medical-animation-market
Related Report: https://www.credenceresearch.com/report/ecoa-esource-and-clinical-trials-market
Browse Our Blog: https://www.linkedin.com/pulse/north-american-arrhythmia-management-system-ams-market-singh
Browse Our Blog: https://tealfeed.com/north-american-arrhythmia-management-system-ams-scifx
About Us -
Credence Research is a viable intelligence and market research platform that provides quantitative B2B research to more than 10,000 clients worldwide and is built on the Give principle. The company is a market research and consulting firm serving governments, non-legislative associations, non-profit organizations, and various organizations worldwide. We help our clients improve their execution in a lasting way and understand their most imperative objectives. For nearly a century, we’ve built a company well-prepared for this task.
Contact Us:
Office No 3 Second Floor, Abhilasha Bhawan, Pinto Park, Gwalior [M.P] 474005 India
0 notes
Text
Cardiovascular Biomaterial Market Research Report, Growth, Analysis and Forecast 2028
Global Cardiovascular Biomaterial Market, By Type (Natural, Ceramic, Metallic, Polymer), Product (Catheters, Stents, Implantable Cardiac Defibrillators, Pacemakers, Sensors, Heart Valves, Vascular Grafts, Guidewires, Ventricular Assist Devices), Country (U.S., Canada, Mexico, Germany, Italy, U.K., France, Spain, Netherland, Belgium, Switzerland, Turkey, Russia, Rest of Europe, Japan, China, India, South Korea, Australia, Singapore, Malaysia, Thailand, Indonesia, Philippines, Rest of Asia- Pacific, Brazil, Argentina, Rest of South America, South Africa, Saudi Arabia, UAE, Egypt, Israel, Rest of Middle East and Africa) Industry Trends and Forecast to 2028.
An expert team performs systematic, object-oriented and complete market research study to provide the facts associated with any subject in the field of marketing via Cardiovascular Biomaterial marketing report. The report has a lot to offer to both established and new players in the Cardiovascular Biomaterial industry with which they can completely understand the market. SWOT analysis and Porter’s Five Forces analysis methods are used wherever applicable, while generating this report. One of the most important parts of an international Cardiovascular Biomaterial market report is competitor analysis with which businesses can estimate or analyse the strengths and weaknesses of the competitors.
Key Players
The major players covered in the cardiovascular biomaterial market report are DSM, Wright Medical Group N.V, Zimmer Biomet, Bayer AG, BASF SE, CRS Holdings Inc., Invibio Ltd., Foster Corporation, CVD Equipment Corporation, Abbott, Baxter, Medtronic, Johnson & Johnson Private Limited , Boston Scientific Corporation , Edwards Lifesciences Corporation, BD, Molnlycke Health Care AB, Smith+Nephew, Integra LifeSciences, Messe-Düsseldorf GmbH, AnteoTech, and ANYGEN among other domestic and global players. Market data is available for global, North America, South America, Europe, Asia-Pacific (APAC) and Middle East and Africa (MEA) separately. DBMR analysts understand competitive strengths and provide competitive analysis for each competitor separately.
Browse More Info @ https://www.databridgemarketresearch.com/reports/global-cardiovascular-biomaterial-market
With the help of credible Cardiovascular Biomaterial market analysis report, businesses can make out the reaction of the consumers to an already existing product in the market. The report includes estimations of recent state of the market, CAGR values, market size and market share, revenue generation, and necessary changes required in the future products. A wide-ranging competitor analysis helps build superior strategies of production, improvement in certain product, its advertising or marketing and promotion for the business. Exhaustive and comprehensive market study performed in the wide ranging Cardiovascular Biomaterial market report offers current and forthcoming opportunities that put light on the future market investment.
Key questions answered in the report:
Which product segment will grab a lion’s share?
Which regional market will emerge as a frontrunner in coming years?
Which application segment will grow at a robust rate?
Report provides insights on the following pointers:
Market Penetration: Comprehensive information on the product portfolios of the top players in the Cardiovascular Biomaterial Market.
Product Development/Innovation: Detailed insights on the upcoming technologies, R&D activities, and product launches in the market.
Competitive Assessment: In-depth assessment of the market strategies, geographic and business segments of the leading players in the market.
Table Of Content
Part 01: Executive Summary
Part 02: Scope Of The Report
Part 03: Global Market
Part 04: Global Market Size
Part 05: Global Market Segmentation By Product
Part 06: Five Forces Analysis
More Reports:
Diuretic Drugs Market
Patient Engagement Technology Market
Healthcare Business Intelligence Market
Chinese Hamster Ovary cells (CHO) Market
Anti-cancer Drug Market
About Us:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market
Contact:
Data Bridge Market Research
Tel: +1-888-387-2818
Email: [email protected]
0 notes
Text
Primary Lithium Batteries for Medical Market Outlook on Key Growth Trends, Factors and Forecast 2032

Primary Lithium Batteries for Medical Market Overview:
The primary lithium batteries for medical market refers to the sector that provides non-rechargeable lithium batteries specifically designed for medical devices and applications. These batteries offer reliable and long-lasting power to various medical devices, ensuring uninterrupted operation in critical healthcare settings. The Global Primary Lithium Battery Market Size Reached USD 3035.2 Million in 2022. It is Expected to Grow at a CAGR of 4.3% 2023-2030. Here is an overview of the primary lithium batteries for medical market, including key factors that drive its growth:
Key Factors:
Medical Device Applications: Primary lithium batteries are widely used in a range of medical devices, including implantable medical devices, patient monitoring systems, infusion pumps, defibrillators, hearing aids, insulin pumps, neurostimulators, and diagnostic equipment. These batteries provide a safe and dependable power source for essential medical functions.
Reliability and Longevity: Medical devices require a consistent and reliable power supply to ensure accurate measurements, continuous monitoring, and proper functioning. Primary lithium batteries are known for their stable voltage output and extended shelf life, ensuring the reliability and longevity of medical devices during critical operations.
Compact and Lightweight: Primary lithium batteries offer a high energy density, meaning they can store a significant amount of energy in a compact size. This characteristic is crucial for medical devices, which often require small and lightweight power sources to be portable and non-intrusive.
Safety and Regulatory Compliance: Primary lithium batteries for medical applications are designed and manufactured to meet strict safety standards and regulatory requirements, including those specified by organizations such as the International Electrotechnical Commission (IEC) and the Food and Drug Administration (FDA). These batteries undergo rigorous testing and validation to ensure their safety in medical environments.
Shelf Life and Storage Stability: Primary lithium batteries have a long shelf life and can retain their charge for extended periods. This is important for medical devices that may have intermittent usage or require stockpiling for emergencies. The ability to store these batteries for an extended time without significant loss of capacity or performance is a key advantage in the medical field.
Durability and Performance in Extreme Conditions: Medical devices may operate in various environments, including high or low temperatures, humidity, and mechanical stress. Primary lithium batteries are designed to withstand these challenging conditions, offering excellent performance and durability to ensure uninterrupted operation in critical healthcare settings.
Increasing Medical Device Adoption: The growing adoption of medical devices and technological advancements in healthcare drive the demand for primary lithium batteries. The rising prevalence of chronic diseases, an aging population, and the need for advanced diagnostic and treatment methods contribute to the increasing use of medical devices that rely on reliable power sources.
Technological Advancements: Ongoing advancements in primary lithium battery technology, including improvements in energy density, safety features, and integration with medical devices, further propel the growth of the market. These advancements enhance the performance, efficiency, and usability of medical devices, driving the demand for primary lithium batteries.
Emphasis on Portable and Wearable Devices: The trend towards portable and wearable medical devices, such as remote patient monitoring systems and wearable biosensors, requires compact and long-lasting power solutions. Primary lithium batteries provide the necessary energy supply for these devices, supporting the shift towards personalized and patient-centric healthcare.
In summary, the primary lithium batteries for medical market is driven by the wide range of medical device applications, reliability, longevity, compactness, safety compliance, shelf life, durability, technological advancements, increasing medical device adoption, and the emphasis on portable and wearable devices. As the healthcare industry continues to evolve and demand reliable power solutions, the market for primary lithium batteries for medical applications is expected to grow.
Demand:
Primary lithium batteries are widely used in medical devices due to their long shelf life, high energy density, and reliable performance. These batteries provide a stable power source for various medical applications, including implantable devices, diagnostic equipment, monitoring devices, and portable medical devices.
Implantable medical devices such as pacemakers, defibrillators, neurostimulators, and drug delivery systems often rely on primary lithium batteries. These batteries are preferred in implantable devices because they provide a long operational life, minimizing the need for battery replacement surgeries.
Diagnostic equipment such as glucose meters, blood pressure monitors, and various medical sensors also utilize primary lithium batteries for their compact size and high energy capacity. These batteries offer reliable power for accurate readings and prolonged use.
Portable medical devices, such as infusion pumps, portable oxygen concentrators, and handheld monitors, often utilize primary lithium batteries as well. These batteries provide the necessary energy for these devices to be portable and convenient for patients.
The demand for primary lithium batteries in the medical market is influenced by factors such as technological advancements in medical devices, an aging population, increasing prevalence of chronic diseases, and the overall growth of the healthcare industry. To obtain the most up-to-date information on the specific demand for primary lithium batteries in the medical market, it would be best to consult industry reports, market research, and battery manufacturers who specialize in medical applications.
We recommend referring our Stringent datalytics firm, industry publications, and websites that specialize in providing market reports. These sources often offer comprehensive analysis, market trends, growth forecasts, competitive landscape, and other valuable insights into this market.
By visiting our website or contacting us directly, you can explore the availability of specific reports related to this market. These reports often require a purchase or subscription, but we provide comprehensive and in-depth information that can be valuable for businesses, investors, and individuals interested in this market.
“Remember to look for recent reports to ensure you have the most current and relevant information.”
Click Here, To Get Free Sample Report: https://stringentdatalytics.com/sample-request/primary-lithium-batteries-for-medical-market/6710/
Market Segmentations:
Global Primary Lithium Batteries for Medical Market: By Company
• EVE Energy
• SAFT
• Hitachi Maxell
• GP Batteries International
• Energizer
• Duracell
• Varta
• Changzhou Jintan Chaochuang Battery
• Vitzrocell
• FDK
• Panasonic
• Murata
• Wuhan Lixing (Torch) Power Sources
• Newsun
• Renata SA
• Chung Pak
• Ultralife
• Power Glory Battery Tech
• HCB Battery
• EEMB Battery
Global Primary Lithium Batteries for Medical Market: By Type
• Li/SOCL2
• Li/MnO2
• Li-SO2
• Others
Global Primary Lithium Batteries for Medical Market: By Application
• Portable Medical Electronics
• Insulin Pumps
• Portable Ultrasound Equipment
• Others
Global Primary Lithium Batteries for Medical Market: Regional Analysis
All the regional segmentation has been studied based on recent and future trends, and the market is forecasted throughout the prediction period. The countries covered in the regional analysis of the Global Primary Lithium Batteries for Medical market report are U.S., Canada, and Mexico in North America, Germany, France, U.K., Russia, Italy, Spain, Turkey, Netherlands, Switzerland, Belgium, and Rest of Europe in Europe, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, China, Japan, India, South Korea, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), and Argentina, Brazil, and Rest of South America as part of South America.
Visit Report Page for More Details: https://stringentdatalytics.com/reports/primary-lithium-batteries-for-medical-market/6710/
Reasons to Purchase Primary Lithium Batteries for Medical Market Report:
• To gain insights into market trends and dynamics: this reports provide valuable insights into industry trends and dynamics, including market size, growth rates, and key drivers and challenges.
• To identify key players and competitors: this research reports can help businesses identify key players and competitors in their industry, including their market share, strategies, and strengths and weaknesses.
• To make informed business decisions: this research reports provide businesses with data-driven insights that can help them make informed business decisions, including strategic planning, product development, and marketing and advertising strategies.
About US:
Stringent Datalytics offers both custom and syndicated market research reports. Custom market research reports are tailored to a specific client's needs and requirements. These reports provide unique insights into a particular industry or market segment and can help businesses make informed decisions about their strategies and operations.
Syndicated market research reports, on the other hand, are pre-existing reports that are available for purchase by multiple clients. These reports are often produced on a regular basis, such as annually or quarterly, and cover a broad range of industries and market segments. Syndicated reports provide clients with insights into industry trends, market sizes, and competitive landscapes. By offering both custom and syndicated reports, Stringent Datalytics can provide clients with a range of market research solutions that can be customized to their specific needs
Contact US:
Stringent Datalytics
Contact No - +1 346 666 6655
Email Id - [email protected]
Web - https://stringentdatalytics.com/
#Non-rechargeable Batteries#Long Life Batteries#High Energy Density Batteries#Battery Power#Medical Sensors#Medical Diagnostics#Cardiac Devices#Neurostimulators#Hearing Aids#Infusion Pumps#Portable Medical Devices#Remote Monitoring#Medical Wearables.
1 note
·
View note
Text
Home Healthcare Market to be Worth $514.5 Billion by 2030 - Exclusive Report by Meticulous Research®
According to a new market research report titled, ‘Home Healthcare Market by Offering (Fertility, Pregnancy, Cholesterol Tests, Oxygen Delivery, Dialysis, IV, Patient Support Equipment), Services (Rehabilitation, Telehealth, Nursing, Hospice), Application (Cardiovascular, Diabetes)- Global Forecast to 2030,’ published by Meticulous Research®, the home healthcare market is projected to reach $514.5 billion by 2030, at a CAGR of 7.9% from 2023 to 2030.
Population aging is the dominant demographic trend of the twenty-first century—a reflection of increasing longevity, declining fertility, and the progression of a large number of individuals to older ages. The OECD forecasts that, globally, the population aged 60 and over is expected to reach 2.1 billion by 2050. As per the World Health Organization (WHO) statistics, the global population in the age group of 60 years and above was expected to increase from 1 billion in 2020 to 1.4 billion in 2022. The growth in the aging population has resulted in the increased adoption of home healthcare services due to the high costs of hospital healthcare services.
Download Free Report Sample Now @ https://www.meticulousresearch.com/download-sample-report/cp_id=1280
The geriatric population is highly susceptible to diseases such as high blood pressure, diabetes, arthritis, and cardiovascular diseases. Therefore, the growing global geriatric population, coupled with the fact that approximately 70% of home health patients belong to the age group of 65 years and above, is expected to drive the demand for home healthcare products and services during the forecast period.
Impact of COVID-19 on the Home Healthcare Market
The COVID-19 pandemic positively impacted the home healthcare market. The pandemic has increased the preference for home healthcare due to the risks of acquiring infections during hospital and clinic visits for treatment and related purposes. The pandemic has also increased the adoption of telehealth, making it a primary source of care for many patients. According to the 2021 World Economic Forum’s 5G Outlook Series report, there has been an increase of 490% in telemedicine visits for urgent cases. Furthermore, the outbreak of the COVID-19 pandemic increased the demand for home healthcare infrastructure and increased investments from governments in promoting homecare services. For instance, in August 2022, Fresenius Medical Care North America, the North American division of Fresenius Medical Care AG & Co. KGaA (Germany), acquired Interwell Health U.S. This acquisition was aimed at bringing Fresenius Health Partners’ expertise in kidney care and value-based performance and contracting.
The home healthcare market is segmented based on Offering (Home Tests and Patient Monitoring Equipment [Fertility Tests & Aids, Pregnancy Tests, Gender, DNA, and Parental Tests, Cholesterol Tests, HIV Tests, Holter Monitors, Blood Pressure Monitors, Oximeters, Heart Rate Monitors,
Thermometers, Stethoscopes, Defibrillators, Pedometers, Scales & Body Fat Monitors, Peak Flow Meters, Apnea Monitors, Baby Monitors, Coagulation Monitors, Diabetes Management]), Home Therapeutic Equipment ({Home Respiratory Therapy Equipment, [Continuous Positive Airway Pressure Equipment (CPAP Machines, CPAP Accessories & Consumables)], Oxygen Delivery Equipment, Nebulizers & Accessories, Ventilators & Accessories}, Home Dialysis Equipment, {Home Peritoneal Dialysis Products, Home Hemodialysis Products}, Home IV Equipment {IV Pumps, Other Home IV Equipment}, Other Equipment), Patient Support Equipment, {Mobility Assist Equipment, [Wheelchairs], Walking Assist Devices {Walking Assist Devices, [Walkers and Rollators, Canes & Walking Sticks, Crutches], Mobility Scooters, Medical Furniture and Accessories, Bathroom Safety Equipment), Services (Rehabilitation Services, Infusion Services, Skilled Care/Nursing, Unskilled Care Services, Telehealth Services, Hospice Care Services, Respiratory Therapy Services, Other Services), Application (Cardiovascular Diseases & Hypertension, Diabetes, Respiratory Diseases, Pregnancy, Mobility Disorders, Cancer, Wound Care, and Other Applications) and Geography. The study also evaluates industry competitors and analyzes their market shares at the country and regional levels.
Speak to our Analysts to Understand the Impact of COVID-19 on Your Business : https://www.meticulousresearch.com/speak-to-analyst/cp_id=1280
Based on offering, in 2023, the home therapeutic equipment segment is expected to account for the largest share of the home healthcare market. The growth of this segment is attributed to increasing cases of chronic kidney and respiratory diseases, the high costs of therapeutic services in hospitals, and the availability of rental products, including ventilators, nebulizers, and oxygen delivery equipment.
Based on services, the skilled care/nursing segment is projected to register the highest CAGR during the forecast period. The growth of this segment is attributed to the wide range of daily tasks performed by nurses to provide adequate care for patients receiving homecare, the rising adoption of home-based treatments that require technical knowledge of operating healthcare products which includes home dialysis equipment, and home IV equipment, home respiratory therapy equipment, and the increasing demand for home care for the elderly population.
Based on application, in 2023, the diabetes segment is expected to account for the largest share of the home healthcare market. The growth of this segment is attributed to the rising number of diabetic patients globally who are more prone to diseases such as cardiovascular diseases and Alzheimer's disease. Furthermore, the growing need to monitor the heart rate, hemoglobin, cholesterol, and blood pressure levels of diabetic patients contributes to the growth of this market segment.
“Exciting news! We are thrilled to announce an incredible opportunity for you!
For a limited time only, we are delighted to offer an exclusive flat 30% discount on all our remarkable research reports. It's our way of showing appreciation for your continuous support and commitment to staying informed. This remarkable discount allows you to unlock a wealth of valuable insights and expert analysis at an unbeatable price. Whether you're a business professional, an academic researcher, or an avid learner, this offer is tailor-made to enhance your knowledge and empower your decision-making.”
Quick Buy – Home Healthcare Market - Global Opportunity Analysis And Industry Forecast (2023-2030), Research Report: https://www.meticulousresearch.com/Checkout/85264114
Based on geography, in 2023, North America is expected to account for the largest share of the home healthcare market, followed by Europe and Asia-Pacific. Furthermore, in 2023, the U.S. is expected to be the largest shareholding market in North America. The market growth in the U.S. is attributed to the high disposable incomes of the population, the rising number of patients with chronic diseases, and government investments in promoting home healthcare. However, Asia-Pacific is expected to witness a rapid growth during forecast period. The growth of this regional market is attributed to the increasing geriatric population, the rising prevalence of chronic diseases in developing nations, the increasing investments in home healthcare in developing countries such as China and India, and the higher preference for home-based treatments due to the high costs of hospital services. Furthermore, government initiatives promoting homecare and the launch of advanced homecare products are driving the growth of the home healthcare market in Asia-Pacific.
The report also includes an extensive assessment of the key growth strategies adopted by leading market players in the past three to four years. In the last couple of years, the home healthcare market has witnessed various developments.
Some of the key players operating in the home healthcare market are Abbott Laboratories (U.S.), Amedisys, Inc (U.S.), Owens & Minor (U.S.), B. Braun SE (Germany), Baxter International, Inc. (U.S.), BAYADA Home Health Care (U.S.), Convatec (U.K.), Halyard Health, Inc. (U.S.), Covidien (Ireland), Fisher & Paykel (New Zealand), Fresenius Medical (Germany), GE Healthcare (U.S.), Johnson & Johnson (U.S.), and F. Hoffmann-La Roche AG (Switzerland).
To gain more insights into the market with a detailed table of content and figures, click here: https://www.meticulousresearch.com/product/home-healthcare-market-1280
Scope of the Report:
Home Healthcare Market, by Offering
Home Tests and Patient Monitoring Equipment
Fertility Tests & Aids
Pregnancy Tests
Gender, DNA, and Parental Tests
Cholesterol Tests
HIV Tests
Holter Monitors
Blood Pressure Monitors
Oximeters
Heart Rate Monitors
Thermometers
Stethoscopes
Defibrillators
Pedometers
Scales & Body Fat Monitors
Peak Flow Meters
Apnea Monitors
Baby Monitors
Coagulation Monitors
Diabetes Management
Home Therapeutic Equipment
Home Respiratory Therapy Equipment
Continuous Positive Airway Pressure Equipment
CPAP Machines
CPAP Accessories & Consumables
Oxygen Delivery Equipment
Nebulizers & Accessories
Ventilators & Accessories
Home Dialysis Equipment
Home Peritoneal Dialysis Products
Home Hemodialysis Products
Home IV Equipment
IV Pumps
Other Home IV Equipment
Other Equipment
Patient Support Equipment
Mobility Assist Equipment
Wheelchairs
Walking Assist Devices
Walkers and Rollators
Canes & Walking Sticks
Crutches
Mobility Scooters
Medical Furniture and Accessories
Bathroom Safety Equipment
Home Healthcare Market, by Services
Rehabilitation Services
Infusion Services
Skilled Care / Nursing
Unskilled Care Services
Telehealth Services
Hospice Care Services
Respiratory Therapy Services
Other Services
Home Healthcare Market, by Application
Cardiovascular Diseases & Hypertension
Diabetes
Respiratory Diseases
Pregnancy
Mobility Disorders
Cancer
Wound Care
Other Applications
Home Healthcare Market, by Geography
North America
U.S.
Canada
Europe
Germany
France
Italy
U.K.
Spain
Rest of Europe (RoE)
Asia-Pacific
China
Japan
India
Rest of Asia-Pacific
Latin America
Brazil
Mexico
Rest of Latin America
Middle East & Africa
Download Free Report Sample Now @ https://www.meticulousresearch.com/download-sample-report/cp_id=1280
1 note
·
View note
Text
Emergency Room Equipment Industry Current Trends and Challenges Analysis by 2023-2030

The emergency room equipment market refers to the global industry involved in the manufacturing and distribution of medical devices and equipment specifically designed for use in emergency departments or emergency rooms (ERs) of hospitals and healthcare facilities.
For Sample Report Click Here:- https://www.marketinforeports.com/Market-Reports/Request-Sample/485270
Emergency rooms are critical areas where patients with acute illnesses, injuries, or life-threatening conditions receive immediate medical attention. To effectively manage these situations, ERs require specialized equipment and devices to provide rapid assessment, diagnosis, and treatment. The emergency room equipment market encompasses a wide range of products that are essential for the functioning of an emergency department.
Some common types of emergency room equipment include:
Diagnostic equipment: This includes devices such as X-ray machines, CT scanners, ultrasound systems, electrocardiograms (ECGs), and vital signs monitors. These tools help healthcare professionals quickly evaluate patients and diagnose their conditions.
Life support equipment: Emergency rooms are equipped with various life support devices, including ventilators, defibrillators, cardiac monitors, and infusion pumps. These devices are crucial for stabilizing patients in critical condition or cardiac arrest.
Surgical instruments: Emergency room equipment also includes a variety of surgical instruments and supplies for performing emergency procedures. These may include surgical trays, sterile gloves, sutures, forceps, and scalpels.
Trauma care equipment: ERs are often equipped with specialized equipment for managing trauma cases, such as trauma beds, immobilization devices, cervical collars, traction devices, and splints.
Resuscitation equipment: Automated external defibrillators (AEDs), airway management devices (such as endotracheal tubes and laryngoscopes), and other resuscitation equipment are essential for responding to cardiac arrests and maintaining airway patency.
Patient monitoring systems: These systems provide continuous monitoring of patients’ vital signs, such as heart rate, blood pressure, oxygen saturation, and temperature. This allows healthcare providers to track patients’ conditions and respond promptly to any changes.
The emergency room equipment market is driven by factors such as the increasing number of emergency visits, rising demand for advanced diagnostic tools, and technological advancements in medical devices. The market includes several key players, including medical device manufacturers, distributors, and service providers. These companies strive to develop innovative and efficient equipment to enhance patient care in emergency settings.
0 notes
Text
Electric Coils and Solenoids: A Guide to Suppliers and Manufacturers

Electric Coils and Solenoids: A Guide to Suppliers and Manufacturers
Electric coils and solenoids are essential components in various industries, including automotive, medical, and aerospace. These electromechanical devices convert electrical energy into mechanical energy, and vice versa. In this article, we will explore the different types of Encapsulated Electric Coils and solenoids, their applications, and the top suppliers and manufacturers in the industry.
Types of Electric Coils and Solenoids
There are several types of electric coils and solenoids, each designed for specific applications. Here are some examples:
Magnetic coils: These coils are used in electric motors and generators, and they generate a magnetic field when an electric current flows through them.
Custom solenoid coils: These coils are designed for specific applications, and they can be customized to meet the client's requirements.
Voice coils: These coils are used in speakers and headphones, and they convert electrical signals into sound waves.
One of the most significant trends in the electric coils and solenoids market is the adoption of digitalization and automation in the manufacturing process. Digital platforms and software tools are being used to design, simulate, and test electric coils and solenoids, resulting in faster and more accurate production. Another trend is the use of sustainable materials and manufacturing processes in the production of electric coils and Custom Solenoid Coils. Manufacturers are exploring new materials and techniques to reduce waste and minimize environmental impact.
Applications of Electric Coils and Solenoids
Electric coils and solenoids have various applications in different industries. Here are some examples:
Automotive industry: Electric coils and solenoids are used in car engines, fuel injection systems, and transmission systems.
Medical industry: These devices are used in medical equipment like MRI machines and defibrillators.
Aerospace industry: Electric coils and solenoids are used in aircraft engines, landing gear, and hydraulic systems.
Electric coils and solenoids are essential components in various industries, and their applications are diverse. Choosing the right supplier or manufacturer for electric coils and solenoids is crucial to ensure quality and reliability. The top suppliers and manufacturers mentioned in this article are just a few examples of the many options available in the market. With the right electric coils and solenoids, industries can improve efficiency, reliability, and performance in their applications.
For More Info:-
Electric Coils Suppliers
Electromagnetic Coils Manufacturer
High-quality Self-supported Electric Coil Supplier
Customizable Electromagnetic Coil Manufacturer
0 notes
Text
Advanced ceramics market growth & demand analysis till 2031
The global advanced ceramics market. According to the study, the market reached a valuation of around US$ 60 Billion in 2020, which amounts to around 20% share of the overall ceramics market.
Sales of advanced ceramics are slated to rise at a CAGR of 7% to top US$ 120 Billion by 2031. Demand for alumina ceramics is set to increase at a CAGR of 6% across the forecast period of 2021 to 2031.
Download Sample Copy of This Report:
https://www.factmr.com/connectus/sample?flag=S&rep_id=2993?VM
The readability score of the Advanced Ceramics Market Demand report is good as it offers chapter-wise layout with each section divided into a smaller sections.
The report encompasses graphs and tables to show the entire assembling. Pictorial demonstration of the definite and estimated values of key segments is visually appealing to readers.
This Advanced Ceramics Market outlook report explicates on vital dynamics such as the drivers, restraints and opportunities for key players and competitive analysis of Advanced Ceramics Market along with key stakeholders as well as emerging players associated with the manufacturing of product.
The Key trends Analysis of Extended Oral Antibiotics Market also provides dynamics that are responsible for influencing the future Sales and Demand of over the forecast period.
What differences can the Advanced Ceramics Market report make on the revenue impacts and strategies of businesses?
Fact.MR strives to provide comprehensive assessments of opportunities in various regions and technology segments. The study also offers an uncluttered data-driven insights into the growth avenues of the Advanced Ceramics Market and all its segments. Some of the ways the study can make a discernible impact are by offering evidence-based perspectives on:
Attractiveness quotient of emerging product/technology types in various products in the Automated External Defibrillators Market.
Micro-economics factors that may hamper the prospects of some of the key segments
Recent spate of research and development (R&D) funding on key Advanced Ceramics Market
New business models paving way for disruptions in demand dynamic of key segments
Regional markets that will be future engine of growth and the industry trends that will support these markets
Challenges overcoming which may offer industry players competitive edge
Competitive Landscape
Leading companies in the market for advanced ceramics are putting a lot of effort into creating innovative technologies for unique applications.
Competition in the market has been observed to intensify in recent years. Major players in the market for advanced ceramics are investing in improving their product offerings as well as expanding their production capacities to cater to rising demand.
Key Segments Covered in Advanced Ceramics Industry Research
· Material
Alumina Ceramics
Ceramic-based Components
Titanate Ceramics
Zirconia Ceramics
Silicon Carbide Ceramics
Ceramic Filters
Others
Silicon Nitride Ceramics
Magnesium Silicate Ceramics
Pyrolytic Boron Nitride Ceramics
Aluminium Nitride Ceramics
Electroceramics
Structural Ceramics
Technical Ceramics
High-tech Ceramics
Ferrite Ceramics
Transparent Ceramics
· Class
Ceramic Matrix Composites
Ceramic Coatings
Monolithic Ceramics
· Application
Electrical Equipment
Catalyst Support
Electronic Devices
Wear Parts
Engine Parts
Filters
Bioceramics
· End User
Electrical & Electronics Sector
Transportation Sector
Medical Sector
Defense & Security Sector
Environmental Sector
Chemical Sector
Get Full Access of Complete Report:
https://www.factmr.com/checkout/2993
Questionnaire answered in the Market outlook Report of advanced ceramics include:
What is the key strategy deployed by large players to maximize Automated External Defibrillators Market growth?
What are the main challenges faced by players in the Advanced Ceramics Market Demand?
With the advent of technological advancement, how will the Advanced Ceramics Market landscape change over the forecast period?
What does player bring to the table which is unique as a strategy, and is easy to emulate for new investors in the Advanced Ceramics Market size?
How will be insights and market estimations provided in the Fact.MR report on the Demand of advanced ceramics make a difference?
The study takes a closer look at the major economic turmoil, with a focus on the recent COVID-19 pandemic disruptions
The assessment of key growth dynamics highlights the attractiveness of new automation technologies and offers readers insight on the prospect of these during the forecast period
The study tries to offer a balance perspective of the opportunities in mature and the most lackluster markets
Provides scrutiny of the industry trends that have shaped recent government policies
Provides an account of major breakthroughs in all segments that might change the course of the market considerably
Provides an incisive analysis of socio-political milieu in which the key markets operate, and how will that influence the lucrativeness of the overall Automated External Defibrillators Market
Analyzes how collaborations and partnerships among players from different industries shape the key growth dynamics in the near future
Evaluates the role of various stages of funding on new growth avenues in key regional markets
Contact:
US Sales Office : 11140 Rockville Pike
Suite 400 Rockville, MD 20852
United States
Tel: +1 (628) 251-1583
E-Mail: [email protected]
0 notes
Text
Did you Know the Future of Medical Kits
Medical kits refer to the segment of the healthcare industry that produces and sells pre-packaged kits containing various medical supplies and equipment. These kits are designed to be used in a variety of medical settings, including hospitals, clinics, ambulances, and in-home care.
Medical kits can be specialized for a particular medical condition, such as diabetes or asthma, or they can be more general and contain a variety of basic medical supplies, such as bandages, gauze, and antiseptics. Some medical kits may also contain more advanced equipment, such as defibrillators, airway management devices, and respiratory therapy devices.
Upcoming Trends: Medical Kit Industry
The global medical kits market is expected to grow in the coming years, driven by factors such as the increasing prevalence of chronic diseases, the growing demand for home healthcare services, and the need for emergency medical services.

0 notes