#dry hcl gas generation system
Text
Impact of Innovations in HCL Gas Generator Design
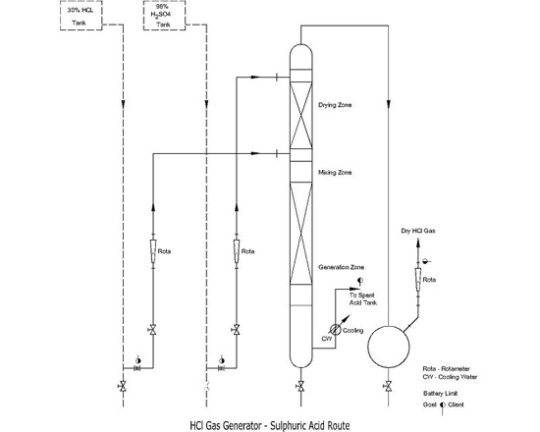
The generation of hydrogen chloride (HCl) gas is essential for a wide range of chemical operations in various industries. From pharmaceutical manufacture to mineral processing, HCl plays an important function as a reactant, catalyst, and in a variety of other applications. Traditionally, HCl gas was produced using traditional processes, which were often inefficient, dangerous, and had a negative influence on the environment. However, recent advances in HCl gas generator technology are poised to transform the way this important gas is produced, providing considerable benefits that are redefining chemical operations.
Improved Safety and Risk Mitigation
One of the most notable benefits of modern HCL Gas Generator is increased safety. Cutting-edge designs include strong safety measures like fail-safe systems, automated shutdowns, and real-time monitoring capabilities. These developments serve to reduce the dangers involved with handling dangerous gases by safeguarding personnel and lowering the possibility of accidents or leaks. Furthermore, many new generators are designed to comply with demanding industry standards and regulations, providing operators with peace of mind.
Greater Effectiveness And Productivity
Traditional methods for producing HCl gas were frequently inefficient, resulting in increased operating costs and lower production. Innovative HCl gas generators overcome these issues by optimizing the generating process, resulting in increased yields and lower energy use. Advanced control systems, better reactor designs, and improved heat transfer mechanisms all help to drive efficiency increases, which translate into cost savings and increased profitability for chemical processing facilities.
Environmental Sustainability
As environmental issues continue to dominate headlines, the chemical industry is under increasing pressure to embrace sustainable practices. Innovative HCl gas generators are meeting this demand by prioritizing eco-friendly designs and reducing environmental effects. Many contemporary generators use innovative scrubbing technology to trap and neutralize hazardous pollutants, lowering the carbon footprint of HCl production. Furthermore, some generators are built to use renewable energy sources, which enhances their environmental credentials.
Flexibility and Durability
The chemical processing sector is dynamic, with changing manufacturing demands and requirements. Innovative dry hcl gas generation system are built with flexibility and scalability in mind, allowing for easy adaption to changing requirements. Modular designs allow for easy expansion or downsizing, while advanced control systems enable precise and fast modifications to production rates. This versatility means that chemical processing plants can handle variable needs effectively while maintaining quality and safety.
Better Dependability and Service
Unplanned downtime can be costly for chemical processing plants, causing production delays and financial losses. Innovative HCl gas generators prioritize dependability and optimize uptime with strong designs, predictive maintenance tactics, and remote monitoring capabilities. Advanced diagnostics and real-time data analysis allow for preventative maintenance, reducing the danger of unexpected faults and assuring a consistent, constant HCl gas supply.
Conclusion
The impact of innovations in HCl gas generator technology extends beyond the boundaries of individual chemical processing facilities. By enabling safer, more efficient, and environmentally responsible HCl production, these advancements contribute to the overall sustainability and competitiveness of the chemical industry. As technological progress continues, we can expect even more groundbreaking developments in HCl gas generation, further enhancing chemical processing capabilities and driving progress across various sectors.
Source URL: https://medium.com/@gohiljadav95/impact-of-innovations-in-hcl-gas-generator-design-d62b5c3a8ac1
0 notes
Link
0 notes
Text
Juniper Publishers- Open Access Journal of Environmental Sciences & Natural Resources

Investigations of Physico-Chemical Composition of Groundwater in Otuoke and Environs, Bayelsa State, Nigeria
Authored by Abadom DC
Abstract
The study was designed to investigate the groundwater quality in Federal University of Otuoke and environs, Bayelsa State. A total of fourteen groundwater samples were acquired and analyzed for their physico-chemical and heavy metal parameters. Heavy metals were all analyzed with the Atomic Adsorption Spectrometer. The result revealed that for the heavy metals, iron was most predominant, ranging from 0.004 to 10mg/L with mean and SD of 0.95±2.63mg/L. Manganese ranged from 0.01 to 0.91mg/L, with mean and SD of 0.18±0.21mg/L. Copper was below the machine detectable limit in six samples, but ranged from 0.006 to 0.013mg/L, with a mean of 0.01mg/L. All other heavy metal including lead, arsenic, cobalt, boron and barium were below the detectable limit of the machine (< 0.001mg/L).In order of decreasing magnitude, the average cationic concentrations in the groundwater are in the order; Na (101.71mg/L) >Ca (29.04mg/L) > Mg (10.01mg/L) > K (2.31mg/L); and anionic concentrations; Cl (25.88mg/L) > HCO3 (1.65 mg/L) > PO4 (0.42 mg/L) > NO3 (0.08mg/L) > SO4 (0.05mg/L).Assessment of groundwater in the area for drinking purposes revealed that the water is predominantly acidic (pH=6.37), with iron (0.95mg/L) and manganese (maximum =0.91mg/L) contents exceeding regulatory guidelines (WHO, 2011; NSDWQ, 2007) for potable drinking water in most locations. This shows that the groundwater sources are unsafe for consumption purposes. All other measured chemical parameters were within regulatory requirements. Constant monitoring and quality assessment is necessary to ensure that groundwater in the area is within regulatory requirements.
Keywords: Physico- chemical; Groundwater quality; Heavy metals
Abbreviations: GPS: Geographic Positioning System; AAS: Atomic Absorption Spectrophotometer; SD: Standard Deviation; TDS : Total Dissolved Solids; APHA: American Public Health Association
Introduction
Groundwater quality is determined by the solutes, flow paths and soil gases dissolved in the water, as well as the matter suspended in and floating on the water. Hence, groundwater quality is a consequence of the natural physical and chemical state of the water as well as any alteration factors that may have occurred as a consequence of human activity and microbial activities in soils [1]. The quality of groundwater is of vital concern, since it is directly linked with health and human welfare. Ranjana 2010 clearly stated that the quality of public health depends greatly on the quality of groundwater. Groundwater in the preferred source of potable water in the Niger delta, because it is less prone to contamination as a result of its natural filtration [2]. Contamination of groundwater from heavy metals may occur due to factors including irrigation with contaminated water, transportation, industrial emissions, the use of fertilizers and metal based pesticides, etc. [3]. The presence of heavy metal, even at small concentrations in water is an indication of contamination and the persistent consumption of such water could result to adverse health effects.
Although groundwater quality is more preffered when compared to surface water, its quality is the sum of natural and anthropogenic influences [4]. Water quality parameters reflect the level of contamination in water resources and show whether water is suitable for human consumption, irrigation and/or industrial usage. Drinking contaminated water is unacceptable because of its adverse health effects [5]. There are two basic contaminant indicators whose presence or absence helps determines the quality of water in any given area (elemental and microbial). Microbial contaminants in water includes; fungi, pathogenic bacteria and viruses. Elemental contaminants include; physicochemical parameters, metals as well as organic chemical contaminants such as; pesticides and radioactive contaminants Akunobi and Chibuzor 2012. The quantity of water may not be an issue in a terrain such as Niger Delta but its quality is of utmost importance. A substantial part of the study area is motor able and is close to Yenagoa the State capital. This has led to continuous influx of people and increased business activities in the area over the last decade with strong dependence on groundwater. Predominant anthropogenic activities in the area which can pose severe risk to the groundwater resources includes gas flaring from exploitation of oil and gas resources, leakages and corrosion of pipelines, septic tanks and possible effluents from industries, open dumping, etc. Therefore, it becomes obligatory to undertake a groundwater hydro chemical survey in the area to ascertain its quality for drinking, domestic use and other purposes.
Description of the Study Area
The study area Federal University Otuoke and environ is located within the lower section of the upper flood plain deposits of the sub-aerial Niger Delta [6]. Geographically, it lies between latitudes 40 46'N and 50 51'N and longitudes 60 15'E and 60 2 3'E (Figure 1). The area is bounded on the North by Yenagoa, the capital of Bayelsa State and on the south by Brass and Nembe Local Government Areas of Bayelsa State, to the West by southern Ijaw and Ahoada-west local government areas of Bayelsa State and Rivers State respectively. The area can be accessed from the north by the Mbiama-Yenagoa road and on the south by the Nembe and Brass Rivers [7]. Most part of the area is motor-able; hence there is a network of roads that links the different parts of the area.
Brief Geology and Hydrogeology of the Area
The study area lies in the coastal Niger Delta sedimentary basin. The geology of the Niger Delta has been described in details by various authors. The formation of the Delta started during Early Paleocene and resulted mainly from the buildup of fine grained sediments eroded and transported by the River Niger and its tributaries. The Tertiary Niger Delta is a sedimentary structure formed as a complex regressive off-lap sequence of clastic sediments ranging in thickness from 9,000-I2,000m [8]. Starting as separate depocenters, the Niger Delta has coalesced to form a single united system since Miocene. The Niger Delta is a large and ecologically sensitive region, in which various water species including surface and sub-surface water bodies exist in a state of dynamic equilibrium [8]. Stratigraphically, the Niger Delta is sub-divided into Benin, Agbada and Akata Formations in order of increasing age. The Benin Formation is the water bearing zone of the area (Table 1). It is overlain by Quaternary deposits (40-I50m thick) and generally consists of rapidly alternating sequence of sands and silty clays with the latter becoming increasingly more prominent seawards [9]. The clayey intercalations within the Benin formation have given rise to multi-aquifer system in the area [9]. The first aquifer is commonly unconfined while the rest are confined. The study area has been noted to have poor groundwater quality due to objectionable high concentration of certain groundwater parameters and encroachment of saltwater or brackish water into the freshwater aquifers [10-12]. The static water level in the area ranges from 0-2m during the rainy season and I-3m during the dry season [13]. The main source of recharge is through direct precipitation where annual rainfall is as high as 3000mm [14,15]. The water infiltrates through the highly permeable sands of the Benin Formation to recharge the aquifers [16,17]. Groundwater in the area occurs principally under water table conditions [18].
Materials and Methods
Groundwater samples were collected from fourteen boreholes in Federal University of Otuoke and its environs during the rainy season. The boreholes utilized for this study were selected from eight communities at random. Both private and public water sources were sampled in this study. Sterilized water bottles were used to collect representative water samples to prevent contamination. At each borehole location, the sample bottles were washed and rinsed thoroughly with the sample water before being sampled. The samples were collected close to the well head to maintain the water integrity. The boreholes were allowed to flow for about 3 minutes to ensure stable conditions before samples were collected. The bottle was filled to the brim with the sample water, and the lid immediately replaced to minimize oxygen contamination and escape of dissolved gases. Sampling was done using two sets of prelabelled bottles of one litre capacity for ionic and heavy metals analysis respectively Water samples for the determination of cations were stabilized by adding few drops of diluted HCl to them after collection. To maintain the integrity of the water samples, physico-chemical parameters sensitive to environmental changes such as pH, conductivity and temperature were measured and recorded in- situ using portable digital meters. The co-ordinates of all the sampling locations were recorded using a Garmin 78 model Geographic Positioning System (GPS). The samples were later transported to the laboratory in an ice chest for chemical analysis. Table 1 shows the borehole sampling locations along with the geographic cordinates. Heavy metals were determined using an Atomic Absorption Spectrophotometer (AAS) as described in APHA 3111B and ASTM D3651 [19-22]. This involved direct aspiration of the sample into an air/acetylene or nitrous oxide/acetylene flame generated by a hollow cathode lamp at a specific wavelength peculiar only to the metal programmed for analysis. For every metal investigated, standards and blanks were prepared and used for calibration before samples were aspirated. Concentrations at specific absorbance displayed on the data system monitor for printing. The equipment limit of detection is <0.001mg/L. Table 2 shows the equipment and analytical methods used for groundwater samples analysis.
Results and Discussion
Groundwater temperature in the study area ranges from 24.60 to 29.5oC with mean of 27.31±1.12oC and variance of 1.25 (Table 3). Groundwater pH which is a measure of acidity or alkalinity, ranges from 4.78 to 7.01 with mean, standard deviation (SD) and variance of 6.27±0.56 and 0.32. The highest pH values were obtained from BH7 (7.01) and BH8 (6.83) whereas the lowest pH was obtained at BH3 (4.98) (Figure 2); (Table 3). This shows that the water in the area is predominantly acidic. The EC ranges from 53.20 - 130.30 |iS/cm with mean of 94.81±22.12|iS/cm and variance of 489.13. The high standard deviation and variance shows that there is wide degree of variability in the EC of the groundwater resources in the area. Groundwater turbidity ranges from 3.71 - 5.14 NTU, with mean, SD and variance of 4.37±.46 NTU and 0.21 respectively (Tables 3 & 4). Total soluble solids ranged from 4.72 to 13.02mg/L while hardness ranged from 32.0 to 61.0mg/L. Water is said to be hard when it contains large amount of dissolved salts, such as calcium and magnesium ions. Total Dissolved Solids ranges from 4.11 to 92.10mg/L with mean, SD and variance of 36.02±23.30 and 542.92 respectively (Table 4). Alkalinity ranged from 10.34 to 12.01mg/L with mean and SD of 11.19±0.35mg/L
The ionic concentrations in were in the order; Na >Ca> Mg> K, and Cl> PO4> HCO3> SO4> NO3 (Table 4). Schoeller diagram which is a graphical presentation of cations and anions shows that cations predominate over anions in the groundwater (Figure 3). For cations, sodium ranged from 93 to 112mg/L with mean and SD of 101.70±5.0mg/L. Calcium which is the second most dominant cation in groundwater in the area ranged from 14.99 to 55.03mg/L, with mean, SD and variance of 29.04±9.77mg/L and 95.36mg/L respectively. The highest calcium concentration was obtained from BH13 and the lowest was from BH12. Magnesium and potassium ranged from 7.82 to 15.05mg/L and 0.76 to 4.65mg/L, with mean and SD of 10.01±2.14 and 2.31±1.19mg/L respectively (Table 4). Chloride was the most dominant cation in the groundwater, ranging from 14.33 to 47.80mg/L, with mean and SD of 25.88±10.46mg/L. The highest chloride concentration was obtained from BH13 while the lowest was obtained from BH3. Although the concentrations of bicarbonate were relatively low, it was the second most dominant anion in the groundwater, ranging from 0.40 to 4.80mg/L with mean and SD of 1.65±1.15mg/L. Phosphate concentration ranges from 0.01 to 5.01mg/L, with mean and SD of 0.42±1.32mg/L while Nitrate ranged from 0.01 to 0.21mg/L with mean and SD of 0.08±0.07 mg/L. Sulphate had the lowest anionic concentration, ranging from 0.01 to 0.09mg/L with mean and SD of 0.05±0.03mg/L. A map showing the distribution of cations and anions in the study is presented in Figure 4. The map shows sodium is the most predominant cation while chloride is the most predominant anion in the groundwater.
For the heavy metals, iron was most predominant, ranging from 0.004 to 10mg/L with mean and SD of 0.95±2.63mg/L. Manganese ranged from 0.01 to 0.91mg/L, with mean and SD of 0.18±0.21mg/L. Copper was below the machine detectable limit in six samples, but ranged from 0.006 to 0.013mg/L, with a mean of 0.01 mg/L. All other heavy metal including lead, arsenic, cobalt, boron and barium were below the detectable limit of the machine (< 0.001mg/L) (Table 4).
Suitability for Drinking Purposes
The suitability of groundwater for drinking purpose was achieved by comparing the acquired groundwater geochemical results with regulatory guidelines for potable water. The average pH of the groundwater shows that the water is acidic (pH=6.37) and deviates from both WHO (2011) and NSDWQ (2007) guidelines of 6.5-8.5 [23,24], and hence is unfit for drinking. Prolonged consumption of acidic water over long periods of time may result in derangement of the balance of acid to base in the human body, which results in metabolic acidosis [25]. Also, the average concentration of iron (0.95mg/L) exceeds both WHO 2011 and NSDWQ 2007 guidelines of 0.30 mg/L,concentration can kill organisms directly, and while continued exposure over long periods of time to lower concentrations can lead to stunted growth, lower reproduction rates, deformities, and even mortality (Lewis and Clark, 1996). Based on Freeze and Cherry [26] classification scheme, the groundwater samples are classified as soft, with hardness values < 100 in most of the boreholes. Based on total dissolved solids (TDS), Davis, Deweist [27] classify groundwater in the study area as 'desirable for drinking' having TDS values all below 500mg/L. Figure 5 is the cross plot of EC against TDS for the groundwater while Figure 6 is the cross plot of pH against temperature for the groundwater. Figure 7 is the cross plot of Na against Cl for the groundwater in the area [28-31].
Summary and Conclusion
In order of decreasing magnitude, the average cationic concentrations in the groundwater are in the order; Na (101.71mg/L) >Ca (29.04mg/L) > Mg (10.01 mg/L) > K (2.31mg/L); and anionic concentrations; Cl (25.88mg/L) > HCO3 (1.65mg/L) > PO4 (0.42mg/L) > NO3 (0.08mg/L) > SO4 (0.05mg/L). For the heavy metals, iron was most predominant, ranging from 0.004 to 10mg/L with mean and SD of 0.95±2.63 mg/L. Manganese ranged from 0.01 to 0.91mg/L, with mean hence the water is unfit for drinking, unless treated for iron. Manganese is relatively higher than WHO (2011) and NSDWQ (2007) of 0.2mg/L in BH8 (0.91mg/L), and must be treated before consumption. Because of the toxic nature of heavy metals, exposure to a high concentration can kill organisms directly, and while continued exposure over long periods of time to lower concentrations can lead to stunted growth, lower reproduction rates, deformities, and even mortality (Lewis and Clark, 1996). Based on Freeze and Cherry [26] classification scheme, the groundwater samples are classified as soft, with hardness values < 100 in most of the boreholes. Based on total dissolved solids (TDS), Davis, Deweist [27] classify groundwater in the study area as 'desirable for drinking' having TDS values all below 500mg/L. Figure 5 is the cross plot of EC against TDS for the groundwater while Figure 6 is the cross plot of pH against temperature for the groundwater. Figure 7 is the cross plot of Na against Cl for the groundwater in the area [28-31]. and SD of 0.18±0.21 mg/L. Copper was below the machine detectable limit in six samples, but ranged from 0.006 to 0. 013.g/L, with a mean of 0.01mg/L. All other heavy metal including lead, arsenic, cobalt, boron and barium were below the detectable limit of the machine (< 0.001mg/L). Assessment of groundwater in the area for drinking purposes revealed that the water is predominantly acidic (pH = 6.37), with iron (0.95mg/L) and manganese (maximum = 0.91mg/L) contents exceeding regulatory guidelines WHO, 2011; NSDWQ, 2007 for potable drinking water in most locations. This shows that the groundwater sources are unsafe for consumption purposes. All other measured chemical parameters were within regulatory requirements. Constant monitoring and quality assessment on the groundwater is necessary to ensure that groundwater in the area is within regulatory requirements.
To know more about Juniper Publishers please click on: https://juniperpublishers.com/manuscript-guidelines.php
For more articles in Open Access Journal of Environmental Sciences & Natural Resources please click on: https://juniperpublishers.com/ijesnr/index.php
#Juniper Publishers PubMed Indexed Journals#Juniper Publishers Review#Ecological psychology#Molecular Ecology#Oceanography#Geo Morphology
0 notes
Text
Acacia Decurrens (Wild) an Invasive South Africa Tree: Chemical Profile, Antibacterial and Antioxidant Activities-JuniperPublishers
Journal of Chemistry-JuniperPublishers
Abstract
The present study describes the profile, antimicrobial and antioxidant potential of the stem bark of Acacia decurrens. The methanol and hexane fractions had 20% and 0.2% extract yield respectively. The GC-MS result of the hexane, chloroform, and ethyl acetate fractions confirm the presence of 52 compounds and the ICP analysis of the stem bark was found to contain high levels of Co, Zn, Mn, Ca, Ni, Mg, Cr, K and Fe; which is an indication of hyper accumulation capacity. The UV-Visible spectra of showed various peaks which are indication of important radical scavenging Chromophores. Phytochemical screening indicated that the alkaloid (0.6-3.3%) and saponins (5.1-8.6%) contents of the various fractions were significantly lower than the tannin (30.9-55.8 mg TAE/g), steroid (13.92-41.2%), phenol (40.6-65.5 mg GAE/g) and flavonoids (210.2-284.9 mg RUE/g) contents. The disk and well diffusion methods were used to determine the sensitivity and MIC of the fractions. The ethyl acetate and methanol were the most potent antibacterial fractions with 75% and 65% inhibition respectively with MIC of 12.5 μg/ml compared with ampicillin. The antioxidant activity of the fractions was analysed by different methods and revealed good antioxidant potential with different IC50 values of 42.2-49.6 mg/mL for ABTS and 37.8-75.0 μg/ml for DPPH respectively, compared to standard antioxidants.
Keywords: A. decurrens; antioxidant; antibacterial; DPPH; ABTS; ICP-OES; GC-MS, UV/visible
Abbreviations: TC: Tannin Content; TFC: The Total Flavonoids; ZI: Zones Of Inhibition; MHB: Muller-Hinton Broth Medium
Introduction
Acacia decurrens (Willd), commonly known as black wattle or early green wattle is a perennial tree or shrub, in the subfamily Mimosoideae of the pea family Fabaceae. They are present in all terrestrial habitats, including alpine settings, rainforests, woodlands, grasslands, coastal dunes and deserts [1] and due to their prolific nature; they are being classified as invasive in some countries. The Acacia is repeatedly mentioned in the Book of Exodus, perhaps referring to Acacia raddiana, in regards to the construction of the Tabernacle [2]. The edible flowers and seed pods are used to produce dyes, while the trees are grown for firewood, fast-growing windbreak or shelter tree [3] and the edible gum from the trunk of the is used as a low-quality Arabic gum. In South Africa, Acacia decurrens is classified as an invasive species [4], threatening native wildlife [5]. Native wildlife may not have evolved defenses against the invader or they cannot compete with a species that has no predators and these can be exploited for its pharmacological potentials (such as antiproliferative, antimicrobial and antioxidant activities) against native infections.
In this study, a preliminary investigation into the chemical composition, antimicrobial and antioxidant potential of A. decurrens will be conducted, for the first time. Major chronic and degenerative diseases such as atherosclerosis, ischemic heart disease, ageing, diabetes mellitus, cancer, immunosuppression, neurodegenerative diseases are caused by oxidative stress [6] and are one of the most important routes for producing free radicals in foods, drugs and even in living systems [7]. Antioxidative defense mechanisms have proven over time to be the most effective method of combating oxidative stress caused by free radicals. Recently, there has been an upsurge of interest in the pharmacological potentials of plants as antioxidants in reducing oxidative stress-induced tissue injury [8-9]. This research is focused on the generic protocol using a bioassay- guided approach based on straight forward testing of the plant fractions followed by in vitro biological activity testing.
Material And Methods
Chemicals
Ethanol, methanol, hexane, ethyl acetate, chloroform, DMSO, ampicillin, nutrient broth sodium hydrogen carbonate, gallic acid, rutin hydrate, 2,2-dyphenyl-1-picrylhydrazyl (DPPH), 2,2'-azinobis(3-ethylbenzothia-zoline-6-sulfonic acid) (ABTS), potassi⌝um persulfate (di-potassium peroxdisulfate), potassium persul⌝fate, Folin-Ciocalteu's phenol reagent, ascorbic acid, aluminium chloride were obtained from Sigma-Aldrich Chemicals Co., St Louis, MO, USA. All reagents used in this study are of analytical grade.
Equipment
The fresh stem bark of A. decurrens was harvested around October 2016 near Vaal Dam Road, Heidelberg (26.5033°S, 28.4397°E), and South Africa, diced, and dried at an ambient temperature at a relatively low humidity. Authentication of the of A. decurrens stem bark was carried out by the South Africa National Biodiversity Institute, Pretoria, and voucher specimen number: 1200-1, and was deposited at Pretoria National Botanical Garden. The extraction was performed by serial maceration using 4 L of each solvent: hexane, chloroform, ethyl acetate, and methanol with slight agitation at 111 revs/min for seven days. The solvents were removed.
Plant Materials
The fresh stem bark of A. decurrens was harvested around October 2016 near Vaal Dam Road, Heidelberg (26.5033°S, 28.4397°E), and South Africa, diced, and dried at an ambient temperature at a relatively low humidity. Authentication of the of A. decurrens stem bark was carried out by the South Africa National Biodiversity Institute, Pretoria, and voucher specimen number: 1200-1, and was deposited at Pretoria National Botanical Garden. The extraction was performed by serial maceration using 4 L of each solvent: hexane, chloroform, ethyl acetate, and methanol with slight agitation at 111 revs/min for seven days. The solvents were removed.
Extraction and Determination of plant yield
The A. decurrens was diced to facilitate drying at a relative humidity of 55% at ambient temperature and the dried stem barks were pulverized using a hammer mill. The powder was stored in polyethene bags to prevent moisture absorption and contamination. Exactly 2 kg of the powdered stem bark were macerated at ambient temperature in 4 L of hexane, chloroform, ethyl acetate and methanol sequentially for seven days each with slight agitation at 111 revs/min. Afterwards, the extracts were filtered through Whatman filter paper No. 42 (125 mm) then through cotton wool and dried to constant weight in open air.
Qualitative Phytochemical Screening
The Phytochemical screening of the fractions was carried out using standard qualitative procedures [10].
Quantitative Phytochemical Screening
The Total Phenolic Content (TPC) was determined as described by Vuong, Hirun, Roach, Bowyer, Phillips, Scarlett [11], the results were expressed as mg of gallic acid equivalents per g of sample (mg GAE/g). The Total Flavonoids (TFC) was measured as described by Zhishen, Mengcheng, Jianming [12], the results were expressed as mg of rutin equivalents per g of sample (mg RUE/g). The Tannin Content (TC) was determined as described by Rajeev, Pawan, Gagandeep [13], and the results were expressed as mg of tannin equivalents per g of sample (mg TAE/g). The alkaloid content was determined as described by Fazel, Hamidreza, Rouhollah, Mohammadreza [14], saponins content determined by the method of Makkar, Siddhuraju, Becker [15] and the terpenoids content Ferguson [16] and expressed as percentage respectively
Elemental Analysis
Aqua regia digestion (HCl-HNO3): A mixture of concentrated HCl and HNO3, in the ratio of 3:1 is used as the aqua regia mixture. According to Muinde, Nguu, Ogoyi, Shiundu [17], 1.0 g of diced stem bark was digested in 10 ml of aqua regia mixture in digestion tubes for 3 hrs at 60 °C. After cooling the entire digest were filtered and transferred into 50 ml standard volumetric flask with deionized water Reagent blanks were prepared similarly to the samples. All sample solution was clear and diluted 10 times before analysis.
ICP-OES measurement: The measurement was calibrated by the method of external standards with Rh, Re as the internal standard. The reagent blank solution contained 1% of concentrated HNO3. Mixed standard solutions containing 7 elements, Cr, Ni, Zn, Fe, Co, K and Ca were prepared in reagent blank solutions [18].
Spectroscopic Analysis
UV-Visible Spectroscopic Analysis Of The Fractions
The fractions of A. decurrens were analysed in the UV- Visible range between 200-900 nm using PerkinElmer Spectrophotometer and the characteristic peaks were recorded.
GC - MS analysisof the fractions
The analysis was carried out on Clarus 500 PerkinElmer Gas Chromatograph with an Elite -5 (100% Dimethylpolysiloxane) column coupled to a mass spectrometer detector The GC-MS method used in the study involves setting the column at an initial temperature of 110 oC and held for 2 min. After 2 mins the oven temperature has raised the rate of 5 oC/min, to 280 oC, and held for 9 min. The carrier gas (He) flow rate was maintained 1 ml/ min while the injection port temperature was kept at 250 oC. The solutions of the fractions were injected in split mode as 10:1. Mass spectral scan range was set at 45-450 (m/z).
Antimicrobial Assay
Preparation of Inoculum
The test organism Micrococcus luteus (ATCC 26883) Staphylococcus aureus (ATCC 25923), Escherichia coli (NCTC 11954), Salmonella typhi (ATCC 29692), Klebsiella pneumonia (BAA 1706), Shigella sonnei (ATCC 25931), Staphylococcus epidermis (ATCC 12228), Listeria monocytogenes (ATCC(R) BAA- 751TM) and Enterococcus faecalis (ATCC 22735) were obtained from the Department of Biotechnology, Vaal University of Technology, South Africa. Stock culture was maintained at 4°C on slants of nutrient agar. The active stock culture was inoculated in fresh tubes of Muller-Hinton Broth Medium (MHB) and the bacteria were incubated for 24 h at 37°C.
Screening for the Antimicrobial Potential of the fractions
The bacteria cultures were grown in nutrient broth liquid medium at 37 °C. After 24 h of growth, each microorganism, at a concentration of 106 cells/mL, was inoculated on the surface of nutrient broth plates. A 6 mm in diameter disk impregnated with 1000|ig/ml of the fractions was placed on the surface of the inoculated Petrie dish and incubated at 37 °C for 24 h. The Zones Of Inhibition (ZI) were measured after 24 hrs and fractions with ZI more than 7 mm were reported as being sensitive [19]. In this study, the 2% DMSO and Tween 80 were used to dissolve the fractions in the culture media and were used as the negative control. And they showed no inhibitions in this study. These tests were performed in triplicate.
Determination of the Minimum Inhibitory Concentration (MIC)
The fractions with ZI more than 7 mm were subjected to further antimicrobial assay to determine theMinimal Inhibitory Concentration. Serial dilution of the active fractions of concentrations about 12.5-50 μg/ml were prepared and was used to impregnate the disk overnight. The impregnated disk was placed on the inoculated plates and incubated overnight at 37°C. After 24 hrs, the MIC of each fraction and ampicillin sodium salt (positive control) was determined [20]. These tests were performed in triplicate.
Antioxidant Assay
DPPH assay
The antioxidant activity of the fractions was examined on the basis of the scavenging effect on the stable 2,2-diphenyl-1- picrylhydrazyl (DPPH) free radical [21]. Freshly prepared 300 μl of 0.05 mM ethanolic solution of DPPH was added to 40 |il of each fraction with concentrations of 0.02 - 2 mg/ml. To the mixture, 2.7 ml of 96% ethanol was added and vortexed vigorously. The mixture was left incubate for 5mins at ambient temperature and absorbance measured spectrophotometrically at 517 nm. A blank sample was prepared and all determinations were performed in triplicate. The radical scavenging activities of the tested fractions expressed as a percentage of inhibition were calculated according to the following equation [21].
Present (%) Inhibition of DPPH Activity = [(AB-AA) / AB] x 1 00
Where AA and AB are the absorbance values of the test and of the blank, respectively. A percent inhibition versus concentration curve was plotted and the concentration of fractions required for 50 % inhibition was determined and represented as LD50 (μM) for each of the test solutions. All determinations were carried out in triplicate.
ABTS assay
Experiments were performed according to Re, Pellegrini, Proteggente, Pannala, Yang, Rice-⌝Evans [22], with small modifications. ABTS of 7 mM and potassium persul⌝fate of 2.45 mM were prepared in distilled water and these two solutions mixed. The mixture was allowed to stand in the dark at ambient temperature for 16 hrs to generate the ABTS radical (ABTS•+). The ABTS radical solution was diluted with distilled H2O to an absorbance of 1.00 at 734 nm and the fractions of concentrations 0.02 - 2 mg/mL were added to diluted ABTS•+ solution and the absorbance reading was taken after 6 min of incubation at 734 nm. Results are presented as the ability of extract to scavenge 50 % of free radical ABTS•+ (1C50). All determinations were carried out in triplicate. Ascorbic acid was used as a positive control.
Go to
Results And Discussion
Influence Of Solvent On The Recovery Yield Of Secondary Metabolite
The fractions obtained from the four solvent extractions were different in colours and nature (Table 1). The methanol fraction gave the greatest yield (20 %), whereas the hexane fraction had the lowest yield (0.2 %) (Figure 1) and there was a significant difference in extraction yield between the methanol and the other fractions. This implies that most of the secondary metabolite is highly polar. These results are consistent with the previous studies of materials, which reported that extraction solvents significantly affect the recovery yields of a secondary metabolite from the plant (Table 1).
According to some researchers, aqueous, methanol and ethanol have been proven as effective solvents to extract phytochemical compounds from different plants [23]. The differences in the dielectric constants and polarities of the solvents used, result in different extraction yields of the secondary metabolite. These findings further confirm that extraction solvent plays an important role in extractability of secondary metabolite from the materials and each material has a more suitable solvent for extraction of secondary metabolite [24] (Figure 1).
Preliminary Phytochemical Screening
The results provide evidence of the presence of terpenoids, phenols, tannins, flavonoids, saponins, alkaloids in various fractions of A. decurrens stem bark. The major outcome of the present investigation revealed that the samples tested contained high concentrations of health-enhancing phytochemical constituents which are an indication that the plant has a medicinal value other than the tree being grown for firewood, or as a fast-growing windbreak or shelter tree [25] (Table 2).
NA, absent; +, low in abundance; ++, moderate in abundance; +++, high in abundance
The compounds identified in the fractions include steroids and tannins, which are known to mediate cardiotonic (Table 2) and possesses insecticidal, antioxidant, antimicrobial activities [26], phenols and flavonoids which may possess antioxidative, antidiabetic, anticarcinogenic, antimicrobial, antiallergic, antiinflammatory and antimutagenic activities [26-27] Ghani A [28] reported that ethnomedicinally the stem bark is being used for the treatment of diarrhoea and as an astringent.
Quantitative Phytochemical Screening
The alkaloids were present in the hexane and chloroform (0.6 - 3.3) %, steroids in hexane, chloroform, ethyl acetate and methanol (13.92 - 41.2) % and saponins in ethyl acetate and methanol (5.1 - 8.6) % (Figure 2). Alkaloids, saponins, and steroids have not been implicated as potent antioxidantcompounds because of their mechanism of action while phytochemical compounds like phenols, flavonoids, and tannins have been greatly implicated as potent antioxidant compounds [29] (Figure 2).
The total phenolic content of the chloroform, ethyl acetate, and methanol, calculated from the calibration curve (R2 = 0.9903), ranges from 406 ± 1.70 - 655 ± 0.35 mg GAE/g (Figure 3) , total flavonoid content of the ethyl acetate and methanol (R2 = 0.9986) were 210.24 ± 2.20 - 284.92 ± 1.02 mg RUE/g (Figure 4) and total tannin content of the hexane, chloroform, ethyl acetate and methanol of A. decurrens stem bark fractions (R2 = 0.9921) were 30.87 ± 1.73-55.80 ± 2.00 mg TAE/g (Figure 5). Phenolic compounds have redox properties, which allow them to act as antioxidants [30]. There is a significant rise in the amount of tannin and flavonoids extracted as the polarity of the solvent changes from hexane, chloroform, ethyl acetate to methanol because tannins are water soluble polyphenol compounds [31] and flavonoids are composed of a simple skeleton containing two phenol rings connected by a propionic chain [32]. The hypertolerance for heavy metals by the plant is justified by a lot of secondary metabolites with chelating potentials which the lone pairs of an electron can be deposited in the empty d-orbitals, keep the metals in an inactive state (Figures 3-5).
UV-visible absorption of the A. decurrens fractions
The fractions have similar absorption maxima with the hexane and ethyl acetate fractions having an additional absorption maximum in the visible region at 410(0.116) and in the UV region 390(0.648) and 345(0.663) nm respectively. There is a remarkable difference in the absorption pattern of the methanol fraction compared to the other fractions indicating a different structural feature of its compositional compounds, as confirmed in the report of Masayoshi [33], that there is a close relationship between the absorption of the organic compound and its structure. The three absorption maxima of the methanol fraction are all in the UV region, which is an indication of the absence of extensively conjugated compounds. The UV-visible absorption results are in conformity with the qualitative phytochemical screening (Table 2) result, with similar secondary metabolites present.
Elemental composition of A. decurrens stems bark
The concentration of nine metals were analysed using PerkinElmer Elan DRC II inductively coupled plasma mass Altec Mercury Analyzer AMA 254 and the elemental concentrations are presented in Figure 6. The stem bark was found to have high concentrations of heavy metals such as Co, Zn, Mn, Fe, Ni and Cr due to bioaccumulation. This is an indication that the plant has a high phyto- tolerance capacity and can be employed for green remediation [34] (Figure 6). The hyper-accumulation capacity for heavy metals due to A. decurrenshyper-tolerance, or phytol tolerance potential; explains the adaptive evolution of A. decurrens to hostile environments through many generations which have earned its invasive classification in South Africa [4]. The sorption potential is high and will have an adverse effect on native wildlife and vegetation but can be exploited as a source of controlled green-herbicide.
GC-MS analysis of the A. decurrens fractions
The GC-MS analysis of the stem bark clearly stems bark shows the presence of fifty-two major compounds in the hexane, chloroform, and ethyl acetate fractions in which most of the other compounds are presence in less than 20% composition of the fractions. The hexane, chloroform and ethyl acetate fractions contain eight, fourteen thirteen majort compounds respectively with retention time and m/z values presented in Table 3. The hexane and ethyl acetate fractions showed similar absorption maxima in the visible region at 410(0.116) and in the UV region 390(0.648) and 345(0.663) nm respectively due to the presence of possible chromophores on the major compounds in the hexane and f ethyl acetate fractions. There is also a remarkable similarity in the free radical scavenging activities of the hexane, similarity of the secondary metabolites but with different IC50 chloroform, and ethyl acetate fractions due to the structural because of some distinct compounds present (Table 3).
Evaluation of the antimicrobial potential of plant fractions
The data pertaining to the antimicrobial potential of the plant fractions are presented in Table 4 and Table 5. All the fractions from A. decurrens presented antimicrobial activity to at least one of the tested microorganisms with the fractions from ethyl acetate and methanol presenting the highest activities,tested and ampicillin was active against all the organisms expect for E coli and K pneumonia. Such results were not totally unexpected since these bacteria form resistance to penicillin is mediated by penicillinase, rendering the antibiotic ineffective (Table 4).
Among the fractions, the ethyl acetate fraction showed the highest antimicrobial activity comparable to the control. However,no activity against resistant bacteria likes E coli and K pneumonia due to the absence of potent compounds against or due to the presence of resistance genes in plasmids of the organism [35].On the other hand, E faecalis, S aureus, S sonnei, S epidermis and L monocytogenes which are also resistant to different antibiotics,had their growth inhibited by the ethyl acetate and methanol fractions. M luteus was observed to have shown susceptibility all the fractions despite the fact that M luteus survive in oligotrophic environments for extended periods of time [36] (Figure 7), the sensitivity is an indication that the extracts contain potent compound for the treatment of opportunistic infection caused by M luteus such as recurrent bacteremia, septic shock, septic arthritis, endocarditis, meningitis, intracranial suppuration, and cavitating pneumonia in immunosuppresed patients.
The data obtained, through the determination of MIC, shows that the polar fractions of the extract contain potent antibacterial compounds (Table 5). The ethyl acetate and methanol fractions showed anti-bacteria activity towards the same set of tested organism (Table 4) with MIC of 12.5 μg/ml. This observed MIC for the polar fractions is comparable with the MIC of ampicillin, this is an indication that the polar extracts act as an irreversible inhibitor of the enzyme transpeptidase, which is needed by bacteria to make the cell wall just like ampicillin [37] (Figure 7). The two critical stages of bacterial cell wall production in binary fission is usually breached by Ampicillin ultimately leading to cell lysis; therefore, ampicillin, ethyl acetate, and methanol fractions can be referred to as being bacteriolytic [37]. The hexane and chloroform fractions show very low and similar activity MIC with the hexane fraction being more potent against E coli, S typhi and M luteus with MIC of 25 -50 5 μg/ml. The similarity in activity can be justified by the presence of closely related compounds as indicated in the GC-MS result.
Antioxidant Assay
The fractions exhibited moderate radical scavenging activity. The hexane fraction and chloroform fractions showed comparative antioxidant activities and are more potent when compared with the radical scavenging activities of the ethyl acetate and methanol fractions. The radical scavenging potential in the form of their IC50 values revealed that ascorbic acid (IC50 = 31.7 mg/mL) >chloroform fraction (IC50 = 37.8 mg/mL) >ethyl acetate fraction (IC50 = 46 mg/mL) >methanol fraction> (IC50 = 48.6 mg/mL) >hexane fraction (IC50 = 75 mg/mL). Similar results were observed using ABTS analysis, the radical scavenging decreases in the order chloroform fraction (IC50 = 42.2 mg/mL) >methanol fraction (IC50 = 44.6 mg/mL) >ethyl acetate fraction (IC50 = 49.6 mg/mL) > ascorbic acid (IC50 = 54.7 mg/mL) >hexane fraction (IC50 = 54.7 mg/mL (Figure 8).
Ascorbic acid standard acts as a chain breaking the free radical chains [38-39]. The scavenging activity observed for the different fractions is a function of the secondary metabolites present and their hydrogen-donating ability which reduces the blue-green ABTS* and purple DPPH *coloured solutions. The phytochemical screening showed that the chloroform fraction with IC50 3 7 mg/mL against the DPPH* and IC50 42.2 mg/mL against the ABST* is the most potent due to the relatively high phenolic (65.5 mg GAE/g) and tannin (47.5 mg TAE/g) contents, which is in agreement with the work of RE Beyer [38], which reported direct correlation between the phenolic and tannin contents to the antioxidant activity.
The ethyl acetate and methanol fractions were potent antioxidant compounds with relative good IC50 due to the presence of flavonoids, phenols and tannins, while the antioxidants activity of the hexane fraction with IC50 75 mg/mL against the DPPH* and IC50 57.8 mg/mL against the ABST* is the least potent due to the absence principle antioxidant metabolites (Figure 8) [39]. The A. decurrens stem bark extracts showed higher antioxidant activity with DPPH when compared to ABTS, with more potent activity than the positive control (ascorbic acid) in the ABTS assay, Brand-Williams, et al. [40] reported similar slow reaction of most antioxidants which were tested with the ABTS. The phytochemical screening of the chloroform fraction indicates the presence of phenols and GC-MS spectrum with fourteen distinct compounds from the hexane fraction which are most likely phenols. This distinct set of compounds has greatly impacted on the antioxidant activity of the chloroform fraction, as the free radical scavenging ability is facilitated by their hydroxyl groups.
Flavonoids, including flavones, flavonols, and condensed tannins, are plant secondary metabolites present in the ethyl acetate and methanol fractions owe their antioxidant activities to the presence of free OH groups, especially 3-OH hence, the trend of scavenging activity observed in the extracts. Flavonoids have antioxidant activity in vitro and also act as antioxidants in vivo depending onto the presence of free OH groups, especially 3-OH [41,42]. A lot of phenols and flavonoids justify the comparative scavenging potential of the extracts and not necessarily a number of flavonoids alone.
Go to
Conclusion
The findings from this study confirm chloroform as the most effective solvent for extracting potent antioxidant compounds while the ethyl acetate and methanol as the most effective solvent for extracting potent anti-bacteria compounds. The phytochemical constituent presence in the chloroform fraction were sterols, flavonoids, alkaloids, tannins and phenols which are known to exhibit antioxidant activity while the ethyl acetate and methanol were discovered to contain terpenoids, phenols, tannins, flavonoids, saponins, glycosides. The GC-MS of the hexane, chloroform, and ethyl acetate extracts confirms the presence of fifty-two compounds with the methanol fraction excluded due to the poor peak resolution as a result of the large quantity of polar biologically active phytochemical substances.
Plant ethyl acetate and methanol fractions have great potential as antimicrobial compounds against the tested microorganisms with MIC of 12.5 μg/ml. Thus, they can be used in the treatment of infectious diseases caused by resistant microbes. The efficiency of antioxidants in chloroform, ethyl acetate, and methanol fractions, studied using ABTS and DPPH radical scavenging assay, indicated that the IC50 for chloroform and the other fractions is dependent on the quantity of phenolic, tannins and the presence of free OH groups, especially 3-OH compounds present in the flavonoids. The free radical scavenging activity in the chloroform fraction is comparatively stronger than other fractions. This is the first report on the antioxidant activity and the hyperaccumulator capacity for heavy metals by A. decurrens.
To know more about Journal of chemistry,
Click here: https://juniperpublishers.com/omcij/index.php
To know more abour juniper Publishers,
click here: https://juniperpublishers.com/index.php
#Juniper Publishers Indexing Sites List#JuniperPublishers#Juniper Publishers group#Juniper Publishers#Juniper Publisher Reviews#chemistry#organic chemistry#inorganic chemistry#chemistry journal#open access journals#Open access Journal of chemistry#drug
0 notes
Text
Useful Ayurvedic Treatment of Acidity

Acidity is a condition that occurs due to the over production of the acid by gastric glands of the stomach. The stomach naturally generate the gastric or hydrochloric acid (HCL) to digest and break down the food but excess production of this acid is due to trigger factors such as the dehydration, acidic food, and stress. Eating unhealthy and spicy foods or lying down instantaneously after meal causes of acidity. Infection with the bacterium called Helicobacter pylori is also a causes of the acidity in some cases. Symptoms of acidity include irritation in stomach, difficulty in swallowing, throat and the heart problems, abdominal pain, other conditions such as regurgitation, restlessness, nausea, bad breath, indigestion, prolonged sour taste in the mouth, constipation, etc.
Here are some useful Ayurvedic Treatment of Acidity that can assist in curing the symptoms of acidity and controlling the symptoms of acidity.
Mint leaves - This is the best herbal Ayurvedic Treatment of Acidity. Mint leaves are an very effective herbal medicine for acidity. The drug is made of mint extract which assist in drying the stomach acid reflux. Peppermint is an useful natural coolant. Boil some mint leaves and drink this water when it cools down.
Ginger - Ginger is the best herbal treatment for the acidity problem or acid reflux condition as it keeps the pH level of the body balanced. It relaxes the stomach by reducing the acid levels of the stomach. For better results, add some lemon juice to ginger juice and chomp through it once daily for about 1 month.
Cold milk - Cold milk is capable to treating acidity the problem like heartburn and acid reflux. The calcium present in it provides the instant relief from stomach irritation. It prevents the formation of acid in the stomach and also absorbs unnecessary acid. Drink cold milk once daily. This is the best Ayurvedic Treatment of Acidity at your home.
Tulsi or holy basil - Holy basil or Tulsi is the best Ayurvedic Treatment of Acidity. It helps a person suffering from the acidity problem by reducing the effects of gastric acid and excess gas production. Regularly 2-3 tulsi leaves reduces the acidity problem.
Jaggery - Jaggery is the best home treatment for acidity trouble and digestive system. This decreases the acidity in the stomach, making it more alkaline. Make a habit of eating jaggery after the meal.
Aloe Vera - Aloe Vera can also be considered as helpful home remedies for Ayurvedic Treatment of Acidity. Aloe Vera has the anti-inflammatory properties ease abdominal bloating and soothe the digestive system. Aloe Vera juice is consumed to cure heartburn.
Coconut water - Coconut water is a very useful Ayurvedic treatment of acidity. Water helps in normalizing the emission of stomach acid. The high fiber content in this Coconut water prevents the dyspepsia and acid reflux.
Fennel seeds - People often scoff fennel seeds or fennel after dinner, as these seeds are very good for proper digestion and are beneficial to protect the stomach from the acidity problem and any kind. They enclose minerals, vitamins, and fiber that support the digestive procedure.
Cumin - Cumin is the best Ayurvedic Treatment of Acidity. These seeds act as a prevailing acid neutralizer that improves digestion and minimize the abdominal pain. Take this useful remedy once regularly for about 10-15 days.
Amla - Amla is ordinary antioxidant and very effective source of vitamin C. It also improves the anyone immune system. Amla is considered to be best used herb. The cheering effects of this food help in a usual way to treating the acidity. It also cures the damaged stomach lines and the esophagus. To avoid acidity, take a teaspoon of Amla powder regularly. It is best Ayurvedic Treatment of Acidity.
Lemon water - Lemon water is a very high quality home treatment for treating the acidity, and improves digestion. This helps counteract the effects of the acid present in your stomach. Drinking this water before foods will help you promote proper digestion. Lemon water is very effective Ayurvedic Treatment of Acidity.
Buttermilk - Buttermilk is naturally very effectual remedy with food and treating the symptoms of acid reflux. For best results, you can use pepper powder, add salt, and cumin powder to your butter milk.
If you want to know more about Ayurvedic Treatment of Acidity, you can visit our website or you can also call on +919015100300.
Resource:
https://bit.ly/2Cdd0vX
0 notes
Text
Get the best HCL gas generator at Goel Impex. Our dry HCL gas generation systems are designed for efficiency and reliability, making them perfect for industrial applications. Look into our advanced dry HCL gas generator plants and experience superior performance. Whether you need dry HCL gas generation for your processes, our advanced technologies provide the perfect solution. Visit us today at https://goelequipments.com/hcl-gas-generator/ or call 9825318944 to learn more!
#hcl gas generator#dry hcl gas generation systems#dry hcl gas generator plants#dry hcl gas generation plants#dry hcl gas generator#hcl gas generation unit
1 note
·
View note