#iron oxide nanoparticles coprecipitation method
Text
COALS Controls Model for FGD System
COALS Controls Model
In order to develop a COALS Controls Model for FGD system, we first have to understand the power plant in question. Then, we need to understand the emission levels and associated control methods. This will enable us to develop a model that is applicable to a wide range of power plants.
The COALS Controls Model can provide useful guidance to design an efficient FGD system for a WTE plant. By modeling the emissions, COALS can estimate the concentration of a range of trace elements. The simulated data can be used to estimate FGD wastewater concentrations more closely.
The COALS Controls Model simulates the total mass of trace elements entering the FGD system and the mass entering the coal-fired boilers. It then multiplies the total mass of coal by the county-level distribution of trace elements. COALQual data provides the best nationwide coverage.
Wet Circoclean(r)
Wet Circoclean(r) system is designed to remove pollutants from flue gas in WTE plants. It is suitable for plants burning biomass, refuse-derived fuels, domestic waste, or industrial waste. The system uses a circulating fluidised bed to separate acid gases, dioxins, and heavy metals. It also uses a high-velocity process that results in high separation efficiency.
Organosulfides for metals precipitation
Metals can be precipitated in a wastewater treatment plant (WTE) using a variety of chemicals. Typically, lime, dolomite, sodium carbonate, and sodium hydroxide are used. Other chemicals that can precipitate metals include calcium salts and fluoride.
Metals can also be precipitated using a variety of organic compounds, including sodium sulfide, potassium sulfide, or sodium hydrosulfide. These agents are used in continuous or batch processes. They are usually slightly soluble.
DTCs can precipitate various metals by altering their chemical structures. Increasing the number of dithiocarbamate groups in the DTC can improve the efficiency of the process. In a study published by Fu et al., the use of disodium N,N-bis-(dithiocarboxy)piperazine improved heavy metal precipitation in water with a high concentration of recycled sludge.
Researchers found a correlation between copper ion removal and BDP/Cu2+. A 1:1 ratio of BDP to Cu2+ allowed copper to be reduced from 50 to 0.04 mg/L. These results indicated that the precipitation process can be efficient in a wide pH range. In addition, further studies revealed that BDP was effective at removing dyes.
Iron coprecipitation
Iron coprecipitation is a process whereby iron oxide nanoparticles grow in a hydrogel network. These particles perturb the local and gradient magnetic fields and dephase water proton spins. The final density of the iron oxide nanoparticles can be tuned by varying the concentration of iron chlorides.
To model the process accurately, coupled reaction-diffusion equations must be solved. They must account for the diffusive transport and removal of OH-1 ions during the in situ coprecipitation process. The finite capacity of effective sinks complicates the analytic evaluation of the diffusion constant. Once the iron precursor has been converted into iron oxide, it no longer serves as a sink for the OH-1 ions. To address these limitations, numerical models of the process are used.
Another technique that is useful for monitoring the iron coprecipitation process is magnetic resonance imaging (MRI). This technique allows visualization of the growth of iron oxide nanoparticles in the hydrogel network. MRI images reveal a dark contrast due to the growth of iron oxide nanoparticles, which are nucleated by diffusion of precipitating agent into the gel.
0 notes
Text
PROJECT TOPIC- SYNTHESIS AND CHARACTERIZATION OF TIN (IV) OXIDE NANOPARTICLES BY SOL-GEL PROCESS
PROJECT TOPIC- SYNTHESIS AND CHARACTERIZATION OF TIN (IV) OXIDE NANOPARTICLES BY SOL-GEL PROCESS
PROJECT TOPIC- SYNTHESIS AND CHARACTERIZATION OF TIN (IV) OXIDE
NANOPARTICLES BY SOL-GEL PROCESS
CHAPTER ONE
INTRODUCTION
1.1 BACKGROUND OF THE RESEARCH
Miniaturization is a general aim of the technological development that is taking place to produce smaller, faster, lighter and cheaper devices with greater functionality, while using less raw materials and consuming less energy. Research on…
View On WordPress
#cerium oxide nanoparticles applications and prospects in nanomedicine#iron oxide nanoparticles coated with dextran#iron oxide nanoparticles coprecipitation method#iron oxide nanoparticles dextran coating#metal oxide nanoparticles in organic solvents synthesis formation#zinc oxide nanoparticles for selective destruction of tumor#zinc oxide nanoparticles in modern sunscreens
0 notes
Text
Synthesis and Characterization of Nickel Doped Iron Oxide Nano Particles for Biomedical Application _ Crimson Publishers
Synthesis and Characterization of Nickel Doped Iron Oxide Nano Particles for Biomedical Application by Arshad Javid Muhammad in Integrative Journal of Conference Proceedings
Abstract
The objective of this work is to synthesize and characterization of nickel ferrite as MRI contrast agent to improve the signal intensity of T2 weighted images for biomedical application. For structural analysis, XRD was revealed that Ni-doped Fe2O4 have a cubic spinal structure Having Miller Indices (hkl) values of (220), (311), (222), (400), (331), (442), (333), (440) and (531). From XRD data, the grain size of Ni Fe2O4 was observed to be (17.12nm) after 20wt.% Ni-doped Fe2O4, and its further increases up to19.36nm for 40wt.% Ni Fe2O4, respectively. The XRD pattern confirmed that doping of Ni metal increased the grain size of nanoparticles. SEM was performed to study the morphology of prepared samples. EDX was performed to confirm the elemental analysis. EDX spectra depicted the desired peaks of Fe, O, Cl, and Ni for Ni-doped Fe3O4. Saturation magnetization (Ms) was improved with concentration of dopant material Ni (20wt.%, 40wt.% ) in magnetic nanoparticles. In essence, this study demonstrates the very easy way of synthesis of Ni doped iron oxides nanoparticles for biomedical applications as MRI contrast agents
Keywords: Nickel ferrites; Magnetic nanoparticles; MRI contrast agents; T2-Weighted
Introduction
Magnetic nanoparticles were significantly studied for biomedical research such as drug delivery, hyperthermia in cancer, protein separation, biosensing and Magnetic Resonance Imaging (MRI) [1-3]. Since the late 1990s, iron oxide-based nanoparticle contrast agents have been explored and clinically used as T2-weighted contrasts agents. They compose magnetic nanoparticle core and biocompatible coating material, preventing aggregation and sedimentation and allowing high biological tolerance [4]. Recently, researchers have focused on nickel ferrite nanoparticles as MRI contrast agents due to their high magnetic susceptibility, biocompatibility, biodegradability and nontoxicity characteristics [5]. Several studies have investigated the nickel-based nanoparticles as an alternative to gadolinium for reducing the risk of toxicity [6]. Nickel metal also possesses a high spin quantum number and proton exchange kinetics [7]. MRI has several blessings over unique imaging modalities due to excessive spatial selection, amazing clean tissue evaluation and non-utilization of radioisotopes. Paramagnetic gadolinium complexes are commonly used as MRI contrast agent [8]. However, gadolinium-based complexes have low sensitivity and have toxic outcomes that incorporate Nephrogenic Systemic Fibrosis (NSF) [9]. Moreover, most gadolinium complexes are designed to circulate time, precluding excessive decisions and focusing on MRI quickly. The signal intensity is a function of T2 relaxation, i.e., I∼Mo e-t⁄T2 was used to determine the T2 relaxation times. In this research work, nickel dopped iron oxide nanoparticle have been synthesized using co-precipitation method to enhance its sensitivity as T2-W contrast agents.
Experimental
Ferric chloride hexahydrate (FeCl3.H2O), ferrous chloride tetrahydrate (FeCl2.H2O), nickel chloride hexahydrate (NiCl2.H2O) and ammonium hydroxide (NH4OH) were used for the preparation of Fe3O4 and NiFe2O4 superparamagnetic nanoparticles using coprecipitation method. Distilled water was used as a solvent to remove the impurities in the final product. Oleic acid was used as a surfactant [3]. First, the solution of NiCl2.6H2O was prepared in distilled water and stirrered for 1 hour at at 50 ℃ approximately. Then the solution of FeCl2.4H2O was prepared in the distilled water and stirrered for 1 hour at 50 ℃. Then solutions of NiCl2.6H2O and FeCl2.4H2O were mixed with continuous stirring at 70 ℃. Then NaOH was added drop wise upto pH 12. Oleic acids were added in the same solution as a capping agent and surfactant. The precipitation was washed out with distilled water and dried in the oven at 80 ℃ for 6 hours. The synthesized nickel ferrites were grinded into a fine powder. The chemical reaction of the NiFe2O4 has been mentioned as in equation-1
Results and Discussion
X-Rays Powder Diffraction (XRPD)
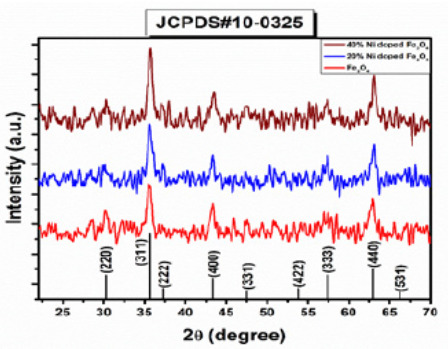
Nidoped ferrites was analyzed with X-ray diffractometer using Cu as a targeted source of X-rays production with Kα_1 radiation having the wavelength of λ=1.54 Å. The powder sample was evaluated in the angle range of 2θ=10110° at the scanning speed of 0.02°/min and step size of 0.031° and step time of 0.3 sec. Powder samples of undoped and Ni-doped ferrites showed crystalline nature as shown in Figure 1. XRD pattern for Ni-doped iron oxide Ni0.2 Fe2.8 O4 and Ni0.4 Fe2.6 O4. was shown in Figure 1. Diffracting peaks of all prepared samples were depicted in Figure 1 at 2θ = 29.94°,35.57°,37.13°,43.32°,47.33°,54.11°,57.21° and 62.95° with miller indices (220), (311), (222), (400), (331), (422), (333) and (440) respectively (Table 1).
The XRD diffraction peaks of the Ni0.2 Fe2.8 O4 (S2), and Ni0.4 Fe2.6 O4 (S3), belongs to the FCC structure, which can be well-matched with (JCPDS) card no (000100325). Diffraction peaks and their sharpness define the degree of crystallinity. There are no other extra secondary phases, suggesting that the ions of Ni2+ are entirely diffused into the Asite which is Fe2+ in Fe3O4. For the calculation of the lattice parameter following relation was used:
SEM analysis
The surface morphology of Nidoped Fe3O4 was studied through SEM model Instrument JSM5910, Japan at 20.0kV. SEM confirmed that particles are spherical in shape and most of them are in flask shape [10]. The density of the particles was also increased with the increase in the concentration of Ni in Fe (Figure 2).
Vibrating Sample Magnetometer (VSM)
Magnetic properties of prepared samples such as saturation magnetization were measured at room temperature using Dexing Magnet Tech Co, Model (VSM100), China. Nidoped Fe2O4 nanoparticles did not depicted hysteresis curve. This saturation magnetization confirmed that all the samples have superparamagnetic behavior in nature. The magnetization curve showed high saturation magnetization and low coercive force. The saturation magnetization was increased from 48.96emu/ cm3 to 126.7emu/cm3. When high concentration of Ni2+ shifts the Fe3+ ions from tetrahedral site to the octahedral site, then the tetrahedral site-to-octahedral site interactions increases, and the octahedral-to-octahedral interactions decreases. The total magnetic moment of the system is increased and therefore the magnetization of the system also increases. It was observed that the coercivity of the system decreases with the increasing content of Ni substitution. When the 20wt.% and 40wt.% nickel is incorporated in ferrite the maximum saturation magnetization was increased up to 115.55emu/cm3 and 126.7emu/cm3 respectively also the remanence was increased to 19.92emu/cm3 and then decrease to 19.50emu/cm3 for 20% Ni dope Ni and 40% Ni doped ferrite, respectively. There is no detectable change observed in coercive field values that are 0.0094 and 0.0095 T for 20% Ni-doped Ni and 40% Ni-doped ferrite, respectively. The Maximum saturation magnetization (Ms), Remanence (Mr), the ration of Mr/Ms, and coercive field values for undoped and Ni doped ferrites was listed in Table 3.
Where Hc represents the coercive field and Ms shows saturation magnetization, while anisotropy constant value K depends upon the concentration of dopant material. It means that the anisotropy constant of the system increases with the increasing content of Ni (Figure 3).
Conclusion
In this study, Nidoped iron oxides nanoparticles were prepared using co-precipitation method at room temperature. The structural conformation was done with XRD which exhibit spinal cubic structure of magnetic nanoparticles. From XRD data, the average grain size of Ni Fe2O4 from 17.12nm to 19.36nm after doping of Ni with 20wt.% and 40wt.%, respectively. The surface morphology of samples revealed that particles depicted the flat surface and have negligible agglomeration in SEM analysis. The saturation magnetization for NiFe2O4 was enhanced 115.55, to 126.7emu/cm3 after Ni doping with 20wt.% and 40wt.% Ni, respectively. Therefore, this study concludes that nickel ferrites may be used in diagnostic modality to see the pathology of the organ as T2-W contrast agents for biomedical applications.
https://crimsonpublishers.com/icp/fulltext/ICP.000549.php
For more open access journals in Crimson Publishers
please click on https://crimsonpublishers.com/
For more articles in open access Integrative Journal of Conference Proceedings
please click on: https://crimsonpublishers.com/icp/
Follow On Publons: https://publons.com/publisher/6342/crimson-publishers
Follow On LinkedIn: https://www.linkedin.com/company/crimsonpublishers
#Crimson Publishers LLC#crimson publishers#Crimson ICP#conference proceedings#Integrative Journal of Conference Proceedings#Open Access Journal in Conference Proceedings
0 notes