#iso 13845
Text
PR 369: MD-QMS ISO 13485:2016 Lead Auditor Course, Applicational pending with CQI-IRCA for Approval
This Lead Auditor course provides the MD QMS Lead Auditor with the confidence, audit tools, and techniques to be able to effectively audit an MD QMS based on ISO 13485:2016 in accordance with the audit protocol of ISO 19011:2018.
0 notes
Text
What is the difference between ISO 9001 certification in Saudi Arabia and ISO 13485 certification in Saudi Arabia ?

An ISO 9001 Certification in Saudi Arabia serves as evidence that your supplier has carried out a systematic method to fine control and manages its operations in a way that ensures comprehension, reputation, and fulfilment of your necessities.
Particularly in Saudi Arabia, a fine control system that contributes to compliance with laws and rules governing services or products which are important to meet the needs of customers and different pertinent events is needed to hold ISO 9001 Certification.
What advantages does Saudi Arabia derive from ISO 9001 certification?
Organisations can structure their work approaches and gain a competitive edge with the assistance of ISO 9001.
The implementation of ISO 9001 can increase a corporation's likelihood of securing business or government contracts.
Enhancing a organisation's worldwide visibility increases the likelihood of achieving better earnings margins.
After cautiously examining the foundational concepts and blessings of ISO 9001 Certification in Saudi Arabia, one can not assist however be intrigued with the aid of the certification technique.
What is the distinction between ISO 13485 and ISO 9001 certifications for Saudi Arabia?
ISO 9001 Certification ISO 13485 Certification is a international popular for best control systems and a country wide requirement in Saudi Arabia. The application of the ISO 9001 preferred ensures that customers are provided with products and services of the utmost fine.
ISO 13485 Certification is ascribed to a high-quality management gadget for medical devices in Saudi Arabia. It was the only obsolete ISO 9001 machine present, placing it other than the relaxation. It is widely held that ISO 13485 will ultimately be built upon the inspiration of Saudi Arabia's ISO 9001 Certification.
The documentation for Saudi Arabian ISO 9001 certification accommodates the following statistics:
One of the numerous similarities among ISO 13845 and ISO 9001 is their shared awareness on product high-quality.
Fifthly, the two collections of structures are diverse inside the following methods:
Specifics regarding the products.
Rules should be adhered to.
Keeping data is an absolute necessity.
Customer contentment.
Constant development.
As part of the ISO 13485 quality management gadget, product monitoring and measurement are required. Consumer health statistics have to not be made to be had to unauthorised 1/3 parties.
Rework have to be documented, and permitted personnel have to be identified according with ISO 13485 standards for concessions. IECTR8345% has extensively greater stringent documentation requirements than ISO9001. Organisations that attain acclaim for scientific gadgets are obligated to combine a risk assessment methodology into their product development techniques.
The documentation referring to medical devices incorporates the subsequent information:
Exhaustive justification.
Specific demands.
Producing a final product.
An software set up is in progress.
The techniques utilised to deliver services.
Traceability is additionally obligatory for documentation and documents concerning purchases. The documentation of the foundation's qualification, in addition to the employed techniques and methods, is vital. The documentation of the product's pure or infection-unfastened repute is required.
Projects require recordkeeping in the course of the verification, installation, and preservation phases. Maintain information for every load of sterilisation.
It is important to preserve statistics of the validation tactics for pc software, sterilisation, and product identity, as well as the traceability of lower back items. The implementation of a traceability procedure need to be documented.
Charts detailing the additives, materials, and operational parameters of clinical gadgets requiring implants, as well as the employees accountable for their inspection, have to be maintained in a steady repository.
How does one gain ISO 9001 and ISO 13485 certificate in Saudi Arabia?
To steady ISO certification, an enterprise have to satisfy the prerequisites of the ISO 9001 control machine, region purchaser pleasure as a pinnacle precedence, and enhance the control device.
In Saudi Arabia, obtaining ISO 9001 practitioner certification ought to take between 3 and 6 months.
In relation to scale, no difference may be made between businesses or industries. Numerous enterprises put into effect the ISO 9001 trendy; certification is needed for best the following:
ISO certification can beautify the credibility of an business enterprise by way of assuring that its services or products will meet or exceed consumer expectancies. Certification is usually mandated through law or, in a few times, decree.
The utility of requirements for ISO 9001 Certification in Saudi Arabia is an essential element of the certification system. Prior to acquiring certification, the agency is required to complete the registrar's audit, which serves as an attestation of its adherence to the conditions above.
While ISO 13845 certification is nice in Saudi Arabia, acquiring 1/3-celebration certification from an accepted organisation might be even more advantageous. In order to accumulate certification, a employer have to satisfy specific criteria.
What factors caused Saudi Arabia granting Factocert ISO compliance certification?
On average, the performance of our Saudi Arabian ISO 9001 Certification experts is superb. Due to the outstanding show of each machine head's call, the corporation can maintain operations in spite of its departure. In a way diagram, moves have not but all started to conclude in this way.
Major cities, such as Riyadh, Jeddah, Dammam, Al Khobar, Dhahran, Buraidah, Al-Ahsa, Qatif, and Jubail, offer green ISO 9001 consulting offerings. In addition to utility education, audit registration, and additional ISO 9001 standards, the business enterprise presents ISO 22000, 17025, and 45001. All ISO policies are followed through the devices, consisting of ISO 14001 and ISO 27001.
The economic system of Saudi Arabia may want to benefit from support for ISO 9001. We are providing you with an approximation of the certification fee at this time.
Visit for more information: ISO 9001 Certification in Saudi Arabia.
Related links:
ISO Certification in Saudi Arabia
ISO 14001 Certification in Saudi Arabia
ISO 27001 Certification in Saudi Arabia
ISO 45001 Certification in Saudi Arabia
ISO 22000 Certification in Saudi Arabia
ISO 13485 Certification in Saudi Arabia
HALAL Certification in Saudi Arabia
CE Mark Certification in Saudi Arabia
ISO 9001 Certification in Saudi Arabia
0 notes
Text
Why does your business need ISO Certification in Canada?

ISO Certification in Canada: ISO is an independent company that develops standards to guarantee your businesses or organizations’ quality, safety, and efficiency. Obtaining ISO certification in Canada allows businesses and organizations to demonstrate effective and efficient products and services. Recently, ISO certification has become easier than ever; it can be achieved through a few clicks on the internet. I want to get certified and go for Online ISO Certification in Canada ISO 9001 certification in Canada
.
ISO Certification and its Benefit
By increasing the ability of your processes, ISO Certification can take your company to the next level.
Your organization will be able to reduce work-related risks and environmental threats.
In the organization, it emphasizes balancing energy use and encouraging cost savings.
As a result, all customer requirements are met and fulfilled.
Organizations are required to maintain environmental, health, and safety measures.
This enables products and services to be delivered effectively and efficiently.
How to acquire ISO Certification in Canada
ISO 9001 Certification in Canada
For any business to succeed, quality is the only secret ingredient. Implement ISO 9001 QMS Certification in Canada to guarantee product and process quality by establishing a quality management system ISO 14001 certification in Canada
.
ISO 14001 Certification in Canada
Sustainable development requires a healthy environment. By obtaining the ISO 14001 EMS Certification in Canada, organizations can implement environmental management systems and ensure a positive environmental impact ISO 45001 certification in Canada
.
ISO 45001 Certification in Canada
When your workforce is safe, your company can achieve great things. Obtain ISO 45001 Certification in Canada and ensure the health and safety of your staff against work-related injuries and illnesses. Ontario, Alberta, and Québec also offer ISO 45001 certification.
ISO 13485 Certification in Canada
In Canada, ISO 13485 Certification provides a framework for enforcing a quality management system for medical devices. From manufacturing to transportation, storage, and retail, it ensures consistency in quality.
ISO 22000 Certification in Canada
Our well-being depends heavily on the quality and safety of our food. The ISO 22000 Certification in Canada is essential for any organization in the food supply chain. Producers, manufacturers, distributors, storage facilities, retailers, and even restaurant owners can use it to ensure their food is safe to consume.
ISO 27001 Certification in Canada
Data security has become increasingly important in the age of digitalization. Data breach or loss has enormous implications for national security and privacy. Businesses can enforce information security management systems and prevent data misuse with ISO 27001 Certification in Canada.
ISO 13845 Certification in Canada
Specifically designed for organizations that offer educational products and services, ISO 21001 is an international standard published by the International Organization for Standardization. In Canada, ISO 21001 certification helps educational providers meet students’ needs. By implementing ISO 21001, educational institutions ensure that students and learners receive the best instruction possible.
ISO 50001 Certification in Canada
Obtaining ISO 50001 certification allows you to sustainably utilize energy, which reduces business risks and improves operations. With ISO 50001 Certification in Canada, organizations can pursue a proficient framework that encourages them to improve their energy management system and its effectiveness continuously.
ISO 22301 Certification in Canada
Many types and sizes of organizations can benefit from ISO 22301, a management system standard for Business Continuity Management. By becoming ISO 22301 Certified, organizations enforce to legislators, regulators, customers, prospective consumers, and other interested parties that they adhere to sound business continuity management practices. Businesses can also demonstrate to stakeholders that they have achieved ISO 22301 Certification in Canada.
Certification by the CE Mark
For products manufactured, designed, and sold in the European Economic Area (EEA), CE stands for conformity with health, safety, and environmental protection. Organizations outside the European Economic Area can adopt and achieve the CE Mark. The CE Mark is not mandatory, but 25 Directives and Regulations (list of products) by the EEA do. CE Mark certification demonstrates reliability, coherence, and efficiency. Additionally, CE Mark certification ensures that your product won’t endanger people.
Apply for ISO Certification in Canada
Factocert is one of the leading ISO certification bodies offering other related services to organizations. Obtain ISO Certification in Canada to demonstrate your organization’s efficiency and effectiveness.
ISO Training in Canada
Learn how to assess ISO standards by enrolling in training courses provided by Factocert. As a result, you can plan and conduct an audit systematically. Factocert is the right option for anyone conducting first-party, second-party, or third-party audits. When designing, implementing, supervising, or auditing ISO Training in Canada, choose SIS certification.
Cost-effective ISO Certification
Factocert’s team of well-qualified auditors will help you reduce your certification costs by negotiating. Your company or organization will be more cost-effective if you obtain your required standards from Factocert within your domestic territory.
Why choose Factocert for Applying for ISO Certification? The only thing you need to know
By using all the right processes, Factocert provides international management system certification services to all organizations regardless of their type, size, and methods. As part of our accreditation process, we are certified by the International Accreditation Forum (IAF), the International Organization for Accreditation Services (IOAS), and the International Accreditation Service (IAS).
By choosing Factocert for your company, you will reap many benefits. Listed below are some of the most significant benefits:
There is no limit to organizations’ types, sizes, or processes that can be certified by Factocert.
As no intermediary is involved, you can receive direct certificates for the required standards if you choose the SIS Cert.
Every client is essential to Factocert, the only certifying body that emphasizes them.
To certify any organization, Factocert uses all the appropriate resources.
Factocert does not follow biases in the certification process, whether the client is a well-established business or not.
We provide the best ISO consultants in Canada, Who are very knowledgeable and provide the best solution. And to know how to get ISO certification in Canada. Kindly reach us at [email protected]. ISO Certification consultants support organizations in complying with ISO standards and help them quickly implement ISO certification in Canada with proper documentation and audit.
For more information, visit ISO Certification in Canada.
Related Article: ISO Consultants in Canada
0 notes
Text
How to acquire different types of ISO certification in Canada?

Introduction
ISO Certification in Canada, ISO is an independent company that develops standards to guarantee your businesses or organizations’ quality, safety, and efficiency. Obtaining ISO certification in Canada allows businesses and organizations to make sure that their products and services are effective and efficient. Recently, ISO certification has become easier than ever; it can be achieved through a few clicks on the internet. I want to get certified and go for Online ISO Certification in Canada.
ISO Certification and its Benefit
By increasing the ability of your processes, ISO Certification can take your company to the next level.
Your organization will be able to reduce work-related risks and environmental threats.
In the organization, it emphasizes balancing energy use and encouraging cost savings.
As a result, all customer requirements are met and fulfilled.
Organizations are required to maintain environmental, health, and safety measures.
This enables products and services to be delivered effectively and efficiently.
How to acquire ISO Certification in Canada
ISO 9001 certification in Canada
For any business to succeed, quality is the only secret ingredient. Implement ISO 9001 QMS Certification in Canada to make sure the quality of your products and processes by establishing a quality management system.
ISO 14001 certification in Canada
Sustainable development requires a healthy environment. By obtaining the ISO 14001 EMS Certification in Canada, organizations can implement environmental management systems and ensure a positive environmental impact.
ISO 27001 certification in Canada
When your workforce is safe, your company can achieve great things. Obtain ISO 45001 Certification in Canada and ensure the health and safety of your staff against work-related injuries and illnesses. Ontario, Alberta, and Québec also offer ISO 45001 certification.
ISO 13485 Certification in Canada
In Canada, ISO 13485 Certification provides a framework for enforcing a quality management system for medical devices. From manufacturing to transportation, storage, and retail, it ensures consistency in quality.
ISO 22000 Certification in Canada
Our well-being depends heavily on the quality and safety of our food. The ISO 22000 Certification in Canada is extremely important for any organization in the food supply chain. Producers, manufacturers, distributors, storage facilities, retailers, and even restaurant owners can use it to ensure their food is safe to consume.
ISO 27001 Certification in Canada
Data security has become increasingly important in the age of digitalization. Data breach or loss has huge implications for national security and privacy. Businesses can enforce information security management systems and prevent data misuse with ISO 27001 Certification in Canada.
ISO 13845 Certification in Canada
Specifically designed for organizations that offer educational products and services, ISO 21001 is an international standard published by the International Organization for Standardization. In Canada, ISO 21001 certification helps educational providers meet students’ needs. By implementing ISO 21001, educational institutions ensure that students and learners receive the best instruction possible.
ISO 50001 Certification in Canada
Obtaining ISO 50001 certification allows you to sustainably utilize energy, which reduces business risks and improves operations. With ISO 50001 Certification in Canada, organizations can pursue a proficient framework that encourages them to improve their energy management system and its effectiveness continuously.
ISO 22301 Certification in Canada
Many types and sizes of organizations can benefit from ISO 22301, a management system standard for Business Continuity Management. By becoming ISO 22301 Certified, organizations enforce to legislators, regulators, customers, prospective consumers, and other interested parties that they adhere to good business continuity management practices. Businesses can also demonstrate to stakeholders that they have achieved ISO 22301 Certification in Canada.
Certification by the CE Mark
For products manufactured, designed, and sold in the European Economic Area (EEA), CE stands for conformity with health, safety, and environmental protection. Organizations outside the European Economic Area can adopt and achieve the CE Mark. The CE Mark is not mandatory, but 25 Directives and Regulations (list of products) by the EEA do. CE Mark certification demonstrates reliability, coherence, and efficiency. Additionally, CE Mark certification ensures that your product won’t endanger people.
Apply for ISO Certification in Canada
Factocert is one of the main ISO certification bodies offering other related services to organizations. Obtain ISO Certification in Canada to demonstrate your organization’s efficiency and effectiveness.
ISO Training in Canada
Learn how to assess ISO standards by enrolling in training courses provided by Factocert. As a result, you can plan and conduct an audit systematically. Factocert is the right option for anyone conducting first-party, second-party, or third-party audits. When planning, implementing, supervising, or auditing ISO Training in Canada, choose SIS certification.
Cost-effective ISO Certification
Factocert’s team of well-qualified auditors will help you reduce your certification costs by negotiating. Your company or organization will be more cost-effective if you obtain your required standards from Factocert within your domestic territory.
Why choose Factocert for Applying for ISO Certification: The only thing you need to know
By using all the right processes, Factocert provides international management system certification services to all organizations regardless of their type, size, and processes. As part of our accreditation process, we are certified by the International Accreditation Forum (IAF), the International Organization for Accreditation Services (IOAS), and the International Accreditation Service (IAS).
By choosing Factocert for your company, you will reap many benefits. Listed below are some of the most significant benefits:
There is no limit to organizations’ types, sizes, or processes that can be certified by Factocert.
As no middleman is involved, you can receive direct certificates for the required standards if you choose SIS Cert.
Every client is important to Factocert, the only certifying body that emphasizes them.
To certify any organization, Factocert uses all the appropriate resources.
Factocert does not follow biases in the certification process, whether the client is a well-established business or not.
We provide the best ISO consultants in Canada, Who are very knowledgeable and provide you with the best solution. And to know how to get ISO certification in Canada?Kindly reach us at [email protected] Certification consultants follow the guidelines set by the international organization for standardization and help the organization to implement ISO certification in Canada in an easy way with proper documentation and audit.
For more Information visit: ISO Certification in Canada
0 notes
Photo

#IEC #62304 #ISO #13845 #Functional #Safety #Medical #Devices #Medical #Software #Development #Lifecycle #Quality #Management https://www.instagram.com/p/CbvW2MfK63f/?utm_medium=tumblr
#iec#62304#iso#13845#functional#safety#medical#devices#software#development#lifecycle#quality#management
0 notes
Text
Jal Medical Singapore- The Emerging Leader for Biomedical Solutions
Jal Medical, with a truly integrated biomedical manufacturing facility, has a wide array of advanced rapid in-vitro immunodiagnostic test systems and electrical bio sensing systems. In this fast moving world, many individuals do not find the necessary time to get tested for the various diseases that they are or might be suffering from. Gone are the days when people used to visit testing laboratories as a precautionary measure. Jal Medical Singapore comes with a solution for this problem by providing various rapid testing kits through which, people can perform tests at their respective homes. Additionally, these test kits also provide results within minutes and thus saves the individuals from the trouble of visiting laboratories again to get their test results.
We, at Jal Medical, envision to provide safe, reliable and cost effective solutions in the market, globally. Our offerings include Blood glucose meters, STD rapid screen tests, DOA rapid screen tests, Infectious diseases rapid screen tests, Fertility rapid screen tests, and ABO & RhD blood grouping test card. The FDA, ISO 13845 and GMP certification proves the company’s competence to provide efficient and reliable products.

Products Offered at Jal Medical
Blood Glucose Meters
A blood glucose meter is a medical device that is used for determining the glucose level in the blood. It is mostly preferred by the people who are diagnosed with diabetes, as they have to regularly keep a check on their blood sugar & glucose level. The glucometer draws the blood from by piercing the skin and then tests the blood for the sugar and glucose level. Usually, the most preferred site for piercing in order to draw blood is the finger. The blood glucose monitoring aids in keeping a track of continuous variation in glucose level. It thus helps in proper planning of meals, activities, medication, etc.
Jail Medical supplies kits for glucose test that generates quick and reliable result. It has a large display that makes it a user-friendly device. The blood glucose monitor produces result in 6 seconds with a very small blood sample of 0.7uL. The fact that the device has a warranty of 5 years makes it a durable equipment to have.
youtube
STD Rapid Screen Test Kits
STDs, or sexually transmitted diseases, are one of the dangerous diseases that have the potency to claim lives. The STDs have the tendency to go down unnoticed and after a substantial amount of time, it becomes very difficult to treat it. Jal Medical offers a solution to this problem by supplying STD rapid screen test kits that help in detecting the problem early. People feel shy to undergo tests for STDs at medical centers. Even if they do, these people are not able to gather the courage to collect the result.
1 note
·
View note
Text
Why is Quality Assurance needed for Medical Devices?

Medical devices have become the backbone of the healthcare industry as they help healthcare professionals diagnose conditions or ailments and treat patients. These devices have reduced the probability of ‘assumption or chance’ in diagnosis and added a modicum of certainty. Just think of a doctor making a correct diagnosis of a patient’s illness after thoughtfully studying the MRI scan report instead of merely going by the symptoms. Also, medical devices have greatly facilitated the functioning of telemedicine during the pandemic and allowed healthcare professionals to treat patients virtually.
This way, many patients were spared the ordeal of visiting overcrowded hospitals or healthcare facilities and contracting the virus. And to ensure the effectiveness of such devices and meet the rigorous regulatory standards, manufacturers are implementing quality assurance. According to markets and markets, the global medical device software testing market is likely to touch $11.8 billion by 2025, at a CAGR of 4.8 percent. The driving factors for growth in testing medical devices are:
Harmonization of regulatory and quality standards
Growing usage of medical devices across the world
Growing need for verification and validation of medical devices
Application of stringent government and industry regulations
Outsourcing QA medical devices to a medical device testing company
Since medical devices have a direct impact on the lives of patients, they need to be tested rigorously on all parameters, including adhering to regulatory standards such as ISO 13845:2016. Medical devices bring with them a high level of responsibility and liability on the part of the manufacturers. As the technology and processes have become highly complex, the quality of medical equipment testing services needs the implementation of an automated quality management system.
What are QA medical devices all about?
Given the critical role of medical devices in the healthcare domain, they are highly regulated by a multitude of regulatory bodies. Further, they need to meet a set of standardizations and compliances and deliver accurate readings, effective performances, and safety. For instance, a CT scan should not emit harmful X-ray radiation beyond the safety limits, and the probes comprising electrodes for EEG should be safe to record the electrical impulses of the brain. To ensure these, there should be a proper strategy for medical devices validation in place. Medical device software testing ensures the device’s software and systems are fully compliant and compatible with various regulatory frameworks.
What is the importance of testing medical devices?
The benefits of applying QA to ensure medical devices validation are as follows:
Upholds quality compliance with various regulatory frameworks and standardizations including HIPAA, FDA, and PCI DSS, among others.
Ensures various components of medical devices, such as CRMs, software, and database management systems, among many others, function seamlessly under different scenarios.
Proper connectivity of medical devices with third-party tools can be verified and validated using integration testing.
Helps to enhance the clinical efficiency and effectiveness of medical devices.
Ensures superior patient experiences and optimal patient care.
Eliminating or mitigating any risk of failure by implementing end-to-end medical device software validation.
Identifies and fixes glitches that can otherwise lead to false diagnoses.
Reduces market time for medical devices.
Elimination of wasteful and non-value-added activities.
How to mitigate the future risks associated with medical device software testing?
It is not only about mitigating the present risks but also anticipating and securing the devices against potential risks in the future.
Cybersecurity concerns: With the integration of medical devices into cloud platforms, there is an increased risk of cybersecurity concerns. For instance, threat actors can break into medical devices and cause them to malfunction or give erroneous results. To avoid such situations, the FDA is coming up with a pre-market regulation wherein manufacturers of such devices will have to incorporate stringent data safety measures. Besides, manufacturers will be required to submit a ‘software bill of material’ to validate product information for customers.
Promoting innovation: With smart technologies on the anvil, medical devices need to incorporate them to improve efficiency, usability, accuracy, and safety. These technologies have the vast potential to be deployed across the healthcare ecosystem. The new-gen technologies can facilitate device upgrades and automation with respect to factors such as accessibility, operation, and safety. However, not everything is hunky-dory with these next-gen technologies, for there can be complications as well. These relate to the sharing of data in the cloud and the integration of apps with medical devices. Remember, the effectiveness and sustainability of medical devices are directly proportional to the integrity of the data they offer. This is where the FDA is contemplating ways to build streamlined pathways to mitigate risks and enhance the quality of medical devices in the future.
Conclusion
Quality assurance in medical devices testing verifies whether the procedures and processes are compliant with regulatory standards such as ISO 13485:2016, MDSAP, or USFDA. It verifies facets such as medical device design, supplier management, risk management, clinical data, product labeling, complaint handling, and storage and distribution, among others.
Resource
James Daniel is a software Tech enthusiastic & works at Cigniti Technologies. I'm having a great understanding of today's software testing quality that yields strong results and always happy to create valuable content & share thoughts.
Article Source: medium.com
0 notes
Text
Behind the Scenes with Bonpara
Behind the Scenes with Bonpara @Bonnou_Paradox #homicidols
This is super neat, you guys! Bonnou Paradox, the idols making the level best effort to directly replace Guso Drop in the chikasphere, released this pretty comprehensive mini-documentary from the making of their “NO=LIMIT” video and accompanying time: You know what’s great about dual monitors and private work spaces? Being able to let this play with impunity, seeing how the sausage gets made,…
View On WordPress
8 notes
·
View notes
Text
What is ISO 13485, and what are the Key benefits of the ISO 13485 Certification?
ISO 13485 is an internationally recognized quality standard that states the wants of the standard Management System (QMS) for the planning and manufacture of Medical Devices throughout the globe. ISO 13845 is helpful for several organizations and may be employed by suppliers and external parties that are involved with providing medical device products. Requirements of ISO 13485 apply to organizations despite their size and despite their sort except were explicitly expressed. where needs are such as applying to medical devices, they apply equally to associated services as provided by the organization. the wants could vary based mostly upon the category of medical device – from a chair to a pacemaker.
The processes needed by ISO 13485 Registration in Saudi Arabia apply to the organization, however, aren't performed by the organization, are the responsibility of the organization, and are accounted for within the organization’s quality management system by observation, maintaining, and dominant the processes. If applicable regulative needs allow exclusions of style and development controls, this will be used as a justification for his or her exclusion from the standard management system. These regulative needs will offer various approaches that are to be addressed within the quality management system. it's the responsibility of the organization to make sure that claims of conformity to ISO 13485: replicate any exclusion of style and development controls.
Key benefits of the ISO 13485 Certification:
As the most recognized standard for quality management systems within the medical device trade worldwide, organizations that have achieved ISO 13485 certification will demonstrate to potential customers that they follow best practices and guarantee quality. what is more, when certification associate degree organization can usually be considered competent below their scope. These edges and additional ought to quickly recoup the outlay required to get and maintain certification.
The advantages of ISO 13485 Certification in Dubai aren't exaggerated. Organizations of all sizes have acknowledged however helpful certification has been, in addition to different benefits that a product quality management system unremarkably includes. we'll additionally make a case for six edges that ISO 13485 will bring for your organization:

1. Improves your Organization’s credibility: Certification to the ISO 13485 customary establishes you as a high-quality organization within the medical device trade. Certification proves to customers that your organization ensures quality, and you'll have an enforced system and certification to prove it. a high-quality Management System or QMS is often an improbably effective selling tool, and it's become a requirement for several countries because it promises quality. Therefore, ISO 13485 certification provides a larger chance for additional business opportunities.
2. Improves client Loyalty: The ISO 13485 customary is predicated on the standard management principles, one in every of that is client focus. this will be accomplished by observing client desires and coming up with and ensuring their desires are met. Customers expect quality and cannot as simply have interaction with a provider that may not be certified. to boot, satisfying your customer’s desires ensures there's a larger chance for retention, and it permits you to supply different products or services to new purchasers. this may translate to larger financial gain.
3. Improves your Procedures: By ISO 13485 Services in Bahrain utilizing the procedural approach counseled within the ISO 13485 customary, quality is created easier and more consistently. you may enjoy fewer mistakes, potency enhancements, and maybe encourage investment interest in your organization.
4. Evidence-based decision making: Another quality principle of ISO 13485 is that the employment of evidence-based deciding. after you implement evidence-based deciding, your decisions are going to be higher aligned with the objectives of your organization. Another profit may be a higher understanding of the effectiveness or deficiencies of your systems and therefore the effectiveness of enhancements created to your systems.
5. A Culture of Continuous Improvement: An additional profit that comes from ISO 13485 certification is that the plan of continuous improvement. after you are certified, senior management and workers are going to be needed to seem out for opportunities to boost your systems, product, and services. By establishing effective procedures, you scale back problems and avoid additional stress on your organization. there'll be less energy and time invested in breakdown mistakes, supplying you with enlarged time to produce quality merchandise or services.
6. Higher worker Commitment: When workers are asked to look for methods to boost procedures, they regularly offer the most effective recommendation, as they need additional personal expertise with the procedures. attributable to this, they're going to expertise additional job satisfaction and increase their commitment to the organization. The additional your worker's expertise quality in their job capacities and business processes, the additional connection there, that prompts enlarged potency and profit.
How to get ISO 13485 Consulting services in Bangalore?
If you are wondering how to get ISO 13485 Consultants in Bangalore, never give it a second thought approaching Certvalue with a 100% track record of success without any fail in the certification process. ISO 13485 in Bangalore are easy and simple with Certvalue. You can easily reach Certvalue by simply visiting www.certvalue.com where you can chat with an expert or you can also write an enquiry to [email protected] so that one of our experts shall contact you at the earliest to provide the best possible solution available in the market.
0 notes
Text
ISO 9001 & ISO 13485

ISO 13485 for medical device quality control having several similar nature with ISO 9001.
Today, we are looking at the differences and similarities between these two criteria, and if life sciences firms and associated services require both certificates. ISO 13485 is a quality system for the medical device industry and it effectively covers ISO 9001 with some additional requirements.
It is that comparing ISO 9001 and ISO 13485 is a valuable exercise. By understanding the differences between these two standards, you have to learn where device manufacturers need to raise the bar on quality.
Similarities between ISO 9001 and ISO 13485
ISO 9001 & ISO 13485 both are basically about precisely the exact same things- helping in businesses to create always safe, higher quality solutions.
Risk- reduction: These two criteria highlights the need for associations to integrate risk into production and design.
Plan-Do-Check-Act: They utilize the Plan-Do-Check-Act procedure strategy regardless of how the two criteria don’t share exactly the same structure.
Client focus: ISO 9001 and ISO 13485 are constructed around ensuring customer expectations are met.
QMS needs: Organizations will need effective procedures and resources such as document control, Employee training, Audits and Corrective Action.
ISO 13485 builds on the requirement of ISO 9001 by addressing the responsibility of the manufacturer for “maintaining the quality management system.”
Include regulatory document with system documentation.
The QMS includes a file for identifying product-related documents.
A master record device must specifically define QMS requirement.
Changes to QMS documentation must be reviewed and approved by the original approving functions.
Manufacturer is required to analyse data standards based on product and its requirements.
Differences between ISO 9001 and ISO 13845:
In a lot of ways they are alike, but there are important ways that they’re different.
Aims and results: ISO 9001 requirements are skewed heavily towards ensuring client focus and satisfaction. ISO 13485 puts emphasis on medical devices for safety.
Continuous focus: ISO 9001 currently requires producer to reveal constant improvement and on the other side, ISO 13485 just needs demonstrating effective implementation and maintenance of the grade system.
Documentation: For ISO 13485’s documentation demands are a lot more extensive than people at ISO 9001.
Regulatory compliance: ISO 13485 is closely connected to regulatory demands, particularly towards criticism handling, regulatory alarms, and post-market surveillance.
What are the additional requirements in ISO 13485-:
Quality Management System
Effectiveness of the QMS.
QMS documentation changes must be evaluated for their impact.
Control and management of outsourced processes.
Documented procedure for software validation.
Documented procedure for software validation.
An organization should maintain each medical device file.
Management Responsibility
Ensure effectiveness of the QMS by the management commitment.
Promote awareness of regulatory requirements.
Management review for revised regulatory requirements.
Resource Management
Requirements for work environment, including cleanliness of clothing, temporary work conditions, and product control.
Product Realization
Risk management procedures are associated with product realization planning.
Advisory notices added to customer communication center.
Development and Design procedures need to be documented
Measurement, Analysis and Improvement
The documented procedure required for a feedback system.
Additional requirements of inspection of product.
Feedback included in analysis of data.
Including update of documents in the taken actions.
Including investigation records.
For, ISO 9001 and ISO 13485 certification you can easily reach to one of the best ISO consultant for training, consultant, development, and certification provider, i.e. FINECERT.
Our presence is globally and waiting to serve you for your business to get ISO certified under standards. We will help you in every step until your business get certified. ISO 9001 certification in Saudi Arabia, Oman, Nigeria, Qatar, South Africa; ISO 13485 consultant in Saudi Arabia, Oman. Qatar, South Africa, Nigeria. For more ISO certification inquiries, you can write to us at [email protected] or visit us at www.finecert.com.
0 notes
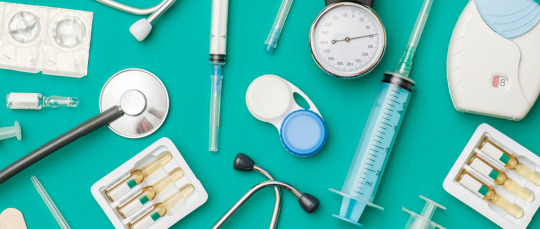
Photo

ISO 13485 Certification-consultant |operon strategist
operon strategist consultant for ISO 13845. It is beneficial for many organisations, and can be used by suppliers and external parties that are involved with providing medical device products.
Contact details –
Phone no - 9325283428
Mail - [email protected]
Visit - https://www.operonstrategist.com/iso-13485-medical-device-certification-consultant/?utm_source=image%20submission&utm_medium=image%20page&utm_campaign=image%20submission
0 notes
Text
You Can't Buy Happiness But Can Marry A Haitian shirt
You Can’t Buy Happiness But Can Marry A Haitian shirt
Creepy to the core. I think you epitomized a creepy, stalker psychopath perfectly. Devoid of logic, emotions, and even good sense. The fact that he chalked you out reveals that he probably intended to meet out the same treatment to you as he did to the Hispanic family. However, why would he document his crime and let you control evidence You Can’t Buy Happiness But Can Marry A Haitian shirtcould…
View On WordPress
0 notes
Text
Difference between ISO 13485 and ISO 9001 Certification

ISO 13485 Certification is quality management for medical devices shares many similarities and differences with ISO 9001 Certification, the leading quality management standards developed by the International Organization for standardization.
While most revised standard follows ISO 9001’s new high-level structure Annex SL Structure, but ISO 13485 does not follow HLS, even though it was released after ISO 9001 Certification standard.
Today we’re viewing some similarities between these two ISO standards, and few areas major in which ISO 13485 and ISO 9001 get differentiated from one another.
What Is ISO 13485?
An ISO standard that has been specifically designed and published for the safety and quality assurance of medical devices and related industries. This has successfully implemented the rock-solid instrument to all the manufacturers or producers of Medical Device industries for achieving reliability, trust, and unbreakable commitment from their end-user and customers to human safety and the quality of medical devices being manufactured and their acceptance worldwide.
What ISO 9001 Implies?
ISO 9001 is one of the international standards too developed and published by the International Organization for Standardization that has vividly specified the guidelines to establish, maintain, monitor, and continual improvement in a Quality Management System (QMS) of an organization.
Also, Check ----> ISO 13485 Certification in Sri Lanka
Similarities Between ISO 9001 and ISO 13485
When you get down to brass tracks, ISO 9001 Certification and ISO 13485 Certification are both essentially and identically similar. These both standards act as a helping hand to companies for creating consistently safe, high-quality products for the consumer and end-user. ISO 9001 also shares other similarities with ISO 13485, such as:
Risk identification and risk mitigation: More so than previous versions, both these ISO standards highlight the need for organizations to incorporate risk involved in design and production and mitigate earlier.
Plan-Do-Check-Act: Even though the two standards do not share the same structure, but they both use the Plan-Do-Check-Act process approach system for implementation.
Customer-oriented: Both ISO 9001 and ISO 13485 are built around ensuring customer satisfaction and meet their requirements and expectations.
QMS requirements: To comply with either standard, organizations will require effective processes, maintain quality and enhance quality and tools for Documents, Employee Training, Internal Audits, and Corrective Action.
Differences Between ISO 9001 and ISO 13845
Even though they are similar in various terms, it’s important to note that there are significant ways in which these two are different.
Aims and outcomes: ISO 9001 Certification requirements are skewed heavily towards ensuring customer needs and satisfaction of their requirements, while ISO 13485 Certification puts more emphasis on the safety and efficacy of medical devices with the concern of public health.
Continuous improvement focus: ISO 9001 now requires manufacturers to show continuous improvement in the management system. ISO 13485, on the other hand, only requires demonstrating effective implementation and maintenance of the quality system by improving the devices as per advanced technology.
Documentation: ISO 13485’s documentation requirements are much more extensive, as they relate to public health and safety than those in ISO 9001.
Risk management: Organizations certifying to the ISO 13485 medical device standard will also need to incorporate risk management principles into product realization and post-market feedback after the usage.
Regulatory compliance: ISO 13485 is closely linked to regulatory requirements, especially concerning complaint handling systems due to defaults and errors in the devices, regulatory notifications, and post-market surveillance.
Do You Need Both Certifications for your organization?
Situations where you might consider certifying to both certificates just when medical devices represent just part of your business. For ex. organizations providing medically related services such as contract manufacturers, suppliers or distributors might need to certify to ISO 13845 Certification as well as ISO 9001 compliance.
0 notes
Text
Turkey: there were mysterious huge dips in the ground
Turkey: there were mysterious huge dips in the ground
US, WASHINGTON (ORDO NEWS) — In Konya silt in the center of Turkey, about 300 major soil failures formed. Giant pits dotted the plain and became a local attraction.
Dips in the soil appeared in the form of cones and deep craters, which look like foundation pits and traces of a projectile or meteorite.
Scientists believe that such a large number of dips are caused by natural geological processes…
View On WordPress
0 notes
Text
$29.99 ITG.digital Online Illustrations Builder Pro Lifetime Subscription
$29.99 ITG.digital Online Illustrations Builder Pro Lifetime Subscription
The provider offers you the ITG.digital Online Illustrations Builder Pro: Lifetime Subscription for only $29.99. 677+ Upvotes on Product Hunt! Customize Thousands of Illustrations to Your Preferred Elements, Style & Colors with Zero Design Knowledge.
Details
Length of access: lifetime
This plan is only available to new users
Redemption deadline: redeem your code within 30 days of purchase
Max…
View On WordPress
0 notes
Link
If you are looking for a reliable ISO accreditation agency in Australia then GCC is a perfect choice for complete process. We have many types of ISO certificate services like ISO 9001 QMC, ISO 14001 Environment Management, ISO 13845 Medical Devices, AS/NZS 4801-OHSMS, OHSAS 18001-OHSMS etc.
0 notes