#peptide API
Explore tagged Tumblr posts
Text
API Peptide – High-Quality Active Pharmaceutical Ingredients
Discover premium API peptides for pharmaceutical and research applications. Ensure high purity, stability, and efficacy with top-rated peptide APIs from trusted suppliers. Find reliable sources for drug development and biotechnology advancements today!
0 notes
Text
Superior Small Molecules and API Peptides
Discover Asymchem's superior peptides and small molecules solutions. We specialize in API peptides and advanced intermediates for pharmaceutical innovation.
0 notes
Text
Is Retatrutide Peptide Legal? Regulatory Landscape for Clinical Use and Research
Target Keywords: GMP Retatrutide, Clinical Retatrutide Regulation, Retatrutide peptide legal status, research peptides FDA compliance Internal Link: 👉 Trusted Retatrutide Supplier with GMP Certification
Introduction: Why Regulatory Clarity Matters for Retatrutide Peptide
Retatrutide, a promising triple agonist peptide under clinical development, has gained global attention for its dramatic impact on obesity and metabolic disease. But before clinics, CROs, or pharmaceutical developers decide to purchase or administer it, a crucial question remains:
Is Retatrutide legal for research or clinical use in the U.S., EU, and other key regions?
In this article, we’ll break down the regulatory status of Retatrutide Peptide across major jurisdictions, and offer clear guidelines for sourcing and handling this compound within a compliant framework.
1. Retatrutide’s Current Development Status
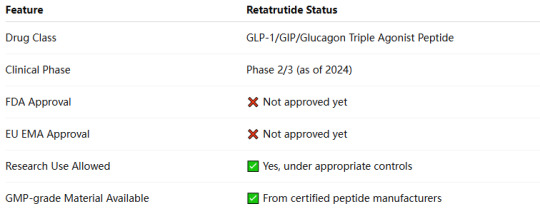
Although Retatrutide is not FDA- or EMA-approved for human therapeutic use, it can be legally used in non-human research and clinical trials under specific regulations.
2. Understanding Regulatory Terms: GMP, API, RUO

Procurement teams must ensure the Retatrutide they acquire:
Comes from a GMP-compliant source
Is labeled appropriately as RUO
Accompanied by COA, MSDS, and batch records
3. Is Retatrutide FDA-Compliant for Clinical Supply?
🧪 U.S. Regulatory Snapshot (FDA)
❌ Not approved for prescription or therapeutic use
✅ Allowed for use in:
Pre-IND studies
Clinical trials with IND exemption
Animal model research
Must be labeled: “For research use only. Not for human consumption.”
🇪🇺 EU EMA Regulatory Snapshot
❌ No marketing authorization granted
✅ Research material accepted under:
Clinical trial applications (CTAs)
Laboratory and formulation development
Requires adherence to EU GMP Directives (EudraLex Vol. 4)
🌏 Other Regions
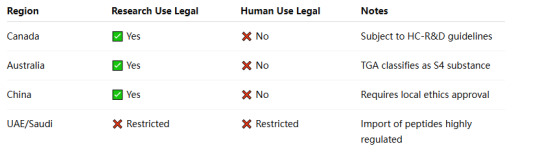
4. How to Stay Compliant When Sourcing Retatrutide
To avoid legal or safety issues, here’s a checklist for compliant Retatrutide procurement:
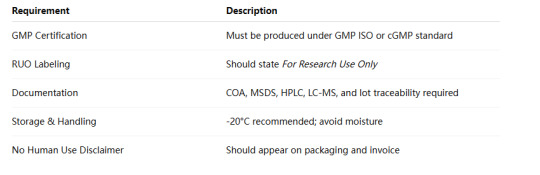
📌 Pro Tip: Always choose a peptide supplier who discloses GMP status, regulatory documentation, and origin of synthesis.
🔗 Explore GMP Retatrutide Peptide Options
5. Legal vs. Practical Use: What Clinics & Labs Must Know
Even though Retatrutide is not yet approved, many legitimate clinics and research labs are:
Using it in IRB-approved studies
Collaborating on pilot safety trials
Exploring combination therapy in pre-market formulations
🚫 However, selling or prescribing it for weight loss treatment outside of trials is prohibited in the U.S., EU, and most markets.
6. Final Recommendations for Labs, CROs, and Clinical Buyers
✅ Do:
Work with a GMP-certified Retatrutide Supplier
Keep clear documentation on file for import audits
Ensure staff is trained on handling, documentation, and labeling
❌ Don’t:
Use Retatrutide in human trials without IND or ethics board approval
Re-label research-grade product for off-label sale
Ignore local customs/import peptide restrictions
🔍 Summary Table: Legal Status of Retatrutide by Region
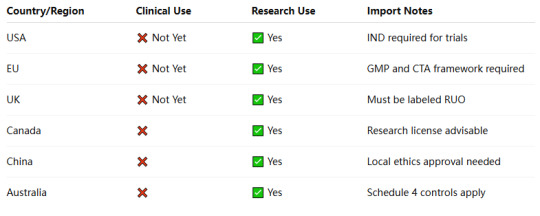
📌 Conclusion
Retatrutide is a cutting-edge metabolic peptide, but its legal use is strictly limited to preclinical and clinical trial contexts. It’s crucial to source GMP Retatrutide from trusted peptide suppliers and comply with regional regulations.
Whether you're a procurement lead at a CRO, clinical coordinator, or pharmaceutical buyer, understanding the legal status of Retatrutide Peptide protects your team—and your research integrity.
👉 Looking for a compliant Retatrutide Supplier? Explore GMP-certified options here: 🔗 https://retatrutidesupplier.com/retatrutide
📌 Conclusion
Retatrutide is a cutting-edge metabolic peptide, but its legal use is strictly limited to preclinical and clinical trial contexts. It’s crucial to source GMP Retatrutide from trusted peptide suppliers and comply with regional regulations.
Whether you're a procurement lead at a CRO, clinical coordinator, or pharmaceutical buyer, understanding the legal status of Retatrutide Peptide protects your team—and your research integrity.
👉 Looking for a compliant Retatrutide Supplier? Explore GMP-certified options here: 🔗 https://retatrutidesupplier.com/retatrutide
0 notes
Text
BJ Madan: Trusted Channel Partner for Leading Peptide Manufacturers in India
BJ Madan – Connecting Research and Industry to the Best Peptide Manufacturers in India
In the rapidly growing fields of biotechnology, pharmaceuticals, diagnostics, and cosmetics, peptides have become essential components for drug development, therapeutic research, and innovative treatments. The demand for high-purity peptides has surged across India, and BJ Madan & Co. plays a pivotal role in fulfilling this need by partnering with reputed peptide manufacturers and making their products accessible to researchers, pharma companies, and healthcare institutions.
The Growing Role of Peptides
Peptides, which are short chains of amino acids, are used in:
Cancer immunotherapy
Hormone therapy
Vaccine development
Diagnostic kits
Skincare and anti-aging formulations
The complexity and sensitivity of peptide-based products demand that they be manufactured with rigorous quality standards and delivered under tightly controlled conditions.
BJ Madan: Your Link to Quality Peptide Supply
With over six decades of experience in medical and pharmaceutical distribution, BJ Madan serves as a trusted channel partner to leading peptide manufacturers, ensuring seamless delivery of:
Research-grade and clinical-grade peptides
Custom synthesized peptides
Peptide APIs for pharma formulations
Peptides for diagnostic and laboratory use
BJ Madan’s extensive industry network and logistical strength allow it to serve clients across India, from research institutes and CROs to biotech firms and diagnostic labs.
Why Choose BJ Madan?
Access to Certified Manufacturers: GMP- and ISO-compliant production facilities
Expert Procurement Support: Custom synthesis and technical consultation available
Regulatory Compliance: Documentation and certifications for R&D and clinical use
Reliable Distribution: Temperature-controlled shipping and prompt delivery across India
Enabling Innovation Across Healthcare and Life Sciences
BJ Madan continues to support India's innovation ecosystem by ensuring that high-quality peptide products are readily available to those who need them—whether for clinical research, product development, or advanced diagnostics.
Visit:- https://www.bjmadan.com/peptides.html
0 notes
Text
0 notes
Text
Trusted Tirofiban Manufacturer For High-Quality APIs
Tirofiban Manufacturer is a famous antiplatelet drug used in the manipulation of acute coronary syndrome (ACS). As a non-peptide glycoprotein IIb/IIIa inhibitor, it prevents platelet aggregation and is essential subsequently for percutaneous coronary interventions (PCI). With its growing call for in cardiology and emergency remedy, sourcing Tirofiban from a reliable and GMP-compliant producer is vital for pharmaceutical corporations, hospitals, and studies establishments.
What Is Tirofiban?
Tirofiban is normally to be had as Tirofiban hydrochloride monohydrate, a white to off-white powder soluble in water. It’s formulated into injectable answers used to save you blood clots in sufferers with coronary coronary coronary heart conditions. The drug works via inhibiting fibrinogen binding to platelet receptors, thereby lowering the hazard of clot-related sports activities collectively with coronary coronary coronary coronary coronary heart attacks.
Key Considerations When Choosing a Tirofiban Manufacturer
1. Regulatory Compliance and Certifications
The most important trouble at the same time as deciding on a Tirofiban Manufacturer in China is compliance with global pharmaceutical requirements. Look for businesses that take a look at Good Manufacturing Practices (GMP) and characteristic certifications which includes WHO-GMP, US FDA, EU GMP, or ISO 9001. These certifications make certain the producing environment is sterile, controlled, and meets terrific requirements critical for human-use prescribed drugs.
2. API and Formulation Capabilities
Some producers' reputation certainly on producing the Active Pharmaceutical Ingredient (API), at the same time as others moreover offer formulated injectables. Depending to your goals, choose a manufacturer that offers the famous product form at the facet of precise documentation collectively with the Certificate of Analysis (COA), Drug Master File (DMF), and Stability Data.
3. Research and Development Support
For pharmaceutical organizations growing new cardiovascular treatment options, having R&D guidance from the manufacturer is a primary advantage. Leading producers regularly provide custom synthesis, stability research, and device development offerings. This partnership can boost up scientific trials and regulatory submissions.
4. Quality Control and Assurance
A reliable Tirofiban Manufacturer can also have in-residence analytical laboratories to carry out rigorous trials out of every batch. This consists of exams for impurities, balance, and performance. Make terrific they comply with ICH suggestions and provide batch records and complete traceability.
5. Global Supply and Logistics
Tirofiban is a sensitive drug that calls for proper cold chain logistics and ordinary packaging. A specific producer ought to provide inexperienced export services, help with regulatory filings, and a robust logistics community to make sure properly timed deliveries ultimately of regions. Local warehousing alternatives additionally may be useful for consistent delivery.
6. Pricing and Transparency
While price is a problem, in no manner compromise is incredible. Be careful of alternatively low expenses as they will advocate compromised manufacturing practices or loss of regulatory compliance. Always request samples, validate the COA, and conduct audits or 0.33-celebration inspections if crucial.
Final Thoughts
Finding a reliable Tirofiban Manufacturer China consists of extra than evaluating expenses. Quality, compliance, documentation, and issuer help are essential at the identical time as sourcing this form of essential cardiovascular drug. Whether you are a pharmaceutical commercial enterprise organisation or a medical institution procurement crew, making an funding time in vetting your corporation will ensure affected person safety and regulatory peace of thoughts.
0 notes
Text
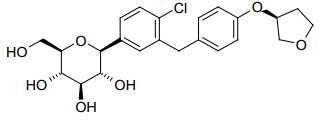
Empagliflozin API is a sodium-glucose co-transporter 2 (SGLT2) inhibitor that reduces glucose reabsorption in the kidneys, leading to increased urinary glucose excretion. It's indicated for improving glycemic control in adults with type 2 diabetes mellitus and for reducing the risk of cardiovascular death in adults with type 2 diabetes and established cardiovascular disease.
Dr. Reddy's Laboratories, headquartered in Hyderabad, India, is a leading manufacturer and supplier of high-quality, affordable Active Pharmaceutical Ingredients (APIs), including Empagliflozin. With a strong presence in the US, Europe, Brazil, Latin America, Japan, China, Korea, the Middle East, and other emerging markets, Dr. Reddy's API business leverages over 30 years of technical expertise in developing complex APIs. Their capabilities encompass steroids, peptides, complex long-chain molecules, and highly potent APIs (HPAPIs), supported by robust intellectual property and regulatory proficiency.
0 notes
Text
Expansions at sites in Le Mans and Mourenx, France have solidified the CDMO’s position in targeting the hot antibody-drug conjugate and peptide purification markets. As the demand for complex active pharmaceutical ingredients (APIs) continues to grow, Germany-based contract development and manufacturing organization Axplora is investing in the infrastructure to meet the needs of customers in antibody-drug conjugates (ADCs) and GLP-1 receptor agonists (GLP-1s) — two of the biopharma industry’s hottest areas. Martin Meeson, the former CEO of Fujifilm Diosynth Biotechnologies who took the helm of Axplora in April 2024, contends that to handle the complexities of API development and manufacturing for such innovative drugs there is a need for high-purity standards and chemical processes, as well as the ability to scale production. “It’s really about scaling out the things that we are really good at,” Meeson told Pharma Manufacturing. “In ADCs and GLP-1s, we’re scaling in terms of volume and in areas where it’s not as visible, such as skills around the adjacent services like conjugation and the increasing complexity within the synthetic chemistry molecules.” Formed in 2022 from the integration of PharmaZell, Farmabios, and Novasep CDMO, Axplora operates nine API manufacturing sites in Europe and India. PharmaZell and Farmabios have expertise in APIs, highly potent compounds, and regulatory excellence, while Novasep CDMO’s core competencies are in small molecule and ADC manufacturing. Axplora has expanded commercial ADC payload production at its Le Mans, France site. The expansion includes a new GMP-compliant payload manufacturing workshop, designed to enhance capacity and meet the industry’s growing demand for ADCs. “I don’t think people expected the volumes that were needed within the ADCs that we’re now seeing today as an industry,” Meeson said. “So, we’ve invested in a much larger facility in Le Mans to be able to support those larger demands from the customers, both at a clinical stage and commercial stage.” The expanded Le Mans site — part of the France 2030 national program to encourage the development of biopharmaceuticals in the country — now includes six ADC workshops dedicated to clinical and commercial production, as well as bioconjugation, supported by high-performance chromatography purification lines. The Le Mans project strengthens Axplora’s position as a global manufacturer supplying 40% of the world’s marketed ADCs and 50% of FDA-approved ADCs, according to the company. Axplora is also making an investment in GLP-1 manufacturing at its Mourenx, France site. The company says the capital expenditure will solidify its capabilities in peptide purification in biologics and support the development of next-generation therapies, including GLP-1s. Construction and infrastructure development has started, with the first supplies of GLP-1 therapies expected in 2026. “You maybe wouldn’t think of us in the GLP-1 space,” Meeson acknowledged. “We don’t promote ourselves as a peptide manufacturer but in some of the capabilities we have scale that many CDMOs don’t have. A core expertise of our organization is purification.” GLP-1s for diabetes and obesity are “really impactful therapies that we’re able to work on,” Meeson said. “We have a co-investment in the purification of a major GLP-1. It just shows how purification is one of the key pillars within the organization. It’s a pillar that underpins the work that we do in ADCs as well.” With the current geopolitical tensions, shifting trade policies, and potential disruptions in global supply chains, Meeson believes that Axplora is well-positioned when it comes to regional production and localized manufacturing. “We have some sites in low-cost manufacturing out in India and we have a strong platform of sites across Europe and it’s a very good position to be in,” Meeson said. “We are constantly looking at where things are coming from, where we are single, and where we are double source to ma...
0 notes
Text
Overcoming the “Choke Points” in Semaglutide Side Chain Synthesis with Core Technologies to Enable Efficient GLP-1 Drug Manufacturing Semaglutide, a groundbreaking product in the GLP-1 drug class, owes its extended half-life and enhanced receptor affinity largely to its unique side chain, Ste-Glu-AEEA-AEEA-OSU (CAS: 1169630-40-3). This side chain covalently modifies the peptide backbone, significantly improving pharmacokinetics and therapeutic performance. However, its complex structure presents two critical synthetic challenges: - Precise Assembly of Repetitive AEEA Units:The side chain features consecutive AEEA (aminoethoxyethoxyacetic acid) units, which require stepwise coupling via highly activated intermediates (e.g., AEEA-AEEA). Any impurities or deviations compromise downstream reaction efficiency and may trigger irreversible byproducts. - Stereochemistry and Stability of Glutamic Acid (Glu):The glutamic acid component must maintain strict L-configuration, and its carboxyl groups require directional protection (e.g., OtBu) to preserve biological activity. Leveraging deep expertise in peptide chemistry and industrial-scale manufacturing, Watson has successfully broken through the synthetic bottlenecks of these two key intermediates—AEEA series and glutamic acid derivatives—emerging as an “invisible champion” in global semaglutide API production. I. AEEA Series Intermediates: From Molecular Design to Industrial Scale-Up As the hydrophilic spacer in the side chain, the quality of AEEA units directly impacts drug solubility and metabolic behavior. Watson’s innovations have set new industry standards through: 1. Dual Activation Strategies for Flexible Supply To address the diverse process requirements in semaglutide side chain synthesis, Watson offers two intermediate options: - Fmoc-AEEA (CAS: 166108-71-0):Featuring Fmoc-protected amino and pre-activated carboxyl groups, this option allows direct use in solid-phase synthesis, eliminating 2-3 activation steps and shortening production cycles by over 30%. - AEEA-AEEA (CAS: 1143516-05-5):Retains a non-activated hydroxyl end for custom activation approaches. Both approaches leverage precision molecular engineering to ensure superior product consistency and performance. 2. Ultra-High Purity and Batch Consistency Given the hygroscopic and oxidation-sensitive nature of AEEA intermediates, Watson employs “inert gas protection + low-temperature crystallization” purification processes to achieve purity >99.0% and limit single impurities to 99.0% Chemical Purity: The Foundation of Compliance and Cost Reduction Semaglutide side chain synthesis demands >99.0% chemical purity, as residual trace impurities (e.g., oxidative byproducts) from lower-grade materials can amplify exponentially in downstream steps, jeopardizing entire batches. Watson employs gradient crystallization and supercritical chromatographic purification combined with a 200+ impurity database and end-to-end monitoring, ensuring purity exceeds 99.0%—enhancing coupling yields by 8%-12% and reducing purification costs. 2. 99.5% Optical Purity: Uncompromising Chiral Control Watson’s Fmoc-Glu-OtBu (CAS: 84793-07-7) and Glu-OtBu (CAS: 45120-30-7) products achieve >99.5% optical purity through asymmetric synthesis and dynamic kinetic resolution. In-line process analytical technology (PAT) ensures batch-to-batch consistency in stereochemistry, completely eliminating risks of drug inactivation from isomer contamination. 3. Tight Control of Single Impurities: Watson’s “Dual-Standard” Approach While many suppliers focus solely on overall purity, neglecting the toxicological risks of specific impurities, Watson sets a new industry benchmark by limiting maximum single impurity to Read the full article
0 notes
Text
Anhydrous HOBt Market, Global Outlook and Forecast 2025-2032
Anhydrous HOBt Market, Global Outlook and Forecast 2025-2032
The global Anhydrous HOBt market is gaining significant traction in pharmaceutical and chemical synthesis applications, with its valuation reaching USD 68 million in 2023. Industry analysis projects steady growth at a CAGR of 4.6%, pushing market value to approximately USD 101.93 million by 2032. This organic compound derived from benzotriazole has become indispensable for peptide synthesis, where it prevents racemization and enhances coupling efficiency.
Anhydrous HOBt serves as a critical reagent in producing activated esters and amides, particularly in drug development and specialty chemical manufacturing. Its demand continues to rise as pharmaceutical companies increase investments in peptide-based therapeutics and complex organic synthesis.
Download FREE Sample Report: https://www.24chemicalresearch.com/admin24cr/download-sample/288449/global-anhydrous-hobt-forecast-market-2025-2032-683
Market Overview & Regional Analysis
North America currently leads the Anhydrous HOBt market with a 28% revenue share, driven by robust pharmaceutical R&D activities and the presence of major peptide manufacturers. The region accounted for USD 19.14 million in 2023, with growth sustained by increasing adoption in bioconjugation and combinatorial chemistry applications.
Europe follows closely, where strict quality standards for pharmaceutical intermediates propel demand for high-purity Anhydrous HOBt. Meanwhile, Asia-Pacific emerges as the fastest-growing region, with China and India expanding their pharmaceutical API production capabilities and investing in peptide synthesis technologies.
Key Market Drivers and Opportunities
The market is primarily driven by the growing peptide therapeutics sector, which has seen over 60 products receive FDA approval and more than 150 in clinical trials. Anhydrous HOBt's crucial role in solid-phase peptide synthesis (SPPS) positions it favorably as drug developers increasingly focus on biologics and targeted therapies.
New opportunities are emerging in bioconjugation applications and the production of peptide-drug conjugates. The compound's ability to facilitate efficient amide bond formation makes it valuable for developing antibody-drug conjugates (ADCs) and other next-generation biopharmaceuticals. Furthermore, increasing academic and institutional research in peptide science continues to expand the application horizons.
Challenges & Restraints
While the market shows promise, it faces several challenges including regulatory scrutiny of chemical reagents in pharmaceutical manufacturing. The compound's hygroscopic nature also creates handling and storage difficulties, potentially affecting product quality and shelf life.
Competition from alternative coupling agents like HATU and HBTU presents another restraint, though Anhydrous HOBt maintains advantages in cost-effectiveness for many applications. Additionally, the need for specialized expertise in handling sensitive peptide synthesis reagents may limit adoption among smaller research facilities.
Market Segmentation by Type
Purity 98%
Purity 99%
Download FREE Sample Report: https://www.24chemicalresearch.com/admin24cr/download-sample/288449/global-anhydrous-hobt-forecast-market-2025-2032-683
Market Segmentation by Application
Peptide Synthesis
Others
Market Segmentation and Key Players
GenScript Biotech
Suvchem
BuGuCh and Partners
Thistle Scientific
Apexbio Technology
SRL Chemical
Changzhou Hubin Medicine Raw Materials
Zhejiang Wild Wind Pharmaceutical
Report Scope
This comprehensive report provides detailed analysis of the global Anhydrous HOBt market from 2023 to 2032, featuring:
Market size estimates and growth projections
Detailed segmentation by type and application
Competitive landscape with market share analysis
Regional demand patterns and growth opportunities
Supply chain analysis and pricing trends
The study includes in-depth profiles of key manufacturers, covering their production capacities, product portfolios, and strategic initiatives. It examines technological developments in peptide synthesis and evaluates their impact on Anhydrous HOBt demand.
Get Full Report Here: https://www.24chemicalresearch.com/admin24cr/reports/288449/global-anhydrous-hobt-forecast-market-2025-2032-683
About 24chemicalresearch
Founded in 2015, 24chemicalresearch has rapidly established itself as a leader in chemical market intelligence, serving clients including over 30 Fortune 500 companies. We provide data-driven insights through rigorous research methodologies, addressing key industry factors such as government policy, emerging technologies, and competitive landscapes.
Plant-level capacity tracking
Real-time price monitoring
Techno-economic feasibility studies
With a dedicated team of researchers possessing over a decade of experience, we focus on delivering actionable, timely, and high-quality reports to help clients achieve their strategic goals. Our mission is to be the most trusted resource for market insights in the chemical and materials industries.
International: +1(332) 2424 294 | Asia: +91 9169162030
Website: https://www.24chemicalresearch.com/
Follow us on LinkedIn: https://www.linkedin.com/company/24chemicalresearch
0 notes
Text
Active Pharmaceutical Ingredient Market to Expand to $415.3B by 2033 💉💊
Active Pharmaceutical Ingredient (API) market is set to expand significantly, growing from $245.2 billion in 2023 to $415.3 billion by 2033, registering a CAGR of 5.6%. This growth is driven by rising demand for generic drugs, advancements in biotechnology, and increasing prevalence of chronic diseases.
To Request Sample Report: https://www.globalinsightservices.com/request-sample/?id=GIS21460 &utm_source=SnehaPatil&utm_medium=Article
Key Market Drivers & Trends
✅ Rising Demand for Generic Medications
Cost-effective treatments are fueling the adoption of generic APIs. ✅ Growth in Biotech APIs & Personalized Medicine
Monoclonal antibodies & peptide-based drugs gaining traction. ✅ Oncology & Cardiovascular APIs Leading
Increased focus on cancer treatments & heart disease therapies. ✅ Stringent Regulatory Frameworks Driving Quality Standards
Compliance with FDA & EMA guidelines shaping API production.
Regional Insights
🌎 North America Leads — Strong R&D investments & a well-established pharmaceutical sector. 🇪🇺 Europe Expands — Growing biosimilar adoption & favorable regulatory policies. 🇮🇳 India Surges — Top exporter of cost-effective generic APIs.
Market Segmentation & Performance
📌 Synthetic APIs Dominate (55%) — Cardiovascular & oncology drugs drive demand. 📌 Biotech APIs Rising (30%) — Biopharmaceutical advancements support growth. 📌 Generic APIs Gaining Traction (15%) — Expanding access to affordable medicines.
Top Industry Players & Competitive Strategies
🏭 Teva Pharmaceutical Industries — Cost-effective API production leadership. 🏭 Pfizer Inc. — Expanding R&D investments & innovative therapies. 🏭 Novartis AG — Focus on biotech APIs & high-value drug formulations.
Future Outlook & Challenges
🔬 With a 10% projected increase in R&D expenditure, the API market will see major advancements in biotech APIs, AI-driven drug development, and nanotechnology-based APIs. However, regulatory complexities, high production costs, and competition from low-cost manufacturers pose challenges. The shift toward sustainable API manufacturing and AI-driven drug synthesis will create new growth opportunities.
💊🌍 #APIMarket #PharmaceuticalInnovation #DrugDevelopment #BiotechAPI #GenericDrugs #OncologyAPI #CardiovascularTreatment #AIinPharma #SmartPharmaceuticals #ChronicDiseaseCare #HealthcareAdvancements #FDARegulations #EMACompliance #BiopharmaGrowth #APIManufacturing #R&DInvestment #PharmaIndustryTrends #NanotechnologyInDrugs #FutureOfMedicine #PharmaSupplyChain #Biosimilars #MedicalBreakthroughs #LifeSciences #PrecisionMedicine #SustainablePharma
0 notes
Link
0 notes
Text
BJ Madan – Trusted Partner for Leading Peptide Manufacturers in India
BJ Madan – Connecting You with World-Class Peptide Manufacturers in India
Peptides are at the forefront of innovation in pharmaceuticals, diagnostics, and research. As the demand for high-purity, lab-grade, and therapeutic peptides rises across healthcare and biotech sectors, the need for reliable sourcing becomes critical. BJ Madan & Co., a trusted name in healthcare and scientific supply, serves as a key distributor and facilitator for peptide manufacturers in India.
The Rising Importance of Peptides
Peptides play a vital role in a wide range of applications including:
Cancer research and treatment
Hormonal therapies
Vaccine development
Cosmetic and dermatological formulations
Metabolic and cardiovascular research
Their specificity and bioactivity make peptides a powerful tool in both clinical and research settings.
Why BJ Madan?
With a legacy of over six decades, BJ Madan is known for its deep-rooted connections in the pharmaceutical and life sciences industries. Acting as a critical supply chain link, BJ Madan works closely with top peptide manufacturers in India and abroad to provide high-quality peptide products that meet international standards.
What BJ Madan Offers:
Custom synthesis solutions for research peptides
Bulk supply for pharmaceutical formulations
cGMP-compliant peptide products
Peptide APIs for drug development and clinical trials
Supporting Innovation Through Reliable Supply
Whether you're a pharmaceutical company, biotech startup, academic researcher, or contract manufacturing organization (CMO), BJ Madan ensures timely access to peptides with complete documentation, batch consistency, and technical support.
Partnering for Progress
BJ Madan continues to empower R&D labs, hospitals, and pharmaceutical innovators by bridging the gap between cutting-edge peptide manufacturing and end-user requirements. With a reputation built on trust and quality, BJ Madan is your dependable partner in advancing science and medicine.
Visit:- https://www.bjmadan.com/peptides.html
0 notes