#pharmaceutical compliance
Text
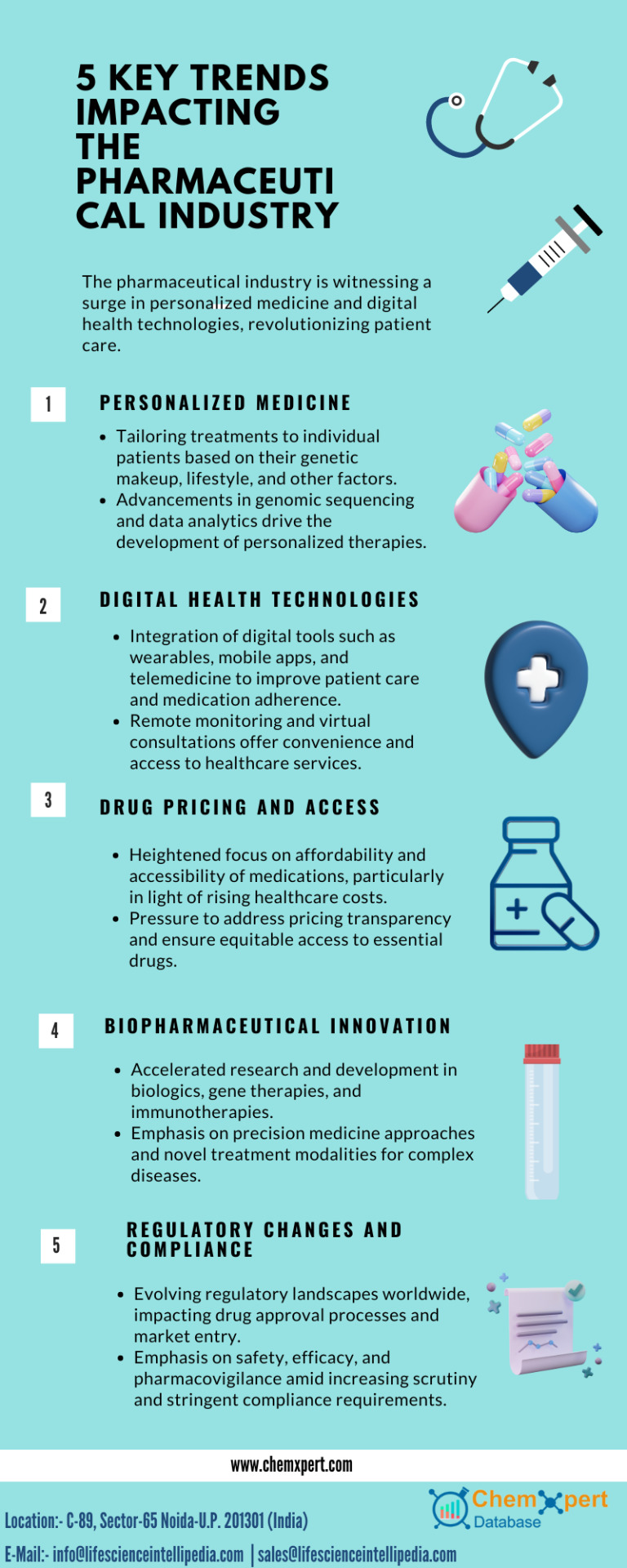
Comprehensive Pharmaceutical Solutions with Chemxpert Database
Chemxpert Database offers robust solutions for pharmaceutical online needs, facilitating cutting-edge pharmaceutical research and seamless connections with third-party medicine manufacturers. Our platform streamlines the drug manufacturing process, ensuring quality and efficiency. Additionally, we provide targeted pharma advertising opportunities to enhance your brand's reach. Trust Chemxpert Database for a comprehensive approach to all your pharmaceutical requirements, from research and manufacturing to marketing.
#pharmaceutical manufacturing process#pharmaceutical development#nitrosamine risk assessment#pharmaceutical companies in Netherlands#pharmaceutical compliance
1 note
·
View note
Text
Pharma Compliance: Essential Strategies for Marketing and Sales Excellence
The pharmaceutical industry is a key player in advancing patient care. Through their development of novel diagnostics and therapeutics, physicians and other medical professionals are able to detect and treat health disorders and diseases more effectively. However, like any industry, pharma is not free from legal and ethical standards that must be adhered to. And in the context of pharma marketing and sales, compliance is of vital importance.
Why? Because compliance helps ensure that patient care remains a top priority, above all else. Not to mention, compliant organizations face fewer liabilities. Non-compliance in pharma marketing and sales efforts can result in serious consequences ranging from regulatory fines and lawsuits to reputational harm. There are even criminal considerations.
In March 2023, the Department of Justice (DOJ) updated its Evaluation of Corporate Compliance Programs (ECCP). This document1 is meant to serve as a guidance document for prosecutors when evaluating corporate compliance programs. Among other updates, the ECCP Guidance now includes a new directive for assessing how companies govern employees' use of personal devices, communication platforms and messaging applications.
It’s a lot to take in, and we feel your pain! So, to help executives cut through the compliance clutter, we’re doing a deep dive into why it plays such a crucial role in pharma’s sales and marketing efforts. We’ll look at what compliance means, discuss the risks involved with non-compliance, and outline a framework for achieving compliance in pharma marketing and sales.
0 notes
Text
AI for Pharmaceutical Compliance: Revolutionizing Innovation
Discover how AI is revolutionizing Pharmaceutical Compliance at LighthouseAI. Explore the benefits and latest advancements in this rapidly evolving field, helping companies ensure adherence to regulatory standards. Unleash the potential of AI for innovation in Pharmaceutical Compliance.

0 notes
Text
Unlocking the Secrets of Pharmaceutical Validation: A Comprehensive Training Guide
Introduction:
Welcome to the world of pharmaceutical validation, a critical arena where precision meets compliance to ensure the safety and efficacy of medicinal products. In this full training session, we will navigate through the core stages of validation: Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). Each stage…

View On WordPress
#acceptance testing#DQ#IQ#OQ#performance verification#pharmaceutical compliance#PQ#quality audit#system description#system qualification#test evidence#Validation process
0 notes
Text
0 notes
Text
Market Forecast: Predicting Growth Trajectory and Opportunities in the Softgel Capsules Industry
Unveiling the Lucrative Realm of Softgel Capsules Market
Introduction
In the ever-evolving landscape of pharmaceuticals, softgel capsules have emerged as a pivotal player, revolutionizing drug delivery systems. These encapsulated wonders offer a myriad of benefits, ranging from enhanced bioavailability to improved patient compliance. At the heart of this innovation lies a bustling market, brimming with opportunities and challenges alike. In this comprehensive guide, we delve deep into the dynamics of the softgel capsules market, unraveling its nuances and potential for growth.
Understanding Softgel Capsules
What Are Softgel Capsules?
Softgel capsules represent a sophisticated dosage form characterized by a gelatin shell enclosing liquid or semi-solid fillings. This versatile encapsulation technique facilitates the administration of various pharmaceuticals, including oils, suspensions, and solutions, with unparalleled precision and efficacy.
Key Advantages
Enhanced Bioavailability: The unique composition of softgel capsules promotes rapid absorption and bioavailability of active ingredients, ensuring optimal therapeutic outcomes.
Improved Stability: The hermetic seal of softgel capsules shields delicate compounds from environmental factors, prolonging shelf life and maintaining product integrity.
Ease of Swallowing: Unlike traditional dosage forms, softgel capsules boast a sleek, easy-to-swallow design, enhancing patient comfort and compliance.
Market Landscape
Growth Trajectory
The softgel capsules market has witnessed exponential growth in recent years, fueled by advancements in pharmaceutical technology and rising consumer demand for convenient drug delivery systems. With an expanding geriatric population and escalating prevalence of chronic diseases, the market shows no signs of slowing down.
Key Players
Leading pharmaceutical companies are capitalizing on the burgeoning demand for softgel capsules, leveraging innovative formulations and strategic partnerships to gain a competitive edge. From multinational giants to niche players, the market is teeming with diverse stakeholders vying for market share.
Market Segmentation
By Product Type
Gelatin-based Softgel Capsules: Traditional gelatin formulations continue to dominate the market, owing to their versatility and cost-effectiveness.
Non-gelatin Softgel Capsules: With growing concerns regarding religious and dietary restrictions, non-gelatin alternatives such as plant-based and fish-derived capsules are gaining traction among health-conscious consumers.
By Application
Pharmaceuticals: The pharmaceutical sector accounts for the lion's share of softgel capsules usage, driven by the need for targeted drug delivery and enhanced patient adherence.
Nutraceuticals: The burgeoning nutraceutical industry relies on softgel capsules to encapsulate vitamins, minerals, and dietary supplements, catering to wellness-conscious consumers seeking convenience and efficacy.
Market Dynamics
Drivers
Technological Advancements: Continuous innovation in encapsulation technology, coupled with the advent of novel excipients, is expanding the horizons of the softgel capsules market.
Consumer Preference for Oral Dosage Forms: The growing preference for oral dosage forms over conventional tablets and injections is fueling the demand for softgel capsules, driving market growth.
Challenges
Regulatory Hurdles: Stringent regulatory frameworks governing pharmaceutical manufacturing pose a significant challenge for market players, necessitating compliance with stringent quality standards and guidelines.
Price Volatility of Raw Materials: Fluctuations in the prices of gelatin and other raw materials used in softgel capsules production can impact profit margins and operational efficiency, posing a challenge for manufacturers.
Future Outlook
The future of the softgel capsules market appears promising, buoyed by ongoing research and development efforts aimed at enhancing product efficacy and safety. With increasing investments in precision medicine and personalized healthcare, softgel capsules are poised to play a pivotal role in shaping the future of drug delivery systems.
Conclusion
In conclusion, the softgel capsules market represents a dynamic and thriving sector within the pharmaceutical industry, driven by innovation, consumer demand, and regulatory developments. As market dynamics continue to evolve, staying abreast of emerging trends and opportunities is paramount for industry stakeholders seeking to capitalize on this lucrative market segment.
#Softgel Capsules#Pharmaceutical Innovation#Drug Delivery Systems#Nutraceuticals#Gelatin Capsules#NonGelatin Capsules#Regulatory Compliance#Precision Medicine#Sustainability#Personalized Healthcare#Pharmaceutical Packaging#Biodegradable Capsules#Targeted Drug Delivery#Formulations#Product Integrity#Dietary Restrictions#Religious Restrictions#Cosmetic Industry#Market Forecast#Growth Opportunities
1 note
·
View note
Text
Medical waste disposal- Stay up to date with the currents regulations and compliances

#compliance#regulations#online safety#icd10#hipaa compliance#MSDS#online safety audits#medical waste disposal#medprodisposal#waste management#medical waste#pharmacy waste disposal#medical waste disposal solutions#medicines#biohazard sharps container#pharmaceutical waste management#disposal
0 notes
Text
Compliance Matters: Elevating Pharma Marketing Through Ethical Practices
In the pharmaceutical industry, compliance isn't merely a legal obligation; it's a fundamental pillar ensuring trust and safety in healthcare marketing. Enter ZING, a groundbreaking solution designed to streamline Pharma Marketing Compliance. ZING provides a robust engagement suite enabling secure, compliant communication, allowing pharmaceutical companies to effectively engage healthcare professionals (HCPs) while adhering to stringent regulatory standards.
What is Pharma Compliance?
Pharma compliance involves adhering to legal, ethical, and regulatory standards established by governing bodies to ensure that promotional activities and communications about pharmaceutical products are accurate, responsible, and focused on patient safety. These guidelines govern advertising, product disclosure, and interactions with HCPs, maintaining public trust and integrity within the pharmaceutical industry.
Why Pharma Compliance Matters
Understanding the landscape of regulations is vital for anyone involved in Pharma Marketing Compliance. Here are some key guidelines and standards:
FDA Guidelines: The Food and Drug Administration (FDA) sets pivotal standards for pharma marketing in the U.S., requiring all marketing efforts to comply with rigorous safety and efficacy criteria.
DOJ's ECCP: The Department of Justice's Evaluation of Corporate Compliance Programs (ECCP) emphasizes the importance of ethical conduct in pharma marketing.
GDPR: The General Data Protection Regulation (GDPR) governs the handling of personal data within the EU and EEA, demanding clear consent for data processing and ensuring data security.
Challenges in Achieving Pharma Compliance
Navigating the world of Pharma Marketing Compliance can be complex due to several challenges:
Complex Regulations: Laws vary widely by country and region.
Training Needs: Staff must be continuously trained in the latest regulations.
Digital Communication: Ensuring all messages meet compliance becomes a significant challenge.
Consequences of Non-Compliance: Non-compliance can lead to hefty fines and legal action.
The Importance of Pharma Compliance
Adhering to compliance in pharmaceutical industry is crucial for several reasons:
Patient Safety: Accurate communication ensures that HCPs and patients receive the right information.
Building Trust: Compliance demonstrates a commitment to ethical practices and respect for patient well-being.
Competitive Advantage: Prioritizing compliance positions companies as leaders in ethical marketing.
How ZING Facilitates Compliance and Simplifies Communication
ZING makes compliance straightforward and efficient:
Automated Compliance Checks: ZING reviews communications for compliance automatically.
Data Protection: ZING protects sensitive information with state-of-the-art encryption.
Audit Trails: Every action and communication is logged, simplifying compliance reporting.
Benefits of SMS Communication with ZING
Direct and Immediate: SMS messages reach HCPs directly, facilitating timely engagement.
High Open Rates: 98% of text messages are opened, typically within 3 minutes of delivery.
Strong Conversion Rates: Text messages boast an average response rate of 45%.
Boosting Sales: SMS marketing campaigns can increase sales by up to 20%.
Simple Yet Secure: ZING’s SMS platform combines simplicity with security.
Conclusion
By placing compliance in pharma marketing strategies, companies can ensure their efforts contribute positively to public health, build trust, and secure a competitive edge. With ZING, achieving compliance becomes less about navigating complexity and more about effective, impactful communication. This innovative solution ensures engagements with HCPs are not only compliant but also impactful, fostering trust and adherence to the highest standards of communication.

0 notes
Text
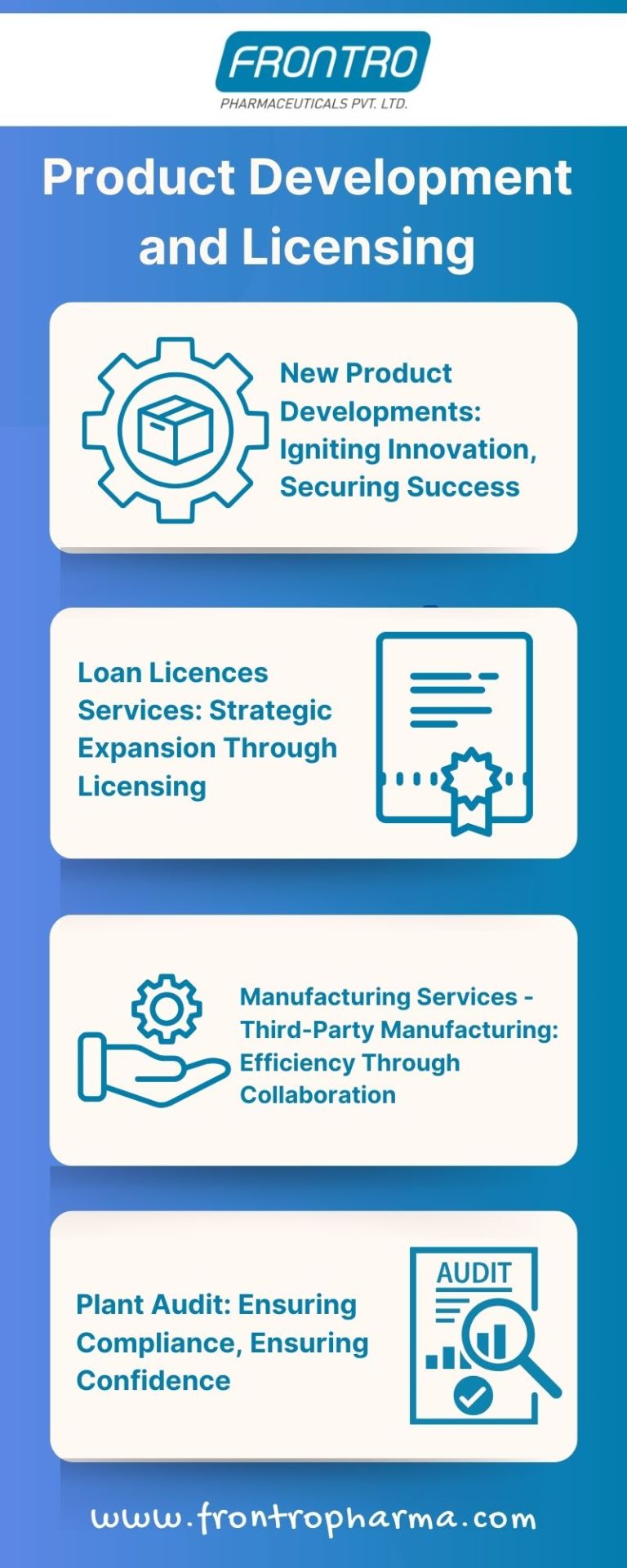
Empowering Pharmaceutical Excellence: Product Development & Licensing Services in India
Frontro Pharma leads the charge in empowering pharmaceutical excellence through its comprehensive Product Development & Licensing services in India. With a focus on pioneering innovation and securing success, we accelerate new product developments, ensuring quality control certifications that meet stringent regulatory standards. Our expertise extends to strategic expansion through loan licenses, facilitating seamless acquisition and compliance. Additionally, our third-party manufacturing services optimize efficiency through collaboration, while comprehensive plant audits instill confidence in regulatory compliance. Partner with Frontro Pharma to navigate the dynamic landscape of the Indian pharmaceutical industry and propel your company towards excellence.
#Product Development & Licensing in india#Regulatory Approvals and Compliance Associates#New Product Development & Licensing#Pharmaceutical Regulatory Affairs Consultants#Regulatory Services For New Product Development & Licensing
0 notes
Text
Easy Way to Reduce Costs in Drug Manufacturing | Chemxpert Database
In the realm of pharmaceuticals, where innovation saves lives and every molecule counts, the cost of drug manufacturing is a critical factor. Drug manufacturing companies are constantly seeking ways to optimize their processes to reduce costs without compromising quality or safety. Moreover, with the ongoing debates surrounding drug pricing, pharmaceutical companies face increasing pressure to find efficient methods of production that translate into affordable medications for consumers. In this blog post, we explore some strategies and technologies that can help reduce costs in drug manufacturing, benefiting both drug manufacturers and consumers alike.
#pharmaceutical compliance#low cost medicine#pharmacy distributor#API meaning pharma#top pharma companies in USA#contract manufacturing pharma
1 note
·
View note
Text
Clinical Trials : Holistic Exploration of the Current State and Future Outlook
The global clinical trials market size is expected to reach USD 123.5 billion by 2030, expanding at a CAGR of 6.49 from 2024 to 2030, according to a new report by Grand View Research, Inc. An increase in the volume and complexity of clinical trials has been witnessed lately, which plays an important role in the R&D of new drugs and products. The market witnessed a decline of 6% in 2020 owing to the COVID-19 pandemic. However, the market is projected to recover from 2021 onwards. In addition, clinical trials have become increasingly costly, adding to the overall cost of developing a drug.

Clinical Trials Market Report Highlights
The phase III clinical trials segment dominated the market with a 53.3% share in 2023. This can be attributed to the complexity of this phase
The interventional studies segment dominated the market in 2023. It is one of the most prominent methods used in clinical trials in the study design segment owing to the increasing demand for the intervention for clinical trials by researchers
North America held 50.3% of the market share in 2023. Favorable government initiatives and the presence of a large number of players in the U.S. that offer advanced services are responsible for market growth
Asia Pacific region is anticipated to grow at the fastest CAGR over the forecast period owing to the increasing patient pool and cost-efficient services.
For More Details or Sample Copy please visit link @: Clinical Trials Market Report
The increasing need for developing new drugs for chronic diseases, such as cancer, respiratory disorders, diabetes, cardiovascular diseases, and others, is creating immense pressure on the healthcare industry. The COVID-19 pandemic and the increasing demand for developing a suitable treatment are driving the market. The high number of people affected by the disease further depicts an increasing need for therapeutics & vaccines. Currently, there are 288 therapeutics and 106 vaccines under development, out of which, nearly 7.0% of therapeutics are in Phase IV, 21.0% in Phase III, and 43.0% & 13.0% in Phase II & Phase I, respectively.
The pandemic has resulted in the global disruption of traditional onsite clinical trials. Hence, regulatory bodies worldwide have undertaken various initiatives for fast-tracking clinical trials for the development of innovative solutions. One such instance is Solidarity, an international clinical trial launched by the WHO to find effective treatment against COVID-19. Although the pandemic has forced many medical device & drug developers to revise the approach to such crises, integrating best practices within clinical trial procedures & adapting to virtual trials, which can support the continuous development of therapeutics.
ClinicalTrials #HealthcareResearch #MedicalInnovation #DrugDevelopment #PatientRecruitment #Biopharmaceuticals #ClinicalResearch #RegulatoryCompliance #DataManagement #PatientEngagement #PrecisionMedicine #TherapeuticTrials #CROs #ClinicalResearchOrganizations #GlobalHealth #ClinicalStudyDesign #PharmaceuticalIndustry #BiotechResearch #ClinicalEndpoints #HealthTechIntegration
#Clinical Trials#Healthcare Research#Medical Innovation#Drug Development#Patient Recruitment#Biopharmaceuticals#Clinical Research#Regulatory Compliance#Data Management#Patient Engagement#Precision Medicine#Therapeutic Trials#CROs#Clinical Research Organizations#Global Health#Clinical Study Design#Pharmaceutical Industry#Biotech Research#Clinical End-points#HealthTech Integration
0 notes
Text
Optimizing Pharmaceutical Supply Chain Regulation Compliance
LighthouseAI enhances pharmaceutical supply chain regulation compliance using AI. This technology streamlines the management of complex regulatory requirements, ensuring safety and efficacy in the pharmaceutical industry.
#Pharmaceutical Supply Chain Regulation Compliance#Pharmaceutical Supply Chain Regulation#Pharmaceutical Supply Chain
0 notes
Text
Pharmaceutical Promotion Regulation: Ensuring Health & Trust
In today’s fast-paced era of medical breakthroughs, ensuring that pharmaceutical products are promoted accurately and safely is more critical than ever. Not just for the industry, but for the very consumers it serves. This isn’t merely about scrutinizing a captivating advertisement or dissecting the claims made by a catchy commercial. It’s about pharmaceutical regulatory compliance and the pivotal role it plays in our healthcare framework.
A robust adherence to these regulations isn’t just a nod to procedural formalities; it’s the bedrock of ensuring both our health and the trust we place in those promising to safeguard it. So, as we delve deeper into this topic, let’s shed light on why pharmaceutical regulatory compliance is so instrumental.
The Core of the Issue: Safeguarding Health
When it comes to our health, we shouldn’t take shortcuts. Neither should pharmaceutical companies. Medications, whether prescribed or over-the-counter, impact the lives of millions daily. If a company, in its promotions, exaggerates claims or hides side effects, it could potentially harm patients. This is where pharmaceutical regulatory compliance steps in.
Regulations are not mere guidelines. They are carefully constructed mandates ensuring that every medication promoted passes rigorous standards. These standards are there to guarantee that the medications we take are safe and effective. By adhering to pharmaceutical regulatory compliance, companies ensure the well-being of their consumers.
Trust: An Irreplaceable Pillar
Beyond just health, there’s a more intangible yet equally significant element at play: trust. The relationship between a patient and the pharmaceutical industry should be built on trust. When we pick up a medication, there’s an implicit trust that it will work as promoted and won’t harm us. Pharmaceutical regulatory compliance upholds this trust.
But how do these regulations promote trust? Simple. By ensuring that companies provide factual, well-researched information about their products. If a company breaches this trust, they not only risk backlash but also legal repercussions. For more details visit us at https://gmppros.com/.
The Impacts of Non-compliance
Skipping pharmaceutical regulatory compliance isn’t a minor oversight. Non-compliance can lead to severe consequences, both for the companies and the patients. For companies, they risk sanctions, hefty fines, and a damaged reputation. But it’s the patients who face the real dangers: misinformation, health complications, or even life-threatening side effects.
Moreover, non-compliance can erode public confidence, not just in a specific brand but in the pharmaceutical industry as a whole. It underscores the importance of always ensuring that promotions and advertisements adhere to pharmaceutical regulatory compliance.
Bridging the Gap: How Companies Can Uphold Standards
The path to ensuring pharmaceutical regulatory compliance is clear-cut. Companies must remain vigilant, always keeping up with updates and changes in regulations. Regular training sessions for staff, especially those involved in promotions, are crucial.
Third-party audits can also be beneficial. By having an external body review your processes and promotions, you can ensure you’re meeting all standards set for pharmaceutical regulatory compliance.
Moreover, transparency is key. Companies should be open about their processes, research, and findings. This not only upholds pharmaceutical regulatory compliance but also bolsters trust with consumers.
Consumer’s Role: Staying Informed
For consumers, the best defense is staying informed. While pharmaceutical regulatory compliance works to ensure companies promote their products truthfully, it’s also up to consumers to research and understand the medications they take.
Reading labels, asking physicians questions, and even participating in community discussions can arm consumers with the knowledge they need. Trust in the pharmaceutical industry is a two-way street, and informed consumers play a pivotal role in upholding it.
Conclusion
In the vast expanse of healthcare, pharmaceutical regulatory compliance represents more than just bureaucratic checkboxes. It embodies a solemn promise made by the pharmaceutical industry—a vow to put the well-being and trust of its consumers at the forefront. With every genuine promotion of medication, every transparent revelation of side effects, and every corporate decision that tilts in favor of patients over mere profits, the essence of pharmaceutical regulatory compliance shines through.
As we march ahead into the future, it becomes imperative to keep reminding ourselves of the crucial role these regulations fulfill. They are not just guiding principles but foundational pillars ensuring a future where health and trust coalesce seamlessly for everyone’s benefit.
Learn More:
Principles of Pharmaceutical Supply Chain
0 notes
Text
Clinical research is a complex and crucial field in the realm of medicine. Suppose you are interested in learning about the intricate workings of clinical research. In that case, we hope to provide you with a comprehensive overview of the life of a clinical research associate (CRA).
0 notes
Text
Effective Pharmaceutical Waste Management Solutions in Florida with ACRS Waste Solutions

Ensuring proper disposal of pharmaceutical waste is crucial. ACRS Waste Solutions offers comprehensive services in Florida, prioritizing environmentally friendly practices. Trust us for compliant and sustainable Pharmaceutical Waste Management in Florida, safeguarding both public health and the environment.
Visit:- https://www.acrswastesolutions.com/
Call- (855) 543-5500
#pharmaceutical Waste Management in Florida#medical waste disposal daytona beach florida#disposal of medical waste northeast florida#medical waste disposal jacksonville florida#Medical waste compliance Georgia
0 notes