#register trademark eu
Text
The Importance of Registering a Trademark in Europe
In today’s highly competitive global market, securing your brand is more crucial than ever. For businesses looking to establish or expand their presence in Europe, it is essential to register trademark Europe. Doing so not only protects your brand but also enhances its value across a vast and diverse market.

Why Register a Trademark in Europe?
When you register a trademark in Europe, you gain exclusive rights to your brand in all 27 European Union member states. This means you are legally protected from unauthorized use, imitation, or infringement throughout a significant portion of the global market. Whether you’re selling products or services, having your trademark registered in Europe ensures that your brand is safeguarded against competitors.
The Benefits of European Trademark Registration
Comprehensive Protection:
A European trademark registration offers coverage across all EU countries, making it an efficient way to protect your brand across multiple jurisdictions with a single application.
Cost-Effective:
Instead of registering trademarks individually in each country, you can register a trademark in Europe through a single application process, saving both time and money.
Brand Recognition:
A registered trademark in Europe helps establish and reinforce your brand identity, making it easier for consumers to recognize and trust your products or services across the continent.
How to Register a Trademark in Europe
To register a trademark in Europe, you must file an application with the European Union Intellectual Property Office (EUIPO). The application should include a clear representation of your trademark and specify the goods or services it covers. Once approved, your trademark will be protected across all EU member states.
Conclusion
Registering a trademark in Europe is a strategic move for any business aiming to protect its brand and thrive in one of the world’s most lucrative markets. By securing your trademark, you not only safeguard your intellectual property but also strengthen your brand’s reputation and market position across Europe.
#Register Trademark Europe#eu trademark#register trademark eu#trademark search eu#trademark registration europe
0 notes
Photo

You can register trademark in EU
Registration is confirmed by the issuance of the trademark registration certificate. A trademark owner has the right to use the trademark, sell or license it and prevent others from using his trademark in relation to goods and services for which it is registered.
See more: https://regimark.eu/eutm-registration/
0 notes
Text
European trademark protection: ipconsulting.EU
A trademark is a critical way to identify your business with high quality and unique personality in the business world. It is more valuable for traders also who want to enable them and possess their products and services to worldwide recognition. Applying for trademarks with our firm is simple, and EU trademark protection allows you to grow your business internationally.
European trademark registration is a scheme that allows individual and business trademark holders to protect their brands in all 27 EU member states.
It is a primary and planned process that provides traders with complete protection of their business rights. Ipconsulting’s expert advisors provide businesses with detailed information on this investment and guide them in all aspects of the registration process.
There are many advantages of EU trademark registration:
This registration offers businesses to easily set-up their goods and services on the global market with the help of this license. There are able to work globally.
Businesses can legally and privately guard their marks against being used unfairly by other parties or enterprises thanks to European trademark protection.
You will be appropriately guided in registering and obtaining your company license with the help of our services. We also assist you with the many steps of the registration process and provide thorough information on the advantages of European trademark protection. You can use our services more quickly and smoothly.
The procedure of requesting European trademark protection can be challenging in and of itself. For traders, approving the application by the numerous rules, techniques, and courses may take a lot of time and work, but IP consultancy can help. You can quickly register your name in the trademark list by consulting the EU's experienced consultants. We provide you with correct and complete documentation information to make your application quickly registered.
One of the benefits of our services is that European trademark protection services give you eligibility to trade and expand your business to countries other than European Union. This gives you more opportunities in the worldwide market to achieve more clients.
Conclusion
In conclusion, the EU trademark protection is the right way for businesses to promote their services worldwide. We are an IP consulting company that provides merchants the full advantages of European trademark protection to grow their business level!
#EU Trademark Protection#EU Registered Community Design Search#European trademark registration#Europe trade mark search
0 notes
Text
Hungarian entity that Taiwanese pager company said it authorised to produce and sell devices denies making them
Lili Bayer in Brussels and Michael Safi report for the Guardian:
The CEO of a Hungarian entity which a Taiwanese company said it had authorised to produce and sell pagers has denied making the devices, saying she was just an “intermediate.”
Gold Apollo, a Taiwan-based company, said in a statement today that it had a partnership with the Budapest-based BAC Consulting KFT, and had authorised BAC “to use our brand trademark for product sales in designated regions, but the design and manufacturing of the products are solely the responsibility of BAC.”
“Regarding the AR-924 pager model mentioned in the recent media reports, we clarify that this model is produced and sold by BAC. Our company only provides the brand trademark authorization and is not involved in the design or manufacturing of this product,” it added.
BAC Consulting was registered in Hungary in 2022 and provided a Budapest address on its website – the same address used by multiple companies.
On its website, which was live early Wednesday but later became unavailable, BAC Consulting provided long yet vague descriptions of its work.
“With over a decade of consulting experience, we are on an exciting and rewarding journey with our network of passionate experts with a hunger for innovation and discovery for the Environment, Innovation & Development, and International Affairs,” according to the company’s LinkedIn page.
Cristiana Bársony-Arcidiacono presents herself on LinkedIn as the CEO of the company. Her LinkedIn page describes her as a native speaker of both Hungarian and Italian.
Bársony-Arcidiacono and BAC Consulting did not respond to questions from the Guardian. Reached by phone, Bársony-Arcidiacono asked how the paper got the number and then hung up.
However, she confirmed to NBC that her company worked with Gold Apollo.
Asked about the pagers and the explosions, Bársony-Arcidiacono said: “I don’t make the pagers. I am just the intermediate. I think you got it wrong.”
Asked about the Hungarian company, EU foreign affairs spokesperson Peter Stano said at a press conference on Wednesday: “let’s not jump to conclusions at this stage.”
“The reasons and how it was done, how it was organised, needs to be investigated,” he said.
Asked about the CEO’s claim on LinkedIn that she also works for the European Commission, a spokesperson said “she is not a staff member, never been.”
31 notes
·
View notes
Text

Sam Heughan’s Sassenach whisky brand loses the final legal fight in a trademark dispute in the European Union 🇪🇺
Sam Heughan launched his whisky brand "The Sassenach" in 2020, nickname his character uses for his on-screen love interest in the time travel drama "Outlander". Since 2021, Heughan has been embroiled in a legal battle with "Sasse" a German distillery over the name of his whisky brand, arguing that the Sassenach whisky would confuse customers who might think he is linked to them.
The European Union Intellectual Property Office (EUIPO), which resolves trademark disputes, ruled in favour of the German company and issued a decision upholding the opposition saying The Sassenach could not use the name as a whisky brand. After losing the initial decision at the Fifth Board of Appeals in 2021, Heughan's legal team appealed in 2022 to overturn the decision.
His legal team said there was no risk of confusion as Outlander was popular in Germany. Lawyers for the Sasse distillery, however, said: "The television series may be as popular as the other side claims, which we deny, nonetheless it is not sufficient to assume that the average consumer knows the meaning of that term. Both parties in litigation were given time to present evidence and arguments in their defence and after the Examination period, the Opposition Division’s decision was taken this year 2023.
Great Glen Company or its representative never commented on the EU decisions until last October, in New York when Sam Heughan was asked about Sassenach whisky situation in the European Union in a chat with Mark Gillespie at the Whisky Cast podcast and Heughan's response was very limited, deflecting the question talking about the name in dispute but not the EUIPO's decision, regarding Sassenach whisky that was supposedly aware of the official communication from the European Union Intellectual Property Office - Opposition Division- sent to Great Glen Company in July 2023, which considered that the disputed trademark 'The Sassenach' must be rejected for all the contested goods.

It's a bit curious that after the EUIPO decision, Sam Heughan appeared on a surprise visit to New Orleans, which included podcasts, and events with @sgwinespirits on Tales of the cocktail with an unscheduled tasting of his drinks at the Ritz-Carlton in Nola. Later on, he began his Sassenach sales tour around the United States last summer. If these people had known what had happened with his Sassenach brand in the EU would be different?
In addition, Great Glen Company (GGC) applied to register a new trademark with the World Intellectual Property Organisation (WIPO) and the EU, following the EUIPO decision, following its earlier idea to build on all the Outlander ideas, the new trademark is called "LALLYBROCH SPIRITS" (Lallybroch means "lazy tower" in Gaelic). It will not use Midhope/Lallybroch as a distillery. This new trademark has nothing to do with or relate to the grounds of Midhope Castle, the site of a new whisky distillery with a different brand and ownership. Its new application is already registered in the United States.
It's pending resolution in the EU, Canada and the United Kingdom where Heughan requested its registration.
WIPO

EUIPO

THE SASSENACH UNIQUE SPIRITS
The Great Glen Company, Sam Heughan’s firm, applied to register the brand name Scotch whisky THE SASSENACH UNIQUE SPIRITS as a future trademark to sell the whisky across Europe, but Theo Sasse e.K brand distillery in Schöppingen-Germany, objected claiming the name was too close to its trademarked name, which it uses to sell whiskies and brandies spirits.
On 20th July 2023 the Opposition Division takes the following:
DECISION
1. Opposition is upheld for all the contested goods.
2. International registration is entirely refused protection in respect of the European Union.
REASONS
On 24th November 2021 the opponent Theo Sasse e.K filed an opposition against all the goods (Class 33) of international registration designating the European Union. The opposition is based on, inter alia, German trademark registration ‘Sasse’ (word mark). Also, the opponent invoked Article 8(1)(b) EUTMR and Article 8(4) EUTMR.
LIKELIHOOD OF CONFUSION — ARTICLE 8(1)(b) EUTMR
A likelihood of confusion exists if there is a risk that the public might believe that the goods or services in question, under the assumption that they bear the marks in question, come from the same undertaking or, as the case may be, from economically linked undertakings.
The opposition is based on more than one earlier trade mark. The Opposition Division finds it appropriate to first examine the opposition in relation to the opponent’s German trade mark registration.
a) The goods
The goods on which the opposition is based are, inter alia, the following:
Class 33: Alcoholic beverages, excluding beers. Alcoholic beverages, except beer are identically contained in both lists of goods (including synonyms).
b) Relevant public — degree of attention
The average consumer of the category of products concerned is deemed to be reasonably well informed and reasonably observant and circumspect. It should also be borne in mind that the average consumer’s degree of attention is likely to vary according to the category of goods or services in question. In the present case, the goods found to be identical are directed at the public at large.
c) The signs
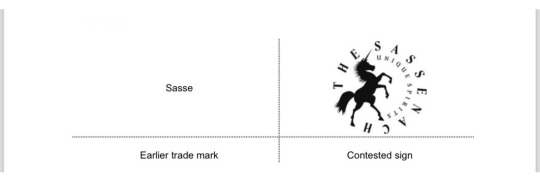
The relevant territory is Germany.
Contested sign The global appreciation of the visual, aural or conceptual similarity of the marks in question must be based on the overall impression given by the marks, bearing in mind, in particular, their distinctive and dominant components. The earlier mark is the word mark ‘Sasse’. The protection of a word mark concerns the word as such and not the specific graphic or stylistic elements accompanying that mark.
The verbal element ‘SASSENACH’ of the contested mark has, contrary to the allegations of the holder, no meaning for the relevant public and is, therefore, distinctive. Likewise, the unicorn device of the contested sign has no particular meaning in relation to the goods and is distinctive.
THE SASSENACH’ in the contested sign are the dominant elements as they are the most eye-catching.
Visually, the signs coincide in ‘SASSE’, which represents the entire earlier mark. The signs differ in the representation of a unicorn and the additional letters ‘-NACH’ (after SASSE) and the non-distinctive elements ‘The’ as well as ‘UNIQUE SPIRITS’ in the contested mark. Thus, the single word element of the earlier mark is fully contained in the most distinctive verbal element of the contested mark. That fact alone is a clear indication of a visual similarity. Therefore, the signs are similar to a below-average degree.

Aurally, the signs coincide in the syllables ‘Sas-se’, which is the sole and distinctive element of the earlier mark and the beginning of the most important verbal element of the contested sign, ‘Sas-se-nach’. The signs differ in the last letters of this word (one syllable), ‘nach’, and in the first verbal element of the contested sign, ‘The’. The fact remains that the earlier mark is entirely included at the beginning of the most important verbal element of the contested sign.
Conceptually, the signs will always be dissimilar as the contested mark will be understood with at least one meaning, namely the unicorn in the contested mark. As the signs have been found similar in at least one aspect of the comparison, the examination of likelihood of confusion will proceed.
d) Distinctiveness of the earlier mark The distinctiveness of the earlier mark is one of the factors to be taken into account in the global assessment of likelihood of confusion. In the present case, the earlier trade mark as a whole has no meaning for any of the goods in question from the perspective of the public in the relevant territory. Therefore, the distinctiveness of the earlier mark must be seen as normal.
e) Global assessment, other arguments and conclusion The goods at issue are identical. They target the general public, who possesses an average degree of attention. The earlier mark has a normal degree of distinctiveness. The signs are visually similar to a below average degree and aurally similar to an average degree since the sole and distinctive element of the earlier mark, ‘Sasse’, is entirely reproduced at the beginning of the contested sign’s only fully distinctive verbal element, ‘Sassenach’. Evaluating the likelihood of confusion implies some interdependence between the relevant factors and, in particular, a similarity between the marks and between the goods or services.
Considering all the above, especially taking into account that the earlier mark is entirely reproduced in the contested sign and used for goods that are identical, the Opposition Division finds that there is a likelihood of confusion on the part of the public. Therefore, the opposition is well founded on the basis of the opponent’s German trade mark registration It follows that the contested trade mark must be rejected for all the contested goods. As the earlier right German trade mark registration leads to the success of the opposition and to the rejection of the contested trade mark The Sassenach for all the goods against which the opposition was directed.
The trademark status was "totally refused", meaning that THE SASSENACH UNIQUE SPIRITS trademark cannot be registered in the EU. If SH's trade mark application is refused, he can file an appeal. He must file his notice of appeal within 2 months from the date of the refusal decision (August-September) and the grounds of appeal must be filed within 4 months from the same date of notification (October-November). But, He did not appeal and last November the EUIPO confirmed by letter the provisional refusal of his trademark and refused its protection in the European Union. The final decision was published on 14 December 2023.

Conclusion
The EUIPO’s decision of the Board of Appeals, regarding its whisky has a “displacement” because Sassenach whisky cannot be registered as a trademark in the EU, the Sassenach trademark was refused. SH must be aware the significance of the total refusal decision regarding its whisky brand. If he was planning to recover from a legal dispute by putting his gin on an impromptu Sassenach tour around US last summer, proving that his recent EU legal battle was a mere bump in the road, he should have thought twice. He lost a legal battle to register his Sassenach whisky brand as a European Community trademark ® in 27 states. It is a big difference. It seems that if Heughan wants to continue selling its whisky, it will have to change the name.
LALLYBROCH SPIRITS registration:
United Kingdom
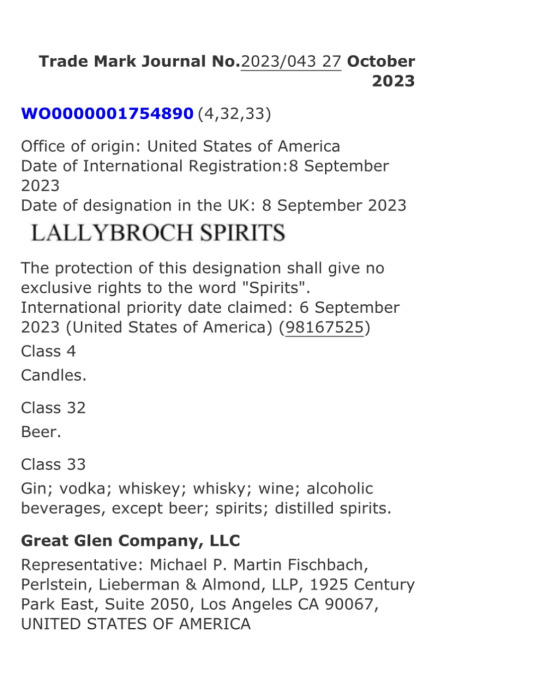
Canada

USA

26 notes
·
View notes
Text
Website : https://en.intertaxtrade.com
Intertaxtrade, established in the Netherlands, excels in facilitating international business and assisting individuals in Europe with integrated solutions in tax, finance, and legal aspects. Registered with the Chamber of Commerce, they offer services like company management in the Netherlands, Dutch company accounting, tax intermediation, international tax planning, business law consulting, EU trademark and intellectual property registration, international trade advice, and GDPR compliance. Their expertise in financial and accounting services ensures clients have a clear financial overview, aiding in business success.
Facebook : https://www.facebook.com/intertaxtrade
Instagram : https://www.instagram.com/intertaxtrade/
Linkedin : https://www.linkedin.com/in/ramosbrandao/
Keywords:
company registration netherlands
legal advice online
comprehensive financial planning
financial planning consultancy
international business services
international business expansion strategies
gdpr compliance solutions
international trade consulting
european investment opportunities
gdpr compliance consulting services
in depth financial analysis
gdpr compliance assistance
cross border tax solutions
netherlands business environment
european union business law
dutch accounting services
tax intermediation solutions
international tax planning advice
eu trademark registration services
investment guidance online
business law consultancy
corporate tax services netherlands
financial analysis experts
business immigration support
startup legal assistance online
european market entry consulting
international financial reporting services
business strategy netherlands
tax authority communication support
international business law expertise
dutch commercial law advice
global business strategy services
european business consulting online
international business services platform
expert legal advice online
efficient company registration netherlands
reliable dutch accounting services
strategic tax intermediation
proactive international tax planning
eu trademark registration support
tailored investment guidance
specialized business law consultancy
dynamic international trade consulting
holistic corporate tax services netherlands
streamlined business immigration support
online startup legal assistance
strategic international business expansion
european market entry planning
innovative cross border tax solutions
navigating the netherlands business environment
european union business law insights
accurate international financial reporting
proven business strategy netherlands
exclusive european investment opportunities
seamless tax authority communication
in depth dutch commercial law advice
comprehensive global business strategy
proactive european business consulting
one stop international business services
personalized financial planning solutions
expert legal advice for businesses
quick company registration in netherlands
trustworthy dutch accounting services
strategic tax intermediation solutions
innovative international tax planning
efficient eu trademark registration
tailored investment guidance online
business law consultancy expertise
comprehensive corporate tax services netherlands
thorough financial analysis support
streamlined business immigration assistance
navigating netherlands business environment
european union business law guidance
international financial reporting accuracy
business strategy for netherlands market
european investment opportunities insights
efficient tax authority communication
international business law excellence
dutch commercial law proficiency
global business strategy implementation
european business consulting excellence
comprehensive international business services
proactive financial planning strategies
expert legal advice on international matters
#company registration netherlands#legal advice online#comprehensive financial planning#financial planning consultancy#international business services#international business expansion strategies#gdpr compliance solutions#international trade consulting#european investment opportunities#gdpr compliance consulting services#in depth financial analysis#gdpr compliance assistance#cross border tax solutions#netherlands business environment#european union business law#dutch accounting services#tax intermediation solutions#international tax planning advice#eu trademark registration services#investment guidance online#business law consultancy
3 notes
·
View notes
Text
The Madrid System for International Registration of Marks

This article on 'The Madrid system for International Registration of Marks' was written by Monika Yadav an intern at Legal Upanishad.
Introduction
In this article, we will know about the Madrid system of trademarks. The Madrid System seems to be a practical and economical way to register and maintain trademarks globally. To file for safety in as many as 128 nations, just submit one application as well as pay a single amount of fee. Utilize a single, centralised system to update, refresh, or increase your global trademark inventory. We will understand the Madrid protocol and also the Madrid agreement. India is also a member, and we see the workings of the system and then conclude our article.
What does it mean?
Two separate treaties, the Madrid Agreement and the Madrid Protocol oversee the registration of trademarks in various jurisdictions worldwide. Although it bears the name "Protocol," it is not a "protocol" to the Agreement but rather a separate agreement. The Madrid System for the International Registration of Marks is what the Agreement and the Protocol are collectively referred to as the Madrid System.
The term "Contracting Parties" refers to all States and organisations that are parties to the Protocol and/or the Agreement. They collectively make up the Madrid Union, which is a Special Union following Article 19 within the Paris Convention. The Madrid System is a method for acquiring a collection of trademark registrations in numerous states that are professionally managed by the International Bureau of the World Intellectual Property Organization (WIPO), effectively establishing a foundation for an "international registration" of marks.
The Madrid Agreement
To enable the need to file, seek, or maintain separate registrations in many countries is eliminated by a single, affordable worldwide trademark registration. The Agreement was established in 1891. Whenever a mark is registered as per the Agreement, having the exact legal impact as if the mark owner had decided to register it in one of the member countries. If the targeted country's trademark office neglects to notify WIPO of a denial of registration within the allotted 12-month period, the trademark will be protected to the same extent as authorized national trademarks in the same nation (but as per Protocol it extends to 18 months).
The Agreement calls for a simplified renewal procedure because WIPO only needs one file for both renewals as well as modifications to the primary registration which impact all of the nations included here by registration.
Regardless of the benefits of registration under the Agreement, the US and other important nations (including Australia, Denmark, Finland, Greece, Iceland, Ireland, Japan, Netherlands, Republic of Korea, Sweden, and the United Kingdom) had not ratified it because of purported structural issues. These so-called flaws allegedly included things like the requirement for home country registration even before the granting of mark safety, infinite limitations on assignability, a chance of a "central attack" on the mark, a brief inquiry time, evaluation costs that were less expensive than those currently charged by the native nation's mark offices.
The Madrid Protocol
To address such alleged flaws within the Agreement, the Protocol was approved in 1989. However, the Protocol upholds the Agreement's original goal, which was to establish a mechanism for quick and affordable worldwide trademark registration. As a result, although there are currently only 57 signatories to the agreement, 74 nations, including the USA, were part of either the Agreement or the Protocol.
India as a member
A trademark holder might also use their Community Trademark (CTM) to preserve their mark through 88 Contracting Parties and the EU under the Madrid system, which is administered by WIPO, by filing a specific application in one of three languages (English, French, or Spanish), in addition to a limited piece of fees in a single currency (Swiss Francs). India adopted the Madrid Protocol on April 8, 2013, and it joined on July 8, 2013, increasing the overall number of parties to 90.

The Madrid system for International Registration of Marks
The Working
With the help of the Madrid System's "Basic Application / Registration" method, a trademark owner who has filed for registration or already has a registration in a member country may receive "International Registration" for their brand from WIPO. After that, the mark's owner has the option of choosing "International Registration," also known as "Designation," to get protection in one or more member nations.
Furthermore, thanks to the Madrid Protocol, a trademark owner can now simultaneously apply for registration across any partner jurisdiction and receive "International Registration" by force of the Madrid Protocol. By submitting a single application in a single jurisdiction with a limited fee, a trademark owner can register their mark for any or even all member states, make any necessary changes to the information (such as their name or address), and renew their registration in all relevant jurisdictions across a single official bureaucratic system.
The natural reversal of the previously stated Madrid System/Protocol feature is, nevertheless, that if a "Basic Registration/Application" is rejected, cancelled, or revoked, the global registration will likewise be rejected, cancelled, or rescinded to the same level. For illustration, if the "Basic Application" for shampoo is cancelled, the "International Registration" of the exact mark for shampoo is going to be cancelled. This applies to applications for registration of marks for herbal products such as cleansers, face washes, and hair products. The "International Registration" will also be rejected if the "Basic Application" is rejected.
The process of testing a "Basic Application" for registerability is known as a "Central Attack." An "International Registration" (also known as a "Transformation") is split up into several applications in every jurisdiction to reduce the impacts of a successful central attack. This overpriced process is justified, however, in addition to this benefit but also because the applications that arise will use the registration date of the global registration as their date of filing.
Conclusion
The Madrid Express database would be a free tool offered by WIPO to look up international marks. It is modified every day. The database includes interfaces for both basic and structured searches. All global registrations which are active at the moment or have recently been terminated are included in Madrid Express.
WIPO makes all efforts to guarantee about the database accurately portrays the information contained in the Global Registration, but users should be aware that the Gazette is still the only authoritative source and that the only reports issued by the International Bureau about the components of the Global Registration for a specific world registration are the approved substances by the Register recognised upon recommendation.
References
- Vicenc Feliu, International Trademark Law- The Madrid System, Available at: https://www.nyulawglobal.org/globalex/International_Trademark_Law.html (Accessed: November 29, 2022).
- Sharad Saini, The Madrid System for the International Registration of Marks, Available at: https://www.khuranaandkhurana.com/2013/07/16/the-madrid-system-for-the-international-registration-of-marks-madrid-system/?amp=1 (Accessed: November 29, 2022).
- Madrid- The International Trademark System, Available at: https://www.wipo.int/madrid/en/. (Accessed: November 29, 2022).
Read the full article
2 notes
·
View notes
Text
Trademark-R | Register Trademark in UK,EU,USA,Australia, Canada
Are you looking to register trademark in UK, Europe (EU), USA, Australia, Canada, China, Ireland or London? We offer Trademark Registration in UK at very affordable price.
Trademark-R #Trademark #TrademarkUK #UKTrademark #UKBusiness #TrademarkEU #TrademarkUSA
Trademark-R, Trademark, Trademark UK, UK Trademark, UK Business, Trademark EU, Trademark USA

0 notes
Text
Leading Intellectual Property Law Firm for Global Brands

As the premier intellectual property law firm in the UK, Brandsmiths has built a reputation for excellence by acting on behalf of some of the world’s most prominent brands. Specializing in Intellectual Property (IP), we help businesses protect their valuable assets, from trademarks and patents to copyright and designs. With a deep understanding of the complexities of IP law, we provide strategic legal solutions tailored to our clients' unique needs.
Comprehensive IP Protection and Enforcement
At Brandsmiths, we offer comprehensive intellectual property services to safeguard your creations and innovations. Our expertise spans across various industries, including technology, fashion, pharmaceuticals, and consumer goods. We assist clients in registering trademarks, patents, and designs while also offering expert advice on IP strategy and portfolio management. Should your IP rights be infringed, our skilled litigators act swiftly to enforce your rights through negotiations, cease-and-desist letters, and, if necessary, litigation.
Trademark Services for Global Brands
Trademarks are the cornerstone of brand identity, and protecting them is essential for maintaining a strong market presence. Our firm offers extensive trademark services, from registration to enforcement, ensuring that your brand is protected on a global scale. We handle trademark disputes, opposition, and revocation proceedings, leveraging our in-depth knowledge of UK, EU, and international trademark law. By working with Brandsmiths, you gain a trusted partner committed to safeguarding your brand's integrity and reputation.
Expertise in Litigation and Commercial Law
Our firm is also renowned for its expertise in litigation and commercial law. We represent clients in complex legal disputes, ensuring that their interests are protected in court. Whether it’s a breach of contract, shareholder dispute, or commercial litigation, our team of experienced litigators delivers results. We are also skilled negotiators, working diligently to resolve disputes through mediation or arbitration when possible, saving our clients time and resources. In the realm of commercial law, we advise on a range of matters, including corporate governance, mergers and acquisitions, and contractual agreements.
Specialized Legal Services in Sport, Media, and Entertainment
Brandsmiths has a niche specialization in sport, media, and entertainment law, offering legal advice to some of the biggest names in the industry. From sponsorship agreements and broadcasting rights to player transfers and image rights, our team has an unparalleled understanding of the legal complexities involved in this dynamic field. We help clients navigate the commercial and legal challenges in these sectors, ensuring that their intellectual property is protected and their commercial interests are secured.
Conclusion
As the UK's leading intellectual property law firm, Brandsmiths is committed to protecting the rights and interests of global brands. With expertise in Intellectual Property, Trademarks, Litigation, Commercial Law, and Sport, Media & Entertainment, we deliver tailored legal solutions that empower our clients to thrive in a competitive market. Our unwavering focus on excellence and innovation makes us the go-to law firm for safeguarding your brand's future.
0 notes
Text
Trademark Registration Process
The legal process of securing the owner's exclusive right to use a specific symbol, term, phrase, design, or combination to identify and set their goods and services apart is known as trademark registration. This is a thorough description of the steps involved in registering a trademark:

Initial Trademark Search Steps:
Make sure the trademark is distinct and not currently in use by conducting a comprehensive search. By doing this, disputes and other legal problems are avoided.
Submitting the Application Preparing the Application:
Choose a regular character mark (text only) and a special form mark (logo, design) for your trademark.
Determine the products or services that are linked to the trademark and are categorized using the Nice Classification system.
Send in the Application
Apply to the appropriate trademark office, such as the European Union Intellectual Property Office (EUIPO) in the EU, the United States Patent and Trademark Office (USPTO) in the US, or other national/regional offices.
Give the necessary details, such as the owner's identity, a list of the goods and services, a representation of the trademark, and the reason for filing (use in commerce or intent to use).
Exam Procedure
Official Review: The application is examined by the trademark office to make sure it conforms with official specifications.
Comprehensive Analysis: The office verifies that the application is unique and looks for trademark disputes.
Publishing and Counterpublications:
The public is informed when an application passes an examination and is published in a journal or official gazette.
Time of Opposition: a predetermined window of time, usually between 30 and 3 months, during which outside parties may submit an objection if they disagree that the trademark should be registered.
Enrollment:
Absence of Resistance or a Viable Resolution:
The trademark moves on to registration if no opposition is lodged or if opposition is dismissed in the applicant's favor.
The awarding of a certificate:
A registration certificate, which confers exclusive rights to the trademark, is issued by the trademark office.
Post-Registration Upkeep and Extension:
Generally, trademarks must be renewed regularly (every ten years in many jurisdictions).
Certain countries need periodic proof of use to keep the registration active.
Observation and Implementation:
Keep a regular eye out for any possible violations in the market.
Protect your trademark rights by going to court if required.
Madrid System for International Trademark Registration:
Regulated by the World Intellectual Property Organization (WIPO), which enables the submission of a single application to request protection across several of its member nations.
just a simple application and registration in the nation of origin.
Submit a worldwide application via the trademark office of your native nation.
The application is sent by WIPO to the chosen member nations for review and registration.
Businesses and individuals can obtain trademark protection by following these steps, guaranteeing that their brand identity is legally protected and solely theirs.
To know more information visit at www.corpbiz.io or can Contact on 9121230280
0 notes
Text
Expanding Your Brand Globally: A Guide to International Trademark Registration
Expanding your brand into international markets is an exciting and lucrative venture. However, with the global reach comes the crucial responsibility of protecting your brand identity. Trademarks, which include logos, slogans, and brand names, are fundamental assets that distinguish your products or services from competitors. Securing international trademark registration is essential to safeguard your brand across borders.
This comprehensive guide will walk you through the process, delivering valuable insights and practical steps for successful international trademark registration.

Understanding Trademarks and Their Importance
What is a Trademark?
A trademark is a symbol, word, or group of words legally registered or established by use as representing a company or product. It can include names, logos, slogans, sounds, and even colors that distinguish your goods or services from others in the market.
Why are Trademarks Important?
Brand Identity: Trademarks help consumers identify and differentiate your products from others, fostering brand loyalty.
Legal Protection: They provide legal protection against unauthorized use of your brand elements.
Business Asset: Trademarks are valuable intangible assets that can be appreciated over time.
Market Expansion: They enable smoother entry into new markets by protecting your brand in foreign jurisdictions.
Preparing for International Trademark Registration
Conducting a Trademark Search
Before filing for trademark registration, it’s crucial to conduct a comprehensive trademark search in your target markets. This step helps to ensure that your trademark is unique and not already in use, preventing potential legal disputes and rejection of your application.
Steps to Conduct a Trademark Search:
Identify Classes: Trademarks are categorized into different classes based on the type of goods or services. Identify the appropriate classes for your trademark.
Use Online Databases: Utilize online trademark databases such as the World Intellectual Property Organization (WIPO) Global Brand Database, the United States Patent and Trademark Office (USPTO) database, and other national databases to search for existing trademarks.
Professional Assistance: Consider hiring a trademark attorney or a professional search service to ensure a thorough search.
Evaluating Trademark Eligibility
Not all trademarks are eligible for registration. Your trademark must be distinctive, not descriptive or generic, and must not conflict with existing trademarks. Additionally, it must not contain misleading or offensive content.
Choosing the Right Markets
Identify the countries where you plan to expand and where trademark protection will be beneficial. Consider factors such as market potential, brand relevance, and local trademark laws. Prioritize countries with significant market opportunities and those where your brand is most likely to face competition or imitation.
Pathways to International Trademark Registration
There are several pathways to register your trademark internationally, each with its advantages and disadvantages. The choice depends on your business needs, budget, and target markets.
National Applications
You can file individual trademark applications directly with each country’s trademark office. This method allows for tailored applications according to specific national laws but can be time-consuming and expensive if you target multiple countries.
Pros:
Customized protection tailored to each country’s laws.
Direct communication with national trademark offices.
Cons:
High costs and administrative burden.
Varied registration procedures and timelines.
Regional Applications
For regions with unified trademark systems, such as the European Union (EU) and the African Intellectual Property Organization (OAPI), you can file a single application that covers all member countries.
Pros:
Simplified process for multiple countries within the region.
Cost-effective for regional protection.
Cons:
Limited to specific regions.
If opposed in one country, it can affect the entire application.
The Madrid System
The Madrid System, administered by WIPO, offers a centralized solution for registering trademarks in multiple countries through a single application. It currently covers over 120 countries, making it an efficient choice for broad international protection.
Pros:
Single application and fee for multiple countries.
Simplified management and renewal process.
Flexibility to designate additional countries later.
Cons:
Dependent on the basic application/registration in the home country for the first five years.
Not all countries are members.
Steps to Register Your Trademark Internationally Using the Madrid System
Basic Application or Registration
To use the Madrid System, you must first have a trademark application or registration in your home country (known as the basic application or basic registration). This serves as the foundation for your international application.
Filing the International Application
Submit your international application through your home country’s trademark office (Office of Origin). The application must include:
Your details and signature.
Representation of the trademark.
List of goods and services categorized according to the Nice Classification.
Designation of member countries where you seek protection.
Examination by WIPO
WIPO examines the application for compliance with formal requirements and then publishes it in the WIPO Gazette of International Marks. It then forwards the application to the trademark offices of the designated countries.
Examination by Designated Countries
Each designated country’s trademark office examines the application according to its national laws. They may approve, partially refuse, or completely refuse the application. You must respond to any refusals or objections within the given timeframe.
Registration and Protection
Once approved by a designated country, your trademark is protected in that country as if it were registered directly with its trademark office. The protection period is generally 10 years, with the possibility of renewal.
Managing and Enforcing International Trademarks
Renewing Your Trademark
Trademark registrations must be renewed periodically, typically every 10 years. The Madrid System allows you to manage renewals centrally, simplifying the process for multiple countries.
Monitoring and Enforcement
Regularly monitor the use of your trademark in international markets to detect unauthorized use or infringement. Take swift legal action against infringers to protect your brand’s integrity. Consider using trademark watch services and engaging local legal counsel for enforcement actions.
Licensing and Franchising
Consider licensing or franchising your trademark to third parties in foreign markets. This can provide additional revenue streams while expanding your brand’s presence. Ensure that license agreements are carefully drafted to protect your trademark rights and maintain quality control.
Tips for Successful International Trademark Registration
Seek Professional Assistance
Trademark registration, especially internationally, can be complex. Engage experienced trademark attorneys or agents who specialize in international trademark law to navigate the process effectively.
Plan Ahead
Trademark registration can take time, often several months to years. Plan your registration strategy well in advance of entering new markets to ensure your brand is protected when you launch.
Be Consistent
Maintain consistency in your trademark usage across all markets. This includes using the same logo, slogan, and brand name. Consistent usage strengthens your brand identity and legal protection.
Understand Cultural Differences
Be aware of cultural differences and language translations that may affect your trademark. Ensure that your trademark does not have unintended negative connotations in different languages or cultures.
Keep Records
Maintain comprehensive records of your trademark registrations, renewals, and enforcement actions. This documentation is vital for managing your trademark portfolio and defending your rights.
Final Words
Expanding your brand globally presents tremendous opportunities for growth and increased market presence. However, it also requires meticulous planning and proactive measures to protect your brand identity. International trademark registration is a critical step in this process, safeguarding your brand from infringement and ensuring legal protection in foreign markets.
By conducting thorough trademark searches, understanding the various pathways to registration, and effectively managing your trademarks, you can navigate the complexities of international trademark registration with confidence. Seek professional assistance, plan, and stay vigilant in monitoring and enforcing your trademark rights. With the right strategy, your brand can thrive on the global stage, enjoying the benefits of expanded reach and recognition.
Embark on your journey of global expansion with the assurance that your brand is well-protected, paving the way for sustained success and growth in the international arena.
0 notes
Text
Why You Should Register a Trademark in the EU
Registering a trademark in the European Union (EU) is a strategic move for businesses looking to protect their brand across a vast and economically powerful market. The process to register trademark EU offers comprehensive protection, covering all 27 member states with a single application. This not only simplifies the registration process but also provides significant cost savings compared to registering trademarks in individual countries.

Benefits of EU Trademark Registration
When you register a trademark in the EU, you gain exclusive rights to your brand across the entire European Union. This means your trademark is legally protected from infringement in all member states, offering a robust shield against unauthorized use. With the rise of e-commerce and the globalization of business, having a trademark registered in the EU ensures that your brand is secure in one of the world’s largest markets.
Another advantage of EU trademark registration is its simplicity. Instead of filing multiple applications in different countries, you submit a single application to the European Union Intellectual Property Office (EUIPO). Once approved, your trademark is protected across the entire EU. This streamlined process not only saves time but also reduces administrative burdens and costs.
The Registration Process
To register a trademark in the EU, you need to submit an application to the EUIPO. The application should include a clear representation of your trademark and a list of the goods or services you want it to cover. After submission, the EUIPO examines the application, and if no objections or oppositions are raised, your trademark is registered.
Conclusion
For any business looking to expand or operate within Europe, it’s essential to register a trademark in the EU. It’s an investment in your brand’s future, providing the protection and peace of mind needed to grow your business confidently across multiple countries.
#register trademark EU#eu trademark#eu trademark registration#trademark search eu#uk trademark registration
0 notes
Text

A Guide to Starting Your Own Skin Care Products Business
In recent years, the skincare industry has experienced exponential growth, driven by increasing awareness of skin health and beauty. If you're passionate about skincare and dream of starting your own business in this field, now is an opportune time to turn your aspirations into reality. Here's a comprehensive guide to help you kickstart your journey into the world of skincare product entrepreneurship.
Research and Planning
Market Analysis: Begin by researching the skincare market to identify trends, target demographics, and potential competitors. Analyze consumer preferences, emerging ingredients, and popular product categories to understand where your business can thrive.
Identify Your Niche: With a saturated market, finding a unique selling proposition (USP) is crucial. Consider specializing in organic, vegan, or cruelty-free products, targeting specific skin concerns like acne or aging, or catering to a particular demographic segment such as men or teenagers.
Product Development: Invest time and resources in developing high-quality skincare formulations. Partner with experienced chemists or formulators to create effective and safe products using proven ingredients. Conduct thorough testing to ensure efficacy, safety, and compliance with regulatory standards.
Legal and Regulatory Compliance
Business Registration: Register your skincare products business with the appropriate authorities in your country or region. Choose a legal structure such as sole proprietorship, partnership, or LLC (Limited Liability Company) based on your preferences and legal requirements.
Intellectual Property Protection: Consider trademarking your brand name, logo, and product formulations to safeguard your intellectual property rights. Consult with a legal expert to understand patent requirements and other legal considerations specific to the skincare industry.
Compliance with Regulations: Familiarize yourself with regulations governing the manufacturing, labeling, and marketing of skincare products. Ensure compliance with standards set by regulatory bodies such as the FDA (Food and Drug Administration) in the United States or the EU Cosmetics Regulation in Europe.
Branding and Marketing
Brand Identity: Develop a compelling brand identity that resonates with your target audience. Choose a memorable brand name, design a visually appealing logo, and craft a compelling brand story that communicates your values, mission, and commitment to skincare excellence.
Product Packaging: Invest in attractive and functional packaging that reflects your brand aesthetic and appeals to consumers. Consider eco-friendly packaging options to align with sustainability trends and attract environmentally-conscious customers.
Online Presence: Establish a strong online presence through a professional website and active presence on social media platforms like Instagram, Facebook, and Pinterest. Share engaging content, including skincare tips, product tutorials, and user-generated content to foster community engagement and brand loyalty.
Distribution and Sales
Retail Partnerships: Explore opportunities to collaborate with retail partners such as beauty salons, spas, boutiques, and specialty stores to distribute your skincare products. Attend trade shows and industry events to network with potential partners and showcase your products.
E-commerce Platforms: Leverage e-commerce platforms like Shopify, WooCommerce, or Amazon to sell your skincare products online. Optimize your product listings with high-quality images, detailed descriptions, and customer reviews to maximize visibility and sales.
Direct Sales Channels: Consider implementing a direct-to-consumer (DTC) sales model through your website or subscription-based services. Offer incentives such as discounts, loyalty programs, and free samples to attract and retain customers.
Continuous Improvement
Customer Feedback: Listen to customer feedback and reviews to identify areas for improvement and innovation. Use surveys, focus groups, and social media monitoring tools to gather valuable insights into customer preferences and expectations.
Stay Informed: Stay abreast of industry trends, scientific research, and technological advancements in skincare ingredients and formulations. Attend seminars, workshops, and conferences to expand your knowledge and network with industry experts.
Adapt and Innovate: Remain flexible and adaptable to changes in consumer preferences, market dynamics, and regulatory requirements. Continuously innovate your product offerings, marketing strategies, and business processes to stay ahead of the competition and sustain long-term growth.
0 notes
Text
Getting A European Trademark Attorney Through The Confusing World Of Trademark Law
Trademarks are very useful for ensuring that a brand's identity is protected and sticks out in the global market. However, when you enter new areas like Europe, the laws about trademarks require more work and are complicated to understand. Hiring a European Trademark Attorney can be a good idea if your business wants to protect its brand and grow into the European market.
What You Need To Know About Why You Need A European Trademark Attorney
A lawyer in Europe who specializes in trademark law will know everything there is to know about EU trademark law and how the EUIPO works. They give you strategic advice on protecting your brand, walk you through the complicated process of registering your trademark, and ensure you follow all local rules.
What You Can Do With The Help Of A European Trademark Attorney
It Is Now Easier To Register Trademarks.
To successfully register a trademark in Europe, you must learn about the European Union Intellectual Property Office (EUIPO) and the state trademark offices.
Streamlined Ways To Settle Disagreements
Brand name fights often cost a lot of time and money. European Trademark Attorneys can help you with opposition processes or infringement actions. Because they are experts in this field, businesses can quickly protect their trademarks and settle disagreements.
Using A Plan To Handle Trademarks
It Is Easy To Look For Trademarks.
Before applying for a trademark, carefully looking for any rights already out there is essential. To get a trademark, a European trademark attorney will search the internet for any possible issues and advise on the best way forward.
Always Keeping An Eye On Trademarks
It takes steady attention to keep a trademark safe. European Trademark Attorneys give monitoring services around the clock to help you find any trademark infringement or illegal use cases. This precaution helps keep the trademark's validity and value over time.
To Sum Up
A European trademark attorney can help you keep your name safe in a constantly changing market. It's a good investment for the growth of your business. You can learn more about the skilled trademark services a European trademark attorney offers and how they can help your business by visiting IP Consulting.
0 notes
Text
MDCG Guidance for Manufacturers of Class I Medical Devices

A medical device’s intended use and inherent risks must be considered when determining its MDR classification. Class I devices pose less risk to patients and end users, as under the previous MDD. The new Regulation EU MDR 2017/745 has added extended rules, leading some devices to fall under Class IIa, IIb, or even III.
This document is intended to guide Class I medical device manufacturers (excluding custom-made devices) that sell products bearing their name or trademark on the Union market to comply with MDR requirements. This guidance should also apply when an importer, distributor, or any other legal entity takes on the obligations owed by manufacturers.
The class I manufacturer must also hire a Notified Body (NB) to perform a conformity evaluation if medical devices are to be provided sterile, have measuring functions, or are reusable surgical instruments. For the NB to be qualified to conduct such a conformity assessment, the specific medical device in question must come within the purview of the Notified Body’s designation.
Large and mid-sized medical device manufacturers must also properly designate at least one individual in charge of regulatory compliance, but micro and small companies can function better by having a regulatory expert.
It is also important to designate an authorised representative residing within the EU if the manufacturer is registered outside of the EU. A foreign manufacturer must specifically issue a mandate outlining the representative’s duties and authority. It is also crucial to note that not all responsibilities associated with the medical equipment can be assigned.
The manufacturer must adequately preserve all the records required to verify compliance with the relevant requirements so they may be made available to the regulatory body upon request.
Steps to placing Class I medical devices on the market:
Manufacturers must ensure compliance with each of the following requirements if they plan to commercialise Class I medical devices. Please be aware that some of the outlined requirements are interconnected and can be completed in a different sequence than that which is shown.
The manufacturer will carry out a gap analysis for Class I devices that have already been released onto the market in compliance with the MDD to ensure that all of the below-listed requirements were satisfied at the time the MDR was applied.
The whole procedure includes a set of mandatory steps, including:
Step 0: Integrate MDR in the Quality Management System (QMS)
The manufacturer’s QMS should be appropriately linked with the standards outlined in the MDR. To ensure compliance with the following requirements, this will enable the correct assessment or decision to be taken and the appropriate documented evidence to be produced.
Step 1: Confirm product as a medical device
The intended purpose and the product’s mode of action are reviewed as per Article 2(1) of the MDR to ensure that the product qualifies as a medical device. If a product is assigned multiple intended purposes, it will qualify as a medical device only if all the intended purposes are covered in Article 2(1).
In the case of products identified as more than one category, the relevant legislation requirements will have to be followed. Despite not being medical devices in and of themselves, accessories to medical devices are subject to MDR regulations and qualify as devices under the MDR definition.
However, the MDR does not include accessories to devices covered by it as per Annex XVI of the MDR.
Step 2: Confirm product as a Class I medical device
The product has to be confirmed if it can be classified as a class I device as per Annex VIII of the MDR. Products previously classified as class I under MDD should be reviewed according to the classification rules of MDR to confirm if reclassification is required.
The MDD guideline cannot be used for devices that were moved from Class I to higher risk classes by the adoption of the MDR. The intended use of the device and any inherent risks related to the period of use, the part of the body, whether it is active or not, and whether it is invasive or non-invasive will determine how the classification standards are applied.
The classification rule with the highest class should be applied if the device in question falls under the purview of more than one classification rule due to its attributes.
Step 3: Procedures before placing on the market
a) Meet the General Safety and Performance Requirements (GSPR)
The devices will adhere to the general safety and performance standards outlined in Annex I of the MDR, considering the intended purposes that their manufacturers had provided.
The Risk management system is a continuous iterative process throughout the entire lifecycle of the product, established by the manufacturer that will enable the identification and analysis of the risks related to each device, the estimation and assessment of the risks associated with those risks, the elimination or control of residual risks, and the evaluation of the adopted measures based on the data gathered from the PMS system.
When a harmonised standard is in place, but a manufacturer chooses to use another reference, implementing that reference should, at the very least, ensure the same level of performance and safety.
Compliance with the relevant harmonised standards will give rise to a presumption that the MDR’s requirements, or portions of them, are also complied with.
If there are available standard specifications, the manufacturer must adhere to them unless they can adequately demonstrate that they have chosen a solution at least as effective and safe. The clinical evaluation processes, risk management, and PMS must be interdependent and updated regularly.
b) Conduct clinical evaluation
As part of the MDR’s technical documentation requirements, all devices—regardless of risk classification—require a clinical evaluation. The level of clinical evidence required to show compliance with the pertinent general safety and performance requirements listed in Annex I, which is obtained by considering the characteristics of the device and its intended purpose, shall be specified, and justified by the manufacturer.
Manufacturers must prepare, carry out, and record a clinical evaluation in compliance with Article 61 and Part A of Annex XIV to accomplish this.

Consideration of available alternative treatment options, the incorporation of clinical data and the acceptability of the benefit-risk ratio are required for carrying out a clinical evaluation.
Additional clinical data will be acquired or developed by clinical investigations if the currently available clinical data are insufficient to establish compliance with the MDR.
If the clinical data currently available for a device that is currently certified under Directive 93/42/EC is insufficient to show compliance with MDR, then post-market clinical follow-up studies of the device may be used to gather more clinical data.
Even data from the general post-market follow-up may occasionally be enough to close the difference.
a) Prepare technical documentation
The manufacturer is responsible for creating and maintaining the technical documentation to prove that their products adhere to the MDR’s technical specifications. Under Annex II and III, this technical documentation must be prepared before the EU declaration of compliance is drawn.
The manufacturer will develop and provide the technical documentation and, if applicable, it’s summary in a way that is unambiguous, clear, well-organised, and easy to search.
The manufacturer shall provide the CA, the authorised representative (where applicable), and NB with access to the technical documents (when applicable).
After reviewing the general safety and performance standards, as well as the pertinent technical provisions of the MDR, the technical documentation will be developed.
b) Request Notified Body involvement
Class I devices do not require the engagement of an NB for MDR compliance. But the manufacturer must follow the guidelines outlined in Chapters I and III of Annex IX or Part A of Annex XI of MDR when the product is a sterile equipment, measuring instrument, or reusable surgical tools.
For the relevant codes and corresponding types of devices as established by Regulation (EU) 2017/2185, manufacturers may select any NB designated in accordance with the MDR.
c) Prepare Instructions for Use and Labelling
Any safety and performance information required for a device’s safe usage and identification of the device should be provided along with the device by the manufacturer and/or the authorised representative, considering the possible users’ training and knowledge.
This data is included on the label, in the device packing, and in the instructions for use. Class I devices do not need instructions for use if they may be operated effectively and securely without them, deviating from the general rule.
Since Class Ir devices will need instructions for reprocessing (cleaning and sterilisation), an exception is most likely proposed.
Labelling and instructions for use must be written in accordance with national language regulations. There will be versions of the labelling and IFU in the technical documentation (in each pertinent national language).
The requirements regarding the information to be supplied with the device will be found in Annex I, Chapter III (23).
Step 4: Check compliance with general obligations for manufacturers
The manufacturer shall ensure that the general obligations for manufacturers outlined in Article 10 are met before releasing a device for sale.
Implementing a suitable QMS that will most effectively assure compliance with the MDR, such as through an internal audit, will receive special consideration. The QMS shall be documented, applied, maintained, updated regularly, and developed continuously, and it will at least include the following elements:
A strategy for regulatory compliance
Identification of applicable general safety and performance requirements and exploration of options to address those requirements
Responsibility of the management
Resource management, including selection and control of suppliers and sub-contractors
Risk management
Clinical evaluation, including post-market clinical follow-up (PMCF)
Product realisation, including planning, design, development, production and service provision
Verification of the UDI assignments
Setting up, implementation and maintenance of a post-market surveillance system
Handling communication with competent authorities, notified bodies, other economic operators, customers and/or other stakeholders
Processes for reporting serious incidents and field safety corrective actions in the context of vigilance
Management of corrective and preventive actions and verification of their effectiveness
Processes for monitoring and measurement of output, data analysis and product improvement.
According to applicable Union and national law, either natural or legal persons may seek compensation for harm brought on by a defective equipment.
In order to protect themselves against potential responsibility under Directive 85/374/EEC, manufacturers must take precautions that are commensurate to the risk class, type of device, and size of the business. These steps must not compromise further protective measures under national legislation.
Step 5: Draw-up the EU Declaration of Conformity
The process by which the manufacturer, who complies with the standards established by Article 52(7), certifies that the devices in question comply with the requirements of the MDR that apply to them is referred to in Article 19 as the EU declaration of conformity.
The CA will have access to the declaration of compliance, which must include, at a minimum, all of the data referred to in Annex IV. The manufacturer will regularly update the EU declaration of conformity and translate it into the official language(s) required by the Member States where the product is sold.
Suppose a device is subject to additional Union laws in addition to the MDR that also call for an EU declaration of conformity. In that case, the manufacturers will create a single EU declaration of conformity that refers to all applicable Union laws.
By creating the EU declaration of conformity, the manufacturer accepts liability for the device’s regulatory compliance with all relevant Union legislation.
For class Ir, Im, and Is devices, the manufacturer must obtain an EC certificate from NB per Annex IX, Chapters I and III, or Annex XI, Part A before applying a CE mark.
Step 6: Affix the CE marking
All Class I medical devices on the market must display the CE conformity label, which must be visible, readable, and permanent. It may be applied to the item or its sterile packaging. The CE marking must be applied to the package in cases where such affixing is impossible or unwarranted due to the nature of the device. The CE certification must be visible on all sales packaging and the instructions for use.
Placing the CE mark on a medical device signifies that it complies with all applicable safety and performance standards and is approved for marketing in the EU. The CE marking will be accompanied by the identification number of the relevant NB in the case of Class I medical devices placed on the market in a sterile condition, devices with measurement functions, and/or reusable surgical tools.
Affixing marks that could lead third parties astray about the significance of the CE marking is banned. Other extra markings may be applied to the product, the packaging, or the usage instructions, but they must not obscure or obstruct the CE marking.
The CE marking format will follow Annex V requirements. The minimum dimensions required for the CE mark may not apply to very small devices.
Step 7: Registration of devices and manufacturers in Eudamed
A streamlined approach might be used for medical devices that were previously put on the market per the Directives, provided that the manufacturer evaluates the gap and ensures that all regulations are properly followed.
A Class I medical device manufacturer must register the product with Eudamed before putting it on the market. Suppose the information listed in Section 1 of Part A of Annex VI has not already been registered in accordance with Article 31. In that case, the manufacturer must submit it to the electronic system in order to register the device. The information referred to in Section 1 of Part A of Annex VI will be given to that electronic system before applying to the NB in situations where the conformity assessment method necessitates the engagement of an NB in accordance with Article 52.
Following the CA’s validation of the manufacturer’s data in Eudamed, the manufacturer will receive an SRN from the aforementioned electronic system. To fulfil its duties under Article 29, the manufacturer will use the SRN when submitting an application to an NB for a conformity assessment and to gain access to Eudamed.
The registration of a device in Eudamed by the manufacturer includes:
Assigning a UDI-DI (with a Basic UDI-DI) as described in Part C of Annex VI to the device in accordance with the issuing entity’s policies mentioned in Article 27(2) and adding the UDI-DI (with a Basic UDI-DI) to the UDI database along with the other core data elements pertaining to that device mentioned in Part B of Annex VI.
Entering the data referred to in Section 2 of Part A of Annex VI, excluding Section 2.2 thereof, or, if previously provided, validating the data, and then keeping the data current up to date.
This document summarises the information presented above and outlines how Class I medical devices should be introduced to the EU market. The guidance’s scope also includes reusable surgical instruments (Class Ir), sterile medical devices (Class Is), and devices with measurement capabilities. These medical devices all need a Notified Body to be involved in pre-marketing procedures.
Originally Published at: https://omcmedical.com/mdcg-guidance-manufacturers-class-i-medical-devices/
0 notes
Text

Does Alex have any relationship with Nobleman magazine? Alex’s business relationship has simply become SH’s public relations. He does not offer solutions or services for the alcohol business they have lost. There’s not much He knows how to do. It’s just SH’s tool 🤷♀️

@directorlady If you are interested, I already wrote about his situation you can scroll through my blog. SH’s Sassenach mark was totally refused in the European Union 🇪🇺 (27 states). He can’t register his booze with that name. Also in Europe, in Germany 🇩🇪 (Deutsches Patent-und Markenamt) his trademark “The Sassenach” received total refusal in respect of all goods and services.
@directorlady You’re welcome. The business they have lost has nothing to do with profits but with the market. He may not have a healthy cash book. He thought it was about getting an easy market in the EU, and spent two years in a costly legal battle that he lost and as a consequence did not obtain the expected income that he was counting on. He created misleading information about SS on his company website while he was still in litigation, that SS was available to order in the EU and also mentioned Canada, where SS also does not reach. As you know, trademark rights (like patent rights) are territorial and must be affirmatively established in each country (or region such as the EU) of interest. So unless it’s a super famous brand (which SS isn’t), you’re out of luck in Europe. Its brand is similar to another company previously registered in the EU. They have to change the name of the product to enter the market.
15 notes
·
View notes