#DSCSA Software
Explore tagged Tumblr posts
Text
Track And Trace Solutions Market Is Anticipated to Witness High Growth Owing to Enhanced Supply Chain Visibility

The Global Track And Trace Solutions Market is estimated to be valued at US$ 4,622.4 Mn in 2025 and is expected to exhibit a CAGR of 14.79% over the forecast period 2025 to 2032.
Track And Trace Solutions enable companies across pharmaceuticals, food & beverage, electronics, and automotive sectors to monitor product movement in real time, ensuring authenticity, safety, and compliance. These systems combine barcode scanning, RFID tags, IoT sensors, and cloud-based software to capture and analyze data at each checkpoint—manufacturing, warehousing, distribution, and retail. The solutions deliver advantages such as reduced counterfeiting, minimized recall costs, improved regulatory adherence, and heightened consumer trust. Track And Trace Solutions Market Insights an era of complex global supply chains, businesses require end-to-end traceability to respond rapidly to disruptions, manage market challenges, and glean actionable market insights. Increasing regulatory mandates, such as the FDA’s DSCSA in the U.S. and the EU Falsified Medicines Directive, have accelerated adoption of these platforms. Moreover, integration with advanced analytics and AI-driven predictive models supports proactive decision-making and business growth. As organizations seek to optimize inventory, enhance transparency, and bolster brand reputation, the need for scalable track and trace solutions grows.
Get more insights on,Track And Trace Solutions Market
#Coherent Market Insights#Track And Trace Solutions#Track And Trace Solutions Market#Track And Trace Solutions Market Insights#Hardware Systems#Software Solutions
0 notes
Text
AI and IoT Integration Transform the Track and Trace Solutions Market
Track and Trace Solutions Market Growth & Trends
The global Track and Trace Solutions Market is experiencing significant growth, driven by various factors, with a projected size of USD 14.3 billion by 2030, expanding at a CAGR of 19.3% from 2023.
Key Drivers:
Brand Protection from Counterfeit Products and Theft: This is a major factor, especially for pharmaceutical and biopharmaceutical companies, as track and trace solutions help enhance distribution channel efficiency and reduce the prevalence of fake products.
Regulatory Compliance: Governments and regulatory authorities worldwide are increasingly implementing stringent regulations (e.g., US Drug Supply Chain Security Act (DSCSA), EU Falsified Medicines Directive (FMD)) that mandate serialization and traceability to ensure product authenticity, safety, and combat illegal supply chains.
Enhanced Supply Chain Transparency and Efficiency: These solutions provide real-time visibility into product movement, aiding in inventory management, managing recalls, and improving overall supply chain operations.
Technological Advancements: The introduction and integration of advanced technologies like RFID, 2D barcodes, IoT, AI, and blockchain are significantly boosting the capabilities and adoption of track and trace systems.
Rising Adoption by Healthcare Manufacturers: The growing medical device and pharmaceutical industries are increasingly deploying these solutions to safeguard their products and reputation.
COVID-19 Outbreak: The pandemic highlighted the critical need for efficient monitoring and tracing technologies, especially for medical supplies and vaccines, leading to increased adoption and innovation in the market. Seizures of false COVID-19 tests and PPE, and initiatives like Smartrac's partnership with SUKU and OPTEL's collaboration with Bureau Veritas for vaccine supply chain logistics, demonstrate this impact.
Challenges:
High Deployment Cost: The initial cost of implementing serialization and aggregation solutions, including hardware, software, and integration with existing systems, can be a significant barrier, particularly for small and medium-sized enterprises (SMEs).
Lack of Common Regulations and Standards: Inconsistent regulations across different geographies can complicate implementation and hinder market growth in some developing regions.
Data Security and Privacy Issues: Handling large volumes of sensitive product and supply chain data requires robust cybersecurity measures to prevent breaches and unauthorized access.
Key Trends:
Serialization as a Prime Method: Serialization, which involves assigning a unique identifier to each product, is a crucial method facilitating easy track and trace globally.
Growth in Software Solutions: Software solutions hold a significant market share due to their ability to provide end-to-end traceability, data management, and compliance with global standards.
Increasing Use of 2D Barcodes and RFID: These technologies are gaining traction due to their ability to store more data and provide fast, secure reading.
Strategic Collaborations and Partnerships: Companies are actively forming alliances to enhance their offerings and expand their market presence.
Overall, the global track and trace solutions market is on a strong growth trajectory, driven by the increasing need for product authenticity, supply chain transparency, and stringent regulatory mandates across various industries, particularly pharmaceuticals and healthcare.
Curious about the Track and Trace Solutions Market? Download your FREE sample copy now and get a sneak peek into the latest insights and trends.
Track and Trace Solutions Market Report Highlights
The software solutions product segment was the largest revenue-generating segment in 2022. This is mainly because of the regulatory mandates for the execution of serialization and aggregation in the healthcare sector
The serialization solutions application segment was the largest grossing segment in 2022. Increasing application of serialization in pharmaceutical and medical device packaging will be a vital factor contributing to the segment growth
The RFID technology segment is expected to exhibit the fastest growth rate during the forecast period. The key factors contributing to the growth of the segment are technological advantages, such as high durability and reusability, more data storage capacity, and no requirement of the line of sight
North America led the global market in 2022. The rising implementation of regulatory standards and regulations, along with the high adoption rate of track and trace solutions by consumers, is anticipated to contribute to the market growth
Track and Trace Solutions Market Segmentation
Grand View Research has segmented the global track and trace solutions market on the basis of product, technology, application, end-use, and region:
Track And Trace Solutions Product Outlook (Revenue, USD Million, 2017 - 2030)
Hardware Systems
Printing & Marking Solutions
Monitoring & Verification Solutions
Labeling Solutions
Others
Software Solutions
Plant Manager Software
Line Controller Software
Bundle Tracking Software
Others
Track And Trace Solutions Technology Outlook (Revenue, USD Million, 2017 - 2030)
Barcode
RFID
Track And Trace Solutions Application Outlook (Revenue, USD Million, 2017 - 2030)
Serialization Solutions
Bottle Serialization
Label Serialization
Carton Serialization
Data Matrix Serialization
Aggregation Solutions
Bundle Aggregation
Case Aggregation
Pallet Aggregation
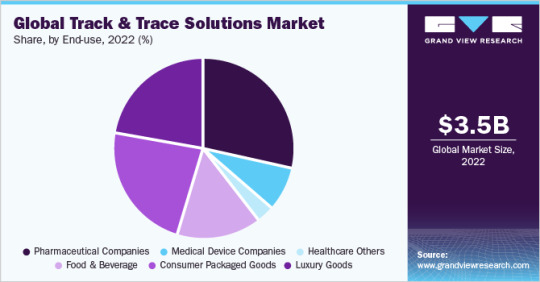
Track And Trace Solutions End-use Outlook (Revenue, USD Million, 2017 - 2030)
Pharmaceutical Companies
Medical Device Companies
Healthcare Others
Food and Beverage
Consumer Packaged Goods
Luxury Goods
Download your FREE sample PDF copy of the Track and Trace Solutions Market today and explore key data and trends.
0 notes
Text
North America Healthcare Logistics Market Size, Share, Trends, Demand, Future Growth, Challenges and Competitive Analysis
"North America Healthcare Logistics Market - Size, Share, Demand, Industry Trends and Opportunities
North America Healthcare Logistics Market, By Type (Cold Chain and Non-Cold Chain), Component (Hardware, Software, and Services), Temperature Type (Ambient, Chilled/Refrigerated, Frozen, and Cryogenic), Logistics (Transportation, Packaging, Storage, and Others), Logistic Type (Sea Freight Logistics, Air Freight Logistics, Overland Logistics, and Contract Logistics), Application (Medicine, Bulk Drug Handlers, Vaccine, Chemical and Other Raw Material, Biological Material and Organs, Hazardous Cargo, and Others), End User (Biopharmaceutical Companies, Hospitals and Clinics, Research Institutes, and Others) – Industry Trends.
Get the PDF Sample Copy (Including FULL TOC, Graphs and Tables) of this report @
**Segments**
The North America Healthcare Logistics Market can be segmented based on the type of services, including transportation, warehousing, and others. The transportation segment encompasses air freight, ocean freight, road freight, and rail freight services. Warehousing services involve inventory management, order processing, and distribution center management. Other services may include cold chain logistics, medical equipment logistics, and pharmaceutical logistics, among others. The market can also be segmented by end-user, such as hospitals, clinics, pharmaceutical companies, medical device companies, and others.
**Market Players**
- DB Schenker - DHL International GmbH - FedEx - Kuehne+Nagel - XPO Logistics, Inc. - UPS Supply Chain Solutions - C.H. Robinson Worldwide, Inc. - Agility - DSV - Penske Logistics
The North America Healthcare Logistics Market is witnessing significant growth due to various factors. One of the key drivers is the increasing demand for efficient healthcare services and the need for streamlined logistics operations in the healthcare industry. The rising prevalence of chronic diseases, coupled with the aging population in the region, is driving the demand for healthcare logistics services. Moreover, advancements in technology, such as real-time tracking and temperature-sensitive monitoring, are enhancing the efficiency and reliability of healthcare logistics operations.
The market is also experiencing a shift towards specialized logistics services, such as cold chain logistics for temperature-sensitive pharmaceuticals and medical devices. This trend is driven by the growing focus on maintaining the integrity and efficacy of healthcare products throughout the supply chain. Additionally, the increasing adoption of e-commerce in the healthcare sector is creating new opportunities for logistics providers to offer customized and on-demand services to healthcare companies.
However, the North America Healthcare Logistics Market is not without its challenges. One of the major hurdles facing the market is the complex regulatory environment governing the transportation and storage of healthcare products. Compliance with regulations such as Good Distribution Practice (GDP) and the Drug Supply Chain Security Act (DSCSA) requires significant investments in infrastructure and technology, which can pose a barrier to entry for smaller logistics companies.
Another challenge is the rising operational costs associated with healthcare logistics, particularly in terms of maintaining specialized facilities and equipment for handling sensitive medical products. Fluctuations in fuel prices and transportation costs also impact the profitability of healthcare logistics providers, highlighting the need for efficient route planning and cost management strategies.
In conclusion, the North America Healthcare Logistics Market is poised for steady growth driven by the increasing demand for specialized logistics services in the healthcare industry. By adapting to evolving market trends and addressing key challenges, market players can capitalize on the opportunities presented by this dynamic sector.
Access Full 350 Pages PDF Report @
The report provides insights on the following pointers:
Market Penetration: Comprehensive information on the product portfolios of the top players in the North America Healthcare Logistics Market.
Product Development/Innovation: Detailed insights on the upcoming technologies, R&D activities, and product launches in the market.
Competitive Assessment: In-depth assessment of the market strategies, geographic and business segments of the leading players in the market.
Market Development: Comprehensive information about emerging markets. This report analyzes the market for various segments across geographies.
Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the North America Healthcare Logistics Market.
This study answers to the below key questions:
What are the key factors driving the North America Healthcare Logistics Market?
What are the challenges to market growth?
Who are the key players in the North America Healthcare Logistics Market?
What are the market opportunities and threats faced by the key players?
Browse Trending Reports:
Europe Flight Data Recorder Market Asia Pacific Flight Data Recorder Market Middle East And Africa Flight Data Recorder Market North America Flight Data Recorder Market Europe Potato Processing Market Asia Pacific Potato Processing Market Middle East And Africa Potato Processing Market North America Potato Processing Market Australia And New Zealand Concrete Admixture Market Saudi Arabia q Pcr Reagents Market Australia Specialty Gas Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email: [email protected]
"
0 notes
Text
Global Manufacturing Execution Systems (MES) Market 2025: Key Trends, Growth Drivers, and Regional Analysis
Market Overview
The global Manufacturing Execution Systems (MES) market is expanding due to the increasing complexity of manufacturing processes, growing adoption of industrial automation in both process and discrete industries, and the importance of regulatory compliance. Additionally, the integration of MES with enterprise resource planning (ERP) and product lifecycle management (PLM) solutions, along with rising MES applications in the pharmaceutical industry, are expected to present significant opportunities for market players. However, challenges such as the complexities of MES deployment in various industries and the need for substantial capital investment, along with ongoing maintenance costs, may hinder market growth.
Get Sample Copy @ https://www.meticulousresearch.com/download-sample-report/cp_id=5446
Impact of COVID-19 on the MES Market
The COVID-19 pandemic disrupted multiple sectors, leading to temporary shutdowns or reduced operations for many manufacturers. The lockdowns and supply chain disruptions created uncertainties, making it difficult for businesses to predict market recovery. The pandemic led to structural shifts with lasting implications for the MES market. Due to halted manufacturing activities and reduced productivity, several market players experienced significant losses. Many manufacturing facilities closed, particularly in sectors like power generation, oil and gas, and automotive. As industries adapted to new operational guidelines post-pandemic, the demand for MES is expected to rise to ensure smooth and efficient operations.
Key Market Drivers and Opportunities
Pharmaceutical Industry Adoption The pharmaceutical industry is increasingly adopting MES to manage evolving trends, reduce production costs, and comply with regulatory standards. MES systems help improve product traceability, manage quality through barcodes and RFID tags, and meet global track and trace regulations. Compliance with the European Union's Falsified Medicines Directive (FMD) and the U.S. FDA's Drug Supply Chain Security Act (DSCSA) is driving this demand. These systems ensure safe manufacturing processes, reducing risks associated with counterfeit drugs.
Service Segment Growth The MES market is divided into software and services based on the offering. The services segment is expected to record the highest compound annual growth rate (CAGR) during the forecast period. The post-implementation services, including software upgrades, training, and ongoing support, are crucial for maintaining MES systems' efficiency. Improved manufacturing processes, reduced waste, and shorter output times are contributing to this segment's growth.
Hybrid Deployment Gaining Traction MES deployment modes include on-premise, on-demand, and hybrid models. The hybrid deployment model is projected to see the fastest growth, particularly in oil & gas and energy & power industries. Hybrid models offer real-time monitoring capabilities and additional storage capacity, enhancing data protection and operational efficiency.
Quality Management as a Leading Application Among MES applications, quality management is set to achieve the highest growth. MES systems are used extensively in regulated industries like food & beverages and pharmaceuticals to monitor and control production processes, ensuring high-quality outputs. The ability to integrate MES with quality control systems allows manufacturers to maintain compliance with stringent quality standards.
Process Industry to Lead Market Growth The market is categorized into process and discrete industries. The process industry segment, including sectors like food & beverages, oil & gas, chemicals, pharmaceuticals, and energy, is expected to grow the fastest. The need for adaptable manufacturing processes to meet dynamic consumer demands is a key growth driver in this segment.
Get Full Report @ https://www.meticulousresearch.com/product/manufacturing-execution-systems-market-5446
Regional Market Insights
The global MES market is segmented into North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa. Asia-Pacific is expected to register the highest CAGR during the forecast period, driven by:
Technological investments in industrial tools and machinery production.
Growth in the semiconductor industry.
Increasing MES adoption in chemical and automotive industries.
Expanding R&D investments in countries like China and India.
China's significant investments in artificial intelligence (AI) for the pharmaceutical sector are also boosting the MES market. The rising number of manufacturing facilities across various industries, such as automotive, textiles, power, and pharmaceuticals, further supports market expansion.
Segment Analysis
1. By Offering:
Software: Core MES solutions enabling real-time production management and control.
Services: Implementation, software upgrades, training, and other support services, ensuring MES systems' effective operation.
2. By Deployment Mode:
On-Premise: MES software hosted on local servers, providing control over data but requiring higher initial investment.
On-Demand (Cloud-Based): Offers scalability and reduced infrastructure costs but depends on internet reliability.
Hybrid: Combines on-premise and cloud benefits, preferred for critical industries needing robust data management and flexibility.
3. By Application:
Monitoring: Real-time tracking of production processes.
Production Control & Documentation: Streamlines production workflows and maintains accurate records.
Inventory & Maintenance Management: Enhances supply chain efficiency and equipment reliability.
Quality Management: Critical for maintaining product standards, particularly in highly regulated sectors.
4. By End-use Industry:
Process Industries: Includes food & beverages, pharmaceuticals, chemicals, energy, and water & wastewater.
Discrete Industries: Encompasses automotive, aerospace, consumer packaged goods, and medical devices.
Leading Market Players
The MES market features several key players focusing on product innovation and strategic expansions. Notable companies include:
Siemens AG (Germany)
Rockwell Automation, Inc. (U.S.)
SAP SE (Germany)
ABB Ltd (Switzerland)
Dassault Systèmes S.A. (France)
AVEVA Group Plc (U.K.)
Applied Materials Inc. (U.S.)
Oracle Corporation (U.S.)
General Electric Company (U.S.)
Emerson Electric Co. (U.S.)
Epicor Software Corporation (U.S.)
Infor Equity Holdings LLC (U.S.)
Eyelit Inc. (Canada)
Aegis Industrial Software Corporation (U.S.)
Critical Manufacturing, S.A. (Portugal)
These companies are adopting strategies like mergers, acquisitions, and new product launches to enhance their market share.
Future Market Trends
Increasing Use of AI and IoT: Enhancing MES functionalities, predictive maintenance, and operational efficiency.
Growing Demand in the Automotive Sector: MES helps manage complex production lines and supports the industry's shift towards electric vehicles.
Adoption in Small and Medium Enterprises (SMEs): Cloud-based MES solutions are making advanced manufacturing capabilities accessible to smaller players.
The MES market is poised for robust growth, driven by advancements in industrial automation, regulatory pressures, and the need for efficiency in manufacturing processes. As industries continue to digitize and streamline operations, MES systems will play a crucial role in achieving operational excellence. Asia-Pacific presents significant growth opportunities due to its expanding industrial base and increasing technological investments. Overcoming challenges related to deployment complexities and high costs will be critical for market players to fully capitalize on emerging opportunities.
Get Sample Copy @ https://www.meticulousresearch.com/download-sample-report/cp_id=5446
0 notes
Text
Regulatory Challenges in the Digital Age: Key Discussions from the 2025 Pharma Congress
Regulatory Challenges in the Digital Age: Key Discussions from the 2025 Pharma Congress Introduction
The pharmaceutical industry is undergoing a transformative shift driven by digital innovations, AI-powered drug discovery, and blockchain-based supply chain management. However, these advancements bring regulatory challenges that require a dynamic and forward-thinking approach. The 15th Digital Pharmaceutical Innovations Exhibition & Congress (May 14–16, 2025) will serve as a critical platform to discuss these evolving challenges.
Regulatory bodies worldwide are working to keep pace with new technologies, balancing patient safety with innovation. As part of the Silver Sponsorship at this prestigious event, we will explore the most pressing regulatory hurdles and share expert insights on how the industry can adapt to this rapidly changing environment.
Key Keywords:
Digital Transformation in Pharma
Regulatory Challenges in Drug Discovery
AI and Compliance in Pharmaceuticals
Blockchain in Pharma Supply Chains
FDA & EMA Digital Regulations
Data Privacy and Cybersecurity in Pharma
Future of Digital Pharma Compliance
Global Regulatory Harmonization
Digital Health Regulation
Ethical AI in Pharma
Key Discussions from the 2025 Pharma Congress
1. AI and Machine Learning in Drug Development: Regulatory Dilemmas
Artificial Intelligence is revolutionizing pharmaceutical R&D, but it also raises concerns about data integrity, algorithmic transparency, and regulatory oversight. Regulators like the FDA and EMA are working on guidelines for AI validation and compliance, but there’s still a long way to go.
One of the key discussions at the congress will be the need for explainability in AI-driven drug development. Regulators are pushing for "glass-box" AI models over "black-box" systems to ensure transparency and accountability in clinical decisions. Companies leveraging AI will need to integrate robust validation and monitoring mechanisms to comply with evolving regulations.
2. Blockchain and Supply Chain Transparency: Compliance Hurdles
Blockchain technology promises tamper-proof, transparent tracking of drugs from manufacturing to end users. However, global regulatory frameworks are still catching up with the legal and technical implications of decentralized ledgers in the pharmaceutical supply chain.
For example, the U.S. Drug Supply Chain Security Act (DSCSA) mandates serialized tracking of prescription drugs. Blockchain can support compliance, but regulatory agencies require interoperability and governance standards. The congress will explore best practices for integrating blockchain with existing regulatory frameworks.
3. Digital Therapeutics and Personalized Medicine: Approval Pathways
As digital therapeutics and AI-driven personalized medicine gain traction, regulatory agencies must refine approval processes. The challenge is determining how traditional drug approval frameworks can adapt to software-based treatments and AI-driven diagnostics.
A key concern is the Software as a Medical Device (SaMD) classification. Regulators must define risk-based categories for digital therapeutics, ensuring they meet safety and efficacy standards without stifling innovation. The congress will host discussions on accelerating regulatory approvals while maintaining patient safety.
4. Data Privacy, Cybersecurity, and Compliance Risks
With the rise of digital health records and cloud-based drug development platforms, data security is a major concern. The pharma industry must navigate stringent regulations such as GDPR, HIPAA, and the evolving AI Act to ensure compliance without hindering innovation.
Cybersecurity threats, including ransomware attacks and data breaches, pose significant risks to pharmaceutical companies handling patient data. Regulatory agencies are introducing stricter penalties for non-compliance, making it crucial for companies to implement robust cybersecurity frameworks. Experts at the congress will discuss proactive strategies to mitigate digital security threats.
5. Real-World Evidence (RWE) and Regulatory Acceptance
Regulatory agencies are increasingly looking at real-world evidence (RWE) to support drug approvals, but there are gaps in standardization and validation. The congress will explore how regulatory bodies are shaping policies to integrate RWE into decision-making.
One of the key challenges is ensuring data integrity and minimizing bias in real-world data collection. The congress will feature case studies on successful RWE adoption and discuss strategies for standardizing methodologies across global markets.
6. Global Regulatory Harmonization: Bridging the Gaps
Pharmaceutical companies operating in multiple markets face challenges in complying with diverse regulatory requirements. The lack of harmonized guidelines can slow down drug approvals and increase operational complexity.
The International Council for Harmonization (ICH) is working on global regulatory alignment, but there are still inconsistencies in areas like AI governance, digital therapeutics approval, and data privacy. The congress will highlight efforts to streamline regulatory compliance across regions and foster greater collaboration between agencies.
Q&A: Addressing the Benefits and Challenges
Q1: How do regulatory bodies approach AI in drug development? A1: The FDA and EMA are actively working on guidelines to standardize AI validation, focusing on transparency, bias mitigation, and data security. Companies must ensure their AI models comply with evolving regulatory frameworks.
Q2: What are the major compliance risks associated with blockchain in pharma? A2: While blockchain enhances transparency, it poses challenges in regulatory recognition, cross-border compliance, and data governance. Companies must align with regulatory guidelines to avoid legal complications.
Q3: How does data privacy regulation impact pharmaceutical digitalization? A3: Regulations such as GDPR and HIPAA impose strict data protection rules. Pharma companies need robust cybersecurity strategies to safeguard patient data and ensure regulatory compliance.
Q4: Why is real-world evidence (RWE) becoming crucial in regulatory approvals? A4: RWE provides valuable insights into a drug’s real-world performance, improving regulatory decision-making. However, standardizing RWE collection and analysis remains a challenge.
Q5: How can global regulatory harmonization benefit the pharma industry? A5: A unified regulatory framework can reduce approval timelines, cut compliance costs, and facilitate faster market entry for innovative drugs. The congress will address collaborative initiatives toward harmonization.
Conclusion
The digital age presents both opportunities and regulatory challenges for the pharmaceutical industry. As companies embrace AI, blockchain, and digital therapeutics, compliance strategies must evolve in parallel. The 15th Digital Pharmaceutical Innovations Exhibition & Congress is the perfect forum to discuss these critical issues and shape the future of digital pharma regulations.
Join the conversation and register today: https://pharmacy.utilitarianconferences.com/registration
Hashtags:
#DigitalPharma #PharmaRegulations #AIinPharma #BlockchainPharma #PharmaCompliance #DrugDiscovery #DigitalTransformation #PharmaTech #PharmaCongress2025 #RegulatoryHarmonization #RealWorldEvidence #SaMD #CybersecurityInPharma
👉 Register here: https://pharmacy.utilitarianconferences.com/registration
Website: https://utilitarianconferences.com/
Twitter: @UCGConferences LinkedIn :https://www.linkedin.com/feed/
To know more abouts topics:- https://youtu.be/qHB0286VJSI?si=rGRqgamVnV7ZNkyT
0 notes
Text
Investing in the Track and Trace Solutions Market: Key Considerations and Opportunities
The global track and trace solutions market revenue is set for significant growth, with a projected compound annual growth rate (CAGR) of 19.1% from 2023 to 2030. Valued at USD 3.51 billion in 2022, the market is expected to reach USD 14.21 billion by 2030. The increasing need for effective tracking systems across various industries is driving this robust expansion.
Track and trace solutions are designed to monitor the movement of products throughout the supply chain, from manufacturing to the end consumer. These systems enable businesses to track goods in real time, ensuring transparency, reducing counterfeit risks, and maintaining compliance with regulatory requirements. The solutions play a vital role in sectors such as pharmaceuticals, food & beverages, consumer goods, and logistics.
Key Factors Driving Market Growth
The surge in demand for track and trace solutions is primarily fueled by the increasing need for transparency and accountability across supply chains. In the pharmaceutical industry, for instance, stringent regulations around the world mandate the use of track and trace systems to prevent counterfeit drugs from entering the market. Governments and regulatory bodies are enforcing strict compliance standards to ensure product safety, which has led to widespread adoption of these solutions by manufacturers and distributors.
Moreover, the growing global trade of consumer goods has also emphasized the need for sophisticated track and trace technologies. Companies across the world are investing in systems that help them maintain end-to-end visibility of their supply chains, ensuring that products are delivered safely and efficiently. Technologies such as RFID, barcode scanners, and cloud-based tracking software are increasingly being integrated to provide seamless tracking and monitoring of goods.
Get Free Sample Report: https://www.snsinsider.com/sample-request/1902
Technological Advancements and Integration
The track and trace solutions market is experiencing rapid technological advancements, particularly with the integration of artificial intelligence (AI) and the Internet of Things (IoT). AI-powered analytics can help companies predict potential disruptions in the supply chain, enabling them to make proactive decisions. Meanwhile, IoT devices facilitate real-time tracking, offering precise information on the location and condition of products during transit.
Blockchain technology is also gaining traction in this market, as it offers secure, tamper-proof tracking systems that ensure data integrity and transparency. Companies are increasingly adopting blockchain-based solutions to enhance trust and improve collaboration across the supply chain.
Regional Market Insights
North America currently dominates the track and trace solutions market, driven by stringent regulatory frameworks and the high adoption rate of advanced technologies. The United States, in particular, has seen significant investments in the implementation of track and trace systems in the pharmaceutical sector to comply with the Drug Supply Chain Security Act (DSCSA).
Europe is also a prominent market, with strong government regulations aimed at combating counterfeit goods. However, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period. Factors such as expanding pharmaceutical manufacturing, rising exports, and increased adoption of digital technologies are contributing to this growth. Countries like China, India, and Japan are investing heavily in track and trace technologies to ensure product authenticity and safety.
Future Outlook
The track and trace solutions market is poised for sustained growth, supported by rising concerns over product safety, the need for efficient supply chain management, and increasing regulatory compliance requirements. With the expansion of global trade, companies are more than ever seeking robust systems that provide visibility, traceability, and assurance of product quality throughout the supply chain.
In conclusion, the track and trace solutions market, which was valued at USD 3.51 billion in 2022, is on track to reach USD 14.21 billion by 2030, reflecting a strong CAGR of 19.1% over the forecast period. The adoption of advanced technologies, coupled with regulatory mandates and the need for effective supply chain management, will continue to drive market growth in the years to come.
Other Trending Reports
Liver Cancer Therapeutics Market Size
Biotechnology Reagents & Kits Market Size
Automated Liquid Handling Technologies Market Size
Biotechnology Market Size
0 notes
Text

What is EPCIS 2.0?
The world of pharmacy distribution is constantly evolving, driven by technological advancements that promise to enhance efficiency and traceability. One such groundbreaking innovation is EPCIS 2.0. But what exactly is EPCIS 2.0, and how can it benefit pharmacy distributors? In this blog post, we'll explore the intricacies of EPCIS 2.0, its applications, and why it's pivotal for the pharmaceutical industry.
Understanding EPCIS 2.0
EPCIS, or Electronic Product Code Information Services, is a global standard for sharing supply chain visibility data. The updated EPCIS 2.0 enhances data sharing and interoperability, allowing businesses to track product movements and statuses with detailed information. This is especially valuable for pharmacy distributors to ensure accurate tracking from manufacturer to patient.
Why EPCIS 2.0 Matters
EPCIS 2.0 boosts supply chain transparency and efficiency in the pharmaceutical industry. It offers real-time tracking to reduce counterfeit risks and automates data collection to streamline processes, resulting in fewer errors and faster deliveries.
Improved Data Sharing
EPCIS 2.0 enables seamless data sharing between stakeholders, crucial for maintaining supply chain integrity in the pharmaceutical sector. It offers traceability for defective medication, aiding compliance with regulations. A DSCSA study found over 90% of pharmacy distributors experienced better compliance and reduced administrative burdens after adopting EPCIS standards.
Interoperability with Existing Systems
EPCIS 2.0 is designed to integrate smoothly with existing enterprise systems. For pharmacy distributors, this means they can leverage their current infrastructure while reaping the benefits of enhanced data visibility. Whether it's an ERP system, warehouse management software, or transportation management system, EPCIS 2.0 ensures compatibility and easy implementation.
Real-World Applications
Several leading pharmacy distributors have already begun to implement EPCIS 2.0, showcasing its real-world benefits. For example, Drugzone, a prominent name in the pharmaceutical industry, reported a 25% increase in operational efficiency and a 15% reduction in shipment errors after integrating EPCIS 2.0 into their processes.
Boosting Competitive Advantage
In a competitive market, staying ahead often means adopting the latest technologies. EPCIS 2.0 not only improves operational efficiency but also provides a competitive edge. Pharmacy distributors that utilize this standard can offer better service reliability, which can be a significant differentiator in the market.
Conclusion
EPCIS 2.0 is a crucial advancement in pharmacy distribution, boosting transparency, efficiency, and compliance. For pharmacy distributors aiming to stay competitive, adopting EPCIS 2.0 is essential. Learn more about its benefits and start your journey at Drugzone.
#drugzone#pharmaceutical distribution#pharmaceutical wholesalers#pharmaceutical distributor#pharmacy wholesale suppliers
0 notes
Text
DRUG SUPPLY CHAIN SECURITY ACT: COMPLIANCE IS NOT AS DIFFICULT AS YOU THINK

DSCSA Compliance, Difficulties, and Solutions
Among a lot of reasons that are presently surfacing around FDA track and trace capability, most primitive ones are misconceptions around the definition of product identification and verification. The other is financial, commercial, and infrastructural gaps between large, small, and mid-size companies.
Verification? Product Identification? Definitions Under DSCSA
There are essential issues in how the modern pharmaceutical industry interprets product identification and verification. It is often misunderstood that verification of product identification means that the manufacturer, repackager, is expected to verify whether they make the product. Or there is an identification on the counterfeit in a particular Pharma supply chain automatically point in time.
As per DSCSA identification and verification definition, the manufacturers and the higher-level partners in a supply chain have been bound to ensure (at any time) that a particular request a product verification helps them verify that the product. It includes an identification number, lot number, and other details that are the same as available on the human-readable label and the one submitted to the USFDA regulators.
Resource & Commercial Gaps Among Companies
The commercial, financial, and business capabilities of a well-established pharmaceutical giant, a mid-scale company, or a freshly approved Pharma company would be different. DSCSA compliance is facing significant hurdles. The large-cap companies are capable of undertaking the serialization process and make the necessary changes in their production facilities. But, they are lacking the right business leadership and the required information for successfully carrying out the compliance process. The mid-scale and small pharmaceutical companies are having significant trouble in building a commercially feasible manufacturing and supply chain model. The focus is on embedding the serialization process via contacted manufacturers or a portion of it that remains in the house.
No Standardization of the Serialization Process
One of the hurdles faced by the DSCSA serialization process is that there is no standardization of the serialization process. New-age startups and well-established companies and their CMOS or CIOs are coming out with consultancy services ‘Serialisation as a Service’ concept consulting the short, less resourceful time to managing to get through these processes. Industry experts and thought leaders have outrightly dishonored the idea of one to one consultancy on a global matter, and it is not advisable.
Benefits of
DSCSA Compliance
and Why Every US Pharma Business Needs It?What Is The Real Solution?
DSCSA Compliance can be best managed with strategized, well-structured intervention of technology product that is capable of foolproof security, safety, and data security compromise concerns. It should be bringing all the primary and secondary stakeholders of a farmer supply chain to a single solution. Standardization of the serialization process and decentralization of information is a must. The centralization of vigilance should tope it and policing efforts. They are more critical for a sustainable, successful stride of DSCSA Compliance with the help of DSCSA Software.
What Makes DSCSA Compliance More Feasible?
There have been various entrant Technology Solutions that are getting on and off the Pharmaceutical Landscape. A completely foolproof, robust Blockchain-Based Serialization Solution, DSCSA Software is all you need. Fortunately, the Blockchain is one Technology that is capable of documenting Transactions on a Decentralized database with 99.99% data immutability at all ends. It is the perfect solution allowing a vast number of private or permissioned vendors to register over a Blockchain Platform voluntarily.
It verifies the profile and makes them a part of a wholly secured, highly transparent Pharma Supply Chain Solution. It is a great Solution that Allows Real-time Transactions and foolproof consensus management across all regulators and the leading supply chain partners. It helps in executing supplies across the pharmaceutical consumer base.
Business Benefits of using a
DSCSA Compliance Software
No FDA penalties
Keeping all Pharmaceutical Supply Chain operations abiding by the Serialization process drastically reduces the chances of ending up FDA sanctions and suspension of Services.
Complete Security
Blockchain day, The Traceability Solutions, and DSCSA Software bring SCM operations a lot closer to the SCM compliance eliminating all possibilities of stolen, counter feet, and low activity Drugs.
Secured Scalability
Expanding wide across more Rx rugs for Manufacturing deep down the consumer base is more affordable and feasible with an end to end Serialization Solution.
Business Beyond Boundaries
The power of Blockchain-Based Private and Formation Registries over the Blockchain Platform to successfully impaneled vendors and officially communicate with other partners in the Supply Chain anywhere across the world.
Bulletproof data security
Blockchain Technology is capable of building a decent life Database with imitable Data records in the form of Smart Contracts. All the product-related information and Realtime Transactions are highly secured.
Reduced Compliance Costs
DSCSA Software Solution is capable of drastically reducing the overall compliance cost, which in turn causes higher profitability in the long run.
Eight Times Better Fault Tolerance
Blockchain-Based DSCSA Software enables suppliers and partners and regulators to identify notice, red flag, quarantine that are passed into the Supply Chain just with the help of a click.
Cost-Effective SCM
The high efficient DSCSA Compliance Software enables companies to focus on cutting costs department wise.
Higher Productivity
The Highly Secured and process includes resource utilization rates and makes the human resource capital way more efficient than ever before.
Conclusion
Forceful intrusions of Technology in Pharmaceutical Traceability and Serialization can make DSCSA Compliance way more comfortable than it looks from the outside. Highly robust, new-age Blockchain-Based DSCSA Software eliminates the hassles of building and managing a Technology Platform. Now you can Setup Serialization Solutions with leading Blockchain Development Companies that are making efforts towards standardizing the process Drug Serialization on a Global Scale.
#DSCSA compliance software#Pharma Serialization#DSCSA Software#Blockchain-Based DSCSA Software#Serialization Solutions#Private Blockchain#Pharma Supply Chain Solution
0 notes
Link
These online training courses are approved by the California State Board of Pharmacy. Sign-up for a course now to earn a training affidavit for your California Designated Representative license application:
California Designated Representative Wholesalers
California Designated Representative 3PL
California Designated Representative Reverse Distributors
1 note
·
View note
Text
Achieving DSCSA Compliance and Patient Safety with Our Comprehensive Solutions
DSCSA compliance is a critical issue for businesses in the pharmaceutical industry. The Drug Supply Chain Security Act (DSCSA) is a federal law that aims to improve the security and traceability of pharmaceutical products as they move through the supply chain. Compliance with the DSCSA is crucial for ensuring that only legitimate and safe products reach patients.
Our company specializes in providing solutions for DSCSA compliance. We understand the complexities of the pharmaceutical supply chain and have the expertise to help your business comply with the DSCSA requirements. Our solutions include software for tracking and tracing products, as well as training and consulting services to ensure that your business is fully compliant.
Our software is user-friendly and can be easily integrated into your existing systems, making it easy to keep track of your products and ensure compliance. Our training and consulting services will help your employees understand the requirements of the DSCSA and how to implement them.
Don't risk non-compliance with the DSCSA. Contact us today to learn how we can help your business stay compliant and protect the safety of your patients.
Need to Know more about DSCSA Compliance and Patient Safety, visit our website.
0 notes
Text
US DSCSA serialisation service providers consider creating robust, secure, and scalable hardware and software solutions to increase supply chains and defend brands against reproduction while deriving benefit from involving cases and increasing health outcomes.
URL: -
For More Detail Visit:-
https://www.pharmasecure.com
0 notes
Text
6 Essential features of Pharmacy management system needed in 2021
1. Reports & Analytics
The pharmacy management system generates reports to check the wholesales’ performance. Reporting & analytics prepare for the pre-requirement of drugs. It also automatically manages the inventory. The feature calculates the number of needed medications. The reporting system enhances sales and ROI (Return of Investment). Moreover, it provides a full report of the pharma business activities.
2. Centralized Database
Data is a crucial element of the pharma business. Furthermore, a centralized database holds the patient’s data at a centralized location. The pharmacy management system provides easy data retrieval. It also manages transactional records. The system eliminates data loss. Additionally, it stores data about drugs and medications. Thus, the set-up provides information for drug availability.
3. Feature of Restore & Backup System of pharmacy management system
What happens when the data is lost suddenly? The business operations may stop abruptly. Data such as patient records and inventory is always at risk. A restore and backup system avoids unnecessary data loss. This system eliminates technical errors while retrieving data quickly.
Read More : Click Here
4. Customer Management System
Customers are at the core of any system designed for businesses. Pharmacy management systems must have an automated solution to manage patients. CMS also keeps a record of the patient’s data. Furthermore, it is a dynamic solution that adds new customers. Well-organized CMS holds customer responses and feedback. Hence, Pharmacists develop business strategies to manage customer requirements.
5. Integration with third-party software
Integration with third-party software manages back-office operations. Enables efficient data flows for prescription and inventory data. US pharmacists integrate with prescription drug monitoring programs. EHR/EMR integration to access patient records. E-prescribing software integration to control medical receipts.
6. Regulatory & Compliance of pharmacy management system
The compliance regulations cover each dimension of pharma practice. POS solutions address compliance for drug ordering and e-prescription.
US and EU regulators use advanced technologies to standardize the dispense of medication. The federal law Drug Supply Chain Security Act (DSCSA) protects against the dispensing of fake prescribed medications. In the EU, various drug suppliers ensure drug authenticity using a ‘point-of-dispense’ verification system. NAPRA is an association that governs pharmacies across Canada.
About Ari
Ari pos, a retail management system, is preferred by many retailers around the globe. Ari has many features and functionalities that benefit a retailer in day-to-day operations. There is a possibility of creating rewards programs and promotional strategies in Ari. You can also design sales promotion strategies and manage them through Ari.
0 notes
Text
How to manage your Inventory between your Pharmaceutical Distribution and 3PL
Moving with a 3PL (third-party logistics) provider model is common practice as businesses seek to exploit value throughout the supply chain. Instead of handling warehouse space, they use to pay a 3PL to handle the pick, pack, receipt, and shipping of product on their behalf. By doing this, the manufacturer ships products directly to the 3PL where orders are then fulfilled on behalf of the Wholesaler or Pharmaceutical Distributors.
This technique is basically for SMEs who do not wish to incur the costs associated with managing and staffing warehouse space, and for businesses that sell across the country and can benefit from multiple locations strategically placed. Before you decide on a 3PL partner, you need to consider all of the features of pharmaceutical ERP software. Below we explore how a Pharmaceutical ERP plays an important role and automates your business processes.
Pharmaceutical products require specialized procedures for their receipt, storage, handling, and distribution to their final destination. Meta-Pharma, an ERP Solution for Pharmaceuticals Industry combines years of experience with a technologically advanced infrastructure for a sense of confidence and commitment that clients rely on and appreciate for the needs of pharmaceutical warehouse and distribution.
Meta-Pharma and Automated Processes
MetaPharma is an ERP Solution for Pharmaceuticals Industry built on Microsoft Dynamics 365 Business Central. It includes robust accounting and inventory management features and advanced functionality. Which work for traceability, lot tracking, regulatory compliance with the DEA, FDA, and DSCSA, and warehouse management. These features work together to automate processes and grow your business, increase transparency, and aid in regulatory compliance.
Meta-Pharma is an ERP Solution for Pharmaceuticals that can integrate with the systems used by your 3PL partners. Just like you need a solution to manage your daily business operations. Meta-Pharma with 3PL provides more chances for automation so that it can easily eliminate manual processes and the risk of human error.
Basically, it can be go through EDI, this integration allows your system to connect directly to the system of each of your 3PL partners. It means that instead of manually sending and receiving information and then manually updating your software, the systems can directly exchange information with one another, and then automatically update data and status’.
Value-Added Solution for Pharmaceutical Distribution
As you can see, integrating your Pharmaceutical ERP system with the software used by your 3PL partners is a much more efficient way of doing business. And, when you compare the costs and time associated with this, you may be happy to find that there is a clear case for electronically exchanging data. Especially, if you have to do a lot of transactions and a high order volume then it must be a better ERP solution for your Pharmaceutical Distribution business.
For more information and a tailored demonstration contact us at Meta-Pharma.
#pharma erp solution#pharmaceutical#3pl#pharmaceutical industry#pharmaceutical distribution#inventory
0 notes
Text
North America Healthcare Logistics Market Size, Share, Key Growth Drivers, Trends, Challenges and Competitive Landscape
"North America Healthcare Logistics Market - Size, Share, Demand, Industry Trends and Opportunities
North America Healthcare Logistics Market, By Type (Cold Chain and Non-Cold Chain), Component (Hardware, Software, and Services), Temperature Type (Ambient, Chilled/Refrigerated, Frozen, and Cryogenic), Logistics (Transportation, Packaging, Storage, and Others), Logistic Type (Sea Freight Logistics, Air Freight Logistics, Overland Logistics, and Contract Logistics), Application (Medicine, Bulk Drug Handlers, Vaccine, Chemical and Other Raw Material, Biological Material and Organs, Hazardous Cargo, and Others), End User (Biopharmaceutical Companies, Hospitals and Clinics, Research Institutes, and Others) – Industry Trends.
Get the PDF Sample Copy (Including FULL TOC, Graphs and Tables) of this report @
**Segments**
The North America Healthcare Logistics Market can be segmented based on the type of services, including transportation, warehousing, and others. The transportation segment encompasses air freight, ocean freight, road freight, and rail freight services. Warehousing services involve inventory management, order processing, and distribution center management. Other services may include cold chain logistics, medical equipment logistics, and pharmaceutical logistics, among others. The market can also be segmented by end-user, such as hospitals, clinics, pharmaceutical companies, medical device companies, and others.
**Market Players**
- DB Schenker - DHL International GmbH - FedEx - Kuehne+Nagel - XPO Logistics, Inc. - UPS Supply Chain Solutions - C.H. Robinson Worldwide, Inc. - Agility - DSV - Penske Logistics
The North America Healthcare Logistics Market is witnessing significant growth due to various factors. One of the key drivers is the increasing demand for efficient healthcare services and the need for streamlined logistics operations in the healthcare industry. The rising prevalence of chronic diseases, coupled with the aging population in the region, is driving the demand for healthcare logistics services. Moreover, advancements in technology, such as real-time tracking and temperature-sensitive monitoring, are enhancing the efficiency and reliability of healthcare logistics operations.
The market is also experiencing a shift towards specialized logistics services, such as cold chain logistics for temperature-sensitive pharmaceuticals and medical devices. This trend is driven by the growing focus on maintaining the integrity and efficacy of healthcare products throughout the supply chain. Additionally, the increasing adoption of e-commerce in the healthcare sector is creating new opportunities for logistics providers to offer customized and on-demand services to healthcare companies.
However, the North America Healthcare Logistics Market is not without its challenges. One of the major hurdles facing the market is the complex regulatory environment governing the transportation and storage of healthcare products. Compliance with regulations such as Good Distribution Practice (GDP) and the Drug Supply Chain Security Act (DSCSA) requires significant investments in infrastructure and technology, which can pose a barrier to entry for smaller logistics companies.
Another challenge is the rising operational costs associated with healthcare logistics, particularly in terms of maintaining specialized facilities and equipment for handling sensitive medical products. Fluctuations in fuel prices and transportation costs also impact the profitability of healthcare logistics providers, highlighting the need for efficient route planning and cost management strategies.
In conclusion, the North America Healthcare Logistics Market is poised for steady growth driven by the increasing demand for specialized logistics services in the healthcare industry. By adapting to evolving market trends and addressing key challenges, market players can capitalize on the opportunities presented by this dynamic sector.
Access Full 350 Pages PDF Report @
The report provides insights on the following pointers:
Market Penetration: Comprehensive information on the product portfolios of the top players in the North America Healthcare Logistics Market.
Product Development/Innovation: Detailed insights on the upcoming technologies, R&D activities, and product launches in the market.
Competitive Assessment: In-depth assessment of the market strategies, geographic and business segments of the leading players in the market.
Market Development: Comprehensive information about emerging markets. This report analyzes the market for various segments across geographies.
Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the North America Healthcare Logistics Market.
This study answers to the below key questions:
What are the key factors driving the North America Healthcare Logistics Market?
What are the challenges to market growth?
Who are the key players in the North America Healthcare Logistics Market?
What are the market opportunities and threats faced by the key players?
Browse Trending Reports:
Europe Flight Data Recorder Market Asia Pacific Flight Data Recorder Market Middle East And Africa Flight Data Recorder Market North America Flight Data Recorder Market Europe Potato Processing Market Asia Pacific Potato Processing Market Middle East And Africa Potato Processing Market North America Potato Processing Market Australia And New Zealand Concrete Admixture Market Saudi Arabia q Pcr Reagents Market Australia Specialty Gas Market
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email: [email protected]
"
0 notes
Text
How is software, technology, and equipment used to secure drug supply from the company?
How is software, technology, and equipment used to secure drug supply from the company?
In your case study paper, you will address the following questions. How is software, technology, and equipment used to secure drug supply from the company? What were the benefits of using the serialization initiative to fulfill state and federal compliance requirements? How will retention of legacy WMS and using the adaptive software tool (STEPlogic) position the company for DSCSA compliancy in…
youtube
View On WordPress
0 notes
Text
How is software, technology, and equipment used to secure drug supply from the company?
How is software, technology, and equipment used to secure drug supply from the company?
In your case study paper, you will address the following questions. How is software, technology, and equipment used to secure drug supply from the company? What were the benefits of using the serialization initiative to fulfill state and federal compliance requirements? How will retention of legacy WMS and using the adaptive software tool (STEPlogic) position the company for DSCSA compliancy in…
youtube
View On WordPress
0 notes