#Microfluidics Solutions
Explore tagged Tumblr posts
Text
Discover A-Gas's innovative range of microfluidics and lab-on-chip solutions, designed for high precision in disease modeling and drug screening. These cutting-edge products leverage the latest technology to offer unparalleled accuracy and efficiency in micro-scale experiments, making them ideal for biomedical and pharmaceutical applications. Learn how our solutions can enhance your research and development efforts in this dynamic field.
#Microfluidics Solutions#Lab on Chip Technology#Biomedical Research Tools#Pharmaceutical Lab Equipment#Precision Disease Modeling#Drug Screening Technology#Advanced Microfluidics Products
0 notes
Text
"Canadian scientists have developed a blood test and portable device that can determine the onset of sepsis faster and more accurately than existing methods.
Published today [May 27, 2025] in Nature Communications, the test is more than 90 per cent accurate at identifying those at high risk of developing sepsis and represents a major milestone in the way doctors will evaluate and treat sepsis.
“Sepsis accounts for roughly 20 per cent of all global deaths,” said lead author Dr. Claudia dos Santos, a critical care physician and scientist at St. Michael’s Hospital. “Our test could be a powerful game changer, allowing physicians to quickly identify and treat patients before they begin to rapidly deteriorate.”
Sepsis is the body’s extreme reaction to an infection, causing the immune system to start attacking one’s own organs and tissues. It can lead to organ failure and death if not treated quickly. Predicting sepsis is difficult: early symptoms are non-specific, and current tests can take up to 18 hours and require specialized labs. This delay before treatment increases the chance of death by nearly eight per cent per hour.
[Note: The up to 18 hour testing window for sepsis is a huge cause of sepsis-related mortality, because septic shock can kill in as little as 12 hours, long before the tests are even done.]
[Analytical] AI helps predict sepsis
Examining blood samples from more than 3,000 hospital patients with suspected sepsis, researchers from UBC and Sepset, a UBC spin-off biotechnology company, used machine learning to identify a six-gene expression signature “Sepset” that predicted sepsis nine times out of 10, and well before a formal diagnosis. With 248 additional blood samples using RT-PCR, (Reverse Transcription Polymerase Chain Reaction), a common hospital laboratory technique, the test was 94 per cent accurate in detecting early-stage sepsis in patients whose condition was about to worsen.
“This demonstrates the immense value of AI in analyzing extremely complex data to identify the important genes for predicting sepsis and writing an algorithm that predicts sepsis risk with high accuracy,” said co-author Dr. Bob Hancock, UBC professor of microbiology and immunology and CEO of Sepset.
Bringing the test to point of care
To bring the test closer to the bedside, the National Research Council of Canada (NRC) developed a portable device they called PowerBlade that uses a drop of blood and an automated sequence of steps to efficiently detect sepsis. Tested with 30 patients, the device was 92 per cent accurate in identifying patients at high risk of sepsis and 89 per cent accurate in ruling out those not at risk.
“PowerBlade delivered results in under three hours. Such a device can make treatment possible wherever a patient may be, including in the emergency room or remote health care units,” said Dr. Hancock.
“By combining cutting-edge microfluidic research with interdisciplinary collaboration across engineering, biology, and medicine, the Centre for Research and Applications in Fluidic Technologies (CRAFT) enables rapid, portable, and accessible testing solutions,” said co-author Dr. Teodor Veres, of the NRC’s Medical Devices Research Centre and CRAFT co-director. CRAFT, a joint venture between the University of Toronto, Unity Health Toronto and the NRC, accelerates the development of innovative devices that can bring high-quality diagnostics to the point of care.
Dr. Hancock’s team, including UBC research associate and co-author Dr. Evan Haney, has also started commercial development of the Sepset signature. “These tests detect the early warnings of sepsis, allowing physicians to act quickly to treat the patient, rather than waiting until the damage is done,” said Dr. Haney."
-via University of British Columbia, May 27, 2025
#public health#medical news#sepsis#cw death#healthcare#medicine#medical care#ai#canada#north america#artificial intelligence#genetics#good news#hope
863 notes
·
View notes
Text
Human Organoids 101: What They Are and Why the Market Is Exploding Right Now

Introduction
The Global Human Organoid Market is undergoing remarkable expansion, driven by cutting-edge advancements in stem cell research, the increasing need for personalized medicine, and the rising adoption of organoids in drug discovery and disease modeling. In 2024, the human organoid market was valued at USD 1.09 billion and is projected to grow at a CAGR of 16.7% over the forecast period. The widespread use of organoid technology in pharmaceutical research, regenerative medicine, and toxicology is revolutionizing biomedical studies worldwide. Innovations such as CRISPR gene editing, high-throughput screening, and organ-on-a-chip systems are improving scalability, enhancing disease model accuracy, and expediting drug development. With substantial investments from pharmaceutical companies and academic institutions, organoid-based research is reducing reliance on animal testing while increasing the reliability of clinical trial predictions.
Request Sample Report PDF (Including TOC, Graphs & Tables)
Human Organoid Market Overview
The demand for human organoids is surging due to their critical role in disease modeling, drug screening, and regenerative therapies. Unlike traditional 2D cell cultures or animal models, organoids provide a more accurate representation of human tissues, making them invaluable in studying complex diseases such as cancer, neurological disorders, and genetic conditions. Additionally, growing ethical concerns over animal testing are accelerating the shift toward human-relevant alternatives.
Get Up to 30% Discount
Human Organoid Market Challenges and Opportunities
Despite rapid growth, the market faces obstacles such as high production costs, technical complexities, and variability in organoid reproducibility. Scalability remains a key challenge, requiring standardized protocols and advanced biomanufacturing techniques. Regulatory uncertainties, particularly in organ transplantation and gene therapy, also hinder market expansion in some regions.
However, significant opportunities exist, including:
The rise of biobanking, enabling long-term storage of patient-derived organoids for future therapies.
Increased demand for personalized drug testing and precision oncology, driving investments from pharmaceutical firms.
Collaborations between academic institutions, biotech companies, and governments, fostering innovation and cost-effective solutions.
Key Human Organoid Market Trends
Integration with organ-on-a-chip technology, enhancing physiological accuracy for drug screening.
AI-powered analytics, improving real-time monitoring of cellular responses.
Advanced bioengineering techniques, such as synthetic scaffolds and microfluidics, expanding applications in regenerative medicine.
Human Organoid Market Segmentation
By Type
Stem Cell-Derived Organoids (65.4% market share in 2024) – Dominating due to applications in disease modeling and drug discovery.
Adult Tissue-Derived Organoids – Gaining traction in personalized medicine and cancer research.
Organ-Specific Organoids – Specialized models for targeted research.
By Source
Pluripotent Stem Cells (PSCs) (68.5% market share) – Leading due to high-throughput drug screening applications.
Adult Stem Cells (ASCs) – Expected to grow at a CAGR of 14.2%, driven by regenerative medicine.
By Technology
3D Cell Culture – Most widely used for biologically relevant tissue modeling.
Microfluidics & Organ-on-a-Chip – Fastest-growing segment (CAGR of 17.8%).
CRISPR & AI-Driven Analysis – Transforming drug discovery with predictive precision.
By Application
Disease Modeling (CAGR of 16.3%) – Key for studying genetic and infectious diseases.
Regenerative Medicine & Transplantation – Rapidly expanding with tissue engineering advancements.
Precision Oncology – Revolutionizing cancer treatment through patient-specific models.
By End User
Pharmaceutical & Biotech Companies – Largest market share, leveraging organoids for drug development.
Academic & Research Institutes – Leading innovation in disease modeling and genomics.
By Region
North America (44.15% market share) – Strong funding and advanced biotech infrastructure.
Europe – Growing demand for personalized medicine.
Asia-Pacific – Fastest growth due to stem cell research advancements.
Competitive Landscape
The market is highly competitive, with key players including:
STEMCELL Technologies Inc.
Hubrecht Organoid Technology (HUB)
Thermo Fisher Scientific Inc.
Merck KGaA
MIMETAS BV
Recent Developments
July 2024: Merck KGaA launched a new cell culture media line for scalable organoid production.
March 2024: STEMCELL Technologies partnered with HUB to enhance organoid models for drug discovery.
March 2024: Thermo Fisher expanded its 3D cell culture portfolio with specialized organoid growth media.
Conclusion
The Global Human Organoid Market is poised for transformative growth, fueled by advancements in biotechnology and personalized medicine. Despite challenges like high costs and standardization issues, the increasing demand for patient-specific treatments and alternative testing models will sustain momentum. The integration of 3D cell culture, CRISPR, and AI analytics is set to expand organoid applications, paving the way for groundbreaking biomedical discoveries.
Purchase Exclusive Report
Our Services
On-Demand Reports
Subscription Plans
Consulting Services
ESG Solutions
Contact Us: 📧 Email: [email protected] 📞 Phone: +91 8530698844 🌐 Website: www.statsandresearch.com
1 note
·
View note
Text
Undergrad research blast from the past. Here I am in 2020 assembling a micro fluidic flow cell with a gold electrode block. I think I took this video for myself so I knew what to clip to what. This was when I worked with electrochemical sensors, transducing signals via impedance spectroscopy.
A lot of electrochemical techniques rely on measuring voltages or currents, but in this lab we looked at impedance- which is a fancy combination of regular resistance (like the same one from ohms law) and the imaginary portion of the resistance that arises from the alternating current we supply.
I would functionalize different groups on the gold working electrode by exposing the surface to a solution of thiolated biomarker capture groups. Thiols love to form self-assembled mono layers over gold, so anything tagged with thiol ends up sticking. [Aside: Apparently after I left the group they moved away from gold thiol interactions because they weren't strong enough to modify the electrode surface in a stable and predictable way, especially if we were flowing the solution over the surface (which we wanted to do for various automation reasons)]. The capture groups we used were various modified cyclodextrins- little sugar cups with hydrophobic pockets inside and a hydrophilic exterior. Cyclodextrins are the basis of febreeze- a cyclodextrin spray that captures odor molecules in that hydrophobic pocket so they can't interact with receptors in your nose. We focused on capturing hydrophobic things in our little pocket because many different hydrophobic biomarkers are relevant to many different diseases, but a lot of sensors struggle to interact with them in the aqueous environment of bodily fluids.
My work was two fold:
1) setting up an automated system for greater reproducibility and less human labor. I had to figure out how to get my computer, the potentiostat (which controls the alternating current put in, and reads the working electrode response), the microfluidic pump, and the actuator that switched between samples to all talk to each other so I could set up my solutions, automatically flow the thiol solution for an appropriate time and flow rate to modify the surface, then automatically flow a bio fluid sample (or rather in the beginning, pure samples of specific isolated biomarkers, tho their tendency to aggregate in aqueous solution may have changed the way they would interact with the sensor from how they would in a native environment, stabilized in blood or urine) over the electrode and cue the potentiostat for multiple measurements, and then flow cleaning solutions to clean out the tubings and renew the electrode. This involved transistor level logic (pain) and working with the potentiostat company to interact with their proprietary software language (pain) and so much dicking around with the physical components.
2) coming up with new cyclodextrin variants to test, and optimizing the parameters for surface functionalization. What concentrations and times and flow rates to use? How do different groups around the edge of the cyclodextrin affect the ability to capture distinct classes of neurotransmitters? I wasn't working with specific sensors, I was trying to get cross reactivity for the purpose of constructing nonspecific sensor arrays (less akin to antibody/antigen binding of ELISAs and more like the nonspecific combinatorial assaying you do with receptors in your tongue or nose to identify "taste profiles" or "smell profiles"), so I wanted diverse responses to diverse assortments of molecules.
Idk where I'm going with this. Mostly reminiscing. I don't miss the math or programming or the physical experience of being at the bench (I find chemistry more "fun") but I liked the ultimate goal more. I think cross reactive sensor arrays and principle component analysis could really change how we do biosample testing, and could potentially be useful for defining biochemical subtypes of subjectively defined mental illnesses.... I think that could (maybe, possibly, if things all work and are sufficiently capturing relevant variance in biochemistry from blood or piss or sweat or what have you) be a more useful way to diagnose mental illness and correlate to possible responses to medications than phenotypic analysis/interviews/questionnaires/trial and error pill prescribing.
4 notes
·
View notes
Text
Top 10 Peristaltic Pump Brands
Top 10 Peristaltic Pump Brands
When it comes to peristaltic pumps, numerous reputable brands have emerged in the market. Here's a ranking of the top ten high-quality peristaltic pump brands:
1. Watson-Marlow
Watson-Marlow is undoubtedly a leader in the peristaltic pump industry, renowned for its exceptional technology and reliability. Their products find extensive use in medical, pharmaceutical, and laboratory settings, providing users with highly accurate fluid control solutions. Watson-Marlow peristaltic pumps have become synonymous with reliability and performance.
2. Masterflex
Masterflex is another leading player in the peristaltic pump market, with products widely used in various industries such as chemicals, food, and beverages. Known for their excellent adjustability and durability, Masterflex pumps are a preferred choice for numerous industrial applications.
3. JIHPUMP
Chongqing JIHPUMP is dedicated to continuous innovation, offering advanced fluid control solutions to support users in diverse applications. Their products are extensively used in liquid transfer, laboratory automation, pharmaceutical production, and chemical reactions, among other areas. With exceptional performance and reliability, JIHPUMP has gained recognition and trust from customers both domestically and internationally.
4. THOMAS
THOMAS is a well-established brand in the peristaltic pump industry with decades of manufacturing experience. Their products find extensive use in air and gas transfer systems, and are highly trusted for their reliability and exceptional performance.
5. Longer
Longer is a German manufacturer renowned for high-quality and innovative peristaltic pumps. Their products are widely used in engineering, environmental protection, and chemical industries, highly regarded for their excellent craftsmanship and stability.
6. BOXER
BOXER is a leading player in the peristaltic pump industry, with products widely used in medical devices and scientific laboratories. Their peristaltic pumps are known for their compact design and exceptional performance.
7. LeadFluid
Baoding LeadFluid is a Chinese peristaltic pump manufacturer focused on providing high-quality fluid control solutions. Their peristaltic pump products find extensive use in laboratories, medical, chemical, and environmental industries. LeadFluid is trusted by customers for its robust, durable, and high-performance products.
8. Signal
Signal is a leading player in the Chinese peristaltic pump market, with products covering multiple industries, including pharmaceuticals, food, and environmental protection. Known for their high cost-effectiveness and flexibility, they have accumulated rich experience in peristaltic pumps, tubing, OEM products, and filling systems.
9. Kamoer
Kamoer is a Chinese peristaltic pump manufacturer specializing in microfluidic control technology. Their products are compact and precise, suitable for laboratory and research applications, and are favored by scientists and researchers. Kamoer has been deeply involved in the microfluidic field for many years, providing accurate and reliable fluid transfer solutions for medical, environmental, and scientific instrument industries.
10. SHENCHEN
SHENCHEN is a Chinese peristaltic pump manufacturer in Baoding, offering peristaltic pump products for various applications. Their products find extensive use in chemical, environmental, and water treatment industries, and are highly favored by customers for their reliability and cost-effectiveness.
1 note
·
View note
Text
There's a hole in your logic
You who know all the answers...

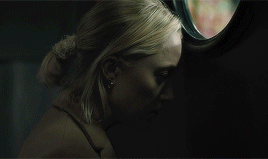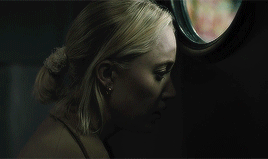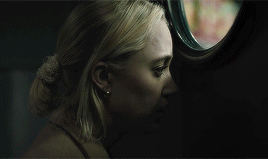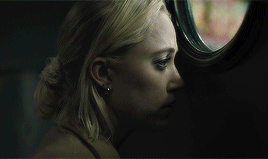
| soundtrack | living space | style
Name: Lucy Archer Bell
Age: 30 (Feb 12)
Identity & Orientation: NB AFAB, they/he/she, disaster pan
Originally From: Clackamas, Oregon
Most Recent Community: Dielectric Research Community, Connecticut
Time in Redwood: New as of February 2043
Resides in: A bungalow near the school
Previous occupation: Biomedical / Neuro-Engineer
Community occupation: Teacher (STEM subjects) / Daycare / Mechanic's assistant
Lucy was born to a mom in (temporary) recovery, and never knew her father. She grew up mostly raised by maternal grandparents while her mother went through several rounds of rehab, additional pregnancies and falls off the wagon. Lucy, meanwhile, became a driven, intelligent young woman - with a real sarcastic wit. She excelled in soccer, but ended up needing financial assistance for college.
She was indignant at needing financial assistance, being teased by classmates for her financial struggle, and eschewed parties to hold down a job around classes. By 2037 Lucy was in her last year of a bachelors program at John Hopkins University in Baltimore, Maryland.
Already having been approached by prestigious biomedical firms, Lucy was starting to feel she'd outrun her crappy circumstance of birth. Around the same time news of a new outbreak was starting one of the firms swooped in and recruited every student on the same track. Promised them the chance to change the world in their lifetime, to save mankind. That they were all needed.
Dielectric was their home for several years as the world shut down. Compartmentalized, kept ignorant of the goings on outside; told it was for the best if they weren't distracted by the outside world.
Small teams worked on different pieces of a greater whole, brainstorming, hypothesizing, testing, making odd supply requests from the men upstairs...
Until supplies stopped showing up.
An intellectual powerhouse, the cell found solutions to problems as they came. Predicted likely future issues and took what preventative measures they could. But eventually, the walls would be permeated.
What researchers survived that inevitable breach fled; they weren't built for brute force fighting. Lucy was never more grateful that she'd been a jock in high school, able to outrun bigger, heavier predators.
A year of another kind of learn-by-doing, and Lucy fell ass-over-teakettle into Redwood. Specifically by setting off a hunting trap.
| Headcanons |
Lucy was on course to earn her Bachelor of Science in Biomedical Engineering; specifically angling for the Neural Engineering Track.
She never lost her snarky personality. Originally a defense mechanism, it eventually simply became a part of her personality. It didn't endear her to superiors at Dielectric, alas.
Before you ask - yes his mom named them Lucy as a Beatles homage. Yes, Lucy finds it tacky. Yes, he knows it's close to Lucille Ball, too.
Doesn't like talking about how they lost their left pinky.
Created "A fully implantable,wireless interface for CNS for electronic recording, stimulation and microfluidic drug delivery."
5 Tattoos: Lunar moth at right hipbone | cobra winding up right arm | Neuron up the exterior left calf | Twilight Zone inspired on left bicep | James Webb hexagon on the back of their neck
Goes by Archer, in addition to Lucy
Birth order: Lucy -> Allison (26) -> Joey (23) -> Nina (20) -> Jordan (16) -> Jimmy (12)
| Wanted Connections |
STEM nerds (open!) - Do you speak science, tech, engineering or math? Then Lucy would love to nerd it up with you. Conversation, projects, wild theories, whatever @orioncarnell , @tamirkamadcr
Chaotic Buds (1/2) - Lucy has high Int, low Wis and obviously would love buds of similar stat block. @aresmelaina ,
Kite String (0/1) - Who's going to tether this girl when she's getting unrealistic, too salty, etc?
Trap Spring (1/1) - She was brought in by a hunter whose trap she set off. Soz, dude. - @davxdalexander
Crossed Paths (2/3) - She was extremely behind the 8 ball on the whole apocalypse thing, running a couple years behind everyone else. Did you encounter her in the last year? Helped her out or took advantage? @frankxausting , @harry-thompson
3 notes
·
View notes
Text
North America Point of Care Diagnostics Market Strategies, Sales, Revenue, Gross Margin, Share by Top Companies till 2028

The urgency to control the spread of the virus and manage a rapidly increasing number of cases created a surge in demand for fast and reliable diagnostic solutions. This situation highlighted the importance of decentralized testing, enabling healthcare providers to detect and isolate cases promptly. As a result, the region witnessed accelerated adoption of molecular and antigen-based point of care testing technologies, along with the introduction of innovative diagnostic kits capable of delivering results within minutes. The pandemic also led to increased public awareness about the importance of diagnostics and prevention, further solidifying the role of point of care testing in everyday healthcare. Strategic insights into the North America Point of Care Diagnostics Market reveal the importance of data-driven decision-making and regional analysis. Businesses operating in this domain can gain a competitive edge by identifying unmet needs, analyzing market trends, and tailoring their offerings to specific patient populations. Leveraging analytics allows stakeholders to forecast future demands, refine product development strategies, and streamline distribution channels to reach underserved areas more effectively. Companies can also focus on enhancing the user-friendliness and portability of diagnostic devices, catering to both professional healthcare providers and patients engaged in self-monitoring.
The North America Point of Care Diagnostics Market is experiencing a transformative shift driven by technological advancements, growing demand for rapid and accurate diagnostic solutions, and increasing healthcare awareness among the population. As healthcare systems strive for quicker patient management and improved outcomes, the North America Point of Care Diagnostics Market continues to gain traction across various clinical and non-clinical settings. This market encompasses a broad range of diagnostic tools designed for near-patient testing, including glucose monitoring, infectious disease testing, pregnancy testing, and cardiac markers, all of which contribute to streamlined clinical decision-making.
One of the key factors fueling the North America Point of Care Diagnostics Market is the rising prevalence of chronic diseases such as diabetes, cardiovascular disorders, and respiratory illnesses. With the aging population in countries like the United States and Canada, the demand for easy-to-use, portable diagnostic devices is accelerating. These tools are enabling healthcare professionals to make timely and informed decisions, which is particularly critical in emergency settings. Consequently, the North America Point of Care Diagnostics Market is expanding not only in hospitals but also in ambulatory care centers, home healthcare, and retail clinics.
Moreover, the increasing focus on personalized healthcare is propelling the growth of the North America Point of Care Diagnostics Market. The ability to tailor treatments based on rapid diagnostic results is enhancing patient satisfaction and improving treatment outcomes. Innovations such as microfluidics, biosensors, and smartphone-integrated diagnostic tools are setting new standards in the industry. These cutting-edge technologies are helping to broaden the application areas of the North America Point of Care Diagnostics Market, making it more accessible and efficient.
The COVID-19 pandemic significantly accelerated the adoption of point-of-care diagnostic tools, highlighting their importance in managing infectious outbreaks. Rapid antigen and molecular testing played a crucial role in early detection and containment efforts. This increased reliance on decentralized testing is expected to have a lasting impact, further reinforcing the growth trajectory of the North America Point of Care Diagnostics Market. Governments and private sectors alike are investing heavily in diagnostic infrastructure, thereby strengthening the market ecosystem.
Additionally, the rise in patient-centric care models is reshaping the North America Point of Care Diagnostics Market. Patients today are more informed and proactive about their health, seeking quicker and more convenient diagnostic services. This shift is encouraging manufacturers to develop intuitive and cost-effective solutions that can be easily used outside traditional lab environments. As a result, home-based diagnostic kits and mobile testing units are becoming more prevalent in the North America Point of Care Diagnostics Market.
In conclusion, the North America Point of Care Diagnostics Market is poised for substantial growth in the coming years. With continuous advancements in diagnostic technology, growing disease burden, and increasing emphasis on preventive healthcare, this market will remain a cornerstone of modern healthcare delivery. Stakeholders across the healthcare continuum are expected to benefit significantly from the innovations and opportunities emerging within the North America Point of Care Diagnostics Market.
𝐓𝐡𝐞 𝐋𝐢𝐬𝐭 𝐨𝐟 𝐂𝐨𝐦𝐩𝐚𝐧𝐢𝐞𝐬
Abbott
BD
bioMerieux SA
BIO-RAD LABORATORIES INC.
Danaher
HOFFMANN-LA ROCHE LTD.
Johnson and Johnson Services, Inc.
Nova Biomedical
Polymer Technology Systems, Inc. (PTS)
Siemens AG
In case of COVID-19, North America is highly affected specially the US. The outbreak of the COVID-19 pandemic situation shown some favorable scenario for players operating in the point of care diagnostics market. Significant population of North America is infected with coronavirus. For instance, the US is a highly affected country in the North American regions. The effect of the corona is mild in adults; however, it is adverse in older people. The infection of corona among older people has resulted in severe complications and has accounted for a good number of deaths in the country. But during the pandemic situation, life science companies engaged in development of novel drugs for the treatment of life threaten diseases. In addition, increasing demand for rapid testing equipment is also playing a prominent role in the growth of the North America point of care diagnostics market. Furthermore, due to the rising numbers of COVID-19 population has positive impact on the adoption of point of care diagnostic kits. This adoption is boosting new product development and launches in the market. For instance, in July 2020, Eurofins U.S. Clinical Diagnostics has announced the availability of its pooled PCR test to detect SARS-CoV-2, which would substantially lower the cost per PCR test for clients. Moreover, in March 2020, Abbott received FDA approval for its molecular point-of-care test for the diagnosis of COVID-19. The test is capable to deliver positive results in five minutes and negative result in less than fifteen minutes. Such product launches are expected to accelerate the growth of the North America point of care diagnostics market.
North America Point of Care Diagnostics Strategic Insights
Strategic insights for the North America Point of Care Diagnostics provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
North America Point of Care Diagnostics Regional Insights
The geographic scope of the North America Point of Care Diagnostics refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
About Us-
Business Market Insights is a market research platform that provides subscription service for industry and company reports. Our research team has extensive professional expertise in domains such as Electronics & Semiconductor; Aerospace & Defense; Automotive & Transportation; Energy & Power; Healthcare; Manufacturing & Construction; Food & Beverages; Chemicals & Materials; and Technology, Media, & Telecommunications.
0 notes
Text
Apoptosis Assays Market Size, Innovations & Restraints To 2032
Apoptosis Assays Market Overview The global apoptosis assays market is witnessing significant growth, currently valued at approximately USD 450 million in 2025. Driven by increasing research activities in drug discovery, oncology, and immunology, the market is projected to expand at a compound annual growth rate (CAGR) of around 8–10% over the next 5 to 10 years. The growing prevalence of chronic diseases such as cancer and neurodegenerative disorders has intensified the demand for apoptosis detection tools to better understand cellular mechanisms and develop targeted therapies. Technological advancements in assay formats, including flow cytometry, ELISA-based kits, and high-throughput screening methods, have enhanced sensitivity and accuracy, fueling market expansion. Additionally, integration of multiplex assays and automation in apoptosis testing platforms are key trends influencing the market landscape. The rising investment in personalized medicine and regenerative research further reinforces the demand for sophisticated apoptosis assays globally. Apoptosis Assays Market Dynamics Drivers: The primary growth drivers include the increasing prevalence of cancer and autoimmune diseases, escalating funding for biomedical research, and the expanding biotechnology and pharmaceutical sectors. Rising awareness about apoptosis's role in pathophysiology and drug development encourages adoption of innovative assay kits and instruments. Restraints: Despite robust growth, high costs associated with advanced apoptosis assay kits and instruments, complexity in data interpretation, and limited accessibility in developing regions pose challenges. Stringent regulatory frameworks for assay validation and approval also impede rapid market penetration. Opportunities: Emerging applications in neurodegenerative disease research, stem cell biology, and immunotherapy offer promising avenues. Increasing collaboration between assay manufacturers and research institutes to develop customized solutions is fostering innovation. Additionally, the advent of label-free and real-time apoptosis detection technologies represents untapped potential. Role of Technology, Regulations, and Sustainability: Cutting-edge technologies such as microfluidics, biosensors, and AI-driven data analytics are revolutionizing apoptosis assay methodologies. Regulatory bodies are emphasizing assay standardization and reproducibility, which is shaping product development. Sustainability initiatives are prompting manufacturers to develop eco-friendly assay kits with reduced reagent consumption and waste generation. Download Full PDF Sample Copy of Apoptosis Assays Market Report @��https://www.verifiedmarketresearch.com/download-sample?rid=14453&utm_source=PR-News&utm_medium=380 Apoptosis Assays Market Trends and Innovations The apoptosis assays market is evolving rapidly with the introduction of multiplex assays enabling simultaneous detection of multiple biomarkers, enhancing throughput and data quality. Integration of microplate readers with advanced software analytics is simplifying apoptosis quantification. Innovative labeling techniques, such as fluorochrome-conjugated annexin V and caspase activity probes, are improving assay specificity. Collaborative ventures between biotechnology companies and academic institutions are accelerating the development of next-generation apoptosis assays with improved sensitivity and ease of use. The increasing use of 3D cell culture models and organoids in apoptosis research is also driving demand for assays compatible with complex biological systems. Apoptosis Assays Market Challenges and Solutions The market faces challenges including supply chain disruptions impacting raw material availability, leading to delayed product deliveries and increased costs. Pricing pressures from generic assay kits and competitive vendors reduce profit margins for manufacturers. Regulatory hurdles related to clinical validation slow down product launches and increase compliance costs.
To address these issues, companies are diversifying supplier networks to mitigate supply chain risks and adopting cost-efficient manufacturing practices. Strategic partnerships and licensing agreements help share regulatory burdens and accelerate approval processes. Additionally, continuous investment in R&D to develop robust, cost-effective, and user-friendly assays supports sustainable growth. Apoptosis Assays Market Future Outlook Looking ahead, the apoptosis assays market is expected to maintain a strong upward trajectory, driven by expanding research in personalized medicine and targeted therapies. The integration of AI and machine learning for apoptosis data interpretation will enhance assay utility and accuracy. Growth in emerging economies with expanding biomedical research infrastructure will contribute significantly to market expansion. Furthermore, increasing adoption of high-content screening and label-free technologies will redefine apoptosis detection standards. Strategic collaborations and innovations focused on assay multiplexing, miniaturization, and automation are set to dominate market developments. Overall, the apoptosis assays market is poised for dynamic growth, underpinned by technological advancements and rising global healthcare demands. Key Players in the Apoptosis Assays Market Apoptosis Assays Market are renowned for their innovative approach, blending advanced technology with traditional expertise. Major players focus on high-quality production standards, often emphasizing sustainability and energy efficiency. These companies dominate both domestic and international markets through continuous product development, strategic partnerships, and cutting-edge research. Leading manufacturers prioritize consumer demands and evolving trends, ensuring compliance with regulatory standards. Their competitive edge is often maintained through robust R&D investments and a strong focus on exporting premium products globally. Danaher Corporation BioTek Instruments Promega Corporation PerkinElmer Abcam plc Abnova Creative Bioarray Thermo Fisher Scientific Merck KgaA Becton GE Healthcare Dickinson and Company Geno Technology GeneCopoeia Inc and Sartorius Get Discount On The Purchase Of This Report @ https://www.verifiedmarketresearch.com/ask-for-discount?rid=14453&utm_source=PR-News&utm_medium=380 Apoptosis Assays Market Segments Analysis and Regional Economic Significance The Apoptosis Assays Market is segmented based on key parameters such as product type, application, end-user, and geography. Product segmentation highlights diverse offerings catering to specific industry needs, while application-based segmentation emphasizes varied usage across sectors. End-user segmentation identifies target industries driving demand, including healthcare, manufacturing, and consumer goods. These segments collectively offer valuable insights into market dynamics, enabling businesses to tailor strategies, enhance market positioning, and capitalize on emerging opportunities. The Apoptosis Assays Market showcases significant regional diversity, with key markets spread across North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa. Each region contributes uniquely, driven by factors such as technological advancements, resource availability, regulatory frameworks, and consumer demand. Apoptosis Assays Market, By Product • Kits• Reagents• Instruments Apoptosis Assays Market, By Technique • Flow Cytometry• Fluorescence Microscopy• Spectrophotometry• Other Techniques Apoptosis Assays Market, By Assay Type • Caspase Assay• DNA Fragmentation Assay• Cell Permeability Assay• Mitochondrial Assay Apoptosis Assays Market, By End-Use • Pharmaceutical & Biotechnology Companies• Hospital & Diagnostic Laboratories• Academic & Research Institutes Apoptosis Assays Market By Geography • North America• Europe• Asia Pacific• Latin America• Middle East and Africa For More Information or Query, Visit @ https://www.verifiedmarketresearch.com/product/apoptosis-assays-market/
About Us: Verified Market Research Verified Market Research is a leading Global Research and Consulting firm servicing over 5000+ global clients. We provide advanced analytical research solutions while offering information-enriched research studies. We also offer insights into strategic and growth analyses and data necessary to achieve corporate goals and critical revenue decisions. Our 250 Analysts and SMEs offer a high level of expertise in data collection and governance using industrial techniques to collect and analyze data on more than 25,000 high-impact and niche markets. Our analysts are trained to combine modern data collection techniques, superior research methodology, expertise, and years of collective experience to produce informative and accurate research. Contact us: Mr. Edwyne Fernandes US: +1 (650)-781-4080 US Toll-Free: +1 (800)-782-1768 Website: https://www.verifiedmarketresearch.com/ Top Trending Reports https://www.verifiedmarketresearch.com/ko/product/dissolvable-frac-plugs-market/ https://www.verifiedmarketresearch.com/ko/product/furosemide-injection-market/ https://www.verifiedmarketresearch.com/ko/product/battery-operated-grease-gun-market/ https://www.verifiedmarketresearch.com/ko/product/form-in-place-gasket-market/ https://www.verifiedmarketresearch.com/ko/product/isolated-gate-drivers-market/
0 notes
Text
Buy PMMA Filler Online: PrimeFill® Injections Long-Lasting Volume
For clinics and aesthetic professionals looking to buy PMMA filler online, PrimeFill® offers a technologically advanced, clinically proven solution. Developed by Dermax, a global leader in medical aesthetics, PrimeFill® combines biocompatibility with long-lasting volumizing effects, making it an ideal choice for practitioners seeking safe, stable, and sustained results.
Ready to expand your treatment portfolio? Click here to buy PMMA injections online with fast shipping and expert support.
What Is PrimeFill® PMMAFiller?
PrimeFill® is a next-generation injectable filler composed of 20% polymethyl methacrylate (PMMA) microspheres suspended in 80% cross-linked hyaluronic acid (HA). Designed for facial contouring, wrinkle correction, and long-term volumization, it provides immediate lift while stimulating the body’s own collagen production over time.
PrimeFill® pmma filler is developed using Microfluidic Tech™, an advanced process that ensures microsphere uniformity, smooth surface texture, and optimized particle distribution. This innovation minimizes adverse reactions and ensures a stable, natural-looking outcome.
Looking to buy PMMA filler that delivers both immediate and durable results? PrimeFill® pmma filler is available online through our trusted platform. Contact us today to place your order or speak with a product specialist.

Key Features and Benefits of PrimeFill® PMMAInjections
Uniform Particle Size and Controlled Distribution
PMMA microspheres in PrimeFill® measure 20-50 μm with high uniformity, reducing risks of migration and inflammation.
The smooth surface of the microspheres supports integration with surrounding tissues and stimulates fibroblast activity, enhancing long-term collagen regeneration.
HA-Based Gel Carrier for Immediate Volume
The 80% cross-linked hyaluronic acid component provides instant volume enhancement and hydration.
Acts as a cushioning medium, supporting the microspheres while allowing for easier and more precise injection.
Strong Tissue Support with High Elastic Modulus
With an elasticity modulus of approximately 1200 Pa, PrimeFill® delivers excellent lifting power.
The formulation is designed to maintain shape under pressure, making it suitable for areas that require structural enhancement like the nose, chin, and jawline.
Clinics wanting to buy PMMA injections online can trust PrimeFill® for its predictable integration and superior patient satisfaction.

How PrimeFill® PMMAFillerWorks: Dual-Action Mechanism
PrimeFill® offers a time-phased treatment process that starts with immediate visual improvement and leads to biologically driven tissue renewal:
Stage 1: Immediate Volume
The cross-linked HA delivers immediate plumping and smoothing to facial depressions, while holding the PMMA microspheres in place.
Stage 2: Collagen Stimulation (Weeks 2–8)
The HA gradually metabolizes, and PMMA microspheres become encapsulated by fibroblasts. This natural response triggers collagen synthesis and tissue remodeling.
Stage 3: Long-Term Volume Support (6 Months+)
A stable fibrous matrix forms around the microspheres, providing lasting volume for up to 5 years, depending on the treatment area and individual factors.
Interested in a product that performs over time? Buy PMMA filler online from Dermax and experience sustained aesthetic outcomes.

Indicated Treatment Areasfor PrimeFill® PMMAInjections Online
PrimeFill® is suitable for both superficial and deep-layer injections in multiple facial zones:
Nose bridge and radix
Nasolabial folds
Marionette lines
Chin projection
Jawline contouring
Tear troughs
Forehead and glabellar lines
Its versatility makes it ideal for clinics aiming to offer a full range of facial volumization and anti-aging procedures.

PrimeFill® PMMA Filler Before and After Photos
PrimeFill® has been shown to deliver:
Natural, harmonious enhancements with smooth contours
Sustained correction of facial lines and volume loss
Visible improvement immediately after treatment with continued enhancement over several months

Pmma injections before-and-after photos from real cases show PrimeFill®’s impact on areas like the chin, nasolabial folds, and nose bridge. These results demonstrate both its sculpting capacity and longevity.
Why Buy PrimeFill® PMMA Filler Online Over Other Fillers?
· Combines the long-term collagen stimulation of PMMA with the immediate volumization of HA
· Uniform microsphere size supports a safer, more predictable treatment experience
· Developed by Dermax, a trusted manufacturer with over 20 years in the aesthetic field
· Distributed globally with multilingual customer support and regional fulfillment warehouses
Looking for a PMMA solution you can trust? Buy PMMA injections online directly from Dermax and benefit from consistent quality, responsive service, and international logistics support.
About Dermax: Trusted Manufacturer for Buy PMMA Filler Injections Onine
Founded in 2003, Dermax has grown into a globally recognized name in the medical aesthetics industry. With manufacturing plants in Germany and Asia, and offices across Europe and Korea, Dermax supplies high-performance aesthetic injectables to over 80 countries.
With a focus on innovation, safety, and clinician education, Dermax remains the preferred partner for thousands of aesthetic professionals worldwide.
Want to buy PMMA filler from a company you can rely on? Contact us now and let our team assist you with product selection, pricing, and international delivery.

Source: https://www.dermaxmed.com/buy-pmma-filler-online-primefill-injections-long-lasting-volume.html
0 notes
Text
3D Cell Culture Market Regulatory Landscape and Investment Opportunities 2032
In 2024, the global 3D cell culture market was worth around USD 2.54 billion. It’s expected to grow steadily, reaching about USD 6.29 billion by 2032, with an annual growth rate of 12.1%. North America led the market in 2024, holding the biggest share at 45.15%, thanks to strong research activities and advanced healthcare infrastructure.
The 3D cell culture market is witnessing significant growth as researchers and pharmaceutical companies increasingly shift toward more physiologically relevant models for studying cell behavior, disease progression, and drug responses. Unlike traditional 2D cultures, 3D cell culture systems better replicate in vivo environments, making them valuable tools in areas like cancer research, tissue engineering, and drug screening. The 3D cell culture market is segmented by product type, technology, application, and end user, offering tailored solutions for diverse research needs. While high costs, complexity of techniques, and standardization challenges act as restraining factors, continuous innovation, automation, and growing support from regulatory and academic institutions are helping overcome these barriers. Regionally, the 3D cell culture market is led by North America and Europe, while Asia Pacific emerges as a rapidly expanding region due to increased research funding and biotechnological advancements.
Continue reading for more details: https://www.fortunebusinessinsights.com/3d-cell-culture-market-109009
Market Segmentation
By Product Type: Includes scaffold-based systems, scaffold-free systems, bioreactors, and cell culture media—defining segments within the 3D Cell Culture Market.
By Cell Type: Human, animal, and stem cells represent major categories shaping the 3D Cell Culture Market.
By Application: Drug screening, disease modeling, regenerative medicine, and tissue engineering form critical applications of the 3D Cell Culture Market.
By End-User: Pharmaceutical & biotech companies, academic research institutes, CROs, and hospitals are primary stakeholders in the 3D Cell Culture Market.
By Technology: Segments include hydrogel-based scaffolds, microcarrier beads, spheroids, organoids, and lab-on-a-chip platforms within the 3D Cell Culture Market.
List Of Key Companies Profiled:
Sartorius AG (Germany)
Thermo Fisher Scientific Inc. (U.S.)
Corning Incorporated (U.S.)
Merck KGaA (Germany)
Avantor Inc. (U.S.)
MIMETAS B.V. (Netherlands)
REPROCELL Inc. (Japan)

Market Growth
The 3D Cell Culture Market is witnessing robust expansion driven by rising demand for more physiologically relevant in vitro models.
Technological advancements like bioprinting, scaffold-free systems, and microfluidics are accelerating innovation within the 3D Cell Culture Market.
Growth in the 3D Cell Culture Market is supported by increasing R&D expenditure in pharmaceuticals and biotechnology sectors.
Strategic collaborations between life sciences companies and academic institutions are fueling development in the 3D Cell Culture Market.
Enhanced adoption in drug discovery, regenerative medicine, and oncology research is propelling momentum in the 3D Cell Culture Market.
Retaining Factors
High demand for accurate, predictive cellular models supports continuous investment in the 3D Cell Culture Market.
Standardization of protocols and reproducibility improvements foster confidence and retention in the 3D Cell Culture Market.
Integrations with AI-driven analytics, imaging, and automation technologies enhance platform stickiness in the 3D Cell Culture Market.
Ongoing training and technical support for researchers ensure steady adoption in the 3D Cell Culture Market.
Regulatory support for non-animal testing models and growing ethical concerns about animal use reinforce growth in the 3D Cell Culture Market.
Regional Analysis
North America: Leads the 3D Cell Culture Market with significant research funding, advanced infrastructure, and strong biotech presence.
Europe: Well-established academic clusters and regulatory incentives make it a key hub in the 3D Cell Culture Market.
Asia Pacific: Rapidly growing region in the 3D Cell Culture Market, driven by expanding biotech industry, government funding, and CRO activity.
Latin America: Emerging adoption of 3D cell culture platforms in academic and pharmaceutical sectors is shaping this region's share in the 3D Cell Culture Market.
Middle East & Africa: Early adoption in research and innovation hubs reflects potential for growth in the 3D Cell Culture Market, backed by increased funding and infrastructure development.
Contact us:
Fortune Business Insights™ Pvt.
Phone: USA: +1 833 909 2966 (Toll-Free),
United Kingdom: +44 808 502 0280 (Toll-Free)
APAC: +91 744 740 1245
Email: [email protected]
0 notes
Text
What Is Driving the Global Digital PCR and qPCR Market Toward $14.8 Billion by 2029?

The Strategic Shift in Precision Diagnostics
The global Digital PCR (dPCR) and Real-Time PCR (qPCR) market is poised for strong and sustained growth, rising from US$9.4 billion in 2023 to a projected US$14.8 billion by 2029, advancing at a CAGR of 8.1%. This dynamic expansion is no accident—it reflects a broader shift in how global healthcare systems approach diagnostics, disease management, and therapeutic development.
Fueled by precision medicine, the surge in infectious diseases, and the demand for faster, more reliable diagnostic solutions, dPCR and qPCR technologies are becoming indispensable. But the market also faces distinct structural and strategic hurdles. In this blog, we explore what’s really powering this market, where growth is concentrated, and how stakeholders—from diagnostics firms to pharma executives—can capitalize.
Download PDF Brochure
Why Is the Digital PCR and qPCR Market Gaining Momentum?
1. Rising Burden of Infectious and Genetic Diseases With the global rise in infectious diseases (like COVID-19 and its variants), tuberculosis, and rare genetic disorders, there's a mounting demand for high-sensitivity diagnostics. Both dPCR and qPCR offer fast turnaround, accurate pathogen quantification, and early detection—critical tools in combating outbreaks and managing chronic conditions.
2. Strategic Role in Biomarker Discovery As precision medicine moves into the mainstream, biomarker-driven diagnostics are becoming foundational. Real-time PCR is essential for gene expression studies, while dPCR provides absolute quantification, enabling more accurate companion diagnostics. The techniques are widely used in clinical trials, personalized therapy development, and oncology diagnostics.
3. Point-of-Care (PoC) Evolution qPCR and dPCR technologies are increasingly integrated into portable, PoC diagnostic platforms, allowing testing in non-laboratory settings such as rural clinics or emergency departments. This shift addresses global healthcare inequities and strengthens pandemic preparedness.
Where Are the Highest-Growth Opportunities?
Asia Pacific: The Fastest Growing Region
Asia Pacific is emerging as a hotbed of growth, thanks to:
Expanding pharma-biotech R&D in India, China, and South Korea
Heavy investment by CMOs and CDMOs
Government initiatives supporting molecular diagnostics
A large untapped market for PoC applications and cancer screening tools
However, instrument affordability and reimbursement gaps remain challenges that must be addressed to unlock full potential.
North America: The Market Leader
North America commands the largest share, led by:
Presence of dominant players like Thermo Fisher Scientific, Bio-Rad, and Danaher Corporation
Mature regulatory landscape supporting innovative diagnostics
High adoption of PCR in clinical diagnostics, biotech, and public health surveillance
How Do Instrument Innovations Drive Market Leadership?
Among instruments, droplet digital PCR (ddPCR) is leading the digital PCR sub-segment. Its ability to partition reactions into thousands of droplets, each acting as a mini PCR reaction, offers:
High precision
Inhibitor tolerance
Quantification without need for standard curves
This makes it ideal for oncology, liquid biopsy, viral load monitoring, and cell therapy R&D.
Who Are the Key Stakeholders in the Market Ecosystem?
The ecosystem spans multiple nodes:
Stakeholder
Role
Raw Material Suppliers
Reagents, enzymes, and microfluidics components
Instrument Manufacturers
ddPCR, chip-based, and real-time PCR platforms
End-Users
Hospitals, diagnostic labs, CROs, CDMOs, pharma-biotech firms, forensic labs
This diverse mix creates opportunities for strategic partnerships, co-development deals, and vertical integration.
What’s Holding the Market Back?
1. Reimbursement and Regulatory Complexity Despite technological advances, limited reimbursement coverage, particularly for advanced PCR tests, discourages widespread adoption. For example, the US CMS policy revisions in 2023–2024 caused confusion around billing for transplant-related diagnostics, underscoring the need for policy clarity.
2. Competition from Emerging Technologies Alternatives like Next-Generation Sequencing (NGS), CRISPR diagnostics, and ELISA are gaining traction. While PCR remains a gold standard, these methods offer greater scalability, faster throughput, and in some cases, lower operational costs.
3. Labor-Intensive Workflow and Standardization Issues Sample preparation and post-PCR analysis still involve manual steps, increasing time-to-result and introducing variability. There's a clear opportunity to innovate through automation and AI integration.
What Opportunities Can C-Level Executives Leverage?
1. Invest in Companion Diagnostics RT-PCR-based companion diagnostics are critical for pharma firms developing targeted therapies. By embedding these diagnostics into drug development pipelines, companies can accelerate regulatory approvals and boost patient stratification precision.
2. Explore Untapped Markets Emerging economies in Southeast Asia, Latin America, and parts of Africa offer immense opportunity. Strategic local partnerships and distribution models can help overcome infrastructure and cost barriers.
3. Adopt Platform Thinking Building scalable PCR platforms that integrate AI, cloud data, and IoT can revolutionize disease monitoring. This will create long-term value for health systems and open recurring revenue streams via software and data analytics.
Conclusion: Precision Diagnostics Is the Next Frontier
As healthcare increasingly moves toward precision, decentralization, and real-time decision-making, digital and real-time PCR technologies are central pillars. However, success in this market depends not only on technological superiority but on strategic alignment—from regulatory navigation and reimbursement advocacy to platform innovation and global expansion.
For More information, Inquire Now.
0 notes
Text
Negative Photoresists: Tailored Solutions for Complex Designs
Explore how negative photoresists deliver precision, durability, and high aspect ratio patterning for electronics, MEMS, photonics, and PCB manufacturing. Discover tailored solutions for microfabrication excellence with A-Gas Electronic Materials. Contact us today to learn more.
#negative photoresists UK#microfabrication photoresists#high aspect ratio photoresists#photolithography resists#PCB photoresist materials#microfluidic device photoresists#optical device photoresists#advanced microfabrication solutions#A-Gas Electronic Materials
0 notes
Text
Europe Point-Of-Care-Testing Market Size, Share, Trends, Growth Opportunities and Competitive Outlook
Executive Summary Europe Point-Of-Care-Testing (POCT) Market :
Europe Point-Of-Care Testing (POCT) Market size was valued at USD 21.63 billion 2024 and is projected to reach USD 10.65 billion by 2032, with a CAGR of 9.3% during the forecast period of 2025 to 2032.
Europe Point-Of-Care-Testing (POCT) Market business market research report help you stay up-to-date about the whole market and also give holistic view of the market. Market research analysis provides the insights which help to have a more precise understanding of the market landscape, issues that may impinge on the industry in the future, and how to position specific brands in the best way. With this report one can focus on the data and realities of the industry which keeps them on the right path. The insights covered in this Europe Point-Of-Care-Testing (POCT) Market report will guide for an actionable ideas, better decision-making and better business strategies.
With Europe Point-Of-Care-Testing (POCT) Market international market research report it becomes easy to do estimations about the investment in an emerging market, expansion of market share or success of a new product. Market research analysis makes the professional reputation better in the field, builds more credibility in the work and helps other participants to have more assurance and trust in your conclusions. This market report guides all sizes of businesses by providing informed decisions on the different aspects of business. Europe Point-Of-Care-Testing (POCT) Market report has been formulated by understanding the significance of sound facts and figures required for any research.
Discover the latest trends, growth opportunities, and strategic insights in our comprehensive Europe Point-Of-Care-Testing (POCT) Market report. Download Full Report: https://www.databridgemarketresearch.com/reports/europe-poct-market
Europe Point-Of-Care-Testing (POCT) Market Overview
**Segments**
- Based on product, the Europe Point-Of-Care-Testing (POCT) market can be segmented into glucose monitoring kits, infectious disease testing kits, cardiometabolic monitoring kits, coagulation monitoring kits, pregnancy and fertility testing kits, tumor/cancer markers, urinalysis testing kits, cholesterol test strips, hematology testing kits, drug-of-abuse testing kits, fecal occult testing kits, and others. - On the basis of mode of prescription, the market can be categorized into prescription-based testing and OTC testing. - Depending on platform, the POCT market in Europe consists of lateral flow assays, dipsticks, microfluidics, molecular diagnostics, immunoassays, and others. - By end-user, the market is classified into hospitals, home care settings, clinics, diagnostic laboratories, and others.
**Market Players**
- Some of the key players operating in the Europe POCT market include Abbott, F. Hoffmann-La Roche Ltd, Siemens Healthcare GmbH, BD, bioMérieux SA, Johnson & Johnson Services, Inc., PTS Diagnostics, Werfen, QIAGEN, Sekisui Diagnostics, and Trividia Health, Inc.
The Europe Point-Of-Care-Testing (POCT) market is a dynamic and rapidly evolving sector that offers a wide range of opportunities for growth and innovation. One of the key trends shaping the market is the increasing demand for convenient and rapid diagnostic solutions, particularly in settings such as hospitals, clinics, and home care environments. The shift towards personalized medicine and the need for quicker and more accurate test results are driving the adoption of POCT devices across Europe.
One of the significant drivers of the Europe POCT market is the rising prevalence of chronic diseases such as diabetes, cardiovascular disorders, and infectious diseases. POCT devices play a crucial role in the early detection and monitoring of these conditions, leading to better patient outcomes and reduced healthcare costs. The focus on preventive healthcare and the emphasis on early diagnosis are also fueling the demand for POCT solutions in the region.
The competitive landscape of the Europe POCT market is characterized by the presence of a diverse range of players, including multinational corporations, medium-sized enterprises, and start-ups. Companies such as Abbott, F. Hoffmann-La Roche Ltd, Siemens Healthcare GmbH, and BD are among the key players driving innovation and technological advancements in the market. These companies are investing in research and development activities to launch new products and enhance their existing portfolio to cater to the evolving needs of healthcare providers and patients.
Regulatory factors also play a significant role in shaping the Europe POCT market. Stringent regulations governing the approval and commercialization of POCT devices are influencing the market dynamics and competition among players. Companies that can navigate the regulatory landscape effectively and ensure compliance with standards and guidelines are better positioned to succeed in the market.
The increasing adoption of digital health solutions and telemedicine platforms is creating new opportunities for POCT market players to expand their reach and offer integrated healthcare solutions. The convergence of healthcare technology, data analytics, and artificial intelligence is paving the way for innovative POCT devices that can deliver real-time insights and personalized recommendations to users.
Overall, the Europe POCT market holds immense potential for growth and innovation, driven by factors such as the increasing prevalence of chronic diseases, the emphasis on preventive healthcare, technological advancements, and regulatory developments. Market players that can differentiate their products, forge strategic partnerships, and stay ahead of the curve in terms of innovation are likely to experience sustained growth and success in the dynamic and competitive landscape of the Europe POCT market.The Europe Point-Of-Care-Testing (POCT) market is experiencing significant growth and evolution driven by various factors such as the demand for convenient and rapid diagnostic solutions, increasing prevalence of chronic diseases, and the shift towards personalized medicine. The market segmentation based on product diversity reflects the wide array of applications for POCT devices, ranging from glucose monitoring and infectious disease testing to pregnancy and fertility testing. This variety indicates the versatility and adaptability of POCT solutions to cater to different healthcare needs and diagnostic requirements.
The mode of prescription segmentation further showcases the different channels through which POCT devices are accessed, whether through prescription-based testing or over-the-counter availability. This distinction highlights the accessibility and flexibility of POCT devices, catering to both healthcare professionals and consumers seeking quick and reliable diagnostic results. The market players in the Europe POCT market consist of industry giants such as Abbott, Roche, and Siemens, along with other key players driving innovation and technological advancements in the sector.
The competitive landscape within the Europe POCT market underscores the importance of research and development activities in driving product innovation and differentiation. Companies investing in cutting-edge technologies and regulatory compliance are better positioned to capitalize on the growing demand for POCT solutions in the region. The integration of digital health solutions and telemedicine platforms further amplifies the opportunities for market expansion and the delivery of integrated healthcare services using POCT devices.
Regulatory factors play a crucial role in shaping the market dynamics and influencing competition among players. Compliance with stringent regulations and standards is essential for market players to navigate the regulatory landscape effectively and ensure the commercial success of their POCT devices. The convergence of healthcare technology, data analytics, and artificial intelligence presents new avenues for developing innovative POCT devices that provide real-time insights and personalized recommendations to users.
In conclusion, the Europe POCT market offers immense potential for growth and innovation, driven by the increasing prevalence of chronic diseases, technological advancements, regulatory developments, and the emphasis on preventive healthcare. Market players that focus on product differentiation, strategic partnerships, and continuous innovation are likely to thrive in the competitive landscape of the Europe POCT market. The evolving market trends and consumer demands present opportunities for companies to enhance their product offerings and create value-added solutions that address the evolving healthcare needs across Europe.
The Europe Point-Of-Care-Testing (POCT) Market is highly fragmented, featuring intense competition among both global and regional players striving for market share. To explore how global trends are shaping the future of the top 10 companies in the keyword market.
Learn More Now: https://www.databridgemarketresearch.com/reports/europe-poct-market/companies
DBMR Nucleus: Powering Insights, Strategy & Growth
DBMR Nucleus is a dynamic, AI-powered business intelligence platform designed to revolutionize the way organizations access and interpret market data. Developed by Data Bridge Market Research, Nucleus integrates cutting-edge analytics with intuitive dashboards to deliver real-time insights across industries. From tracking market trends and competitive landscapes to uncovering growth opportunities, the platform enables strategic decision-making backed by data-driven evidence. Whether you're a startup or an enterprise, DBMR Nucleus equips you with the tools to stay ahead of the curve and fuel long-term success.
Key Pointers Covered in the Europe Point-Of-Care-Testing (POCT) Market Industry Trends and Forecast
Europe Point-Of-Care-Testing (POCT) Market Size
Europe Point-Of-Care-Testing (POCT) Market New Sales Volumes
Europe Point-Of-Care-Testing (POCT) Market Replacement Sales Volumes
Europe Point-Of-Care-Testing (POCT) Market By Brands
Europe Point-Of-Care-Testing (POCT) Market Procedure Volumes
Europe Point-Of-Care-Testing (POCT) Market Product Price Analysis
Europe Point-Of-Care-Testing (POCT) Market Regulatory Framework and Changes
Europe Point-Of-Care-Testing (POCT) Market Shares in Different Regions
Recent Developments for Market Competitors
Europe Point-Of-Care-Testing (POCT) Market Upcoming Applications
Europe Point-Of-Care-Testing (POCT) Market Innovators Study
Browse More Reports:
Global Digital Holographic Display Market Global Animal Feed Acidifier Market Global Polyvinyl Butyral Market Global Lyme Disease Drug Market Asia-Pacific Reverse Logistics Market Middle East and Africa Remote Patient Monitoring and Care Market Global Hepatitis C Diagnosis and Treatment Market Global Sleep Tech Devices Market Global Paperboard Beverage Packaging Market Global Single-use Bioprocessing Systems Market Global Nanobots Market Global Oxidative Stress Assay Instruments Market Global Plastic Baby Food Packaging Market North America Conversational AI Market Global In-Memory Computing Market North America Reverse Logistics Market Global Imaging Infrared Light Emitting Diode (LED) Market Global Inherited Retinal Diseases Market Global Broadband Internet Access Services Market Global Plant Based Food Packaging Market Global Rare Earth Elements Market Global Automotive Natural Gas Vehicle Market North America Automotive DC-DC Converters Market Europe Non-stick Cookware Market Global Connected Care Market Global Triple A Syndrome Treatment Market North America Fluoroscopy - C Arms Market Global Video Conferencing Systems Market Global Automotive DC-DC Converters Market Global High Performance Lubricant Market North America Clinical Chemistry Analyser Market Global Performance Chemicals Market Global Vehicle Diagnostics Market Global Meatless Flavor Additives Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us: Data Bridge Market Research US: +1 614 591 3140 UK: +44 845 154 9652 APAC : +653 1251 975 Email:- [email protected]
0 notes
Text
Asia-Pacific Emerges as Fastest-Growing Region in Microsphere Adoption
The Polymethyl Methacrylate (PMMA) Microsphere Market is witnessing strong growth due to expanding applications across medical, cosmetic, coatings, and electronics sectors. In 2024, the market was valued at USD 389.5 billion and is expected to rise to USD 857.1 billion by 2033, growing at a compound annual growth rate (CAGR) of 8.7%. The growth is fueled by advancements in microencapsulation, high-performance coatings, drug delivery systems, and light-diffusing technologies.

To Get Sample Report: https://www.datamintelligence.com/download-sample/polymethyl-methacrylate-microsphere-market
Key Growth Drivers
Healthcare and Drug Delivery Applications PMMA microspheres are widely used in drug delivery, diagnostic imaging, and embolization procedures. Their biocompatibility and controlled-release properties make them ideal for targeted therapeutics. As minimally invasive procedures become more common in the U.S. and Japan, the demand for PMMA-based embolic agents and microbeads is rising.
Cosmetics and Personal Care Innovation The cosmetics industry increasingly uses PMMA microspheres to provide skin-blurring effects, improve spreadability, and offer a silky, matte finish. These microspheres are incorporated into skincare, foundations, and anti-aging products. In Japan and the U.S., premium cosmetic brands are using PMMA for next-gen formulations due to its non-toxic and biodegradable features.
Optical and Electronics Demand Due to their excellent refractive index and optical clarity, PMMA microspheres are essential in optical coatings, LED lenses, and display devices. Japan and South Korea continue to innovate in this space, integrating PMMA in compact electronics and smart devices, fueling demand in the Asia-Pacific region.
Industrial Coatings and Automotive Sector In coatings, PMMA provides scratch resistance, UV protection, and light diffusion, particularly in automotive and architectural segments. As the global automotive market evolves toward lighter materials and durable coatings, PMMA is being increasingly adopted for both aesthetic and functional uses.
Sustainability and Bio-Based Trends Sustainability is a major trend in the PMMA microsphere industry. Companies are investing in bio-derived or eco-friendly microspheres to meet stricter regulations on microplastics. Japanese firms are leading in this space by commercializing plant-based PMMA products.
Recent Trends and Developments
3D Printing Applications PMMA microspheres are now used as lightweight fillers in 3D printing filaments for enhanced structural stability and reduced material usage. The U.S. has seen a rise in such applications in the aerospace and medical sectors.
Medical Imaging and Diagnostics PMMA microspheres, due to their defined size and optical properties, are becoming essential in fluorescent imaging and microfluidic diagnostics.
Nanotechnology Advancements Researchers are developing nano-sized PMMA microspheres for high-precision drug delivery and implant coatings. These innovations offer improved bioavailability and better patient outcomes.
Automotive Paint Enhancers Automotive manufacturers use PMMA microspheres to develop finishes that are both light-diffusing and scratch-resistant, improving the aesthetic longevity of vehicles.
Regional Insights
United States The U.S. is a major player in the PMMA microsphere market, driven by its advanced healthcare, beauty, and automotive industries. Major pharmaceutical and cosmetic brands are adopting PMMA-based formulations. Furthermore, the FDA's evolving regulatory framework supports the adoption of biodegradable microspheres in therapeutic solutions.
Japan Japan is at the forefront of microsphere innovation, particularly in biodegradable PMMA technologies. With leading chemical manufacturers and a strong focus on clean, functional cosmetics, Japanese industries are shaping the future of PMMA in Asia-Pacific. Japanese electronics and med-tech industries are also investing heavily in microfluidics and biosensing applications.
Asia-Pacific The region is expected to exhibit the fastest CAGR during the forecast period. Factors such as rapid industrialization, growing healthcare access, and increased electronics manufacturing in China, India, and South Korea contribute significantly to regional growth.
Europe European regulations on microplastics are pushing companies to innovate in sustainable PMMA microsphere production. Several EU-based firms are partnering with biotech startups to develop environmentally safe alternatives.
Challenges
High Production Costs Advanced polymerization techniques and stringent quality requirements increase manufacturing costs.
Regulatory Scrutiny Environmental concerns over microplastics have prompted regulators, especially in Europe and Japan, to tighten policies on the use of synthetic microspheres in cosmetics and food applications.
Competition from Alternatives Other materials such as glass microspheres or biodegradable polymers are emerging as competitors, particularly in environmentally sensitive markets.
Key Players
Some of the key companies in the PMMA microsphere market include:
Cospheric LLC
Kuraray Co. Ltd.
Nippon Shokubai Co. Ltd.
Sekisui Kasei
Dynea AS
Trinseo
Kobo Products
HEYO Enterprises
Microbeads AS
Imperial Microspheres
These players are focusing on R&D, sustainability, and capacity expansion to maintain their market positions.
Outlook
The PMMA Microsphere Market is poised for strong growth over the next decade, supported by:
Rising healthcare and cosmetic applications
Demand for sustainable, bio-compatible materials
Growth in the Asia-Pacific region
Technological breakthroughs in manufacturing and material sciences
As global industries pivot toward performance-based, light-weight, and environmentally safe solutions, PMMA microspheres will remain integral across a wide range of applications from skincare to surgical technologies.
0 notes
Text
Why Peristaltic Pumps Are the Best Choice for Shear-Sensitive Fluids?
When dealing with sensitive biological materials, viscous chemicals, or sensitive emulsions in an industrial or lab environment, the nature of the pump used can be the deciding factor to the process. Shear-sensitive liquids must be handled with care in order to preserve integrity, activity, and consistency.
This is where peristaltic pumps shine with accuracy, cleanliness, and little mechanical stress. On this blog here, we demonstrate how peristaltic pumps are ideally suited for the handling of shear-sensitive fluids and how Microlit products can provide fluid purity and accuracy for industries and laboratories.
What Are Shear-Sensitive Fluids?
Shear-sensitive fluids are fluids that degrade or lose their properties when they come in contact with high shear forces. They are:
Biological fluids (e.g., blood, enzymes, proteins)
Emulsions (e.g., cosmetic creams, lotions)
Cell suspensions (e.g., bacterial or mammalian cells)
Polymer solutions
When under pressure from traditional mechanical pumps like gear pumps or centrifugal pumps, these fluids can denature, lyse cells, or emulsify. It is important to preserve their structural integrity, particularly in biomedical and pharmaceutical use.
How Peristaltic Pumps Work?
A peristaltic pump works by squeezing an elastic tube with rolling rollers in such a manner that it creates a peristaltic motion. Compression of the liquid in the forward direction is created by this process without ever touching the internal mechanical parts of the pump.
That is the reason why this mechanism will find its optimal application in sensitive fluids:
No fluid-to-component contact
Low shear force with strength.
Minimize risk of contamination
Simple control of flow-rate and reversible flow
Easily maintained and tubed
The Shear Advantage: Gentle Yet Effective
Conventional pumping systems shear fluids as they pass through tiny gear teeth or impellers. Such forces can:
Lyse cell membranes within biological specimens
Break emulsions apart into their phases
Change the chemical composition or the viscosity of multifaceted formulations
On the other hand, peristaltic pumps achieve smooth and consistent pressure so that cells are not damaged and formulations are not interrupted. Therefore, they are extremely suitable for application in biotechnology, pharmaceutical, cosmetics, and food & beverage.
Applications That Depend on Shear-Safe Pumping
Let us focus more specifically on industries and applications that make use of the peristaltic pump's soft flow behavior:
Pharmaceutical and Biotechnology Laboratories
Fermentations and bioreactors will likely employ sensitive mammalian or bacterial cell cultures. Growth, activity, or yield can be influenced by minimal perturbation. A laboratory peristaltic pump supplies convenient sampling and nutrient addition with no cell breakage, and the results are high-purity.
Diagnostics and Healthcare
In analytical equipment or automated liquid-handling stations, the accuracy and non-contaminating function of peristaltic pumps provide reliable dispensing of patient samples or reagents. Peristaltic pumps are able to dispense small volumes of liquids carefully, which is particularly important in microfluidic platforms and analysers for immunoassay.
Cosmetics and Personal Care
Emulsions of creams, lotions, and serums consist of sophisticated formulations which break down when stressed. These can be transferred in filling and processing without disrupting their form by utilizing a micro peristaltic pump, thereby maintaining texture and performance.
Accuracy, Control, and Contamination-Free Transfer
One of the major advantages of peristaltic pumps is that they offer fluid isolation. Since the fluid never comes into contact with anything inside the pump except the tubing, cross-contamination is impossible. This makes them highly applicable to:
Aseptic procedures
Single-pump multi-fluid handling by a single pump
Recurrent fluid resuscitation
And their ability to precisely deliver microlitre to litre-scale quantities is an analytical process revolution. When combined with an e-dispenser or automated fluidics, peristaltic pumps offer scalable and programmable solutions for laboratories of all sizes.
The Role of Tubing: A Critical Factor
While the pump mechanism is doing the moving, the tubing is doing all of the work in the way of contacting the fluid. Selecting the proper tubing material, i.e., silicone, Tygon, or pharma-grade, is required to provide chemical compatibility and minimal shear stress.
Microlit systems are equipped with biocompatible and sturdy tubing solutions that are suitable for sensitive applications. Low absorption, long tubing life, and high flexibility are ensured in our design to provide maximum pump performance.
Microlit's Commitment to Precision and Purity
At Microlit, we appreciate the finesse of today's laboratory procedures. Our peristaltic pump systems find application with clients requiring precision, simplicity, and security in dealing with sensitive or high-value liquids.
Whether you are in a regulated laboratory, cleanroom manufacturing facility, or research environment, Microlit has:
Touchscreen operation for simplicity
Variable speed motors for precise control of flow
Calibration is used for reproducibility
Space-conserving design to make optimal use of valuable bench real estate
And to address ultra-high-precision liquid handling, our micropipette offerings complement peristaltic systems with direct-volume transfer in high-priority applications like sample preparation, titrations, or micro-dosing.
Best Practices for Operating Peristaltic Pumps with Shear-Sensitive Fluids
To get the best out of your pump system, adopt the following guidelines:
Use the right tubing: Choose tubing diameter and material based on fluid type and flow rate requirement.
Avoid sudden turns: Gentle curves minimize resistance and stress on the fluid.
Maintain moderate speeds: Peristaltic pumps operate at high speeds, but moderate speeds are ideal for shear-sensitive fluids.
Tubing checks at regular intervals: Regularly replace tubing to prevent fatigue or leaks.
Regular calibration: Maintain flow accuracy by periodically calibrating your system, especially when a change of fluids is made.
Conclusion
To laboratories, R&D units, and manufacturing facilities dealing with sensitive liquids, a peristaltic pump is not a privilege but a necessity. Its pumping maintains fluid integrity, averts contamination, and requires minimal maintenance, so it's the best when dealing with sensitive materials.
At Microlit, precision engineering comes to your bench. Our comprehensive portfolio of fluid handling tools, ranging from micro peristaltic pumps to micropipettes, is engineered to meet the challenging needs of laboratories today and tomorrow's advances. Whether you are scaling up a biotech process or optimizing an analytical assay, rely on Microlit because every drop counts.
0 notes