#X-ray diffraction
Explore tagged Tumblr posts
Text

Using JADE software to perform some analysis on the general measurement scans that were run for the samples using the X-Ray Diffraction machine
#material science#mechanical engineering#engineering#JADE software#XRD#X-ray diffraction#general measurement x-ray#grad school
6 notes
·
View notes
Text
Conversation with Professor Albert Berghuis on Antiobiotics and the Only Synchrotronin Canada: Professor, Department of Biochemistry, McGill University
Publisher: In-Sight Publishing Publisher Founding: March 1, 2014 Web Domain: http://www.in-sightpublishing.com Location: Fort Langley, Township of Langley, British Columbia, Canada Journal: In-Sight: Independent Interview-Based Journal Journal Founding: August 2, 2012 Frequency: Three (3) Times Per Year Review Status: Non-Peer-Reviewed Access: Electronic/Digital & Open Access Fees: None…
View On WordPress
#Albert Berghuis#antibiotic resistance#antibiotics#Canadian Light Source#McGill University#Synchrotron#University of Saskatchewan#X-ray diffraction
0 notes
Text
Because it seems like I can't go back to writing my thesis report unless I get cezhou out of my system (for today), rereading the book made me realize how utterly well written it is in terms of how cezhou dynamics changes. I know they get disgustingly cute later on, I know xiao chiye dotes on shen zechuan to the point where he mostly forgets everyone around them. I know they are utterly besotted with each other.
Yet, reading the early chapters when xcy was directly against szc, everytime xcy figures something out which could potentially harm szc, I feel a chill up my spine. Which is useless cause I know nothing will happen, I know the story. And yet, the writing is so immaculate I cannot help but fear xcy. Every single instance where xcy sees through szc's plan a little voice in my head goes "oh fuck". And I think this is one of the best feats an author could achieve. Tang Jiu Qing please personally end me because how do you write characters and a story so well-
#qiang jin jiu#qjj#ballad of sword and wine#danmei#tang jiu qing#t97#someday I will make a long post about the relationship cezhou have before they get together#today I will go back to analysing my x ray diffraction peaks#sigh
29 notes
·
View notes
Text

X-ray diffraction enables measurement of in-situ ablation depth in aluminum
When laser energy is deposited in a target material, numerous complex processes take place at length and time scales that are too small to visually observe. To study and ultimately fine-tune such processes, researchers look to computer modeling. However, these simulations rely on accurate equation of state (EOS) models to describe the thermodynamic properties—such as pressure, density and temperature—of a target material under the extreme conditions generated by the intense heat of a laser pulse. One process that is insufficiently addressed in current EOS models is ablation, where the irradiation from the laser beam removes solid material from the target either by means of vaporization or plasma formation (the fourth state of matter). It is this mechanism that launches a shock into the material, ultimately resulting in the high densities required for high pressure experiments such as inertial confinement fusion (ICF).
Read more.
12 notes
·
View notes
Text
In 1953, using X-ray diffraction photograph of DNA fibres (figure 7.50) obtained by Rosalind Franklin and New Zealand-born Maurice Wilkins, James Watson and Francis Crick came to the conclusion that the DNA structure consists of the now-famous double helix (see figure 7.51).
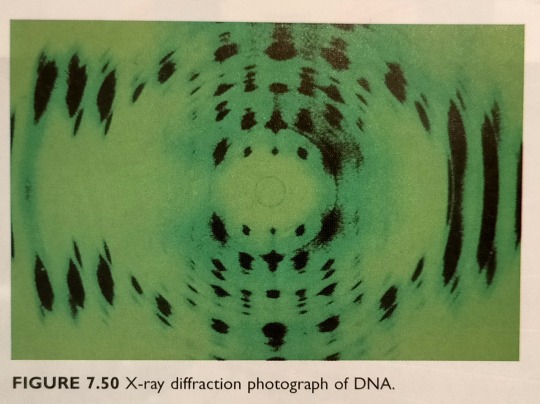
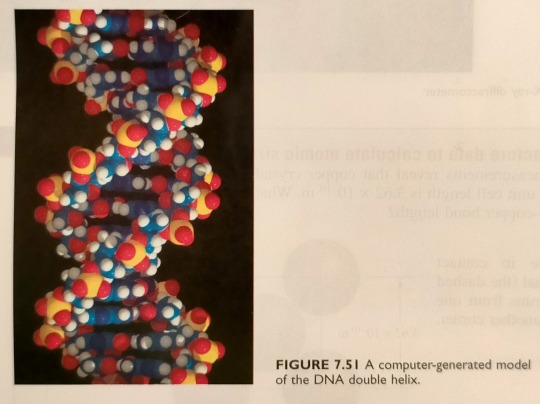
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quotes#chemistry#nonfiction#textbook#50s#1950s#20th century#x ray diffraction#dna#double helix#rosalind franklin#maurice wilkins#james watson#francis crick
8 notes
·
View notes
Text
The efforts, however, are well rewarded because calculations give very accurate locations for the atoms within the unit cell.
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
0 notes
Text






Welcome back to our Trailblazer Series!
Today, we’re honouring Rosalind Franklin, the brilliant chemist whose X-ray diffraction work was key to discovering DNA’s double helix —though she wasn’t properly credited in her time. She also made major contributions to RNA, viruses, and carbon science. Her legacy as a pioneer in molecular biology continues to inspire!
Unpacked Media
56 notes
·
View notes
Text
A new technique that produces 3D models of individual crystals has opened a window for scientists to see the subtle deviations that emerge in their otherwise perfect patterns. Researchers from New York University (NYU) went back to the drawing board on how to look deep inside solids made of repeating units, and determine how they grow. With a short wavelength roughly the same size as many of the repeating units that make up crystals, X-rays have long allowed scientists to infer how a crystal's components fit together by measuring the angle at which the rays are diffracted.
Continue Reading.
76 notes
·
View notes
Text
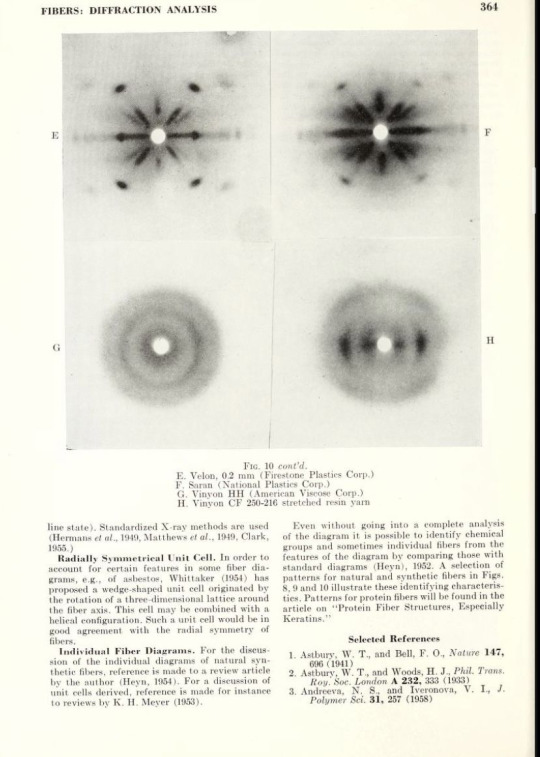
Diffraction patterns for natural and synthetic fibers
Encyclopedia of X-rays and gamma rays, 1963
54 notes
·
View notes
Text



PhDay 14: what am I doing? a badly drawn explanation
My PhD is in the general area of condensed matter physics and extreme conditions, which means I look at solids/liquids, specifically metals, at really high pressure and high temperature. Because under exciting conditions, materials do exciting things!
I get stuff to high pressures by squeezing them between two diamonds inside a small metal container called a diamond anvil cell (DAC), and heating it up essentially by electricity, called resistive heating. (first picture, diamonds in blue and big arrows for direction of pushing the diamonds together)
Diamonds???, I hear you say. Yes, diamonds are more than just pretty and expensive. They are really hard and transparent which means I can look at the structure of my stuff between them by x-ray diffraction. For crystalline stuff like metals, we can decode what the crystal structure is if we shoot x-rays at it that diffract off it in predictable ways, in a synchrotron, a big circular accelerator that makes x-rays. (middle picture, the outside of a synchrotron)
If heat it up enough, stuff melts!! The temperature it melts at changes when you increase the pressure, and I can visualise this on a phase diagram, a graph with T for temperature and P for pressure and drawing a melting curve. (last picture)
#studyblr#stemblr#phdblr#gradblr#phd journey#university#physics#science#if you read all that well done#i was told to stop saying “stuff” in lab reports#so now im saying “stuff” as much as possible
26 notes
·
View notes
Text
there are two types of anime power systems:
"i survived the fall because of the power of love! :3"
and
"Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well.[1][2] Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide (metal carbonyls), cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are representative members of this class. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.[3]
Organometallic compounds are widely used both stoichiometrically in research and industrial chemical reactions, as well as in the role of catalysts to increase the rates of such reactions (e.g., as in uses of homogeneous catalysis), where target molecules include polymers, pharmaceuticals, and many other types of practical products.
Most organometallic compounds are solids at room temperature, however some are liquids such as methylcyclopentadienyl manganese tricarbonyl, or even volatile liquids such as nickel tetracarbonyl.[1] Many organometallic compounds are air sensitive (reactive towards oxygen and moisture), and thus they must be handled under an inert atmosphere.[1] Some organometallic compounds such as triethylaluminium are pyrophoric and will ignite on contact with air.[6]
As in other areas of chemistry, electron counting is useful for organizing organometallic chemistry. The 18-electron rule is helpful in predicting the stabilities of organometallic complexes, for example metal carbonyls and metal hydrides. The 18e rule has two representative electron counting models, ionic and neutral (also known as covalent) ligand models, respectively.[7] The hapticity of a metal-ligand complex, can influence the electron count.[7] Hapticity (η, lowercase Greek eta), describes the number of contiguous ligands coordinated to a metal.[7] For example, ferrocene, [(η5-C5H5)2Fe], has two cyclopentadienyl ligands giving a hapticity of 5, where all five carbon atoms of the C5H5 ligand bond equally and contribute one electron to the iron center. Ligands that bind non-contiguous atoms are denoted the Greek letter kappa, κ.[7] Chelating κ2-acetate is an example. The covalent bond classification method identifies three classes of ligands, X,L, and Z; which are based on the electron donating interactions of the ligand. Many organometallic compounds do not follow the 18e rule. The metal atoms in organometallic compounds are frequently described by their d electron count and oxidation state. These concepts can be used to help predict their reactivity and preferred geometry. Chemical bonding and reactivity in organometallic compounds is often discussed from the perspective of the isolobal principle.
A wide variety of physical techniques are used to determine the structure, composition, and properties of organometallic compounds. X-ray diffraction is a particularly important technique that can locate the positions of atoms within a solid compound, providing a detailed description of its structure.[1][8] Other techniques like infrared spectroscopy and nuclear magnetic resonance spectroscopy are also frequently used to obtain information on the structure and bonding of organometallic compounds.[1][8] Ultraviolet-visible spectroscopy is a common technique used to obtain information on the electronic structure of organometallic compounds. It is also used monitor the progress of organometallic reactions, as well as determine their kinetics.[8] The dynamics of organometallic compounds can be studied using dynamic NMR spectroscopy.[1] Other notable techniques include X-ray absorption spectroscopy,[9] electron paramagnetic resonance spectroscopy, and elemental analysis.[1][8]
Due to their high reactivity towards oxygen and moisture, organometallic compounds often must be handled using air-free techniques. Air-free handling of organometallic compounds typically requires the use of laboratory apparatuses such as a glovebox or Schlenk line.[1]
Early developments in organometallic chemistry include Louis Claude Cadet's synthesis of methyl arsenic compounds related to cacodyl, William Christopher Zeise's[10] platinum-ethylene complex,[11] Edward Frankland's discovery of diethyl- and dimethylzinc, Ludwig Mond's discovery of Ni(CO)4,[1] and Victor Grignard's organomagnesium compounds. (Although not always acknowledged as an organometallic compound, Prussian blue, a mixed-valence iron-cyanide complex, was first prepared in 1706 by paint maker Johann Jacob Diesbach as the first coordination polymer and synthetic material containing a metal-carbon bond.[12]) The abundant and diverse products from coal and petroleum led to Ziegler–Natta, Fischer–Tropsch, hydroformylation catalysis which employ CO, H2, and alkenes as feedstocks and ligands.
Recognition of organometallic chemistry as a distinct subfield culminated in the Nobel Prizes to Ernst Fischer and Geoffrey Wilkinson for work on metallocenes. In 2005, Yves Chauvin, Robert H. Grubbs and Richard R. Schrock shared the Nobel Prize for metal-catalyzed olefin metathesis.[13]
Organometallic chemistry timeline
edit
1760 Louis Claude Cadet de Gassicourt isolates the organoarsenic compound cacodyl
1827 William Christopher Zeise produces Zeise's salt; the first platinum / olefin complex
1848 Edward Frankland discovers diethylzinc
1890 Ludwig Mond discovers nickel carbonyl
1899 John Ulric Nef discovers alkynylation using sodium acetylides.
1909 Paul Ehrlich introduces Salvarsan for the treatment of syphilis, an early arsenic based organometallic compound
1912 Nobel Prize Victor Grignard and Paul Sabatier
1930 Henry Gilman invents lithium cuprates, see Gilman reagent
1940 Eugene G. Rochow and Richard Müller discover the direct process for preparing organosilicon compounds
1930's and 1940's Otto Roelen and Walter Reppe develop metal-catalyzed hydroformylation and acetylene chemistry
1951 Walter Hieber was awarded the Alfred Stock prize for his work with metal carbonyl chemistry.
1951 Ferrocene is discovered
1956 Dorothy Crawfoot Hodgkin determines the structure of vitamin B12, the first biomolecule found to contain a metal-carbon bond, see bioorganometallic chemistry
1963 Nobel prize for Karl Ziegler and Giulio Natta on Ziegler–Natta catalyst
1973 Nobel prize Geoffrey Wilkinson and Ernst Otto Fischer on sandwich compounds
1981 Nobel prize Roald Hoffmann and Kenichi Fukui for creation of the Woodward-Hoffman Rules
2001 Nobel prize W. S. Knowles, R. Noyori and Karl Barry Sharpless for asymmetric hydrogenation
2005 Nobel prize Yves Chauvin, Robert Grubbs, and Richard Schrock on metal-catalyzed alkene metathesis
2010 Nobel prize Richard F. Heck, Ei-ichi Negishi, Akira Suzuki for palladium catalyzed cross coupling reactions
Subspecialty areas of organometallic chemistry include:
Period 2 elements: organolithium chemistry, organoberyllium chemistry, organoborane chemistry
Period 3 elements: organosodium chemistry, organomagnesium chemistry, organoaluminium chemistry, organosilicon chemistry
Period 4 elements: organocalcium chemistry, organoscandium chemistry, organotitanium chemistry, organovanadium chemistry, organochromium chemistry, organomanganese chemistry, organoiron chemistry, organocobalt chemistry, organonickel chemistry, organocopper chemistry, organozinc chemistry, organogallium chemistry, organogermanium chemistry, organoarsenic chemistry, organoselenium chemistry
Period 5 elements: organoyttrium chemistry, organozirconium chemistry, organoniobium chemistry, organomolybdenum chemistry, organotechnetium chemistry, organoruthenium chemistry, organorhodium chemistry, organopalladium chemistry, organosilver chemistry, organocadmium chemistry, organoindium chemistry, organotin chemistry, organoantimony chemistry, organotellurium chemistry
Period 6 elements: organolanthanide chemistry, organocerium chemistry, organotantalum chemistry, organotungsten chemistry, organorhenium chemistry, organoosmium chemistry, organoiridium chemistry, organoplatinum chemistry, organogold chemistry, organomercury chemistry, organothallium chemistry, organolead chemistry, organobismuth chemistry, organopolonium chemistry
Period 7 elements: organoactinide chemistry, organothorium chemistry, organouranium chemistry, organoneptunium chemistry
Organometallic compounds find wide use in commercial reactions, both as homogenous catalysts and as stoichiometric reagents. For instance, organolithium, organomagnesium, and organoaluminium compounds, examples of which are highly basic and highly reducing, are useful stoichiometrically but also catalyze many polymerization reactions.[14]
Almost all processes involving carbon monoxide rely on catalysts, notable examples being described as carbonylations.[15] The production of acetic acid from methanol and carbon monoxide is catalyzed via metal carbonyl complexes in the Monsanto process and Cativa process. Most synthetic aldehydes are produced via hydroformylation. The bulk of the synthetic alcohols, at least those larger than ethanol, are produced by hydrogenation of hydroformylation-derived aldehydes. Similarly, the Wacker process is used in the oxidation of ethylene to acetaldehyde.[16]
Almost all industrial processes involving alkene-derived polymers rely on organometallic catalysts. The world's polyethylene and polypropylene are produced via both heterogeneously via Ziegler–Natta catalysis and homogeneously, e.g., via constrained geometry catalysts.[17]
Most processes involving hydrogen rely on metal-based catalysts. Whereas bulk hydrogenations (e.g., margarine production) rely on heterogeneous catalysts, for the production of fine chemicals such hydrogenations rely on soluble (homogenous) organometallic complexes or involve organometallic intermediates.[18] Organometallic complexes allow these hydrogenations to be effected asymmetrically.
Many semiconductors are produced from trimethylgallium, trimethylindium, trimethylaluminium, and trimethylantimony. These volatile compounds are decomposed along with ammonia, arsine, phosphine and related hydrides on a heated substrate via metalorganic vapor phase epitaxy (MOVPE) process in the production of light-emitting diodes (LEDs).
Organometallic compounds undergo several important reactions:
associative and dissociative substitution
oxidative addition and reductive elimination
transmetalation
migratory insertion
β-hydride elimination
electron transfer
carbon-hydrogen bond activation
carbometalation
hydrometalation
cyclometalation
nucleophilic abstraction
The synthesis of many organic molecules are facilitated by organometallic complexes. Sigma-bond metathesis is a synthetic method for forming new carbon-carbon sigma bonds. Sigma-bond metathesis is typically used with early transition-metal complexes that are in their highest oxidation state.[19] Using transition-metals that are in their highest oxidation state prevents other reactions from occurring, such as oxidative addition. In addition to sigma-bond metathesis, olefin metathesis is used to synthesize various carbon-carbon pi bonds. Neither sigma-bond metathesis or olefin metathesis change the oxidation state of the metal.[20][21] Many other methods are used to form new carbon-carbon bonds, including beta-hydride elimination and insertion reactions.
Organometallic complexes are commonly used in catalysis. Major industrial processes include hydrogenation, hydrosilylation, hydrocyanation, olefin metathesis, alkene polymerization, alkene oligomerization, hydrocarboxylation, methanol carbonylation, and hydroformylation.[16] Organometallic intermediates are also invoked in many heterogeneous catalysis processes, analogous to those listed above. Additionally, organometallic intermediates are assumed for Fischer–Tropsch process.
Organometallic complexes are commonly used in small-scale fine chemical synthesis as well, especially in cross-coupling reactions[22] that form carbon-carbon bonds, e.g. Suzuki-Miyaura coupling,[23] Buchwald-Hartwig amination for producing aryl amines from aryl halides,[24] and Sonogashira coupling, etc.
Natural and contaminant organometallic compounds are found in the environment. Some that are remnants of human use, such as organolead and organomercury compounds, are toxicity hazards. Tetraethyllead was prepared for use as a gasoline additive but has fallen into disuse because of lead's toxicity. Its replacements are other organometallic compounds, such as ferrocene and methylcyclopentadienyl manganese tricarbonyl (MMT).[25] The organoarsenic compound roxarsone is a controversial animal feed additive. In 2006, approximately one million kilograms of it were produced in the U.S alone.[26] Organotin compounds were once widely used in anti-fouling paints but have since been banned due to environmental concerns.[27]"
#these are not mutually exclusive#anime#animanga#anime and manga#anime & manga#shounen anime#shonen anime#shonen#shounen manga#shonen manga#shoujo anime#shoujo manga#shojo#shoujo#shounen#mahou shoujo#shojo manga#shojo anime#magic system#hard magic#soft magic
7 notes
·
View notes
Text

Solus (golden retriever) So, a little status update on my manga! I did the storyboard for around 60 pages, which took around 3 evenings. I'm currently occupied from 9:00 - 18:00 with lab work (x-ray diffraction) + occasionally another few hours of data analysis. However, I'm guessing that I can meet my own deadline of drawing the storyboard for another 140 pages before April starts. Meaning, if university doesn't stress me out too much, I might actually be able to publish the first 20 pages in early May! Thanks for listening to my yapping <3
10 notes
·
View notes
Text


Rosalind Franklin (UK, London, 1920 - 1958)
Photo 51, showing X-ray diffraction pattern of DNA On 6 May 1952, at King´s College London, Rosalind Franklin photographed her fifty-first X-ray diffraction pattern of deoxyribosenucleic acid, or DNA. Photograph 51, or Photo 51, revealed information about DNA´s three-dimensional structure by displaying the way a beam of X-rays scattered off a pure fiber of DNA. Franklin took Photo 51 after scientists confirmed that DNA contained genes.
The X-ray crystallographer and biophysicist provided much of the experimental evidence for the structure of DNA, in the form of Photo 51 (below), before switching her focus to viruses at Birkbeck College. She died of cancer at the age of 38.
https://www.sciencefocus.com/the-human-body/understanding-dna-five-key-scientists-who-unravelled-the-helix
https://www.royalmint.com/stories/collect/a-crucial-contribution-the-life-of-rosalind-franklin/
https://embryo.asu.edu/pages/photograph-51-rosalind-franklin-1952
13 notes
·
View notes
Text

Machine learning uses X-ray diffraction data from polymers to predict the behavior of new materials
Polymers such as polypropylene are fundamental materials in the modern world, found in everything from computers to cars. Because of their ubiquity, it's vital that materials scientists know exactly how each newly developed polymer will perform under different preparation conditions. As described in a new study, which was published in Science and Technology of Advanced Materials, scientists can now use machine learning to determine what to expect from a new polymer. Predicting the mechanical properties of new polymers, such as their tensile strength or flexibility, usually involves putting them through destructive and costly physical tests. However, a team of researchers from Japan, led by Dr. Ryo Tamura, Dr. Kenji Nagata, and Dr. Takashi Nakanishi from the National Institute for Materials Science in Tsukuba, showed that machine learning can predict the material properties of polymers. They developed the method on a group of polymers called homo-polypropylenes, using X-ray diffraction patterns of the polymers under different preparation conditions to provide detailed information about their complex structure and features.
Read more.
#Materials Science#Science#X Rays#Diffraction#Polymers#Machine learning#Materials characterization#Computational materials science#Mechanical properties
12 notes
·
View notes
Text
In 1913, William Henry Bragg and his Australian-born son William Lawrence Bragg discovered that just a few variables control the appearance of an X-ray diffraction pattern.
"Chemistry" 2e - Blackman, A., Bottle, S., Schmid, S., Mocerino, M., Wille, U.
#book quote#chemistry#nonfiction#textbook#10s#1910s#20th century#william henry bragg#william lawrence bragg#william bragg#x ray#x ray diffraction#patterns
0 notes

