Welcome to Mr. Cheung's Classroom Webpage. HS Chemistry - 6th Grade Earth Science - Zoology Elective
Don't wanna be here? Send us removal request.
Text
February 2019 - Chemistry
Here is the tentative schedule for February 2019. Should Chemistry be offered 5 to 6 Periods a week, Lab might be moved to the Double Period (Tuesday) [?]

1 note
·
View note
Text
Chemistry - HW#13
!! ONE QUESTION PER BOX !! in the Homework-style Paper. 8 Question Total. At least 1 Heuristic Decision Tree filled-in on the back !! ----------------------------------------------------------------------------------------- Part A: Read pages 189 to 198 of HOLT Chemistry. Copy the Questions and Answer, neatly Section Review # 1, 3, 5, 6, 7 —— on page 198. Answer, using at least 2 complete sentences for each one. ----------------------------------------------------------------------------------------- Part B: Choose any 3 Regents Problems at the end of the Class-notes/Slides - for **Atomic Concepts* AND **The Periodic Table**. Copy the question, Answer the question, and !! Write out an explanation for your answer, using at least 2 complete sentences for each one. !! ----------------------------------------------------------------------------------------- Reminders: Finish your Lab Reports - Periodic Table Finish your Question and Answer - Do Now Compilation(s) Start your Summary Cheat Sheet for the 2nd Quarter Benchmark Exam. It will cover all topics we covered during the 2nd Quarter - Atomic Concepts to a little bit of Bond Polarity. Chemistry Benchmark - Friday. January 25 ----------------------------------------------------------------------------------------- This is due January 22, 2019
0 notes
Text
Chemistry - HW#12
Part A: Read pages 158 to 165 of HOLT Chemistry. Copy the Questions and Answer, neatly Section Review # 1, 3, and 4 ------ on page 165. Read pages 166 to 175 of HOLT Chemistry. Copy the Questions and Answer, neatly Section Review # 1, 2, and 6 ------ on page 175. ----------------------------------------------------------------------------------------------- Part B:
Choose any 3 new Problems at the end of the Class-notes/Slides - for **The Periodic Table**. You may choose the Regents Questions, if you wish. Copy the question, Answer the question, and Write out an explanation for your answer, using at least 2 complete sentences for each one. ----------------------------------------------------------------------------------------------- Reminders: Finish your Lab Reports - Flame Test Finish your Question and Answer - Do Now Compilation(s) Start your Summary Cheat Sheet for the Periodic Table Test. Start your Summary Cheat Sheet for the 2nd Quarter Benchmark Exam. It will cover all topics we covered during the 2nd Quarter - Atomic Concepts to (upcoming) Bond Polarity. -----------------------------------------------------------------------------------------------
This is due January 15, 2019
0 notes
Text
January 2019 - Chemistry
Tentative Schedule - Please NOTE Test and Benchmark Exam likely dates.

0 notes
Text
Chemistry - HW#11
1) Read pages 124 to 131 of HOLT Chemistry. Read pages 132 to 141 of HOLT Chemistry. Copy the Questions and Answer, neatly Section Review # 1 , 3, 5, 10, and 12 on page 141. -----------------------------------------------------------------------------------
2) Choose any 3 new Problems at the end of the Class-notes/Slides - for **The Periodic Table**. You may choose the Regents Questions, if you wish. Copy the question, Answer the question, and Write out an explanation for your answer, using at least 2 complete sentences for each one. ----------------------------------------------------------------------------------- 3) Finish the Post-Lab Questions and Sample Calculations for the last 2 Labs --- Cooling Curve of Lauric Acid --- and --- Analyzing Bright Line Spectrum ----------------------------------------------------------------------------------- 4) REMINDER: Be sure to complete and reflect on your (STAMPED) Do Now and Bonus Questions Sheet. You will finish copying the pertinent information as you study (when reviewing the slides/work at home), and re-do the problem until it is absolutely correct. SHOW ALL WORK, or else it will be incorrect (and consequently, you will lose points on it).
----------------------------------------------------------------------------------
5) Project #2 - Bright Line Spectrum - Short Story Working with your Team,
Copy and color the Bright Line Spectrum of your assigned Elements. Then, write a short story from the point of view of an ELECTRON, going from ground state to excited state, back to ground state, releasing energy to produce a bright line spectrum. ---------------------------------------------------------------------------------- This is due January 2 or 8, 2019 - the week we come back, from the holiday.
0 notes
Text
Chemistry - HW#10
1) Fill in your Summary Cheat Sheet, and hand it in by Wednesday, so that I can check it for accuracy ---- and staple it to your Test. Your (Atomic Concepts) Test is on Friday, December 14. Study for the Test, by going over Do Now’s / Bonus Questions / End of Slides - Regents Questions. The Extra Credit Question will be a Heating/Cooling Curve Calculation Problem, as it relates to Lab #10. -----------------------------------------------------------------------------------------
2) Read pages 114 to 131 of HOLT Chemistry. Copy the Questions and Answer, neatly Section Review # 3 and 7 on page 122. Section Review # 1 and 2 on page 131.
-----------------------------------------------------------------------------------------
3) Bohr Model Diagram Project is also due Tuesday, December 11, 2018. ----------------------------------------------------------------------------------------- As usual, your lab reports are ideally due the following Thursday. Finish graphing the cooling curve and complete Post-Lab questions and calculations.
0 notes
Text
HS Chemistry - Dec. 2018
Here is the most recent tentative schedule. Note: Test and Quiz Dates, on upcoming Fridays.
As usual, Labs will be on Thursdays.

0 notes
Text
Chemistry - HW #9
1) Choose any 3 new (different from the ones your chose last time) Problems at the end of the Class-notes/Slides. You may choose the Regents Questions, if you wish. Copy the question, Answer the question, and Write out an explanation for your answer, using at least 2 complete sentences for each one. ---------------------------------------------------------------------- 2) Read pages 90 to 98 of HOLT Chemistry. Copy the Questions and Answer, neatly Section Review # 1, 2, 3, and 9 on page 89. Copy the Questions and Answer, neatly Interpreting Graphs # 10 page 113. ---------------------------------------------------------------------- EXTRA HINT for #10: s levels hold 2 electrons, p levels can hold 6 electrons, d levels can hold 10 electrons, and f levels can hold 14 electrons.
You know that the first orbital (n=1) can only hold 2 ( s, only ) electrons, the second orbital (n=2) can hold 8 ( s + p ) electrons, and the third orbital (n=3) can hold 18 ( s + p + d ) electrons. Even though you don’t have to memorize the different levels specifically, you can figure out which one contains the very last electron, since you are filling in each level with 1 electron at a time --- knowing a S can hold 2 max, a P level can hold 6 max, and a D level can hold 10 max.

This is due Tuesday, 12 / 4 / 2018.
0 notes
Text
Chemistry - HW#8
1) Finish Copying and Completing all recent Do Now’s and Bonus Questions.
2) Complete and hand-in all past Lab Reports, excluding the most recent Filtration - Percent Composition - lab. 3) Read, complete, and Sign the Classroom Rules/Syllabus contract, and Lab Safety Contracts. You may be excluded from labs, if you do not sign the Lab Safety Contract. You also have to take the new Lab Safety Quiz (scheduled on 11/20/2018) --------------------------------------------------------------------------------------------------
3) Choose any 3 Problems at the end of the Class-notes/Slides. You may choose the Regents Questions, if you wish. Copy the question, Answer the question, and Write out an explanation for your answer, using at least 2 complete sentences for each one. -------------------------------------------------------------------------------------------------- 4) Copy the Questions and Answer, neatly, the following two problems: Show one or two potential numerical setups for the average atomic mass, given: A]

B] Important Review: It will be Extra Credit on the next Quiz/Test.

This is due Tuesday, 11 / 27 / 2018.
0 notes
Text
Chemistry - HW#7
Read pages 79 to 89 of HOLT Chemistry. Copy the Questions and Answer, neatly Practice Problems # 4 and 5 on page 89.
Copy the Questions and Answer, neatly, the following two problems: Show one or two potential numerical setups for the average atomic mass, given: A]

B] The table below shows data for three hypothetical isotopes of the same element.

This is due Tuesday- 11/20.
0 notes
Text
Chemistry -
For your viewing pleasure, here are the alpha particle scattering simulations (for Rutherford, and also a Plum-Pudding version of it, with no dense nuclei)
https://phet.colorado.edu/sims/html/rutherford-scattering/latest/rutherford-scattering_en.html
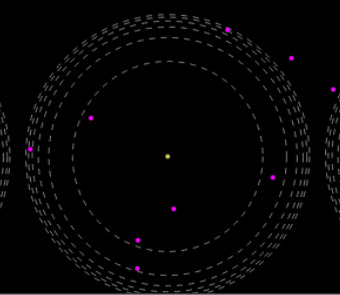

0 notes
Text
HS Chemistry - Nov. 2018
Take Note of Potential Upcoming Quiz/Test Dates!!

0 notes
Text
Chemistry - HW#6
Read pages 79 to 89 of HOLT Chemistry. Copy the Questions and Answer, neatly Practice Problems # 1 and 2 on page 86.
Copy the Questions and Answer, neatly Practice Problems # 1, 2 and 3 on page 89. This is due Tuesday- 11/13.
0 notes
Text
Chemistry - HW#5
Choose any 6 Problems at the end of the Class-notes/Slides. You may choose the Regents Questions, if you wish. Copy the question, Answer the question, and write out an explanation for your answer, using at least 2 complete sentences for each one.
This is due Tuesday, 11/6/2018.
0 notes
Text
Chemistry - Textbook HW#4
Read pages 60 to 61 of HOLT Chemistry. Copy the Questions and Answer, neatly Practice Problems # 1 and 2 on page 61.
Copy the Questions and Answer, neatly Practice Problems # 9, 10, and 11 on page 71.
------------------------------------------------------------------------------ You should use your Notes Packet for reference, to go over what the book does not cover. ------------------------------------------------------------------------------ SHOW ALL WORK,
or else you will lose points on the assignment. As usual, be sure to write in complete sentences.
This is due Tuesday- 10/30
------------------------------------------------------------------------------

^ Temperature (Degrees Celsius)
0 notes
Text
Chemistry Quiz - Friday, 11-2
Topics include Moles, Atomic Mass, Molecular Mass, Avogadro’s Number-----Dimensional Analysis - type problems WITH Liters of Gas conversions. (40%) Calculations from Heat Diagrams, Labeling of Heat Diagrams / Cooling Diagrams, and Entropy are also on the Quiz. (60%)
You can start studying for the first major cumulative test/exam (tentatively scheduled for the week of November 5 ) [Very Likely on November 9], by doing the Practice Questions at the end of the newest set of slides. A lot of it is Review material, so you can get started now.
0 notes
Text
Chemistry - Textbook HW#3
Read pages 38 to 45 of HOLT Chemistry. Copy the Questions and Answer, neatly Practice Problems # 2, 3, 5, 7, 8, and 13 on page 45.
SHOW ALL WORK, or else you will lose points on the assignment. As usual, be sure to write in complete sentences.
This is due Tuesday- 10/23
0 notes