Text
Necessity of Microbiological Testing Laboratory To Keep Save Yourself
It isn't sufficient to simply distinguish your organic entity. You additionally need to understand what antimicrobial specialists your living being is helpless to. There are a few strategies to decide this.
Dilution testing is utilized to quantitatively decide the negligible fixation (in mg/ml) of antimicrobial specialists to restrain or kill the microorganisms. This is finished by adding two-overlay weakenings of the antimicrobial specialist straightforwardly to an agar pour, a stock cylinder, or a miniature stock board. The most reduced level that restrains the noticeable development of the life form is viewed as the Minimum Inhibitory Concentration (MIC).
The agar pour strategy is viewed as the reference test system in Europe. The stock weakening technique is all the more broadly acknowledged in North America. The E test (AB Biodisk) is a plastic strip with a slope centralization of antimicrobial specialists impregnated in it. The strip is put straightforwardly on the outer layer of an immunized plate. The MIC is perused from the strip where the development hindrance catches the plate. These strips are somewhat costly.
Numerous doctors anyway needn't bother with no know the specific MIC, yet which anti-infection agents the microorganism is vulnerable, middle of the road, or impervious to. The Kirby-Bauer agar dissemination technique is proven and factual and is the normalized strategy for deciding antimicrobial defenselessness. White channel paper circles (6 mm in breadth) are impregnated with known measures of antimicrobial specialists. Each plate is coded with the name and centralization of the specialist. For instance, 10 µg of Ampicillin is demonstrated on the plate by AM-10. The code is recorded on the Disk Zone Diffusion Diameter Chart.
The impregnated circles are put on an immunized Mueller Hinton Agar (MHA) plate. The medication diffuses through the agar. The plates are brooded for 16-24 hours. The agar might be enhanced with blood or you might involve blood agar for fussy creatures. The width of the apparent zone of hindrance is estimated and contrasted with reference values. There ought to be adequate microorganisms to frame a noticeable grass of development where it isn't restrained by the medication.
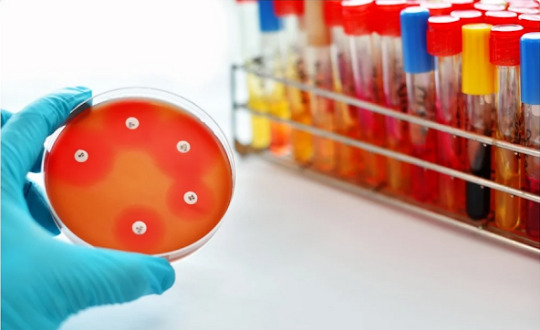
The outcomes are deciphered subjectively as safe, halfway, or defenseless. The standard convention should be adhered to precisely for you, or any clinical lab, to dependably decipher the outcomes.
There might be a few hindrances of development and the creature may as yet be viewed as impervious to that antimicrobial specialist in the event that the zone width is more modest than the reference values recorded on the graph. Likewise note that different antimicrobial specialists have various estimations for safe, transitional, and powerless.
A zone of restraint might be viewed as helpless for one antimicrobial specialist and not for another. For instance, for ampicillin (AM-10) to be a compelling antimicrobial specialist, the zone of hindrance for enterics and most steps should be more prominent than 16 mm while for staphs it should be more prominent than 28 mm.
While figuring out which antimicrobial specialists are best for treatment when different zones of hindrance are available, make certain to check out at the general zone of restraint for that specific antimicrobial specialist and contrast your estimations with that.
For instance, suppose that your intestinal creature has a zone of hindrance around the Polymyxin B circle of 20 mm and a zone of restraint around the Tetracycline plate of 20 mm. Since these estimations are bigger than the powerlessness zones recorded on the Disk Zone Diffusion Diameter Chart, both of these anti-toxins would be considered as opportunities for treatment.

In any case, when we look all the more carefully, we see that a 20 mm zone of restraint for Tetracycline is just 1 mm bigger than whatever is expected to be vulnerable while a 20mm zone of hindrance for Polymyxin B is 8mm bigger than the base vulnerability estimation required. In this specific case then, at that point, Polymyxin B and Tetracycline would both be sufficient for treatment, yet the Polymyxin B would be the most ideal decision.
What kind of tests are done in Microbiological testing laboratory?
The Clinical Microbiology Laboratory relies on usual diagnostic processes like Gram Stains, Culturing, Examination,Identification of Microorganisms containing Yeasts, Bacteria, & Fungi, and Biochemical Testing. This test has an important role in effective IPC (Infection Prevention and Control).
Microbiology Lab Testing Services
Make sure your food items are safe from microbiological contaminants. it is one of the most vital steps you can take before distribution them out to market.

Microbiological Testing In The Food Industry
Biosan performs all the major bacterial pathogen and microorganisms tests that may threaten your food items facility. Our wide research procedures allow us to identify them and isolate indicator organisms. They are:
• Aerobic Plate Count
• Anaerobic Plate Count
• Coliforms
• Lactic Acid Bacteria
• E.coli
• Enterobacteriacea
• Lactobacilli
• Yeast
• Mold
For Antimicrobial testing and Microbiological Testing Laboratory, you can explore Biosan Laboratories INC.
0 notes
Text
Antimicrobial testing, Microbiological testing laboratory
Most of the irresistible sicknesses are bacterial in the beginning. With the revelation of research center strategies to develop these microorganisms utilizing a suitable development medium known as "culture," deciding the responsiveness and obstruction of explicit microbes to a large number of antimicrobial specialists becomes fundamental so medical services suppliers can promptly organize legitimate therapy regimens to their patients.
Antimicrobial testing is a lab strategy performed by clinical technologists (clinical lab researchers) to distinguish which antimicrobial routine is explicitly powerful for individual patients. For a bigger scope, it supports the assessment of treatment administrations given by clinics, centers, and public projects for the control and counteraction of irresistible illnesses. As of late, scientists have needed to execute persistent observation exercises for obstruction designs because of the transformations in bacterial DNA.
Clinical research facilities right now utilize a few techniques relying upon the lab test menu that they give. These methodologies incorporate the circle dissemination and least inhibitory fixation (MIC) techniques. Business frameworks additionally opened up across wellbeing focuses and emergency clinic offices, using both phenotypic and genotypic portrayal of bacterial obstruction. While routine antimicrobial vulnerability testing for gram-positive (e.g., Staphylococcus aureus) and gram-negative microorganisms (e.g., Pseudomonas aeruginosa) are ordinarily accessible in fringe labs, drug helplessness testing (DST) for Mycobacterium tuberculosis are normally completed inside additional mind boggling offices like reference research facilities. In spite of the distinctions in the procedures for vulnerability tests, all research centers should be basic on each step of the examining and testing process so that experimental outcomes are reachable with reliably elevated degrees of precision and unwavering quality.

Procedure
Both circle dispersion and MIC strategies utilize the phenotypic distinguishing proof of vulnerability, and consequently, requires the accompanying system:
• Planning of a normalized inoculum from a bacterial culture:
• Picking very much detached settlements
• Making a bacterial suspension (inoculum)
• Normalizing the bacterial suspension utilizing McFarland principles
• Weakening of bacterial suspension (just for MIC technique)
• Vaccination of bacterial suspension to one of the accompanying:
• A specific development medium (e.g., Mueller Hinton Agar, MHA for circle dispersion)
• A MIC board
• Expansion of antimicrobial circles (just for plate dispersion)
• Brooding of plates (circle dispersion) or boards (MIC)
• Estimating the zone of restraint or perusing MIC board
• Understanding of AST results
Indications
Susceptibility testing for antimicrobials is essential for patients who raise doubt of contamination with explicit microorganisms in light of sickness signs and clinical connection. Antibacterial specialists are then used to recognize awareness or opposition from microbes. Albeit the reason for this audit is principally towards the vulnerability testing for bacterial microbes, it is vital to take note of that antifungal helplessness tests additionally exist for tending to contagious disease (e.g., Candida, Aspergillus spp.). Besides, antiviral powerlessness tests are additionally accessible (e.g., flu) by means of sub-atomic advances including sequencing investigation like Sanger and pyrosequencing techniques.
Potential Diagnosis
A one of a kind effect of AST on tolerant administration is the distinguishing proof of the particular conclusion, and furthermore, focusing on the specific etiologic specialist causing the infection. No two patients can be overseen also, particularly on the off chance that they have similar signs and side effects (illness indication) however with various treatment regimens on the grounds that a similar causative creature can have different opposition designs. For instance, two patients might give a common type of Staphylococcus aureus versus methicillin-safe Staphylococcus aureus (MRSA); and another model would be patients with drug-vulnerable (DS-TB) and medication safe tuberculosis.

Normal and Critical Findings
For circle dissemination, estimating the zone of restraint is finished by utilizing a committed caliper. Accurately measure the distance across by the edges of the restraint zone. For MIC boards, perusing each arrangement of wells for an antimicrobial medication is finished. MIC assurance is by either a reasonable or slight whiteness on the well. Announcing the aftereffects of the hindrance zones and MIC breakpoints is made utilizing either the expressions ``powerless" or "safe" in view of the set cut-off range for zone distance across in the closest entire millimeter and microgram per milliliter, separately. The Clinical Laboratory Standards Institute (CLSI) and the European Committee on Antimicrobial Susceptibility Testing (EUCAST) created master endorsed rules on breakpoints for detailing consequences of these techniques.
Interfering Factors
A few variables influence the consequence of the helplessness testing which covers the entire inspecting, testing, and revealing systems. Any deviation from the standard AST methodology can fundamentally affect succeeding areas of the research center work process which thus would later influence patient conclusion, treatment, and the board. Emotionally supportive networks of the lab work process require severe observation, and lab staff ought to be thoroughly prepared and skilled enough to carry out the technique.
Complications
Irregularities in the AST results should be researched and followed up on right away. No outcomes ought to be delivered when quality control measures are not good. Delivering incorrect medication defenselessness or opposition results can incur more damage to the patients, prompting extreme clinical circumstances and unfortunate visualization. A more terrible outcome in conveying bogus AST results can bring about off-base treatment of the executives' plans which could make further transformations of these irresistible life forms, uncovering the patients and the local area to a higher gamble.
Patient Safety and Education
Patients ought to be sufficiently educated about the AST and its signs, patient necessities, and its clinical use for patient administration. Medical services suppliers like doctors, lab work force, attendants, and pharmacologists are urged to spread right data about the test. In any case, understanding of the AST results should happen between the patient and the doctor to work with great consistency with the recommended prescriptions and to forestall self-drug. With the ascent of antimicrobial obstruction, the significance of AST requires an accentuation on clinical, research center, and nursing staff, as well as patients and their relatives, and the entire local area prompting a bound together methodology.
Clinical Significance
When antimicrobial vulnerability results become accessible, treatment regimens for every patient can be created by medical services suppliers. Recommended prescriptions of proper anti-toxins need individualization for every patient determined to have an irresistible infection. Besides, obstruction from essential medications will require a more significant level of antimicrobial stewardship, including judicious utilization of second-line drugs.
0 notes
Text
Antimicrobial Testing Laboratory of Water, Drug, and Food
Antimicrobial Testing Laboratory is a microbial science research center and it offers an extensive variety of testing capacities, which are performed by experienced and concentrated microbiologists. This research facility performs different item viability tests to help organizations, which make antimicrobial items. Clients gets genuinely necessary help from this research center, which incorporate planning basic confirmation of-idea screening tests to leading complicated, enormous scope GLP-agreeable examinations for FDA and EPA.
Numerous strategies can be utilized for testing antimicrobial articles, materials and surfaces. These techniques vary regarding similarity with the degree of normalization, aversion to antimicrobial movement and test material. To come by the exact outcome, you should utilize the best test technique. Administrations presented by an antimicrobial testing research center include:
* Disinfectants/Santizers
* Medical Devices
* Antimicrobial Surfaces
* Virucidal Disinfectants
* Preservatives
* Antimicrobial Devices

Disinfectants/Santizers
The testing lab can give a total EPA, ASTM, AOAC and other test techniques to help organizations, which produce germicidal synthetics. The lab is GLP consistent and EPA examined.
Clinical Devices
Legitimate Antimicrobial Testing is fundamental for forestalling contaminations. Clinical gadgets can be utilized antimicrobial specialists, so the testing research center can perform GLP antimicrobial adequacy reads up for supporting organizations.
Antimicrobial Surfaces
The quantity of research centers that test items against infections is extremely negligible in the US. Nonetheless, antimicrobial testing labs can keep a huge assortment of pathogenic infections for viability testing.
Virucidal Disinfectants
The quantity of research centers that test items against infections is extremely negligible in the US. Nonetheless, antimicrobial testing labs can keep a huge assortment of pathogenic infections for viability testing.
Additives
Additives can likewise be tried in this testing research center. By and large, additives guarantee the microbiological steadiness of beauty care products, cleaners and reagents. Testing labs can likewise embrace different additive test techniques.
Antimicrobial Devices
The testing lab can likewise perform antimicrobial gadget testing, which range from room UV sterilization gadgets to foggers.
Disinfectants
Sanitizers and sanitizers are synthetic substances, which are utilized for inactivating microorganisms on lifeless surfaces. Sanitizers and anti-toxins are different however the two of them are utilized for killing microorganisms. The two of them are likewise thought to be as antimicrobial. Sanitizers can obliterate a large number of microorganisms, yet they are undependable to infuse or ingest. Then again, anti-toxins disturb at least one pieces of microorganisms and they are generally negligible poisonousness to body.
Most sanitizers contain various fixings, which incorporate cleansers, aromas and water. These rack steady, complete arrangements are called definitions. Most sanitizers are phenomenal cleaners too. The dynamic fixing is the synthetic that kills microorganisms. These sanitizers are utilized for killing microorganisms in your body. In such case, these are called cleaning agents. Dynamic fixings, that are utilized on hands, are called hand sanitizers. A few strong dynamic fixings are utilized in high fixation to disinfect clinical hardware like careful endoscopes, without heat. The plan, which disinfects clinical hardware is known as cold sterilants.

Lifeless surface sanitizers and sanitizers are controlled by the US Environmental Protection Agency (EPA) as pesticides. EPA thinks about organisms, parasites, infections and microorganisms as nuisances. The completed items should be tried for antimicrobial viability to decide how and where the item is expected to be utilized. Time span of usability, sturdiness and safeguarding difficulties are a few contemplations while choosing an antimicrobial testing research center for a particular industry or item. Consequently, you should be extremely cautious while choosing a research center for antimicrobial testing.
Microbial Science
Microbial science is a significant area of examination for the drug business. Microbial science becomes essential because of broad association of microorganisms in different illnesses. Therefore, advancement of immunizations to symptomatic clinical gadgets is straightforwardly or in a not entirely set in stone by microbiological studies.
Drug quality, wellbeing and adequacy are the main parts of Microbiological Testing Laboratory of drug items. The presence of any pathogenic microbes, yeasts, molds or bacterial poisons delivered by microorganisms is completely managed microbial science testing research facilities to guarantee insignificant or zero gamble.

Microbiology
At the microbial science testing research centers at Precise Analytics Lab., we direct antimicrobial viability testing of drug items rigorously according to pharmacopeia rules and utilizing high-innovation assets. Our microbiological testing lab is exceptional with Clean Room Partition, Air Handling Unit, Laminar Air Flow, Biosafety Cabinet, Deep Freezer, from there, the sky is the limit, to perform extensive variety of microbiological testing.
Microbiological Testing of Food and Agricultural Products
In a world loaded up with microorganisms, species differing from valuable to unsafe to deadly for people, there are sufficient chances for food items to be polluted "from ranch to fork".
To guarantee security of food items, microbiological tests, for example, testing for microorganisms and deterioration creatures are required. Through this, the gamble of tainting under ordinary use conditions can be inspected and food contamination flare-ups can be forestalled. Testing of food items and fixings is significant along the entire store network as potential blemishes of items can happen at each phase of creation.
Our exceptional microbial science testing lab and very capable, experienced microbiologists guarantee exactness and dependability of results.
0 notes
Text
Why Legionella Testing ismost important for us?
With regards to water safety at home or at work, perhaps the most effective way to distinguish unsafe microbes in water is through Legionella testing. Legionella is a danger that should be tended to. Your office runs wellbeing and legitimate dangers in neglecting to do routine Legionella testing.
You likewise need to comprehend that the advantages of consistently guaranteeing that your water systems are liberated from Legionella incorporate forestalling a possibly lethal flare-up of Legionnaires' illness. In performing routine water testing, you are better ready to defend the prosperity of individuals who use and work in your office. You are additionally ready to safeguard yourself from suit with the ensured research center experimental outcomes you can use as reported verifiable evidence if there should arise an occurrence of charges of disease against your office.

In actuality, standard testing supplements your support endeavors as well as shows an unmistakable activity of an expected level of effort from you. Testing is likewise especially significant in situations where full consistence with the public authority's wellbeing principles can't be for all intents and purposes achieved. This will permit you to carry out receptive techniques where the more continuous routine support and checking assignments are exceptionally difficult to execute. Standard testing will show progressing ingenuity in the battle against Legionella.
Assuming that you are maintaining a working environment or a business-associated premise, you have a lawful commitment to complete a Legionella risk evaluation in your water frameworks. This lawful necessity is characterized by some regulations. These regulations connect with the overall structure of overseeing wellbeing, security, and government assistance at work, which covers the gamble of Legionella, to which individuals might be uncovered throughout work exercises.
The HSE is the implementing body engaged with undertaking any arraignment appropriate to the regulation in regards to Legionella disease. While the HSE's ACOP isn't real regulation, your gamble of openness to suit is expanded when you neglect to take on it.
Without a doubt, consistently testing for Legionella is a fundamental piece of this code of training. In the event that you are as yet not satisfactory regardless of whether you really want to complete routine Legionella testing, the straightforward response is yes. We are discussing a lawful necessity, so you are committed to going along. Toward the day's end, the panic of the suit is only a little piece of it. You truly don't need the culpability of Legionella contamination on your soul, so carefully complete routine Legionella testing to stay away from such repulsiveness and guarantee your wellbeing and the strength of individuals working for yourself and with you.

Indeed, even at home, anybody could be in danger. Various elements in the circulation, stockpiling, or utilization of water might in any case think twice about the nature of the water, in any event, when the specialists execute severe guidelines in the inventory of compact, safe-quality water to homes. In any case, it is lucky that nowadays there are currently effectively accessible as well as reasonable water testing units that can give quick and straightforward ways of assisting individuals with making that what they have is water that is fit to savor their homes. The packs can decide if there are destructive synthetic substances or microscopic organisms in the water supply from the tap.
The water you utilize regularly for family exercises, for example, the water emerging from your waters, ought to likewise be tried. Showerheads are really among the most inclined to scale and microbes arrangement in most of properties authorities on the matter agree, and furthermore, has the most elevated risk for tainting of Legionella microscopic organisms. The utilization of Legionella testing packs is strongly suggested, particularly those that are explicitly intended for boiling water frameworks like showers. Regularly, topping off the furnished sterile container with water from hot taps and showers is all you need to do. Then the provider will gather the example and run some research facility tests on it. When the lab results are delivered, you will be given a declaration.
It is possible to combine water testing kits with state-of-the-art solutions that can present multiple alternatives, just like BIOSAN LABORATORIES, INCwhich can disinfect, descale and eliminate Legionella bacteria and Water Testing Kit all at the same time. For more info on BIOSAN LABORATORIES, INC, check out this website www.biosan.com.
0 notes
Text
Health Benefits of Water Testing With Bacteria and Legionella Test Kit
For many years, it has been a great challenge for universal leaders to work on the quality of water in each and every household. The problem of water safety has directed the UNMDG (United Nations Millennium Development Goals) to oversee access to water and make sure it is safe for cooking, drinking, and personal hygiene. According to current reports, about 1.6 billion people don’t have access to safe water sources, and about 470,000 have deceased from diseases carried about by unsafe water.
It is one of the most popular issues the globe faces today. To best resolve the problem, landowners are stimulated to take responsibility for their water supply and make sure that from the source to the house directly, the water flow is free from the impurity of harmful bacteria.
There is no abode that is completely safe for your water system. Usually, contamination is present everywhere, and it can come from your showerhead, pipeline, tub, and from other unclean areas. Contaminated water leads to serious illness and bacteria born from there. Among its effects is Legionnaire's Disease. This is the kind of ailment that attacks directly the respiratory system and other main organs.

The water you make use of daily for domestic activities, like the water coming out of your waters, should be tested. According to experts, showerheads are truly among the most prone to scale and microbes formation in the majority of properties. And, also it has the highest risk for contagion of Legionella bacteria. The use of Bacteria Test Kit and Legionella Testing Kits are highly recommended, particularly those that are exactly designed for hot water systems such as showers. Generally, filling up the provided bottle of sterile water from hot showers and taps is all you have to do. After that, the supplier will accumulate the sample and track some laboratory tests on it. As soon as the lab outcomes are released, they will provide a certificate.
What you will do to make sure that your water is kept safe from such ailment is stop contamination. You can make utilize a Bacteria Test Kit and Legionella Laboratory Testing. Areal kit for water testing, the legionella test kit is significant in ensuring that you are away from the Disease of Legionnaire.
It is the topmost to invest in a kit that makes sure your safety of the family. You have to find a source offline and online where you can test if the water supply is indeed positive of the microorganisms bringing the infection. You should send the water sample to a laboratory, and you just have to wait for the outcomes. A steadfast water testing provider will be able to offer you the awareness and steps on how to monitor microorganisms and control their growth.
If not disallowed, the Legionnaire's Ailment can cause death. Though you have to treat it with proper medical care, it can be most dreadful for children or family members who have respiratory conditions or allergies. Nothing equals the harmony of mind you will have for ensuring that your water remains safe for your whole family. You never wish harm to come from your own home. You definitely have to do something about it. Uses of Bacteria Test Kit and Legionella Laboratory Testing are the key to avoiding such health risks. Find the best steadfast aid you can find to ask for guidance and techniques when it comes to testing water.

It is probable to combine water testing kits with advanced solutions that can present many alternatives. Contact with the BIOSAN LABORATORIES, INC. that can eliminate, disinfect, and descale Legionella bacteria all at the same time.
BIOSAN LABORATORIES is situated in Warren, Michigan. It specializes in independent Legionella laboratory testing for building water systems and cooling towers. Additionally, to about 40 years of lab experience in eco-friendly microbiological testing. It is also certified by the CDC ELITE Program for Legionella laboratory testing.
We will help you with the whole Legionella Testing method, which contains shipping sample bottles to the locality of your choice free of cost. Contact BIOSAN for additional information at 800-253-6800 or at [email protected] or visit our website https://www.biosan.com/.
Our all staffs are excellent in their work and have aware of the importance of purifying water. The purity of water leads to zero contamination and keeps away from all kinds of diseases. Any type of question in your mind regarding water testing using Bacteria Test Kit and Legionella Laboratory Testing, call us.
0 notes
Text
Why Legionella and Microbial Laboratory Testing Is Important?
What is Legionella Testing?
Legionella is a kind of bacteria. It leads to a severe form of pneumonia known as Legionnaires' disease. Legionella Laboratory Test looks for these bacteria in sputum, urine, or blood. In 1976, this disease got its name after a people joining an American Legion convention became ill with pneumonia.
What is Microbial Testing?
Analysis of microbiological properties of food products utilizes molecular, biological, chemical, and biochemical methods for the discovery, enumeration, or identification of microorganisms in a material like food, environmental drink, or clinical sample. It is frequently applied to disease-producing and damaging microorganisms.
• When water safety at work or home comes, the best way to detect harmful bacteria in the water is through Legionella Laboratory Testing and Microbial Laboratory Testing. Legionella is called a menace that requires to be addressed. Your ability runs legal risks and health in failing to convey out routine Legionella testing.

• Also, you should understand that the assistances of often ensuring that your water systems are free of Legionella and Microbial includes avoiding a potentially fatal outbreak of Legionella and Microbial ailment. In performing routine testing of water, you will be better able to protect the well-being of the people who utilize and work in your facility. You can also able to protect yourself from lawsuits with the certified laboratory test reports you will use as documented old proof in case of charges of contagion against your facility.
• Basically, regular testing enhancements your maintenance exertions and demonstrates a clear action of due attentiveness on your part. Legionella Laboratory Testing, Microbial Laboratory Testing is also particularly important in cases where full obedience to the government's health standards cannot be practically proficient. It is letting you implement reactive processes where the more frequent routine monitoring and maintenance tasks are tough to implement. Regular testing demonstrates ongoing attentiveness in the fight against Legionella.
• If you are running a business-connected premise or an office, you should have a legal responsibility to carry out Legionella and Microbial risk valuation in your water systems. This kind of legal requirement is demarcated under some legislation. This legislation relating to the general outline of managing safety, health, and welfare at work covers the risk of Legionella and Microbial, to which people can be exposed in the course of work actions.

• The HSE (Health, Safety, and Environment) is the enforcing body contained in undertaking any prosecution relevant to the legislation regarding Legionella and Microbial infection. While the HSE's ACOP is not real legislation, your peril of exposure to litigation is enhanced when you are failing to accept it.
• Absolutely, regularly Legionella and Microbial Laboratory Testing is a vital part of this code of practice. If you are still not clear about it whether you need to convey out routine Legionella and Microbial Testing or not, the simple answer is yes. We have a discussion about a legal requirement; therefore, you are obligated to comply. Finally, the fright of litigation is just a minor part of it. You truly don't wish the guilt of Legionella and Microbial infection on your conscience. To accurately carry out routine Legionella Testing and Microbial Testing to avoid such unpleasantness to make sure your health and the health of the persons working for you and with you.
Do you conscious about your health and your family health also?Kindly visit our website https://www.biosan.com/for knowing more about Legionella and Microbial Laboratory Testing. Or else you can reach Biosan Laboratories, Warren, USA. We and our teamare providing high-quality services at an affordable price.
0 notes
Link
Coliform bacteria potentially contaminate the water in various ways thus it is necessary to test the water Properly. The experts at microbial testing services strongly recommend annual coliform bacteria testing for private water wells as they can be contaminated any time without changing the taste or odor of the water.
0 notes
Text
A Complete Guide to Coliform Bacteria Testing
The experts at microbial testing services strongly recommend annual coliform bacteria testing for private water wells as they can be contaminated any time without changing the taste or odor of the water. Coliform bacteria potentially contaminate the water in various ways thus it is necessary to test the water at right time.
You need to remember the below cases to remain safe always.
• Carry out coliform bacteria test at least once a year for all private residential water wells.
• Conduct the test when a new well has been constructed.
• Do not forget to have coliform test after something has been done on the well.
• When you suspect or see any indication that contamination has taken place, such as if the well was covered with flood water, you should immediately opt for coliform testing.
What Is Coliform Bacteria?
There are two categories of coliform bacteria in general that are found in well water - total coliform and fecal coliform or Escherichia coli (E. coli).

The first one, Total coliforms, are found naturally in the environment and are located in the soil, in water, which are influenced by surface water, and in animal or human waste. The occurrence of total coliform, by itself, does not mean that the resource is infected; however, it can show that if not more of the more serious types of harmful bacteria, such as fecal or E. coli bacteria, could be there.
Fecal coliforms belong to the total coliforms group that are thought to be present especially in the gut and feces of warm-blooded animals and are considered a more precise indication of human or animal waste than the total coliforms.
E. coli is the key species in the fecal coliform group and is treated as the best indicator of fecal pollution and the possible existence of pathogens. You need to remember that most coliform bacteria do not cause disease.
Why Should We Do Coliform Testing?
In order to know the standard of your water i.e. whether safe for human use, you must carry out bacteria testing. It is impossible to tell by the look, taste, or smell of the water if disease-causing organisms are in it. That's why it is recommended that you test water for coliform bacteria at least once a year and more frequently if bacteria have been a problem earlier.
Concentrations of pathogens usually from fecal contamination are tiny, and the count of different possible pathogens is large. Thus this is not practical to test for pathogens in every water sample collected. Instead, the existence of pathogens is recognized with indirect evidence by testing for an “indicator” organism like coliform bacteria.
The test for total coliform bacteria is the most basic test for bacterial contamination of a water supply. Total coliform numbers provide a general indication of the sanitary condition of the water supply.

Which Microbial Testing Lab Do We Prefer?
Even though the at-home test is easy, fast, and effective for determining if your well water has coliform and E.coli or not, you should go for a well-known Microbiological testing laboratory to get more precise solutions. The microbiologists at the laboratory are smart enough to conduct high-end tests to find the coliform bacteria using the modern equipment and latest technologies in the industry.
In case, you wish to do the test on your own, you can simply by using various test kits available in the market. The mechanism of test turns the media into blue-green if coliform bacteria are detected.
How Do We Collect Water Sample For Test?
You need to keep in mind some points while going for collecting a water sample and send it to the nearby microbial testing laboratory. Get started.
• Clean your hands with soap and warm water.
• Take the sample from a cold water tap. Use a tap without a screen or remove the screen before collecting the sample.
• Open the tap freely for a couple of minutes prior to collecting the sample.
• Hold the bottle near the tap base to collect the sample. Fill it at least 200 ml and never overfill the bottle.
• Tight the bottle and it is secure, but do not over-tighten it.
• Place the identification label from the requisition form on the bottle.

The requisition form should contain the following things:
• Name and contact number
• Mailing address and postal code
• Details of Collection site e.g. kitchen sink
• Legal land description and/or civic address
• Time and date of sample collection
What Should We Do if Coliform Bacteria are Detected in our Water?
If E.coli or fecal coliform are identified in the water, the first step should be an emergency chlorination that usually lasts two to five days. During that period it is recommended that you should vigorously boil the drinking and cooking water for one-two minutes before using it.
In fact, it seems inevitable to continue testing the water because if anything happens to the chlorine residual, or if the chlorine-demand changes and the consumers fail to know about it, the water can be unsafe again. In case, fecal coliform or E. coli is detected in well water, your primary step should not merely be to disinfect the system but to identify the source of your bacterial contamination.
0 notes