Don't wanna be here? Send us removal request.
Text
If you're in chemistry, pharmaceuticals, or just love science, understanding enantiomeric excess is key! It's a measure of how much one enantiomer in a chiral compound is present in excess over the other-crucial for drug safety, potency, and effectiveness.
Whether you're a student, researcher, or professional, this quick read will refresh your grasp on Enantiomeric Excess (EE) and how it's measured.
#Chemistry#Pharmaceuticals#ChiralChemistry#ScienceExplained#organic chemistry#science side of tumblr#pharmaindustry#chromatography
0 notes
Text
Artificial Intelligence in Drug Discovery: The Future is Now

The future of pharmaceuticals is being reshaped by Artificial Intelligence (AI) — and it's happening faster than we imagined.
From identifying new drug targets to predicting molecular behavior, AI is reducing time and cost in drug development. It’s not just speeding up research — it’s making smarter, safer medicine possible.
🔍 What’s Inside:
How AI is transforming early-stage research
Predictive modeling in drug design
Real-world applications from leading biotech companies
Ethical & regulatory challenges ahead
💡 AI isn’t replacing scientists — it’s becoming their most powerful tool.
🔗 Read the full post here
#DrugDiscivery#AI#ArtificialIntelligence#Pharma#Biotech#MachineLearning#HealthcareInnovation#pharmaceutical#science side of tumblr#organic chemistry#innovation
0 notes
Text
The Biopharmaceutics Classification System (BCS) is a widely recognized framework used to categorize pharmaceutical compounds based on their solubility and intestinal permeability. These two key properties are critical in determining the absorption and oral bioavailability of drug formulations. By classifying drugs into four distinct categories, the BCS offers valuable insights into their in vivo behavior, enabling scientists and formulators to better predict a drug’s performance within the body. This system plays a pivotal role in guiding the design and development of effective oral dosage forms, streamlining the drug development process, and ultimately optimizing therapeutic outcomes.
0 notes
Text
Nitrosamine impurities have become a critical concern in the pharmaceutical industry due to their potent toxicity and the extremely low acceptable limits imposed by regulatory authorities. Unlike typical impurities, nitrosamines present unique analytical challenges—they are often not detectable using standard techniques such as conventional chromatography or spectroscopy. Despite having no intended use in drug manufacturing, their unintended presence in pharmaceutical products can pose significant health risks, including carcinogenicity.
In this article, I will leverage my expertise to explore the key aspects of nitrosamine impurities. Topics covered will include the various types of nitrosamines, their toxicological profiles, pathways of formation during drug manufacturing, and proven strategies for detection and control. I will also highlight relevant case studies and address frequently asked questions to provide practical insights for professionals seeking to enhance their understanding and mitigation of nitrosamine-related risks in pharmaceutical production.
Continue reading for a comprehensive overview of this critical issue.
0 notes
Text
How To Calculate Solubility BY HPLC?

Solubility—the ability of a substance (solute) to dissolve in another substance (solvent)—is a fundamental property that plays a critical role in pharmaceutical sciences. It directly influences bioavailability, bioequivalence, and the overall efficacy and safety of a drug. As such, solubility is central to chemical and analytical research, as well as to quality control processes in the pharmaceutical industry.
In this article, you'll explore the concept of solubility in depth. Topics include solubility classification, the principles governing solubility, how to calculate solubility using High-Performance Liquid Chromatography (HPLC), and how to interpret a solubility curve. We’ll also discuss key factors that affect solubility, strategies for selecting appropriate solvents in drug development, practical applications in formulation science, and essential terminology. Case studies and FAQs will provide real-world context and further insights.
Checkout the link to continue reading and deepen your understanding of this essential pharmaceutical concept:
Understanding Solubility in Pharmaceuticals: A Comprehensive Guide
#pharmaceutical#organic chemistry#science side of tumblr#chromatography#university#student#pharmaindustry#innovation#development#chemistry#big pharma#drug companies#pharmacy
0 notes
Text
What Is System Suitability Test (SST) In HPLC And GC Analysis: 11 Minutes Easy Learning
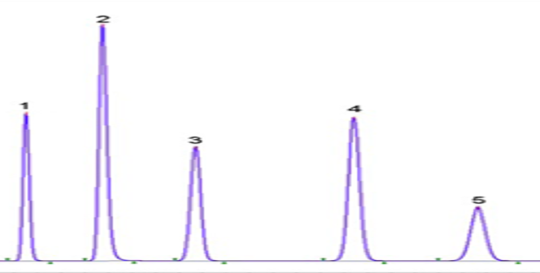
System Suitability Test (SST) is a critical step in ensuring that chromatographic systems - whether HPLC, GC, LCMS, GCMS, or TOC - are operating optimally for sample analysis. Before performing routine sample or standard injections, a system suitability solution is first injected into the system, and the results are evaluated against pre-defined criteria. Only if these criteria are met can further analysis proceed. Read complete article here:
Suitability Test (SST) In HPLC And GC Analysis
1 note
·
View note
Text
0 notes
Text
#pharmaceutical#organic chemistry#science side of tumblr#university#chromatography#pharmaindustry#development#innovation
0 notes
Text
#pharmaceutical#development#university#science side of tumblr#organic chemistry#pharmaindustry#innovation
0 notes
Text
https://pharmaknowledgeforum.com/pharmaceutical-reference-standards/
0 notes
Text
0 notes
Text
Specialty Chemicals play a unique role in many industries like pharmaceuticals food, cosmetic, agriculture etc., Which is why I decided to share my skill-based knowledge on this topic. In this article, you will learn definition, applications, manufacturing and FAQS of Specialty Chemicals.
1 note
·
View note
Text
1 note
·
View note
Text
#pharmaceutical#development#university#science side of tumblr#innovation#organic chemistry#student#pharmaindustry#chromatography
0 notes
Text
#pharmaceutical#development#innovation#science side of tumblr#pharmaindustry#organic chemistry#chromatography
0 notes
Text
he UV visible spectrophotometer is one of the traditional Analytical techniques, which is widely used in the pharmaceutical industries for both quantitative(such as assay and content test) and qualitative analysis (such as identification test and to find out wavelength maxima of pharmaceuticals.
#organic chemistry#science side of tumblr#innovation#university#pharmaindustry#pharmaceutical#spectroscopy
0 notes