female. 25. part time dreamer, part time chemistry phd student. lazy. lost. nostalgic. pessimistic. lastfm twitter livejorunal goodreads
Don't wanna be here? Send us removal request.
Photo




Working with gaseous could be really different compared to other “classical’ methods.
In this case I made a reaction with a perfluoroalkyl-iodide what is gaseous at room temperature, however it dissolved readily in the reaction mixture. I flushed the balloon on the Schlenk flask a few times with argon, removing air and anything else, what could cause problems (first two gifs) and dissolved the fluorous reagent in the reaction mixture causing a slight yellow discoloration. The dissolved amount was measured from the weight gain of the flask.
As time goes on, the transparent reaction mixture turned foggy (third gif) and after a little time my product separated from the reaction mixture (last pics) as an oily upper layer.
230 notes
·
View notes
Photo



fireworks on sunday it was so beautiful and kind of looks like space
52K notes
·
View notes
Photo


icelandic evening by marina weishaupt (500px / flickr / instagram)
555 notes
·
View notes
Video
youtube
My favorite thing from this year: playing with the glowstick reaction using TCPO (bis(2,4,6-trichlorophenyl) oxalate).
The glow stick contains two chemicals and a suitable dye. One of the chemicals is a diaryl oxalate (in this case TCPO, or bis(2,4,6-trichlorophenyl) oxalate), the other one is an oxidizer, usually hydrogen peroxide. By mixing the peroxide with the oxalate ester, a chemical reaction takes place, releasing energy that excites the dye, which then relaxes by releasing a photon, emitting light. The color of the emitted light depends on the structure of the dye.
Using Nile Red:

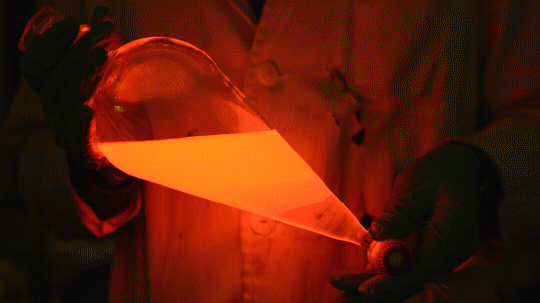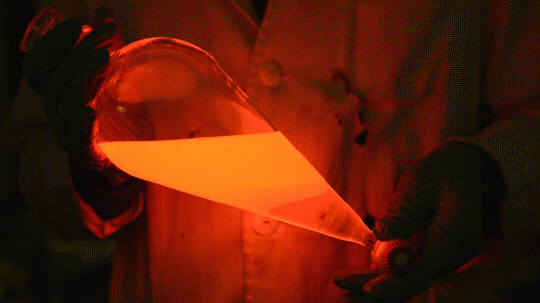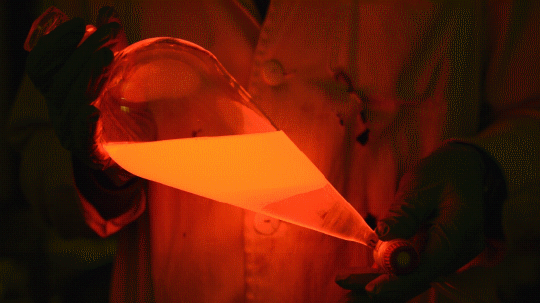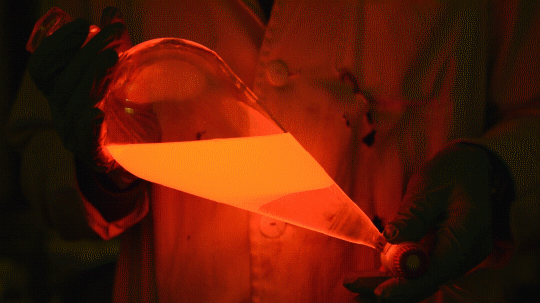
Mixing it with Perylene:


During this reaction I used two type of dyes, on of them was Nile Red, what is a quite special, solvatofluorescent dye and Perylene what is a polyaromatic hydrocarbon. When used in pure form the perylene emits a bright blue light, but when its combined with Nile Red, it emits this nice pinkish-purple as seen on the gifs above and on the video.
343 notes
·
View notes
Photo






our host galaxy | the milky way
I was flipping through my photo catalog and discovered I had enough decent Milky Way photos to make a Tumblr photoset. Enjoy.
© Lorenzo Montezemolo // instagram | tumblr | flickr | website
4K notes
·
View notes
Quote
Why do people think being with someone is the answer to everything?
Elizabeth Scott, Love You Hate You Miss You (via wordsnquotes)
93K notes
·
View notes
Quote
I get way too sensitive when I get attached to someone. I can detect the slightest change in the tone of their voice, and suddenly I’m spending all day trying to figure out what I did wrong.
Humans of New York - Amman, Jordan (via 5000letters)
1M notes
·
View notes
Photo

CHEMICAL SANDWICH
Ferrocene is such an exciting molecule that it spawned a whole subfield of chemistry: organometallics, the study of metal atoms bound directly to carbon atoms. The excitement continues today in labs across the world, where chemists make beautiful crystals of the compound and its derivatives. Ferrocene consists of an iron atom sandwiched between two aromatic, five-membered rings, a structure deduced by Harvard chemists Geoffrey Wilkinson and Nobel prize winner Robert Burns Woodward (J. Am. Chem. Soc. 1952, DOI: 10.1021/ja01128a527). Ernst Otto Fischer of the Technische Hochschule in Munich independently came to the same conclusion also that year.
Submitted by Ryoji Tanaka
Do science. Take pictures. Win money. Enter our photo contest here.
Want to know more about the history and chemistry of this remarkable molecule? Hop down the ferrocene rabbit hole with C&EN via the links below.
100 years of X-ray crystallography: Ferrocene
Ferrocene turns 50
Self-Assembling Ferrocene Complex Spawns Quasicrystals
X-ray examination of iron biscyclopentadienyl
129 notes
·
View notes
Photo




Dissolving white phosphorous in a really special solvent.
Phosphorus is a chemical element with symbol P and atomic number 15. As an element, phosphorus exists in two major forms - white phosphorus and red phosphorus - but because it is highly reactive, phosphorus is never found as a free element on Earth. From the two major allotropes, white phosphorous is the less popular, since it is HIGHLY toxic, ignites easily on air and it leaves really nasty marks after it gets on the skin.
When exposed to oxygen, white phosphorus glows in the dark with a very faint tinge of green and blue. It is highly flammable and pyrophoric (self-igniting) upon contact with air. Owing to its pyrophoricity, white phosphorus is used as an additive in napalm.
When white phosphorus is dissolved in normal organic solvents, like benzene, toluene, ect, it ends up with a transparent solution, it may have a slight yellow discoloration. However in this case it is highly colored as seen on the gifs, it’s due radical formation and depending on the concentration the color of the solution could highly vary.
Really important note: do not try to work with white phosphorus, it really toxic and it could easily ignite.
258 notes
·
View notes
Quote
When you grow up as a girl, the world tells you the things that you are supposed to be: emotional, loving, beautiful, wanted. And then when you are those things, the world tells you they are inferior: illogical, weak, vain, empty.
Stevie Nicks (via wnq-music)
7K notes
·
View notes
Quote
To be honest, I still think about you. I always wonder how you’re doing or if you’re okay. Sometimes, I wonder if you ever think about me, but I doubt it. I never really stopped loving you, I only gave up because you did. Also, just because we don’t talk anymore, doesn’t mean I don’t care anymore.
(via r2--d2)
25K notes
·
View notes
Photo






My new favorite: Solvatofluorescence of Nile Red
Solvatochromism is the ability of a chemical substance to change color due to a change in solvent polarity, so it has different color in different solvents.
Also in some cases, the emission and excitation wavelength both shift depending on solvent polarity, so it fluoresces with different color depending on the solvent what it’s dissolved in. This effect is solvatofluorescence.
On the video the highly solvatochromic organic dye, Nile Red was added to different organic solvents and was diluted with another, different polarity organic solvent. As the polarity of the solution changed, the emitted color from the fluorescent dye also varied as seen on the gifs above and as seen on the video:
youtube
To help the blog, donate to Labphoto through Patreon: https://www.patreon.com/labphoto
982 notes
·
View notes
Photo

@nasa-official @unofficially-nasa What did we do to deserve a beige universe?
379 notes
·
View notes