Don't wanna be here? Send us removal request.
Text
Chemistry Sem 4 End - Mod 1
Lanthanide contraction
aka trends in atomic and ionic radii
There is a regular decrease in the atomic and ionic radii with each increase in atomic number as we go from La to Lu.
This decrease in size is called Lanthanide contraction.
The configuration of Lanthanides show that the added electrons enters 4f-orbitals.
The shielding effect of 4F orbital is said to be imperfect due to its diffused shape.
Thus as the anomic no. increases, the nuclear charge increase by unity at every step, while there is no comparable increase in shielding effect of 4f e-.
This causes a contraction in the size of 4f orbitals consequently. The atomic+ionic radii goes on decreasing as we go from La to Lu.
Consequences of lanthanide contraction include: - Steady decrease in size from La to Lu. - Gradual increase in electronegativity, hence the reactivity increases. - Steady increase in standard reduction potentials.
Major consequences:
1) Occurrence of Yttrium (a transition element) with heavier lanthanides: - Since the size of Y3+ ion is comparable to the heavier ions like Tb3+, Dy3+, Ho3+, and Er3+, it occurs with them in natural minerals. - The crystal structure, solubility and chemical properties of the yttrium compounds are so close, these heavier lanthanides are known as yttrium earths
2) Close resemblance of properties of II and III transition series: - Zr and Hf, Mo and W, Ru and Os, all have the same size. - Transition elements of the third series have virtually the same atomic and ionic sizes as the corresponding elements just above them in their respective sub-groups. - Hf and Zr were expected to have different sizes but because of lanthanide contraction, Hf has contracted so much that it is the same size as that of Zr. They resemble very closely in their physical and chemical properties. - Therefore they occur together in the earth's crust and their separation is difficult, same for Mo and W and Ru and Os.
3) Basicity of oxides and hydroxides: - Basicity is defined as tendency to lose electrons. The greater the size, the greater the basicity. - From La to Lu, as size decreases, basicity of oxides and hydroxides decreases. Hence La(OH)3 is more basic and Lu(OH)3 is less basic.
4) Tendency to form complexes: - From La to Lu as size decreases, ionic character decreases and covalent character increases. Greater the covalent character, greater the tendency to form complexes. - Complex formation tendency of Lanthanides is comparatively less than the d-block elements because of their low charge density, i.e., change to size ratio. - The tendency to form complexes and the stabilities of complexes increases with increase in size in case of lanthanides. More stable complexes are formed in higher O.S. than in lower O.S.
--- II and III transition series compared with their 3d analogues

--- Transition elements, complex formation, catalytic susceptibility
Transition Elements

d-d Transition (ik it's not asked)


Complex Formation
All transition metal ions have vacant d-orbitals in which they accept electron pairs donated by the ligands.
They act as electron pair acceptors or Lewis acids.
The species which donates the pair of electron is called as ligands.
Ligands are electron pair donors or Lewis bases.
The most important property of transition elements is to form complexes or coordination compounds because - All transition elements are smaller in size, they have very high effective nuclear charge. - Greater the effective nuclear charge, the greater is the tendency to form complexes. - They have incompletely filled or vacant d-orbitals to accept electrons donated by the ligands.
The tendency to form complexes and the stability of complexes across the transition series increases with increase in atomic number or decreases in size of the ion.
With a particular metal ion and particular ligand, stable complexes are formed in higher oxidation states than lower O.S.
-
Catalytic Properties
A catalyst is a substance which increases the speed of the reaction without itself undergoing any change.
Ex of transition metal catalysts used in industrial processes:
i) TiCl4 (Titanium tetrachloride) - it is used as a Ziegler-Natta catalyst in polymerization of ethene or ethylene to produce polyethene.
ii) V2O5 (Vanadium pentoxide) - Used in oxidation of SO2 to SO3 in the manufacture of H2SO4 in contact process.
iii) MnO2 (Manganese dioxide) - Used in decomposition of KClO3 to give KCl and O2.
iv) Fe-Mo (Ferro Molybdenum) - Iron along with promoter molybdenum is used in production of NH3 by Haeber's process.
v) Pd - Used in hydrogenation of phenols.
vi) Pt - Used in Ostwald's process for manufacture of HNO3.
--- Gouy's Balance and Magnetic Properties of D-block
Gouy's
Powdered sample is taken into a pyrex glass cylinder and is suspended between the poles of electromagnet
The cylinder is uniformly packed with the sample and is vertically suspended from the beam of a balance.
The bottom of the substance coincides with the center of the magnet field.
The length of the cylinder is about 10cm such that the magnetic field is zero at the upper point.
When the magnetic field is on, the sample is either repelled by or attracted into the magnetic field.
The force required to maintain the position of the sample is measured by the weight that must be added or removed from the other pan of the balance to maintain equilibrium.
If the sample is paramagnetic, it will be drawn into the field, therefore it shows an increase in weight.
If the sample is diamagnetic, it will be repelled from the field, showing a decrease in weight.
The weight of the sample in presence of magnetic field and absence of magnetic filed is determined, let the difference in the weight of the sample under two conditions be ΔW.
Gouy's balance allows us to determine magnetic susceptibility with the magnetic moment formula.
n is the number of unpaired electrons, n can be determined w Gouy's balance.

-
Magnetic prop
On the basis of magnetic properties, there are 2 types: paramagnetic and diamagnetic.
Paramagnetic substances: - Weakly attracted to magnetic field. - Lose magnetism on the removal of magnetic field. - Caused by the presence of unpaired electrons. - Since most transition metal atoms have unpaired d-electrons, they are paramagnetic in nature.
Diamagnetic substances: - Repelled from the magnetic field. - Have filled e- subshells - All elements (except H) have at least 1 filled e- shell, - therefore some degree of diamagnetism is shown in all elements.
A paramagnetic rod in a magnetic field takes up a parallel position to magnetic field.
A diamagnetic rod in a magnetic field takes up a perpendicular position to the magnetic field.
Most transition metals show paramagnetic behavior, exceptions include Sc3+ and Zn2+ because the do not have any unpaired electrons.
Compounds that display paramagnetically when placed in a field are pulled. The extend of the pull can be measured with Gouy's magnetic balance.
Paramagnet in Gouy's balance shows an increase in weight
Diamagnet in Gouy's balance shows a decrease in weight
Since each unpaired electron is regarded as a micromagnet with a certain value of magnetic moment, the total magnetic moment of a cation depends upon the number of unpaired electrons and is given by

(the formula in red, idk how to type whole square rooted formulas)
where n = no of unpaired electrons.
--- Compare and Contrast Lanthanides and Actinides
Similarities:
In both, the differentiating electron enters f-orbitals.
The stable oxidation states of both is +3.
The atomic radii regularly decreases from La to Lu which is called as lanthanide contraction. Also there is regular decrease in size from Ac to Lr which is called actinide contraction.
Their perchlorates, nitrates, and sulphates are water soluble.
Their carbonates, bicarbonates, and fluorides are water insoluble.
Differences:

--- Titanium triad
Titanium: Ti = 22 [Ar]3d^2 4s^2
Zirconium: Zr = 40 [Kr]4d^2 5s^2
Hafnium: Hf = 72 [Xe]4f^14 5d^2 6s^2
They constitute IV B group of the periodic table.
General electronic configuration is (n-1)d^2 ns^2
The common and stable oxidation state is +4 in which their elements has d^0 configuration
Hence they are diamagnetic and colorless
These elements form covalent compounds: ex TiCl4
Reactivity:
Oxides and halides of +III state (+3) - +3 O.S. acts as a strong reducing agent - They have 1 unpaired electron, paramagnetic - In +3 only one d-d transition, single peak in spectrum. - +3 is more basic than +4 in Ti Ti -hot HCl-> TiCl3 <-650C- TiCl4 - Solutions of TiCl3 and R3Al form Ziegler-Natta catalyst. - Zr (III) and Hf (III) are unstable in water and only exist as solids.
Oxides and halides of +IV state (+4) - They from stable ionic oxides of formula MO2 which are non volatile, insoluble in water. - Basic character of oxides increases with increase in atomic number. This TiO2 is amphoteric, ZrO2 and HfO2 can be prepared by reacting metal with halogen.

- With excess of water TiCl4 is hydrolyzed completely, while ZrCl4 hydrolysis stops at ZrOCl2. - TiCl4 - colorless, diamagnetic, covalent forming liquid. - ZrCl4 - white solid. -These halides act as good acceptors, forming octahedral complexes: TiF4 -conc.HF-> [TiF6]^2-
--- Free electron theory
Proposed by Drude and Lorentz to explain the high electrical and thermal conductivity of metals.
Postulates: - Metal atoms have several unoccupied electron orbitals in their outer shell - Generally the ionization energies of metal atoms are less so they lose one of more valence electrons and form positive ions called metal nuclei. - These electrons are free to move throughout the metal as the metals contain a large number of vacant orbitals. - This these free moving electrons are said to be delocalized. -Thus according to this, a metal may be regarded as an assembly of positive ions (cations) immersed in a sea of mobile electrons or a sea of negative charge cloud. - Therefore this model is aka electron sea model. - Electron pulls the cation towards themselves in all direction and holds the cations close in turn giving metal it's solid structure - Free Electron Theory explains the following:
Non-Directional Bond
Bonds in metals are nondirectional because the electrons are not shared with one atom in one direction
However, they are shared with many other neighboring atoms in all directions.
Weak Bond
Valence electrons are attracted simultaneously by a large number of atoms.
Net binding energy is very small and the bond formed is weak.
Thermal Conductivity
When a part of metal is heated, the electrons in that part absorb energy from the heat source.
These excited electrons move to the cooler part of the metal, transferring the kinetic energy to the electrons present in that part by colliding into them.
This process continues till the temperature of all parts of the metal becomes the same.
-
Electrical Conductivity
Electrical conductivity is the ability of a material to conduct electric current
Metals are good conductors due to the presence of mobile electrons in metals
When potential difference is applied across a metal sheet, free electrons start to move towards the anode.
The electrons act as negative charge carriers, allowing electrical energy to flow through the metal.
New electrons are discharged from the negative electrode leading to continuous flow of electrons, that is, current starts to flow from negative to positive poles.

-
Ductility and Malleability
This is due to the non-directional nature of the metallic bond.
Because of the fact that on application of force, the metal ions can easily move from one lattice to another leading to the change in shape of metal
Limitations - Fails to explain why tungsten has very high melting point while mercury melts at -39°C. - Fails to explain why some metals have greater conductivity than others, ex: silver is a better conductor than lead - Fails to explain why osmium metals are extremely hard.
--- magnetic and spectral props of lanthanides
Magnetic Properties:
Due to the presence of unpaired electron in the 4f orbitals, most of the lanthanide ions are paramagnetic in nature.
Except for ions like La^3+ - 4f^0 which is diamagnetic in nature.
since 4f electron do not participate in bonding, the orbital motion of electron is not restricted. In such a case magnetic moment can be calculated by spin orbital coupling formula

-
Spectral Properties:
Pure lanthanide metals are colorless but their the positive ions are colored. The color of the ion depends upon the number of unpaired electron present in the f-orbitals.
The unpaired electron of f-orbitals undergo f-f transition which appear as short bonds in the UV-visible region, Lanthanide ions with f^0, f^7, f^14 configuration are colorless because of vacant, half filled and fully filled f-orbitals.
Ce4+ is colored in spite of f^0 configuration. It is not due to f-f transition but it is attributed to charge transfer transitions. (transitions between ligand and metal).
---
What are semi-conductors? Diff Extrinsic + Intrinsic Def conductors, insulators, with ex, def p-type + n-type
Insulators
Have a completely filled valence band and empty conduction band
There is a large energy gap between the valence and conduction band
Electrons lack the energy to jump across energy gap, therefore electricity cannot be conducted through insulators.
ex: rubber
Conductors
Have little to no energy gap between the valence and conduction bands
The two bands may even overlap each other
Since the energy requirement is so low, electrons can easily flow freely from the valence band to the conduction band.
Therefore electricity can easily be conducted through conductors
ex: gold
Semi-conductors
Semiconductors are materials with a conductivity in between insulators and conductors.
There is a small to moderately sized energy gap between the valence and conduction bands.
Since there is a considerable amount of energy required to cross the energy gap, only promoted electrons in the conduction band and unpaired electrons in the valence band can conduct electricity.
Holes, the absence of electrons, are formed in the valence band due to the transfer of electrons from valence to conduction band.
The probability of promoting electrons rises with temperature, thus the conductivity of semiconductors increases with temperature.

Semiconductors can be classified into 2 types: - Intrinsic - Extrinsic
Intrinsic Semiconductors
Pure semiconductors, a single pure element.
They do not conduct electricity at standard temperatures, but as temp increases, so does conductivity.
This is because at low temp there isn't enough energy for electrons to cross the moderately sized energy gap
These materials act as both poor conductors and poor insulators
ex: Silicon, Germanium
Extrinsic Semiconductors
Impure semiconductors, have been doped with a suitable extremely small doping agent
Depending on the type of doping agent, there are two subtypes: - n-type - p-type
n-type Semiconductors
n type implies the overall negative charge of the semiconductor.
There is an excess of electrons from the addition of a donor impurity.
Donor impurities are pentavalent elements such as phosphorus, add excess electrons
The majority carriers of charge are electrons while holes are the minor carriers.

p-type Semiconductors
p type implies the overall positive charge of the semiconductor.
There is a lack of electrons from the addition of an acceptor impurity
Acceptor impurities are trivalent elements such as boron, creates holes.
The majority of carriers of charge are positively charged holes while the minor carriers are electrons.

--- Explain stability of variable oxidation states of d-block elements,
Oxidation States
The most important property of transition elements is their tendency to exhibit variable oxidation states.
This property can be explained in terms of participation of 's' as well as 'd' electrons in bonding because the energy difference between ns and (n-1)d electrons is very less,
therefore both ns and (n-1)d electrons are available for bonding.

In some transition elements all of the (n-1)d electrons are not involved during bond formation.
ex: Fe (3d^6, 2s^2), It should have +8 as its highest O.S, but is it only +6 which is also in rare cases. (+2 and +3 are common for Fe)
During bond formation, only the unpaired electrons of the 3d subshell take part in bond formation.
In iron there are 4 unpaired and 2 paired 3d electrons, hence effective electrons in 3d orbitals are 4. 4 electrons from 3d and 2 e- from 4s = +6.
Maximum O.S. in the second and third transition series in +8 exhibited by Ru.

Relative Stability of Oxidation States
The +2 oxidation state becomes more stable across the period while +3 becomes less.
The relative stabilities of various oxidation states of 3d series elements can be correlated with the extra stability of 3d^0, 3d^5 and 3d^10 configuration to come extent. Ti4+ (3d^0) is more stable than Ti3+ (3d^1) Mn2+ (3d^5) is more stable than Mn3+ (3d^4) Fe3+ (3d^5) is more stable than Fe2+ (3d^6)
This generalization is not true in case of 4d and 5d series.
---
0 notes
Text
Chemistry Sem 4 End - Mod 2
Precipitation and neutralization of liq. ammonia
Liquid NH3 contains a strongly electronegative element, nitrogen, which causes hydrogen bonding. NH3 has sizable dielectric constant and has a high ionizing capacity.
Precipitation Reaction:
The solubility of different substances in liq. NH3 and H2O are different.
A number of reaction which are not possible in water can occur in liq. ammonia.
Silver nitrate gives AgCl ppt. in water
KCl + AgNO3 <-H2O-> KNO3 + AgCl ppted
While on the other hand, KNO3 and AgCl react in liq. ammonia to give KCL ppt.
AgCL + KNO3 <-Liq. NH3-> KCl ppted + AgNO3
-
Neutralization Reaction:
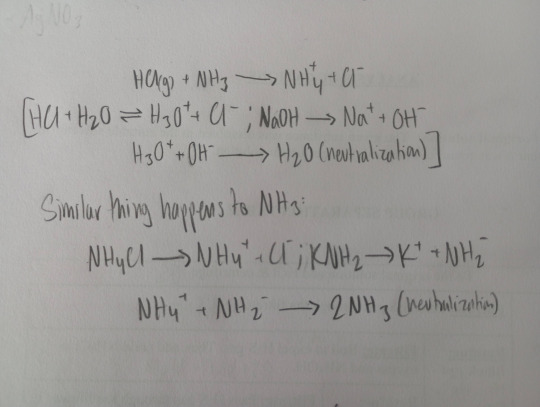
The role of HCl in water is the same as NH4Cl in ammonia.
The role of KOH in aqueous solution is the same as KHN2 in NH3.
NH3Cl is a strong acid and KNH2 is a strong base in ammonia solution.
--- toxicity of As, Hg, Pb
Arsenic Poisoning
Arsenic is an essential ultra-trace element present in red algae, chicks, humans, and some other mammals.
In chicks, deficiency of arsenic results in depressed growth.
But when present in more than ultra trace quantities, it is moderately toxic to plants and highly toxic to mammals.
Lewisite is a deadly poisonous gaseous arsenic compound: - Cl2-AsCH=CHCl It binds to enzymes in living species and weakens its activity.
Arsenic contaminated drinking water can cause severe damage to our skin, liver, and kidneys.
Arsenic compounds are used as insecticides by farmers. Such pesticides, mining operations, and the burning of coal are the chief sources of arsenic pollution in the air and water.
-
Mercury Poisoning
Mercury has no biological function.
Hg is highly toxic to fungi, plants, and animals. It is a contaminated poison for mammals.
The intake of Hg-contaminated fish can cause serious issues.
If Hg is dissolved into the blood and carried to the brain, it can cause irreversible damage to the central nervous system.
Monomethyl and dimethyl mercury ([CH3Hg] and [(CH3)2Hg)] cause nervous disorders in marine life.
Contact with mercury causes nervousness, fear, inability to make decisions, heaviness, irritability, headaches, pessimism, fatigue, sleeplessness, trembling of limbs, falling teeth, and diarrhea. Also genetic changes.
Mercury is primarily from two sources: natural volcanic eruption or weathering of mercury-containing rocks, and as a waste product in industrial processes.
Animal charcoal and penicillamine both act as antidotes for Hg poisoning.
-
Lead Poisoning
Lead has no biological function.
Pb is a cumulative poison as it continuously accumulates in the tissues of organisms.
Lead in very low concentrations such as 0.2ppm in the human body can cause metabolic disturbances.
Lead can bind to cellular enzymes, this disrupts the functioning of cells and organs of the muscular, circulatory and nervous systems.
Lead damaged organs such as the liver, kidneys, and intestine.
Lead develops issues and abnormalities in fertility and pregnancy.
Lead causes coagulation of proteins.
Lead can be absorbed by skin, resulting in many skin diseases.
The deposition of lead in bones and teeth causes tiredness, headache, loss of appetite, anemia, muscular weakness, etc.
Excessive intake of lead causes disruption of hemoglobin synthesis, loss of appetite, anemia, kidney disfunction, nervous disorders, and brain damage.
Air gets polluted by PbBr2 and PbCl2 through the combustion of petrol.
Lead pollution is also caused by industries involved in lead mining, extraction, purification, and the manufacture of alloys and paints.
2PbCO3.Pb(OH)2, a lead based pigment causes serious health hazards.
Even prolonged use of lead utensils causes 'lead sickness.'
In the case of lead poisoning, intravenous injection of CaNa2.EDTA can treat the patient.
--- Hemoglobin structure, how does it carry oxygen
Hemoglobin or Hb is the red pigment which is present in red blood cells in the human body.
For each 100ml of blood in a normal human male there is around 15g of Hb.
About 65% of iron in the human body is present as Hb.
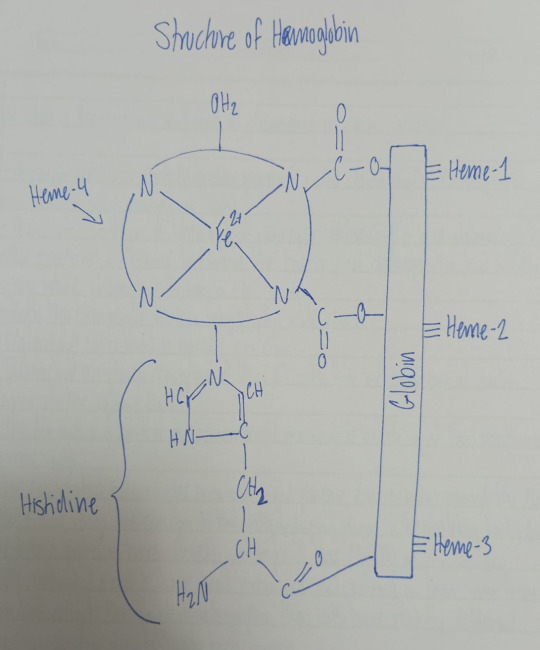
Hb is Fe(II) porphyrin, it contains four identical units arranged roughly tetrahedrally.
Each histidine unit has one heme group attached to it.
The molar mass of Hb is about 64500. Each Hb molecule has 4 heme groups: heme-1, heme-2, heme-3, heme-4, they are bound to globin on its surface.
Therefore Hb is a heme containing protein
The 4 heme groups present in the structure of Hb are the subunits of Hb.
Hb is an octahedral complex of Fe(II). Fe(II) occupies the central position and the four corners of the square base are occupied by the four N-atoms of heme group. One axial position is occupied by N-atom of histidine and the other axial position is occupied by H2O molecule.
The 4 subunits are linked together through salt bridged present between the four polypeptide chains. it is now believed that the salt bridges present in between the polypeptide chains of Hb introduces stain in the molecule of Hb.
Hb which has not been oxidized is called deoxy-hemoglobin or just hemoglobin while Hb which has been oxidized is called oxygenated-hemoglobin or oxyhemoglobin.
Fe(II) present in Hb can be oxidized to Fe(III) to form Fe(III) protein called met-hemoglobin. Fe(III) protein is responsible for the brown color of old meat and dried blood.
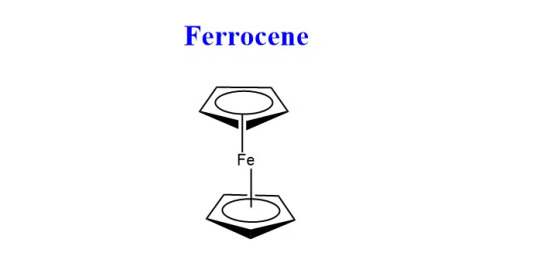
--- Synthesis of Ferrocene + properties
Aka dicyclopentadienyl iron, has the chemical formula (C5H5)2Fe
Ferrocene is the first discovered metallocene sandwich compound.
Metallocene is derived from “Metallo”- transition metal and “Cene” - benzene.
The cyclopentadienyl ligands C5H5 bond in such a way that iron is present in the center of the complex resulting in a sandwich structure
Primarily ferrocene is used as a catalyst in the industrial world
-
Synthesis
Can be prepared: - From Grignard's reagent - From sodium cyclopentadienide - From cyclopentadiene
Grignard's: When the Grignard reagent is treated with iron chloride(II), ferrocene is formed
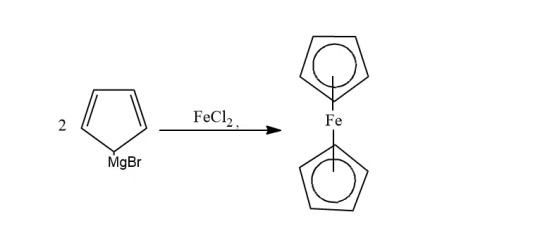
Sodium Cyclopentadienide: Cyclopentadiene is treated with sodium metal to form cyclopentadienide, which on reacting with FeCl2 forms ferrocene.
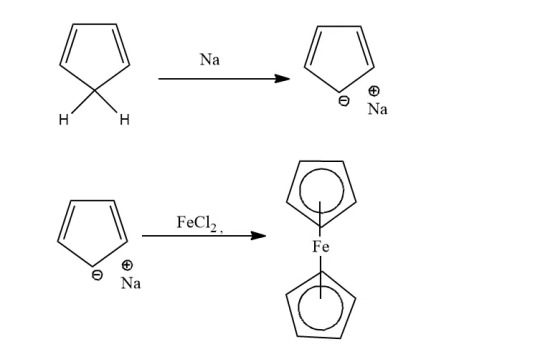
Cyclopentadiene: When cyclopentadiene is treated with iron chloride in the presence of a strong base, ferrocene is obtained. Lab method.
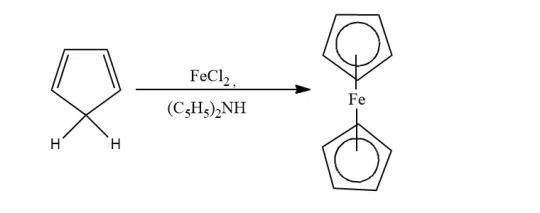
-
Physical Properties
Orange crystalline solid at room temp w a camphor like odor.
Stable organometallic compound w melting point 173-174°C and boiling point 249°C.
less soluble in water but soluble in organic solvents like acetone.
-
Chemical Properties
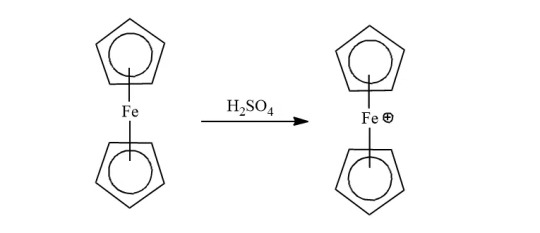
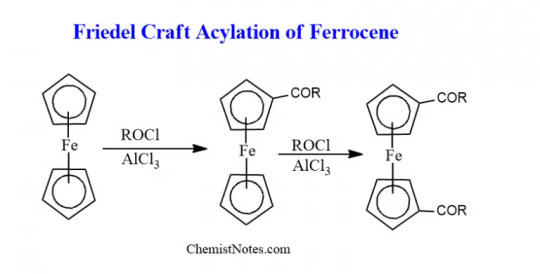
Ferrocene can undergo numerous electrophilic reactions faster than benzene. But, it can not undergo an electrophilic reactions with electrophiles which are strong oxidants like H2SO4 or HNO3.
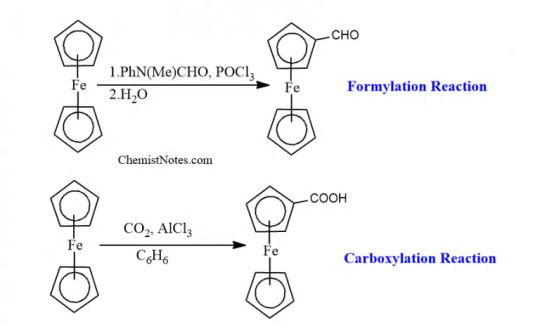
It can also undergo formylation and carboxylation reactions to give mono-functionalized derivatives, since the functional group extensively deactivates the ferrocenyl group.
Friedel craft acylation reaction can be given by Ferrocene.
--- Solubility Product + Common ion effect
Solubility Product
When a saturated solution of a salt, for example AB, is in contact with the solid phase, the following equilibrium forms:
AgCl (undissolved solid) <--> AgCl (dissolved but unionized) <--> Ag⁺ + Cl⁻ (ions)
AgCl is sparingly soluble in water
The solid is in equilibrium with the ions of solution and this applying the law of mass action, in general: [Ag⁺][Cl⁻]/[AgCl] = K
Now since the solution is saturated and is in contact with the solid, the concentration of the unionized salt molecules should be constant at constant temp.
Hence: [Ag⁺][Cl⁻]/Constant = K where [Ag⁺][Cl⁻] = new constant, [Ag⁺][Cl⁻] = Ksp
Thus, the product of the ionic concentration in a saturated solution of a soluble electrolyte at a constant temp in a constant quantity called solubility product (Ksp)
Ex: using excess reagent, since BaSO4 is insoluble in H2O a small amount would remain in solution if equal amounts of two reagents, like BaCl2 + H2SO4 are taken.
Therefore when carrying out complete precipitation, the precipitant must always be added in a little excess such that the ionic products of compound to be precipitated far exceeds its solubility product.
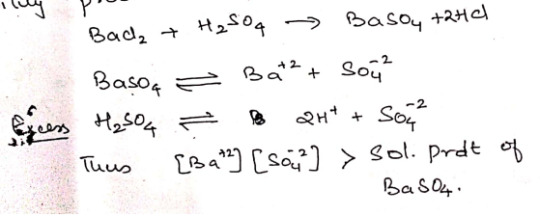
-
Common Ion Effect
Consider dissociation of a weak electrolyte in water, there exists an equilibrium between ions and unionized molecules to which the law of mass action can be applied.
ex: AB <--> A⁺ + B⁻, [A⁺][B⁻]/[AB] = K (ionization constant)
If the above electrolyte solution added another strong electrolyte with a common ion, either A⁺ or B⁻, the cation or anion will increase.
In order to keep 'K' constant, [AB] should increase in concentration.
This suppression in dissociation is due to a common ion, hence the phenomenon is called common ion effect.
Thus, the suspension of the dissociation of a weak electrolyte in presence of a strong electrolyte which have a common ion with each other is called Common Ion Effect.
-
In group II, cations are ppted as sulphides in acidic medium while in group IV, the cations are ppted as sulphines in basic medium.
The weak electrolyte H2S ionizes in aqueous solution to give sulphide ions in sufficient amounts to exclude the solubility product of group II and the group IV radicals.
But when the solution is made acidic with the addition of dil. HCl, H⁺ ions from the HCl suppresses the ionization of H2S due to the common ion effect
This causes sulphide ion concentration to decrease, thus only the sulphides of group II with a much lower solubility product than the group IV are ppted.
In the presence of NH4OH, the OH- ions combine with the H+ of H2S, forming unionized water, resulting in greater ionization of H2S molecules to give H+ and S-.
This increases the concentration of S- ions, it becomes so high that the solubility product of group IV sulphides is exceeded and this ppted out.
H2S <--> 2H⁺ + S⁻2 is feebly ionized
HCl <--> H⁺ + Cl⁻ is highly ionized
---
Flame test + brown ring test
Flame Test
Flame test is a quick test used in semi-micro analysis to identify the presence of a relatively small number of metal ions in a compound
Flame test is effective in indicating the presence of group I and group V cations
- Principle:
If you excite an atom or an ion by strongly heating, electrons can be promoted from their normal unexcited state into higher orbitals.
As they fall back down to lower levels, energy is released as light.
Each jump involves a specific amount of energy being released as light, and each corresponds to a particular wavelength.
As a result of all these jumps, a spectrum of lines will be produced, some of which will be in the visible part of the spectrum.
The visible color is a combination of all those individual colors.
- Procedure:
To the salt mixture taken in a watch glass, add 2 drops of conc. HCl. Mix with a glass rod to make a paste, expose the end of the rod to the flame of the bunsen burner.
If an apple green color is observed, barium cation from group V may be present.
If a brick red color is observed, calcium cation may be present.
If a crimson red color is observed, strontium cation may be present
-
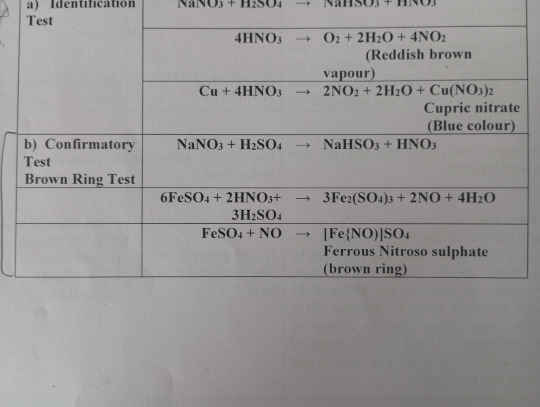
Brown Ring Test
Brown ring tests is a common nitrate test which determines the presence of nitrate anion in any solution.
- Principle
The nitrate ion functions as an oxidizing agent.
Iron (II) reduces the nitrate ion in the reaction mixture, and iron (II) is oxidized to iron (III).
When nitric oxide is reduced to NO-, ferrous nitroso sulphate complex is formed, which creates a brown ring at the intersection of two layers
- Procedure
A small portion of Na2CO3 extract is taken in a semi-micro test tube
It is acidified by adding a few drops of dil. H2SO4 till no more effervescence is evolved, then followed by a dilute solution of FeSO4.
By holding the test tube at an inclined position, conc. H2SO4 is slowly added along the inner wall of the test tube.
If a brown ring is formed at the junction of the two layers, the presence of nitrate ion is confirmed.
---
Classify organometallic compounds
Organometallic compounds, or organometallics, are compounds where the central metal atom is linked directly with the C atom of the organic ligand.
These compounds contain 1 or more M-C bonds.
The metal atom may be a transition metal, lanthanide, actinide, or a main group element.
Although B, Si, Ge, As, etc. are all non-metallic elements, they are also classified as organometallic compounds.
Cyanides and carbides have M-C bonds, but these compounds are not organometallic compounds because metal is not attached to the carbon of alkyl group.
Classification on the bases of nature of M-C bond: - Ionic bonded - σ-bonded - π-bonded - Bridge bonded
Ionic bonded - The organometallic compounds of highly electropositive metals are usually ionic in nature - In these compounds, the hydrocarbon residue exists as a carbanion with a negative charge, it is attracted to the metal atom by non-directional electrostatic forces. - Colorless, non-volatile, very reactive solids, insoluble in organic solvents. - ex: OMCs with alkali (no lithium), alkaline earth metals, etc. - R2M (M=Ca or Ba), R⁻Na⁺
σ-bonded - C atom of organic ligand bonds to the metal by a 2e⁻, 2 centered covalent bond. - Metals with low electropostive nature form sigma-bonded covalent bonds. - Formed by most elements with values of electropositivity > 1, such as non-metals and weakly electropositive metals. - M and C share a pair of electrons, forming a covalent bond. - ex: (CH3)2Zn
π-bonded - Specific to transition metals - First π-bonded OMC was prepared by ferrocene: (C5H5)2Fe - Ferrocene has a sandwich structure in which the iron atom lies between 2 planar C5H5 rings:

- Bonding involves overlap of π-electrons of the cyclopentadienyl rings with unfilled d-orbitals of the metal.
Bridge-bonded - The compounds in which a loosely bonded electron deficient species exists with the coordination of metals like Li, Be, Al, etc. - This group includes the organometallic compounds with bridging alkyl groups. - Ex: Al2Me6
--- Reactions in HF
Anhydrous HF is a strong acidic solvent
It has high dielectric constant = 84
High dipole moment = 1.9D
Boiling point = 19.4°C and Freezing point = -83°C
It acts as a better solvent than water.
-
Auto Ionization:
Aka self ionization, refers to the process in which a molecules can donate a proton to another molecule of the same species, resulting in the formation of ions.
HF + HF -> H2F⁺ + F⁻
Only a few substances can donate H⁺ to HF:

Precipitation:
Perchlorates, sulphates of non-alkali metals get precipitated when their fluorides dissolved in liq. HF are treated with solution of alkali metal sulphate, perchlorate, etc. NaClO4 + TiF -HF-> TiClO4 ppted + NaF Na2SO4 + NiF2 -HF-> NiSo4 ppted + 2NaF
Acid-Base Reactions:
3HF <--> H2F⁺ (acid) + HF2⁻ (base)
All substances which can form H2F⁺ ion will behave as an acid and substances which yield HF2⁻ ion behave as a base. HNO3(base) + HF -> H2NO3+ + F- BF3(acid) + 2HF -> BF4- + H2F+ HClO4(acid) + HF -> ClO4- + H2F+ HClO4(base) + HF -> H2ClO4+ + F-
Protonation:
Organic compounds like ethanol, benzene, etc. get protonated when dissolved in HF. C2H5OH + HF -> C2H5OH2+ + F- C6H6 + HF -> C6H7+ + H-
---
(5M) Solvolysis reaction of liq. ammonia w ex
Solvolysis is a type of nucleophilic substitution or elimination reaction where the nucleophile is a solvent molecule.
Ammonolysis or ammolysis is solvolysis by ammonia
The attachment of one or more solvent molecules to a solute species through any chemical linkage.
Its products are called solvates H2O -> Hydration -> Hydrates liq. NH3 -> Ammonation -> Ammonites BF3 + NH3 -> BF3.NH3 SiF4 + 2NH3 -> SiF4.2NH3
--- (5M) Bio significance of Na, K, Mg, Cl
Sodium:
Na⁺ cation is a major ion present in the extracellular fluids of organisms, it can be found in large quantities in bones as phosphate. May also be present in chloride and bicarbonate.
Na⁺ ion activates some enzymes in animals.
Imp source for sodium is common table salt (NaCl), which is used in all cooking.
Excessive intake of Na⁺ ions can cause hypertension, for example when fish take in too much saline water where NaCl is in large amounts.
In biological systems, Na⁺ ions regulate acid-base equilibrium.
Sodium is important for the formation of HCl acid in the stomach., the conduction of nerve impulses, and muscle contraction
Since herbivorous animals fail to receive enough Na⁺ ions from their diets, they look out for 'salt-lick' to bridge the gap in their Na⁺ requirements.
Sodium also helps in maintaining osmotic pressure of the body fluid, protecting the organism from fluid loss.
Sodium helps in the prevention of normal irritability of muscle and permeability of cells
Injectable medicines are dissolved in NaCl before they can be administered to humans
Na⁺ cations are essential for both nerve action and heart function.
Na⁺ cation is also responsible for the transport of glucose and amino aids into the cell.
-
Potassium:
K⁺ cation is primarily in intra-cellular fluid as well as extra-cellular fluid.
Imp sources is almost all foods like coffee, tea, cocao, dried beans, molasses, leafy green veg, milk, fish, chicken, pork, dried apricots and peach, banana, orange juice, etc.
K⁺ ion is essential for nerve impulse and muscle contraction.
K⁺ ions are important for all organisms with the potential exception of blue green algae.
When injected intravenously potassium is moderately toxic to mammals.
Much like sodium, potassium also influences acid-base equilibrium in extracellular fluid.
Potassium controls osmotic pressure and water retention
Potassium is essential for metabolic functions such as protein biosynthesis by ribosomes.
Enzymes such as glycolytic enzyme pyruvate kinase requires K⁺ for optimization.
K⁺ ions are required in cells for glucose metabolism, protein synthesis, and activation of some enzymes.
-
Magnesium
Mg is an important non-transition element to all organism. It is present in great concentration in red blood cells.
Imp sources are almonds, cereals, beans, green veg, potatoes, and cheeses.
Mg(II) specifically plays an essential role in many metallo-enzymes
Mg2⁺ cation is present in chlorophyll of plants, enabling the process of photosynthesis.
Constipation, obesity, liver, and gall bladder disorders can all be treated with Mg(II) salts.
Mg forms a vital complex with ATP which is required for most enzymatic reaction involving ATP within the cell.
Magnesium ions are present in important enzymes such as phosphate and aminopeptidase.
Magnesium deficiency is induced by tetanus like cramps and abnormal swelling of the atrial walls in plants. Mg deficiency destroyed the green color of leaves.
-
Chlorine
Cl⁻ ion is an essential electrolyte, commonly found in the body's fluids and blood.
An imp source for sodium is common table salt (NaCl), which is used in all cooking.
Cl⁻ may be present as chlorides (HCl, NaCl, KCl, etc.)
Chlorine is primarily absorbed from the gastrointestinal tract.
Cl⁻ ion helps the body to maintain normal hydration and osmotic pressure, prevents excessive fluid loss.
Cl salts aid in the maintenance of normal acid-base equilibrium.
Chloride ions play an important role in the gaseous transport of CO2.
NaCl and KCl maintain the correct viscosity of blood.
Gastric HCl is an important digestive fluid.
Chlorine ions are mostly excreted by the kidneys in urine.
Deficiency of chlorine in the body leads to hypochloremia: poor appetite, increased urination, irritability, muscle twitching
Excess of chlorine in the body leads to hyperchloremia: diarrhea, vomiting, fatigue, dehydration
--- (5M) Reactions for identification of CO3(2-) and NH4+ ions
Analysis of NH4⁺ cation:
Ammonium salts when titrated with NaOH solution produces ammonia gas with a pungent odor.
NH4Cl + NaOH -> NaCl + NH3 + H2O
The evolution of NH3 gas allows us to confirm that NH4⁺ is present.
To confirm the evolved gas in NH3:
i) Expose the gas to a filter paper paper dipped in CuSO4 solution - If the paper turns blue, the gas in NH3 and HN4⁺ is initially confirmed. - CuSO4 + 4NH3 -> [Cu(NH3)4]SO4 - [Cu(HN3)4]SO4 is tetramine copper complex, it is a deep blue color.
ii) Expose the gas to a filter paper dipped in Nessler's reagent. - If the paper turns brown, NH4⁺ is confirmed. - Ammonium chloride reacts with Nessler's reagent to form a brown ppt called iodide of Millon's base.

-
Analysis of CO3(2-) anion
Carbonate salts when treated with dilute HCl acid produces carbon dioxide gas along with brisk effervescence.
Na2CO3 + 2HCl -> 2NaCl + CO2 +H2O
Confirming that the gas emitted is CO2 will confirm the presence of CO3(2-).
If we pass the gas through lime water and the lime water turns milky, the gas if confirmed to be CO2 and CO3(2-) is confirmed.
CO2 + Ca(OH)2 (lime water) -> CaCO3 + H2O
---
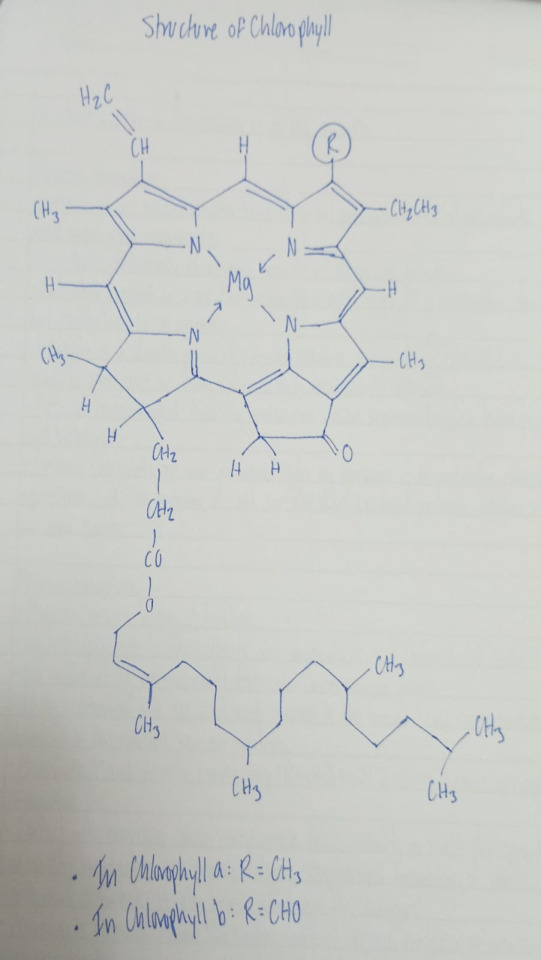
Chlorophyll Structure

---
Grignard's Reagent


0 notes
Text
Chemistry Sem 4 End - Mod 3
Kirchhoff's law

--- Gibbs and Maxwell Relation



--- Joule Thomson effect


--- Gibbs free energy + Helmholtz free energy
These are two equations derived by Gibbs and Helmholtz called Gibbs Helmholtz equations.
One of the equations can be expressed in terms of changes in free energy (ΔG) and enthalpy (ΔH)
This equation is called Gibb’s Helmholtz equation in terms of free energy and enthalpy change at constant pressure.
It is generally employed and is applicable to all processes, chemical or physical, but in a closed system.

--- Carnot Cycle (5 or full 10 marks)



--- First law of Thermodynamics
Statement: Energy can be neither created nor destroyed, it can only be transferred from one form to another.
Explanation: When a system having internal energy U1 absorbs energy q from it's surroundings, then a part of the absorbed energy is utilized in increasing the initial energy of the system to U2 and the rest of the energy is used in doing expansion work. Therefore the amount of heat energy absorbed is equal to the increase in the internal energy and the work done by they system.
Equation + 5 Cases:

---
Reversible + Irreversible processes
Processes can be classified into Reversible and Irreversible
Reversible: A process which takes place extremely slowly through a series of small steps in such a way that the direction of the process can be reversed at any instant by making small changes in the state of the system. At the initial, final, and all intermediate stages, the system is in equilibrium state.
Irreversible Processes: A process which is carried out rapidly from the initial to the final state in a single step. It cannot be carried out in the reverse order. The system is in equilibrium at the beginning and at the end, but not at the points in between.
Differences between the two:

---
(5M)Def system + surroundings and diff types of system
System is the part of the universe which is under study and has definite boundaries.
Surroundings are the remaining part of the universe other than the system
The surroundings are limited to the immediate vicinity of the system.
ex: When studying a glass of water, water is the system, glass is a boundary, and the area around the glass of water is the surroundings.
There are a few types of systems depending on the nature of boundary: - Real System - Ideal System - Open System - Closed System - Isolated System
Open System: - The boundary is open and not insulated - Therefore an open system is one which can transfer both energy and matter to and from its surroundings. - ex: Hot water in a beaker placed on a table, the water vapor (matter) and heat (energy) both can escape from the beaker and be transferred to it's surroundings.
Closed System: - The boundary is closed and not insulated - Therefore a closed system is one which cannot transfer matter but can transfer energy to and from it's surroundings. - ex: Hot water in a closed container, the water vapor (matter) can not escape the system, however the heat (energy) can transfer through the walls of the container to the system's surroundings.
Isolated System: - The boundary is closed and insulated - Therefore an isolated system is one which cannot transfer either matter nor energy to and from it's surroundings. - ex: Hot water in an insulated thermos, neither the water vapor (matter) nor heat (energy) have a chance to escape the system.
---
Process and Types


---
State and Path Functions

---
Joule's Law

---
Work Done in Isothermal Reversible Expansion of Ideal Gas


#notes#science#send help#long post#long reads#chemistry#physical chemistry#physics#i don't wanna do this anymore
0 notes
Text
Chemistry Sem 4 End - Mod 4
Arrhenius eq
We know that the kinetic energy of a gas is directly proportional to its temperature.
Thus as the temperature of a system increases, more and more molecules acquire the necessary energy greater than Ea to cause productive collisions, This increases the rate of reaction.
In 1889, Arrhenius suggested as simple relationship between the rate constant, k, for a reaction and the temperature of the system:
formula
This is the Arrhenius equation where - A is an experimentally determined frequency factor - Ea is activation energy - K is rate constant - R is gas constant - T is Kelvin temperature

Arrhenius equation can be used to calculate the activation energy, Ea.

---
Rate constant eq for first order reaction, define order of a reaction
Order of a reaction is defined as the sum of the powers of concentrations in the rate law.
Consider the example of a reaction which has the rate law: rate=k[A]^m[B]^n The order of such a reaction is (m+n).
The order of a reaction can also be defined with respect to a single reactant, thus the reaction order with respect to A is m and with respect to B it is n.
The overall order of reaction (m + n) may range from 1 to 3 and can be fractional.
Reactions may be classified according to the order. If m+n = 1, it is first order reaction m+n = 2, it is second order reaction m+n = 3, it is third order reaction


---
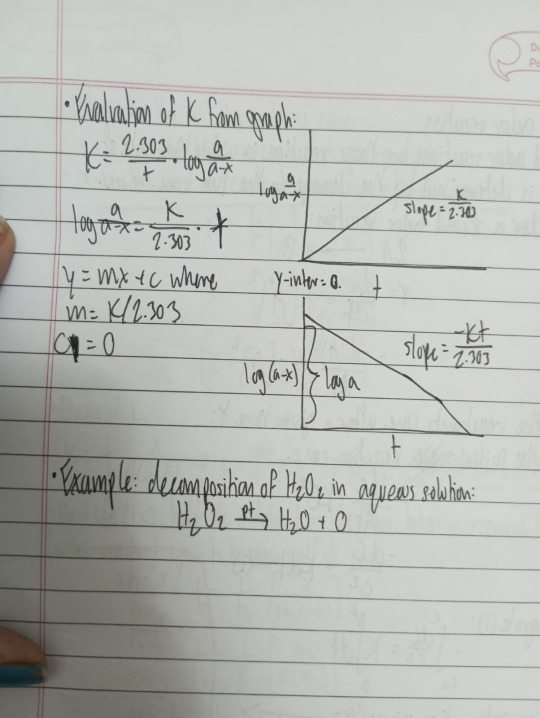
Jablonski diagram + explain phosphorescence, fluorescence, and ISC
According to Grothus-Draper law, only the light which is absorbed by a system can bring about a photochemical reaction.
The light absorbed may be re-emitted along in one or more steps. This phenomenon is called fluorescence. The emission in fluorescence ceases with the removal of light source.
Sometimes the light absorbed is given out slowly and even long after removal of light source. This is call phosphorescence.
The phenomena of both fluorescence and phosphorescence can be explained with Jablonski's diagram.
The quantity 2S+1 where S is the total electron spin in most of the molecules have even no. of electrons and all the electrons are spin paired, Here S gives Spin Multiplication.
If spins are paired (↑↓) unpaired orientation is cancelled by downward spin. therefore S=0 S1 = +1/2, S2 = -1/2 S = 1/2 - 1/2 = 0
Hence (2S+1) = 2x0+1 = 1 or singlet ground state
When absorption of photon of suitable energy hv one of the paired electrons goes to the higher energy level. The spin orientation of two single electrons may be parallel or antiparallel

If spins are parallel: S = phospherS1+S2 = 1/2+1/2 = 1 (2S+1) = (2x1)+1 = 3 Spin multiplicity of molecules is known as triplet excited state.
If spins are antiparallel: S = S1+S2 = 1/2-1/2 = 0 (2S+1) = (2x0)+1 = 1 Spin multiplicity of molecules is known as single excited state.
Since electrons can jump to any of the higher electronic states depending upon the energy of the photon absorbed, we got a series of singlet excited states: Sn where n=1,2,3,4,...
This S1, S2, S3, etc. are known as 1st singlet excited state, 2nd singlet excited state, 3rd singlet excited state, so on.
Similarly T1, T2, T3, etc. are know as 1st triplet excited state, 2nd triplet excited state, 3rd triplet excited state, etc.
Accordingly, energy sequence is as follows: ES1>ET1, ES2>ET2,etc
On absorption of light photon, the electron of the absorbing molecule may jump from: S0 -> S1 or S2 or S3 (Singlet excited state) depending upon the energy of light photon absorbed as shown in diagram.
For each singlet excited state (S1, S2, S3, etc) there is a corresponding triplet excited state (T1, T2, T3, eTc)
The molecule is said to be activated whether the singlet or triplet excited state A + hv -> A*
'A' molecule in the ground state on absorption gets A* excited state molecule (Activated molecule)
The activated molecule remains in the ground state by dissipating its energy through the following types of processes: - Non-radiative - Radiative
Non-radiative transitions
These transitions involve the return of the activated molecule from higher excited states to the first excited state. These reactions do not involve in the emission of any radiations and are this referred as non-radiative transitions.
The energy of activated molecule disappears in the form of heat through molecular collisions. This process is called Internal Conversion (IC)
The molecules also lose energy by another process called Intersystem Crossing (ISC)
ISC involves in transition between states of different spins and different multiplicity, ie: S2 -> T2 S1 -> T1
These transitions are also non-radiative spectroscopically, such transition are forbidden.
Radiative transitions
Fluorescence: (S1->S0)
The transition from the single excited state to ground state
Such transitions are accompanied by the emission of radiation. Spectroscopically the transition from S1 -> S0 is allowed and occurs in about 10^-8 seconds
The emission of radiation is this transition is called fluorescence.
Phosphorescence: (T1->S0)
The transition from the triplet excited state to ground state.
It is rather slow since it is a forbidden transition.
The emission of radiation in this transition is called phosphorescence.
The life times of phosphorescence are much longer being of the order of 10^-3 seconds or greater since the transition involves spin inversion which needs time for it's occurrence.
Both fluorescence and phosphorescence radiations are of shorter frequencies than existing light as some part of energy of molecule disappears in the form of heat during "non-radiative transitions."

---
Grothus-Draper principle of photochem activation
There are two basic laws of photochemical reactions - Grothus-Draper law - The Stark-Einstein law of Photochemical Equivalence
Grothus-Draper:
When light falls on a cell containing a reaction mixture, some light is absorbed and the remaining light is transmitted.
Only the absorbed component of light that is capable of producing the reaction, the transmitted light is ineffective chemically.
This is Grothus-Draper law and may be stated as follows:
When light falls on an object, a part of it is reflected and a part of it is transmitted. The rest is absorbed by the reacting system which is effective in bringing about a chemical change.
However, this does not mean that the absorption of radiation must necessarily be followed by a chemical reaction.
When the conditions are not favorable for the molecules to react, the light energy remains unused. It may be re-emitted as heat or light.
-
The Stark-Einstein law of Photochemical Equivalence
Stark and Einstein noted that each molecule taking part in a reaction absorbs only a single quantum or photon of light.
The molecule that gains one photon-equivalent energy is activated and enters into reaction.
They thus proposed The Stark-Einstein law of photochemical equivalence, which may be stated as:
In a primary photochemical process, each molecule is activated by the absorption of one quantum or radiation (one photon)

molecule ‘A’ absorbs a photon of radiation and becomes activated. The activated molecule A* then decomposes to yield B.
---
Quantum efficiency of the photochem combination of H2 and Cl2
Quantum yield or the quantum efficiency of a photochemical process is defined as the no. of molecules reacted or formed per photon of light absorbed.
∅ = no of molecules reacted/no of photons absorbed OR
∅ = no of molecules reacted/no of Einsteins of radiation absorbed
For product formation:
∅ = no of molecules of product formed/same two denominators ^
Is the 2nd law is correct, then quantum yield should be unity? but this is very rare.
The quantum yields may be as high as as 10^6 or as low as 10^-2 for several photochemical reactions.
-
Cause of high quantum yields:
When one photon is absorbed by molecules and it decomposes to form more than one product molecule then the ∅ of molecule is greater than 1 and is said to be high.
Hydrogen-chloride reaction is a good example of high quantum yield.
But before explaining, photochemical process has 2 steps:
1) Primary Process: It proceeds through absorption of radiation by molecule to form excited atom or molecule. - ex: A (molecule) + hv (photon) -> A* (excited molecule)
2) Secondary Process: The activated molecules may react with the other molecules to form product - Or, the activated molecule may emit the radiation of either the same or different frequency.
-
ex: HCl Reaction
This is a photochemical chain reaction
A mixture of hydrogen and chlorine is exposed to light of wavelength 4000Å or less than 480nm.
The hydrogen and chlorine react rapidly to form HCl.
This is followed by secondary reactions. 1) Cl2 + hv -> Cl + Cl - primary process 2) Cl +H2 -> HCl + H - secondary process 3) Cl2 + H -> HCl + Cl - secondary process 4) Cl + Cl -> Cl2 - secondary process - Step 1 is chain initiation - Step 2 and 3 are chain propagation - Step 4 is chain termination
The reason for higher quantum yield is one photon of light absorbed in step 1 forms large number of HCl molecules in steps 2 and 3, where in step 4 Cl atoms lose their excess energy and recombine at the walls of the vessel by chain terminating.
The number of HCl molecules formed for a photon of light is very high, as a result quantum yield of reaction varies from 10^4 to 10^6
---
zero-order kinetics

---
determine order of reaction w differential method
This method was suggested by van’t Hoff and, therefore, it is also called van’t Hoff’s differential method.
According to it, the rate of a reaction of the nth order is proportional to the nth power of concentration.

---
(5M) rate of reaction
The quantity of reactant species consumed on the quantity of product species formed in unit time in a chemical reaction is called the rate of reaction. A -> B
With the passage of time, the concentration of A goes on decreasing, while the concentration of B goes on increasing.
The rate of reaction at any moment may be expressed as follows: Rate of Reaction (ɣ) = amt of A consumed/time taken OR Rate of reaction (ɣ) = amt of B produced/time taken
If in a unit time, the amount of A consumed or B produced is large, the reaction is said to be a first order reaction.
However the rate of reaction cannot be determined by dividing the total change in concentration by total time taken, this is because the rates of reactions are not uniform.
Rate of reaction depends on the concentration of the reactant.
With the passage of time, the concentration of reactant species decreases progressively and hence the rate of reaction goes on decreasing.
Therefore, the rate of reaction at a given moment can be expressed by dividing the small change in concentration dx by small time interval Rate of reaction at a given moment = dx/dt
Hence the rate of reaction may be defined as: The instantaneous change in concentration of a reactant/product species at a given moment of time
Consider that change in concentration of the reactant species in an infinitesimally small time. Hence rate of reaction = dx/dt
1) If the rate is expressed in terms of the change in concentration of any reactants, there will be decrease in concentration with time. This, rate of reaction = -dx/dt
2) if the rate is expressed in terms of the change in concenreation of any one of the products, there will be an increase in concentration with time Therefore rate of reaction - +dx/dt
Units of Rate of Reaction: moles per liter per second
Factors affecting the rate of reaction - Nature of reactants and products - Concentration of reactants and products - Temperature - Surface area of reactants - Presence of a Catalyst - Light
1) Nature of Reactants:
Reaction between nitric oxide and oxygen is faster than the reaction between carbon monoxide and oxygen under the same conditions of temperature. NO(g) + 1/2O2(g) -> NO2(g) - fast CO(g) + 1/2O2(g) -> CO2(g) - slow
This is due to the difference in the nature of the reactants, that is NO and CO.
Since the bonds are broken and new bonds are formed in a chemical reaction, it seems reasonable that the rate of reaction should depend on the types of bonds involved.
2) Effect of Concentration:
The greater the concentration of reactants, the greater the inter-molecular collisions, and the greater the rate of reaction.
ex: A candle burns normally in air (20% O2) but burns more brightly in a jar of pure oxygen (100% O2) due do the higher concentration of oxygen.
3) Effect of Temperature:
Temperature has marked effect on rate of chem reaction. In most of the cases, the rate of reaction becomes double on triple for a 10°C rise in temperature
Large increase in reaction rate is due to the increase in the collision frequency and increase in effective collisions which can bring about a chemical change.
4) Effect of Surface Area:
Powdered sugar dissolved more readily in water than a crystalline sugar, This is because in the case of powdered sugar, more of it's surface area comes in contact with water in comparison to crystalline.
Similarly, powdered charcoal burns explosively in air while large pieces of coal burn slowly.
Thus, surface area of reactants in contact is an important factor for rate consideration.
5) Effect of Catalyst:
Rate of reaction may be increased by adding a suitable catalyst.
Inhibitor hinders the catalyst action while promoters increase the activity of the catalyst.
A catalyst takes part in the reaction. It is consumed in one step and regenerated in the subsequent step.
It is believed that a catalyst somehow provides a new path to the reaction.
6) Effect of Light:
The rates of certain chemical reactions are greatly influences by the light or radiations.
Absorption of light may exercise or energize a reacting molecule.
Sometimes, the molecules are broken down into more reactive intermediates.
---
(5M) Diff thermal + photochem processes

---
(5M) Activation energy
We know that for each reaction a certain energy barrier must be surmounted.
The reactant molecules must possess the activation energy, Ea, for the reaction to occur.
The catalyst functions by providing another pathway with lower activation energy, Ecat.
Thus a much larger number of collisions becomes effective at a given temperature.
Since the rate of reaction is proportional to effective collisions, the presence of a catalyst makes the reaction go faster, while all other conditions remaining the same.
It may be noted from the diagram that although a catalyst lowers the activation energy, the energy difference, ΔE, between products and reactants remains the same.

---
2nd order reaction


---
Methods for Determination of Order of Reaction




#exam season#notes#science#send help#long post#long reads#chemistry#physical chemistry#physics#chemical kinetics#photochemistry
0 notes
Text
Zoology Sem 4 End - Mod 1
Cell regulation
https://www.tumblr.com/i-should-have-studied/771736523452399616/zoology-hw-mitosis-and-cell-regulation?source=share
---
Chromosome Structure and Types
Chromosomes are the part of cell that carry hereditary information in the form of genes
In the nucleus of each cell, the DNA molecule is packaged into compact thread-like structures called chromosomes.
Each chromosome is made up of DNA tightly coiled many times around proteins called histones
They appear as rod-shaped dark stained bodies during the metaphase stage of mitosis when cells are stained with a suitable dye like acetocarmine and viewed under microscope.
Every chromosome has two arms that are short (p arms) as well as two more long arms (q arms) and a centromere that holds the entire thing together in the center.
Humans have 23 pairs chromosomes or 46 total, half from mum and half from dada.
-
Structure of Chromosome:
Animal chromosomes typically measure 0.5-4um in length, but size carries between species.
Each chromosome typically has one centromere and 1-2 arms that project from the centromere.
Structurally, chromosome is differentiated into three regions: - Pellicle: the thin outer envelope around the substance of chromosome - Matrix: The ground substance of chromosome which contains chromonemata - Chromonemata: Embedded in matrix, two identical spirally coiled threads, so tightly coiled they appear as a single thread.
During mitotic metaphase, the following structural features are visible under microscope:
1) Centromere: The area of the chromosome where spindle fibers are connected. Also called kinetochore. It divides the chromosome into p arm and q arm.
2) Chromatid: One of two distinct longitudinal subunits that make up the chromosome. Chromatids come in two varieties: sister chromatids and non-sister chromatids.
3) Secondary Constriction: Some chromosomes have a secondary constriction along with centromere, forms a satellite.
4) Telomere: The two ends of a chromosome, very stable, do not correct to the telomeres of the other chromosomes.
5) Chromomere: The chromosomes in certain species have tiny beads-like structures known as Chromomeres.
6) Chromonema: Threadlike coils found in chromosomes and chromatids (plural term for chromonemata)
7) Matrix: The fluid element which houses chromonemata.
-
Types of Chromosomes:
A) Autosomes and Sex Chromosomes:
Human chromosomes are of 2 types, auto and sex.
Genetic traits which are linked to the sex of a person are passed through the sex chromosomes or allosomes.
The last pair of chromosomes in humans are the two sex chromosomes, XX in female and XY in male.
The rest of the genetic information is carried in autosomes.
There are 23 pairs of chromosomes total the first 22 pairs are all autosomes.
B) Based on the Number of Centromeres
- Monocentric: Has one centromere
- Dicentric: Has 2 centromeres
- Polycentric: Has more than 2 centromeres
- Acentric: No centromeres, these are freshly broken chromosomes that don’t last long.
- Diffused: Have indistinct centromeres spread across the length of the chromosome
C) Based on the Location of Centromere
- Metacentric: Centromere located in the middle of chromosome, p and q arms are equal in length.
- Submetacentric: Centromere is slightly offset from the center, leads to an asymmetry of both arms.
- Acrocentric: Centromere is very off from the center, sub-terminal in position. Results in a very long arm and an extremely short arm.
- Telocentric: Centromere located at the at the very end of the genome, terminal in position. Humans don’t have Telocentric chromosomes, present in mice.
---
5M: Plasma Membrane
Plasma Membrane is the biological membrane which separates the interior of a cell from its outside environment.




---
5M: Giant Chromosomes
Huge chromosomes, they over 100x thicker than mitotic chromosomes.
Found in certain tissues of varies plants and animals.
Easily visible under light microscope.
There are 2 types: polytene and lampbrush
-
Polytene Chromosomes:
Giant chromosomes 50-200 times larger than typical chromosomes, but relatively smaller than lampbrush chromosomes.
Found in the larvae of certain dipterans, specifically in the larval salivary glands, midgut epithelium, rectum, and malpighian tubules of general such as drosophila.
The salivary cells are so large in size that they can be seen with the lens power of a dissection microscope, nuclei of the cells are around 25µ in diameter.
Discovered by E.G. Balbiani in 1881.
Polytene chromosomes get their name from the fact that they are formed by many parallel chromatids, often more than a thousand bands which do not separate from one another following duplication.
Along each chromatid strand, some regions of chromatin are tightly coiled and other regions are less coiled, thus they appear to be dark and light bands respectively when observed under microscope.
During larval development, specific areas become uncoiled. They form localized regions called puffs. Puffs are regions of active RNA synthesis, they project out in the form of loops.
Regions which show larger puffs than others are called Balbiani rings.
Functions of Polytene Chromosomes: - primarily carries genes which ultimately control physiology of an organism. The genes are formed of DNA molecules. - Indirectly helps in protein synthesis
-
Lampbrush Chromosomes:
Largest known chromosome, can be seen with the naked eye, may reach up to 5900µ in length
Found in the yolk rich oocyte nuclei of certain vertebrates like fish, birds, amphibians, etc.
First discovered by Flemming in 1882.
Have characteristic shape of lateral loops which give it a brush-like appearance.
Consists of a longitudinal axis formed by a single DNA molecule along with several hundred bead like chromosomes which are distributed in a linear manor.
From each chromosome, there emerge two symmetrical lateral loops, one from each chromatid. They are able to expand or contract in response to various environmental conditions.
Around 5-10% of the DNA is found in the lateral loops.
Functions of Lampbrush Chromosomes: - Synthesis of RNA and proteins at the thin insertion, then carried around the loops to the thick insertion. - Formation of yolk material for egg.
---
5M: Mitosis
Mitosis is a type of cell division in which single cell divides into two daughter cells that are exactly the same as the parents cell, including the same ploidy (n->n, 2n->2n).
Mitosis occurs in somatic cells of plants and animals.
The process of mitosis consists of the following stages: - Interphase - Karyokinesis - Cytokinesis
Interphase:
Technically not a stage of mitosis, it is the phase between two successive cell divisions.
Interphase appears dormant, but it is the most metabolically active, the cell copies its DNA in preparation for mitosis.
Interphase consists of: - G1/Gap-1 phase - S/Synthesis phase - G2/Gap-2 phase.
Karyokinesis:
The division of the nucleus, consists of 4 phases: - Prophase - Metaphase - Anaphase - Telophase
Prophase: - The first visible stage of karyokinesis, also the longest. - At first the chromosomes appear as long coiled threads called chromatids. - The chromatin condenses to form shorter, thicker, and more visible chromosomes. - The centrioles separate and move to the opposite poles of the cell. - Spindle starts to form and microtubules expands in the nuclear area. - Each chromosome splits longitudinally to form 2 sister chromatids, they are attached to each other at the centromere. - The nuclear membrane and nucleolus disappears.
Metaphase: - The 2nd stage of mitosis. - Chromosomes align along the equatorial plane to form metaphasic plate. - Centrioles project spindle fibers which attach to the centromere of each chromosome.
Anaphase: - 3rd and shortest stage of mitosis. - The centromere of each chromosome splits into two sister chromatids, forms 2 daughter chromosomes. - Spindle fibers contract, pulling each daughter chromosome to the opposite poles. - During polar movements the chromosomes shows different shapes like L,U,V,J,I. - At the end of anaphase, each pole will get one set of daughter chromosomes.
Telophase: - The 4th and final stage of mitosis. - Daughter chromosomes at each pole decondense to become thin and long chromatin strands. - Spindle fibers disappear, nuclear membrane and nucleolus reappear. - 2 nuclei form with the same number of chromosomes as parents cells.
Cytokinesis:
Division of cytoplasm leads to the separation of a cell into two by mitosis end.
In plant cells, cell plate forms. In animal cells, cleavage/furrow formation starts in anaphase, continues through telophase.
---
Meiosis




1 note
·
View note
Text
Zoology Sem 4 End - Mod 2
DNA Replication with Enzymes Required
DNA replication is the process of producing two identical copies of DNA from an original DNA molecule.
It is vital for cell division, growth, and the repair of damaged tissues. It ensures that every new cell inherits an accurate copy of the genetic material.
DNA replication is semi-conservative. Each newly formed DNA molecule has one strand from the original molecule and one newly synthesized strand.
There are 4 steps of DNA Replication: - Formation of Replication Fork - Initiation - Elongation - Termination
Formation of Replication Fork - Before DNA can replicate, this double-stranded molecule must unwind into two single strands. - DNA unwinds when the complementary base pairing between the double-stranded is broken - The site to initiate unwinding is denoted by specific Adenine and Thymine rich regions. - These specific coding regions are called Origin of Replication (Ori), this is where the replication process begins by the action of initiator proteins. - Within this replication protein complex is an enzyme, DNA helicase, which starts to unwind the DNA from its Ori to expose two strands resembling a Y-like structure called replication fork. - Helicase activity causes stress to the un-winded strand forming supercoiled DNA, this stress is relieved by Topoisomerase enzyme by negative supercoiling.
Initiation - The replication fork is bidirectional - The strand which runs from 5′->3′ direction towards the replication fork is referred to as leading strand - the strand runs from 3′->5′ away from the replication fork and is referred to as lagging strands. - To this exposed single-stranded DNA, SSB proteins are adhered to prevent recoiling of DNA and to stabilize it. - Then enzyme DNA primase comes into action to synthesize a short stretch of RNA primer. - This provides a free 3′ hydroxyl group where DNA polymerase can add nucleotides and extend the new chain of nucleotides.
Elongation - Now that primer is added to unzipped two single-stranded DNA, they can act as a template for synthesizing new DNA. - The enzyme DNA polymerase synthesizes new nucleotide to match the template and add on to the free 3′ hydroxyl group. - The leading strand runs from 5′->3′, therefore the addition of nucleotides by DNA polymerase happens from 5′->3′ direction. - As the replication fork progresses the addition of nucleotide is continuous thus only requiring the primer once on leading strand. - However, lagging strands is antiparallel, runs from the 3′->5′ direction, therefore the continuous addition of nucleotides is not possible as the replication fork progresses on lagging strand - DNA polymerase cannot add complementary nucleotides to the 5′ end. Therefore, multiple primers are required. - Due to this, the DNA nucleotides synthesis from lagging strands occurs in fragments. These fragments are called Okazaki fragments. - The leading strand uses one primer synthesizes nucleotides continuously, while the lagging strand uses multiple primers and synthesizes nucleotides discontinuously.
Termination - RNA primers of both leading and lagging strands are degraded by nucleases enzyme activity of DNA polymerase - The gaps so formed are filled and sealed by the enzyme DNA ligase. - DNA polymerase also shows proofreading activity to check, remove, and replace any errors. - Eventually, the replication forks terminate at terminating recognizing sequences (ter). - The ter sequences are of around 23 base pairs which facilitate as the binding sites for TUS protein. - The ter-TUS complex arrests replication fork and terminates replication.
-
Enzymes and Proteins required
Nucleases: cleaves the phosphodiester bonds between nucleotides.
DNA Polymerases: synthesize new DNA strands by adding nucleotides to a template strand. 3 types in prokaryotes: - DNA polymerase I (repairs and processes Okazaki fragments). - DNA polymerase II (a repair polymerase). - DNA polymerase III (primary polymerase for DNA replication).
DNA Ligase: bonds DNA strands by catalyzing the formation phosphodiester bonds between nucleotides, requires ATP.
DNA Helicase: unwind double-stranded DNA by breaking hydrogen bonds, forms replication fork, requires ATP.
DNA Primase: synthesizes short RNA primers, providing the necessary 3′ hydroxyl group for DNA polymerases to begin nucleotide addition.
DNA Topoisomerase: relieve torsional strain during DNA replication by creating transient nicks in the DNA backbone.
Single Strand Binding Proteins (SSBPs): binds to single-stranded DNA, stabilizes it and prevents the strands from re-annealing or degrading.
---
Genetic Code
The genetic code is the set of rules by which a linear sequence of nucleotides specifies the linear sequence of a polypeptide.
That is, the specify how the nucleotide sequence of an mRNA is translated into the amino acid sequence of a polypeptide.
There are 7 important properties of genetic code: Triplet, Universal, Comma-less, Non-overlapping, Non-ambiguous, Degenerate, Has polarity.
1) The Code is Triplet: - In a triplet code, 3 RNA bases code for one amino acid. - A triplet code has 64 codons which is enough to code for 20 amino acids along with start and stop signals. - 4x4x4=64 or 4^3=64 - Ex: AUG, UAA, UAG, UGA
2) The code is Universal: - Universality of the code means that the same sequence of 3 bases encode the same amino acid in all forms of life. - The same genetic code applies to all modern organisms with only minor exceptions such as yeast, mitochondria, and mycoplasma.
3) The code is Comma-less: - Genetic code is continuous and comma-less, there is no signal to indicate the end of one codon and the beginning of the next. - The deletion of a single base in a code alters the entire sequence of amino acids after the point of deletion. - ex: ATG CAT (G)GT AAA TAC -> ATG CAT GTA AAT AC_
4) The code is Non-Overlapping: - Adjacent codons do not overlap, this means that no base can take part in the formation of more than one codon. - Each individual base is only read once. - In non-overlapping code, 3 bases code for one amino acid and 6 bases code for 2 amino acids. - Theoretically, in overlapping code, 6 bases may code for 4 amino acids. - ex: picture.
5) The code is Non-Ambiguous: - There is no ambiguity about a particular codon - Each particular codon will always code for the same amino acid - The same codon can never code for 2 or more different amino acids. - Ex: AUG will always code for methionine (Met)
6) The code is Degenerate: - The occurrence of more than one codon for a single amino acid. - Any particular amino acids can be coded for the polypeptide chain by more than one base triplet. - Degeneracy provides protection against harmful mutations, If one codon is mutated, there are other codons which can code for the same amino acid. - Ex: Leucine has 6 synonymous codons while methionine only has one.
7) The code has Polarity: - The code is always read in a fixed direction: 5'->3'. - If the code were to be read in the opposite direction (3'->5'), it would specify different amino acids since the codon would have reversed base sequence. - Ex: AAC CUA GCC UUA ≠ AUU CCG AUC CAA
---
DNA and RNA Structures, polymorphism of DNA
DNA Structure
- DNA is made up of nucleotides, each nucleotide is made up of a pentose sugar back bone (deoxyribose), a nitrogenous base (A,C,G,T) attached to the 1' end, and a phosphate group attached to the 5' end of one sugar and the 3' end of adjacent sugar ring through a phosphodiester bond.
Therefore DNA is a polynucleotide structure.
DNA is made up of 2 helical chains that intertwine with each other to form a double helix.
The helical chains run anti-parallel to each other, one polynucleotide runs from 5'->3' while the other runs from 3'->5'. The chains are connected to each other via nitrogenous bases through hydrogen bonds.
Hydrogen bonding contributes to the specificity of base paring. Adenine pairs with Thymine through double bonds (A=T) and Cytosine pairs with Guanine through triple bonds (C≡G).
The base pairs A=T and G≡C are complementary base pairs, therefore the amount of A is equal to the amount of T and the same for C and G.
The geometry of the helix is stabilized by the hydrophobic and hydrogen bonding interactions between nitrogenous bases.
The double-helical structure of DNA is highly regular, each turn of the helix measures 10 base pairs.
The distance between each turn is 3.4 nm
The diameter of double helix is 2nm
The regularity of the helical structure forms two repeating and alternating spaces: major and minor grooves.
The major groove occurs when the backbones are far apart from each other and the minor groove occurs when they are close.
These grooves act on base-pair recognition and binding sites for protein, the major groove contains base pair specific info while the minor groove is largely base-pair nonspecific, caused by protein interaction in the grooves.
The major groove is 2.2nm wide and the minor groove is 1.1nm wide.
-
RNA Structure
RNA is a typical single-stranded bipolymer of ribonucleotides bonded with each other via a phosphodiester bond.
Each RNA strand is synthesized in the 5'->3' direction from a locally single-stranded region of DNA.
It has ribose sugars which are attracted to 4 bases: A,C,G,U.
The uracil base in RNA replaces the thymine base in DNA.
Overall, RNA is composed of a ribose sugar, phosphate, and nitrogenous base.
Ribose sugar in RNA has an extra OH-group at the 2' carbon in comparison to the deoxyribose sugar of DNA.
The extra OH-group in DNA has led them to be synthesized for short term functions.
The 3d structure of RNA is critical to its stability and function.
RNA being a single stranded molecule can form a complex structure by allowing its ribose sugars and bases to be modified on the action of cellular enzymes (which attach to the chemical groups) to perform different functions.
RNA is capable of folding onto themselves and showing intramolecular hydrogen bonding between complementary strands, making a double stranded molecule to exhibit specific functions.
-
DNA Types
Based on location there are 2 types:
Nuclear DNA - Located within the nucleus of eukaryote cells. - Usually has two copies per cell. - The structure of nuclear DNA chromosomes is linear with open ends - Includes 46 chromosomes containing 3 billion nucleotides. - Nuclear DNA is diploid, ordinarily inherited from both parents. - The mutation rate for nuclear DNA is less than 0.3%
Mitochondial DNA - located in the mitochondria. - Contains 100-1,000 copies per cell. - Small and circular structures - Has 16500 base pairs, encodes proteins specific for mitochondria. - Haploid, comes only from the mother. - The mutation rate for mitochondrial DNA is higher than nuclear DNA
Based on forms, there are 3 main types:
A-Form: - Confirmation of the deoxyribose sugar ring: in the C3′ endoconformation in A-form. - Placement of base-pairs within the duplex: displaced away from the central axis and closer to the major groove. - Ribbon-like helix with a more open cylindrical core. - Right-handed double helix - 11 bp per turn; 0.26 nm axial rise; 28o helix pitch; 20o base-pair tilt - 33o twist angle; 2.3nm helix diameter - Narrow and deep major groove and wide and shallow minor groove. - This form of DNA is favored by low hydration
B-Form: - The standard structure of DNA, described by Watson and Crick. - a right-handed double helix. - The two strands of the DNA molecule are plectonemic coil meaning that these two strands are coiled around the same axis and are intertwined with each other. - The distance between the base pairs is 0.34 nm. One turn of the helix contains 10 base pairs with a length of 3.4 nm. - This form of DNA is 2nm in diameter. The wide and shallow major groove of 2.2 nm, and narrow and minor groove of 1.1 nm. - B-DNA is narrower than A-DNA.
Z-Form: - Left-handed helix - The backbone is not a smooth helix but an irregular zig-zag, which is resulted from alternating sequences of purines and pyrimidines. - The B form DNA can take the Z form when proteins are bound to DNA in one helical conformation and force the DNA to adopt a different conformation. - Longer and thinner than the B and A forms. - The helical width is 1.8 nm, being the smallest. - The distance between the base pairs is 0.37 nm. One turn of the helix contains 12 base pairs with a length of 4.56 nm. - The major groove is flat and the minor groove is narrow and deep. - Plays an important role in protection against viral diseases
There are also less common types such as C-Form, D-Form, E-Form.
---
PCR
PCR stands for polymerase chain reaction.
It is a laboratory technique in which a specific region of DNA is replicated over and over in order to yield many copies of a particular sequence.
It is an enzymatic method carried out in vitro.
DNA amplified by PCR can be sent for sequencing, visualized by gel electrophoresis, or cloned into a plasmid for further experiments.
-
Requirements
The key ingredients for a PCR are template DNA, primers, Taq polymerase, and nucleotides.
These ingredients along with cofactors of enzyme and water as buffer undergo repeated cycles of heating and cooling to allow DNA synthesis.
-
Taq Polymerase
PCR requires a DNA polymerase enzyme which can make new strands of DNA using pre-existing strands as templates.
The first and most commonly used polymerase is the Taq polymerase from the thermophilic bacterium, Thermus aquaticus.
T. aquaticus lives in hot springs and hydrothermal vents.
Its DNA polymerase is heat-stable and most active around 70°C, a temp at which human and E.coli DNA polymerase would be nonfunctional.
Taq polymerase's heat-stability makes it ideal for PCR.
-
PCR Primers
Taq polymerase can only make DNA in the presence of a primer, a short sequence of nucleotides (~20) which gives a starting point for DNA synthesis.
PCR needs two primers, a forward one reverse primer, they are designed such that they flank the target region.
Primers bind to the template with complementary base paring.
-
Procedure
There are 3 steps,
1) Denaturation - The DNA template is heated to 95-96°C - This denatures the hydrogen bonds that hold DNA strands together, allowing the strands to separate to form single stranded DNA.
2) Annealing: - The mixture is cooled to 50-60°C - This allows the primers to bind or anneal to their complementary sequence in the template DNA.
3) Extension: - The temperature is raised to 72°C - This allows the Taq polymerase to extend the primers synthesizing new strands of DNA.
The cycle repeats 25-30 times in a typical PCR reaction, which generally takes 22-24 hours, depending on the length of the DNA.
If efficient, the target region can go from one copy to billions.
Since there are many copies of primers and Taq polymerase, the no. of DNA molecules can roughly double each cycle.
-
Applications:
DNA fingerprinting
Paternity testing
Criminal identification
Identification of microorganisms.
Fossil study
Gene cloning
Gene sequencing
Vaccine production
Drug discovery
Mutation study
Human Genome Project
---
Electrophoresis
Electrophoresis is the migration of charged particles under the influence of an electric field.
Various essential biological molecules like amino acids and proteins have ionizable group, which at a given pH exist in solution as electrically charged cations (+ve) and anions (-ve).
Cations and anions are separated by electrophoresis under the influence of electric field. The charged particles migrates either to cathode or anode depending on their net charge.
-
Principle:
When a potential difference is applied, the molecules with different net charge will begin to separate according to their different electrophoretic mobility.
Even the molecules with similar charge will begins to separate if they have different molecular sizes.
Therefore, some forms of electrophoresis rely on the different charges on the molecules for separation while some other form exploits difference in molecular size.
Electrophoresis is considered an incomplete form of electrolysis since the electric field is removed before the molecules in the samples reaches the electrode.
The separated samples are then located by staining with an appropriate dye like ethidium bromide.
-
Agarose Gel Electrophoresis
There are many types of electrophoresis, but one of the most important types is agarose gel electrophoresis.
Widely used technique in molecular biology and biochemistry for the separation and analysis of nucleic acids and proteins.
It is particularly suitable for the separation of linear nucleic acid fragments, such as DNA.
Agarose is a natural linear polysaccharide derived from red seaweed agar.
To prepare an agarose gel, a casting tray with a comb is used to create slots or wells where the samples will be loaded.
In gel electrophoresis, the gel serves as the medium through which the separation of molecules takes place. The gel forms a matrix with small pores.
Negatively charged nucleic acid molecules(DNA and RNA) migrate through the gel with shorter molecules moving faster and farther due to a sieving effect where they have easier passage through the gel pores.
Proteins are separated primarily based on their charge in agarose gels since the pores of the gel are too small to effectively sieve proteins.
-
Applications
DNA sequencing
Estimation of the size of DNA molecules
Analysis of PCR products
DNA fingerprinting
Pathogen detection
Vaccine development
Medical research
Protein research
Agricultural testing
---
5M: t-RNA
Transfer RNA or t-RNA is a type of RNA which helps to decode information present in mRNA sequences into specific proteins.
It is enclosed by DNA in the cell nucleus and transcribed with the help of RNA polymerase III.
The structure of tRNA folds upon itself and creates an intra complementary base paring which gives rise to hydrogen-bonded stems and associated loops that contains nucleotides with modified bases.
The structure in 2d resembles a cloverleaf with three loops and an open end. It is usually 79-80 ribonucleotides in length.
Each of these loops consisting of arms has a distinct name and function. The 3 loop consisting arms are namely: - DHU or D arm, which has recognition side for specific enzyme amino-acyl tRNA synthetase - T arm that consists of ribosome recognition site, and - Anticodon arm that recognizes and binds to mRNA present in ribosome.
The open end with no loop is the site for attachment of amino acid, via 3' OH bonding with COOH group of the amino acid.
In general, tRNA reads the code on the mRNA sequence in ribosome and translates specific amino acid, it does so along the length of the mRNA and gives out a polypeptide chain of amino acids (proteins) in association with other important enzymes like aminoacyl tRNA synthetase and peptidyl transferase.
1 note
·
View note
Text
Zoology Sem 4 End - Mod 3
Law of Segregation
Mendel’s Law of Segregation explains how the alleles responsible for a specific trait separate during the formation of gametes and how they are passed onto the offspring.
Each individual possesses two alleles for a particular trait, one inherited from each parent. During gamete formation, the alleles separate from each other, so that each gamete carries only one allele for each trait.
Since each gamete carries only one allele for a trait, they are considered pure for that particular characteristic.
This law is significant as it introduced the concept of hereditary factors that remain as separate entities even when present together with other similar entities.
The law was used to disprove the blending traits theory encoded by recessive alleles in the F1 generation.
This law enables the use of Punnett squares for the estimation of resulting genotypes from a cross as it is based on the equal segregation of alleles.
- pic
This law is based on the first phase of the meiosis, where the homologous chromosomes with two copies of the same gene are segregated into individual daughter nuclei.
The division of homologous chromosomes during meiosis can account for the segregation of the alleles at the gene locus to form different gametes.
-
Example of Mendel's Law of Segregation
Monohybrid cross between tall and drawf pea plants.
Assume the tall homozygous pea plant parent has the alleles TT and the short plant parent has tt genotype.
The parent with TT genotype produces gametes with a single T allele while the parent with tt genotype produces singular t gametes.
As a gamete can only have a single chromosome of a homologous pair, each gamete carries one allele.
During the cross, the gametes fuse together to form a heterozygous plant with Tt genotype with both dominant and recessive alleles.
As a result of dominance, the dom T allele expresses itself in the hybrid of the first gen while the rec t allele remains unexpressed.
In heterozygous individuals, both the alleles remain together but do not interfere or affect each other.
diagram ig
---
Inborn Errors of Metablolism
Inborn errors of metabolism are genetic diseases which effect the metabolic pathways.
Also called inherited metabolic disorders.
They involve the failure of break-down or storage of carbohydrates, fatty acids, and proteins due to an enzyme defect.
These diseases are rare, occur in 1/2500 births.
The cause of the majority of these are due to defects in single genes which code for enzymes that facilitate conversion of various substrates into products.
Due to decreased enzyme activity, these disorders cause specific compounds to accumulate in the body to toxic levels.
These metabolic disturbances, if untreated, may result in cognitive impairment, organ failure and ultimately death.
They can being to present at any age.
Awareness of these diseases, their presentations, and their evaluation is critical for their management.
Many IEM’s are screened for at birth via the Heel Prick Test, newborn screening allows early identification and initiation of treatment, improving patient outcomes
-
One Gene-One Enzyme Hypothesis:
Originally hypothesized by Beadle and Tatum
Principle: - All biochemical processes in all organisms are under genetic control. -The overall biochemical process are resolved into a series of individual step-wise reactions. - Each single reaction is controlled by a single gene. In every case a 1:1 correspondence of gene and biochemical reaction exists - Mutation of a single gene results only in an alternation in the ability of the cell to carry out a single primary chemical reaction. - The underlying hypothesis is that each gene controls the reproduction, function, and specificity of a particular enzyme:

ex: Albinism
It is a rare autosomal recessive genetic condition caused due to mutation in the tyrosinase gene (TYR)
TYR is on chromosome 11q14 There is a complete lack of tyrosine activity characterized by hypopigmentation at birth
Melanin controls skin, eyes, and hair pigmentation.
People with albinism have extremely pale skin, hair and also affects the vision.
The TYR gene product, tyrosinase, normally hydroxylates tyrosine to DOPA and oxidizes DOPA to melanin. Loss of this function leads to an inability to synthesize melanin.
People with albinism should avoid prolonged UV light exposure.
Corneal lenses should have UV protection. Bifocals and low vision aids may be considered in older children and adults.
---
Monohybrid and Dihybrid Cross
Monohybrid Cross
A monohybrid cross is a type of genetic cross between two individuals who differ in only one trait.
It is the study of inheritance patterns for a singular trait, with a focus on a single gene with two opposing alleles.
Two heterozygous individuals for a specific trait are crossed. One allele is inherited from each parent, the progeny are observed to determine the inheritance and expression of that specific trait.
In monohybrid crosses, the dominant allele is expressed in the phenotype while the recessive allele is only expressed in the absence of the dominant allele. This allows us to identify which allele is dom over the other.
Monohybrid crosses follow the principles of Mendelian inheritance, established by Gregor Mendel.
Monohybrid crosses are often illustrated using a Punnett square, an illustration which helps to predict the ratio of phenotypes and genotypes in offspring.
-
Ex of Monohybrid Cross: Mendel's Peas Experiment
George Mendel used the monohybrid cross to determine the dominant and recessive traits in the case of peas.
An example of one of the experiments is the height of the plant. Some plants were taller while the others are shorter.
The homozygous genotype for the tall pea plant is TT, and the homozygous genotype for the dwarf pea plant is tt.
A monohybrid cross between the two plants resulted in the production of heterozygous genotype Tt in the F1 generation.
The phenotype of the F1 generation plants was tall, indicating that the tall allele is dominant over the short allele.
Then, the F1 gen plants were self crossed to produce F2 generation where a consistent ratio of tall:dwarf plants was observed.
Around 75% of the plants displayed the tall phenotype, while the remaining 25% exhibited the short phenotype.
Phenotypic Ratio: 3:1
Genotypic Ratio: 1:2:1
cross
-
Dihybrid Cross
A dihybrid cross is a type of genetic cross between two individuals who differ in two traits simultaneously.
More complex than monohybrid crosses, involves two genetic traits where the parents can be either homozygous or heterozygous.
The number of gametes and offspring formed during a dihybrid cross is more than those during a monohybrid cross. These have more phenotypic variation than the ones obtained from monohybrid crosses.
Dihybrid crosses follow the principles of Mendelian inheritance, established by Gregor Mendel, specifically it is a good example of the law of independent assortment.
But it only works in the case of genes that are not linked and are present on different chromosomes.
Dihybrid crosses are often illustrated with a Punnett square with four rows and four columns. It helps to predict the genotypes and phenotypes of the offspring
draw
-
Ex of Dihybrid Cross: Mandel's Pea Experiments again
Cross between homozygous pea plant with round yellow seeds and homo plant with wrinkled green seeds.
The round yellow seeds are represented by RRYY genotype, the wrinkled green seeds are represented by rryy.
The gametes formed from these alleles are RY and ry respectively.
On crossing, F1 forms with yellow round seeds and RrYy genotype.
The four alleles can combine into four different combinations: RY, Ry, rY, and ry.
The four alleles are assorted randomly to produce four types of gametes.
The gametes unit at random during fertilization to produce sixteen types of individuals in the F2 generation, the ratio being 9 round yellow, 3 round green, 3 wrinkled yellow, and 1 wrinkled green.
Phenotypic Ratio: 9:3:3:1
Genotypic Ratio: 1:2:1:2:4:2:1:2:1
cross
---
Linkage and Crossing Over
Linkage is the phenomenon of genes staying together throughout inheritance through several generations without any change or separation due to their presence on same chromosomes.
It refers to genes that are located on the same chromosome.
Linked genes do not show independent assortment.
Unlinked genes show independent assortment
Linkage involves at least 2 genes which are found on the same chromosomes in a linear fashion.
It reduces variability.
It usually involves those genes which are located close to each other.
The strength of linkage depends on the distance between the linked gene, lesser the distance greater the strength of linkage.
The types of linkage can be classified on the bases of: - Crossing over - Chromosome involved - Gene involved.
On the Basis of Crossing Over:
Complete Linkage - If at least two traits are inherited together and consistently appear in two or more generations in their original or parental combinations - These genes do not produce non-parental combinations. Genes showing complete linkage are closely located on the same chromosome. - It is rare but has been reported in Drosophila male.
Incomplete Linkage - Exhibited by those genes which produce some percentage of non-parental combinations. - Such genes are located distantly on the chromosome. - It is due to accidental or occasional breakage of chromosomal segments during crossing over. - Incomplete linkage has been observed in peas.
On the Basis of Chromosomes Involved:
Autosomal linkage - Linkage of genes on autosomes
Allosomal linkage - Linkage of genes on sex chromosomes
On the Basis of Genes Involved:
Depending on whether all dominant or some dominant and recessive alleles are linked together
Coupling Phase - when genes come from the same parent, they enter the same gamete and inherited together. - Dominant alleles and recessive alleles present on the same chromosomes shows coupling phase
Repulsion Phase - when genes are inherited separately they come from different parents and enter different gametes. - Dominant alleles of same genes are linked with recessive alleles of other genes on same chromosomes shows repulsion phase
-
Crossing over is the exchange of genetic material, leading to genetic recombination
It occurs between non-sister chromatids. One chromatid from each of the two homologues chromosomes is involved in crossing over.
Leads to re-combinations or new combinations between linked genes.
Crossing over of two genes generally yields two recombinant types or crossover types and two parental types or non-crossover types.
Generally leads to exchange of equal segments or genes and recombination is always reciprocal.
It begins at pachytene stage of prophase-I of meiosis
There are 2 ways to classify crossing over: - Based on Cell Type - Based on Number of Chiasmata
Based on Cell Type:
Somatic or Mitotic Crossing over - Occurs in the chromosomes of somatic cells during mitosis. - Relatively rare, does not have significant genetic implications.
Germinal or Meiotic Crossing over - occurs in germinal cells during meiosis, specifically during the formation of gametes - Universal, plays a crucial role in genetic diversity. - Involves the exchange of genetic material between homologous chromosomes - Essential for proper chromosome segregation and genetic variation.
Based on Number of Chiasmata:
These are all types of equal crossing over in germinal cells
Single Cross Over - Formation of a single chiasma - genetic material is exchanged between two chromatids out of the four present in a tetrad. - Relatively straightforward genetic recombination
Double Cross Over - Formation of two chiasmata - Can involve two, three, or all four chromatids. - Results in a more complex recombination of genetic material - Leads to a higher variability in the resultant chromatids
Multiple Cross Over - Formation of more than two chiasmata - Relatively rare, occurs less frequently than single and double cross overs. - Multiple cross overs can involve extensive genetic recombination.
---
Mendel's Laws of Inheritance
There are 3 laws of inheritance: -Law of Dominance -Law of Segregation -Law of Independent Assortment
1) Law of Dominance
When there are 2 alternate forms or alleles of a particular trait present in an organism, one allele will be dominant and the other recessive.
In heterozygous genotypes, only the dominant allele is expressed, while the recessive allele remains masked away.
This law explains how the traits of the parents are expressed in the offspring during a monohybrid cross.
ex: When crossing a homozygous tall pea plant (TT) with a short pea plant (tt), a heterozygous tall pea plant is observed (Tt). Despite the presence of a t allele, only the tall trait is shown.
cross
-
2) Law of Segregation
Also called the law of purity of gametes.
This law explains how the alleles responsible for a specific trait separates during the formation of gametes and how they are passed onto the offspring.
Each individual possess two alleles for a particular trait, one inherited from each parent. During gamete formation, the alleles separate from each other, so that each gamete carries only one allele for each trait.
Since each gamete carries only one allele for a trait, they are considered pure for that particular characteristic.
ex: During the same monohybrid cross between tall and dwarf pea plants, the gametes T and t are retained throughout the crosses
cross
-
3) Law of Independent Assortment
Alleles for different traits separate and are inherited independently during the formation of gametes.
The alleles for one trait is not linked or influenced by the alleles for other traits.
ex: Mendel's dihybrid cross (round yellow + wrinkly green)
cross
---
Chromosomal Mutations
Chromosomal mutation refers to change in the structure or number of chromosomes within a cell, impacting genetic information and potentially leading to various genetic disorders.
Chromosomal mutations often arise from mistakes during cell division, crossing over, mutagen exposure, and even simply inherited from previous generations.
They can be detected by microscopic examinations or genetic analysis.
Chromosomal mutations may be categorized as Chromosomal Mutations I and II.
-
Chromosomal Mutations I:
Structural mutations which arise as a result of alterations in the structure of the chromosomes.
Structural mutations are further divided into 4 different types - Inversion - Deletion - Duplication - Translocation
Inversion: - occurs when a chromosome segment breaks off, rotates 180 degrees, and reattaches. - This reverses the order of the genes within that segment. - There is no net loss or gain of genes, simply a rearrangement of the sequence. - This structural rearrangement can disrupt gene function and may lead to genetic disorders or affect the chromosome’s ability to pair properly during meiosis. - ex: Inversion of chromosome 12 created the tallest teenager in the world at 7ft 9in.
Deletion - involves the loss of a chromosome segment due to breakage. - results in the absence of certain genes. - can lead to a deficiency in genetic material, which may cause developmental abnormalities or genetic diseases if crucial genes are missing. - chromosomes that have undergone deletion cannot revert back to normal and, if transmitted to the next generation, can be hereditary. - ex: Deletion of the short arm of chromosome 5 in humans results in ‘cri du chat’ syndrome where babies cry like cats.
Duplication/Amplification - occurs when a chromosome segment is copied and inserted into the genome - results in multiple copies of that segment - An increase in gene dosage can disrupt normal cellular function, may contribute to cancer or other genetic disorders - ex: duplication of a segment of the X-chromosome, called section 16A, in Drosophila. Codes for bar traits like narrower, oblong, bar-shaped eye with a few facets.
Translocation - involves the exchange of chromosome segments between nonhomologous chromosomes. - No net gain or loss of chromosomes or genes during translocation but a rearrangement - The rearrangement can create novel gene combinations and may result in genetic disorders or increased susceptibility to cancer due to disrupted gene function.
The exchange of chromosome sections generates new linkages with possible new phenotypes.
ex: translocation of chromosome 21 onto the 14th chromosome causes down syndrome.
-
Chromosomal Mutations II:
Includes mutations that are caused by alterations in the number of chromosomes in a cell.
Change in the no of whole chromosomes is called heteroploidy.
It produces phenotypic changes, modifications of phenotypic ratios, and alteration of linkage groups.
Heteroploidy can be further classified into: - Aneuploidy - Polyploidy
Aneuploidy - characterized by an abnormal number of chromosomes in a cell, either through an excess or deficit of chromosomes. - Aneuploidy resulting from the loss of chromosomes is called hypoploidy - Resulting from the addition of chromosomes is called hyperploidy. - Hypoploidy occurs due to the loss of a single chromosome (monosomy) or a pair of chromosomes (nullisomy). - Hyperploidy, might involve the addition of a single chromosome (trisomy) or the addition of a pair of chromosomes (tetrasomy). - Aneuploids are caused by a result of nondisjunction during mitosis or meiosis. - Aneuploidy in animals causes some genetic imbalance leading to higher mortality or reduced fertility. - ex: Down's syndrome, trisomic condition of chromosome no 21.
Polyploidy - The presence of more than two complete sets of chromosomes in a cell. - Polyploidy includes different combinations like triploid (3n), tetraploid (4n), pentaploid (5n), hexaploid (6n), and octoploid (8n). - Polyploidy higher than tetraploid is not common in natural environments, but can be observed in some plants - Polyploidy can be further divided into autopolyploids and allopolyploids. - Autopolyploids have the same basic set of chromosomes but multiplied to form multiple sets. - Allopolyploids result from the doubling of chromosome number in a hybrid from two different species. - ex: Doob grass, triploid and sterile, propagates vegetatively.
---
5M: Sex-Linked Inheritance
The inheritance of a trait that is determined by a gene located on one of the sex chromosomes.
The genes which occur exclusively on the X chromosome are called X-linked genes.
The genes which exclusively occur in Y chromosome are called Y-linked or holandric genes.
Genes occurring only in the X chromosomes are represented twice in female, because females have XX genotyping and only once in male because of the XY genotyping.
In males, the differential region of each chromosome has genes with no counterparts on the other kind of sex chromosome. These genes, whether dominant or recessive, show their effects in the male phenotype. Such genes in the differential regions are called hemizygous in males.
-
Inheritance of X-Linked Recessive Genes
In X-linked recessive inheritance, the genetic trait is linked to the X chromosome and recessive.
Since males have only one X chromosome inherited from their mother, any recessive gene present on it will be expressed, since there is no second X chromosome to mask the effect.
Since females have two X chromosomes, they must carry the affected allele on both their chromosomes in order to express the recessive trait.
Women can carry a defective gene without showing symptoms if the other X chromosome has a normal copy of the gene. This makes these women carriers of X-linked recessive traits.
Therefore, X-linked recessive disorders are more frequently observed in men than in women.
An example of an X-linked recessive disorder is red-green color blindness.
-
Inheritance of X-Linked Dominant Genes
In X-linked dominant inheritance, the genetic trait is linked to the X chromosome and is dominant.
X-linked dominant disorders are less common compared to X-linked recessive disorders.
The presence of just one copy of the dominant gene on the X chromosome is enough to express the trait in both males and females.
However, the phenotypic expression is typically less severe in females compared to males due to the compensatory effects of the second X chromosome
Women can receive the trait from either papa or mother while men can only receive it from mommy
ex: Vitamin D-resistant rickets
-
Inheritance of Y-Linked Genes
Also called Holandric Inheritance
Less common since the Y chromosome contains far fewer genes than the X chromosome.
Y-linked genes are passed directly from father to son and are responsible for male-specific traits
The most notable gene on the Y chromosome is the SRY gene, which plays a pivotal role in determining male sex by initiating the development of male reproductive organs.
ex: Excessive development of hair on ear
---
Non-Mendelian Laws of Inheritance



2 notes
·
View notes
Text
Zoology Sem 4 End - Mod 4
Fertilization
Fertilization is the process of fusion of sperm and ovum to form a zygote.
Sperm is the haploid male gamete and ovum is the diploid female gamete.
In humans and other mammals, fertilization is internal. Usually takes place in the ampulla of fallopian tube.
Fertilization is external in the case of frogs.
Male discharges semen into the vagina of the female during sexual intercourse, from the vagina, sperms reach the ampulla by the movement of their tails.
Sperm can survive in the female’s reproductive tract for 1-3 days, it can fertilize the ovum in the 12-24 hours following ovulation.
During sexual intercourse, nearly 300 million sperms are introduced into the vagina, but only a few hundred of them reach near the ovum.
The gametes have proteins on their surfaces, many reactions occur on the surfaces of the gametes to ensure each ovum can only be fertilized by one sperm.
The sperm must penetrate the outer layers of the egg, the corona radiata and the zona pellucida. This is facilitated by the enzymes released from the acrosome of sperm head.
Once a sperm enters the egg, the membrane of the egg changes to prevent other sperm from entering and ensuring diploidy.
The entry of sperm stimulates the second meiotic division of oocyte to form ovum
The genetic material from both the sperm and egg combine, resulting in a zygote with a full set of chromosomes.
The zygote undergoes rapid cell division to being the process of embryonic development.
Both gametogenesis and fertilization ensure genetic diversity and the continuation of life through successive generations.

---
Types and Functions of Placenta in Mammals
Placenta is a temporary organ which establishes a connection between the fetus and mother during pregnancy.
It supports the growth and development of the fetus by providing oxygen and essential nutrients and expels waste products from it's bloodstream.
There are different types of placenta based on various criteria: A) Based on the Involvement of Embryonic Tissue B) Based on the Relationship of Villi with the Uterine Wall C) Based on the Distribution of Villi D) Based on the Degree of Involvement of Fetal and Maternal Tissues
Depending on the involvement of embryonic tissue: - Yolk-Sac Placenta: Highly vascular yolk sac fuses with the chorion ex: Marsupials. - Chorio-Allantoic Placenta: Allantois with its blood vessels fuses with the chorion ex: Eutherian mammals
Depending on the relation between villi and uterine wall: - Non-Deciduate Placenta: Implantation superficial Fetal chorionic epithelium is in loose contact with the uterine epithelium At the time of birth the fetal villi are drawn out completely without tearing or causing injury to the uterine wall, no bleeding occurs ex: Pig, Horse, Cattle - Deciduate Placenta: Intimate implantation Uterine wall become eroded so that the fetal chorionic epithelium may come to lie either in the connective tissue or into the maternal blood. At the time of delivery, there is more or less extensive bleeding and tearing of uterine wall tissue. ex: Man, Dog, Cat - Contra-Deciduate Placenta: Intimate implantation Both fetal and maternal tissue are absorbed in-situ by maternal leucocytes. ex: Mole
Depending on the Distribution of Villi: - Diffused Placenta: Villi evenly dispersed over the surface of chorion, ex: Pig - Cotyledonary Placenta: Villi cluster in large isolated patches, ex: Sheep - Zonary Placenta: Villi arranged in definite band or girdle encircling the middle of chorion, ex: Carnivores like dogs - Discoidal Placenta: Villi are limited to 1-2 discoidal patches, ex: humans
Depending on the Degree of Fetal and Maternal Tissue Involvement: - Epitheliochorial Placenta: The uterine epithelium and the embryo’s chorion remain in simple apposition 6 membrane barriers between fetal and maternal blood streams ex: pig - Syndesmochorial Placenta: The uterine epithelium disappears chorion makes direct contact with either the glandular epithelium or the endometrium 5 membrane barriers ex: sheep and cattle - Vasochorial or Endotheliochorial Placenta: Endometrium and glandular epithelium both vanish Chorion directly contacts the uterine capillary endothelium 4 layers ex: cat and dog - Haemochorial Placenta: Endometrium, glandular epithelium, and endothelium of the capillaries disappear Chorion is bathed in maternal blood 3 layers ex: Humans - Haemoendothelial Placenta: Like haemochorial placenta, the maternal blood capillaries all vanish. But the outer later of the blastocyst also disappears. The fetal endothelium separates the maternal and fetal circulating bloodstreams 2 layers ex: Rats
Function of Placenta:
1) Nutrition: Food materials pass from the mother's blood into fetal blood through the placenta.
2) Digestion: Trophoblasts of the placenta digest proteins before passing them into fetal blood.
3) Respiration: Through the placenta oxygen passes from the maternal blood to the fetal blood, and carbon dioxide passes from fetal blood to maternal blood.
4) Excretion: Nitrogenous wastes such as urea pass from fetal blood into maternal blood through placenta and are filtered out by the kidneys of the mother
5) Storage: Placenta stores glycogen, fat etc. for the fetus before the liver is formed.
6) Barrier: Placenta functions as an efficient barrier which allows useful aerials to pass into fetal blood. But, harmful substances such as nicotine and heroin can pass through placenta. Therefore, pregnant women must avoid cigarettes and drugs. Viruses and bacteria can pass through placenta.
7) Endocrine Gland: Placenta secretes hormones such as oestrogen, progesterone and human chorionic gonadotropin (HCG).
---
Gametogenesis and Spermatogenesis
Gametogenesis is the process of creating gametes, it is the first phase in the sexual reproduction of animals.
Gametogenesis involves a combination of mitotic and meiotic divisions. Mitotic divisions allow the germinal cells to proliferate, while meiotic divisions result in haploidy.
In males, gametogenesis is known as spermatogenesis. It occurs in the testes and leads to the production of sperm.
In females, gametogenesis is called oogenesis. It takes place in the ovaries and results in the formation of ova
-
Spermatogenesis takes place in the male gonads or testis.
Human testes are formed of many seminiferous tubules bordered by germinal epithelial cells and Sertoli cells between them.
Sperms are produced from the cells of the germinal epithelium while Sertoli cells offer nutrients to sperm and anchor the differentiating cells.
Spermatogenesis is a continuous process that can be studied in two distinct stages: Spermatid formation and spermiogenesis.

Spermatid Formation
The germinal cells which dev into spermatids are called primordial germ cells. They undergo the following:
1) Multiplication Phase: - Primordial germ cells are larger and have distinct chromatin-rich nuclei. - They undergo repeated mitotic divisions to produce sperm mother cells or spermatogonia. - Spermatogonia are diploid cells.
2) Growth Phase: - The spermatogonia cells grow in volume, they amass an abundance of nutrients and chromatin. - At this stage, spermatogonia cells are called primary spermatocytes. - Still diploid cells.
3) Maturation Phase: - Primary spermatocytes undergo the first meiotic division to produce secondary spermatocytes. - Secondary spermatocytes are haploid - Each secondary spermatocyte undergoes the second meiotic division to produce two spermatids - Therefore 4 haploid spermatids are formed from one primary spermatocyte. - Since spermatids cannot directly function as gametes, they undergo spermiogenesis.
Spermiogenesis is the transformation of spermatids into spermatozoa.
During spermiogenesis, the following transformations occur: - The large spherical nucleus becomes smaller by losing water and usually changes its shape into elongated structure. - The Golgi bodies condense into a cap called acrosome in front of the nucleus - Nucleus and the acrosome combinedly form the head of the developing sperm while the cytoplasm with mitochondria and centrioles move downwards and form the cylindrical middle piece behind the head. - The two centrioles of middle piece develop axial filaments which are bunched into a single thread and extend behind in the form of a long vibratile tail. Thus, spermatid is transformed into a motile sperm divisible into head, middle piece and tail.

Since spermatozoa are mobile and extremely active, the excess material of developing sperms is eliminated in order to maximize mobility.
---
5M: Fetal membrane in chick embryo
Fetal membrane, or extra-embryonic membrane, is a specialized structure which develops during embryonic development in vertebrates.
They form from embryonic tissue and serve various functions to support and protect the developing embryo, such as providing nutrition, gas exchange, waste removal, and protection
Fetal membranes have evolved to enable vertebrate embryos to develop on land. They provide the necessary support and adaptations for embryonic survival outside of an aquatic environment.
In chicks, there are four fetal membranes: - the amnion, the chorion, the yolk sac, and the allantois
Amnion - Acts as a protective cushion around the embryo - Prevents mechanical shocks and provides a stable environment for development. - Contains a fluid-filled cavity known as the amniotic cavity, in which the embryo floats in amniotic fluid. - This protects the embryo from desiccation and concussions.
Chorion - Aka serosa - The outermost membrane, surrounds the other extra-embryonic membranes and plays a role in gas exchange. - Also provides additional support. - In chick, the chorion fuses with the allantois during later stages of egg development - This forms the chorioallantois, a combined respiratory and excretory organ.
Yolk Sac - Surrounds and nourishes the yolk - Supplies essential nutrients to the developing embryo. - Serves as the first respiratory organ for the embryo, allowing for gas exchange. - Acts as a site of hematopoiesis, similar to the liver, and produces blood cells. - As development progresses, the yolk sac diminishes in size and is eventually incorporated into the chick’s digestive system.
Allantois - Involved in waste management and gas exchange. - Initially forms as a precursor to the urinary bladder, stores embryonic waste and facilitates the exchange of gases. - This allows for the resorption of carbon dioxide and the uptake of oxygen. - In chick, the allantois also participates in the absorption of calcium from the eggshell.
Each of these fetal membranes is composed of two germ layers
The amnion and chorion consist of the extra-embryonic ectoderm and the somatic layer of mesoderm, collectively called the somatopleure.
The yolk sac and allantois are composed of the extra-embryonic endoderm and the splanchnic layer of mesoderm, collectively called the splanchnopleure.
---
5M: Regeneration in Turbellaria
Regeneration is the innate ability of some organisms to replace or restore damaged or absent organs, tissues, cells, or even entire body parts to their fully functional state.
The underlying mechanisms of regeneration are governed by molecular processes, specifically gene regulation. These processes drive cellular activities such as cell proliferation, morphogenesis, and differentiation.
There are 2 laws of regeneration: - The capacity for regeneration is greater in simple animals and it diminished with more complex forms in the animal kingdom. - The ability for regeneration may be greater in the earlier stages of development.
Among platyhelminths, the process of regeneration is exceedingly higher in planarians. Any small piece of planarian worm can develop into a whole animal again.
Planarians are "almost to be called immortal under the edge of the knife."
However among reptiles, the regeneration is restricted to the replacement of lizard tails, proving the first law.
There are 4 types of regulation: - Epimorphosis - Morphallaxis - Heteromorphosis - Super Regeneration
Epimorphosis is contingent upon the growth of novel, appropriately patterned structures - ex: replacement of broken tail in lizard.
Morphallaxis is characterized by minimal growth and primarily relies on the repatterning of existing tissues. - if planaria is cut into 2 or more pieces, each piece will reconstitute itself into a new and complete individual of a diminished size. - even a small piece can regen into a complete whole.
Heteromorphosis is when a particular organ is amputated, the remaining portion may develop into a different organ entirely. - ex: shrimp eye may regen into an antenna
Super Regeneration is a restorative regeneration which encompasses the restoration of the entire organism or specific lost body parts. - In planaria, simply a small wound can cause the development of a new organ. - If there is an incision near the head, an additional head may regenerate.
---
5M: Types of Cleavage
Cleavage is a fundamental process in embryology that occurs during the early stages of embryo development.
It involves the division of cells, leading to an increase in cell number and the formation of a cluster of cells known as blastomeres.
Blastomeres come together to form a compact mass called the morula.
Cleavage ultimately culminates in the formation of the blastula or blastocyst.
Cleavage is initiated by the sperm during fertilization, which activates the zygote.
Cleavage primarily occurs through repeated mitotic divisions, wherein the zygote rapidly undergoes cell division.
Cleavage increases the number of cells and nuclear mass without a corresponding increase in cytoplasmic mass, the ratio of nuclear material to cytoplasmic material increases with each division.
Cleavage is influenced by the concentration of yolk present in the egg. Depending on the amount of yolk, cleavage can be categorized as either holoblastic or meroblastic.
-
Holoblastic Cleavage:
Aka total or complete cleavage
The entire egg undergoes division
2 sub types:
Equal-Sized Holoblastic Cleavage: - When the blastomeres resulting from cleavage are of equal size - This means that each division produces blastomeres that are roughly the same in size - results in a relatively uniform distribution of cytoplasm among the daughter cells.
Unequal-Sized Holoblastic Cleavage: - The production of blastomeres that differ in size. - The resulting blastomeres may have different amounts of cytoplasm and potential developmental fates. - small micromeres and larger macromeres are formed.
-
Meroblastic Cleavage:
Only a portion of the egg undergoes cleavage, while the yolk remains undivided.
Occurs in eggs with a large amount of yolk, preventing complete division of the entire egg.
2 sub-types:
Discoidal Cleavage: - Characterized by the presence of a small disc of cytoplasm, blastodisc, located at the animal pole of the egg - Only the blastodisc undergoes cleavage while the yolk remains undivided - ex: birds and reptiles
Superficial Cleavage: - Segmentation occurs only in the surface layer of the egg, while the central yolk, centrolecithal, remains undivided. - The cytoplasmic region surrounding the yolk is the site of cleavage and gives rise to the embryo. - ex: insects
-
Based on the symmetry of cleavage, there are 5 types: - Radial - Spiral - Bilateral - Biradial - Rotational
Radial: Regular cleavage, divisions perpendicular to each other.
Spiral: Cleavage planes are oblique. The four blastomere of lower plane rotate clock wise or anti-clock wise.
Bilateral: Unequal holoblastic cleavage, blastomere of one lateral side are smaller and the other four lateral blasotmeres are larger in size.
Biradial: First two cleavages are meridional and third is vertical. Four central blastomere are large and four blastomere are small.
Rotational: - The zygote divides vertically into two daughter cells. - One of the blastomeres divides longitudinally, while the other divides latitudinally. - These divisions rotate 90 degrees from each other. - This pattern continues so that the resultant blastomeres end up being smaller than their respective parent cells
#biology#zoology#send help#exam season#notes#science#long post#long reads#is this nsfw?#sorry tumblr
1 note
·
View note
Text
Zoology Practical Sem 4
Q1. Root Tip Slide Preparation
Aim: To study the various stages of mitotic division using onion root tip.
Principle:
Mitosis is an equational cell division occurring in vegetative cells of plants and animals where in a parent cell divides into two daughter cells of same genetic makeup as that of the parent cells.
Actively growing somatic regions of living organisms undergo mitosis at a good rate, leading to the growth and development of the body.
Onion root tips are a good source to study the various stages of mitosis under a microscope.
Acetocarmine is routinely used to stain root tips after properly squashing them. They are then placed on the slide. Acetocarmine can specifically stain the chromosomes.
A typical eukaryotic cell mainly divides by mitosis, which comprises the following stages - Interphase - Prophase - Metaphase - Anaphase - Telophase and Cytokinesis.
Procedure:
Take a small onion root tip on a clean slide and add a drop of acetocarmine stain. Warm gently by passing the slide over a flame a few times.
Tease the stained material with a dissection needle. Place a coverslip and keep a tissue paper over it. Tap or press the material from above so that the stained cells spread out.
Locate a suitable area using a magnification of 10X and 40x. Observe the slide and identify the stage of mitosis based on their features described.
Prophase:
The first stage of mitosis is prophase. The important events during this phase are:
A) Nuclear Changes - Chromatin material of nucleus gradually condenses into distinct chromatin threads by losing water. - The chromatin threads coil like cylindrical springs, and in doing so they gradually becomes shorter and thicker, forming chromosomes. - The proteinaceous matrix gets deposited around the chromosomes, so that this gradually becomes shorter and thicker to form chromosomes. - Each chromosome is already doubled due to the doubling of DNA in interphase. - By the end of prophase, two chromatids of each chromosome become more distinct and appears to be split up length wise. - The nucleus and the nuclear membrane disappear by the end of prophase.
B) Cytoplasm Events - The centriole divides into two and then one of the daughter centrioles moves towards the opposite pole. - Astral rays radiate out from each daughter centriole.
--------------------------------------------------------
Spotters
Lampbrush Chromosome
The largest known chromosomes, can be seen with naked eye and are characterized by their lateral loops.
Found in the yolk rich oocyte nuclei of certain vertebrates like fish, amphibians, reptiles, and birds during first prophase of meiosis.
Loops give it a brush like appearance
Discovered by Elemming in 1882.
Can reach up to 5900μ in length
Consists of a longitudinal axis formed by a single DNA molecule along with several hundred bead like chromosomes distributed in a linear fashion
From each chromosome, there emerge 2 symmetrical lateral loops which can expand or contract in response to external stimuli
5-10% of DNA is in the lateral loops
Functions: RNA syntheses and formation of yolk material
---
Polytene Chromosome
Giant banded chromosomes, smaller than lampbrush chromosomes.
Found in the larvae of certain dipterans.
First discovered by E.G. Balbiani in Chironimus in 1881.
Can occur in the larval salivary glands, midgut epithelium, rectum, and malpighian tubules of various genera like drosophila.
The salivary cells are so large they can be easily seen with a dissecting microscope.
Nuclei very large, around 25μ in diameter, chromosomes 50-200 times larger than typical chromosomes.
Tightly coiled chromatin: dark bands, loosely coiled: light bands.
Puffs: uncoiled regions of active RNA synthesis.
Balbiani rings: regions with larger puffs than others.
Primary function is to carry genes which ultimately control physiology of an organism.
---
T.S. of Testis
Round or oval in shape, externally surrounded by visceral peritoneum, a layer of fibrous connective tissue, and the tunica albuginea.
Each testis has a mass of coiled microscopic seminiferous tubules held together by connective tissue.
The connective tissue has small patches of interstitial cells which secretes the hormone testosterone responsible for male secondary sexual characters.
Stratified germinal epithelium lines the seminiferous tubules.
Germinal cells give rise to sperm.
Sertoli cells between germinal cells, nourish the developing sperm.
---
T.S. of Ovary
Ovary is solid and oval in shape.
It consists of the stroma that is differentiated into peripheral cortex and central medulla.
Lying in the cortex are ovarian follicles, corpora lutea, corpora albicans and interstitial cells. Medulla only contains blood vessels
Primary follicles arise from germinal epithelium into the cortex and each contains a central oocyte surrounded by a single layer of follicle cells.
Sec.follicles contain zona pellucida, several layers of follicle cells and thin connective tissue layer called theca having capillaries.
Mature/Graffian follicle contains large central follicular cavity, antrum externally lined by membrana granulosa.
Corpus luteum - ruptured follicle has solid mass of cells - secretes progesterone.
---
Cleavage of Zygote of Frog - 2 celled stage
The first cleavage plane is meridional.
The pattern of cleavage is holoblastic and unequal type.
The first cleaving furrow appears near the animal pole and extends towards the vegetal pole.
The cleaving furrow cuts the egg through its medium animal vegetal polar axis vertically into two equally sized blastomeres.
The mode of division of the zygote is mitotic.
---
Cleavage of Zygote of Frog - 4 celled stage
The second set of cleavage is also meridional which starts before the completion of the first.
The second cleavage is at right angle to the first, resulting in 4 blastomeres.
All the 4 blastomeres are equal-sized.
The grey crescent, animal pole, and vegetal pole show no change in their position and size.
---
Cleavage of Zygote of Frog - 8 celled stage
The third cleavage results into 8-celled stage.
The third cleavage is holoblastic but unequal.
The third cleavage is horizontal but well above the equator towards the animal pole.
It results into 8-blastomeres of two distinct types.
The upper 4 smaller pigmented blastomeres at the animal pole are called micromeres.
The lower 4 larger yolk-ladder cells at vegetal pole are called macromeres.
---
Cleavage of Zygote of Frog - 16 celled stage
The fourth cleavage plane is oriented from animal to vegetal pole.
The fourth set of cleavage is also vertical, therefore, produces a 16-celled stage.
The micromeres divide more rapidly.
The yolk-ladder macromeres divide slowly.
The yolk shows its influence on cleavage rate.
---
Cleavage of Zygote of Frog - Morula
The fifth cleavage is irregular but it is usually double oriented parallel to third cleavage plane resulting into 32-celled stage.
The fifth cleavage separates the micromeres and macromeres in complete two sets of 16 each.
The blastomeres assume spherical shape.
The surfaces of blastomeres in contact to each other are flattened due to their mutual pressure.
The whole embryo acquires a characteristic appearance reminiscent of a mulberry, hence the name morula.
---
Blastula of Frog
It is a hollow ball of different sized cells representing blastula stage in the development of frog.
The cavity is known as blastocoel.
Blastocoel is eccentric and placed towards the animal pole
Blastocoel is surrounded by micromeres and macromeres.
Micromeres are smaller, found in the anterior animal pole, and have dark pigments.
Macromeres are larger, found in the posterior vegetative pile, and are larger in the yolk.
---
Gastrula
Embryo developed from blastula
Oval in shape, contains three germ layers: ectoderm, endoderm, and mesoderm.
Internally the gastrula has a cavity called the archenteron.
It opens to the exterior through blastopore.
A mass of endoderm cells protrudes through the blastopore, these cells constitute the yolk plug.
The floor and the lateral sides of the archenteron are formed from the endoderm.
The roof of the archenteron is formed from the chordomesoderm.
Externally the gastrula is covered by ectoderm.
The ectoderm that lies on the mid-dorsal line develops into the nervous system, hence it is called neurectoderm.
The remainder of the ectoderm is the epidermal ectoderm.
---
Chick Embryo - 18 hours of incubation
After 18 hours of incubation the notochord has become markedly elongated, forming a prominent structure.
Notochord extends towards cephalic region in the mid-line from Hensen's node.
18 hours incubated embryo is spoken of as being in the head process stage.
As the tip of the notochord neural plate develops, caudal and cephalic ends are seen.
In front of notochord and neural plate there is a space called as pro-amnion.
Embryonic area, anterior border of mesoderm area pellucida and area opaca become more prominent.
Primitive streak gradually decreases in size.
---
Chick Embryo - 24 hours of incubation
The folding of the neural plate is clearly marked. In stained and transparent preparation of entire embryo neural folds appear as a pair of dark bands formed of neural groove.
Neural folds at cephalic are more prominent than at caudal end.
Foregut is established, the gut caudal to foregut called as midgut and the anterior intertinal portal appear.
In the middle, four pairs of somites are seen.
Hensen's nod is pushed caudally and primitive streak is further reduced.
---
Chick Embryo - 33 hours of incubation
Shows some of the fundamental steps in the formation of central nervous system and circulatory system.
Various neuromeric enlargements from brain regions.
Brain differentiated into prosencephalon, mesencephalon, and rhombencephalon.
Optic vesicles established.
Infundibulum formed.
12 pairs of somites formed.
Mid-region of heart is considerably dilated and bent to the sight.
Primitive streak becomes shorter due to lengthening of neural tube.
---
Chick Embryo - 48 hours
Embryo shows cranial flexure and twisting of head over the right side.
Fore, mid, and hind brain distinct, fore brain takes the anterior most places.
Heart differentiated into ventricular arterial, and sinus region.
Second aortic arched develop.
Vitelline arteries and veins are distinct.
Somites are 26 pairs.
---
Chick Embryo - 96 hours
The entire body bends 90°, the embryo lies with it's left side on the yolk.
At the end of the 96 hours, the body folds have undercut the embryo so it only remains attached to the yolk by a slender stalk.
The yolk stalk becomes elongated, allowing the embryo to become straight first in the mid-dorsal region and then convex dorsally.
Optic cub shows more developed lens.
Endolymphatic duct arises from the auditory vesicle.
Visceral arches have become very much thickened.
Appendage bugs increase rapidly in size, become elongated.
No of somites increases to 41 pairs.
---
Mitosis: Prophase
The first stage of mitosis.
Chromosomes appear as long coiled threads called chromatids.
Chromatin becomes shorter, thicker and more visible due to the condensation of DNA to form chromosomes.
Stability of nucleus increases.
Each chromosome starts to splits longitudinally into two sister chromatids. These sister chromatids are attached with each other at centromere.
Nuclear membrane and nucleolus disappear by the end of prophase.
---
Metaphase
The 2nd phase of mitosis
Appearance of spindle fibres
Spindle fibres attached to the centromere of chromosome.
The chromosomes are arranged on the equatorial plane.
The process of gathering of chromosomes in equator is called congression and plate formed is called metaphasic plate.
---
Anaphase
The third and shortest phase of mitosis
The centromere of each chromosome splits into two sister chromatids and forms two daughter chromosomes.
The daughter chromosomes are pulled towards the poles due to the contraction of spindle fibers.
During polar movement, the chromosomes shows different shapes like J,U,V,L or I shaped in appearance.
At the end of anaphase, each pole will get one set of daughter chromosomes.
---
Telophase
The fourth and final stage of mitosis.
The daughter chromosomes reach respective poles, they uncoil and become thin, long and visible.
The spindle fibers start to and finally disappear.
The nuclear membrane and the nucleolus reappear.
Two nuclei are formed at the end of telophase. Both the nuclei have the same number of chromosome as parent cell.
---
Meiosis: Prophase-I
The longest stage of meiosis-I
Divided into 5 sub-stages:
Leptotene: size of cell and nucleus increases, fine thread like chromomeres present
Zygotene: Synapsis occurs, the pairing of homologous chromosomes.
Pachytene: Tetrads form from bivalents, crossing over starts.
Diplotene: Crossing over occurs, homologous chromosomes begin to sperate but are held together by chiasmata.
Diakinesis: Chiasmata move towards the end of chromosomes, nuclear membrane and nucleolus disappear
---
Metaphase-I
2nd phase of meiosis
The spindle fibers organize between two poles and get attached to the centromere of chromosomes.
Chromosome moves to equator
The bivalent chromosomes are arranged in the equatorial plate in such a way that 2 metaphasic plates are formed
---
Anaphase-I
3rd phase of meiosis
Spindle fibers contract, pulls chromosomes to the polar region.
The separated chromosome is known as dyads
No splitting of chromosomes occurs so the centromere of each homologous chromosome does not divide. Thus, the chromosome number of the daughter nuclei is reduced to half.
Now the separated chromosome moves toward opposite poles
---
Telophase-I
Two groups of chromosome formed at each pole and organized into nuclei.
The nuclear membrane and nucleolus reappears.
The chromosomes get uncoiled into chromatin thread.
The spindle fibers disappear.
---
Prophase-II
First stage of meiosis-II
The dyads chromosome becomes thicker and shorter
Nuclear membrane and nucleolus disappear
Spindle fibre starts to form
---
Metaphase-II
The dyads chromosomes comes to equatorial plane
Spindle fibres organize between poles and attaches to centromere of chromosome.
---
Anaphase-II:
Centromere of each chromosome divides and sister chromatids separates to form two daughter chromosome
Spindle fibre contracts and pull the daughter chromosome apart towards opposite pole.
---
Telophase-II:
Chromosome become organize at respective pole into nuclei
Chromosome elongates to form thin networks of chromatin
Nuclear membrane and nucleolus reappears
0 notes
Text
IA Prep: Botany (sem 4)
Anomalous Secondary Growth ---
What is it?
Anomalous Secondary Growth is the growth resulting
Boerhaavia
A transverse section shows epidermis, collenchymatous hypodermis, parenchymatous cortex, endodermis and single layered pericycle.
The primary vascular bundle are conjoint collateral, endarch, and open.
These bundles occur in 3 concentric rings.
Inner most ring consists of 2 large vascular bundles present in the pith, opposite to each other.
Middle ring consists of 6-14 small vascular bundles.
The outer ring consists of 15-20 smaller vascular bundles, these are the normal vascular bundles.
Middle and inner rings of bundles are considered medullary bundles.
Secondary Growth
During secondary growth, the two central bundles and the middle ring of bundles increase in size due to the activity of fasicular cambium.
Inter-fasicular cambium do not develop.
The outer ring of vascular bundles are involved in the secondary growth.
The intra and interfasicular cambial strips join to form complete cambial ring.
This complete cambial ring forms the secondary xylem to the inner side and secondary phloem to towards the outside only in fasicualar region.
In between the bundles the cambium produces parenchymatous cells (thick walled) called conjuctive tissue.
After sometime this cambium ring stops activity.
Then a new cambial ring is formed external to the secondary phloem.
The cells in pericycle is involved in the formation of second cambial ring. This is called as accessory cambium.
This also produces secondary xylem to the inner side and secondary phloem to the outer side.
After a period of activity this cambium also stops its function. Another ring of cambium arises outside which behaves in the same pattern.
In this way 4-5 rings are produced and embedded in the conjuctive tissue.
Periderm- protective covering in outer stelar region.
Bignonia
Bignonia is a woody climber
A young stem shows ridges and furrows.
Epidermis is the outermost layer with thick cuticle.
Collenchyma is prominent below the ridges.
The cortex is made up of layer of parenchyma cells.
Endodermis is not very distinct
Pericycle is in the form of sclerenchymatous patches.
The vascular bundles are conjoint, collateral, endarch and open.
They are arranged in the form of ring around the periphery of the
pith.
Secondary Growth:
The inter and intra fasicular cambial strips become active to form a normal ring of cambium.
In the initial stages, the cambial behavior is normal, it makes more of secondary xylem and less secondary phloem.
Subsequently, at four corner points the cambium begins to produce more of secondary phloem and relatively small amount of secondary xylem.
Resulting in the formation of 4 deep wedges of phloem projecting into secondary xylem.
Initially the number of phloem furrows are 4 in number, but later additional furrows may be formed in older stems.
In this way, the xylem cylinder is broken into vertical plates, separated by furrows of secondary phloem.
Periderm is produced in cortex region due to the activity of phellogen.
---
Wood Structure
Teak
Scientific: Tectona grandis
Telugu: Adaviteeku
Hindi: Sagun
Family: Verbenaceae
One of the most important timber plants in the world, well known for both durability and strength
Occurrence:
- Native to India, Java, Sumatra, and Indonesia
- In India, teak forests mostly found in M.P, Maharashtra, Gujarat, and Karnataka.
- Other states include A.P, Rajasthan, U.P, Tamil Nadu, and Orissa.
Morphology:
- Tree height: 30m
- Girth: 2-4m
- Leaves: Opposite, simple, elliptical to obovate, 25-50cm long.
- Flowers: Small, arranged in panicles.
- Bractiate, Bisexual, and Hypogynous.
- Fruit: Hard, 4 lobed nut, 1-2 seeds.
Cultivation:
- Quick growing, temp requirement: 26-30˚C, annual rainfall: 130-300 cm.
- Growth is stunted in nitrogen deficient soil.
- Plantation time of seedlings: April-May.
- Good quality timber obtained from: 80 years old trees.
Prop of wood:
- Scented oil in wood acts as its preservative.
- Sapwood white in color, susceptible to termites and fungi
- Heartwood is golden yellow -(air)-> dark brown and resistant to fungal attacks.
- Ring is porous, presence of vessels.
- Tyloses are present.
Uses of Teak:
- Timber is durable, hard and resists decay, used in furniture and plywood.
- Used to make agricultural implements.
- Wood waste is raw material for paper pulp.
- Wood oil: used to treat eczema
- Flowers: relieves kidney problems
- Root bark: used for coloring mats.
Red Sanders
Scientific: Pterocarpus santalinus
Telugu: Erra chandanamu
Hindi: Lal chandan
Family: Fabaceae
This tree is valued for the rich red color of its wood, not aromatic.
Occurrence:
- Grows in southern India.
- Common in Cuddapah, Nellore and southern Kurnool districts.
Morphology:
- Grows in height to 10-11m.
- Leaves imparipinnate, leaflets pulvinous, 3 or rarely 5.
- Flowers bright yellow in short racemes.
- Pedicellate, Bisexual, and Zygomorphic.
- Flowering occurs during April-June
- Pods are winged with 1-2 seeds.
Props of wood:
- Bark is blackish-brown, wood is hard close grained.
- Differentiated into sap wood and heart wood. Sap wood is white and heart wood is black in color.
- Pores are medium sized and scattered
- Medullary rays are numerous and equidistant.
Uses of wood:
- Used in making agricultural implements.
- Gives a blood red colored die on distillation.
- Extensively used in carvings, statues, and picture frames.
Neem
Scientific: Azadirachta indica
Telugu: Yepa
Tamil: Vepa
Family: Meliaceae
Occurrence:
- Native to India, grows wildly.
- Also grows in Nepal, Bangladesh, Pakistan, and Sri Lanka.
Morphology:
- A fast growing tree which can reach a height of 15-20m.
- Evergreen, but in severe drought it may shed most or all of its leaves.
- Leaves: opposite pinnate with 20-30 medium dark green leaflets.
- White and fragrant flowers, arranged in more-or-less drooping axillary panicles.
- Each inflorescence has 150-250 flowers, they are protandrous and bisexual.
- Fruit: smooth olive-like drupe, it encloses one, rarely two or three,
elongated seeds (kernels)
Properties of wood:
- Bark: grey with numerous scattered tubercles.
- Sapwood is grey while heartwood is red.
- Pores are large/moderate sized and oval shaped; medium to coarse textured.
- Medullary rays are numerous and prominent.
- Wood is scented.
Uses of wood:
- Construction of cart axles and wheels, agricultural implements, and furniture.
- Bark contains tannins which are used in tanning, dyeing etc.
- Compounds extracted from the bark are used in production of some dental-care products like toothpaste.
- The bark acts as an insect repellent and it also has anti-bacterial
properties.
- Wood has been used as firewood and charcoal for a long time.
-------------------------------------------------
Stomatal Types ---
Stomata are minute pores which occur in the epidermis.
Stomata are used for the exchange of gases from the plant and the atmosphere, each stoma opens into a sub-stomatal chamber or a respiratory cavity to facilitate this function.
Evaporation of water takes place through the stomata.
Each stoma is surrounded by two kidneys shaped guard cells.
The stomata may occur on any part of a plant except the roots.
The epidermal cells bordering the guard cells are called accessory cells or subsidiary cells
There are 4 types of stomata based on the accessory cells and their arrangements:
- Anomocytic
- Anisocytic
- Paracytic
- Diacytic
Anomocytic Stomata:
Also called Type-A, Ranunculaceous type, or irregular-celled type stomata
No accessory cells are present
The stoma remains surrounded by ordinary epidermal cells which are arranged irregularly
Commonly found in dicotyledons such as Tridax.
picture
Anisocytic Stomata:
Also called Type-B, Cruciferous type, or unequal-celled type stomata.
The stomata is surrounded by three accessory cells.
Of the three accessory cells, one of them is distinctly smaller than the other two cells.
Commonly found in genera such as Brassica.
picture
Paracytic Stomata:
Also called Type-C, Rubiaceous type, or parallel-celled type stomata.
At least two accessory cells are present.
The accessory cells lie parallel to the long axis of the pore and guard cells
Commonly found in the members of Rubiaceae.
picture
Diacytic Stomata:
Also called Type-D, Caryophyllaceous type, or cross-celled type.
Each stoma is surrounded by a pair of accessory cells.
The common walls of each accessory cell remains at a right angle to the long axis of the guard cell.
Commonly found in Ocimum.
picture
There are two more types of stomata:
- Gramineous
- Coniferous
Gramineous Stomata:
The stoma have guard cells where in which the middle portions are much narrower than the ends, making the cell appear to be dumb-bell shaped
Commonly found in Gramineae
pic?
Coniferous Stomata:
The stomata are sunken and appear as if the accessory cells are suspended and arching over them.
In the median parts, the guard cells are have narrow lumina and the section is elliptical in shape.
At the ends, they lumina is wider and the section is triangular.
The walls of these guard cells and the accessory cells are partly lignified and partly non-lignified.
---
Simple Tissues ---
Simple permanent tissues are tissues which are composed of identical cells which together perform a common function.
Simple tissues can be divided into three groups:
- Parenchyma
- Collenchyma
- and Sclerenchyma.
=====
Parenchyma is made up of living cells.
Each cell is either spherical, ovular, rectangular, polygonal, elongated, or irregular in shape.
The cell wall is thin, made up of cellulose, hemicellulose, and pectin.
Young parenchyma cells are loosely arranged
There is intercellular space present in parenchyma
Parenchymatous cells can store reserve food material
Parenchymatous cells are found in all parts of plant such as cortex, pith, palisade, mesophyll, flower, seed, etc.
Parenchyma is also found in vascular tissues.
-
There are three types of parenchyma based on structural modifications and specialized functions:
- Prosenchyma
- Aerenchyma
- Chlorenchyma
Prosenchyma are thick-walled fiber-like elongated cells which provide rigidity and strength to the plant, ex: pericycle
Aerenchyma have large intercellular spaces filled with air, it helps in the buoyancy of the plant, ex: cortex of hydrophytes
Chlorenchyma are cells which have chloroplasts and perform photosynthesis, ex: palisade of leaves
-
Functions of parenchyma:
Photosynthesis - chlorenchyma has chloroplasts which perform photosynthesis
Storage - parenchyma can store food in the form of starch, proteins, oils, and fats
Buoyancy - aerenchyma helps aquatic plants float
Transportation - Parenchyma of xylem and phloem helps in the transport of nutrition and water
Mechanical support - prosenchyma provides mechanic support
diagram
=====
Collenchyma is a living tissue
The shape of each collenchyma cell is somewhat elongated
Collenchyma cells have a thick wall due to the deposition of hemicellulose and pectin in intercellular spaces
Intercellular spaces may or may not be present in collenchyma
-
There are three types of collenchyma based on the deposition of hemicellulose and pectin:
- Angular collenchyma
- Lacunar collenchyma
- Plate or lamellar collenchyma
Angular collenchyma have a thick cell wall at the corner of the cell, does not have intercellular space.
Lacunar collenchyma have a thick cell wall at the border of the cell, has intercellular space.
Plate or Lamellar Collenchyma have a thick cell wall at tangential wall, does not have intercellular space.
-
Functions of collenchyma:
Collenchyma provides mechanical support and elasticity to the stems of dicots.
Collenchyma has chloroplasts, therefore it can carry out photosynthesis.
Provides strength and flexibility to the plant body.
diagram
=====
Sclerenchyma is made up of dead cells.
The shape of sclerenchyma cells are elongated and pointed at both ends.
The cell wall of sclerenchyma is thick and lignified, it encloses an empty cavity called 'Lumen'
Sclerenchyma cells lack protoplasm.
Sclerenchyma provides strength and rigidity to the plant body.
-
There are two types of sclerenchyma:
- Fibers
- Sclereids
Fibers are thick walled, elongates, spindle shaped dead cells with pointed ends.
Cell walls enclose narrow lumen with simple round pits and lignified secondary wall.
Present in xylem, phloem, cortex and pericycle.
They made up the covering of fruits.
Fibers provide mechanical support.
There are three types of fibers:
- Surface Fibers: On fruit wall + seed coat (ex: coconut)
- Wood fibers: associated w xylem
- Bast fibers: associated w phloem, cortex, and pericycle.
Sclereids are very thick walled cells with either spherical, ovular, or dumbbell shape.
They are also called stone cells.
Sclereid cell wall has simple pits.
Present in hard part of plants and the pulp of fruit.
Provide local mechanical support.
5 Types based on shape:
- Stone cells: Isodiametric, similar to parenchyma in shape.
- Macrosclereids: Rod like shape
- Osteo sclereids: Bone like shape
- Astro sclereids: Star like shape
- Filiform sclereids: Hair like elongated cell with branches
-
Functions of Sclerenchyma:
Sclerenchyma is made up of dead and lignified cells which provides mechanical support.
Provides hardness to stony fruits like nuts, coconut, almond etc.
---
Complex Tissues ---
Complex permanent tissues are composed of 2 or more types of cells
All the cells contribute to a common function.
They are aka vascular tissues.
Vascular tissues are primarily associated with conduction of water, minerals, and food in plants.
There are 2 types of vascular tissues: xylem and phloem
===
Xylem
Primary function of xylem is to conduct water and minerals from the roots to leaves.
Additional function involves providing mechanical support. (secondary xylem)
-
Two types of xylem: primary and secondary.
Primary xylem:
- Xylem in primary plant body
- Developed from pro-cambium
- Organized as bundles along with phloem
- Main function: water and mineral conduction
- 2 types: protoxylem + metaxylem
Secondary xylem:
- Xylem in secondary plant body
- Developed from vascular cambium
- Main function is the same
- Additional function: provide mechanical support.
-
Elements of Xylem: Tracheids, Vessels, Xylem fibers, and Xylem parenchyma.
Tracheids:
- Elongated tube like cells occurring along the long axis of the plant.
- Cells are devoid of protoplast, and hence is a dead component.
- Has a cavity, an empty lumen, and tapering ends.
- Round or polyhedral in cross-section.
- Average cell length is 5-6mm
- The most primitive and fundamental element in xylem element, found in the fossils of seed-plants.
- In modern plants they occur predominately in lower vascular plants, pteridophytes and gymnosperms
- Has secondary cell wall which is hard, thick and extremely lignified.
- Secondary walls are deposited in different patterns:
- annular: rings, most primitive
- spiral: helical spirals
- scalariform: ladder like
- reticulate: net like
- pitted: pits evenly distributed all over
Vessels:
- Aka trachea
- short dead cells devoid of protoplast
- have hard and lignified cell-wall
- forms a row of cylindrical cells arranged in longitudinal series like a tube.
- Each cell has perforation at the end walls, rarely occur on the lateral walls
- Distinct ‘perforate’ bodies makes translocation of solutes easy.
- Perforations remain either mostly in parallel series like bars called scalariform perforation, or in a network known as reticulate perforation, or even may form a group of circular holes called foraminate perforation.
- The perforation occurs in form of a single large circle, referred to as simple perforation
- Has various types of secondary thickenings such as:
- annular, spiral, scalariform, reticulate or pitted.
- The pits are mostly of bordered types.
- Present in angiosperms, usually absent in pteridophytes and gymnosperms.
- Does not occur in some parasitic and aquatic plants.
Xylem Fibers:
- Also called xylary fibers
- Fibers are very much elongated, dead cells with thick lignified cell walls.
- Primarily give mechanical support.
- Two types: fiber traeheids and libriform fibers.
- Fiber tracheids are intermediate forms between fibers and tracheids, have bordered pits.
- Libriform fibers are narrow with highly thickened secondary wall, have simple pits.
- Gelatinous fibers in tension wood are a type of xylem fiber.
Xylem Parenchyma:
- Living cells w cytoplasm + prominent nucleus
- Cells are thin-walled, lignified secondary wall absent.
- Parenchyma in secondary xylem rarely undergoes secondary growth.
- Two types: Axial parenchyma + Ray parenchyma
- Meant for storage of starch and fatty food, tannins, crystals, etc.
- They are involved in conduction of water and solutes and mechanical support.
===
Phloem
Primary function is to transport food to the different parts of the plant body
2 types of phloem: Primary (dev from pro-cambium during 1' growth) + secondary (dev from vascular cambium during 2' growth).
4 elements: Sieve elements, companion cells, phloem parenchyma, phloem fibers and sclereids.
-
Sieve Elements: divides into sieve tubes and sieve cells.
Sieve Cells:
- Less specialized and more primitive than tubes
- Occur in lower vascular plants and gymnosperms
- Narrow elongated cells with steeply inclined end walls
- Sieve areas are located on the lateral walls and very rarely at the end walls.
Sieve Tubes:
- More specialized and advanced than cells.
- Occurs in angiosperms
- Long tube-like structures formed arranged in longitudinal series.
- end-walls of Sieve tube are perforated in a sieve-like manner.
- The perforated end-walls are called the sieve plates.
- Through sieve plates, cytoplasm connections are established between adjacent cells.
Companion Cells:
- Occurs abundantly in angiosperms, particularly in the monocotyledons, absent in some primitive dicotyledons.
- Pro-cambial mother cell divides longitudinally into two daughter cells, one of which serves as the sieve element and the other one becomes the companion cell.
- Hence companion cell is considered as sister cell to sieve tube.
- Companion cells remains associated with the sieve tubes of angiosperms
- These are smaller cells, having dense cytoplasm and prominent nuclei without starch grains.
- The wall between the sieve tube and companion cell is thin and provided with primary pit fields.
- Function: controls the activity of sieve tube.
Phloem Parenchyma:
- Occurs in both primary and secondary phloem
- Thin walled living cells
- Have primary pit fields
- Some parenchyma cells store starch.
- Two types: Axial and Ray parenchyma
Phloem Sclerenchyma:
- Aka Phloem fibers or Bast fibers
- Sclerenchyma of phloem
- Dead cells with lignified cell wall
- Have simple or slightly bordered pits.
- Septate fibers occurs in Vitis
- In some plants, phloem fibers are very long.
- rare in pteridophytes and some spermatophytes
- Provides mechanical strength to the plant.
- Used for the manufacture of ropes and cords.
2 notes
·
View notes
Text
Zoology hw: Mitosis and Cell Regulation
Mitosis
Mitosis is the process of cell division in which one mother cell divides to form two genetically identical daughter cells. This results in the duplication of cells and their reproduction. The number of chromosomes is consistently preserved in both the daughter cells as 2n. Mitosis occurs in somatic cells such as in the skin and bone marrow. Strassburger first observed mitosis in plant cells in 1870 while Boveri and Flemming observed them in animal cells in 1879. The term mitosis was coined by Flemming in 1882, it was derived from the Greek term "mitos' which means 'thread' in english. Continuous mitosis results in an increase in the of number of cells, allowing an organism to grow from a single cell into a complex living organism.
Mitosis is a part of the cell cycle which is preceded by interphase. Interphase is when the cell gets ready for mitosis by growing and replicating it's DNA before division. Also the metabolism of the cell increases. Interphase consists of three phases: G1 phase, S phase, and G2 phase in respective order.
Mitosis is comprised of two steps, karyokinesis and cytokinesis. After interphase, mitosis starts with karyokinesis. Karyokinesis is the nuclear division of the parent cell. The term 'karyokinesis' is derived from the Greek words 'karyon' which means nucleus and 'kinesis' which means movement. It is comprised of 4 phases: prophase, metaphase, anaphase, and telophase in that order.
Prophase is the first stage of karyokinesis, it is also the longest of the four. This phase can be sub divided into early prophase and late prophase. During early prophase the chromatid fibers condense to form elongated chromosomes, the nuclear membrane and nucleolus start to disappear, the centrioles separate and start to move towards opposite ends of the cell, and the spindle starts to form. During late prophase the nuclear membrane completely disappears, the centrioles have finished moving to the opposite poles, and the spindle has formed completely as the microtubules expand in the nuclear area.
Metaphase is the second stage of karyokinesis. During metaphase the chromosomes align along the equatorial plane to form the metaphasic plate. Also, the centrioles at polar ends project spindle fibers which attach to the centromere of each chromosome.
Anaphase is the third stage of karyokinesis, it is the shortest of the four. During anaphase the centromere of each chromosome splits into two sister chromatids to form two daughter chromosomes. The daughter chromosomes are separated and pulled towards the opposite poles due to the contraction of the spindle fibers. While the chromosomes move, they appear in various shapes such as I, U, L, J, or V. By the end of anaphase, each pole will receive one full set of daughter chromosomes.
Telophase is the fourth and final stage of karyokinesis. During telophase the daughter chromosomes arrive at each pole and decondense to become thin and long chromatin strands. The spindle fibers disappear while the nuclear membrane and nucleolus reappear. At the end of telophase two nuclei are formed, both of which have the same number of chromosomes as the original parent cell, 2n.
Cytokinesis is the division of cytoplasm, the separation a cell into two by the end of mitosis. It occurs differently in plant and animal cells. In plant cells cytokinesis occurs by the formation of a cell plate while in in animal cells it occurs by cleavage or furrow formation. In animals cleavage formation first starts in the anaphase stage and continues through telophase. Cytokinesis ensures two genetically identical daughter cells are formed.
------------------------------
Cell Regulation
Cell regulation refers to the control of the process of cell division, it is a complex process which must occur precisely. Since all of the processes occur in a sequence, the cell must know when to proceed, hold, and stop each step. Every aspect of a cell is checked by an internal mechanism before cell division can continue. The cell cycle has numerous checkpoints for this purpose, the cycle halts till every condition is properly met at each checkpoint. Checkpoints halt the process of cell division incase there is genetic damage, these pauses allow the cell to repair the damage before it's too late. If the damage is not repaired, the cell undergoes apoptosis and if the checkpoint mechanism failed the cell will become cancerous.
A protein complex called Maturation Promoting Factor or MPF is essential in the cell cycle. MPF is composed of two proteins, cyclin and cyclin-dependent kinase or CDK. When both cyclin and cdk are activated, they allow the cell cycle to pass checkpoints. There are three checkpoints in the cycle with involve CDKs, each of which has its own specific cyclin molecule to initiate each phase of the cell cycle. Other checkpoints do not involve CDKs but also occur at the cell cycles transition phases. The three CDK checkpoints are G1, G2, and M checkpoints respectively.
The G1 checkpoint determines whether the conditions are favorable for the cell cycle to proceed into S phase. This checkpoint is the restriction point at which the cell fully commits to the cell division process. The external factors such as size and growth are checked at this point along with adequate energy reserves and any damage to the DNA. If the cell meets all the G1 checkpoint requirements, the cell may enter S phase and begin replicating DNA. If the cell failed to meet the requirements, the cell can either halt the cycle and fix the problems or the cell can enter the G0 phase and rest till conditions improve. The G1 checkpoint is operated by signals given by cyclin D and CDK4.
The G2 checkpoint ensures all the chromosomes have been correctly replicated without any damage before the cell may enter the mitotic phase. If the checkpoint mechanism detects any issues with the DNA, the cycle must halt as the cell tries to either complete replication or repair damaged DNA. If the DNA has been replicated entirely and correctly, the CDKs may signal the start of the mitotic phase. The G2 checkpoint is operated by signals given by cyclin E/A and CDK2
The M checkpoint occurs at the end of metaphase during mitosis. It ensures all the sister chromatids are properly attached to the spindle fibers before the cell enters anaphase, hence it is also called the spindle checkpoint. Since anaphase is an irreversible stage in the cell cycle, it is essential to have a checkpoint right before it. The M checkpoint is operated by signals given by cyclin B and CDK1.
Regulation of the cell cycle is carried out by the regulator molecules of the cell cycle, they ensure cell division occurs properly. Regulator molecules can be categorized into two main groups, positive regulators and negative regulators. Positive regulators promote the progression of the cell cycle while negative regulators pause the cycle until all the conditions are met.
Positive regulation of the cell cycle is primarily governed by the two proteins, cyclins and CDKs or cyclin-dependent kinases, they are responsible for promoting the cell cycle. MPF or maturation promoting factor is a protein complex composed of both of them.
Cyclins are cell signaling proteins that fluctuate throughout the cell cycle in a predictable pattern. There are 4 main types of cyclins: D, E, A, and B, each of which is associated with a different phase of the cell cycle. Once the cell moves from one stage of the cycle to another, the cyclins which were active in the previous stage degrades. Cyclins can only regulate the cell when they are bound to a CDK molecule, and to fully activate the cyclin-CDK complex must be phosphorylated at specific sites.
Cyclin-dependent kinases or CDKs are enzymes which can phosphorylate other proteins or enzymes. Phosphorylation activates proteins by changing their shape, these activated proteins are involved in the advancement of the cell cycle. The level of CDK proteins remains consistent throughout the cell cycle since they do not degenerate like cyclin proteins.
Negative regulation of the cell cycle is integral for cell maintenance and prevention of uncontrolledly cell division. If a negative regulator protein is damaged or fails to function, it results in uncontrolled cell division leading to tumor which may or may not be cancerous. The most studied negative regulators include retinoblastoma protein (Rb), p53, and p21.
Retinoblastoma protein or Rb is a tumor-suppressor which can be observed in many cells, it monitors the overall size and readiness of the cell for mitosis. P53 is a multi-functional protein. It is activated during the G1 phase when DNA damage is present, it employs the mechanism to handle said damage. If the DNA can be repaired, p53 recruits enzymes to do so, if it cannot be repaired p53 may trigger apoptosis to prevent the duplication of faulty chromosomes. As p53 levels rise, the production level of p21 does too. P21 is an inhibitor protein which binds to and acts on cyclin-CDK complexes, it enforces a pause on the cell cycle as indicated by p53. This makes sure the cell can not progress into the S phase with any damaged DNA present.
0 notes
Text
Mod 1 Botany Sem End
Principles of Plant Systematics
Taxonomy is the classification of organisms.
It is the identification and naming group of plants and animals.
The word systematics comes from the latin word systema.
It means the study of the diversity and the history of organism and the evolutionary relationship between them.
In 1737, Linnaeus wrote "Systema Naturae"
The goal of systematics is - to know the evolutionary history of an organism - and to convey info through classification
---
The significance and aims are systematics:
It is the fundamental of biology, it enhances the understanding of evolution.
It helps in understanding conservation of organisms.
It helps in predicting the medicinal properties of relates plant species.
It provides a scheme of classification
And provides suitable methods for identification, nomenclature and discovery of plants.
Provides inventory of plant taxa that suits local, regional and continental levels.
Provide insight into diversity and evolutionary process in plants
It allows us to collect and preserve plant taxa in herbaria for future reference.
---
There are 3 basic components to taxonomy: - Identification - Nomenclature - Classification
Identification:
To identify unknow plant specimens through similarities and dissimilarities
Comparison can be made with authentic herbarium or indirectly with the help of literature.
New taxon may be discovered.
Floras, monographs, manuals and journal are sources of literature one may refer to.
Computer punch card keys are modernly used for identification.
Royal Botanical Garden and the Botanical Survey of India are both very important for plant identification.
Nomenclature:
The correct scientific name to an identified plant
ICBN is the governing body for scientific names.
ICBN - International Code for Botanical Nomenclature
The International Botanical Congress sets rules and regulations.
We follow a binomial nomenclature for scientific names of organism, starting with a capitalized generic epithet and followed with a lowercase specific epithet.
Classification:
There are 3 widely accepted systems of classification: - Artificial - Natural - Phylogenetic
Artificial: - Classification is based on habit, color, number, and forms (herbs, shrubs, trees, etc.) - Based off of Thephrastus's book Historia plantarum - Expanded upon by Linnaeus, the father of systematic botany. He counted the number of stamens and observed floral characters.
Natural: - Based on the constancy of species. - By Bentham and Hooker - Classification is based on the phenetic relationship with data of morphology, anatomy, embryology, ultrastructure, and phytochemistry. - A larger number of characters is shared by different taxa, they are more closely related (based on numerical taxonomy)
Phylogenetic: - Proposed by Darwin after his theory of evolution. - Based on genetic and evolutionary relationships - Form a phylogenetic tree or cladogram. - Engler and Prantl System of classification - Classification is vertically configurated - Common ancestor: same group (Monophyletic) or more than one group (Polyphyletic).
------------------------------------------------------
Bentham and Hooker Classification
Also called Natural System of Classification
The work of G. Bentham and J.D. Hooker.
They have grouped together advanced seed bearing plants into a major division called Spermatophyta.
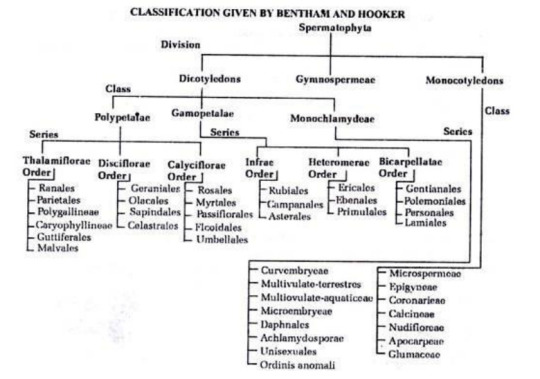
Class Dicotyledonae: angiosperms in which the seed bears two cotyledons, leaves exhibit reticulate venation. Divides into 3 subclasses:
Subclass Polypetalae: flowers contain perianth (calyx and corolla), in the corolla petals are free. Divides into 3 series: - 1 Series Thalamiflorae: Thalamus is conical, elongated or convex, flowers hypogynous. - 6 orders and 34 families. - 2 Series Disciflorae: Flowers hypogynous due to presence of ring like disc below ovary - 4 orders and 22 families. - 3 Series Calyciflorae: Flowers epigynous or perigynous - 5 orders and 27 families.
Subclass Gamopetalae: Flowers with distinct calyx and corolla. In the corolla petals are fused. Divides into 3 series: - 1 Series Inferae: Epigynous flower, either regular or zygomorphic - 3 orders and 9 families. - 2 Series Heteromerae: Ovary superior with more than two carpels with regular flowers - 3 orders and 12 families. - 3 Series Bicarpellatae: Superior, bicarpellary ovary. Flowers actinomorphic to zygomorphic - 4 orders and 23 families.
Subclass Monochlamydae: Flowers with either absence of or one non-essential whorl (perianth). Divides into 8 series: - 1 Series Curvembryae: Usually single ovule, embryo coiled around the endosperm. - 6 families - 2 Series Multiovulate Aquaticae: Aquatic plants with a syncarpous ovary and many ovules. - 1 family - 3 Series Multiovulate Terrestris: Terrestrial plants with syncarpous ovary and many ovules. - 3 families - 4 Series Microembryae: one ovule, small, tiny embryo endospermic seed. - 4 families -5 Series Daphnales: one carpel and one ovule - 5 families - 6 Series Achlamydosporae: Ovary inferior, 1 to 3 ovules, unilocular - 3 families - 7 Series Unisexuales: Flower unisexual, perianth usually absent - 9 families - 8 Series Ordines Anomali: Plants with uncertain systematic position but close to unisexuales - 9 families
Class Gymnospermae: gymnosperms in which seeds are not enclosed in fruits,
Places in between dicots and monocots, could not recognize the phylogenetic significance.
Divided into 3 families: Gnetaceae, Conferaceae and Cycadaceae.
Class Monocotyledonae: Includes angiosperms in which the seed bears only one cotyledon. Leaves exhibit parallel venation. Consists of Closed Vascular Bundles. Divides into 7 series': - 1 Microspermae: Ovary inferior; seeds minute and non-endospermic. - 3 families - 2 Epigynae: Ovary inferior, seeds large and endospermic. - 7 Families - 3 Coronarieae: Ovary superior, perianth petalloid. 8 Families - 4 Calycinae: Ovary superior, perianth sepalloid. 5 Families - 5 Nudiflorae: Perianth reduced or absent. Seeds are endospermic.- 5 Families - 6 Apocarpae: Carpels more than one, free, seeds are endospermic. - 3 Families - 7 Glumaceae: Perianth reduced or absent, scaly bracts present. - 5 Families
In total, Bentham and Hooker classified the angiosperms into 202 families while providing distinct diagnostic key characters to each.
Merits:
1. One of the most valuable contributions is the description of the taxa at all levels. Descriptions are accurate and it is easy to identify plant species up to family level.
Since the descriptions were based on direct observation, they have become models of accuracy.
They placed order Ranales at the beginning of the system, a reasonable choice.
The placement of dicots before monocots is also accepted by all modern taxonomists.
Demerits:
Gymnosperms are more primitive than angiosperms and should not have been placed between dicots and monocots.
The introduction of monochlamydeae is a drawback since the group consists of both advanced and primitive forms.
Among the monocots, Orchidaceae is placed in the beginning with all it's advanced characters.
The subdivision of monocots is based on the position of ovary and characters of perianth. This resulted in an anomalous situation for many families.
----------------------------------------------------------
Engler and Prantl
Also called Phylogenetic System of Classification
The work of Adolf Engler and Karl Prantl, two German botanists.
They classified algae to angiosperms based on their evolutionary trends into 13 divisions.
The 13th division Embryophyta Siphonogama includes the seed plants, which were divided into gymnospermae and angiospermae.
Angiospermae further divides into two classes monocotyledoneae and dicotyledoneae.

Salient Features:
Flowers without perianth or with one whorl of perianth are primitive.
Flowers with two whorled perianth are distinguished into sepals and petals as they are advanced.
Unisexual flowers are primitive and bisexual ones are advanced.
Zygomorphy and epigyny were traced to be advanced.
Apocarpy is regarded as a primitive character while syncarpy is advanced
The evolution of angiosperm is polyphyletic.
Monocotyledons:
The monocots regarded as more primitive than dicots therefore they have been placed before dicots
Monocoots include 11 orders and 45 families.
The orders with Pandanales and Helobiae have naked unisexual flowers.
The order Liliflorae: Liliaceae and Amaryllidaceae, the former has hypogynous and later epigynous flowers.
The order Glumiflorae- Graminae is in the fourth position. Due recognition is not given to its advanced characters
Orchidaceae is considered as the most advanced family and is placed at the end of monocots.
Dicotyledoneae:
divides into 2
1) Archichlamydeae
Includes Polypetalous and Monochlamydeous families: 33 orders
Naked, unisexual, wind-pollinated flowers.
They are grouped as homogenous called Amentiferae
Achlamydeous is followed by monochlamydeous
Families start with Ranales- flowers cyclic or spirocyclic and hypogynous.
Polypetalous ends with Myrtiflorae and Umbelliflorae with epigynous flowers.
2) Metachlamydeae
Sympetalous include gamopetalous families-actinomorphic, bisexual flowers.
Tubiflorae- 7th order under Sympetalae include families of Bicarpellatae-Hypogynous (Bentham and Hooker)
The link between hypo and epigynous flowers
Epigynous flower are placed in orders Rubiales, Cucurbitales, and Campanulatae.
Cucurbitacea-Polypetalae (B&H) is now placed in Sympetalae due to gamopetalous corolla.
Merits
This system of classification is a development over Eichler in many respects.
Gymnosperms kept under separate sub-division.
It is natural system since it was proposed subsequent to the acceptance of “Theory of descent” (the idea that species change over time, give rise to new species, and share a common ancestor).
The large artificial group of Bentham and Hooker's system Monochlamydaceae has been completely abolished.
Compositae and Orchidaceae treated as most advanced families of dicot and monocots respectively.
Demerits
Monocot treated more primitive than dicot.
Amentiferae and Centrospermae placed at the beginning of Ranales.
Helobae is placed in between Pandanales and Glumiflorae.
Derivation of bisexual flower from unisexual.
Parietal placenta is advanced over axile.
Derivation of entomophily from anemophily
--------------------------------------------------------
Angiosperm Phylogeny Group
Also called APG System
The Angiosperm Phylogeny Group is an informal international group of systematic botanist who came together to establish a consensus on the taxonomy of angiosperms that would reflect new knowledge about plant relationships discovered through phylogenetic studies.
An important motivation for them was what they considered deficiencies in prior classifications since they were not based on monophyletic groups.
Angiosperm Classification
In the past, classification systems were typically made by a single botanist or a small group, resulting in many systems. Different countries favored different systems.
Ex: The Engler system in continental Europe and the Bentham and Hooker system in Britain (preferred by Kew)
Before the availability of genetic evidence, angiosperm classification was based on morphology and biochemistry.
After the 1980s, detained genetic evidence analyzed by phylogenetic methods became available. While confirming or clarifying some relationships in existing classification systems, it radically changed others.
The genetic evidence created a rapid increase in knowledge which led to many proposed changes, posing problems for all the classification systems at the time.
The impetus came from a major molecular study published in 1993 based on 5000 flowering plants and a photosynthesis gene rbcL, producing shocking results.
At first there was reluctance to develop a brand new system entirely based on a single gene, but subsequent work continued to support these findings.
The studies involved were a huge collaboration between a very large number of scientists. Instead of naming each person's part individually, they opted to name the entire project Angiosperm Phylogeny Group classification or APG.
The first APG publication was in 1998 and was widely accepted. After that 3 revisions have been published: APG II in 2003, APG III in 2009, and APG IV in 2016.
13 researchers have been credited as authors for the 3 papers and 43 more as contributors.
Principles of the APG system:
principles of the APG's approach to classification were set out in the first paper of 1998, and have remained unchanged.
i) The Linnean system of orders and families should be retained. "The family is central in flowering plant systematics." An ordinal classification of families is proposed as a "reference tool of broad utility".
ii) Groups should be monophyletic. The main reason why existing systems are rejected is because they do not have this property, they are not phylogenetic.
iii) A broad approach is taken to define the limits of orders and families. It is said that a limited number of larger orders will be more useful. Families containing only a single genus and orders containing only a single family are avoided.
iv) Above or parallel to the level of orders and families, the term clades is used more freely.
APG I (1998)
The initial paper was the first to systematically re-classify angiosperms primarily on the basis of genetic characteristics.
The authors' views were that there is a need for a classification system for angiosperms at the level of families, orders and above, but that existing classifications were "outdated".
The main reason why existing systems were rejected was because they were not phylogenetic.
An ordinal classification of flowering plant families was proposed as a "reference tool of broad utility".
The broad approach adopted to defining the limits of orders resulted in the recognition just of 40 orders.
Only a handful of families had been adequately studied, but the primary aim was to obtain a consensus on the naming of higher orders
While the relationship of orders was established, their composition and order was not.
A major outcome of the classification was the disappearance of the traditional division of the flowering plants into two groups, monocots and dicots. The monocots were recognized as a clade, but the dicots were not.
A number of former dicots were placed in separate groups basal to both monocots and the remaining dicots, the eudicots or 'true dicots'.
APG II (2003)
The second paper was published as an update to the classification of 1998.
the focus shifted to the family level, in particular those families generally accepted as problematic.
consensus was achieved relatively easily resulting in an updated classification at the family level
The authors stated that changes were proposed only when there was "substantial new evidence" which supported them
The classification continued the tradition of seeking broad circumscriptions of taxa, trying to place small families containing only one genus in a larger group
The authors stated that they have generally accepted the views of specialists, although noting that specialists "nearly always favor splitting of groups"
APG II continued and extends the use of alternative 'bracketed' taxa allowing the choice of either a large family or a number of smaller ones.
Some of the main changes in APG II were: (important)
i) New orders were proposed, particularly to accommodate the 'basal clades' left as families in the first system.
ii) Many of the previously unplaced families are now located within the system.
iii) Several major families were re-structured. In 2007, a paper was published giving a linear ordering of the families in APG II, suitable for ordering herbarium specimens.
APG III (2009)
The third paper updates the system described in 2003.
The broad outline of the system remains unchanged, but the number of previously unplaced families and genera is significantly reduced.
This requires the recognition of both new orders and new families compared to the previous classification.
The number of orders goes up from 45 to 59; only 10 families are not placed in an order and only two of these (Apodanthaceae and Cynomoriaceae) are left entirely outside the classification.
Authors say that they tried to leave long-recognized families unchanged, while merging families with few genera. They "hope the classification will not need much further change."
A major change is that the paper discontinues the use of 'bracketed' families in favour of larger, more inclusive families. As a result, the APG III system contains only 415 families, rather than the 457 of APG II.
In the same volume of the journal, two related papers were published. One gives a linear ordering of the families in APG III; as with the linear ordering published for APG II, this is intended for ordering herbarium specimens.
APG IV (2016)
In the development there was some controversy over the methodology and the development of a consensus proved more difficult than in previous iterations. In particular Peter Stevens questioned the validity of discussions regarding family delimitation in the absence of changes of phylogenetic relationships.
Further progress was made by the use of large banks of genes, including those of plastid, mitochondrial and nuclear ribosomal origin
The fourth version was finally published in 2016. It arose from an international conference hosted at the Royal Botanical Gardens in September 2015 and also an online survey of botanists and other users
The broad outline of the system remains unchanged but several new orders and families are included, and some previously recognized families are lumped.
This brings the total number of orders and families recognized in the APG system to 64 and 416, respectively.
---------------------------------------------------------------
Herbarium
Meaning
A herbarium is a place where plants are collected from far and wide and preserved in a pressed and dried condition.
They are stores in pigeon hole almirahs according to accepted systems of classification.
The dried plant is pressed onto a sheet. Fleshy plants like Cactaceae (cacti) are preserved in preservatives instead of a dried state.
Herbaria were initiated by an Italian taxonomist Luca Ghini, but the concept of preserving plant specimens in dried form is 450 years old.
The first herbarium of the world was established in 1545 in University of Padua, Italy.
Present day Herbarium sheets have a definite size: 29x41cm. ± 1 cm.
---
Functions
is it an invaluable conservatory of plant material of flora from around the world, they provide one place to study it all.
The labels of herbarium sheets are valuable, they provide data for botanical, ethno-botanical, and phytogeography studies.
The herbarium serves as a helpful aid in teaching botany to students in institutions where they are present.
Preserved specimen are used in almost all types of taxonomic research, it is essential for biosystematics today to correctly identify and name plants.
The specimen are used as a source of material for anatomical, palynological and chemo-taxonomical studies.
They provide important data on places of plant occurrence, time of flowering, and other data for research in embryology, cytology, and ecology.
---
Kinds
Depending upon the interest of the organization or institution: - Herbaria of Organizations - Regional Herbaria - Local Herbaria - Herbaria of institutions, Universities, Colleges, etc.
Depending on categories: - Herbaria of drugs and medicinal plants - Herbaria of crop plants and weeds in cultivated fields etc.
Important Herbaria of the World - Royal Botanic Garden (Kew) - Museum National d’ Historia Naturelle (Paris) - Kemerovo Botanical Institute (Leningrad) - Conservatoire et Jardin Botaniques (Geneva)
Herbaria of India - Botanical survey of India, Andaman and Nicobar circle, Port Blair - Botanical survey of India, Arid zone circle, Jodhpur. - Botanical survey of India, Sikkim Himalayan circle, Gangtok, Sikkim. - Delhi University Herbarium, Delhi. - Lloyd Botanic Garden, Darjeeling. - School of Plant Morphology, Meerut College Meerut. (contains approximately 25,000 specimens).
---
Making of Herbarium
Involves collection, drying, poisoning, mounting, stitching, labeling and deposition.
Collection: Plants are collected first, angiospermic material should have grown leaves, complete inflorescence, flower and fruit etc.
Size of the material depends upon the requirements and availability. Herbaceous small plants may be collected with roots, but for woody plants just 4-6 twigs are enough.
One should not collect diseased, infected or inappropriate plant material.
The collection should be given a field number. The species should have least 4-6 specimens with same field number.
The habit, habitat, flower, color locality interesting features etc. should be noted down in a field note book.
Drying and Poisoning: The specimens should be preserved in blotting paper or newspaper after spreading it correctly.
It should be pressed in field press.
After 2-3 sheet changes, the specimen is dried.
To keep the specimen away from disease or pests poisoning is done. Chemicals like corrosive sublimate (HgCh) are either sprayed or painted on the dried specimen.
Mounting, Stitching, and Labelling: Dried specimen are glued and or stitched onto herbarium sheets made up of thick card sheets of 29 x 41 cm ± 1cm size and labelled.
Labels have all the information about Botanical name, Local name, Locality, time, characters, collector’s names etc.
After identification the sheet is placed in species cover.
All the species of one genus are placed in one genus cover, which finally is kept in family cupboard of Herbarium.
For keeping the specimen for long time, they should be protected from pests and insects like Silver fish and Book worm etc. DDT spray and or copper sulphate solution helps.
Identification and Determination of Plants:
Usually identification is the process through which specimen whose name is not known is recognized by its characters to known plant and given the name.
Now the practices are stopped since no plants are identical. The process is called determination and the slips are marked Determinovit (Det) slip.
For identification, the scientific method is to first study the character of plant, check them with the flora of the region, work keys and, compare with full description and illustration, then it is carefully compared with earlier identified plants of that species or variety.
If s plant does not fit in the key or match in the herbarium, it is compared to species of adjacent floras in large herbaria.
After identification the important process is to use correct nomenclature. Always use the latest nomenclature.
---
Problems in Management: - Lack of knowledge on significance. - Wrong notion that it is simply a storehouse of dead plants. - Lack of sufficient trained man-power - Lack of taxonomists (fucking fair) - Lack of funds
-------------------------------------------------------
ICBN
Nomenclature may be defined as the system of naming objects with one correct name which is scientific.
Binomial nomenclature: this was employed by Linnaeus in the first edition of ‘Species Plantarum’ (1753).
According to this the name of a plant consists of two Latin words. The first is generic epithet and the second species epithet. Ex: Morus alba
The foundations of ICBN are found in Linnaeus ‘ Philosophia Botanica’ (1751) wherein he proposed certain principles of nomenclature.
Augustin de Candolle’s ‘Theorie Elementaire de la botanique’(1813) gives detailed rules on plant nomenclature.
A precise and simple system of nomenclature was put forward by First International Botanical Congress held in 1867 in Paris.
Alphonse de Candolle proposed the laws of botanical nomenclature (Lois de la nomenclature) at this congress which was adopted with some modifications.
These rules are known as de Candolle rules or Paris Code (1867).
Later at the Cambridge congress certain refined changes were made and ICBN was adopted.
The code is divided into three parts: - Principles - Rules - Recommendations
Principles
The Principles form the basis of the system of botanical nomenclature.
The 6 Principles are: - Botanical nomenclature is independent of zoological nomenclature. - Application of names of taxonomic groups (taxa) is determined by means of nomenclatural type. - The nomenclature of taxonomic groups is based upon priority of publication. - Each taxonomic group can bear only one correct name the earliest that is in accordance with the rules except in specific cases. - Scientific names of taxonomic groups are treated as Latin regardless of their derivation. - The Rules of nomenclature are retroactive unless expressly limited.
The Rules give detailed prescription on all the points connected with the naming of plants.
The names contrary to a rule cannot be maintained.
Recommendations
The recommendations are often practical application of rules, their objective is to bring about greater uniformity and clarity.
The code has three appendices: - Appendix I - Appendix II - Appendix III
Appendix I deals with the names of hybrids.
Appendix II includes the names of families which are conserved.
Appendix III lists the names of genera which are conserved against the principle of priority because of their long use.
Rules
Some of the important rules are:
1) Ranks of Taxa:
According to Article 1 of the code the word taxa signifies taxonomic groups of any rank.
The rank of species is basic and the relative order of the ranks of taxa are species, genus, family, order, class, division and plant kingdom.
Thus species is included in a genus, each genus in a family and so on.
2) Typification:
According to Article 7 the application of names of taxa of the rank of family or below is determined by means of nomenclatural types.
The nomenclatural type is that element with which the name is permanently associated.
The type of a species or intraspecific taxon is an individual specimen, however, for small herbaceous plants the type consists of more than one individual specimen mounted on a sheet.
If a specimen cannot be preserved the type may be a description or figure.
The type for a genus is species that for a family is genus.
The following terms are used in the nomenclature of types: - Holotype: the one specimen or other element designated by the author as the nomenclatural type. - Isotype: a duplicate of holotype. Ex: If several herbaceous plants(mounted on separate sheets) or several branches of a tree are collected at the same time one is designated as type and the others become isotypes. - Lectotype: a specimen or other element selected from the original material to serve as a nomenclatural type when no holotype was designated at the time of publication or as long as it is missing - Syntype: Any one of the two specimens cited by the author when no holotype was designated or more than one specimen is designated as type. - Neotype: a specimen or other element selected to serve as nomenclatural type as long as all of the material on which the description of the new species was based is missing.
3) Priority:
Each taxon can bear only one correct name and the correct name is the earliest legitimate one except in case of limitation of priority by conservation.
The principle of priority does not apply to names of taxa above the rank of family.
4) Names of Families:
The name of a family is a plural adjective and is formed by adding the suffix -aceae to the stem of a legitimate name of an included genus ex. Rosaceae
5) Names of Species:
It is a binary combination consisting of the name of the genus followed by the specific epithet.
If an epithet consists of two or more words, these are to be united or hyphened like Hibiscus rosa-sinensis.
Specific epithet should not be a repeat of generic epithet, like Linaria linaria such names are tautonyms.
6) Names of Infraspecific Taxa:
An infraspecific taxon when containing the nomenclatural type or next higher taxon cannot be indicated by epithets.
Article 26 requires that in such case the specific epithet should be repeated unaltered in infraspecific taxon.
Ex: Lobelia spicata var.originalis McVaugh should be named Lobelia spicata Lam.var.spicata.
7) Names of Plants in Cultivation:
Plants brought from the wild into cultivation retain original names.
For example forms of Chrysanthemum parthenium brought into cultivation are not to be renamed.
8) Conditions of Effective and Valid Publication:
According to Article 29 of the code publication of new names and descriptions are effective when the printed matter is distributed to the general public or to at least ten botanical institutions with libraries accessible to botanists generally.
The date of effective publication is the date on which the printed matter became available.
9) Retention of Names of Taxa which are Divided:
When a genus is divided into two or more genera the original generic name must be retained for the genus including the type of species.
For ex. Lychnis dioica L. was divided by Miller into two species which were named as L. dioica L. Emend Mill and L. alba Mill.
10) Changes in Names of Taxa:
This may be required by transferring of the taxon or by its union with another taxon of the same rank or by a change of its rank. (Article 51).
0 notes
Text
Mod 2 Botany Sem End
Fabaceae - Pea Family
Classification: Spermatophyta Angiosperms Dicotyledons Polypetalae Calyciflorae Rosales Fabaceae
---
Derivation of Classification:
Spermatophyta: Seed bearing plants.
Angiosperms: Seeds enclosed within a fruit.
Dicotyledons: 1) Tap root system 2) Dorsiventral leaves 3) Reticulate venation 4) Mostly pentamerous flowers 5) Seed with two cotyledons
Polypetalae: 1) Perianth present; differentiated into calyx and corolla 2) Petals free
Calyciflorae: 1) Flowers actinomorphic or zygomorphic 2) Hypogynous, perigynous, or epigynous 3) Cup shaped thalamus called calyx tube may be present 4) Stamens 10-many, inserted on the calyx tube 5) Monocarpellary gynoecium with marginal placentation
Rosales: 1) Leaves alternate and stipulate 2) Flowers bisexual 3) Actinomorphic or zygomorphic 4) Monocarpellary
---
Family:
Distribution: This family includes 600 genera and 12800 species, cosmopolitan distribution.
Habitat: Terrestrial mesophytes. Exceptions: Aeschynomene - hydrophyte Alhagi maurorum - xerophyte
Habit:
Mostly herbs - Pisum spp., Medicago
Few shrubs - Tephrosia, Crotolaria
Some trees - Butea frondosa (aka flame of the forest), Erythrina
Some herbaceous stem climbers/twiners - Dolichos, Clitoria.
Herbaceous tendril chambers - Pisum spp, Lathyrus spp.
Root System: Tap root system with bacterial nodules. These plants are used in crop rotation and as green manure.
Stem: Aerial, erect or climbing, strong or weak, solid, branched, herbaceous or woody, cylindrical or angular, smooth or hairy.
Leaves: Alternate, mostly compound
Unipinnately compound - Clitoria, Tephrosia, Pisum.
Pinnately trifoliate - Dolichos
Palmately trifoliate - Crotolaria
Simple - Crotolaria retusa
Stipulate or Exstipulate, stipules are foliaceous in Lathyrus and Pisum. They take on the function of photosynthesis.
In Pisum the terminal leaflets are modified into tendrils.
In Lathyrus the entire leaf is modified into tendrils.
In Dolichos and Clitoria the leaflets are stipelate.
Leaf is petiolate, pulvinous leaf base, leaflets – ovate, oblong, margin entire, apex acute, obtuse or retuse, pinnately reticulate venation.
In Desmodium gyrans (Indian telegraph plant) the central leaflets exhibit autonomous movements in response to variation in temperature.
Inflorescence: Axillary or terminal, racemose – simple raceme, solitary in Cicer.
Flower: Bracteate, pedicellate, bracteolate (Crotalaria, Clitoria) or ebracteolate (Dolichos, Tephrosia), actinomorphic, zygomorphic, complete, bisexual, hypogynous, pentamerous.
Calyx: 5 sepals, gamosepalous, valvate aestivation and persistent, odd sepal anterior in position.
Corolla: 5 Petals, polypetalous, papilionaceous corolla or butterfly shaped.
The large outermost posteriorly placed petal is called standard/vexillum; it overlaps the two median lateral petals called wings/alae; which in turn overlaps two antero-lateral petals called keel/carina.
Descendingly imbricate aestivation or vexillary.
Androecium: Ten Stamens,
Mostly diadelphous - Tephrosia, Dolichos and Clitoria.
Crotolaria: five long and five short, monoadelphous condition,
Dalbergia spp: 9 stamens, monoadelphous.
Anthers are dithecous, introrse, basifixed, or dorsifixed.
Gynoecium: Simple, monocarpellary. Ovary superior, long cylindrical (Pisum) or laterally compressed (Crotolaria).
Gynophore may be present. Style long, terminal but at the base in Crotolaria it bends ends in hairy stigma.
Fruit: Legume or pod
Seed: Seeds are endospermic
Floral Formula:
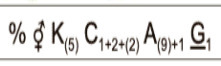
---
Economic Importance:
Most of the crops are used as fodder for cattle.
Pulses: Pisum sativum - Garden pea, Glycine max - Soybean
Vegetables: Phaseolus vulgaris - Kidney bean
Oils: Arachis hypogea - Groundnut
Timber yielding: Dalbergia latifolia – Indian Rosewood,
Indigofera tinctoria – the leaves yield indigo dye used in dyeing and printing cotton and rayon and pigments for paints and ink.
-------------------------------------------------
Embryology in Relation to Taxonomy
Schnarf, a German embryologist, studied the role of embryology in 1931.
The angiosperm embryological characters of importance are: anatropous, double fertilization, triple fusion, post fertilized triploid endosperm, and dicot or monocot conditions.
Embryology in relation to taxonomy can be observed at three levels: above family level, at family level, and at generic level.
---
Above Family Level
1) Caryophyllales
Commonly known as centrospermae
Have trinucleate pollen
Bitegmic crassinucellate ovule
Curved and peripheral embryo
Perisperm with or without endosperm.
2) Helobia
Have helobial type of endosperm
-(a cell wall is laid down between the first two nuclei, after which one half develops endosperm along the cellular pattern and the other half along the nuclear pattern.)-
3) Gentinales
Lack of integumentary tapetum
Have nuclear endosperm
Buddleiaceae and Oleaceae have integuments and cellular endosperm
4) Orchidales
Have an undifferentiated embryo
Very little to no endosperm
---
At Family Level
1) Podostemaceae
Includes perennial aquatic herbs with unique embryo features such as:
Pseudo embryo sac
Bisporic type of embryo sac
Pollen grain in pairs
Tenuinucellate bitegmic ovule
Presence of suspensor
2) Onagraceae
-(THe one where the megaspore divides one less time, only forming 4 nuclei instead of 8)-
Monosporic 4 nucleate, oenothera type embryo sac (with the exception of trapa)
Embryo sac derived from micropylar megaspore of tetrad
Only egg apparatus, 2 synergids, and one polar nucleus form.
Antipodals and one polar nucleus absent.
3) Cyperaceae
Microspore mother cell only gives one funtional microspore
4) Loranthaceae
Actually has 2 sub families, Loranthoideae and Viscoideae
i) Loranthoideae - Triradiate pollen - Polygonum embryo sac - Composite endosperm - Presence of suspensor - Polyembryony
ii) Viscoideae - Spherical pollen - Allium type embryo sac - Non-composite endosperm - Absence of suspensor - Polyembryony - The subfamilies have recently changed to Loranthaceae and Viscaceae respectively
At Generic Level
1) Trapa
Kept under Onagraceae (B&H) and Trapaceae (Englerian)
Evidence for both: -(????)-

2) Paeonia
Used to be included in Ranunculaceae (characters not present), now in Paeoniaceae
Generative cells are longer and elongated
Embryo sac long and narrow
Seed coat is massive
Germination epigeal
3) Exocarpus (Previously Santalaceace)
Initially placed in Exocarpaceae (under gymnosperms) due to the presence of naked ovules and pollen chamber.
But later it was confirmed to be Santalaceae due to the:
Presence of angiospermic flowers
Polygonum embryo sac
Cellular endosperm
And transverse division in zygote
4) Butomus
Polygonum embryo sac
Other genera of the family Butomaceae process Allium type embryo sac
With the exception of Butomus, all other genera have been transferred to Alismataceae
I'm failing
--------------------------------------------------------------------
Cytotaxonomy
Cytology in relation to taxonomy
The application of cytological data in solving taxonomic problems.
The characteristics of the chromosomes, which have proved to be of taxonomic value include: - Chromosome number - Chromosome size - Chromosome morphology - Chromosome behavior
Chromosome Number: - The chromosome no. is constant and same in all individuals of a species. - It is used as a confirmative property to distinguish a species from members of other species. - Can be divided into: - Separation at species level - Separation at interspecific level - Separation at generic level - Separation at family level - Ex: Monotropa hypoptiys and Monotropa hypophegea are two difference species that are morphologically similar, but they are separated on the bases of chromosome no. - M. hypopitys is hexaploid (2n=48) - M. hypophegea is diploid (2n=16) - It is usually seen that closely related plants, like the different species of a genus, show chromosome numbers with reveal an arithmetic relation with one another.
Aneuploids: - Plants with basic no of chromosome with some addition or deletion of a few chromosomes. - Monosomics: 2n-1, like in Datura - Nullisomics: 2n-2, like in Triticum - Tricomics: 2n+1, like in Wheat - Tetrasomics: 2n+2 chromosome
Chromosome Size: - It has already been discovered that evolutionary development involves in addition to alterations in chromosome number - Ex: Cytologically, Cyclea and Cissampelos are seen to be based on 12, while Stephania shows n=13. It is seen that the number n=13 is characteristic of the tribe Cocculeae, which further shows chromosomes of small size. - Large chromosomes, low chromosome no., and symmetrical karyotype represents a primitive status. - Small chromosomes, high chromosome no., and extreme asymmetry indicates advancement.
Chromosome Morphology: - Karyotype of plants is now very useful for the classification of some plants. - Monocotyledons have larger chromosome no. than dicot. - Woody plants have smaller chromosome no. than herbs. - The position of centromere and satellite is considered in classification: - ex 1: Human chromosomes are divided into 7 groups and sex chromosomes: - ex 2: Also, the shape of metaphase chromosome is considered for classification.


Chromosome Behavior - It provides clues about the cause of sterility and fertility among populations - Structural difference in the paternal chromosome is the main cause for the sterility - If the two sets of paternal chromosomes are homologous, the plants seem to be fertile.
---
Significance of Cytotaxonomy:
The role of cytotaxonomy is very important in taxonomic studies.
Cytotaxonomy is more significant, the process is dealing with the comparative study of chromosome and with this method minute variation among the individuals can be detected.
DNA is present in the every chromosome and the variations in each DNA are responsible for the variation among the individuals, species, genus and everything.
When the differences of physiological variations are too less among the individuals of same species and other higher taxa, Cytotaxonomy is a part of taxonomic biology that deals with the classification of organisms.
Cytotaxonomy classifies these organisms based on their function and cellular (DNA) structure. As the cytologic data are directly derived from nucleus, the seat of hereditary material, they may be used for understanding the evolution and relationships of population.
The characteristics of karyotypes are taxonomically useful where the individual chromosomes are large enough for detailed microscopic observation.
In this branch, another useful taxonomic character is the position of centromere. Meiotic behavior may show heterozygosity of in versions. This may be constant for a taxon, offering further taxonomic evidence.
--------------------------------------------------------------
Chemotaxonomy
The chemical constituents of plants differ from one species to another
They are restricted to certain taxa, making them valuable characters for plant classification.
The classification of plants on the basis of chemical contents is called chemotaxonomy or chemical taxonomy.
The following chemicals are present in plants and accounted for classification:
- Non-protein amino acids
- Phenolics
- Betalins
- Alkoloids
- Terpenoids and Steroids
- Crystals
- Immunological reactions
1) Non-protein Amino Acids
There are 300 non-protein amino acids in plants
Some are restricted to certain groups alone
They are used to classify and distinguish the taxa from others
Ex: Lathyrine in genus Lathyrus
Ex: add one more
2) Phenolics
Derivatives of phenolic compounds
Plants are classified on the basis of specific phenolic compounds.
Ex: Flavonols and methoxy cinnamic acid - herbaceous plants
Ex: Leucoanthocynin - woody plants.
3) Betalins
Derivatives of phenols serving as pigments.
They are present in only 10 families.
Ex: The position of the family Cactaceae was disputed for many years, but on the basis of the presence of betalins, it's position in centrospermae has been confirmed.
4) Alkaloids
Nitrogen containing compounds with a heterocyclic ring.
There are about 5000 different alkaloids in angiosperms.
They are used as a source for plant classification.
Ex: Lupin - Fabaceae
Ex: Tropane - Solanaceae
Ex: Morphine - Papaver somnifer
5) Terpenoids and Steroids
Terpenoids are unsaturated hydrocarbons derived from isoprenes
Ex: Carotenoids, iridoids
Steroids are saturated hydrocarbons with four rings in their structure
Ex: Cucurbitins present in Cucurbitaceae
6) Crystals yay
Some plants have raphide crystals in different parts of their body.
The forms of crystals are used to some extent in the classification of plants.
Ex: Presence and absence of raphides are used in the grouping of plants in the family Rubiaceae
Calcium oxalate crystals are present in the ovary walls of the members of Asteraceae.
7) Immunological Reactions
The storage protein or pollen protein is injected from the plant body to a test animal (usually a mouse or rabbit)
The test animal produces antiserum against the protein
The antiserum is mixed with the plant extract to detect the precipitate formed by antigen-antibody reaction.
The nature and amount of precipitate indicate the relationship of the protein to the plant.
High rate of precipitation indicates closeness of the plants.
Low rate shows that the plants are not related.
This type of study is called serotaxonomy.
Ex: Closeness of Delphinium to Aconitumis has been confirmed by serological studies.
--------------------------------------------------------------
Numerical Taxonomy
Numerical taxonomy or taximetrics is taxonomy which uses mathematical methods to find similarities and differences.
It is based oh phenetic evidences (phenotype) published by Sokal and Sneath in 1963.
Basic aspects:
I) Construction of Taxonomic Groups: - Individuals are selected and their characters are spotted - There is no limitation in the number of characters. - The greater the number, the better the approach (at least 100 characters) - General character analysis
II) Discrimination of Taxonomic Groups: - When groups show a overlapping of characters. - The discrimination between them is done by analysis technique
---
Construction of Taxonomic Groups:
Phenetics: - Based on the similarities between phenetic characters - There is no idea of ancestral relationship - Taxa are connected at different levels according to the overall similarity in the form of a tree diagram called phenogram.
Cladistics: - Based on the relationship between individuals to evolutionary history. - There is a common ancestry - The taxa re connected in a tree called a cladogram.
There are 5 steps in the construction of taxonomic groups: i) Operational Taxonomic Units ii) Unit Characters or Attributes iii) Selection of Unit Characters iv) Estimation of Similarity v) Similarity Matrix
1) Operational Taxonomic Units:
Can be written as OTUs
The basic unit of study in numerical taxonomy
Can be individual, species, genus, family, order, or class based.
OTU is not comparable to formal taxonomic units
Comparison made only with equal rank - Genera are compared with different species - Family with different genera
2) Unit Characters or Attributes:
Characters used in numerical taxonomy are called unit characters
According to Sokal and Sneath 1963- “Taxonomic character which exists in one or more states”
Only the presence or absence of phenetic characters are considered.
Divides into two types:
a) Binary Characters: - Unit characters with two contrasting states - Ex: Presence or absence of trichomes - Ex: Fruits dehiscent or indehiscent - Positive Characters can be denoted as + or 1 - Negative Characters can be denoted as - or 0 - Missing characters are denoted as NC (no comparison)
b) Multistate Characters: - Characters in more than 2 states - Characters coded into number of states: i) Qualitative Multistate Characters: - They contain 3 or more contrasting forms, ex: flower color. - They are analyzed by converting them into a series of binaries. ii) Quantitative Multistate Characters: - Measures size on a continuous scale, ex: Length of leaf/height of plant.
3) Selection of Unit Characters:
According to Sneath & Sokal:
They should come from all parts of an organism
Belong to all stages of the life cycle of organism
Variable characters within the group used
Attention is given to characters related to morphology, physiology, ecology, and the distribution of organism
All characters are given equal value
4) Estimation of Similarity
The resemblance between a pair of OTUs
Similarity- % of characters in which they agree
Dissimilarity- % of characters they do not agree
Can be calculated with:
a) The Coefficient of Association (S):

The number of possible different combination of matches and mismatches of characters of 2 OTUs in a conventional 2x2 table:

b) Coefficient of Correlation (r):

c) The Measurement of Taxonomic Distance between OTUs (d):

5) Similarity Matrix
Simple matching indices found for all OTUs
Similarity arranged in a test table
10x10 similarity matrix for 10 OTUs

3 clusters can be regarded as three phenons
Group of similar organism by numerical method are phenos
Phenos are arranged in phenogram or dendrogram
Since this process of rearrangement is based on visual inspection, it is difficult to achieve with more than 10 OTUs.

Sokal and Sneath described shaded similarity matrices
The range of similarity is indicated by squares with different densities of shading

Advantages: - Helps in interpretation of wide range of data - Gives better classification - Re-interpretation possible
Disadvantages: - Selection of characters can be difficult - Numerical species recognized by this method are unacceptable
0 notes
Text
Mod 3 Botany Sem End
Ayurveda
Intro
Ayurveda is derived from the sanskrit words 'ayu' - life and 'veda' - knowledge. It means the 'science of life'
It is the most ancient system of medicine dating back to the Vedic age (1500-800BC)
This medical system is considered to be divine in origin as the father of Ayurveda, Dhanvantari, received knowledge from Lord Brahma, the god of creation
Ayurveda is also called Brahma Sampradaya.
---
Principles
1) Panchmahabhootas
Everything in the universe is made up of 5 basic elements:
- Aakash (Space)
- Prithvi (Earth)
- Jal (Water)
- Agni (Fire)
- Vayu (Air)
2) Tridoshas
The 5 basic elements are represented in the human body as 3 basic forces within:
- Vaata (Vayu + Aakash): Directs nerve impulses, circulation, respiration, and excretion.
- Pitta (Agni + Jal): Governs digestion, visual perception, and hunger.
- Kapha (Prithvi + Jal): Works at a cellular level, maintains stability, and gives the body strength.
Good health depends on the balance and equilibrium of the 3 doshas.
An imbalance leads to disease.
3) Dhatus
There are 7 types of dhatus (tissues) which make up the body:
- Rasa (Plasma, body fluids)
- Rakta (Blood)
- Mamsa (Muscle)
- Meda (Fat)
- Shukra (Hormones and Secretion of Genitals)
- Asthi (Bones)
- Majja (Bone marrow)
4) Malas
There are 3 ways in which the body expels waste:
- Purisa (Feces): For healthy digestion
- Mutra (Urine): Regulated fluid balance and blood pressure
- Sweda (Sweat): Controls body temp. and maintains electrolytic balance in the body
5)Srothas
Includes the pathways through which the nutrients, hormones, and waste flow through the body.
6) Trigunas
The same way the body is governed by the tridoshas, the mind is governed by the trigunas:
- Satwa: Brings about noble, kind, and good thought in a person, making them righteous and spiritual.
- Rajas: Responsible for movement, activity, desire, and passion.
- Tamas: responsible for laziness, oversleeping, and ignorance.
---
Diagnosis
Ayurveda aims at
- Curing the disease
- Enhancing vitality of the body
- Developing immunity towards diseases
Diagnosis begins with a basic pulse examination (Naadi Pariksha), followed by observing abnormalities in the eyes, tongue, skin, and ear.
A detailed account is made with respect to a patient's sleep patters, physical fitness, body measurements, thirst, and appetite.
Treatment can only begin when the physician thoroughly understand the imbalance in the tridoshas.
Ayurvedic drugs are primarily plant based with few drugs having animal and mineral origin.
The drugs are in the forms of powders, pastes, oils, ointments, decoctions, etc.
---
Treatment
There are 4 ways in which a patient can be treated:
1) Shamana Therapy
The disturbed dosha is restored to its original balance without causing any imbalance in other doshas.
This is achieved by administering carminatives (relieves gas that is accumulated in the alimentary canal), or by giving drugs that induces thirst and hunger.
Therapy also includes exercise and exposure to early sun.
2) Shodhana Therapy
Includes drugs that cause
- Emesis (Vomiting)
- Purging (Purifying)
- Enemas (Injection of fluids into rectum to relieve gastro-intestinal discomforts)
- Nasal administration of medicines
- Blood purification
All of the above features clean the body and are called Panchakarma
3) Diet and Exercise
4) Surgery
Only those diseases which cannot be cured by drugs are subjected to surgery.
------------------------------------------------------------
Siddha
Siddha is a traditional system of medicine having its origin in South India.
It stands for “Perfection in life”
It is said to be divine in origin as it is believed that ‘Lord Shiva’ explained this system to sage ‘Agasthya’ (Father of Siddha).
Hence Siddha is also called as ‘Shaiva Sampradaya’.
14 manuscripts on Siddha medicine are preserved on Palm leaves, as classics in the ‘Saraswati Mahal library’ at ‘Tanjore, Tamil Nadu’.
The people who practice this system of medicine are called as ‘Siddhars'
---
Principles
A healthy soul can only be developed from a healthy body.
1) Everything in the universe is made up of 5 basic elements, similar to Panchamahabhootas:
- Aakasham – space
- Thee – Fire
- Munn – Earth
- Vayu – Air
- Neer - Water
2) Like Ayurveda, Siddha also believes in the concept of Tridoshams, 3 humors or the 3 vital forces:
- Vatham – predominant during childhood. occupies regions related to the pelvis and the rectum.
- Pittam – during adulthood. occupies regions related to the stomach and the viscera
- Kapham – during old age. occupies regions related to breath, the throat, and the head.
When the equilibrium of the 3 humors is disturbed, disease arises.
3) The 7 Dhatus (body tissues) include:
- Rasa/Saram (Plasma) – responsible for growth, development and nourishment.
- Cheneer (Blood) – for nourishing muscles, imparting colour and improving intellect.
- Oon (Muscle) – for shape of the body
- Kozhuppu (Fatty tissue) – for oil balance and lubricating joints
- Elumbu (Bone) – for body structure, posture and movement
- Majjai (Bone marrow) – for formation of blood corpuscles
- Sukkilam (semen) and sronitha (for reproduction)
4) The 3 ways through which the body expels waste
- Feces
- Urine
- Sweat
---
Diagnosis and Treatment
The diagnosis of a disease is based on 8 factors:
- Na – tongue
- Naadi – pulse
- Neer – urine
- Sparsham – touch
- Swara – voice
- Kan – eyes
- Varna – skin colour
- Mala – body waste
Urine examination is considered to be very peculiar when it comes to diagnosis in siddha
Urine is collected in a porcelain bowl and a drop of gingelly oil is added. The spread and color change in oil is recorded.
Siddha medicine included drugs that are plant based, mineral based and metallic origin.
Drugs are classified into 6 classes based on their ability to dissolve in water and effect of heat on its nature.
- Uppu (salt) - water soluble, gives out vapor on heating
- Pashanam (rocks) - insoluble in water but give out vapor when heated
- Uparasam (minerals) – similar to pashanam, heat resistant and differ in action, ex: mica
- Loham (metals) – insoluble in water and melt when heated
- Rasam (soft drugs) – soluble in water, ex: mercury
- Gandhakm (sulphur) - insoluble in water
Treatment is aimed at keeping the 3 humors in equilibrium and the maintenance of 7 tissues.
Proper diet, exercise, timely medicine and disciplined life are advised for restoring the equilibrium of humors in diseased condition.
Treatment is classified into:
1) Divine method (Deva Maruthuvam)
The medicines prepared from metals and minerals come under this topic.
A very small dose brings quick recovery even from chronic ailments.
Most of these medicines have no expiry date that as they can be preserved life-long.
Ex: Chendooram - Red color powder (metallic compounds)
2) Rational method (Manida Maruthuvam)
They are herbal medicines which have short definite life span.
Dose may vary accordingly.
Ex: Charu (juice)
3) Surgical method (Asura Maruthuvam)
These are surgical procedures meant for diseases which are not cured alone by internal medicines.
Done by incision, excision, heat application (steam therapy), blood letting (the surgical removal of some of a patient's blood for therapeutic purposes.) etc.
------------------------------------------------------------
Unani
The Unani system of medicine originated in Greece (Yunan in Arabic).
It is the Perso-Arabic system of medicine was based on the teachings of the Greek physicians.
Hippocrates, 460-377 BC is said to be 'father of Unani medicine’.
After the decline of Greek-Roman empire, the medicinal system of the Greeks was upheld by the Muslims as the Unani medicine.
Later, Prophet Mohamed (Founder of Islam) and his successors (caliphs) in the Arabic countries took interest in expanding the Unani medicine.
Jundishapur, on the border of Persia became the center of Unani medicine.
The Unani system was brought to India by Muslims during their rule.
---
Principles
The Unani physicians regarded human body to be composed of 7 natural principles:
1) Al-Anasir (Elements):
- Includes air, water, fire, and earth.
- The human body is also constituted with these four elements:
Naar (fire)
Hawa (air)
Ma (water)
Arz (earth)
Al-Mizaz (Temperments):
- represents the physico-chemical aspects of the body.
- Any change in temperament results in change in person’s health.
- Ex: Hot, Cold, Dry, Wet, combinations of them
Al-Akalt (Humors/Body Fluids):
- includes the structural components of the body.
- There are 4 humors:
Blood (Khoon)
Phlegm (Balgham)
Yellow Bile (Safra)
Black Bile (Sauda)
- These 4 humors are utilized by body as nutrient components for growth and repair of organs and to yield energy.
- Thus ‘humoral balance’ is required to be maintained in the body to remain healthy.
- Any imbalance leads to disease.
Al-Aza (Organs)
- 4 organs of primary importance:
- Heart
- Brain
- Liver
- Testicles/ovaries
- All other organs are governed by them. Thus initially these organs are monitored in case of a disease.
Al-Arwah (Vital Spirit)
there are 3 forces administered in Unani system:
- The vital forces – these are the life forces and they arise in heart
- Natural forces – these forces arise in the liver
- Psychic forces – these forces mediate the behaviour, cause voluntary movement and create sensation. These forces arise in brain.
Al-Quwa (power/energy)
Unani system postulates that the body itself contains a mechanism of healing.
This power restores any disturbance in the body.
Al-Afal (functions)
Includes the physiology of the body including biochemical processes.
---
Diagnosis
Disease diagnosis is carried out in the following steps:
- Body heat: measured by pulse and palpitation (rapid, strong or irregular heart beat)
- Urine examination: indicates the disorders in kidney, liver and digestive organs
- Stool examination: helps in diagnosis of indigestion problems, acidity and presence of worms
- Examination by touch, tapping the body to hear sound: used to diagnose disease of internal organs
- Examination of blood pressure
---
Treatment
The Unani physicians are called as ‘Hakims’ or ‘Tabibs’.
Treatment includes:
1) Diet Therapy
- Treating the ailments by regulating the quality and quantity of food intake.
- Relates to the timing of food intake
- Food intake should not be delayed and also not to be eaten unless there is appetite.
- Some light activity should be practiced after the meal, like walking.
2) Drug Therapy
- Use of naturally occurring drugs of herbal, animal and mineral origin.
- Both single and compound formulations are used in the treatment.
- Examples of drugs include – beetle nut, Chandan, imli, cloves, nutmeg etc.
3) Surgery
- the last therapeutic measure to restore the health.
------------------------------------------------------------
Homeopathy
Introduction
Homeopathy is basically a western system of medicine that became popular in India.
It is derived from 2 Greek words, 'Homois' - similar, and 'Pathos' - suffering.
It is developed by a German doctor, Samuel Hahnemann in the late 1700s
This system of medicine is a 'holistic approach' that takes into consideration the whole person and the relation of the life style to the disease.
Its main aim is to bring back the lost equilibrium of the sick individual by stimulation the defense mechanism.
Hahnemann put forward the Law of Similar: Similia Similibus Curentur, which means 'like cures like'.
He believed that symptoms that are the outward reflection of the body's fight to overcome the illness
A homeopathic doctor does not treat the name of the disease, rather the treatment is targeted against the patient.
Hahnemann published an article titled 'Essay on the new principle for ascertaining the curative power of the drug' in 1796, in this he postulated that 'a drug cured those symptoms of a disease, which it can produce when taken by healthy individual, like cures like.'
ex: Red onion makes your eyes water, that's why it is used in homeopathic remedies for allergies.
Homeopathy in other words means 'like disease'. This means that the medicine given is like the disease that the person is expressing.
---
Homeopathy in India
It was introduced in 1839 when Dr. John Martin Honigberger successfully treated Maharaja Ranjit Singh for paralysis of vocal cords.
Later Dr. A.L. Sircar edited the first homeopathic journal 'Calcutta journal of medicine' in 1868.
In 1881, Dr. P.C. Mujumdar and Dr. D.N. Roy established first homeopathic college named 'The Calcutta homeopathic medicinal college'.
Gradually, homeopathic dispensaries were opened slowly in other cities and states.
---
Principles
1) Similia Similibus Curentur (like cures like)
This law states that which can cause can cure.
The onion which produces tears in eyes and irritation (similar to a cold) can be used as homeopathic medicine to cure cold.
This the the law of cure.
2) Simplex Similimum Minimum
This principle consists of 3 words:
- Simplex:
Simple medicines should be prescribed.
This is the law of single remedy
Compound medicines are not allowed
- Similimum:
The totality of the symptoms of the patient must be taken
This will lead a picture that corresponds to one medicine
- Minimum:
A low dosage of medicine is recommended
Medicines for low potency when given for long duration have better impact.
3) Principle of Individualization
It states that one must treat the patient, not the disease.
Not two human beings are alike, so the medicines use for their treatment need not be alike.
Medicines are prescribed based on 'totality of symptoms' of the individual, so the name of disease is not important to the doctor.
4) Principle of Potentization
Homeopathic medicines are diluted in alcohol or water or milk sugar/lactose to make them more palatable.
The more the medicine is dilutes, the more powerful it becomes.
The process of dilution is called as 'Potentization'
The medicines are referred to as 'potencies'
5) Law of Direction/Direction of Cure
Healing often progresses from more important organs to less important organs, thus the health of the heart or brain improves before that of less vital organs like the stomach or joints.
Healing often follows a downward course, from head to foot. This pathway of symptom movement may be understood by remembering that the head houses the brain.
Symptoms disappear in the reverse order of their appearance.
Ex: The first symptoms to appear will be the last to resolve, while the most recent symptoms will be the first to disappear.
Healing often proceeds from within the organism and extends outwards.
---
Diagnosis and Treatment
The homeopath first tries to study the nature of symptoms of the illness.
In diagnosis, the whole range of patients mental and emotional and physical state is considered to understand the state of patients defense mechanism.
After the process of diagnosis comes the principle of individualization, hence every case is treated as separate individual.
The physician's interest is not to just cure the diseases but also the long term well-being.
These medicines are used in the form of pills, powders, and diluted liquid formulations.
The strength of this treatment lies in the wholistic approach towards the sick individual.
Ex of medicinal plants used in drug prep: Tulasi, Tippateega, Aswagandha, etc.
This system uses mineral salts that are concerned with the functional activities of the human body. There are 12 in number:
- Calc Fluor: Strengthens teeth enamel and bones
- Calc Phos - Restores cells and heals fractures
- Calc Sulph - Purifies blood and reduces infection
- Ferr Phos - Anti-inflammatory and reduces fever
- Kali Mur - Purifies blood
- Hali Phos - Supports nerve health
- Kali Sulph - Heals mucous membrane
- Mag Phos - Eases cramps
- Nat Mur - Balances body fluids
- Nat Phos - Neutralizes activity, aids digestion
- Nat Suplh - cleans kidney, liver, and treats cold and flu
- Silica - Cleanses blood, conditions skin and connective tissue
------------------------------------------------------------
AYUSH
The department of 'Ayurveda, Yoga and Naturopathy, Unani, Siddha, and Homeopathy' abbreviated as AYUSH, is a givernment in India.
The department started 1995 as the department of 'Indian Systems of Medicine and Homeopathy' (ISM&H). AYUSH received its current name in 2003. Then it was operated under Ministry of Health and family Welfare.
The Ministry of AYUSH was formed on 9th November, 2014 by the elevation of department of AYUSH.
AYUSH is a common Hindu name, derived from Sanskrit meaning 'Life'.
---
Bodies Under AYUSH:
Research Councils:
- Central Council for Research in Ayurvedic Medicine Science (CCORAS)
- Central Council for Research in Siddha (CCRS)
- Central Council for Research in Unani Medicine (CCRUM)
- Central Council for Research in Homeopathy (CCRH)
- Central Council for Research in Yoga and Naturopathy (CCRYN)
- Pharmacopoeial Laboratory for Indian Medicine (PLIM)
National Institutes:
- National Institute of Ayurveda (NIA), Jaipur
- National Institute of Siddha (NIS), Chennai
- National Institute of Homeopathy (NIH), Kolkata
- National Institute of Naturopathy (NIN), Pune
- National Institute of Unani Medicine (NIUM), Bangalore
- Rashtriya Ayurveda Vidyapeeth (RAV), New Delhi
---
Roles:
1) Upgradation of educational standards in Ayurveda, Yoga, Siddha, Unani, and Homeopathic colleges in the country.
2) To conduct time bound research programs on identified diseases to strengthen research activities.
------------------------------------------------------------
NMPB
NMBP stands for the National Medicinal Plant Board.
It was set up in Nov 2000, by the gov of India under the Department of AYUSH.
Located in New Delhi.
It looks into all aspects of medicinal plants and also their large scale cultivation.
It has prioritized 32 medicinal plants for cultivation, conservation, and development.
Ex: Amla, Brahmi, Chandan, Pippali, Sarpagandha, Tulasi, etc.
---
Roles:
1) Developing a database having published information on selected medicinal plants -> as a source of information for student, teachers, and researchers as well.
2) Identification and cultivation of medicinal plants.
3) Organizing programs for the growth of trade, export, conservation, and cultivation
4) Undertaking scientific research activities.
5) Guides farmers in procuring quality planting material and their marketing.
6) Takes up the extension activities like training the farmers as well as the students.
7) Popularizes home and school herbal gardens.
8) The board develops protocols for cultivation and quality control.
------------------------------------------------------------
CIMAP
Central Institute of Medicinal and Aromatic Plants is a research institute of CSIR (Council of Scientific and Industrial Research), with its headquarters in Lucknow.
It is involved in the field of science and business of medicinal and aromatic plants.
CIMAP has 4 research centers situated in Bangalore, Hyderabad, Pantnagar, and Purara.
Established originally as CIMPO (Central Indian Medicinal Plants Organization) in 1959.
CIMAP has signed scientific collaboration agreement with Malaysia to promote research.
---
Roles:
1) Engaged in the extraction of crude drugs and essential oils from the plants and their chemical analysis.
2) Developing new agro-techniques for the cultivation of plants on a larger scale.
3) Houses the national gene bank of medicinal and aromatic plants along with their seed banks.
4) Establishment of Bio-village approaches like Artemisia (U.P) is a model of Public Private Partnership (PPP).
5) Development of improved varieties of Withania, Mentha, Catharanthus are undertaken (Products like geranium oil, artemisin at a cheaper rate).
6) Plant tissue culture technology is being used for plant regeneration invitro.
7) Conducting several training programs and interactive meets to common people and research students.
8) Involved in knowledge dissemination through books and bulletins.
------------------------------------------------------------
CDRI
CDRI stands for Central Drug Research Institute
CDRI Lucknow is a multidisciplinary research institute functioning under CSIR
Established in the year 1951. It has 17 research and development (R&D) wings and a few technical and scientific support divisions.
Some of the R&D divisions are:
- Biochemistry
- Botany
- Chemical Medicine
- Endocrinology
- Medicinal Chemistry
- Microbiology
- Parasitology
- Pharmaceutics
- Pharmacology
- Pharmokinetics and Metabolism
- Toxicology
- Fermentation technology
In addition there are 2 data centers and 1 field station to assist in operational support.
---
Roles:
1) Development or formation of drugs and their marketing
2) Employs latest techniques and methodologies for preparing the drugs that later help in preventing diseases.
3) Using cellular and molecular studies to understand the disease process and reproductive physiology.
4) Evaluation of medicinal properties of natural products
5) Disseminates the information in the field of drug research, development, and production through publications
6) CDRI offers expertise to the Indian Pharmaceutical Industry, academic institutes, etc.
7) Conducting training programs and interactive meets to the research students.
------------------------------------------------------------
Tippateega
Botanical name: Tinospora cordifolia
Vernacular name:
- Telugu – Tippateega
- English – Heartleaf moonseed.
Family: Menispermaceae
Morphology:
- Woody climber with succulent stems
- Leaves are cordate and glabrous (smooth)
- Flowers are unisexual (yellow)
- Fruits are red and globose (drupe)
Useful part: Stem (dried)
Chemical constituents:
- Columbin
- Tinosporaside
- Tinosporic acid
- Berberine
- Giloinin
- Gilonin
Uses:
- Antiallergic
- Antidiabetic
- Antipyretic (prevents or reduces fever)
- Antispasmodic (suppresses sudden involuntary contraction of muscles)
- Anti-inflammatory, anticancer.
- Diuretic (increases frequency of urination)
- Aphrodisiac (arouses sexual desire)
- Stomachic (assisting in digestion)
- Anthelminthic
------------------------------------------------------------
Tulasi
Botanical name: Ocimum sanctum
Telugu name: Tulasi
Hindi name: Tulsi
English name: Holy basil
Sanskrit name: Vrinda
Family: Lamiaceae
Morphology: Erect, highly branched, softly pubescent aromatic sub shrub/annual herb.
Leaves range from elliptic to oblong, flowers white to purple, fruits are ellipsoidal nutlets - carcerulus.
Useful parts: The entire plant, especially the essential oil extracted from the leaves.
Chemical constituents: Eugenol (70%), Methyl eugenol (20%), Carvacrol (3%), Caryophylene (1%)
Uses:
- Antibacterial
- Insecticidal
- Diaphoretic (induces perspiration)
- Expectorant (clears lungs from excess mucus)
- Carminative (prevents formation or facilitates expulsion of gas
- Treats Catarrh (excessive discharge of mucus in the nose and throat due to inflammation of the mucous membrane)
- Treats cough, cold, gastric disorders, etc.
- Treats snake bite and scorpion sting.
------------------------------------------------------------
Pippallu
Botanical Name: Piper longum
Telugu Name: Pippallu
English name: Long Pepper
Family: Piperaceae
Morphological features:
- A slender aromatic herb, perennial with woody roots.
- Cultivated on large scale through layering.
- Fruits are small and ovoid, borne on fleshy spikes
Useful part: Roots, stem and fruits
Chemical constituents/Active principle:
- Alkaloids : Piperine, Piperlongumine, Piperlonguminine.
- Piperine analogues : Silvatine, Sitosterol.
Uses:
- Used as a preservative in pickles
- Treatment of urinary tract infections
- Treatment of cough, cold, piles, dysentery
- Used as a sedative
------------------------------------------------------------
Karaka
Botanical Name: Terminalia chebula
Telugu Name: Karaka
Hindi Name: Harda
English Name: Myrobalan
Family: Combretaceae
Botanical features :
- Medium sized tree
- Leaves: ovate to elliptical, sub opposite
- Flowers: white, in terminal panicle
- Fruits: drupe, ellipsoidal, 5 ribbed when dry
Useful part: Fruits (dried)
Chemical constituents/Active principle:
- Chebulic acid (tannin)
- Galloyl glucose
- Carbohydrates : glucose, sorbitol, sucrose and gentiobiose.
During maturation, tannins decrease
Uses:
- Used to make Triphala, an ayurvedic drug
- Astringent (shrink or constrict body tissues)
- Laxative (induces bowel movements)
- Treatment of chronic ulcers
- Dentrifice: Cleanses and polishes teeth (used as tooth powder)
- Wound healer
-Treatment of eye diseases, cough, and bronchial asthma
------------------------------------------------------------
Kalabandha
Botanical name : Aloe vera
Telugu name: Kalabandha
Hindi name: Kumari
English name: Aloe
Family: Liliaceae
Morphology:
- Dwarf succulent plant
- Leaves in rosettes, margin spiny
- Xerophyte
Useful part:
- Juice is extracted from the leaves, concentrated and dried.
- Odor is characteristically unpleasant
- Taste is bitter.
Chemical constituents / Active principle :
- Aloin: Mixture of Aloin A (Barbaloin) and Aloin B (Isobarbaloin).
- Anthroquinone
- Glycocides
- Saponins
Uses:
- Treatment of intestinal worms
- Anti-inflammatory
- Wound healer: burns, insect bites, rashes, acne, sunburn
- Anti-diabetic
- Beauty products and cosmetics
------------------------------------------------------------
Turmeric
Botanical name: Curcuma longa
Telugu name: Pasupu
English name: Turmeric
Hindi name: Haldi
Sanskrit name: Haridra
Family: Zingiberaceae
Useful Part: Rhizome
Primary rhizome:
- Oblong and ovate, often called bulbs or round turmeric.
Secondary rhizome:
- Cylindrical long, branching, tapering at both ends. Commonly called fingers.
- Used as raw material, subjected to further processing.
- Has a characteristic pungent odor and bitter taste.
Chemical Constituents: Curcumin provides the distinct yellow color, three analogs have been detected so far: Curcumin I, Curcumin II, Curcumin III.
The volatile oil contains monoterpenes and sesquiterpenes like zingiberene, turmerone, borneol and cineol.
Uses:
- Antiseptic
- Astringent (tightening of soft body tissues)
- Carminative (prevents formation or expulsion of gas)
- Blood purification
- Treatment of Cough, Cold, Skin Diseases, Jaundice, and Menstrual Cramps.
- Spice
------------------------------------------------------------
Aswagandha
Botanical name : Withania somnifera
Vernacular name: Ashwagandha
Family: Solanaceae
Morphology:
- Highly branched shrub (1 m)
- Stem – hairy
- Leaves – ovate
- Flowers – green
- Fruits – globose and red, embedded in thin persistent sepals
Geographical distribution:
- India – Rajasthan, Madhya Pradesh and Maharashtra
- World – Australia, Sri Lanka and Israel
Useful parts: Roots, leaves, fruits and seeds.
Chemical constituents/Active principles:
- Alkaloids and Steroids
- Withanine
- Withanone
- Withaferin-A
- Anaferine
Uses:
- Sedative
- Antispasmodic and antihelminthic
- Poultice to boils and swelling
- Leaves show insect repellant properties
- Leaf extract is an antibiotic against Staphylococcus aureus and ranikhet virus
- Fruits are diuretic
------------------------------------------------------------
Sarpagandha
Botanical name: Rauwolfia serpentina
Vernacular names: Sarpagandha, Serpentine root
Family: Apocynaceae
Morphology:
- Perennial, erect shrub.
- Leaves – whorled
- Flowers – white or light pink
- Fruits – globose, blue or black
- Roots – alkaline, rarely branched
Geographical distribution:
- Tropical regions
- India: U.P, Bihar, West Bengal, Tamil Nadu, Karnataka, Maharashtra, and Gujarat.
Useful parts: Primary roots and leaves
Chemical constituents/Active principle:
- Reserpine
- Serpentine
- Ajamaline
Uses:
- Hypnotic (sleep inducing)
- Reduces blood pressure
- Increases uterine contractions during parturition (action of giving birth)
- Increases lactation in mothers.
------------------------------------------------------------
Nelausiri
▪ Botanical name: Phyllanthus amarus
▪ Vernacular name: Nela usiri
▪ Family: Euphorbiaceae
▪ Morphology:
- Annual erect herb, branchlets slender
- Leaves - elliptical, obovate or oblong, pinnately compound.
- Flowers axillary, solitary in lower axils.
- Fruit - capsule.
- Occurs as a weed. It grows abundantly during rainy season.
Active principle:
- Phyllanthine (bitter)
- Hypophyllanthine
- Niranthine
Uses:
- Cures Jaundice.
- Effective on Hepatitis B virus, it blocks the DNA polymerase (enzyme responsible to synthesize DNA ) of the virus.
- The decoction of the herb is febrifuge (reduces fever), stomachic and diuretic.
- It improves appetite quickly.
------------------------------------------------------------
Amla
Botanical name: Phyllanthus emblica
Vernacular name: Amla
Family: Euphorbiaceae
Morphology:
- Small deciduous tree
- Leaves – oblong
- Flowers – unisexual, in axillary fascicles. Male flowers in upper axils, female flowers in lower axils.
- Fruit – drupe, globose, fleshy.
- Seeds - trigonous
Active principle: Phyllemblin and tannins.
Chemical constituents : Vitamin C, Iron and Phosphorous.
Uses:
- Essential ingredient of Chyawanprash (health supplement) and Triphala (treatment for dyspepsia (indigestion) and piles).
- Treatment of diarrhea, dysentery, anemia, and jaundice.
- Diuretic and laxative (prevents constipation)
- Promotes hair growth.
------------------------------------------------------------
Brahmi
Botanical name: Bacopa monnieri
Vernacular name: Bramhi
Family: Scrophulariacea
Morphology:
- Annual prostrate herb with ascending succulent branches
- Leaves – sessile, fleshy, obovate, spathulate. When crushed they give a characteristic lemon scent.
- Flowers – solitary, axillary, and white.
Chemical constituents/Active principles: Brahmine, herpestine, Bacosides A and B.
Uses:
- Medhya rasayan – a brain tonic which helps to improve memory and concentration.
- Bacosides A and B enhances body’s anti-oxidant levels.
- Maintains ionic equilibrium
- Treats asthma and epilepsy.
0 notes
Text
Mod 4 Botany Sem End
Pharmacognosy
Intro:
Pharmacognosy is one of the oldest disciplines of pharmaceutical sciences.
Pharmacognosy is the study of crude drugs of plants, animals, or mineral origin.
The term was coined in 1815 by Seydler
Pharmacognosy = pharmakon + gnosis or drug + knowledge
Crude drug refers to the raw form of the product which has not been refined or treated for marketing.
The source of a crude drug may be either from plants, animals, or even parts of both.
The study of crude drugs includes history, distribution, cultivation, collection, processing, evaluation, and preservation.
Crude drugs are obtained from natural sources.
The active principles which are present in plants are used in the prep of medicine.
---
Scope:
Pharmacognosy is an interdisciplinary science which links botany, zoology, and chemistry.
Plants have made a rich contribution to the medical world, leading to the establishment of many pharmaceutical industries.
Pharmacognosy also includes plant taxonomy, plant breeding, plant pathology, and genetics. With this knowledge, one can improve the cultivation methods for both medicinal and aromatic plants.
It gives us knowledge on vegetable drugs under botany and animal drugs under zoology.
It is essential for the evolution for new medicines since crude drugs are used as a source of active principles which are used in medical prep.
Is it an important link between pharmaceuticals and basic science as well as ayurvedic and allopathic systems of medicine.
It is used as a research tools which can help improve the health care facilities around the world.
It is involved in the development of other departments of science associated with it.
Examples:
- Based on Source:
Vegetable source - Fennel, clove, senna
Animal source - Honey beeswax, shark liver oil
Mineral source - Chalk (CaCO3)
- Crude drugs which have received clinical support:
(Scientific/Common - Key constituent: Activity type)
Allium sativum/Garlic - Allin: Reduces cholesterol
Azadirachta indica/Neem - Gedunin: Antimalarial
Bacopa monnieri/Brahmi - Bacosides: Memory enhancer
Curcuma longa/Turmeric - Curcumin: Anti inflammatory
------------------------------------------------------------------------------
Taxol
Generic Name: Paclitaxel
Other trade name: Onxal
An anti-cancer chemotherapy drug (cytotoxic or antineoplastic)
Classified as a "plant alkaloid"
The most well known naturally sourced cancer drug in the US
Derived from the bark of the pacific yew tree (Taxus brevifolia)
Specific pharmacological uses of taxol are it being used for: - breast cancer - ovarian cancer - lung cancer - bladder cancer - prostate cancer
- melanoma
- esophageal cancer
- various types of tumors
Kaposi's sarcoma
Administration of taxol:
Either given as an injection/infusion into the vein (intravenous/IV)
Taxol is an irritant, a chemical that can cause inflammation of the vein through which it is given.
If the medication escapes from the vein, it can cause tissue damage. The nurse or doctor who gives Taxol must be carefully trained.
Since severe allergic reactions have occurred in some people taking Taxol, one needs to take additional medications to prevent an unwanted reaction.
There is no pill form of Taxol.
Drug action:
Cancerous tumors are characterized by continuous cell division, which is no longer controlled as it is in normal tissue.
The cell cycle goes from the resting phase, through active growing phases, and then to mitosis (division).
Usually the drug works by damaging the DNA or RNA of the cell
If the cell is unable to divide, it dies.
The faster the cells are dividing, the more likely it is that chemotherapy will kill them, causing the tumor to shrink.
Taxol also induces cell suicide (self-death/apoptosis)
Chemotherapy is most effective at killing cells that are rapidly dividing.
However, chemotherapy does not know the difference between cancerous cells and normal cells.
"Normal" cells will grow back and be healthy again, but till then, side effects occur.
The "normal" cells most commonly affected by chemotherapy are blood cells, the cells in the mouth, stomach and bowel, and hair follicles; resulting in low blood counts, mouth sores, nausea, diarrhea, and hair loss.
---------------------------------------------------------------------
Artemisinin
Extracted from the plant Artemisia annua, commonly called sweet wormwood.
It is a herb employed in Chinese traditional medicine.
An anti malarial drug.
Highly effective in treating malarial parasites in the body.
Uses:
For severe malaria, the WHO recommends intravenous or intramuscular treatment with the artemisinin derivative artesunate for at least 24 hours.
Artesunate rapidly kills the parasites, but is itself rapidly cleared from the body
It is not used for malaria prevention because of the extremely short activity (half-life) of the drug
To be effective, it would need to be administered multiple times a day, everyday.
Drug Action:
Falciparum malaria is a mass killer that went out of control. These drugs are highly active against all Plasmodium species.
Artemisinin derivatives are the most rapidly acting of all antimalarial drugs and produce the fastest clinical responses to treatment.
A 3-day course of the artemisinin derivative in combination with a slowly eliminated partner drug (elimination half life > 1 day) is required for optimum cure rates.
Two-day and 1-day courses are insufficient because they expose only one asexual cycle to the artemisinin derivative
The drug effects the parasite growth in the human body, reducing the symptoms of disease.
------------------------------------------------------------------
Galathamine
An alkaloid that has been isolated from the bulbs and flowers of a few members of the Amaryllidaceae family
Specifically Galanthus nivalis (Common snowdrop), Galanthus caucasicus (Caucasian snowdrop), and Galanthus woronowii (Voronov's snowdrop).
Isolated for the first time from bulbs of Galanthus nivalis
Treat the symptoms of Alzheimer's disease (a brain disease which slowly destroys the memory and the ability to think, learn, communicate and handle daily activities)
Uses:
treatment of mild to moderate vascular dementia and Alzheimer's disease.
In the US, it is approved by the FDA as safe and effective.
The product is supplied in prescription form in twice-a-day tablets, once-a-day extended-release capsules, and in oral solution.
Drug Action:
It is a competitive and reversible inhibitor of acetylcholinesterase that works to increase acetylcholine levels.
As Alzheimer's disease is a progressive neurodegenerative disorder, galantamine is not known to alter the course of the underlying dementing process.
Galantamine works to block the enzyme responsible for the breakdown of acetylcholine in the synaptic cleft, thereby enhancing cholinergic neuron function and signaling.
Galantamine is sold under the brand name Razadyne, and is available as oral immediate and extended release tablets and solution
--------------------------------------------------------------------------
Flavopyridole
A semisynthetic flavonoid structurally related to a naturally occurring alkaloid
It is isolated from Dysoxylum binectariferum, a plant indigenous to India.
Treats human breast carcinoma cells.
Toxicities include diarrhea, nausea, vomiting
Uses and action:
It has been shown to be a potent cyclin-dependent kinase inhibitor
Studies in human breast carcinoma cells (MCF-7) demonstrated that flavopiridol inhibits cdk1, cdk2, and cdk4 by binding to the ATP-binding pocket of the kinase.
Subsequent studies have shown that as a result of the cdk inhibition, flavopiridol produces potent cell cycle arrest
In clinical studies, flavopiridol was initially administered as a 72 hour continuous infusion every 2 weeks, with a maximally tolerated dose of 40 mg/m2.
This activates the apoptotic mechanism of cell, causing cell death.
-----------------------------------------------------------------------
Adulteration of Crude Drugs
Adulteration: the practice of substituting original crude drugs either partially or whole with other similar substances which are inferior in chemical and therapeutics properties.
Adulterant: some material which is both cheap and easily available in abundance.
Due to the presence of adulterants, the purity of the crude drug is reduced. It is a type of contaminant.
Ex: Varieties like Arabian senna are adulterant for Indian senna.
Adulteration is mainly practiced to make more money and also when the price of the drug is high.
Types of Adulteration:
Deliberate/Intentional Adulteration: normally commercial, mostly done with the intention of enhancement of profits. i) Substitution with an inferior commercial variety. - Ex: Japanese ginger (Zingiber mioga) is used to adulterate medicinal ginger (Zingiber officinale). ii) Adulteration by artificially manufactured substitutes. - Ex: Paraffin wax for beeswax or artificial sugar for honey. iii) Substitution by superficially similar but cheaper natural substances. - May or may not have any therapeutic value - Ex: Leaves of ailanthus are substituted for the leaves of belladonna, senna, and mint. iv) Addition of synthetic principles - These are added to the natural products. - Ex: Addition of citral to lemon oil, both have a strong lemon like smell.
Accidental/Indeliberate/Ignorant Adulteration:
Entirely unintentional adulteration, can happen due to following: i) Family collection - When a person does not know the right time and season for collection of drug plants that contain maximum amount of active principles, it may lead to inferior quality of drugs and thus lead to adulteration. - Ex: Corms of colchicum are to be collected in the summer and wild cherry bark is to be collected in autumn for maximum concentration of active principles. - Ex: Belladonna roots should be 3-4 years old. ii) Collection of drugs with less valuable parts - Plants containing actual genuine drug should be collected, but sometimes due to ignorance, some accessory plants are also collected. - Ex: Senega roots are sometimes collected with mixed stem parts. iii) Collection of allied species - Either due to ignorance or negligence, a person may collect parts from an allies species of the medicinal plant due to superficial morphological resemblances. - Ex: instead of Datura stramonium, a similar plant, Xanthium stramonium, is collected. iv) Incorrect Storage - Many drugs have become invalid due to the loss of active principles due to incorrect methods of storage. - Ex: Datura not to be stored in places of excessive moisture
---------------------------------------------------------------------
Indian Pharmacopoeia
The word pharmacopoeia is derived from the greek words Pharmakon (drug) and Poiea (make).
It is an official publication in the form of a book with an authentic description of the drugs along with the directions of their use.
Is it a "stock of medicinal drugs"
The details about the drug include: - botanical name - common name - vernacular name - geographical distribution - history - uses of drug - action of drug - parts of plant used in preparation - morphology - anatomy - chemical studies, etc.
Various places in the world have their own recorded pharmacopoeia, ex: Indian Pharmacopoeia.
The Indian pharmacopoeia (IP) is an official drug compendium of the Indian subcontinent.
It is the sole authority for all the prescriptions of medicines and other health care products which are manufactured and sold in India.
IP is usually suffixed with the respective year of publication ex: IP 2007; was published by Indian Pharmacopoeia Commission (IPC) which is an autonomous institution under the Ministry of Health and Family Welfare under GOI.
IP was started with the aim to promote public heath care by bringing quality drugs, dosage forms, and medical devices used by health professionals, patients, and consumers.
The first edition of IP was published in 1955, followed by respective editions in 1966, 1985, 1996, 2007, 2010, 2014, 2018, 2022.
The principle of "openness, justice, and fairness" is kept in mind during compiling and editing the contents of each edition.
Public review and comment process for standard development has given special importance to incorporate new ideas.
Headquarters of IPC is at Ghaziabad, UP.
The most recent publication is IP 2022, the 9th edition. It comprises of 4 volumes.
----------------------------------------------------------------------
Types of Plant Crude Drugs
The term plant crude drug implies the raw form of the product obtained form the plant that has not been refined or treated for marketing.
The systematic study of these crude drugs is called Pharmacognosy.
Crude Drugs are divided into 2 groups: - Organized Drugs - Un-Organized Drugs
Organized Drugs:
These are obtained from the plants or animal parts that has a cellular structure and also a definite shape.
These drugs are further classified based on the specific part having medicinal value. They include: - Root Drugs: Boerhavia - Rhizome: Ginger, Turmeric - Bark: Cinnamon - Leaf: Datura - Flower: China rose - Fruit: Coriander - Seed: Cardamom - Whole Plant: Catharanthus
Un-Organized Drugs:
These are obtained from the plant or animal parts that has no specific structure and they are acellular.
They are further classified based on their nature: - Latex: Opium - Juice: Aloe - Extract: Agar - Gum: Acacia - Resin: Asafoetida - Oils and Fats: Arachis
----------------------------------------------------------------------
Collection of Plant Crude Drugs
There are many aspects of collecting the drugs. They are collected from both wild plants as well as cultivated plants.
The crud value mainly depend on 2 factors: - Time of Collection - Method of Collection
Time of Collection:
Drugs obtained from the flower tops should be collected before the flower opens: Clove.
Drugs obtained from the leaf should generally be dried before collected: Digitalis.
The leaves of Aloe are to be collected when they are succulent.
Barks are to be collected when the process secondary growth is active: Cinnamon.
Roots are to be collected when the vegetative growth ceases: Boerhavia.
Rhizomes are to be collected when food is completely stored: Ginger.
Gums and resins are to be collected when they start to ooze out from the metabolic plant by products: Acacia.
Method of Collection
Medicinal plant parts should be harvested/collected under the best possible conditions avoiding rain, dew, or even high humidity levels. This is to prevent the microbial growth that may lead to contamination of the crude product.
Cutting devices, harvesters, and other machines should be kept clean to reduce the risk of contamination. Even the soil particles are to be brushed or washed off after harvesting (for underground parts)
Damaged plant or infected plant parts are to be excluded.
During harvesting, care should be taken to ensure that no toxic weeds are mixed with the medicinal plant parts.
All containers that are used during collecting and storing must be clean and free from contamination.
In the case of Cinchona, the bark is stripped off from the plants by a special process called as coppicing.
----------------------------------------------------------------------
Processing of Plant Crude Drugs
The crude drugs are collection are processed and stored before they are marketed.
Processing includes Drying and Grading.
Drying prevents contamination, prevents enzyme activity, and prevents chemical changes.
The crude drugs after collection are subjected to dehydration by dying and since the chemical constituents vary in their composition and type, application of uniform drying is not possible.
Ex: Plants containing volatile oils need to be dried at a very low temperature or else they may lose their aroma and also their quality, starchy herbs gelatinize if overheated.
Drying is of 2 types: - Open Air Drying - Artificial Drying
Open Air Drying:
Natural type of drying
Plant parts are dried in the sun or shade if sunlight causes discoloration.
The leaves of Digitalis and Clove are shade dried.
The bark of Cinnamon is sun dried.
Artificial Drying:
When the conditions are not suitable for natural drying, the crude drugs are dried artificially.
The leaves of Digitalis are dried in vacuum driers.
Tea leaves are kept in trays dries and dried by providing air at desired temperature.
The latex of Papaya is dried by play drying (Liquid -> dry powder by drying with hot gas)
Grading is the process of selecting the better one among the lot. It is done manually by hand picking, or passing through sieves/meshes. It is also done by grading machines which involves the same principle.
----------------------------------------------------------------------
Storage of Plant Crude Drugs
The crude drugs are collection are processed and stored before they are marketed.
Storage involves packing and preservation.
Packing:
The crude drugs are susceptible to certain physical and chemical deterioration if they are not preserved and packed properly.
Humidity, light, and temp are the physical factors that bring down the quality of the drug during preservation.
Drugs stored in usual containers like sacks, paper bags, cardboard boxes, etc. reabsorb 10-12% moisture. In a few drugs this leads to degradation of the active constituent. Ex: Digitalis.
The type of soil in the storage cellars also affect the quality of the drug.
Usually sandy soils are used in cellars as they lose the moisture and keep the drug dry, whereas the clay soils absorb moisture therefore not preferred.
Volatile substances are directly affected by temp, ex: Ginger
Preservation:
Drug materials are preserved in sealed containers to avoid exposure to humid atmosphere.
The premises and cupboards with the packages must be periodically fumigated to keep away the pests and insects. ex: Carbon disulphide is used as a fumigant.
Drugs can also be preserved in well closed opaque containers.
The packaged drugs should be preserved from the attack of bacteria and other microbes.
----------------------------------------------------------------------
Evaluation of Plant Crude Drugs
Drug evaluation is the determination of identity, quality, and purity of the crude drug, which denotes level of active constituent and extent of foreign material present in the drug.
The correct assessment of the crude drug is done by: - Organoleptic Evaluation - Physical Evaluation - Chemical Evaluation - Biological Evaluation
---
A) Organoleptic Evaluation:
In this type of evaluation, the external and internal characters such as shape, smell, taste, and texture, etc, is evaluated. This evaluation is categorized into: - Morphological Characters - Sensory Characters - Microscopic Characters
1) Morphological Characters:
The morphological characters include shape, size, and external markings. In order to study the above characters, the organized drugs are further classified into: - Bark drugs - Root drugs - Rhizome drugs - etc.
Bark: Outermost tissue of woody stem (cinnamon)
Underground parts: Rhizome, roots and stolons (ginger)
Leaves: Their shape, margin, apex, base, and venation help in their identification (tualsi)
Flowers: These are the reproductive structures of the plant. (The dried stigmas and style of saffron)
Fruits: (Gokhru)
Seeds: (Linseed)
Herbs: The entire play is used as a drug (pudina)
The following may be noted: - The shape of the drug containing plant, like cylindrical - The length and diameter are measured in cm/mm. - External markings on some of the drugs when present (ridges, wrinkles, modules, scars of leaf, root buds, etc.)
2) Sensory Characters:
They include color, odor, taste, and texture. This method of evaluation is applicable to drugs containing volatile oils or pungent active principles.
Color: The usual color range in a drug varies from white, yellow, grey, brown, orange to black.
Odor: The smell of the drug can be aromatic, camphoraceous, etc. Ex: Mentha leaves are identified by smell.
Taste: The taste may be sour/acidic, salty/saline, sweet, bitter, etc. Ex: Licorice is sweet.
Texture: The texture can be fibrous (licorice), oily (nutmeg), etc.
3) Microscopic (Anatomical/Internal) characters:
Drugs in powdered form can be evaluated by microscopic study of internal characters. They mainly include: - Histological examination - Microscopic measurements
Histological examination: Presence and arrangement of various tissues is observed in the section. ex: Stomata, phloem elements, woody tissues, and other tissues.
Microscopic measurements: Includes measurement of size like diameter of starch stain, length of stomata, diameter of phloem fibers, etc. Includes: - Stomatal number: Average number of stomata per sq.mm of epidermis of leaf - Stomatal index: The percentage of stomata calculated by Salisbury's formula (Sx100/E+S where S=no of stomata per unit area and E=no. of epidermal cells in the same unite area) - Palisade ratio: Average no of palisade cells beneath each upper epidermal cell.
---
B) Physical Evaluation:
It is the determination of physical constants for drug evaluation.
1) Swelling Factor: A constant obtained for mucilage containing drugs on account of swelling.
2) Viscosity measurement: An index of the composition of gums.
3) Ash Value: A constant obtained by determining the ash content after incineration. It indicates the residue of mineral substances.
4) Refractory Index: A constant for evaluation volatile oils. It is the ration of velocity of light in vacuum to velocity of the substances.
5) RF Value (Retention factor): A constant obtained for a substance using a definite solvent in chromatography. Identifies if a component is polar or non-polar.
6) Moisture Content: The % of active chemical constituent in crude drug mention on air-dried basis. Determined by heating the drug at 105°C in an oven.
7) Melting Point: Each drug has a specific range of melting point.
---
C) Chemical Evaluation:
Determination of the active principle in a drug by the chemical methods:
1) Instrumental Methods: - Calorimetry: Estimation of alkaloids - Fluorimetry: Estimation of quinine and reserpine - Spectrophotometry: Estimation of lobeline (an alkaloid obtained from tobacco)
2) Chemical Constants: - Like acidity, iodine value which tells the degree of unsaturation, and ester value which is used for evaluation of volatile oils.
3)Chemical Tests: - Iodine test: For starch - Borntrager's' Test: For detecting anthraquinone glycosides in senna. - Vital Morin Test: For detecting alkaloids in datura.
4) Micro Chemical Tests: - These tests are carried out on slides. - Ex: Eugenol in clove oil is precipitates as potassium euginate crystals by adding KOH.
---
D) Biological Evaluation:
This method is employed as a last resort when the above mentioned ones fails to evaluate the drug.
The response produced by the test drug on a living system is compared with that of the standard (bioassay)
Tests are carried out on living systems like rabbits, rats, microbes, etc.
The effects measured in the test animals are different from those observed in human patients.
0 notes
Text
Zoology Practical Spotters
T.S of Thyroid
Comprises of a framework of connective tissue, enclosing numerous rounded thyroid follicles or vesicles of different sizes.
Thyroid is richly supplies with blood vessels and nerves. It is innervated from the sympathetic nerves.
Thyroid secretes thyroxin, which contains an amino acid and 65% iodine.
Thyroxin controls the entire metabolism.
Deficiency of thyroxin causes lowered metabolism.
Hyperthyroxin results in the protrusion of eyeballs.
Removal of thyroid from frog stops metamorphosis.
Thyroid gland is controlled by THS - Thyroid Stimulating Hormone from pituitary.
---------------------------------------------------------
T.S. of Parathyroid Gland
Two parathyroid glands are always found embedded in the substance of thyroid or found on each side of the thyroid gland.
Each parathyroid is enclosed within a capsule.
Each parathyroid is a glandular organ consisting of columns of epithelial cells.
Parathyroid produces a hormone called parathormone, which is devoid of iodine.
Removal of parathyroid causes tetany or death.
Parathyroid gland develops an epithelial outgrowth from the third and fourth branchial clefts of the embryo.
------------------------------------------------------
T.S of Adrenal Gland
Adrenal glands lie at the anterior end of kidneys and are two in number.
--------------------------------------------------
T.S of Pituitary
stfu
-------------------------------------------------
T.S if Pancreas
I'll cry
----------------------------------------------
Ground nest - Lapwings
Lapwings nest on the base ground in open waste lands with place encircled by pebbles.
The nests are a scrape in the ground lined with plant materials.
Usually the nests are in open areas, because the lapwing needs a good all-round view from the nest to spot predators.
Lapwing nests and eggs are camouflaged, they blend in with their surroundings by matching their backgrounds color and pattern.
-----------------------------------------------------
Walled nest - Common Swallow
Common swallows secrete a cement like substance by salivary gland.
They put this secretion on their feathers and make walled nests using cemented feathers.
Both male and female common swallows build the nest cup using mud.
The birds line the cup first with grass, then feathers, and if in colonies they may steal nest-lining materials from neighboring nests.
--------------------------------------------------
Walled nest - House martin
House martins secrete a cement like substance by salivary gland.
They put this secretion on their feathers and make walled nests using cemented feathers.
Traditionally, they would nest on cliff faces. However, now they are mostly associated with manmade structures
They build nests out of mud, gathering it up from ponds and streams
Nests are usually built on houses, hence the bird's name
----------------------------------------------
Floating nest - Coots
Coots make their nests floating on water.
The entire nest is generally a floating structure anchored to upright stalks.
The nest material is woven into a shallow basket with a hollowed interior lined with finer smooth material to hold the eggs
They build nests using reeds and grasses atop floating vegetation in shallow water.
----------------------------------------------------
Floating nest - Jacanas
Jacanas, an aquatic bird, make their nests on floating water
Male jacanas do most of the nest building
Floating plants are uses a base for the nest made of leaves and stems.
The male typically sits on the eggs in the nest too.
-------------------------------------------------------
Nest of Brood Parasitism
Some birds do not build their own nest.
They instead parasite in the nest of other birds.
Koel and Cuckoo for example, put their eggs in the nests of crow.
The host birds are then responsible for raising and feeding the parasite chick
The parasite bird and chick may kill the eggs or chicks of the host bird to reduce competition for resources.
-----------------------------------------
Communal nest - Mouk Parakeets
At least four pairs of the same species of mouk parakeets live together in the communal nest.
Female lay eggs but incubation of eggs and food for young is the duity of male.
Monk parakeets place their nests in deciduous and evergreen trees, palm trees
The bulky nests provide a year-round home for the colony and may be a reason why Monk Parakeets are able to survive cold winters.
-----------------------------------------------------------------
Excavated hallow holes in trees - Wood Pecker
Some woodpeckers, such as this one, make a hollow nest in the trunk of a tree and use it as a nest
Both male and female woodpeckers build nest holes.
Most woodpecker species excavate a new nest cavity every year.
Woodpeckers are very important to ecosystems for their excavation activities, their abandoned nests provide homes to other animals.
------------------------------------------------------------------------------
Ground nest - penguin
Penguins can not fly and do not spend too much time in water, so they lay eggs and nest on ground.
Some penguins make scrape nests by making an indentation in the ground for their egg to sit and add vegetation around it for protection.
Other penguins use only rocks and pebbles to make a nest on flat ground
--------------------------------------
Ground nest - pheasant
Pheasants are arboreal birds that lay their eggs on lonely places of grounds.
The nests are usually surrounded by tall vegetation.
They are often in a natural depression or a hollow that the bird scoops out itself.
Female pheasants find or make the nest.
Females gather grasses, leaves, weed stalks, fine twigs, corn husks, and/or a few feathers from their own breast to line the nest
-----------------------------------------------
Social Insects - Honey Comb
Honey bees are social insects that build combs of wax.
They all live together in large, well-organized family groups
The comb stores honey and pollen, and is where larvae is grown.
The comb acts as a permanent colony for the species.
Female worker bees form the comb.
The honey bee hive consists of several hexagonal chambers or cells, such as
- Attachment cells: for attachment of the comb to any substratum.
- Storage cells: For storage of honey and pollen grains.
- Brood cells: For development of the young.
-------------------------------------------------------------------------
Social Insects - Honey Bee
Honey bees are social insects.
They all live together in large, well-organized family groups.
They have a highly organized division of labor
There are 3 castes of honey bees: queen, drone, and worker bees
Each caste has their own physical features and functions:
- Queen bee: The fertile female bee, she exists solely to lay eggs.
- Drone bees: All male bees are drones, they only exist to copulate with the female bee and fertilize her eggs.
- Worker bees: Sterile female bees. Smallest of the three. Most in number. Do all the work of the colony except laying eggs.
-----------------------------------------------------
Natural Hole & Cavities - Myna
Mynas nest in natural holes or cavities in trees.
The nest of myna are lined by twigs, roots, and rubbish.
Sometimes myna nest in manmade hollow structures such as pipes.
Mynas typically breed from August to March
Some mynas use the abandoned nests of woodpeckers.
-----------------------------------------------------
Excavated Hollow holes - Bee Eater
Bee eaters make hollow nests in the trunk of a tree and use it as a nest.
The tend to dig into vertical sandy banks or dirt to make nests.
They dig out an oval shaped chamber inside the mud to keep their eggs in.
Some bee-eaters nest in colonies.
---------------------------------------------
Natural Hole & Cavities - Kingfisher
Kingfishers nest in natural holes or cavities.
Some Kingfishers nest in tunnels dug into the natural or artificial banks in the ground.
Other kingfishers nest in arboreal termite nests.
The male and the female take turns digging the burrow, with males spending about twice as much time digging as females.
THIS ONE DOESN'T MAKE SENSE
--------------------------------------------------
Pendant or Suspended Nest - Sun Bird
Sun birds build pendant nests out of completely woven fibers.
The nests are often suspended from branches
The nest is tightly bound to the branch with spiders webs
The nests are usually lined with feathers
Only female sunbirds build nests
========================
Ground nest - Duck
Ducks nest on the ground in open waste lands with space encircled by pebbles.
They nest close to water.
The nests are generally concealed under overhanging grass or other vegetation
The female forms a shallow depression or bowl on the ground in moist earth
She pulls vegetation she can reach toward her while sitting on nest.
During egg-laying phase, she lines the nest with grasses, leaves, and twigs from nearby.
----------------------------------------------------------------------------
Pendant or Suspended Nest - Tailor Bird
Tailor birds build pendant nests of completely woven fibers.
A tailor bird neatly stitches the edges of cleverly folded one or more large plant leaves with the threads of wool or cotton by means of its pointed beak.
The funnel shaped nest is ultimately linked by soft fibers, cotton wool, and vegatation so that the nest can become comfortable.
0 notes
Text
Zoology Practical Minor Experiments
Hydrotactic
Aim: To study the hydrotactic behavior of earthworms
Material: Live earthworms, dissection tray, filter paper, blunt forceps, and water.
Principle: A taxis is a behavior response that produces movement either towards or away from a stimulus. Taxis behaviors are classified according to the stimulus producing the response. The presence of stimuli producing the response such as light, gravity, or chemicals can result in taxis behavior. If the organism moves towards the stimulus, then the movement is referred to as a positive taxis. Avoidance or movement away from a stimulus is a negative taxis. Hydrotaxis is in response to water.
Method: Take a dissection tray and spread a filter paper. Divide the filter paper into equal parts A and B. To part A, sprinkle a few water drops to create moist conditions, keep part B dry. Introduce 5 earthworms in the center of the dissection tray. Observe the behavior of worms showing positive or negative response to hydrotactic behavior.
Result: Positive response towards moist side of the dissection tray, all 5 worms moved to side A.
Inference: The earthworms exhibit positive response to the given hydrotaxis.
-----------------------------
Chemotactic
Aim: To study the chemotactic behavior of earthworms.
Materials: Live earthworms, dissection tray, filter paper, blunt forceps, water, pH strips, natural sources for making acidic and basic solutions, q-tips, mortal and pestle, petri-dish.
Principle: A taxis is a behavior response that produces movement either towards or away from a stimulus. Taxis behaviors are classified according to the stimulus producing the response. The presence of stimuli producing the response such as light, gravity, or chemicals can result in taxis behavior. If the organism moves towards the stimulus, then the movement is referred to as a positive taxis. Avoidance or movement away from a stimulus is a negative taxis. Chemotaxis is in response to chemical stimulus.
Method: Take a dissection tray and spread a filter paper. Clean the earthworm with water and place it on the dissection tray with the help of blunt forceps. Take the natural source and prepare a homogenate solution with water. Check the pH of the solution, then treat the earthworm with the prepared solution. Observe the behavior of worms, showing either positive or negative chemotactic behavior.
Results: - Sample 1: Onion - pH 6 -> Negative response - Sample 2: Garlic - pH 6 -> Negative response - Sample 3: Lemon - pH 4 -> Negative response - Sample 4: Tomato - pH 6 -> Positive response - Sample 5: Cucumber - pH 6 -> Positive response - Sample 6: Pineapple - pH 6 -> Positive response
Inference: Earthworms are said to exhibit negative response to both acidic and alkaline medium to the given chemicals
---------------------------------------------
Geotactic
Aim: To study the geotactic behavior of earthworms
Materials: A wooden box with a partition creating two smaller chambers, live earthworms, and moist mud.
Principle: A taxis is a behavior response that produces movement either towards or away from a stimulus. Taxis behaviors are classified according to the stimulus producing the response. The presence of stimuli producing the response such as light, gravity, or chemicals can result in taxis behavior. If the organism moves towards the stimulus, then the movement is referred to as a positive taxis. Avoidance or movement away from a stimulus is a negative taxis. Geotaxis is in response to gravity.
Method: The partitioned wooden box has two sides, A and B. spread moist mud onto both sides. Leave an equal number of earthworms in both the chambers. The partition should have small gateways foe earthworms to be able to move from one side to another. Close the box, leave the experiment for 6 minutes. After the wait, open the box and count the number of earthworms on each side. More in number indicates preference, if there are more on the upper side they are geo-negative, if there are more on the lower side they are geo-positive. Repeat the experiment numerous times by changing the degree of slant/tilt.
Results: - Situation I at 10°: Number of earthworms in chamber A = Number of earthworms in chamber B =
*this but each time ^^
Inference: More number. of earthworms tend to move towards downwards direction. Hence, they are geopositive
---------------------------
Phototactic
Aim: To study the phototactic behavior of earthworms
Materials: Wooden box with a partition creating 2 smaller chambers, moist mud, live earthworms, torch/bulbs fixed in one of the chambers of the box.
Principle: A taxis is a behavior response that produces movement either towards or away from a stimulus. Taxis behaviors are classified according to the stimulus producing the response. The presence of stimuli producing the response such as light, gravity, or chemicals can result in taxis behavior. If the organism moves towards the stimulus, then the movement is referred to as a positive taxis. Avoidance or movement away from a stimulus is a negative taxis. Phototaxis is in response to light.
Method: The partitioned wooden box has 2 sides, A and B. Fix a small torch or a dim bulb inside A, spread moist mud on both sides (just enough for the earthworms to crawl over, they should not get inside the mud) Leve equal number of earthworms in both chambers. The partition should have small gateways for earthworms to move from one side to another. Close the box, light the bulb, leave the experiment for half an hour on a table which is of uniform level. After the wait, open the box and count the number of earthworms on each side. More the number of earthworms indicates preference.
Results: - Situation 1: Chamber A has light and Chamber B has no light. Number of earthworms in A before 30min = 5, Temp = 30°C. Number of earthworms in B before 30min = 5, Temp = 30°C. Number of earthworms in A after 30min = 3, Temp = 30°C. Number of earthworms in B after 30min = 7, Temp = 27°C. Therefore the earthworms prefer no light.
** this a few more times too^^
table
Inference: Higher number of earthworms in a chamber indicates positive response towards that stimulus while lower number indicates negative response.
worm diagram for all ^^
---------------------------
Habituation to touch
Aim: To study habituation in snail.
Materials: Live snails, q-tip, water, stopwatch.
Principle: Habituation is the decrease in probability of a response occurring when an stimulus either is presented repeatedly or it is a gradual fading of a response. This happens a stimulus proves to be safe, neutral, or irrelevant is given repeatedly. If a neutral stimulus (like that which has neither noxious nor beneficial consequences) is repeatedly determined to an organism, it's response to the stimulus tends to decrease gradually and may eventually seize all together. By habituation, animals learn what not to do.
Method: 1. Take the petri dish and add water to it. Put the snail into the petri dish. 2. Touch the snail's waving tentacles with a q-tip, it will draw from its head and close the operculum. 3. Wait, do not disturb the snail till it comes out of its shell and crawls again. 4. Repeat the last two steps numerous times till the snail stops reacting to touch.
Result: - Number of times the tentacles were touched with q-tip: 8 - Number of times the snail withdraws its head and closes operculum: 5
TABLE
Inference: The snail stopped responding to the neutral stimulus after being touched 8 times, which means that the snail got habituated and did not respond to the last two times.
0 notes