Materials Science and Engineering is a relatively new field that involves the study, discovery, and design of materials.
Don't wanna be here? Send us removal request.
Text

Cobalt catalyst rivals platinum in key industrial reaction
Propane dehydrogenation is a key industrial route to producing propylene without relying on oil. However, its current production processes rely heavily on precious-metal catalysts such as Pt-based materials. Developing efficient alternatives using Earth-abundant metals has remained a challenge. In a study published in Nature Catalysis, Prof. Xiao Jianping's group from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, and collaborators, have developed a high-performance cobaltosilicate zeolite catalyst (CoS-1) via a hydrothermal synthesis method. The catalyst has solely tetrahedral cobalt sites and none of the unstable cobalt species, achieving a propylene productivity as high as 9.7 kgC3= kgcat–1 h–1, surpassing that of an industrial PtSn/Al2O3 catalyst.
Read more.
4 notes
·
View notes
Text









Sources of Color: Organic Compounds
Color in many (but not all) organic materials is caused by the presence of particular organic compounds. In plants, chlorophyll is responsible for the green color most of them exhibit, while the colors of many flowers and fruits are caused by organic compounds like carotenoids, flavonoids, betalains. The same mechanism is responsible for color in most organic dyes and pigments, such as the indigo dye that is used to color jeans, as well as pigmentation in animals. Some animals get their coloration, like flamingos, from organic compounds in the food they eat. Melanin is the main pigmentation found in mammals, and can also be found in other species. Albinism is the absence of this pigment.
Sources/Further Reading: (WebExhibits) (University of Wisconsin)
Images: ( 1 and 3-9 ) ( 2 )
10 notes
·
View notes
Text

Cool science: Researchers craft tiny biological tools using frozen ethanol
Imagine drawing on something as delicate as a living cell—without damaging it. Researchers at the University of Missouri have made this discovery using an unexpected combination of tools: frozen ethanol, electron beams and purple-tinted microbes. By advancing a method called ice lithography, the team was able to etch incredibly small, detailed patterns directly onto fragile biological surfaces. While traditional lithography is commonly used to make tiny circuits and other electronic parts for phones and computers, it relies on a liquid process that can easily harm delicate materials, including carbon nanotubes and biological membranes.
Read more.
7 notes
·
View notes
Text
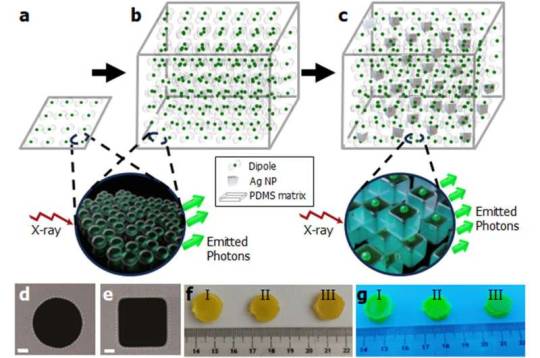
From thin to bulk: Affordable, brighter and faster scanning with high-energy radiation sources
Imagine a medical scanner that works faster and produces clearer images, or a radiation detector that pinpoints tiny traces of radioactive material with unprecedented accuracy. These futuristic possibilities are a step closer to reality thanks to new research by scientists at the Łukasiewicz Research Network—PORT Polish Center for Technology Development. In a publication in Advanced Materials, they reveal how they've scaled up a new type of light-emitting material—known as a scintillator���by embedding it with nano-engineered metallic structures, unlocking performance previously thought unattainable in bulk materials. Scintillators are special substances that emit visible light when exposed to high-energy radiation such as X-rays or gamma rays. They are critical in numerous fields—from medical imaging and security screening to high-energy physics experiments. But traditional scintillators have limitations: They often emit weak signals or respond slowly, making them less efficient for demanding applications.
Read more.
#Materials Science#Science#Radiation#Scintillators#Plasmonics#Nanotechnology#Nanoparticles#Self assembly
5 notes
·
View notes
Text
Rutgers University-New Brunswick researchers have discovered a new class of materials -- called intercrystals -- with unique electronic properties that could power future technologies. Intercrystals exhibit newly discovered forms of electronic properties that could pave the way for advancements in more efficient electronic components, quantum computing and environmentally friendly materials, the scientists said. As described in a report in the science journal Nature Materials, the scientists stacked two ultrathin layers of graphene, each a one-atom-thick sheet of carbon atoms arranged in a hexagonal grid. They twisted them slightly atop a layer of hexagonal boron nitride, a hexagonal crystal made of boron and nitrogen. A subtle misalignment between the layers that formed moiré patterns -- patterns similar to those seen when two fine mesh screens are overlaid -- significantly altered how electrons moved through the material, they found.
Read more.
#Materials Science#Science#Crystals#Electronics#Twistronics#2D materials#Quasicrystals#Rutgers University
9 notes
·
View notes
Text

Pressure and temperature record broken for sCO₂ materials testing
Southwest Research Institute (SwRI) has achieved a significant milestone, reaching new temperature records for testing materials in high-pressure environments. While conducting material testing for a high-pressure, high-temperature supercritical carbon dioxide (sCO2) turbine, SwRI achieved unprecedented conditions of 1,150 degrees Celsius (2,100 degrees Fahrenheit) at 300 bar (4,350 psi). These are the highest published temperature and pressure conditions ever reached in sCO2 materials testing. In 2020, began to design an sCO2 oxy-fuel turbine for a direct-fired sCO2 power plant. The project, led by Senior Research Engineer Michael Marshall and Institute Engineer Dr. Jeff Moore, required materials testing in extreme sCO2 environments. "We evaluated turbine materials at constant temperatures and pressures with 100% sCO2. We assessed the performance of different materials and coatings under extreme conditions," said SwRI's Dr. Florent Bocher, who oversaw materials engineering for the project.
Read more.
7 notes
·
View notes
Text









Sources of Color: Transition Metal Impurities
There are two sources of color that are generally derived from transition metals, known as idiochromatic and allochromatic. This post offers a very brief explanation of allochromatic colors, which derive their color from the presence of impurities. One common way to tell if a mineral is colored from impurities or not is known as the streak test. Scratching the mineral against a rough surface—as if using a piece of chalk—will leave a streak behind of powdered material. If the streak is the same color as the mineral used, the color is likely not derived from impurities.
Sources/Further Reading: (WebExhibits) (University of Wisconsin) (American Mineralogist)
Image sources: ( 1 ) ( 2 and 8 ) ( 3 ) ( 4 ) ( 5 ) ( 6 ) ( 7 ) ( 9 )
4 notes
·
View notes
Text









As with all colors, the color orange can be caused by a variety of different mechanisms:
The color of image one, orange rust, is attributed to transition metal compounds.
The color of image two, an orange sodium lamp, is attributed to gas excitations.
The color of image three, a pile of orange carrots, is attributed to the presence of the organic compound known as carotene.
The color of image four, orange crocoite, is attributed to charge transfer.
The color of image five, orange sodium dichromate, is attributed to charge transfer.
The color of image six, orange orpiment, is attributed to its band gap as a pure semiconductor.
The color of image seven, orange leaves, is attributed to the organic compounds known as carotenoids as well as the decay and partial absence of chlorophyll.
The color of image eight, an orange LED, can be caused by doped semiconductors. Note that the exact material used in the LED show is unknown, but that gallium arsenide phosphide, and aluminium gallium arsenide phosphide are common choices for orange LEDs.
And finally the color of image nine, copper, is a result of its metallic electron configuration.
9 notes
·
View notes
Text

Using sound waves to create a smart T-shirt
Imagine wearing a T-shirt that measures your breathing or gloves that translate your hand movements into commands for your computer. Researchers at ETH Zurich, led by Daniel Ahmed, Professor of Acoustic Robotics for Life Sciences and Healthcare, have laid the foundations for just such smart textiles. Unlike many previous developments in this area, which usually use electronics, the ETH researchers rely on acoustic waves passed through glass fibers. This makes the measurements more precise and the textiles lighter, more breathable and easier to wash. "They are also inexpensive because we use readily available materials, and the power consumption is very low," says Ahmed.
Read more.
#Materials Science#Science#Sound#Smart materials#Wearable technology#Acoustics#Glass fibers#Glass#Fibers#Textiles#ETH Zurich#Waveguides
8 notes
·
View notes
Text

Researchers develop a pressure-induced water-producing material
Researchers at the University of Tsukuba have discovered a phenomenon—applying pressure to a copper-chromium Prussian blue analog, which is a compound featuring crystal voids, causes the discharge of water retained within these voids. This material is expected to serve as a novel onsite water production platform for the extraction of water solely through pressure application, without temperature or humidity control, even in arid regions. Copper-chromium Prussian blue analogs are crystalline compounds containing voids (pores). The researchers found that the water retained in these pores can be expelled by applying pressure to these crystals. Previously reported onsite water production technologies rely on variations in temperature and humidity, and are thus strongly dependent on changes in environmental conditions, thereby often involving long waiting periods.
Read more.
6 notes
·
View notes
Text

Synthetic materials mimic seashells to enhance energy absorption
Millions of years of evolution have enabled some marine animals to grow complex protective shells composed of multiple layers that work together to dissipate physical stress. In a new study, engineers have found a way to mimic the behavior of this type of layered material, such as seashell nacre, by programming individual layers of synthetic material to work collaboratively under stress. The new material design is poised to enhance energy-absorbing systems such as wearable bandages and car bumpers with multistage responses that adapt to collision severity. Many past studies have focused on reverse engineering to replicate the behavior of natural materials like bone, feathers and wood to reproduce their nonlinear responses to mechanical stress. A new study, led by the University of Illinois Urbana-Champaign civil and environmental engineering professor Shelly Zhang and professor Ole Sigmund of the Technical University of Denmark, looked beyond reverse engineering to develop a framework for programmable multilayered materials capable of responding to local disturbances through microscale interconnections.
Read more.
17 notes
·
View notes
Photo









Sources of Color: Transition metal compounds
There are two sources of color that are generally derived from transition metals, known as idiochromatic and allochromatic. This post offers a very brief explanation of idiochromatic—or self-colored—transition metal compounds, which derive their color based on the bulk composition, or, in the case of solutions, the transition metal ion that is most prevalent. Color in transition metal compounds arises from incomplete d orbitals. Because of this, it is not only the transition metal element in question that determines the resulting color, but also its oxidation state. Some of the best known examples are the blue and green of copper compounds.
Sources/Further Reading: (University of Wisconsin) (American Mineralogist) (chemguide) (Science Revision)
Image sources: ( 1 ) ( 2 ) ( 3 ) ( 4 ) ( 5 ) ( 6 ) ( 7 ) ( 8 ) ( 9 )
12 notes
·
View notes
Text

Red-sea-star-inspired polyurethane enables rapid underwater self-healing
A research team has synthesized a novel red sea star-inspired polyurethane, which can achieve rapid underwater self-healing. The study was published in Macromolecules. Self-healing properties enable materials to autonomously repair structural and functional damage, significantly extending their lifespan while enhancing reliability and durability. Self-healing polyurethane has emerged as a promising candidate due to its flexible design and simple synthetic process. However, water molecules disrupt dynamic bond exchange, slowing down the self-healing process. This impairment limits the application of self-healing polyurethane in aqueous environments, including underwater robotics and implantable medical devices.
Read more.
9 notes
·
View notes
Text

Advancing toward cellulose-based materials for effective and sustainable food packaging
Professor Ying-Chih Liao from the Department of Chemical Engineering at National Taiwan University has published a study in the Chemical Engineering Journal, presenting fully biodegradable food packaging films developed through novel integration strategies and highlighting how the synergistic effects of each component provide an effective alternative to petroleum-based plastics. Due to growing market demand, global plastic production has surged. However, excessive single-use habits have led to the massive discharge of petrochemical plastic waste, which degrades into micro- and nanoplastics, posing serious environmental and health risks. Packaging films, which account for approximately 40% of total plastic waste, represent a paramount target for reduction. To systematically address these pressing concerns, both material selection and fabrication methods must be considered comprehensively. Bacterial cellulose (BC), chitosan (CS), and waterborne polyurethane (WPU) were selected as the main components for their alignment with sustainability criteria.
Read more.
#Materials Science#Science#Cellulose#Packaging#Biomaterials#Biodegradable#Chitosan#Polyurethane#Coatings#National Taiwan University
6 notes
·
View notes
Text

A new strategy to fabricate highly performing thin-film tin perovskite transistors
Tin-halide perovskites, a class of tin-based materials with a characteristic crystal structure that resembles that of the compound calcium titanate, could be promising alternatives to commonly used semiconductors. Past studies have explored the possibility of using these materials to fabricate p-channel thin-film transistors (TFTs), devices used to control and amplify the flow of charge carriers in electronics devices. So far, however, the reliable fabrication and integration of thin-film perovskites into commercially available electronics has proved challenging. This is in part due to difficulties encountered when trying to produce uniform perovskite films with consistent electronic properties using scalable and industry-compatible methods. Researchers at Pohang University of Science and Technology recently introduced a new promising strategy for the fabrication of highly performing TFTs based on tin-halide perovskites. Their approach, outlined in a paper published in Nature Electronics, relies on thermal evaporation and the use of lead chloride (PbCl2) as a reaction initiator.
Read more.
4 notes
·
View notes
Photo
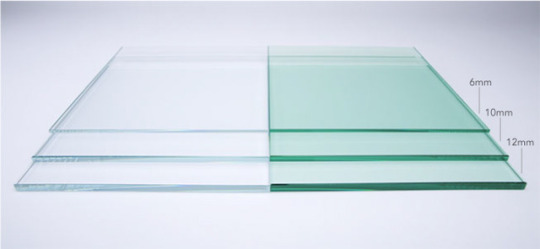
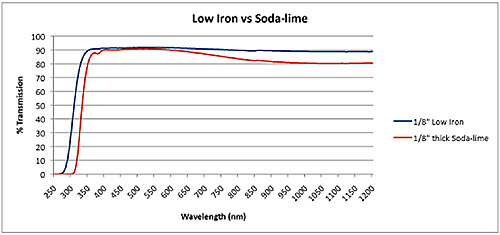


Glasses: Low iron glass
Glass is known for its transparency, or its ability to allow the visible wavelengths of light to pass through it relatively unaltered. However, the primary constituents of silica-based glasses (the most well known type) usually have impurities that can be difficult to completely remove. Impure glass often has a greenish-tint to it, resulting from iron left behind. Low iron glass, therefore, is a form of ultrapure silica glass, in which the iron content has been carefully managed and reduced to allow for increased transparency (often by selecting raw materials that contain less iron).
The difference in transmission can vary, and depends greatly on the thickness of the glass. Thinner sections of low iron glass usually have 2-3% greater transmission of light, but thicker glasses can have even higher percentages. (See chart in the upper right corner above.) There are no set compositions for most types of glass, but low iron glass most always has around 1/10 the amount of iron as other silica glass, or around 0.01% ferric oxide content.
Typical applications of this specialty glass include display, lighting, or architectural applications, as well as optical applications requiring clear visibility and solar panels. Several brand names for this material are UltraClear, Optiwhite, Starphire, Starlite, and Krystal Klear. Low iron glass can technically be used anywhere clear float glass is also used though, and can be processed in much the same ways as well, including lamination, toughening, and other treatments.
Sources/Further Reading: ( 1 - image 1 ) ( 2 - image 2 ) ( 3 - images 3 and 4 ) ( 4 ) ( 5 )
84 notes
·
View notes
Photo

This is Alexandrite, it’s also called “emerald by day, ruby by night”
It changes colour based on whether the light source is from the sun or from a candle.
It does this because Alexandrite strongly absorbs yellow light due to chromium ions in its crystal structure, leaving the other colours behind. Light from the Sun emits all colours, but it peaks in the green, and our eyes are most sensitive to green, so in Sunlight Alexandrite is green.
Incandescent lights are things like candles and filament light bulbs. They also emit all colours of light, but they peak far, far into the red, so there’s not nearly as much green or blue, so under those, Alexandrite is red.
Gemstones are awesome.
75K notes
·
View notes