#... and can tolerate more kinds of charges. case in point Fe 2+ and 3+ are all very common and stable. while a 2+ on carbon would explode)
Explore tagged Tumblr posts
Note
Hi mandizo chemposting the Anon is back. thank u for the long chempost. and for taking the time to explain yayyyy ^_^ this was rlly fun to read (even though it took a Second for me to get it... but i learned smth new!) so cool how everything just clicks and with such a simple formula. thats so satisfying.
i like the havoc duo molecule a lot... its so havoc duo. thank u for showing me this creature. may i ask how the rings are like... Floating there around zirconium??? i've never seen anything like it. (my teacher always says transition metals are just Weird. and now i see this Thing. like whatttt)
and not to dox myself too... im chinese aswell but born and raised in america lmaoo (cant read or write any chinese 🔥🔥🔥) also taking ib chem rn! yeah i took two extremely similar chem courses but erm. anyways. i have free will. def more organic chem (albeit limited to just a handful of reaction mechanisms) and self-guided lab stuff which is my fave part tee bee aych. sooo fun because. Freedom to do whatever ur heart desires... truly something calming about turning ur brain off and getting into the flow of repeating the same titration like 15 times. also dopamine rush when u actually see a trend in your data. (this is one of the simple joys of life. reject gambling. accept being a nerd)
also why did they make potassium permanganate such a pretty colour cause someitmes i just wanna drink it if ykwim.
ty for chatting w me. hope these questions arent annoying im just a silly high schooler that likes chemistry...
HI HI HELLOW YAY SO AWESOME TO HAVE YOU BACK.!!!!!!!!!!!! ^_^^^^^^ ur so right i love the colors of KMnO4... the crystals are so pretty and the solution looks dope too. forbbiden Grape Juice... i was really happy about the neat formula i ended up with as well. before actually working on it i already expected to end up with a cool solution but its still awesome that the analysis ended uup with a simple (and lest we forget a powerful) formula. Such is the awesomeness of thermodynamics... if i knew nothing about thermodynamics and you asked me to determine if a proposed reaction under certain conditions is possible or not i would probably cry. realisticly how would one without thermodynamics knowledge approach this quetsion! not like we can ask the molecules can we... and the beauty of thermodynamics that it enables us to solve such difficult (and important) problems by easily obtaining values of relevant thermodynamic functions (like ΔfH or ΔcH, Sm, and ΔfG) with a calorimeter (but more often and realistically by looking it up on a table) and then doing some simple algebra to come up with solutions to the vital questions "if reactions occur", "under what conditions" and "how much do they occur" which helps us synthesize drugs, produce fertilizer on industrial scales, so on and so forth, which are feats that wouldve otherwise been unimaginable if not for thermodynamics.
THIS IS GETTING TOO LONG AGAIN AHHHHHH SORRY.......... but to answer ur question about the havoc duo molecule :smirk: if you are familiar with the Hit Series "AP Chemistry" youd know that atoms are held in place thanks to Bonds between them. what are bonds but a stick with two atoms on each side right?! get ready to have your mind blown... lets check this epic molecule out and pay close attention to that one bond with Pd (ignore tge two red dots i was having fun with macbook screenshot editing):
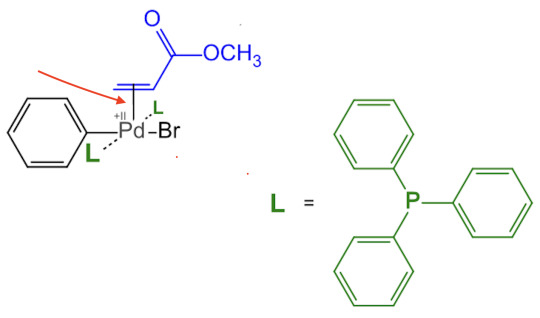
wtf.. why does it look like a C=C pi bond is bonding with a single Pd atom? it looks like that becuasE IT IS THAT. yuipppp... we're looking at a complex with a "polyhapto" ligand. On an intuitive level this thing is straight up nightmare fuel. but on an orbital level this actually makes sense. pi electrons live in pi orbitals which are at the end of the day still orbitals. so whats stopping pi orbitals to interact and bond with empty orbitals of other atoms! nothimg is what ^_^.
lets step it up a bit. we know that double bonds in benzene arent really typical double bonds at all. they are instead delocalized. the double bonds go wherever they please in the ring. So is the case with the cyclopentadienide anion (abbreviated as Cp-).

here comes the shocker: even delocalized bonds such as the one you'd find in benzene are NOT safe from this bonding with transition metals madness. check THIS bond out:
yuippp. thats an entire ass molecule sized ligand bonding to iron with its funky delocalized bonds. and if you thought the silliness would end here, im sorry to report that (purely by coincidence) in 1950, this happened in a lab:

we are looking at a complex consisting of two cyclopentadienyl rings sandwiching (the proper scientific term is really "sandwiching") a central iron atom. this is peak tomfoolery. it looks almost comical. and most awesomely Such sandwich compounds are very stable! havoc duo molecule finds itself in a similar position:
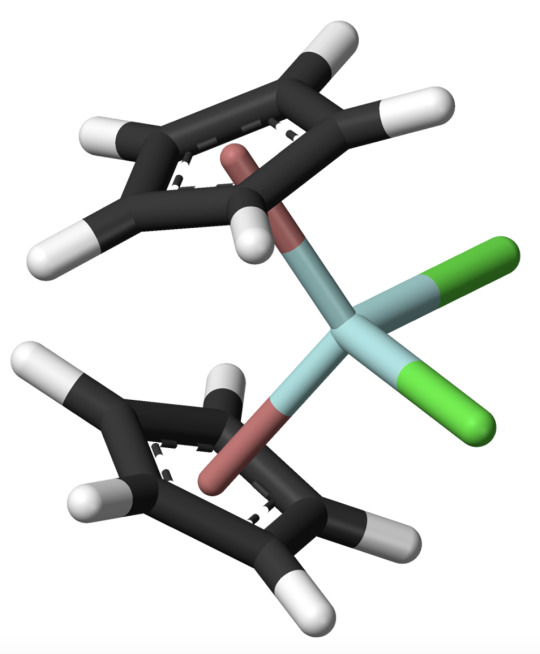
the rings around zirconium arent just simply floating oh nono... They are actively bonding with their entire electron cloud above their ring to zirconium!!
#THANKS FOR TGE ASKS ANON#i think YOURE cool too#FEEL FREE TO TELLME MORE STUFF ANY TIME#if u dont mind the lengths these poasts ...of course#i love talking about and explaining chemistry if u havent noticed#hope you have a good time with ib chem ^_____^^^!!!#the reason why transition metals are so silly like this is becuase of their active d orbitals#d orbitals look silly (aka they have unique geometry) and are quite soft (which means they bond with “soft bases” more easily...#... and can tolerate more kinds of charges. case in point Fe 2+ and 3+ are all very common and stable. while a 2+ on carbon would explode)#titrations... ive done so many titrations#Organic reaction mechanisms are so awesome#if organic reactions were stories then mechanisms are the language in which chemists use to tell them#A REALLY REALLY GOOD BEGINNERS BOOK ON ORGANIC REACTION MECHANISMS (WHICH I OWE MY FOUNDATIONS IN THIS FIELD ENTIRELY TO)#is “Pushing Electrons” 2nd edition by Daniel Weeks#its basically impossible to not follow this book... get a pdf (wink wink) of this book and your off to a great start#chemposting
1 note
·
View note