#Analytical laboratory testing is important for the quality control of drugs. This testing ensures that any patient will not face any side-ef
Text
Crafting Clear Skin: The Precision of Salicylic Acid Manufacturing
Salicylic acid has long been a cornerstone in skincare and pharmaceutical formulations, celebrated for its remarkable efficacy in treating acne, exfoliating the skin, and managing various dermatological conditions. As a leading ingredient in numerous products, the demand for high-quality salicylic acid is unwavering. Salicylic acid manufacturers play a crucial role in meeting this demand, employing advanced technologies, stringent quality control measures, and innovative processes to produce this essential compound. In this blog, we explore the world of salicylic acid manufacturing, highlighting its significance, processes, benefits, and why it’s a cornerstone of modern skincare and pharmaceutical solutions.

The Importance of Salicylic Acid
Salicylic acid is a beta-hydroxy acid (BHA) derived from natural sources like willow bark and wintergreen leaves or synthesized in laboratories. It is renowned for its ability to penetrate pores, exfoliate dead skin cells, and reduce inflammation, making it a powerful ingredient in acne treatments, chemical peels, and dandruff shampoos. Its keratolytic properties help to soften and shed the outer layer of skin, promoting cell turnover and revealing a smoother, clearer complexion.
For more information salicylic acid manufacturer
Advanced Manufacturing Processes
Manufacturing salicylic acid involves sophisticated chemical processes to ensure purity, potency, and safety. The most common method is the Kolbe-Schmitt reaction, which synthesizes salicylic acid from sodium phenoxide and carbon dioxide under high pressure and temperature. This method yields high-purity salicylic acid, suitable for both pharmaceutical and cosmetic applications. Manufacturers utilize advanced equipment and precise control systems to maintain optimal reaction conditions, ensuring consistent quality and yield.
Quality Control and Assurance
Quality control is paramount in salicylic acid manufacturing. Rigorous testing protocols are implemented at every stage of production, from raw material selection to final product packaging. Analytical techniques such as high-performance liquid chromatography (HPLC), gas chromatography (GC), and mass spectrometry (MS) are used to verify the purity, potency, and stability of salicylic acid. These tests ensure that the final product meets stringent industry standards and regulatory requirements, guaranteeing safety and efficacy for consumers.
Customization and Innovation
Salicylic acid manufacturers often work closely with cosmetic and pharmaceutical companies to develop customized formulations tailored to specific product needs. Whether creating a potent acne treatment, a gentle exfoliating cleanser, or an effective dandruff shampoo, manufacturers provide expertise in optimizing salicylic acid concentrations and formulations for maximum benefit. This collaborative approach fosters innovation, resulting in new and improved products that address evolving consumer demands and dermatological advancements.
Sustainability and Ethical Practices
In response to growing environmental concerns, many salicylic acid manufacturers are adopting sustainable and ethical practices. This includes sourcing raw materials from renewable resources, minimizing waste and emissions, and implementing energy-efficient technologies. Some manufacturers are also exploring greener synthesis methods that reduce environmental impact while maintaining high-quality production standards. These efforts align with the broader industry trend toward sustainability and responsible manufacturing.
Meeting Regulatory Standards
Compliance with regulatory standards is a critical aspect of salicylic acid manufacturing. Regulatory bodies such as the FDA (Food and Drug Administration) and EMA (European Medicines Agency) set stringent guidelines for the production and use of salicylic acid in cosmetic and pharmaceutical products. Manufacturers must adhere to Good Manufacturing Practices (GMP) and ensure their products are free from contaminants, properly labeled, and safe for consumer use. Regular audits and inspections by regulatory authorities help maintain compliance and uphold product integrity.
Future Trends and Innovations
The future of salicylic acid manufacturing is marked by continuous innovation and adaptation to emerging trends. Advances in green chemistry, biotechnology, and nanotechnology are poised to revolutionize production methods, enhancing efficiency and sustainability. Additionally, research into new applications and formulations of salicylic acid promises to expand its role in skincare and healthcare, offering consumers even more effective and versatile solutions.

Conclusion
Salicylic acid manufacturers are at the forefront of producing one of the most versatile and effective ingredients in skincare and pharmaceuticals. Through advanced manufacturing processes, stringent quality control, and a commitment to innovation and sustainability, these manufacturers ensure the consistent supply of high-quality salicylic acid. As consumer demand for effective skincare solutions continues to grow, the role of salicylic acid manufacturers remains vital, driving the development of products that promote healthier, clearer skin and improved well-being. Embrace the power of precision and discover the transformative benefits of expertly crafted salicylic acid.
2 notes
·
View notes
Text
Shriram Pharmacy College: Best Pharmacy Education In India

Shriram Pharmacy College, Bankner, stands out as one of India’s premier institutions for pharmacy education. With a strong focus on practical learning, experienced faculty, and state-of-the-art facilities, Shriram Pharmacy College has become synonymous with excellence in pharmacy education. The college offers a comprehensive Bachelor’s program in Pharmacy, covering diverse aspects of the pharmaceutical industry. This blog will explore the key highlights of studying at Shriram Pharmacy College, including career paths, curriculum details, and the unique benefits of choosing this institution.
### 1. **Bachelor’s Program in Pharmacy: A Four-Year Journey to Excellence**
The Bachelor’s program in Pharmacy at Shriram Pharmacy College is a four-year degree program designed to provide students with an in-depth understanding of pharmaceutical sciences. The curriculum is meticulously structured to include theoretical knowledge and practical training, ensuring that students are well-equipped to meet the challenges of the pharmaceutical industry. With a focus on developing technical skills, critical thinking, and ethical practices, the program ensures that graduates are not just academically proficient but also industry-ready professionals.
### 2. **Extensive Training Across Eight Semesters**
The Bachelor of Pharmacy program at Shriram Pharmacy College is spread across eight semesters, each designed to build a strong foundation in core subjects and introduce advanced concepts in pharmaceutical sciences. From pharmacology, medicinal chemistry, and pharmacognosy to biopharmaceutics and clinical pharmacy, the curriculum encompasses all essential topics. Students gain hands-on experience through laboratory sessions, internships, and industry visits, allowing them to apply theoretical knowledge to real-world scenarios and develop critical skills for their future careers.
### 3. **Pharmacy Business: Focus on Customer Service**
One of the critical aspects of pharmacy education at Shriram Pharmacy College is the focus on the pharmacy business and customer service. Students are trained to provide excellent customer service in community and hospital pharmacies, ensuring that they understand the importance of patient care, ethical practices, and communication skills. Courses cover topics such as patient counseling, prescription handling, and customer relationship management, preparing students to excel in pharmacy operations and maintain high standards of care.
### 4. **Hospital Pharmacist: Ensure Accurate Medication Dispensing**
Shriram Pharmacy College places a strong emphasis on training students to become hospital pharmacists, where accuracy in medication dispensing is crucial. The curriculum includes in-depth knowledge of pharmacotherapy, drug interactions, and patient safety protocols. Students learn to work closely with healthcare professionals to ensure patients receive the correct medications and dosages. The practical experience gained through hospital internships and simulation labs helps them understand the complexities of hospital pharmacy and prepares them for a career in healthcare settings.
### 5. **Chemical Technician: Maintain Strict Safety Protocols**
A significant part of the pharmacy education at Shriram Pharmacy College is dedicated to training students as chemical technicians who can work in laboratories and research facilities. The program emphasizes maintaining strict safety protocols, handling chemicals, and using advanced laboratory equipment. Students learn the importance of quality control, analytical testing, and standard operating procedures (SOPs). The hands-on training in state-of-the-art labs ensures that graduates are well-prepared to contribute to the pharmaceutical and chemical industries.
### 6. **Drug Inspector: Conduct Thorough Compliance Checks**
Shriram Pharmacy College prepares its students to pursue careers as drug inspectors, where they are responsible for ensuring that pharmaceutical products meet regulatory standards. The program covers various aspects of drug laws, quality assurance, and regulatory affairs, providing students with the knowledge needed to conduct compliance checks thoroughly. By understanding the regulatory frameworks and industry standards, graduates are equipped to play a vital role in maintaining the quality and safety of pharmaceuticals in the market.
### 7. **Medical Writer: Communicate Complex Information Clearly**
Another career path for graduates of Shriram Pharmacy College is that of a medical writer. The program emphasizes the importance of clear communication, allowing students to convey complex scientific and medical information to various audiences. Courses in technical writing, medical journalism, and content development are integral parts of the curriculum. The training enables graduates to work in research organizations, pharmaceutical companies, and healthcare institutions where effective communication of medical information is essential.
### 8. **Medical Representative: Build Strong Client Relationships**
The Bachelor of Pharmacy program at Shriram Pharmacy College also prepares students to become successful medical representatives who play a crucial role in the pharmaceutical industry. Training focuses on building strong client relationships, understanding product portfolios, and effectively communicating product benefits to healthcare professionals. Students learn the skills needed to thrive in sales and marketing roles, such as persuasive communication, negotiation, and product knowledge, which are vital for success as a medical representative.
### 9. **Unique Benefits of Choosing Shriram Pharmacy College**
Choosing Shriram Pharmacy College for your pharmacy education comes with several unique benefits. The college offers an integrated approach to learning that combines classroom instruction, practical training, and real-world experience. The faculty comprises industry experts and seasoned academicians who provide mentorship and guidance to students. The college also has strong ties with leading pharmaceutical companies and healthcare institutions, facilitating excellent placement opportunities for graduates.
/media/eb0cb48ed6146db932072f4aa2538013
### FAQs
**Q1: What is the duration of the Bachelor of Pharmacy program at Shriram Pharmacy College?**
The Bachelor of Pharmacy program at Shriram Pharmacy College is a four-year degree program, divided into eight semesters. Each semester covers different aspects of pharmaceutical sciences, from fundamental subjects like pharmacology and medicinal chemistry to advanced topics such as clinical pharmacy and drug regulations. The program also includes practical training through lab work, internships, and industry visits, which helps students gain hands-on experience and prepares them for various career opportunities in the pharmaceutical industry.
**Q2: What career opportunities are available after completing a B.Pharm from Shriram Pharmacy College?**
Graduates from Shriram Pharmacy College have a wide range of career opportunities in the pharmaceutical sector. They can work as hospital pharmacists, community pharmacists, drug inspectors, medical writers, chemical technicians, or medical representatives. The comprehensive education provided by the college ensures that students are well-prepared for roles in both clinical and non-clinical settings, research and development, regulatory affairs, and pharmaceutical marketing, among others. The college’s strong industry ties also help in securing placements for graduates.
**Q3: How does Shriram Pharmacy College ensure practical training for its students?**
Shriram Pharmacy College emphasizes practical training through its state-of-the-art laboratories, hands-on workshops, and internships. The college has collaborations with leading pharmaceutical companies and healthcare institutions where students gain real-world experience. Additionally, regular industry visits, guest lectures by professionals, and simulation-based learning modules are integrated into the curriculum. This approach ensures that students are well-equipped with the skills and experience needed to excel in various pharmacy-related roles upon graduation.
**Q4: What makes Shriram Pharmacy College stand out among other pharmacy colleges in India?**
Shriram Pharmacy College stands out due to its commitment to providing a well-rounded pharmacy education. The college offers a robust curriculum that combines theoretical learning with practical application, guided by a team of experienced faculty members. The state-of-the-art facilities, strong focus on research, and industry-oriented training modules further distinguish it from other institutions. Moreover, the college provides excellent placement support and has a track record of producing highly skilled pharmacy professionals who excel in their careers.
**Q5: Are there any research opportunities available for students at Shriram Pharmacy College?**
Yes, Shriram Pharmacy College encourages students to participate in research activities. The college provides opportunities for research in various fields such as pharmacology, medicinal chemistry, and clinical pharmacy. Students can work on innovative projects under the guidance of experienced faculty members, participate in seminars and conferences, and collaborate with industry professionals. These research opportunities not only enhance their learning experience but also prepare them for careers in academia, research, and development sectors.
### **Conclusion**
Shriram Pharmacy College, Bankner, offers a comprehensive and industry-relevant Bachelor of Pharmacy program that equips students with the skills and knowledge needed to excel in the pharmaceutical field. With a curriculum designed to provide both theoretical and practical knowledge, coupled with excellent career support, the college stands as a leading choice for pharmacy education in India. Whether you’re looking to become a pharmacist, a medical writer, or a drug inspector, Shriram Pharmacy College provides the foundation for a successful and rewarding career.
### Stay Connected with Shriram Pharmacy College!
For the latest updates, educational content, and insights into the dynamic field of pharmacy, don’t miss out on the Shriram Pharmacy College YouTube channel. By liking, sharing, and subscribing, you’ll gain access to expert lectures, student testimonials, campus events, and much more. Stay informed about advancements in pharmaceutical sciences and become a part of our vibrant community. Your support helps us grow and continue providing valuable resources to students and professionals alike. Join us today and never miss an update!
#pharmacy#public health#pharmacist#youtube#hospital#shriram medical college#online pharmacy#shriram nursing college#medicine#shriram pharmacy college
0 notes
Text
Ibuprofen Manufacturing Plant Project Report 2024: Setup and Cost

Introduction
Ibuprofen is a widely used nonsteroidal anti-inflammatory drug (NSAID) that provides relief from pain, inflammation, and fever. Its applications range from over-the-counter medications for headaches and arthritis to prescription drugs for more severe conditions. Establishing an ibuprofen manufacturing plant involves a meticulous approach to ensure high-quality production, regulatory compliance, and efficient operations. This article provides a detailed overview of a Ibuprofen Manufacturing Plant Project Report for setting up an ibuprofen manufacturing plant, focusing on key aspects such as site selection, plant design, production processes, quality control, and regulatory considerations. A frequently asked questions (FAQs) section further clarifies common inquiries related to the project.
Project Overview
1. Objective
The main objective of the Ibuprofen Manufacturing Plant Project is to establish a facility that produces high-quality ibuprofen efficiently while meeting stringent pharmaceutical standards. The plant aims to cater to the growing demand for this essential medication by leveraging advanced technology and adhering to best practices in production and quality control.
Get a Free Sample Report with Table of Contents @
https://www.expertmarketresearch.com/prefeasibility-reports/ibuprofen-manufacturing-plant-project-report/requestsample
2. Site Selection
Selecting the optimal location for the ibuprofen manufacturing plant is critical for operational success. Key factors to consider include:
Proximity to Raw Material Suppliers: Ibuprofen production requires specific raw materials, including intermediates and active pharmaceutical ingredients (APIs). Being close to suppliers can reduce transportation costs and ensure a steady supply of necessary materials.
Infrastructure: The site should have access to essential infrastructure such as reliable power, water, transportation networks for shipping products, and communication systems.
Regulatory Environment: The location should offer a favorable regulatory environment with ease of obtaining necessary permits and compliance with environmental and health regulations.
Labor Availability: Access to a skilled workforce is important for operating and maintaining advanced manufacturing equipment and ensuring adherence to Good Manufacturing Practices (GMP).
3. Plant Design and Layout
The design and layout of the ibuprofen manufacturing plant play a crucial role in ensuring operational efficiency and safety. Key areas of the plant include:
Raw Material Storage: Secure storage areas for incoming raw materials and intermediates, with proper inventory management systems to track usage and ensure quality.
Production Areas: Dedicated spaces for various stages of ibuprofen production, including synthesis, formulation, and packaging. The layout should facilitate a smooth workflow and minimize the risk of contamination.
Quality Control Laboratories: Equipped with advanced analytical instruments for testing raw materials, in-process samples, and finished ibuprofen to ensure they meet quality standards.
Packaging and Distribution: Areas for packaging ibuprofen tablets, capsules, or other dosage forms, and preparing them for shipment to customers.
Utilities and Waste Management: Facilities for managing power, water, and waste in an environmentally responsible manner, ensuring compliance with regulatory requirements.
4. Production Process
The production process for ibuprofen involves several key stages:
Raw Material Preparation: The raw materials, including intermediates and APIs, are prepared and pre-treated for the synthesis process.
Synthesis: The core of ibuprofen production involves a chemical reaction where intermediates are processed to produce ibuprofen. This step requires precise control over reaction conditions and reagent handling.
Purification: After synthesis, the ibuprofen product undergoes purification to remove impurities and ensure it meets required specifications.
Formulation: The purified ibuprofen is formulated into tablets, capsules, or other dosage forms, with precise control over dosage and consistency.
Packaging: The finished ibuprofen is packaged into various formats and prepared for distribution to ensure product protection and compliance with labeling regulations.
5. Quality Control
Ensuring the quality of ibuprofen is essential for its safety and efficacy. Key quality control measures include:
Raw Material Testing: Verifying the quality and suitability of raw materials and intermediates before they enter the production process.
In-Process Monitoring: Regular checks during synthesis, purification, and formulation to ensure adherence to established specifications and prevent deviations.
Finished Product Testing: Comprehensive analysis of the final ibuprofen to ensure it meets required specifications for potency, purity, and stability.
6. Regulatory Compliance
Compliance with regulatory standards is critical for operating an ibuprofen manufacturing plant. This includes:
Good Manufacturing Practices (GMP): Adhering to GMP guidelines to ensure that the manufacturing process is consistent and controlled, resulting in high-quality products.
Environmental Regulations: Managing waste, emissions, and resource use in compliance with local and international environmental regulations.
Health and Safety Standards: Ensuring that the plant meets health and safety regulations to protect workers and maintain safe operating conditions.
7. Economic and Environmental Considerations
The economic aspects of setting up an ibuprofen manufacturing plant involve significant capital investment in infrastructure, equipment, and operational costs. However, the potential for substantial returns through product sales and market expansion makes it a viable investment. Environmentally, implementing sustainable practices such as energy-efficient technologies and effective waste management can help minimize the plant’s ecological footprint and enhance its sustainability.
FAQs
1. What is ibuprofen used for?
Ibuprofen is used to relieve pain, reduce inflammation, and lower fever. It is commonly used for conditions such as headaches, arthritis, menstrual cramps, and muscle pain.
2. What are the main stages of ibuprofen production?
The main stages of ibuprofen production include raw material preparation, synthesis, purification, formulation, and packaging.
3. How do I choose the right location for an ibuprofen manufacturing plant?
Choosing the right location involves factors such as proximity to raw material suppliers, availability of infrastructure and skilled labor, favorable regulatory environment, and environmental impact considerations.
4. What are Good Manufacturing Practices (GMP) in ibuprofen production?
Good Manufacturing Practices (GMP) are guidelines that ensure the manufacturing process is consistent and controlled, resulting in high-quality products. GMP includes equipment maintenance, process control, and employee training.
5. What quality control measures are necessary for ibuprofen production?
Quality control measures include testing raw materials, monitoring in-process production, and conducting comprehensive tests on the finished ibuprofen to ensure it meets required specifications for potency, purity, and stability.
6. How can an ibuprofen manufacturing plant minimize its environmental impact?
An ibuprofen manufacturing plant can minimize its environmental impact by implementing energy-efficient technologies, reducing waste, controlling emissions, and adhering to environmental regulations.
7. What is the typical timeline for setting up an ibuprofen manufacturing plant?
The timeline generally includes phases for planning and design, construction and equipment installation, validation and commissioning, and production start-up, spanning approximately 18-24 months.
8. What are the economic benefits of an ibuprofen manufacturing plant?
Economic benefits include job creation, increased industrial output, and potential revenue from product sales. A well-managed plant can significantly contribute to the local economy and provide a strong return on investment.
Related Reports
https://www.expertmarketresearch.com/reports/immersion-cooling-market
https://www.expertmarketresearch.com/reports/automotive-fabric-market
https://www.expertmarketresearch.com/reports/buy-now-pay-later-market
Media Contact:
Company Name: Claight Corporation
Contact Person: Lewis Fernandas, Corporate Sales Specialist — U.S.A.
Email: [email protected]
Toll Free Number: +1–415–325–5166 | +44–702–402–5790
Address: 30 North Gould Street, Sheridan, WY 82801, USA
Website: www.expertmarketresearch.com
Aus Site: https://www.expertmarketresearch.com.au
0 notes
Text
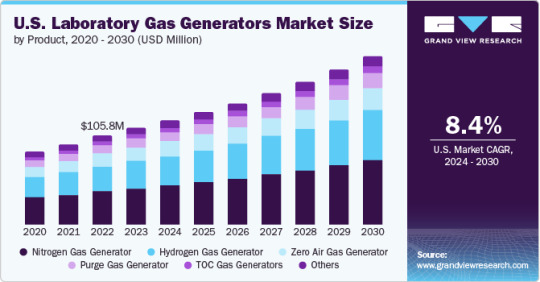
Laboratory Gas Generators Market To Reach $777.4 Million By 2030
The global laboratory gas generators market size is anticipated to reach USD 777.4 million by 2030 and is expected to expand at a CAGR of 8.3% during the forecast period, according to a new report by Grand View Research, Inc. The growth of the market is attributed to the growing importance of analytical techniques in drug and food safety testing, growing safety concerns related to the use of conventional gas cylinders, and increasing R&D spending in the life science sector.
Laboratory gas generators operate across diverse applications, including chromatography and mass spectrometry, wherein they provide high-purity gas essential for accurately identifying various substances within samples, contributing to drug development and quality control. Further in drug detection and analysis, gas supplied from generators ensures efficient and accurate analysis of drug composition, purity, and potential contaminants.
In pharmaceutical packaging, nitrogen gas generators create an inert environment, shielding products from oxidation and degradation during transport and storage, ultimately preserving their integrity and efficacy. However, their role goes beyond mere protection. The nitrogen blanketing facilitated by generators also helps improve the quality of pharmaceutical products. Oxygen exposure can degrade various medications, affecting their potency and shelf life. By creating an oxygen-free environment, generators maintain product quality and extend their usable window.
The laboratory gas generators industry is characterized by intense competition, with numerous manufacturers and suppliers vying for market share. Some of the key players operating in the market include Peak Scientific Instruments, PerkinElmer Inc., Linde plc., VICI DBS, Dürr Technik GmbH & Co. KG, Erre Due S.p.a., and CLAIND srl. Companies are focusing on product innovation, technological advancements, and strategic collaborations to differentiate their offerings and gain a competitive edge. In addition, the growing trend towards customization and modularization in gas generation systems allows end-users to tailor solutions to their specific requirements, further driving market growth and adoption.
Request a free sample copy or view report summary: Laboratory Gas Generators Market Report
Laboratory Gas Generators Market Report Highlights
The nitrogen gas generator segment led the market and accounted for 38% in 2023. The adoption of nitrogen gas generators is driven by their ability to provide high-purity nitrogen on-demand, eliminating the need for gas cylinder handling and storage.
Growing Liquid Chromatography-Mass Spectrometry (LC-MS) field, crucial for drug discovery and metabolomics, drives the need for high-purity nitrogen and helium gas generators for nebulization and collision gas.
The life science sector represents a significant end-user segment for laboratory gas generators, driven by the increasing demand for analytical instruments in drug discovery, development, and quality control processes.
North America dominated the market owing to the increasing investments in the life science, food & beverage, and chemical sectors in the region.
In July 2023, Peak Scientific Instruments introduced nitrogen gas generation solutions with the release of the Corona 1010A. This innovative solution delivers high-purity, filtered nitrogen gas, empowering various applications with a reliable and potent source. Capable of delivering flows up to 5 liters per minute at pressures reaching 80psi.
Laboratory Gas Generators Market Segmentation
Grand View Research has segmented the global laboratory gas generators market based on product, application, end-use, and region:
Laboratory Gas Generators Product Outlook (Revenue, USD Million, 2018 - 2030)
Nitrogen Gas Generator
Hydrogen Gas Generator
Zero Air Gas Generator
Purge Gas Generator
TOC Gas Generators
Others
Laboratory Gas Generators Application Outlook (Revenue, USD Million, 2018 - 2030)
Gas Chromatography
Liquid Chromatography-mass Spectrometry (LC-MS)
Gas Analyzers
Others
Laboratory Gas Generators End Use Outlook (Revenue, USD Million, 2018 - 2030)
Life Science
Chemical & Petrochemical
Food & Beverage
Others
Laboratory Gas Generators Regional Outlook (Revenue, USD Million, 2018 - 2030)
North America
U.S.
Canada
Mexico
Europe
UK
Germany
France
Spain
Italy
Asia Pacific
China
India
Japan
South Korea
Australia
Central & South America
Brazil
Argentina
Middle East & Africa
Saudi Arabia
UAE
South Africa
List of Key Players in the Laboratory Gas Generators Market
Peak Scientific Instruments
PerkinElmer Inc.
Linde plc.
VICI DBS
Dürr Technik GmbH & Co. KG
Erre Due S.p.a.
Tisch Environmental, Inc.
CLAIND srl
Isolcell
OXYMAT
0 notes
Text
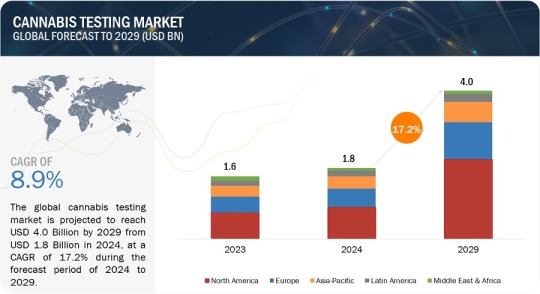
Cannabis Testing Market worth $4.0 billion by 2029
Cannabis Testing Market in terms of revenue was estimated to be worth $1.8 billion in 2024 and is poised to reach $4.0 billion by 2029, growing at a CAGR of 17.2% from 2024 to 2029 according to a new report by MarketsandMarkets™.
The important factors impacting market growth are legalization trends and rising medical applications for cannabis. Rising approvals for medical and recreational cannabis drive the regulations requiring product safety testing which in turn fuel the need for cannabis testing services. Additionally, the rise in the number of cannabis testing labs due to legalization is propelling the demand for analytical instruments is likely to uplift market growth in coming years.
Cannabis Testing Market Trends
Download an Illustrative overview:
Browse in-depth TOC on "Cannabis Testing Market"
439 - Tables
52 - Figures
378 – Pages
Products segment held the highest estimated share of the cannabis testing market.
Based on product & software, the cannabis testing market is segmented into products (analytical instruments {chromatography instruments [liquid chromatography, gas chromatography & other chromatography instruments], spectroscopy instruments [mass spectrometry instruments & atomic spectroscopy instruments] & other analytical instruments} and consumables {chromatography columns, standards and CRMS, sample preparation products and other consumables) and software. The product segment accounted for the largest share of the cannabis testing market in 2023. Market growth is driven by technological advancements providing sophisticated tools for precise analysis, ensuring compliance and quality control, and the expanding legalization of cannabis products. Additionally, increasing requirements for potency labeling and pesticide screening fuel the demand for specialized consumables, further propelling growth in the cannabis testing market. For example, Thermo Fisher Scientific Inc. launched the Thermo Scientific SureSTART consumables portfolio consisting of vials, well plates, caps, inserts, kits, and mats to improve analytical performance and sample security for chromatography and mass spectrometry users in routine and research labs in clinical, food, pharma, biopharma, environmental, and academic sectors.
High growth of services segment attributed to potency testing services.
Based on service, the cannabis testing market is broadly segmented into terpene profiling, microbial analysis, residual solvent analysis, potency testing, heavy metal testing, pesticide screening, and other services. In 2023, potency testing accounted for the largest share of the cannabis testing services market. The high growth of this segment is due to stringent regulatory requirements ensuring accurate THC and CBD levels. This is crucial for product labeling, consumer safety, and compliance, driving demand from producers and dispensaries seeking to meet legal standards and provide reliable, high-quality products.
Services take away the largest estimated share of the end-user segment.
Based on end users, the cannabis testing market is classified into segmented into product & software end users (cannabis testing laboratories {small-scale laboratories, medium-scale laboratories, large-scale laboratories} and research institutes) and service end users (cannabis drug manufacturers & dispensaries, cannabis cultivators/growers). In 2023, the service end users' segment was predicted to account for the highest share of the global cannabis testing market. The large share of this end user segment is due to the rising consumer awareness of quality and safety, rising legalization of cannabis, stringent regulatory standards, and technological advancements in cannabis testing.
During the forecast period, North America displayed lucrative market growth.
North America accounted for the largest share of 65.5% of the cannabis testing market in 2023. The North American market is projected to reach a value of USD 2.6 billion by 2029 from an estimated value of USD 1.2 million in 2024, at a CAGR of 17.3% during the forecast period. The region leads the cannabis testing market due to stringent regulatory standards, robust infrastructure, and widespread legalization of cannabis for medical and recreational purposes. Additionally, North America's advanced R&D capabilities and evolving consumer demand for quality assurance drive its prominence in this growing sector.
Request Sample Pages:
Cannabis Testing Market Dynamics:
Drivers:
Increasing legalization of medical and recreational cannabis
Increasing use of cannabis for medicinal applications
Technological advancements in testing technologies
Restraints:
Lack of standardization
Investment risks due to regularization
Opportunities:
Untapped markets in emerging economies
Research collaborations
Challenge:
High setup costs
Key Market Players of Cannabis Testing Industry:
The global cannabis testing market comprises many key market players competing for markets shares like Agilent Technologies, Inc. (US), Shimadzu Corporation (Japan), Thermo Fisher Scientific Inc. (US), Danaher Corporation (US), Waters Corporation (US), Restek Corporation (US), SGS SA (Switzerland), Merck KGaA (Germany), PerkinElmer, Inc. (US), Hamilton Company (US), Sigma Analytical Services (Canada), SC Labs (US), PharmLabs LLC (US), MCS, Inc. (US), ProVerde Laboratories (US), and Eurofins Scientific (Luxembourg).
The primary interviews conducted for this report can be categorized as follows:
By Respondent: Supply Side- 70%, and Demand Side - 30%
By Designation (Supply Side): Managers - 45%, CXOs & Directors - 30%, Executives- 25%
By Region: North America -40%, Europe -25%, Asia-Pacific -20%, Latin America -10%, MEA- 5%
Recent Developments:
In September 2023, Shimadzu Corporation launched the Brevis GC-2050 gas chromatograph used in various applications including cannabis testing.
In April 2023, SC Labs, one of the leading cannabis testing companies, acquired C4 Laboratories, one of the first Arizona cannabis labs, thus allowing it to be licensed and accredited in five states: California, Colorado, Michigan, Oregon, and Arizona.
In June 2022, Shimadzu Corporation launched the AA-7800 series atomic absorption spectrophotometers. The AA-7800 series is used for quality control in the raw material, food, and for inspecting water quality and hazardous substances in soil thus presenting an advantage in heavy metal testing of cannabis.
Get 10% Free Customization on this Report:
Cannabis Testing Market - Key Benefits of Buying the Report:
The report will help market leaders/new entrants by providing them with the closest approximations of the revenue numbers for the overall cannabis testing market and its subsegments. It will also help stakeholders better understand the competitive landscape and gain more insights to better position their business and make suitable go-to-market strategies. This report will enable stakeholders to understand the market's pulse and provide them with information on the key market drivers, restraints, opportunities, and challenges.
The report provides insights on the following pointers:
Analysis of key drivers (increasing legalization of medical and recreational cannabis, increasing use of cannabis for medicinal applications and technological advancements in testing technologies), restraints (lack of standardization and investment risks due to regularization), opportunities (untapped markets in emerging economies and research collaborations) and challenges (high setup costs) are influencing the growth of cannabis testing market.
Product Development/Innovation: Detailed insights on newly launched products of the cannabis testing market.
Market Development: Comprehensive information about lucrative markets – the report analyses the cannabis testing market across varied regions.
Market Diversification: Exhaustive information about new products, untapped geographies, recent developments, and investments in the cannabis testing market.
Competitive Assessment: In-depth assessment of market shares, growth strategies, and product offerings of leading players include Agilent Technologies, Inc. (US), Shimadzu Corporation (Japan), Thermo Fisher Scientific Inc. (US), Danaher Corporation (US), Waters Corporation (US), Restek Corporation (US), SGS SA (Switzerland), Merck KGaA (Germany), PerkinElmer, Inc. (US), Hamilton Company (US), Sigma Analytical Services (Canada), SC Labs (US), PharmLabs LLC (US), MCS, Inc. (US), ProVerde Laboratories (US), and Eurofins Scientific (Luxembourg) among others in the cannabis testing market.
Get access to the latest updates on Cannabis Testing Companies and Cannabis Testing Industry Growth
#Cannabis Testing Market#Cannabis Testing Industry Size#Cannabis Testing Market Share#Cannabis Testing Market Growth
0 notes
Text
The Importance of Quality Assurance in the Pharmaceutical Industry
https://jpcdn.it/img/051eb779158d87d0dd880a012d758bd3.jpg
Quality assurance (QA) in the pharmaceutical industry is critical to ensuring the safety, efficacy, and quality of drugs and medical products. It encompasses all aspects of the manufacturing process, from the initial research and development stages to the final distribution of the product. A robust QA system ensures that products meet the stringent regulatory standards set by health authorities worldwide.
Key Elements of Pharmaceutical Quality Assurance
1. Regulatory Compliance
Pharmaceutical companies must comply with various national and international regulations, including Good Manufacturing Practices (GMP), Good Laboratory Practices (GLP), and Good Clinical Practices (GCP). Regulatory bodies such as the FDA (Food and Drug Administration) in the United States, EMA (European Medicines Agency) in Europe, and other similar organizations globally, set these standards. Compliance ensures that pharmaceutical products are consistently produced and controlled to quality standards.
2. Quality Management Systems (QMS)
A Quality Management System is a structured system that documents processes, procedures, and responsibilities for achieving quality policies and objectives. It helps in coordinating and directing an organization’s activities to meet customer and regulatory requirements and improve its effectiveness and efficiency on a continuous basis.
3. Risk Management
Risk management is an essential part of QA, involving the identification, assessment, and control of risks throughout the product lifecycle. This includes evaluating potential hazards in the manufacturing process and implementing control measures to mitigate these risks. Techniques such as Failure Mode and Effects Analysis (FMEA) and Hazard Analysis and Critical Control Points (HACCP) are commonly used in risk management.
4. Validation and Verification
Validation ensures that manufacturing processes, equipment, and software perform as intended and produce consistent results. This includes process validation, cleaning validation, and analytical method validation. Verification involves checking and testing to confirm that processes and products meet predefined specifications and standards.
5. Documentation and Record-Keeping
Proper documentation is a cornerstone of QA. It provides evidence that manufacturing processes are performed according to approved procedures and that products meet their quality attributes. This includes batch records, Standard Operating Procedures (SOPs), deviation reports, and audit trails. Accurate and comprehensive documentation is crucial for regulatory inspections and audits.
6. Training and Competency
Ensuring that personnel are adequately trained and competent is vital for maintaining quality standards. Regular training programs and assessments help keep staff updated on current best practices, regulatory requirements, and technological advancements.
7. Continuous Improvement
Continuous improvement is a proactive approach to enhancing quality and efficiency. By analyzing data from audits, inspections, and performance metrics, companies can identify areas for improvement and implement corrective and preventive actions (CAPA). This ongoing process helps in maintaining high standards and adapting to new challenges and opportunities.
Challenges in Pharmaceutical Quality Assurance
Despite the comprehensive systems in place, QA in the pharmaceutical industry faces several challenges:
Complex Supply Chains: With many raw materials and components sourced globally, ensuring quality and compliance throughout the supply chain can be challenging.
Regulatory Changes: Keeping up with evolving regulations and guidelines across different markets requires constant vigilance and adaptation.
Technological Advancements: Integrating new technologies, such as automation and data analytics, into existing QA processes can be complex and resource-intensive.
Counterfeit Drugs: The proliferation of counterfeit drugs poses significant risks to patient safety and requires robust QA measures to detect and prevent them.
Conclusion
Quality assurance in the pharmaceutical industry is a multifaceted and dynamic field that plays a crucial role in safeguarding public health. By adhering to regulatory standards, implementing robust quality management systems, and continuously striving for improvement, pharmaceutical companies can ensure the production of safe, effective, and high-quality products. The commitment to quality assurance not only protects consumers but also enhances the reputation and success of pharmaceutical organizations in a highly competitive market.
0 notes
Text
The Vital Role Of FARE Labs In Pharmaceutical Testing.

Introduction:
In the pharmaceutical industry, where human health is at stake, ensuring the safety and efficacy of drugs is paramount. FARE Labs, a trusted name in testing and calibration, plays a pivotal role in upholding these standards through its comprehensive pharmaceutical testing services. This article explores the critical importance of pharmaceutical testing and highlights how FARE Labs contributes to safeguarding public health and fostering innovation in the pharmaceutical sector.
Comprehensive Pharma Testing Services by FARE Labs:
FARE Labs stands as a beacon of quality and reliability in the realm of pharma testing, offering a wide range of services tailored to meet the stringent requirements of the industry. With accreditation as per ISO/IEC 17025:2017 by NABL and its recognition by esteemed regulatory bodies like the Food Safety & Standards Authority of India (FSSAI), Agricultural & Processed Food Products Export Development Authority (APEDA), Export Inspection Council (EIC), Bureau of Indian Standards (BIS), and MOEF & CC attest to its credibility and adherence to stringent quality standards, FARE Labs employs cutting-edge technologies and adheres to international pharmacopeial standards to conduct meticulous analyses.
The major capabilities and competencies of FARE Labs are as follows:
Raw Material Analysis:
The quality of raw materials used in pharmaceutical formulations directly impacts the safety and efficacy of drugs. FARE Labs specializes in raw material analysis, conducting tests to assess purity, identity, and potency. This ensures that pharmaceutical labs have access to high-quality raw materials, laying the foundation for the production of safe and effective drugs.
Drug Formulation Testing:
Before drugs are released to the market, they undergo rigorous testing to ensure their safety, efficacy, and stability. FARE Labs conducts comprehensive drug formulation testing, examining factors such as dissolution rate, bioavailability, and shelf life. This testing is crucial for pharmaceutical companies to fine-tune formulations and meet regulatory requirements.
Quality Control and Batch Testing:
Maintaining consistent quality across batches is essential in pharmaceutical labs. FARE Labs provides quality control and batch testing services, ensuring that each batch of drugs meets established specifications. This meticulous testing helps pharmaceutical companies identify and address deviations early, maintaining product integrity and compliance with regulatory standards.
Stability Studies:
The stability of drugs over time is a critical aspect of pharmaceutical testing. FARE Labs conducts stability studies to assess how drugs degrade under various conditions such as temperature, humidity, and light exposure. This testing provides valuable data to pharmaceutical companies for establishing expiration dates and storage recommendations.
Regulatory Compliance:
Compliance with regulatory requirements is non-negotiable in the pharmaceutical testing labs. FARE Labs assists pharmaceutical companies in navigating complex regulatory landscapes by ensuring regulatory compliance through thorough testing and documentation. This ensures that drugs meet the stringent standards set forth by regulatory authorities.
Conclusion:
FARE Labs, through its unwavering commitment to pharmaceutical testing, emerges as a trusted partner in the quest for safe and effective drugs. By conducting comprehensive analyses and adhering to regulatory standards, the laboratory ensures that pharmaceutical companies can bring high-quality products to market with confidence. As the pharmaceutical industry continues to evolve, FARE Labs stands ready to provide the analytical expertise and support needed to navigate the complexities of drug development and testing with precision and excellence.
SOURCE- https://farelabs.com/the-vital-role-of-fare-labs-in-pharmaceutical-testing/
#Pharma Testing Labs#PharmaceuticalTesting#Pharmaceuticaltestinglabs#Pharmaceuticaltestingservices#PharmacyLabTesting
0 notes
Text
Maximizing Efficiency with Medical Records Software

In the fast-paced world of healthcare, efficiency is paramount. Medical records software, also known as Electronic Medical Records (EMR) or Electronic Health Records (EHR) systems, plays a crucial role in streamlining operations, enhancing patient care, and reducing administrative burdens. This article explores how healthcare providers can maximize efficiency with medical records software, highlighting its key benefits, features, and best practices for implementation.
The Importance of Efficiency in Healthcare
Efficiency in healthcare is not just about speed; it’s about optimizing resources, reducing errors, and improving patient outcomes. Inefficient processes can lead to long wait times, increased costs, and suboptimal patient care. Medical records software addresses these challenges by digitizing patient records, automating administrative tasks, and facilitating seamless communication between healthcare providers.
Key Benefits of Medical Records Software
Improved Patient Care and Safety:
Medical records software ensures that patient information is accurate, up-to-date, and easily accessible. This reduces the risk of medical errors, such as incorrect prescriptions or missed diagnoses. Additionally, EMR systems can provide clinical decision support, alerting providers to potential drug interactions or recommending evidence-based treatment options.
Enhanced Coordination and Communication:
Efficient communication between healthcare providers is crucial for coordinated care. Medical records software allows for the seamless sharing of patient information across different departments and specialists. This integrated approach ensures that all providers involved in a patient’s care have access to the same information, reducing duplication of tests and procedures and improving overall care coordination.
Streamlined Administrative Processes:
Manual administrative tasks, such as scheduling appointments, billing, and coding, can be time-consuming and prone to errors. Medical records software automates these processes, freeing up staff to focus on patient care. Features like automated appointment reminders, electronic billing, and coding assistance improve accuracy and reduce administrative workload.
Data Analytics and Reporting:
Medical records software provides robust data analytics and reporting capabilities. Healthcare providers can track key performance indicators, monitor patient outcomes, and identify trends. This data-driven approach enables providers to make informed decisions, improve operational efficiency, and enhance the quality of care.
Key Features to Maximize Efficiency
To maximize efficiency with medical records software, it’s essential to choose a system with the right features. Here are some key features to look for:
User-Friendly Interface:
A user-friendly interface is critical for ensuring that staff can quickly and easily navigate the system. Intuitive design reduces the learning curve and minimizes the risk of user errors.
Interoperability:
The ability to integrate with other healthcare systems, such as laboratory information systems and pharmacy management software, is essential for seamless information exchange. Interoperability ensures that all relevant patient data is available in one place.
Mobile Access:
Mobile access allows healthcare providers to view and update patient records on the go. This is particularly useful for providers who work in multiple locations or make home visits.
Customization:
Every healthcare practice has unique needs. Customizable templates and workflows ensure that the medical records software can be tailored to fit the specific requirements of the practice.
Security and Compliance:
Ensuring the security of patient data is paramount. Look for software that complies with industry standards and regulations, such as HIPAA. Features like encryption, access controls, and audit trails help protect sensitive information.
Best Practices for Implementation
Implementing medical records software effectively requires careful planning and execution. Here are some best practices to ensure a smooth transition:
Involve Stakeholders:
Engage all stakeholders, including physicians, nurses, administrative staff, and IT personnel, in the selection and implementation process. Their input is valuable in choosing a system that meets the needs of the entire practice.
Training and Support:
Provide comprehensive training to ensure that all users are comfortable with the new system. Ongoing support and resources should be available to address any issues that arise.
Data Migration:
Plan for the secure and accurate migration of existing patient records to the new system. This may involve data cleaning and validation to ensure the integrity of the data.
Monitor and Optimize:
After implementation, continuously monitor the system’s performance and gather feedback from users. Use this information to make necessary adjustments and optimize workflows.
Conclusion
Maximizing efficiency with medical records software is a multifaceted process that involves selecting the right system, implementing it effectively, and continuously optimizing its use. By leveraging the benefits and features of medical records software, healthcare providers can streamline operations, enhance patient care, and ultimately improve the overall efficiency of their practice. As technology continues to evolve, staying up-to-date with the latest advancements in medical records software will be essential for maintaining a high standard of care and operational excellence.
0 notes
Text
Pharmaceutical distributor | importer | Exporter supplier in india
Contract manufacturing pharma organization
In contract manufacturing, one organization engages an outside party/ parties to handle some of its operations and activities under the contract. Contract manufacturing acts as a helping hand to the manufacturer and the organization (such as hospitals, pharmaceutical companies, etc.), and it is beneficial for both parties. Usually, an agreement is signed by owners (manufacturers and clients) and contract facilities in contract manufacturing.
In the pharmaceutical industry, contract facilities provide various services, operations, and activities, e.g., analytical testing and other laboratory services, packaging and labeling, sterilization or terminal sterilization, cold chain storage and transportation services, and many more.
Ikris Pharma Network is a globally trusted contract manufacturing in the pharmaceutical industry in India. Ikris works according to the FDA’s guidelines of CGMP ( current good manufacturing practice), which includes the implementation of oversight and controls over the manufacture of medicines to ensure quality. It also includes managing the risk of and establishing the safety of raw materials, materials used to manufacture drugs and finished drug products. Ikris have expertise in contract manufacturing and providing such facilities for more than 5 years. It has not limited its services to only contract manufacturing but has mastery in providing other services too.
Global contract manufacturing company
Contract manufacturing in pharma refers to outsourcing the packaging and many more services related to drugs or medicines. An agreement is made between the manufacturers, contract manufacturer and the client regarding operations and activities of the medicine. Operations and activities include packaging and labelling, storage and transportation, cold chain storage, etc.
Contract manufacturers provide facilities to the manufacturing organization so that manufacturers can focus on their core competencies. Contracting can enhance speed and efficiency, provide technological expertise, and expand capacity. This facility is favourable for both manufacturers and clients. Contract manufacturers and manufacturers should adhere to the FDA guidelines always.
Ikris Pharma Network is an India based pharmaceutical contract manufacturing company providing its services worldwide. Ikris strictly adhere to the FDA guidelines and work accordingly. Following these guidelines can benefit the patients who take the drugs manufactured under these arrangements in many ways and avoid the adulteration of drugs. Ikris have expertise in this business, and it works under professionals who have experience from ages. Apart from this, Ikris has excelled in providing other pharmaceutical services too.
Best third party pharmaceutical manufacturer in India
Third-party manufacturing in the pharma industry refers to the organizations which act as a linkage between manufacturers and clients. In third-party manufacturing, pharmaceutical products are outsourced or manufactured from other manufacturing units with your brand name. There is an agreement between the three parties (the manufacturer, the third-party manufacturer, and the client organization). This quality agreement defines the expectations among the three parties regarding the quality of the product, duration of time required, etc.
There are numerous responsibilities of a third-party manufacturer in pharmaceuticals which include: finalizing quantity and composition of the required product, packaging of medicine, delivery of the product, checking of the quality of the product, storage of certain drugs under controlled temperature, etc.
Ikris is one such worldwide trusted third-party pharmaceutical manufacturer in India. Ikris have mastery in this field of third-party manufacturers of medical products. Ikris takes care that FDA guidelines are strictly followed in this process. Based on the FDA standards, Ikris makes the product qualitative and non-adulterated to make it safe for potential patients. Moreover, Ikris provides other major facilities other than third-party pharmaceutical manufacturing. It has been providing services in India and overseas for more than 5 years.
Contract Manufacturing for Pharma products
Contract manufacturing for pharmaceutical products is when a pharmaceutical company engages with other outsourcing organizations to manufacture the products under their brand.
Contract manufacturing is beneficial for both manufacturers and clients (pharmaceutical organizations or hospitals). Usually, an agreement is signed among owners (manufacturers and clients) and contract facilities in contract manufacturing.
Ikris Pharma is an India based pharmaceutical company that deals in contract manufacturing of pharma products. India is known as the largest generic medicine manufacturer in the World, and Ikris works with only certified manufacturers of such medicines. The services of pharmaceutical contract manufacturing companies are not limited to manufacturing only. Ikris provide a host of services which also include: Drug Stability studies, Manufacturing, Development of compliance documents as per FDA regulatory requirement, Developing late-stage clinical trial material, Providing scale-up and registration batches and Pre formulation. Ikris adhere to the FDA regulations and work according to these guidelines benefits the potential patient to have quality medicines with zero adulteration.
Contract manufacturing has become the essential service that a pharmaceutical organization needs. Ikris Pharma provides contract manufacturing of various medicines such as nutraceutical products, generic medicines, etc. Ikris Pharma has been in this industry for more than 7 years supplying drugs and products in India and around the World.
Top Third Party Pharma Manufacturers in India
Here we are talking about third party pharmaceutical manufacturer India, known for the largest generic medicine manufacturing in the World. Third-party manufacturers set up a business association among manufacturers and the clients (hospitals/organizations). When clients search for quality products at reasonable prices and manufacturers want their medicine to reach potential customers, then comes the role of third party manufacturer, quenching the thirst of both parties. Third-party manufacturers make a pact among manufacturers and clients. According to the pact, manufacturers produce products as per the requirements of the client.
Third-party pharma manufacturing is also known as contract manufacturing. A vast number of pharmaceutical organizations use this process for outsourcing manufactured pharma products. Some key factors burden the manufacturers to carry out several activities in-house. Third-party manufacturers decrease their (manufacturers) burden by doing jobs such as packaging and testing before transporting goods to a distribution centre. The involvement of third parties is not only limited to manufacturing and packaging. Product development, specialized processing, such as radiation sterilization, testing, logistics, and medicine transportation at appropriate temperatures.
Ikris Pharma Network is one of the best third party pharmaceutical manufacturers at the international level. Ikris provides this service to hospitals and pharmaceutical organizations, and we work with manufacturers only. There is a list of favours one gets if working with Ikris, a third party manufacturer:
We connect buyers with manufacturing companies. We have more than a hundred manufacturing companies in our Network.
We transport temperature-sensitive medication in cold chain transportation facilities under the required temperature of the medicines.
We always transport the product to the medical sites on time or ahead of time.
One will find 100 % transparency during the whole process.
We work only with approved manufacturers.
Pharmaceutical wholesalers and distributors
The pharmaceutical market is changing rapidly. Due to increasing chronic diseases and after the Covid-19 outbreak, there came a massive boom in the pharma industry. Thus the pharmaceutical organizations and manufacturers began to manufacture and supply medicines and medical equipment faster to meet the requirements of the patients. Pharmaceutical distributors work more than an intermediary and ease the tasks of manufacturers and clients.
Pharma distributors purchase and take legal ownership of pharmaceuticals and manage inventory and credit risk; this allows manufacturers to focus on their core drug development and manufacturing competencies. They are critical players in the healthcare ecosystem. Ninety-two percent of prescription pharmaceuticals flow through a complex, secure ecosystem composed of patients, providers, pharmacies, distributors, group purchasing organizations (GPOs), manufacturers, pharmacy services administration organizations (PSAOs), pharmacy benefit managers (PBMs).
Pharmaceutical distributors continue to evolve beyond their flagship offering of core services to enlarge services to ecosystem stakeholders while optimizing costs. In addition to enabling a secure, transparent, and efficient pharmaceutical supply chain that safeguards patient safety, distributors offer value-added services such as independent pharmacy support, generic sourcing programs, hub services, and innovative partnerships.
Distributors provide services and distribution facilities such as packaging and testing before transporting goods to a required site. Ikris Pharma Network is one of the trusted distributors and wholesalers in the Indian market, exporting medical products and medicines globally. Ikris is a GDP-certified company having licenses for both wholesale and retail drugs. There are temperature-sensitive drugs that require appropriate storage and transportation under the required temperature, and we are best at catering cold-chain supplies of medicines.
We have professionals, sophisticated processes, and impeccable reliability. Our aspiration and liability are to keep up to ensure our clients’ supply chains and businesses both succeed and keep flourishing. We believe in completing the assigned task before or on time. That’s why we are emerging as a top pharmaceutical distributor in India and at the global level.
India: Largest pharmaceutical distributors and manufacturers
A pharmaceutical distributor works as a link between manufacturers, healthcare providers, and eventually the patients. Pharma distributors are the backbone of the healthcare ecosystem. India is known as the largest generic medicine manufacturer and distributor in the World. Distributors manage a complex supply chain, harnessing innovative technologies to ensure safe, secure, and efficient delivery every time.
During Covid-19, distributors played a crucial role by supplying medicines and medical aids on time. Pharmaceutical distributors continuously keep in check the appropriate storage conditions, secure handling of the drugs, and medication delivery under required circumstances. Distributors facilitate the manufacturers to concentrate on their core responsibility by taking care of the distribution system, which is a complex job in a pharmaceutical ecosystem. With passing time, chronic diseases are increasing, and after Covid-19, there is a massive demand for medicines in the pharma sector. And to meet the need of the medication, manufacturers are manufacturing the products at a double rate and delivering the products on time; manufacturers need distributors to do the further job of supplying the client's requirements on time at the required site.
Ikris Pharma Network is known as the trusted pharmaceutical distributor from India. We deal in high-quality drug supply at the global level. Ikris is a certified supplier of generic medicines in India as well as in the World. We have certification from the highest authorities like ISO 9001:2008, GMP, WHO, GDP, etc. Ikris being a pharma distributor, provides services and distribution facilities such as cold chain shipping, packaging, and testing before transporting goods to a required site.
Ikris Pharma Network distributes medicines and medical products in bulk to organizations/hospitals and directly to patients. Our dedicated team and professional leaders make this distribution organization outstanding in the pharmaceutical sector in the World. We have excelled in distributing rare medicines which are not available easily in the market, such as cancer medications, heart disease medications, HIV-related medication, etc. We have served 50000+ patients, supplied more than 5000 products across 150+ countries such as Africa, United States, UK, Gulf countries, Vietnam, Sri Lanka, South East Asia, Philippines, Cambodia, Poland, and many more.
Ikris Pharma Network is a fast-growing global pharmaceutical consultancy and service company whose motto is "access is a right, not a privilege." It is dedicated to delivering hard-to-access medicines to needy patients to improve their quality of life. With Ikris, geographical boundaries are no more constraints for needy patients in getting otherwise unavailable medication.
Top-grade third-party pharmaceutical manufacturer
The third-party pharmaceutical manufacturer refers to when one firm gets medications produced by a manufacturing firm with its brand name. Third-party manufacturers help in making higher quality medicines available to the potential customers manufactured by the manufacturer. Both manufacturer and the third-party manufacturer sign the legal document according to which manufacturer will manufacture the products as per the customers' requirements.
Ikris Pharma Network (IPN) is the best emerging third-party pharmaceutical manufacturer in India, and is a known largest generic medicine manufacturer. IPN comes with a lot of perks as a third-party manufacturer, such as
The entire process becomes cost-effective.
Increase in the efficacy in the core competencies of a pharma firm.
Product development, specialized processing, such as radiation sterilization, testing, logistics, and medicine transportation at appropriate temperatures.
100% transparency in the entire process.
Selects the best manufacturer according to the customers' requirements.
IPN has an established vast network of USFDA-approved manufacturers.
Licensed Private Label Manufacturer
Private label manufacturing refers to the contract or third-party manufacturing in which a private label product is manufactured by another firm on a contract basis and sold under the retailer’s brand. Outsourced products are manufactured according to the demand and requirements of the retailers or the customers. The outsourcing process is not limited to manufacturing and packaging, but private label manufacturers provide facilities such as Drug Stability studies, Manufacturing, Development of compliance documents as per FDA regulatory requirements, Developing late-stage clinical trial material, Providing scale-up and registration batches and Pre formulation.
Ikris Pharma Network or IPN is one of the best private label pharmaceutical manufacturers firm for outsourcing the manufacturing of pharma products and medicines. IPN is already exporting products to more than 150 countries worldwide. It is run by professionals who have had expertise in this industry for more than 20 years. Ikris works with USFDA approved manufacturers only.
Pharmaceutical distributor
India is known for generic medicines manufacturing in large quantities. India is one of the top suppliers and distributors in the World. With increasing cases of chronic or long term diseases, the demand for medicines is also growing. To meet the requirements of the patients' manufacturers manufacture the drugs at twice the previous rate of manufacturing. Distributors help in connecting the manufacturers and health providers. Distribution is a vital stage in the complex supply chain of products in the pharmaceutical industry.
Ikris Pharma Network is a top-level wholesale pharmaceutical distributor from India. In addition, to allow a secure, transparent, and efficient pharmaceutical supply chain that safeguards patient safety, IPN offers value-added services such as independent pharmacy support, generic sourcing programs, hub services, and innovative partnerships. It also has expertise in cold-chain transportation for temperature-sensitive drugs, supplying in India and overseas.
A Pharmaceutical Supplier in Brazil
During the Covid-19 crisis, many countries ran out of rare medicines for patients suffering from chronic diseases like cancers, HIV, etc. India came forward to help such countries by supplying rare drugs to different countries worldwide. India is known as the largest generic medicine. Ikris Pharma Network is a fast-growing global pharmaceutical consultancy and service company dedicated to delivering hard-to-access drugs to needy patients to save their lives. Ikris is also emerging as the best pharmaceutical supplier in Brazil, mainly after it supplied medicines during the pandemic, and IPN is continuously supplying drugs in the Brazilian pharmaceutical sector. It has already provided more than 150 products to Brazil. IPN is a GDP-certified pharmaceutical organization for wholesale as well as retail drugs. We supply to pharmacies, hospitals, pharmacy organizations and many more. It is a reliable brand Worldwide for providing the best quality pharma products. It only works through legal channels in delivering products and works as per the FDA guidelines.
Private label manufacturers in India
Private label manufacturing is similar to contract manufacturing or third-party manufacturing.
This process is beneficial for pharmaceutical manufacturers and customers (hospitals, pharmacies, etc.). In private label manufacturing, the products are outsourced to manufactured by some other pharmaceutical firm under another firm's brand. To meet the increasing demand for medicines and medical equipment, pharmaceutical organizations outsource the in-house operations and activities to other firms to lessen their burden and focus on their core business.
Ikris Pharma Network is one of the best known private label pharmaceutical manufacturers India. Being a certified private label manufacturer, IPN provides the best services, including Drug Stability studies, Manufacturing, Development of compliance documents as per FDA regulatory requirements, Developing late-stage clinical trial material, Providing scale-up and registration batches and Pre formulation. IPN has been supplying internationally in more than 150 countries for more than seven years. It is run by professionals and has a vast network with FDA-approved manufacturers.
Contract Manufacturing Pharma Companies in India
Contract manufacturing refers to the manufacturers who serve other pharmaceutical organizations on a contractual basis to provide services from drug development through drug manufacturing. Contract manufacturing helps pharmaceutical firms focus on drug discovery, drug marketing, etc., reducing their in-house activities. The contract manufacturer and the customer and the manufacturer enter into a written agreement to ensure that the customer’s operations do not face any obstacles during the whole process to have an uninterrupted quality supply of the product within the appropriate time.
Ikris Pharma Network is on top in listed contract manufacturing Pharma companies in India. Do you know what qualities make IPN best? Here are the following benefits of IPN as a contract manufacturer:
Cost optimization
Fast track drug products and speed time delivery to the required site
Meeting current and projected growth trends for drug products
Helps pharmaceutical companies in the cost optimization and ease of burden of hiring and training workers during periods of labour shortage
Services offered by IPN include but are not limited to pre-formulation, formulation development, stability studies, method development, clinical trial materials, formal stability, scale-up, registration batches and commercial production.
Best Third-Party Manufacturing Pharma Companies
Third-party manufacturers mean one firm outsources the products to manufacture by another firm under the brand name of retailers. The complex supply chain in the pharmaceutical ecosystem leads pharmaceutical organizations to opt for third-party manufacturing. An assignment is signed among the manufacturers and third-party manufacturers according to which manufacturer will manufacture the products based on the customers’ requirements.
The need for third-party manufacturers comes when an organization is burdened with many in-house activities and concentrates on its core business. The involvement of third parties is also not limited to manufacturing and packaging: product development, specialized processing, such as radiation sterilization, testing, and logistics.
Ikris Pharma Network is an internationally acclaimed pharmaceutical organization. It is a GDP-certified organization having licenses in both wholesale and retail drugs. IPN is on top of the list of third-party manufacturing pharma companies in India. It provides 100% transparency in the whole process. It has been exporting products across the globe for more than 7 years.
Contract manufacturing pharmaceutical company
Contract manufacturing is a term that defines a process in which a firm produces products for a pharmaceutical firm but does not own the inventory. This kind of partnership benefits the pharma firm, the contract manufacturer, and the customers. The lack of facilities for in-house activities or the burden of many in-house activities leads to the need for a contract manufacturer. Contract manufacturers also provide advanced technologies and high containment capabilities and manufacturing and other operations and activities (Drug Stability studies, Manufacturing, Development of compliance documents as per FDA regulatory requirement, Developing late-stage clinical trial material, etc.).
Ikris Pharma Network is an internationally acclaimed and GDP-certified pharmaceutical organization blooming as among the best pharma contract manufacturing companies in India and globally. They have been in this industry for more than a decade and can find a tailor-made solution according to the needs of the clients. IPN only works with the FDA-approved manufacturers and believes in 100 percent transparency during the whole process.
Third-party manufacturers in Pharmaceutical
Third-party manufacturing is also termed contract manufacturing. Third-party manufacturing is a partnership in which two parties agree to manufacture the products according to the customers' demand within an appropriate time period.
Ikris Pharma Network (IPN) is emerging as the best third-party manufacturing pharma.IPN is run by professionals having years of experience in this industry. Apart from manufacturing and packaging, IPN facilitates many services such as product development, specialized processing, such as radiation sterilization, testing, logistics, and medicine transportation at appropriate temperatures. Outsourcing the products to third-party manufacturers reduces the burden of the pharmaceutical organization in many ways, which results in the concentration of a pharma organization in their core business. IPN as a third-party manufacturer proves to be a good agreement because it adheres to FDA guidelines, ensures the security of the supply, has specialist capabilities, has a license, provides product lifecycle management, has market access, and provides cost optimization.
Need for Contract Manufacturing in the Pharmaceutical Industry
With passing time, the demand for generic medicines is increasing. Because of the increasing demand, the pharmaceutical ecosystem is getting complex, and many burdens are on pharmaceutical organizations to manufacture higher quality products within the appropriate time. For easing the responsibility of the pharmaceutical organization, contract manufacturers came into being to carry out many in-house activities. CMOs have facilities such as quality equipment and a labor force to carry out cost-effective production.
Ikris Pharma Network (IPN) has expertise in pharmaceutical contract manufacturing. It facilitates manufacturing and packaging services with more services (like Drug Stability studies, Manufacturing, Development of compliance documents as per FDA regulatory requirement, Developing late-stage clinical trial material, Providing scale-up and registration batches and Pre formulation, and so on). IPN has been working in the pharmaceutical ecosystem for more than 7 years and delivering products to more than 150 countries worldwide.
Role of contract manufacturing in pharma ecosystem
Third-party manufacturing or private label manufacturing is very similar to contract manufacturing. In these processes, an agreement is signed among the contract manufacturers, the pharmaceutical organization in which manufacturers agree to manufacture the products as per the clients' necessities to pacify the requirements of the potential patients for higher quality medicines available at a reasonable price. Contract manufacturing plays a significant part in the pharmaceutical ecosystem to ease the tasks of the pharmaceutical organizations in in-house activities and operations to manufacture a quality drug.
Ikris Pharma Network is a GDP-certified pharma contract manufacturing organization helping other pharmaceutical organizations to focus on their core competencies by performing some tasks for them. Contract manufacturers perform functions like manufacturing and packaging of the products and offer many other services like cold-chain supply, cost-effective production, drug stability studies, picking up the best manufacturer as per the clients' requirement, and many more.
Top pharmaceutical third-party manufacturing organization
With the growing need for generic medicines, third-party manufacturing is in huge demand offering a range of facilities other than manufacturing and packaging the products. Third-party manufacturing means the agreement in which one firm outsources the drugs for production to another firm under its brand name. So, if one is looking for the best third-party manufacturer, Ikris Pharma Network is the best option one can opt for.
Ikris Pharma Network tops the list of top 10 pharmaceutical third-party manufacturing company in India. Ikris is a GDP-certified pharmaceutical organization that has provided many services such as third-party manufacturing in India and internationally for more than 7 years. Service provided by IPN includes Drug Stability studies, Manufacturing, Development of compliance documents as per FDA regulatory requirement, etc. IPN only works according to the FDA guidelines and works with only those manufacturers approved by the FDA.
Top grade pharmaceutical contract manufacturing companies
The term “Contract manufacturing” refers to a process in which one firm partners with another firm to ease their burden of in-house activities and operations so that they can focus on their core business. Manufacturers and contract manufacturers sign an agreement to manufacture the products according to the clients’ requirements and complete the task in time.
Ikris Pharma Network is in the top 10 pharmaceutical contract manufacturing companies list. IPN is an internationally acclaimed pharmaceutical contract manufacturing company that serves manufacturing and packaging facilities and other services such as cold-chain supply, developing late-stage clinical trial material, providing scale-up and registration batches and pre-formulation, etc. Outsourcing of products is beneficial for both pharmaceutical organizations and contract manufacturers. IPN is already supplying the product Worldwide. It has a wide established network with FDA-approved manufacturers.
3rd Party Manufacturing Pharmaceuticals
Third-party manufacturing in the pharmaceutical ecosystem plays a crucial role in keeping up with the increasing demand for generic medicines due to the increase in the cases of chronic diseases. Most pharmaceutical firms take the help of third-party manufacturers to manufacture products for them under their brand name.
One should look for the qualities before going for a third-party manufacturer:
Works according to the FDA guidelines.
Cost optimization for production of goods.
Cold-chain supply for temperature-sensitive drugs.
Development of compliance documents as per FDA regulatory requirements.
Have expertise in this industry and work with only manufacturers approved by FDA. One can find all these qualities with other facilities from Ikris Pharma Network (IPN), one of the best 3rd party manufacturing pharmaceuticals in India. IPN is an internationally acclaimed pharmaceutical organization having expertise in contract manufacturing service, including other facilities. Ikris can help one build complete indication-wise portfolios, e.g., if you require a portfolio for Transplant, Ikris Pharma will provide all transplants. This includes Cyclosporine, Mycophenolate, Azathioprine, and Sirolimus, etc.
Third-party Manufacturing Pharmaceuticals
Third-party Manufacturing is a process in which an agreement is signed among the manufacturers and pharmaceutical organizations to fulfill the demand of the potential patients of higher quality drugs at a reasonable cost. Most pharmaceutical organizations prefer third-party manufacturers' involvement due to a lack of time, resources, or labor force. A third-party manufacturer eases the burden of any pharmaceutical organization, which results in the smooth running of their core business without any interruption.
And Ikris Pharma Network (IPN) has expertise in this area of the pharmaceutical industry. Therefore, if you are looking for some reliable plus best 3rd party manufacturing pharmaceuticals in the pharma ecosystem, IPN is a promising option. IPN hosts several services other than Manufacturing and packaging such as drug stability studies, Manufacturing, development of compliance documents as per FDA regulatory requirement, developing late-stage clinical trial material, providing scale-up and registration batches, and pre-formulation.
Top Third-party manufacturers in India
India is known as the largest manufacturer of generic medicines and exporting them to the World. To meet the increasing demands of generic medicines with increasing cases of chronic diseases, the pharmaceutical ecosystem is having a burden, and the ecosystem is becoming complex. The supply chain of pharmaceuticals starts from the supply of active ingredients. It goes through several stages to get the final product. The third-party manufacturers play a crucial role in easing the job of pharmaceutical organizations by doing several tasks for them according to the clients' requirements. Manufacturers and third-party manufacturers sign a legal agreement to manufacture products as per the necessities.
Ikris Pharma Network (IPN) is one of the top third-party manufacturers in India providing services worldwide. IPN is already serving in more than 150 countries in the World and works with only FDA-approved manufacturers to deliver quality products at affordable prices. IPN is a GMP-certified pharmaceutical organization providing many services and has expertise in third-party manufacturing, cold-chain supply, and many more.
Contract Manufacturers in Pharmaceutical ecosystem
Contract manufacturers are those organizations who offer manufacturing services with volume capabilities ranging from small amounts for preclinical R&D to larger volumes necessary for clinical trials purposes and commercialization. A contract manufacturer is a company that serves other companies in the pharmaceutical industry on a contract basis to provide comprehensive services from drug development through drug manufacturing. Contract manufacturing eases the job of pharmaceutical organizations to manufacture quality products at a reasonable price within an appropriate time.
Ikris Pharma Network (IPN) is an internationally acclaimed pharmaceutical organization that hosts many services, and contract manufacturing is one of them. IPN is blooming as one of the best pharma contract manufacturing companies in India. As a contract manufacturing company, IPN serves facilities such as drug stability studies, manufacturing, development of compliance documents per FDA regulatory requirement, etc. IPN as a contract manufacturer provides a wide range of services, including manufacturing of Ayurveda, Herbal, and generic medicines with various approved quality levels.

Role of outsourcing the pharmaceutical products
Pharmaceutical organizations outsource the manufacturing of the products to other firms to ease their burden of in-house activities and operations. Outsourcing helps companies to save on resource costs, infrastructure costs, and other overhead expenses. Contract manufacturers, private-label manufacturers, and third-party manufacturers are the manufacturers for the outsourced production of products. Outsourcing helps pharma organizations improve efficiencies, reduce costs, ensure business continuity, better access expertise, reduce staff, allow staff to focus on the core competency, and mitigate risk through using the specialist count.
Ikris Pharma Network is an internationally acclaimed pharmaceutical organization, GDP-certified organization, and one of the best pharma outsourcing companies in India exporting globally. IPN deals in numerous medicines related to oncology, hematology, hepatitis, immunotherapy, transplant medicine, vaccines, etc. All the products with IPN are manufactured by major reputed Indian manufacturers who are compatible and consistent with FDA standards.
Premium Contract Manufacturing Companies in India
Contract Manufacturing in the Pharmaceutical ecosystem is outsourcing the manufacturing of the products to obtain specific scientific, medical or operational expertise or to have a labour force. Contract manufacturing manufactures products as per the customers' requirements and delivers the products at an appropriate time. In contract manufacturing, a legal agreement is signed among the pharma organizations and the contract manufacturers.
Ikris Pharma Network is one of the top contract manufacturing companies in India who is serving abroad also for more than 7 years. They only work with FDA-approved manufacturers, and IPN itself strictly adheres to the FDA guidelines. They provide a host of services other than manufacturing and packaging, which also include: drug Stability studies, manufacturing, development of compliance documents as per FDA regulatory requirements, Developing late-stage clinical trial material, providing scale-up and registration batches and pre-formulation and many more.
Top contract manufacturing pharma company
Nowadays, most pharmaceutical organizations outsource the products manufactured by another firm. They both enter into an agreement that the manufacturer will manufacture and deliver the products within the appropriate time cost-effectively. Improving efficiencies, reducing costs, ensuring business continuity, better access expertise, reducing staff, allowing staff to focus on the core competency, mitigating risk through using specialists count are a few of the drivers associated with Global pharmaceutical contract manufacturing.
If one is searching for contract manufacturing, Ikris Pharma Network is the best option one can go. IPN is one of the top contract manufacturing pharma companies in India. The expertise in their services hosted makes them different from other organizations in this arena of the pharma ecosystem. IPN serves many facilities and manufacturing and packaging such as: Developing late-stage clinical trial material, drug Stability studies, manufacturing, development of compliance documents as per FDA regulatory requirements, providing scale-up and registration batches and pre-formulation, and many more.
Pharmaceutical Contract Manufacturing in India:
A contract manufacturing organization is sometimes also known as a contract development and manufacturing organization. Contract manufacturing means outsourcing products of higher quality to manufacture by another firm on a contractual basis. Outsourcing is the easiest and most cost-effective way to lessen the work burden for a pharmaceutical organization.
Ikris Pharma Network comes at the top of the best pharmaceutical contract manufacturing companies list. IPN is blooming in the pharmaceutical ecosystem because of its expertise in the pharmaceutical services such as contract manufacturing which includes manufacturing, packaging, transportation, and many more responsibilities in manufacturing an outsourced product. IPN is a reliable and GDP-certified pharmaceutical organization. It is already supplying the products in more than 150 countries in the World. And they work only according to the FDA guidelines.
Why choose Ikris Pharma Network for contract manufacturing?
Contract manufacturing is a significant part of the pharma industry; it helps ease the jobs of manufacturers and clients (organisations/hospitals). Contract manufacturing is a term used when pharma organisations outsource the products manufactured by other organisations under their brand. It is similar to private label manufacturing or third party pharmaceutical manufacturing, or outsource manufacturing.
Ikris Pharma Network is the best contract manufacturing in pharmaceutical industry in India. It deals in rare medicines of higher quality. Ikris Pharma helps one to connect with the best of the manufacturers for manufacturing activities. Ikris believes in working with certified manufacturers only, and it is working with more than 50 manufacturers. It provides a host of services, including Drug Stability studies, Manufacturing, Development of compliance documents as per FDA regulatory requirements, Developing late-stage clinical trial material, Providing scale-up and registration batches, and Pre formulation.
Why to go for Ikris Pharma for private label manufacturing?
Private label manufacturing means pharmaceutical organisation partners with a contract or third-party manufacturer to manufacture the product under the retailer’s brand. Medicines or medical products will be manufactured according to the retailer’s needs. The requirement of private label manufacturing comes when an organisation wants to meet the demand of the patients for medicines with the quality available at a reasonable price as generic medicines by innovators are costlier, and versions of generic medications comparatively come at a lower price. So, keeping the stock available for clients retailers requires the need for private label manufacturing.
Ikris Pharma Network is one of the best private label pharmaceutical manufacturers. It eases the job of retailers/clients to concentrate on their core competencies. In contrast, private label manufacturers reduce their tasks by taking over responsibilities like Drug Stability studies, Manufacturing, Development of compliance documents, as per FDA regulatory requirements, Developing late-stage clinical trial material, Providing scale-up and registration batches and Pre formulation.
How is Ikris best for Reference Listed Drugs?
The term reference listed drugs refer to the approved drug product to which new generic versions show bioequivalence (chemically identical, i.e. same active ingredients). When a pharmaceutical organization seeks approval to market, a generically equivalent drug should refer to the Reference Listed Drug(RLD) in its Abbreviated New Drug Application (ANDA). Reference listed drugs can also be called comparator drugs.
Ikris Pharma Network is a top-level RLD Pharma globally that provides facilities to researchers/CRO/ pharmaceutical organizations in accessing products that are not available or approved in a particular country. Ikris has a vast network of pharmaceutical distributors which leads Ikris in obtaining branded innovator samples or reference listed drugs for bioequivalence and clinical trials from most reference countries (such as the USA, Japan, Australia, etc.). Ikris has a specialization in sourcing reference listed drugs (RLD) from single/multiple branches in specific quantities and products that are available with most suppliers.
Why pick Ikris as a third party manufacturer?
The third-party manufacturer is a term given to those organizations which connect manufacturers and the clients. The pharmaceutical ecosystem is complex from the beginning, supplying active ingredients to the transportation of the products at clinical sites. Thus this ecosystem requires helping hands or partners at each stage to ease the burden of manufacturers and customers. An agreement is made among the manufacturers, third-party manufacturers and the clients (hospitals/ pharma organizations). Third-party manufacturing is another term for contract manufacturing. Responsibilities as a third-party manufacturer include packaging and transportation and much more like product development, specialized processing, such as radiation sterilization, testing, logistics, and medicine transportation at appropriate temperatures.
Ikris Pharma Network (IPN) is an international third-party pharmaceutical manufacturer India. The advantages of IPN as a third-party pharmaceutical manufacturer such as IPN work with more than 100 manufacturing companies. As per the requirement of the client, IPN picks up the best manufacturers. Professionals lead it who have years of experience in this industry with 100% transparency in the whole process. It has been supplying products in India and overseas for more than 7 years.
What makes Ikris the best wholesale pharmaceutical distributor?
A pharmaceutical distributor plays an essential role in the pharma industry. It acts as a link between manufacturers and the clients (pharmacist, hospitals, etc.). In the pharma industry, pharmaceutical distribution is an important activity in the integrated supply chain of medical products.
Pharmaceutical distributors have responsibilities other than distribution as handling and storage of pharma products. To reduce their burden and to concentrate on their core business, manufacturers partner with distributors. Distributors serve more than an intermediary; they purchase and take legal ownership of pharmaceuticals and manage inventory and credit risk.
Ikris Pharma Network(IPN) is emerging as the best pharmaceutical wholesale distributor at the global level. During the pandemic crisis, IPN actively participated in supplying required medicines in India and abroad. IPN believes in supporting patient safety, enabling the right product to reach the right patient in a timely and transparent manner. Ikris is a GDP-certified company that has licenses for both wholesale and retail drugs. And it has expertise in cold-chain supplies for temperature-sensitive medicines. IPN is run by professionals with immense experience in this industry.
How did Ikris help Brazil during Covid?
Covid-19 impacted every person in the World in one way or the other way. The outbreak of the pandemic led to a fall in the global economy and public health sector. Moreover, this resulted in the sudden shutdown of many services such as mobility restrictions, closed international border crossings, and declined economic activity. More than half of the population in the World got infected, resulting in a massive number of deaths. But all those patients suffering from chronic diseases or were having cancers faced many difficulties in getting the medicines within the appropriate time. Due to mobility restrictions and lack of adequate transportation systems, deaths were reported in many countries.
Brazil is one such country that India helped in those crucial times. India supplied many medicines which were not available at that time. Ikris Pharma Network is a GDP-certified India-based company with licenses for wholesale and retail drugs, delivering globally. Ikris was one of the organizations which came forward to help Brazil by exporting all the necessary rare medication within time. Ikris strictly follows the FDA rules and regulations to provide a higher quality of medicines with zero adulteration. Ikris is now supplying more rare drugs and is looking forward to emerging as the best exporter of medical products in the Brazilian health sector.
What is all Ikris doing in Brazil's health sector?
Ikris Pharma Network (IPN) is an internationally recognized pharmaceutical wholesaler and distributor from India and supplies products to everyone involved in the supply chain of the healthcare sector. This organization is GDP certified, delivering medicines and medicinal products to wholesalers, distributors, resellers, clinics, NGOs and Pharmacies. Best quality medicine with zero adulteration is the priority of IPN. Hence, it adheres to the FDA rules and regulations.
In the Brazilian pharmaceutical and healthcare sectors, Ikris is achieving new heights in making necessary medicines readily available and emerging as a trusted pharmaceutical distributor in Brazil. In times of the Covid crisis, Ikris also helped Brazil in coping up with those challenging circumstances. IPN has the highest number of FDA approved manufacturers to manufacture high-quality medicines. It specializes in exporting generic drugs from India to any part of the World. Ikris deals in numerous oncology, haematology, hepatitis, immunotherapy, transplant medicine, vaccines, etc. Ikris has already exported more than 100 products in the Brazilian healthcare sector and is still exporting quality products in Brazil.
Ikris is an expert in cold-chain supply and supply products on or ahead of time to access the medicine quickly.
What is the role of IKRIS Pharma in healthcare?
Ikris Pharma Network (IPN) was established in 2014, situated at Noida in India. IPN is an internationally acclaimed pharmaceutical wholesaler and service company. It is one of the most reliable pharmaceutical companies for generic (or branded generic) and branded medication supplies globally, making it a key player in the industry. IPN built an efficient network over the years and is the only company to have a pan India presence certified by GDP and ISO under WHO Guidelines.
Ikris Pharma Network has a wide range of more than 5000 generic and branded medications’ distribution facilities in India and is internationally emerging as a top-grade generic medicine supplier. It deals in every kind of medicine: tablets, pills, injections, capsules, liquid, topical, suppositories, drops, inhalers, implants or patches, etc. IPN has specialization in supplying medicines related to oncology (different kinds of cancers), hematology (leukemia and lymphoma, multiple myeloma), hepatitis (hepatitis B, hepatitis C), immunotherapy (eliglustat, xeljanz, etc.), transplant medicine, vaccines (such as cholera vaccine, ONCO BCG, etc.).
IPN is very promising in delivering products of higher quality on time or ahead of time under appropriate temperature and conditions securely to the required site. Moreover, it works within the parameters of FDA rules and regulations. They keep their customers (pharmacies, hospitals, patients, etc.) updated about pricing, inventory levels, and order status. They work with 100 % transparency with the client, and it is run by professionals having years of experience in this industry.
IPN is best at serving facilities like access to generic medicines, facilitating access for licensed and unlicensed medicines through Named Patient Import services, RLD supply (comparator sourcing), contract manufacturing/private label manufacturing, cold-chain supply services, etc.
Why Ikris is best generic medicine supplier?
With increasing chronic diseases and after a pandemic outbreak, there is an inflated demand for generic medicines globally, which is the golden opportunity for any pharmaceutical company to bloom in the pharma industry. Efficient and inexpensive drugs have massive needs across the pharmaceutical industry.
Ikris Pharma Network is an internationally acclaimed pharmaceutical wholesaler and service organization. IPN was established in 2014 in Noida, India. It is one of the trusted brands in the pharmaceutical sector for supplying generic medications across the globe. It is certified by the highest authorities such as ISO 9001:2008, GMP, WHO, GDP, etc. IPN deals in various rare medicines related to cancers, chronic diseases, heart problems, HIV and many more. It has a reliable network as a generic medicine supplier across more than 100 countries worldwide. IPN has served 50000+ patients and supplied more than 5000 products across 150+ countries, e.g., Africa, United States, UK, Gulf countries, Vietnam, Sri Lanka, South East Asia, Philippines, Cambodia, Poland, and many more.
What does the term Managed access program mean?
Managed access program helps patients and physicians access much-required medicines when other alternative options are exhausted or the particular is not available in a specific country. Managed Access Program is also known as an Expanded Access Program (EAP), Early Access Program, Compassionate Use Program, Named Patient Program (NPP). These programs help especially needy patients improve their quality of life or save patients’ lives. Such programs also facilitate accessing unlicensed medication from another country when patients have no alternative at the local level.
In almost every country in the World, any patient with some chronic disease, life-threatening, has the right to buy, import, or access medication that can improve or save their life. Every country has its own rules and regulations set up for importing such medicines.
Ikris Pharma Network (IPN) is the fastest growing pharmaceutical consultancy and service organization globally, facilitating access to advanced medicines for needy patients in India or any part of the World via the Named Patient Program. They have already successfully assisted more than 600 patients through this program across different geographies in the World.
How is IKRIS Pharma the best in managed access program?
Managed Access Program is a process that helps needy patients access the medicines that are not available in a particular country or alternative options for that medicine have been exhausted. The other known terms for managed access programs are Expanded Access Program (EAP), Early Access Program, Compassionate Use Program, Named Patient Program (NPP).
Ikris Pharma Network (IPN) is a pharmaceutical consultancy and service company helping patients and supplying products globally. IPN helps patients in accessing life-saving drugs through legal procedures, namely the Named Patient Program. It acts as a portal so that every individual out there, no matter who they are or where they are based, has identical, fast, and fair access to the advanced and best healthcare. There are a few parameters that one should fulfill in favor of accessing life-saving medicines and these parameters are as follows:
The life-saving medication has market approval in another country and is unapproved or unavailable in their native country.
There is no alternative option in the market.
The medication is for personal use only.
The respective patient has a valid prescription letter from their healthcare professional in their home country.
Why is Ikris best for contract manufacturing?
With time the pharmaceutical industry seeks a boom, especially after the Covid-19 pandemic, the requirement of medicines increased at twice the previous rate. Contract manufacturing means outsourcing the production of the products by using the facilities of the other organisations for manufacturing the products under their brand name. Contract manufacturers help the manufacturers to focus on manufacturing the quality product, and the rest of the responsibilities are handed over to the contract manufacturers. An agreement is signed among the manufacturers, clients and the contact manufacturer.
Ikris Pharma Network is emerging as the best-known contract manufacturing of pharma products globally. We have expertise in contract manufacturing services, including Drug Stability studies, Manufacturing, Development of compliance documents as per FDA regulatory requirements, Developing late-stage clinical trial material, Providing scale-up and registration batches, and Pre formulation. This process is beneficial to both manufacturers and clients. It delivers the product under appropriate conditions and on or before the time at required sites. It deals with certified manufacturers only to get the best quality of medicines for potential patients.
Why Ikris Pharma for Private label manufacturing?
Private label manufacturing refers to the process in which products are manufactured by the manufacturer for sale under another organization’s brand. An agreement is signed among the manufacturer, private label manufacturer and the client.
To keep up with the increasing demand for generic medicines in the healthcare sector, pharmaceutical organizations prefer to opt for private label manufacturing to ease their burden and partner with other organizations. These organizations help in tasks such as Drug Stability studies, Manufacturing, Development of compliance documents, as per FDA regulatory requirements, Developing late-stage clinical trial material, Providing scale-up and registration batches and Pre formulation.
Ikris Pharma Network is the best private label pharmaceutical manufacturers India, supplying at international level. Private label manufacturing is similar to third party pharmaceutical manufacturing or outsourcing manufacturing. IPN connects you with the best manufacturers to manufacture the product according to the necessities. IPN deals in rare medicines, and it is already exporting products to more than 150 countries across the World.
Why should one pick Ikris Pharma for a Reference Listed Drug?
Due to the enormous demand for generic medicine in the pharmaceutical market, there comes the need for reference-listed drugs; the clinical trials are held to compare the new generic version of the medication with the innovator sample for bioequivalence such as the same active ingredients. Any organization seeking approval to market a new version of generic medicine must refer to the Reference Listed Drug in its Abbreviated New Drug Application (ANDA).
Ikris Pharma Network is the best RLD supplier internationally. Ikris is the right place to get FDA-approved innovator samples (single batch/multiple batches of specific quantities) for clinical trials. IPN specializes in sourcing Reference Listed Drugs/ Innovator samples from across the globe, including India, the USA, Europe, Canada, Australia, New Zealand, Korea, Japan, and Latin America. IPN has a well-established network worldwide, allowing it to get, store and supply the products from all the major pharmaceutical manufacturers and authorized distribution channels to and from any location in the world, specific products or product sets in a bit huge volumes. They only deal with approved sources and validated supply chains.
Why is Ikris Pharma the best as third-party manufacturing?
Third-party manufacturing refers to outsourcing pharma products or getting products manufactured from other manufacturing units with your brand names. Third-party manufacturers act as connectivity among the manufacturers and the clients. The third-party manufacturer, the manufacturer, and the client agree upon an agreement to manufacture and transport the products as per the client's requirement. The pharmaceutical supply chain is very complex and needs multiple organizations to come together and make it possible to manufacture, supply, and sell quality medicine for potential patients.
Ikris Pharma Network is emerging best among the third-party manufacturing pharma companies. Being a third-party manufacturer has listed responsibilities: to ensure the security of supply, the need for specialist capabilities, in-licensing, product lifecycle management, market access, and cost optimization. The involvement of third-party manufacturers is not limited to manufacturing and packaging. Still, it has other tasks such as product development, specialized processing, radiation sterilization, testing, logistics, and medicine transportation at appropriate temperatures. IPN has excellence in providing these services to the other parties.
Why Ikris is the best pharmaceutical wholesale distributor?
Pharmaceutical wholesale distributors play a very crucial role in the pharmaceutical ecosystem. They are the second link in any pharma industry and the backbone of the pharma sector. Wholesaler distributors purchase the products in bulk, store them under the appropriate conditions and temperatures, and transport them from one location to another (such as pharmacies, hospitals, clinics, doctors’ offices, and labs). Pharmaceutical wholesale distributors purchase the drugs (and other equipment) from pharmaceutical companies, store them in warehouses and other holding facilities, and sell them to pharmacies, etc.
Ikris Pharma Network (IPN) is one of the best pharmaceutical wholesale distributors in Noida, India. IPN is supplying products in India and globally. The temperature-sensitive drugs need the right conditions and temperature to store and deliver, and Ikris has expertise in cold-chain supply. They have professionals having years of experience, sophisticated processes, and impeccable reliability. Their aspiration and liability are to keep up to ensure their clients’ supply chains and businesses both succeed and keep blooming.
What makes Ikris one of the best generic medicine suppliers?
With passing time, cases of chronic diseases are increasing rapidly, and the demand for inexpensive generic medicine is growing simultaneously.
Ikris Pharma Network is the best generic medicine company in India, an internationally acclaimed pharmaceutical wholesaler and service company providing a gamut of services specified in medications. IPN is known to supply higher-quality generic medicines. It is certified by the highest authorities like ISO 9001:2008, GMP, WHO, GDP, etc., and has a diversified products line across all the crucial aspects of healthcare. They have already served more than 5000 patients and supplied more than 150 products in different countries such as Africa, United States, UK, Gulf countries, Vietnam, Sri Lanka, South East Asia, Philippines, Cambodia, Poland, and many more.
India is the largest generic medicine manufacturer and has more than 665 USA FDA-approved manufacturing plants in India. And Ikris Pharma Network works with a vast number of reliable manufacturers.
Why to opt Ikris Pharma as contact manufacturers?
Contract manufacturing is an outsourcing process where a firm enters into a contract with another firm to manufacture products under another firm's brand. Contract manufacturing has massive demand in the pharmaceutical sector. Contract manufacturing is beneficial if the firm gets involved with the right firm, which can manufacture quality products within an appropriate time.
After the increase in chronic diseases and pandemic outbreak, there is an inflated demand for generic medicines; that's how outsourcing processes came in need. Outsourcing is the process of using a non-related for another on a contractual basis. An agreement is assigned among the firms to produce products according to the requirements of the clients.
Ikris Pharma Network (IPN) is one of the best pharmaceutical organizations globally in contract manufacturing of pharma products. Its responsibilities are not only limited to the manufacturing of the products. It provides a host of services: Drug Stability studies, Manufacturing, Development of compliance documents according to FDA regulatory requirements, Developing late-stage clinical trial material, Providing scale-up and registration batches, and Pre formulation. Ikris is already exporting pharma products in more than 150 countries.
What makes Ikris Pharma the top third-party manufacturing company?
Third-party manufacturing is a legal process through which a deal is done between the manufacturer and the company. Manufacturers agree to manufacture quality products by a specific date. Third-party manufacturing pharma means the pharma company gets medications produced with its brand name from a manufacturing company.
Many pharmaceutical organizations use this facility of outsourcing the manufacturing of products to ease their burden, which helps them focus on their core competency. The responsibility of third-party manufacturers is not limited to manufacturing and packaging. Still, they also carry activities like product development, specialized processing, such as radiation sterilization, testing, logistics, and medicine transportation at appropriate temperatures, etc.
Ikris Pharma Network is one of the top third-party pharma manufacturers in India. Ikris provides a lot of advantages as a third-party manufacturer. The benefits with Ikris are: they provide a quality product to the customer within a short time, plays as a linkage between the manufacturer and the customers, have a sizeable established network with USFDA approved manufacturers, run by professionals having years of experience in the pharma industry, 100% transparency in work, etc.
Why is Ikris Pharma a top pharmaceutical wholesale distributor?
A distributor is a vital intermediate between manufacturers and the clients (retailers, hospitals, pharmacies, etc.). The scope of a wide range of contacts with clients and manufacturers increases if one is a wholesale distributor because they have connections with retailers, consumers, pharmacies, chemist shops, clinics, etc. Distributors are the backbone of the pharmaceutical industry, as they are meant to transport the medicines and products to the required sites and increase sales.
Ikris Pharma Network is an internationally acclaimed wholesaler pharmaceutical distribution company of generic medicines delivering products worldwide. Ikris is a GDP-certified pharmaceutical organization having licenses for both wholesale and retail drugs. It is a one-stop-shop for generic medication for every kind of chronic disease. IPN has expertise in catering cold-chain supplies of medicines that are temperature sensitive. IPN purchases and takes legal ownership of pharmaceuticals and manages inventory and credit risk to help manufacturers concentrate on their core competencies to manufacture quality generic medicines. It has professionals, sophisticated processes, and impeccable reliability. They have aspiration and liability to keep up to ensure their clients’ supply chains and businesses both prosper and keep flourishing.
Why Ikris Pharma for contract manufacturing?
When one pharma organization arranges for another to manufacture its products, this process is known as contract manufacturing or outsourcing of the products to manufacture. The company provides the manufacturing company with all the details and, if applicable, also with the materials required for the production process. This type of contract sets out the manufacturer's requirements concerning the quality of the products, certification, quantities, conditions, dates of delivery, etc. The parties make an agreement, and the product is manufactured as per the customer's requirement.
Ikris Pharma Network (IPN) is one of the top contract manufacturing companies in India, dealing internationally. IPN comes with several perks as a contract manufacturer. The list of the perks are:
IPN has a vast established network with FDA-approved manufacturers.
IPN is already doing business in more than 150 countries all over the World.
IPN provides contract manufacturing pharma services including manufacturing of Ayurveda, Herbal, and generic medicines with various approved quality levels.
It picks the best-suited manufacturer according to the requirement of the clients.
#Named patient supply#managed access program supplier#Pharmaceutical supplier in India#Global Access to Medicines#generic medicine#pharmaceutical distributors#pharma distributors app#online medicine app#Named patient program supplier in India#Access to Unlicensed or Unapproved Drugs
0 notes
Text
Understanding Lab Certification Services: What You Need to Know
In the realm of scientific inquiry and industrial production, precision and accuracy reign supreme. Whether you're a researcher investigating the molecular underpinnings of disease or a manufacturer ensuring the quality of your products, reliable laboratory testing is paramount. However, how can you be sure that the laboratory conducting these tests meets rigorous standards? This is where lab certification services step in, providing assurance and validation to laboratories and their processes. In this guide, we'll delve into the intricacies of lab certification services, offering insights into what they entail and why they matter.

Navigating the Landscape of Lab Certification Services
Laboratory certification involves a formal assessment process conducted by independent accrediting bodies. These bodies evaluate a laboratory's competence, impartiality, and adherence to specific quality standards. The certification process typically includes a comprehensive review of the laboratory's facilities, equipment, personnel qualifications, quality management system, and testing procedures.
The Importance of Accreditation
Accreditation serves as a hallmark of credibility and competence in the laboratory industry. Laboratories that undergo accreditation demonstrate their commitment to maintaining the highest standards of quality and accuracy in their testing services. Accredited laboratories adhere to internationally recognized standards such as ISO/IEC 17025 for testing laboratories or ISO 15189 for medical laboratories, depending on their scope of testing.
Assurance of Quality and Reliability
By opting for lab certification services, stakeholders gain confidence in the reliability and accuracy of the laboratory's test results. Accredited laboratories follow stringent quality control measures, ensuring that their analytical methods are validated, instruments are calibrated, and personnel are adequately trained. This commitment to quality translates into dependable data, crucial for informed decision-making in various industries, including healthcare, environmental monitoring, food safety, and manufacturing.
Compliance with Regulatory Requirements
In many industries, regulatory bodies mandate that testing be conducted by accredited laboratories to ensure compliance with safety and quality standards. For example, pharmaceutical companies must submit drug product testing data generated by accredited laboratories to regulatory agencies for product approval. Similarly, environmental testing laboratories must adhere to accreditation requirements to meet regulatory obligations for assessing air and water quality.
Facilitating Global Trade
In the era of globalization, products and services cross international borders with ease. Accredited laboratories play a vital role in facilitating global trade by providing assurance of product quality and safety. For exporters, having their products tested by accredited laboratories enhances market acceptance and minimizes the risk of rejection due to non-compliance with import regulations.
Choosing the Right Lab Certification Services
When selecting a laboratory for certification, it's essential to consider several factors to ensure that your needs align with the laboratory's capabilities. Look for accreditation from reputable accrediting bodies recognized within your industry. Additionally, assess the laboratory's expertise in your specific area of testing and inquire about their track record of accuracy and reliability.
Conclusion: Elevating Standards, Ensuring Excellence
Lab certification services are instrumental in upholding the integrity and reliability of laboratory testing. By undergoing accreditation, laboratories demonstrate their commitment to excellence, instilling confidence in the accuracy and reliability of their test results. Whether you're a healthcare provider, a manufacturer, or a regulatory agency, partnering with accredited laboratories ensures that you have access to high-quality testing services that meet the most stringent standards. As you navigate the complex landscape of laboratory testing, prioritize accreditation and choose lab certification services that elevate standards and ensure excellence.
Elevate Your Lab's Credibility with GMCCX Lab Services
At GMCCX Lab Services, we understand the importance of credibility and reliability in laboratory testing. Our comprehensive accreditation ensures that our clients receive the highest quality testing services backed by internationally recognized standards. With state-of-the-art facilities, experienced personnel, and a commitment to excellence, we stand ready to support your testing needs across a diverse range of industries. Partner with GMCCX Lab Services and experience the difference accreditation makes in elevating your lab's credibility and ensuring the accuracy of your test results. Contact us today to learn more about our lab certification services and how we can help you achieve your testing objectives with confidence.
Take the Next Step Towards Excellence
In the ever-evolving landscape of laboratory testing, staying ahead requires a commitment to excellence and adherence to the highest standards of quality. With lab certification services, you can elevate your lab's credibility, ensure the reliability of your test results, and demonstrate your commitment to excellence to stakeholders worldwide. Whether you're seeking accreditation for your laboratory or outsourcing testing to accredited facilities, prioritize quality and reliability at every step of the process. With the right partners and a dedication to excellence, you can navigate the complexities of laboratory testing with confidence and achieve your objectives with precision and accuracy.
1 note
·
View note
Text
Common Challenges in Developing Oral Suspensions and Solutions
Introduction: The development of oral suspensions and oral solutions in the pharmaceutical industry brings forth a multitude of advantages, but it's not without its set of challenges. In this exploration, we'll delve into the common hurdles faced during the formulation and development of these liquid dosage forms, shedding light on how the industry addresses these challenges to deliver safe and effective medications.
Stability Concerns in Liquid Formulations: One of the primary challenges in formulating oral suspensions and solutions revolves around stability. The liquid nature of these dosage forms can make them susceptible to degradation, impacting the shelf life and efficacy of the medication. This blog post will explore strategies and technologies employed to enhance the stability of oral liquid formulations.
Anchor Text: Oral Suspensions and Oral Solutions
Taste and Palatability Issues: Ensuring that oral medications are palatable is crucial, especially when it comes to pediatric and geriatric patient populations. Taste-masking presents a unique challenge in the development of oral suspensions and solutions. We'll discuss innovative approaches and formulation techniques to overcome taste and palatability issues, creating a more patient-friendly experience.
Optimizing Particle Size Distribution: The particle size of active pharmaceutical ingredients (APIs) plays a significant role in the bioavailability and effectiveness of oral suspensions. This post will explore the challenges associated with achieving and maintaining the optimal particle size distribution, ensuring uniform drug delivery and absorption.
Manufacturing Scale-Up Considerations: Taking a formulation from the laboratory to large-scale production introduces its own set of challenges. We'll examine considerations and best practices for successfully scaling up the manufacturing of oral suspensions and solutions, maintaining consistency and quality throughout the process.
Quality Control Measures: Maintaining high-quality standards is imperative in pharmaceutical manufacturing. Our guide will discuss the importance of robust quality control measures in ensuring the safety and efficacy of oral liquid medications, covering analytical techniques and testing protocols.
Anchor Text: Small Molecule CDMO
Addressing Regulatory Compliance: Meeting regulatory requirements is a critical aspect of bringing oral suspensions and solutions to market. This post will provide insights into the regulatory landscape governing these liquid dosage forms, outlining key considerations for compliance throughout the development and approval process.
Incorporating Patient Feedback: Patient-centric design is becoming increasingly crucial in pharmaceutical development. We'll explore how incorporating patient feedback into the formulation process of oral suspensions and solutions can lead to improved adherence and overall patient satisfaction.
Conclusion: Navigating the common challenges in developing oral suspensions and solutions requires a multidimensional approach. This guide aims to provide valuable insights for pharmaceutical professionals and researchers as they work towards creating effective, palatable, and stable oral liquid medications.
0 notes
Text

Shriram Pharmacy College: Best Pharmacy Education In India

Shriram Pharmacy College, Bankner, stands out as one of India’s premier institutions for pharmacy education. With a strong focus on practical learning, experienced faculty, and state-of-the-art facilities, Shriram Pharmacy College has become synonymous with excellence in pharmacy education. The college offers a comprehensive Bachelor’s program in Pharmacy, covering diverse aspects of the pharmaceutical industry. This blog will explore the key highlights of studying at Shriram Pharmacy College, including career paths, curriculum details, and the unique benefits of choosing this institution.
### 1. **Bachelor’s Program in Pharmacy: A Four-Year Journey to Excellence**
The Bachelor’s program in Pharmacy at Shriram Pharmacy College is a four-year degree program designed to provide students with an in-depth understanding of pharmaceutical sciences. The curriculum is meticulously structured to include theoretical knowledge and practical training, ensuring that students are well-equipped to meet the challenges of the pharmaceutical industry. With a focus on developing technical skills, critical thinking, and ethical practices, the program ensures that graduates are not just academically proficient but also industry-ready professionals.
### 2. **Extensive Training Across Eight Semesters**
The Bachelor of Pharmacy program at Shriram Pharmacy College is spread across eight semesters, each designed to build a strong foundation in core subjects and introduce advanced concepts in pharmaceutical sciences. From pharmacology, medicinal chemistry, and pharmacognosy to biopharmaceutics and clinical pharmacy, the curriculum encompasses all essential topics. Students gain hands-on experience through laboratory sessions, internships, and industry visits, allowing them to apply theoretical knowledge to real-world scenarios and develop critical skills for their future careers.
### 3. **Pharmacy Business: Focus on Customer Service**
One of the critical aspects of pharmacy education at Shriram Pharmacy College is the focus on the pharmacy business and customer service. Students are trained to provide excellent customer service in community and hospital pharmacies, ensuring that they understand the importance of patient care, ethical practices, and communication skills. Courses cover topics such as patient counseling, prescription handling, and customer relationship management, preparing students to excel in pharmacy operations and maintain high standards of care.
### 4. **Hospital Pharmacist: Ensure Accurate Medication Dispensing**
Shriram Pharmacy College places a strong emphasis on training students to become hospital pharmacists, where accuracy in medication dispensing is crucial. The curriculum includes in-depth knowledge of pharmacotherapy, drug interactions, and patient safety protocols. Students learn to work closely with healthcare professionals to ensure patients receive the correct medications and dosages. The practical experience gained through hospital internships and simulation labs helps them understand the complexities of hospital pharmacy and prepares them for a career in healthcare settings.
### 5. **Chemical Technician: Maintain Strict Safety Protocols**
A significant part of the pharmacy education at Shriram Pharmacy College is dedicated to training students as chemical technicians who can work in laboratories and research facilities. The program emphasizes maintaining strict safety protocols, handling chemicals, and using advanced laboratory equipment. Students learn the importance of quality control, analytical testing, and standard operating procedures (SOPs). The hands-on training in state-of-the-art labs ensures that graduates are well-prepared to contribute to the pharmaceutical and chemical industries.
### 6. **Drug Inspector: Conduct Thorough Compliance Checks**
Shriram Pharmacy College prepares its students to pursue careers as drug inspectors, where they are responsible for ensuring that pharmaceutical products meet regulatory standards. The program covers various aspects of drug laws, quality assurance, and regulatory affairs, providing students with the knowledge needed to conduct compliance checks thoroughly. By understanding the regulatory frameworks and industry standards, graduates are equipped to play a vital role in maintaining the quality and safety of pharmaceuticals in the market.
### 7. **Medical Writer: Communicate Complex Information Clearly**
Another career path for graduates of Shriram Pharmacy College is that of a medical writer. The program emphasizes the importance of clear communication, allowing students to convey complex scientific and medical information to various audiences. Courses in technical writing, medical journalism, and content development are integral parts of the curriculum. The training enables graduates to work in research organizations, pharmaceutical companies, and healthcare institutions where effective communication of medical information is essential.
### 8. **Medical Representative: Build Strong Client Relationships**
The Bachelor of Pharmacy program at Shriram Pharmacy College also prepares students to become successful medical representatives who play a crucial role in the pharmaceutical industry. Training focuses on building strong client relationships, understanding product portfolios, and effectively communicating product benefits to healthcare professionals. Students learn the skills needed to thrive in sales and marketing roles, such as persuasive communication, negotiation, and product knowledge, which are vital for success as a medical representative.
### 9. **Unique Benefits of Choosing Shriram Pharmacy College**
Choosing Shriram Pharmacy College for your pharmacy education comes with several unique benefits. The college offers an integrated approach to learning that combines classroom instruction, practical training, and real-world experience. The faculty comprises industry experts and seasoned academicians who provide mentorship and guidance to students. The college also has strong ties with leading pharmaceutical companies and healthcare institutions, facilitating excellent placement opportunities for graduates.
/media/eb0cb48ed6146db932072f4aa2538013
### FAQs
**Q1: What is the duration of the Bachelor of Pharmacy program at Shriram Pharmacy College?**
The Bachelor of Pharmacy program at Shriram Pharmacy College is a four-year degree program, divided into eight semesters. Each semester covers different aspects of pharmaceutical sciences, from fundamental subjects like pharmacology and medicinal chemistry to advanced topics such as clinical pharmacy and drug regulations. The program also includes practical training through lab work, internships, and industry visits, which helps students gain hands-on experience and prepares them for various career opportunities in the pharmaceutical industry.
**Q2: What career opportunities are available after completing a B.Pharm from Shriram Pharmacy College?**
Graduates from Shriram Pharmacy College have a wide range of career opportunities in the pharmaceutical sector. They can work as hospital pharmacists, community pharmacists, drug inspectors, medical writers, chemical technicians, or medical representatives. The comprehensive education provided by the college ensures that students are well-prepared for roles in both clinical and non-clinical settings, research and development, regulatory affairs, and pharmaceutical marketing, among others. The college’s strong industry ties also help in securing placements for graduates.
**Q3: How does Shriram Pharmacy College ensure practical training for its students?**
Shriram Pharmacy College emphasizes practical training through its state-of-the-art laboratories, hands-on workshops, and internships. The college has collaborations with leading pharmaceutical companies and healthcare institutions where students gain real-world experience. Additionally, regular industry visits, guest lectures by professionals, and simulation-based learning modules are integrated into the curriculum. This approach ensures that students are well-equipped with the skills and experience needed to excel in various pharmacy-related roles upon graduation.
**Q4: What makes Shriram Pharmacy College stand out among other pharmacy colleges in India?**
Shriram Pharmacy College stands out due to its commitment to providing a well-rounded pharmacy education. The college offers a robust curriculum that combines theoretical learning with practical application, guided by a team of experienced faculty members. The state-of-the-art facilities, strong focus on research, and industry-oriented training modules further distinguish it from other institutions. Moreover, the college provides excellent placement support and has a track record of producing highly skilled pharmacy professionals who excel in their careers.
**Q5: Are there any research opportunities available for students at Shriram Pharmacy College?**
Yes, Shriram Pharmacy College encourages students to participate in research activities. The college provides opportunities for research in various fields such as pharmacology, medicinal chemistry, and clinical pharmacy. Students can work on innovative projects under the guidance of experienced faculty members, participate in seminars and conferences, and collaborate with industry professionals. These research opportunities not only enhance their learning experience but also prepare them for careers in academia, research, and development sectors.
### **Conclusion**
Shriram Pharmacy College, Bankner, offers a comprehensive and industry-relevant Bachelor of Pharmacy program that equips students with the skills and knowledge needed to excel in the pharmaceutical field. With a curriculum designed to provide both theoretical and practical knowledge, coupled with excellent career support, the college stands as a leading choice for pharmacy education in India. Whether you’re looking to become a pharmacist, a medical writer, or a drug inspector, Shriram Pharmacy College provides the foundation for a successful and rewarding career.
### Stay Connected with Shriram Pharmacy College!
For the latest updates, educational content, and insights into the dynamic field of pharmacy, don’t miss out on the Shriram Pharmacy College YouTube channel. By liking, sharing, and subscribing, you’ll gain access to expert lectures, student testimonials, campus events, and much more. Stay informed about advancements in pharmaceutical sciences and become a part of our vibrant community. Your support helps us grow and continue providing valuable resources to students and professionals alike. Join us today and never miss an update!
#pharmacist#public health#hospital#youtube#pharmacy#shriram medical college#online pharmacy#medicine#shriram nursing college#shriram pharmacy college
0 notes
Text
Lab Equipment: Advancements, Essential Tools, and Their Crucial Role in Scientific Discovery
Introduction:
The laboratory setting is a central hub for scientific research and technological progress. At its core, a diverse array of laboratory apparatus supports various fields of science, such as chemistry, biology, and physics. This equipment is indispensable, enabling experiments, data analysis, and the expansion of human knowledge. In this article, we will delve into the importance of laboratory tools, the latest developments in this realm, and their critical significance in advancing scientific exploration.
The Significance of Laboratory Tools:
Precision and Accuracy: Laboratory equipment is specially engineered to provide unmatched precision and accuracy. Tools like pipettes, spectrophotometers, and analytical balances ensure that researchers can obtain reliable and consistent results, a fundamental requirement for accurate scientific findings. Precision is the cornerstone of scientific inquiry, and laboratory equipment empowers researchers to uphold this standard.
Safety: In many cases, laboratory tools are essential for safeguarding researchers and the environment. Equipment such as fume hoods, biosafety cabinets, and eye protection gear play a pivotal role in protecting scientists from hazardous materials, chemicals, and biological agents. Safety measures are integral to laboratory work, and laboratory equipment helps create a secure and controlled environment.
Efficiency: Efficiency is paramount in scientific research, where time is a precious resource. Laboratory equipment is designed to streamline experiments, reduce manual labor, and enhance productivity. Automation, such as automated liquid handlers and robotic systems, accelerates sample processing, allowing researchers to focus on data interpretation and hypothesis testing.
Latest Advancements in Laboratory Tools:
3D Printing: The advent of 3D printing technology has brought a revolution in the manufacturing of laboratory tools. Researchers now have the ability to design and create custom equipment tailored to their specific needs. This innovation has not only reduced costs but also increased accessibility, democratizing the production of laboratory tools.
Internet of Things (IoT) Integration: Laboratory tools are becoming increasingly interconnected through IoT technology. Researchers can remotely monitor and control equipment, access real-time data, and set up automated workflows. This integration improves data accuracy and makes experiments more convenient and efficient.
Nanotechnology: Nanotechnology is redefining the design of laboratory equipment at the molecular level. Nanoscale instruments offer higher sensitivity and precision, enabling groundbreaking research in areas such as material science, biology, and electronics.
The Crucial Role of Laboratory Tools in Scientific Discovery:
Breakthrough Discoveries: Laboratory equipment has been instrumental in numerous scientific breakthroughs throughout history. From the discovery of DNA's double helix structure using X-ray crystallography to the development of the CRISPR-Cas9 gene-editing technique, laboratory tools have paved the way for revolutionary advancements.
Drug Development: The pharmaceutical industry heavily relies on laboratory equipment for drug discovery and development. High-throughput screening systems, mass spectrometers, and chromatography equipment are essential for testing compounds and identifying potential drug candidates.
Environmental Research: In environmental science, laboratory equipment plays a crucial role in monitoring and analyzing various parameters, such as air and water quality, greenhouse gas emissions, and climate data. Instruments like gas chromatographs and spectrometers are vital in understanding and mitigating environmental challenges.
Conclusion:
Laboratory equipment is the unsung hero of scientific progress. Its precision, safety measures, and efficiency have propelled us into an era of exploration and innovation. As technology continues to advance, laboratory equipment will only become more sophisticated, further expanding the frontiers of human knowledge. Scientists and researchers will continue to rely on these tools to unlock the mysteries of the universe, develop life-saving treatments, and address global challenges. Laboratory equipment is not just a means to an end; it is the catalyst for scientific exploration and achievement.
0 notes
Text
Why do you need the best Eiac-accredited laboratory services
The Significance of ENAS-Accredited Laboratory Testing Services
Accredited laboratories undergo a rigorous evaluation and accreditation process by esteemed organizations to ensure they adhere to specific standards and criteria for testing and analysis. The precise standards vary depending on the type of laboratory and the industry it serves.
Laboratory Testing Services: Unveiling the Essence
Laboratory testing services encompass an array of scientific and analytical procedures executed in a controlled setting, typically a laboratory. The primary objective is to scrutinize and assess a diverse range of substances, materials, or samples. These services wield paramount importance in numerous industries, including healthcare, pharmaceuticals, environmental science, manufacturing, food production, and more. Laboratory testing confers indispensable information and data that fuels decision-making, quality control, research, and safety across various domains.

Key Services Rendered by Elite ENAS-Accredited Laboratory Testing Services
The foremost ENAS-accredited laboratory testing services extend an array of vital solutions, including:
Clinical Laboratory Testing: Crucial within the healthcare sphere, these services entail the examination of patient samples, such as blood, urine, or tissue, for disease diagnosis, health monitoring, and treatment guidance.
Material Testing: Laboratories are equipped to evaluate materials for their physical, chemical, and mechanical attributes. This proves pivotal in sectors like manufacturing, construction, and product development.
Environmental Testing: Laboratories scrutinize air, water, and soil samples to identify pollutants, contaminants, and other environmental variables, ensuring safety and regulatory compliance.
Food and Beverage Testing: Food laboratories rigorously analyze products to ascertain compliance with safety and quality benchmarks, encompassing tests for contaminants, nutritional content, and label accuracy.
Pharmaceutical Testing: In the pharmaceutical realm, laboratories undertake comprehensive testing to validate the quality and safety of drugs and pharmaceutical items.
Biological and DNA Testing: These services span genetic testing, paternity testing, and forensic analysis, aiding in individual or relationship identification and crime resolution.
Advantages of Premier ENAS-Accredited Laboratory Services
Opting for ENAS (Emirates National Accreditation System)-accredited laboratory services can yield multiple benefits, ensuring the quality and dependability of laboratory operations. Some key advantages of engaging ENAS-accredited laboratory services include:
Credibility and Trust: ENAS accreditation serves as a testament to a laboratory's alignment with international standards for competence and performance. This credential fosters trust and credibility among customers, clients, and regulatory authorities.
Quality Assurance: Accredited laboratories adhere to stringent quality control and assurance protocols, guaranteeing the precision and reliability of test results and services.
Regulatory Compliance: Accreditation ensures that a laboratory's testing and calibration services align with local and international regulations and standards. This is of paramount importance in sectors such as healthcare, manufacturing, and environmental monitoring.
Interoperability: ENAS accreditation often enjoys international recognition, signifying that test results from a UAE-based accredited laboratory hold global validity and acceptance. This simplifies international trade and collaboration with foreign counterparts.
Experience Reliable Services with Qualitasme
In your quest for reliable laboratory services, Qualitasme stands out as a trustworthy and dependable choice. Please visit our official website for a comprehensive understanding of our offerings and to experience the utmost in reliability and quality assurance.
0 notes
Text
Pharmaceutical Quality Control Market Demand, Growth Rate & Value Analysis
Pharmaceutical Quality Control Market Research Report: Information By Product (Consumable, Instruments, and Services), By Analysis Type (Sterility Testing, Bioburden Testing, Endotoxin Testing, Stability Testing, Extractable & Leachable Testing, Raw Material Testing, and Others), By Products Tested (Vaccines, Plasma Product, and Drugs) and region (North America, Europe, Asia-Pacific, Middle East and Africa and South America).
The global Pharmaceutical Quality Control market size was estimated at USD 4.2 billion in 2022 and is projected to reach USD 9.64 billion in 2030 at a CAGR of 11.2% during the forecast period 2023-2030.

Pharmaceutical quality control is the integration of all processes to ensure the identity and purity of a particular pharmaceutical product. The global pharmaceutical quality control market is growing rapidly due to the growing awareness of pharmaceutical quality control. Increasing development of comprehensive analytical tests is driving the growth of the market. Demand for integrated analytical services covering all stages of drug research, development and manufacturing also continues to drive the market growth. In addition, the growing demand for pharmaceutical quality control due to the global outbreak of the novel coronavirus disease (COVID-19) presents an opportunity for the market. Technical issues are the major factors restraining the growth of the pharmaceutical quality control market. However, complex and time-consuming regulatory guidelines pose challenges to the growth of the pharmaceutical quality control market.
Another important reason behind the growth of the pharmaceutical quality control market is the increasing technological innovations and advancements offered by the various currently-established market players. They focus on constantly updating technology to improve the quality of care for different patients’ pharmaceutical companies are strictly regulated because the health and well-being of consumers is directly affected by drugs, medical devices and other pharmaceutical products. To sell their products, manufacturers must meet several quality criteria. Small and medium pharmaceutical companies are focusing on complying with the law to produce better products and stay competitive in the market due to the rapid expansion of the pharmaceutical industry. The Food and Drug Administration (FDA) regulates the US market, where the majority of companies operating in developing countries like China and India ship their goods. FDA also inspects the facilities and operations of all pharmaceutical manufacturing operations in the United States. such as the overseas operations of companies that regularly sell their products in the United States.
Therefore, these facilities and their processes must comply with current FDA Good Manufacturing Practices (cGMP). To manufacture and market their products, companies must also comply with the International Organization for Standardization (ISO), 21 CFR Part 211, and the ICH Q10 guidelines. Manufacturers are eager to adopt quality management systems due to the increasing importance of regulatory requirements and market expansion. Coronavirus disease has had a significant impact on the growth of the Pharmaceutical Quality Control market. The epidemic has increased the demand for safe and sterile items. Therefore, pharmaceutical companies are now focusing more on the production of high quality products. In addition, significant player growth and investment has driven the Pharmaceutical Quality Control market value.
Get the Free Sample Pages: https://www.delvens.com/get-free-sample/pharmaceutical-quality-control-market-trends-forecast-till-2030
Delvens Industry Expert's Standpoint
The pharmaceutical quality control market is a growing number of accredited clinical laboratories, an increasing acceptance of third-party quality control, and an increasing preference for support outside in the quality assessment. The number of laboratory tests performed has increased due to the increasing prevalence of various diseases around the world. Both the public and private sectors are increasing the number of laboratories to meet this requirement. Clinical laboratories must be accredited by regulatory bodies such as the International Organization for Standardization and other equivalent standards to perform diagnostic tests in the majority of countries. Competent authorities evaluate the laboratory's capacity and quality system against predetermined standards during the accreditation process, thereby generating revenue from the pharmaceutical quality control market.
Key Findings
Pharmaceutical Quality Control market segmentation, based on product, including consumables, instruments, and services. The consumer goods segment will dominate the market by 2022. The main auxiliary materials used in production are consumables. Consumables can be divided into three categories things that don't affect quality, things that do and things that affect directly. According to forecasts, the service sector will experience the fastest expansion. Furthermore, the highest market share for pharmaceutical quality and control is in the instrument sector.
Pharmaceutical quality control market segmentation based on analysis type including sterility testing, microbial contamination testing, endotoxin testing, stability testing, extraction and filtration testing, raw materials raw and others. Bacterial adulteration test segment dominates the market in 2022. Anaerobic counting test, aerobic count test, mold count test, in vitro test, spore count test, endotoxin test factor, LAL and others are the different categories into which bioburden tests are divided. The bacterial population in a sample was analyzed by an aerobic count assay. It helps to show the quality and degree of damage of the product. Wound cultures, also known as anaerobic counting tests, are performed without the use of oxygen.
The Pharmaceutical Quality Control market segmentation, based on Products Tested, includes Vaccines, Plasma Product, and Drugs. Vaccines segment dominated the pharmaceutical quality control market in 2022. Finding essential choices for quality control and vaccine production is really important. The sub-segment for drugs is predicted to increase at the quickest rate overall.
By region, the study provides market insights across North America, Europe, Asia-Pacific, and Rest of the World. The pharmaceutical quality control market in North America leads it by 2022 (45.80%). This is due to the evolving healthcare systems in the United States and Canada, the presence of many large manufacturers of molecular quality control products in the region, and access to advanced technologies. in the area. Furthermore, the US pharmaceutical quality control market holds the largest share, and the Canadian pharmaceutical quality control market is the fastest growing market in the North American region.
Make an Inquiry Before Buying: https://www.delvens.com/Inquire-before-buying/pharmaceutical-quality-control-market-trends-forecast-till-2030
Regional Analysis
North America to Dominate the Market
North America is expected to witness significant growth in the pharmaceutical quality management software market during the forecast period due to factors such as growing demand for management standards. improved production and quality management as well as the increasing adoption of innovative technologies, cloud-based solutions and QMS software in the pharmaceutical industry.
Competitive Landscape
Merck KGaA,
BioMérieux SA,
Charles River Laboratories International, Inc.,
Sartorious AG,
WuXi AppTec,
Thermo Fisher Scientific, Inc.,
SGS S.A.,
Eurofins Scientific,
Toxikon Corporation,
McKinsey & Company,
Esco Micro Pte. Ltd,
Lucideon Limited,
PerkinElmer Inc.,
SOLVIAS AG,
Shimadzu Scientific Instruments,
METTLER TOLEDO,
REMI GROUP,
BRAM-COR SPA,
Panomex Inc.,
Waters Corporation
Recent Developments
January 2023: Palantir Technologies Inc. launched a consistent quality management system to help life science customers using the Foundry platform meet GxP requirements.
December 2022: FILTEC, an in-line inspection solutions provider, has launched a new Remote Visual Inspection (RVI) system, a compact and flexible machine vision solution for inspection.
Reasons to Acquire
Increase your understanding of the market for identifying the most suitable strategies and decisions based on sales or revenue fluctuations in terms of volume and value, distribution chain analysis, market trends, and factors.
Gain authentic and granular data access for the Pharmaceutical Quality Control Market to understand the trends and the factors involved in changing market situations.
Qualitative and quantitative data utilization to discover arrays of future growth from the market trends of leaders to market visionaries and then recognize the significant areas to compete in the future.
In-depth analysis of the changing trends of the market by visualizing the historic and forecast year growth patterns.
Purchase the Research Report: https://www.delvens.com/checkout/pharmaceutical-quality-control-market-trends-forecast-till-2030
Report Scope
Pharmaceutical Quality Control Market Research Report: Information By Product , By Analysis Type, By Products Tested, By Solution Type and region:
Based on Product
Consumables
Instruments
Services
Based on the Analysis type
Sterility Testing
Membrane Filtration
Direct Inoculation
Others
Bioburden Testing
Aerobic Count Testing
Anaerobic Count Testing
Spore Count Testing
Fungi/Mold Count Testing
Endotoxin Testing
In Vitro
Lal
Others
Stability Testing
Extractable & Leachable Testing
Raw Material Testing
Others
Based on the Products Tested
Overview
Vaccines
Plasma Product
Drugs
Others
Based on solution type
Small and medium-sized enterprises
Corrective Action Preventive Action (CAPA) Management
Audit Management
Document Management
Change Management
Training Management
Complaints Management
Regulatory and Compliance Management
Non-Conformances Handling
Supplier Quality Management
Inspection Management
Other solution Types
Based on region
Asia Pacific
Japan
China
India
Australia
South Korea
Vietnam
New Zealand
Philippines
Thailand
Malaysia
Hong Kong
Taiwan
Singapore
Indonesia
Sri Lanka
Rest of Asia-Pacific
North America
U.S.
Canada
Mexico
Europe
Germany
U.K.
France
Italy
Spain
Sweden
Austria
Finland
Belgium
Turkey
Russia
Poland
Hungary
Czech Republic
Switzerland
Netherlands
Rest of Europe
South America
Brazil
Argentina
Chile
Colombia
Rest of South America
Middle East & Africa
South Africa
U.A.E.
Saudi Arabia
Oman
Qatar
Iran
Egypt
Rest of Middle East and Africa
The prominent players in the Pharmaceutical Quality Control market are
Merck KGaA
bioMérieux SA,
Charles River Laboratories International, Inc.,
Sartorious AG,
WuXi AppTec,
Thermo Fisher Scientific, Inc.,
SGS S.A.,
Eurofins Scientific,
Toxikon Corporation,
McKinsey & Company,
Esco Micro Pte. Ltd,
Lucideon Limited,
PerkinElmer Inc.,
SOLVIAS AG,
Shimadzu Scientific Instruments,
METTLER TOLEDO,
REMI GROUP,
BRAM-COR SPA,
Panomex Inc.,
Waters Corporation
About Us:
Delvens is a strategic advisory and consulting company headquartered in New Delhi, India. The company holds expertise in providing syndicated research reports, customized research reports and consulting services. Delvens qualitative and quantitative data is highly utilized by each level from niche to major markets, serving more than 1K prominent companies by assuring to provide the information on country, regional and global business environment. We have a database for more than 45 industries in more than 115+ major countries globally.
Delvens database assists the clients by providing in-depth information in crucial business decisions. Delvens offers significant facts and figures across various industries namely Healthcare, IT & Telecom, Chemicals & Materials, Semiconductor & Electronics, Energy, Pharmaceutical, Consumer Goods & Services, Food & Beverages. Our company provides an exhaustive and comprehensive understanding of the business environment.
Contact Us:
UNIT NO. 2126, TOWER B,
21ST FLOOR ALPHATHUM
SECTOR 90 NOIDA 201305, IN
+44-20-8638-5055
0 notes
Text
Fluticasone Propionate API Manufacturers

Quality Assurance and Compliance in Fluticasone Propionate API Manufacturing
Quality assurance and compliance are vital aspects of the pharmaceutical industry, ensuring the safety, efficacy, and purity of active pharmaceutical ingredients (APIs) such as fluticasone propionate. In the manufacturing of fluticasone propionate API, adherence to stringent regulations and guidelines is essential to maintain product quality and meet regulatory requirements. This article explores the importance of quality assurance and compliance in fluticasone propionate API manufacturing and the measures taken to ensure high standards.
Stringent Regulatory Standards
Fluticasone propionate API manufacturers are subject to rigorous regulations and guidelines set by regulatory authorities such as the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA). These regulatory bodies outline specific requirements for the manufacturing, testing, and documentation of APIs to ensure their safety and efficacy.
Quality Control Processes
Fluticasone propionate API manufacturers implement robust quality control processes to monitor and maintain product quality throughout the manufacturing cycle. Quality control begins with the selection and qualification of raw materials and continues through each manufacturing stage. Regular inspections and audits are conducted to ensure compliance with Good Manufacturing Practices (GMP) and Good Laboratory Practices (GLP).
Testing Methods and Certifications
Accurate and reliable testing methods are crucial to verify the identity, purity, and potency of fluticasone propionate API. Analytical techniques such as high-performance liquid chromatography (HPLC), gas chromatography (GC), and mass spectrometry (MS) are commonly used to analyze the quality of the API. Additionally, fluticasone propionate API manufacturers obtain certifications such as ISO 9001 (Quality Management System) and ISO 14001 (Environmental Management System) to demonstrate their commitment to quality and environmental sustainability.
Batch Record Documentation
Thorough documentation of the manufacturing process is an integral part of quality assurance in fluticasone propionate API production. Manufacturers are required to maintain detailed batch records that document every step of the manufacturing process, including raw material specifications, equipment used, in-process controls, and final product testing results. This documentation ensures traceability, facilitates quality investigations, and enables regulatory authorities to verify compliance during inspections.
Validation and Qualification
Validation and qualification processes are essential to ensure the reliability and consistency of fluticasone propionate API manufacturing. Equipment used in the manufacturing process undergoes qualification to demonstrate its suitability and reliability. Validation studies are conducted to verify and document that manufacturing processes consistently produce API of the desired quality. This includes process validation, cleaning validation, and analytical method validation.
Risk Management and Continual Improvement
Fluticasone propionate API manufacturers prioritize risk management and continual improvement to enhance product quality and patient safety. Risk assessment methods, such as Failure Mode and Effects Analysis (FMEA), are employed to identify potential risks and implement mitigation strategies. Manufacturers also invest in research and development to optimize manufacturing processes, reduce impurities, and improve efficiency while complying with regulatory standards.
Conclusion
Quality assurance and compliance are critical components of fluticasone propionate API manufacturing. Stringent regulatory standards, robust quality control processes, accurate testing methods, batch record documentation, validation and qualification procedures, risk management, and continual improvement efforts ensure the safety, efficacy, and purity of the API. Fluticasone propionate API manufacturers are committed to meeting regulatory requirements, maintaining high standards, and delivering quality products that contribute to the development of safe and effective medications for respiratory conditions.
0 notes