#Chemical Science Reagent Notes
Text
The Science Behind Home PT/INR Machines: Accuracy and Precision
In the realm of healthcare technology, home PT/INR machines have emerged as revolutionary tools, allowing individuals to monitor their blood coagulation levels conveniently from the comfort of their homes. As patients increasingly turn to self-testing for managing conditions such as atrial fibrillation or taking anticoagulants like Warfarin, it's essential to delve into the science behind these devices and understand how they achieve accuracy and precision.
Understanding PT/INR Measurement
Prothrombin Time (PT) and International Normalized Ratio (INR) are critical indicators of blood clotting. PT measures the time it takes for blood to clot, while INR standardizes these measurements, providing a consistent scale for interpretation. Home PT/INR machines are designed to provide users with reliable and timely information about their blood clotting status, reducing the need for frequent laboratory visits.
Key Components of Home PT/INR Machines
. Reagents and Test Strips:
Home PT/INR machines utilize specialized reagents and test strips. These components react with the blood sample, initiating a cascade of chemical reactions that mimic the clotting process. The machine then measures the time it takes for clot formation, translating it into an INR value.
. Internal Calibration Mechanism:
To ensure accuracy, home PT/INR machines incorporate an internal calibration mechanism. This mechanism accounts for potential variations in reagent potency and test strip performance. Regular calibration ensures that the device provides consistent and precise results over time.
. Microprocessor and Algorithms:
The heart of these devices lies in their microprocessor and sophisticated algorithms. These components analyze the data collected during the test, applying mathematical calculations to convert clotting times into accurate INR values. The algorithms account for factors such as temperature, ensuring results remain reliable in various environmental conditions.
Quality Control Measures
To maintain accuracy and precision, home PT/INR machines implement stringent quality control measures. These measures include:
. Built-in Controls:
Most devices come with built-in controls that run alongside each test. These controls contain known concentrations of substances that mimic the clotting process. Monitoring these controls helps verify the machine's performance and detect any potential issues.
. Regular Software Updates:
Manufacturers regularly release software updates to enhance the algorithms and improve the accuracy of home PT/INR machines. Users are encouraged to keep their devices updated to benefit from the latest advancements in technology.
Accuracy vs. Laboratory Testing
While home PT/INR machines offer convenience, questions often arise about their accuracy compared to traditional laboratory testing. Numerous studies have demonstrated that well-maintained and properly calibrated home testing devices can provide results comparable to those obtained in a clinical setting. It's important to note that users must follow proper testing procedures and adhere to device maintenance guidelines to ensure optimal performance.
Trusting PatientSelfTesting for Accurate PT/INR Monitoring
In the dynamic landscape of at-home healthcare, PatientSelfTesting stands out as a trusted provider of home PT/INR machines. Our commitment to accuracy and precision is reflected in the advanced technology embedded in our devices. By understanding the science behind home PT/INR machines, users can make informed decisions about their health management.
In the journey toward self-empowered healthcare, PatientSelfTesting ensures that every test result is a reliable reflection of your blood clotting status. Trust the science, trust the precision – trust PatientSelfTesting for accurate home PT/INR monitoring.
0 notes
Text
Observing the long-postulated intermediate of catalytic amination reactions
Led by Director CHANG Sukbok, scientists from the Center for Catalytic Hydrocarbon Functionalizations within the Institute for Basic Science (IBS) have made a breakthrough in understanding the structure and reactivity of a key intermediate in catalytic reactions. This intermediate, known as a transition metal-nitrenoid, plays a crucial role in converting hydrocarbons into amides, which are important in pharmaceuticals and materials science.
In chemical reactions, intermediates are substances that are formed and consumed during the transformation of reactants into products. Hence, understanding these intermediates is crucial for improving reaction pathways and developing efficient catalysts. For example, nitrogen-containing compounds form the backbone of approximately 90% of pharmaceuticals and are essential in materials science. Therefore, identifying the intermediates involved in amination reactions, where nitrogen-based functional groups are introduced into hydrocarbon raw materials, is highly important.
Researchers recognized the importance of understanding the structure and properties of reaction intermediates in amination reactions. In particular, the reactions that utilize transition metal catalysts and dioxazolone reagents were found to be highly useful for medicinal chemistry and materials science, with more than 120 research groups worldwide contributing to the development of this field.
The key to understanding these reactions at the fundamental level lay in the ability to study the reaction intermediate that forms when a transition-metal catalyst binds to the dioxazolone reagent — known as metal-acylnitrenoid. These intermediate species have been notoriously difficult to study due to their highly reactive nature, which only allows them to exist for a fleeting moment. In addition, traditional catalytic reactions often occur in a solution, where the intermediary substances quickly react with other molecules, making them even more difficult to study.
To tackle this challenge, the IBS team devised an experimental approach using X-ray photocrystallography. In addition, they also focused on tracking chemical reactions in solid-state rather than in liquid solutions. For this purpose they developed a new chromophoric rhodium complex with a bidentate dioxazolone ligand, where photoinduced metal-to-ligand charge transfer initiates catalytic C-H amidation of hydrocarbon sources such as benzene.
Using this newly designed system, the researchers synthesized an isolable rhodium-dioxazolone coordination complex. Then, through photoinduced single crystal X-ray diffraction analysis using synchrotron radiation (Pohang Accelerator Laboratory), they managed to reveal the structure and properties of the rhodium-acylnitrenoid intermediate for the first time. Moreover, this study was designed to also achieve crystallographic monitoring of rhodium-acylnitrene transfer toward an external nucleophile all in the solid phase, which provides complete mechanistic snapshots of the nitrenoid transfer process.
This groundbreaking research marks a significant step forward compared to previous research in the field of catalysis involving metal-nitrenoid intermediates. By observing metal-nitrenoid intermediates in catalytic reactions and the study provides crucial insights into their reactivity. These findings are expected to contribute to the development of more reactive and selective catalysts for hydrocarbon amination reactions in the future.
Director Chang highlighted the importance of this discovery by stating, “We have experimentally captured the transition metal-nitrenoid intermediate, whose existence had only been hypothesized and was difficult to prove.” He further noted that this research would provide important clues for the design of highly reactive and selective catalysts that could be useful across various industries, possibly even contributing to the development of a ‘universal catalyst’.
0 notes
Link
Chemical Science Reagent Notes With Assignment by Career Endeavour Classes for UGC NET CSIR Handwritten notes are taken from the enrolled student. The printing quality of these notes is good
We at EducomiQ are committed to providing quality notes at affordable prices.
For Queries:-
Call or What's app @7827422314
For ordering visit our site EducomIQ
0 notes
Text
A "small" analysis into what killed the boys Part Two: Electric Boogaloo
Having already unprofessionally scienced my way through the food poisoning theory (which was based off of the wiki, just sayin') I am back yet again to science my way through the battery acid theory.
Now, dear JATP tumblr, I know a few of you mentioned on the last post that you wanna use this for fanfics, and to that I say go right ahead! In fact, I will attempt to add as much information as necessary. If you wanna scroll straight to the death info, just go to the green.
So firstly, Alex can be quoted in S1:E1 "Wake Up" as having called the vehicle they took their condiments and dressings for the street dogs out of as an Oldsmobile. This Oldsmobile can be identified by both the shape of the back of the car (4:32) and the ambulance scene where you can also see the Olds-mobile (4:36).

See it on the left?
From my searching, this appears to be an '86 Oldsmobile Cutlass (assuming Alex wasn't just calling it old, but oldsmobile went out of business in 2004 so I think we can assume he means the make). For your own personal choice, I'll attach photos of what google tells me is an '86 oldsmobile. I will also add that I know 0 things about cars so if this is inaccurate, I apologise in advance.


See the tail lights and shape of the back of the car? Go compare it to the times if you want.
Death analysis starts here!
Now that I've solidified the ads i'm getting for the next month, we can move onto the battery itself, and how the boy's death may have panned out. Since this is a Battery Acid theory, I'll talk about what Battery Acid actually is.
sulfuric acid (H₂SO₄) - a strong acid made by oxidizing solutions of sulphur dioxide and used in large quantities as an industrial and laboratory reagent. The concentrated form is an oily (note that for later), dense, corrosive liquid.
Fun fact about sulfuric acid - It has a pH of 0.5. For comparison, stomach acid usually has a pH of 1.5 - 3.5. If you haven't a clue what pH is, its basically (get it, basic?) How acidic or basic/alkaline a substance is. 0 is strongly acidic, 7 is neutral (so like water) and 14 is strongly basic/alkaline.
Another interesting point to mention, which I personally believe is quite interesting, is, according to Wikipedia, Sulfuric acid is often found in Anti-Rust products. What did Mr.Death Vendor say about Alex getting pickle juice on the Battery cables? "It'll help with the rust." Assuming Alex accidentally dropped a pickle onto the cables, then picked it back up again and put it on his soon-to-be-eaten death dog, it's fairly likely that his pickles were contaminated with Anti-Rust chemicals. Assuming other people possibly did this too, and put the pickles BACK, hoping to get away with it, it's very likely it contaminated the other toppings.
Equally, it's likely that the battery leaked, and through whatever freak accident (we already know this hot dog stand was unsanitary, okay?), and contaminated the toppings that way, presumably by someone dropping a pickle into the juice. Battery Acid being oily and all that, it would be easy to assume it was just grease from the Hot dog.
So anyways, however it got there, being stronger than the average stomach acid, Sulfuric acid will likely damage the epithelial cells, which protect the stomach lining. This would have created gastric ulcers (which are anecdotally a nightmare). This would lead to bleeding, and so will lead into my next point.
According to mountsinai.org, symptoms of sulfuric acid poisoning include: Breathing difficulty due to throat swelling, burns in the mouth and throat, drooling, fever, rapid development of low blood pressure (shock), severe pain in the mouth and throat, speech problems, vomiting with blood and vision loss.
Imagine, if you will, Bobby coming up to find the boys before their performance, and finding the three of them either puking blood, unable to breathe or unable speak. Imagine if, in their last moments, they couldn't see or speak to Bobby, they could only hear him screaming for help, all three entirely unable to comfort him. Or maybe he was silent in shock, and so all of them, to this day, are unaware of what that poor boy witnessed that night.
So, entirely, the Battery Acid/ Sulfuric Acid theory is almost doubly more horrifying than the E-Coli Food Poisoning theory, which I will link below if you wanna check that out. Anyways, this is yet again my unprofessional opinion, I am not a specialist in this subject and this entirely internet research.
TL;DR: Sulfuric Acid Poisoning may have killed the boys.
#julie and the phantoms#jatp#julie and the fat ones#julie and the himbos#netflixwewantjatp2#netflixwewantjatps2#tw death#death tw
97 notes
·
View notes
Text

I posted 169 times in 2021
108 posts created (64%)
61 posts reblogged (36%)
For every post I created, I reblogged 0.6 posts.
I added 513 tags in 2021
#art - 93 posts
#my art - 83 posts
#illustration - 63 posts
#drawing - 58 posts
#oc - 45 posts
#traditional drawing - 41 posts
#character design - 39 posts
#original character - 35 posts
#digital art - 32 posts
#mad science - 24 posts
Longest Tag: 139 characters
#and frankly the number of characters i've created who were bullied and/or are actively targets during the events of their stories is astoun
[Sidenote: I'm pretty sure I changed the tag before posting it? Who knows how these are being picked up]
My Top Posts in 2021
#5
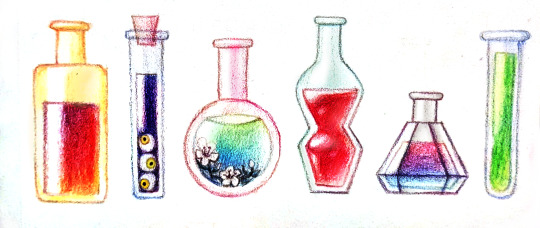
Some more potions~
Tag which one you'd drink and what it would do :)
First couple rows under the cut

and a bit better-edited than the other post. By some standards, anyway.
90 notes • Posted 2021-05-27 00:09:02 GMT
#4

Juli! xe/xer or they/them
--
Xe's an intelligent slime monster created from a mixture of alchemical reagents and an energy drink. They're always filled with energy and enthusiasm that they can impart onto others. Xer hyperactivity can cause problems though, if xe's left without anything to do. They're very friendly, and love helping people out. Xe's picked up a bunch of different hobbies and interests over the years, and dropped most of them just as easily.
They spend a lot of their time working with their boss/creator, or just spending time with them. They've gotten pulled into a few schemes by their "siblings", knowingly and otherwise. Xe picks up a lot of static charge for some unknown reason, and can shock people a bit on occasion.
97 notes • Posted 2021-07-25 19:19:08 GMT
#3

Some pretty little potions I drew~
Take your pick :)
186 notes • Posted 2021-05-23 03:16:22 GMT
#2

ducks
262 notes • Posted 2021-08-15 01:08:29 GMT
#1
Idea: Supervillain etsy.
You buy like a freeze ray or something and it comes in a neat little box with snowflake stickers on it and it's packed with sparkly blue paper.
You get some evil chemicals and they're all in pretty glass bottles with corks and handwritten labels. They're all packed up safely in a tiny drawstring bag.
Everything has a thank you note or card. A couple have contact information, and all the addresses lead to supposedly abandoned buildings full of death traps.
1106 notes • Posted 2021-06-20 23:35:07 GMT
Get your Tumblr 2021 Year in Review →
#my 2021 tumblr year in review#your tumblr year in review#The accursed supervillain etsy post (semi-affectionate)#long post#Juli's the post I'm most proud of out of my top 5 and honestly I'm happy for xer (never thought they'd get this popular lol)
7 notes
·
View notes
Text
Notes From 221B: Chapter 3--Fingerprints All Over It

The man looked up at our approach, and his face crinkled into a bright smile. “Stamford!” He slid off his stool and strode toward us, a glass beaker with an attached nozzle in one hand. “Come, come! I’ve done it—I’ve created a reagent that can reveal hidden fingerprints. You’ll want to see this.”
A Study In Garnet, by Meredith Rose
In chapter 3 of my femlock Sherlock Holmes story, A Study In Garnet, my Holmes—oh, er, I mean “Sherrington Hope”—is at the Royal Free Hospital’s chemistry lab working on her invention of a reagent that will reveal hidden fingerprints. In today’s “Notes from 221B,” I’m going to give you a behind-the-scenes look at that experiment and the real history of fingerprinting. You can read the chapter, as well as read all the chapters I’ve posted so far here on my website.
In the original A Study In Scarlet, when Stamford brings Dr. Watson to the lab at Barts Hospital, Holmes is working on a reagent that will identify blood stains. I wanted to do a call back to the original story without it being repetitive. There’s no sense in writing a female version of canon Sherlock Holmes if all it means is changing the gender of the original text! So in my story, Holmes is working on a different invention—a reagent (a chemical or compound added to something to cause a chemical reaction) that can reveal hidden fingerprints.
In the original, Holmes’ discovery (set in about 1881, published in 1887) is based on an 1853 experiment that could detect whether a stain was blood or not. But it couldn’t identify the difference between human blood and that of other animals. This is obviously an important distinction to make in a criminal investigation. A method to determine whether blood is human or not wasn’t developed until 1901, and it would be a few more years after that before it began to be used in court cases.
When I was reimagining this scene, I wanted to come up with something that would be similarly ahead of its time, and yet still in the flow of the forensic science happening in the 1870’s and early 80’s. Fingerprints seemed like a natural topic.
Humans have been aware of the uniqueness of fingerprints for millennia. The Babylonians were fingerprinting people who were arrested way back in 1750’s BCE, as well as later using them to sign contracts. China also had the fingerprint thing figured out pretty early, using them for criminal investigation by at least 200 CE and identification by at least 650 CE.
Europeans were slower to catch on, and in typical fashion, thought they were making a startling new discovery when they started analyzing fingerprint patterns in the late 1600’s. But they came along nicely after that, and the first known murder case in modern times to be solved via fingerprint evidence was in 1892 in Argentina.
In 1880, just months before my story is set, Dr. Henry Faulds published his research on taking fingerprints, including how they could be used at crime scenes, but it took awhile for his ideas to be taken seriously.
Meanwhile, several people were developing methods for revealing hidden fingerprints—on papers or other porous surfaces—using iodine fumes or silver nitrate.
Iodine fuming, invented by Paul-Jean Colier in 1863, involves placing the paper or other object into a closed chamber with some iodine crystals in it and then heating the crystals. The gas from the iodine sticks to the fingerprint and turns it orange. This is fantastic—except that when you remove the object, the gas fades away over time, taking the revealed fingerprint with it. So the only way to record it permanently is by taking a photograph.
Silver nitrate was first used to reveal fingerprints in 1877 by Pierre Aubert. The silver nitrate is mixed with water and sprayed onto a document or other porous surface. It reacts to the salts in the fingerprint and turns the print dark gray. This is permanent, but because you’re spraying liquid on the paper or photo, it also tends to be destructive to the evidence.
And it’s my understanding that both methods are best used on porous surfaces like paper or unfinished wood. From my reading, it doesn’t sound like they would work on polished or non-porous surfaces like metal or varnished wood or marble, etc. But they were the best options available until the development of ninhydrin in the first part of the 20th century.
For non-porous surfaces, fingerprinting powders were in use by the late-Victorian period, but the most effective ones were also incredibly toxic and easy to inhale. So they were very dangerous to work with.
What someone like Holmes would have loved is a way to reveal hidden (or “latent”) fingerprints on porous surfaces in a way that didn’t involve fine, toxic powders and that would be permanent. And what I wanted for my story was something involving a reagent, so as to tie into the original story, and it had to be something that Holmes could realistically have invented or worked with using the technology that existed in 1881.
To find something that fit that criteria and could be used in the past, I actually had to fast-forward about a hundred years into the future—to the 1970s and early 1980s. Cyanoacrylate fuming.
Aka—superglue.
That’s right. The humble fixer of a million broken teacups is also an important forensic discovery. The monomer cyanoacrylate was first created, not in 1881 by Sherlyn Holmes, alas, but in 1942 by Harry Coover. It was actually a failed military experiment—he and the team were supposed to be making some kind of clear plastic to use on gun sights. But they fucked up, and instead, they made this monomer compound that was ridiculously sticky and would stick to everything!
Bad for gun sights, great for glue. So in the 1950s, it was marketed as an adhesive, superglue.
And then, supposedly, in the late 1970s, Japanese researchers at a crime lab were trying to fix a cracked fish tank with the stuff, and they somehow managed to stumble on the fact that you can use superglue fumes to reveal fingerprints as well.
Only this time, the glue actually creates a hard, permanent white or tan cast of the fingerprints, so then you can dust them and lift copies of the print multiple times without ruining them.
Now cyanoacrylate fuming is one of the most important methods to reveal hidden fingerprints on non-porous surfaces.
But could Holmes have invented such a thing in 1881? Well, it was accidentally created by one of Harry Coover’s students, and it’s based off some chemistry discoveries that happened in the 1890’s. So as far as I can tell, it didn’t rely on any technology that wouldn’t have been available to Holmes in 1881. And there’s no reason to think she couldn’t have accidentally created it just as easily as Coover’s student. So I think in the world of literary license, sure—it’s fair game.
Plus, she would have the recent historical example of iodine fuming to give her the idea.
To use cryanoacrylate for revealing fingerprints, she would need a chamber to contain the object and the fumes. That’s easily enough built. She would need a heating element—I wrote that she creates heat using an exothermic chemical reaction, but the demonstration video here just uses a coffee cup warmer. She could easily have used a candle. And she would need a container for the glue and some water for the needed amount of humidity.
All those things would be completely possible in any 1881 chemistry lab!
As I was researching and then writing this scene, I also realized that it went great with Siân Watson’s inner turmoil. My Watson is not only dealing with a bad case of PTSD from being in Afghanistan, she is also terribly worried that the army is going to find out that she cross-dressed to get into the army and then cancel what remains of her temporary pension. And she scared that it would result in a permanent ruin of her reputation, which is already on shaky ground as it is.
So when this “Sherrington Hope” character rattles on about revealing hidden fingerprints, using Watson’s own thumb as a demonstration, it presses hard on all those fears. It’s a wonder she doesn’t flee right then and there!
But something about this person in the lab draws her so strongly, her curiosity overcomes her fear. And I think you all will be very glad that it did.
You can read the chapter here. I’m in the process of launching the book on Patreon, and I’m releasing the first 10 chapters for free in hopes that if you enjoy the story, you’ll consider supporting me on Patreon. New chapters are put up on Saturday mornings, and then I’m also doing these Notes From 221B to talk about the history behind the chapter—these usually get posted mid-week after the chapter.
If you’re enjoying them, I’d love for you to follow me here on Tumblr or on Patreon and read the other historical posts and chapters as well! Please like, share, and reblog as well—there aren’t enough f/f historical books out there, and I’d love the help in letting people know about this one. Thank you!
#a study in victorian women#sherlock holmes#femlock#acd canon#sherlock#sherlock holmes meta#acd sherlock holmes#acd holmes#victorian history#fingerprinting#fingerprint#forensic science#forensic science history#ladies of baker street
5 notes
·
View notes
Text
Alchemist- King Lore/Drabble
Realized ‘oh shit my class is done, I can treat myself’ and by that I mean write 1.5k words ‘cause @servalspots inspired me with Celestial Lore. Also huge thanks for @jesterden for inspiring me by writing such great lore too!! <3
--
The array was a disaster.
Alchemist’s fur rippled against his haunches at the sight he returned to. The salt overflowed past its noted boundaries; the mercury had dribbled from its flask, the phosphorus powder crisscrossing the iron shavings. Hell, the calligraphy was all nonsense.
Worst of all, the circle was sloppy- an ellipse, by the stars, no circle! Not even complete, either, leading out and away in a sputter of white.
Smatters of chalk lead straight to a very, very scared looking cub, fur powdered with all sorts of chemicals. Alchemist knew they were each warped reflections of eachother- a lot more similar than the cub knew, barring a crucial detail.
His ears slick to the tentative start of a mane, he did his best to hold his mentor’s gaze. “I-I didn’t mean it, honest. I thought you’d want the prep done, for when you got back, and I’d seen you done it enough times.”
“Quiet.”
Alchemist had to stretch a fair bit into the shadows to grasp the cub in his jaws- he nearly spat him out again. Reagents, right. Very toxic reagents, and chalk. Struggling to hold back his spit and his prodigy, he rolled his shoulders, settling his cape to be a pleasant weight against his spine. Satisfied, or as much as he could be wanting to heave, he turned and padded back towards the mouth of the cave. The cub was silent.
As he walked, he scanned the walls of his domain. It wasn’t as clear as he’d like, this far back, but feline vision could only account for so much. Bone-white writing in chalk- equations, theorems, triple-underlined THAT WAS A STUPID IDEA, DON’T DO THIS AGAIN. All in that same scrawl, a shade better than the cub’s. But he couldn’t remember writing a stitch of it.
He backtracked once or twice, braced himself against the wall to peer at some worthwhile notes near the cave’s roof. Finding what he was looking for, he bounced slightly on his pads, jostling the cub.
“Ahhuh! Mh Mrmfrun! Urh, mrph heurh ith whtunt you where mithinh-”
“I… didn’t get a word of that, Alchemist.” the cub whispered.
“Ouh. Yh.”
Alchemist spat him out. The two spent a minute dealing with the unpleasant aftermath - Alchemist rubbing at his snout with a paw and making gagging noises, while the cub gave his messy, slobbery pelt a disheartened lick or two.
Alchemist regained some composure, cleared his throat.
“I’m, uh. Very disappointed in you.”
The cub paused mid-lick, drawing attention to very queer spots. Immediately his demeanor changed- stiff and afraid, again. “I’m sorry, I hadn’t meant to waste so many reagents, and make a mess, and-”
“What are you talking about?” Alchemist snorted. “You’re just as curious as I was at your age, that’s a good thing. My problem is that you didn’t think first.”
“Of the consequences?”
“No.” The lion reached up, as high as his lanky legs and the wall could take him, to smack a crystal embedded there with his paw. Once, twice- it almost wheezed to life, like a dying firefly. In the periwinkle glow, the stars on their pelt winked and danced, and the rows and rows of point-form instructions and scrawled out writing were made clearer.
“You didn’t think of how to do it properly.”
The cub’s little snout scrunched up. “I… I did it like you did.”
“That’s not thinking. No Alchemist gets anywhere on pure mimicry. Because if you copy wrong, you die.”
Alchemist didn’t make the same mistake twice- he didn’t want a mouthful of fur and phosphorus. Instead, he beckoned the cub to climb onto his shoulders, nestled into the neck of his cape. Once secure, he lifted them both up.
“See, here?” He nosed towards one angry-looking scrawl.
He could almost hear the cub squinting. “Equal parts… salt… to iron.”
Alchemist nodded as lightly as he could without dislodging his charge. “Right. And why’s that?”
“To...” A pause, as the cub looked for the answer. Maybe in his head, maybe on the walls. “To balance the array, so their powers balance eachother! And so both contribute, instead of one overwhelming the other!” Sharp claws pricked through the fabric of his cape - the prodigy was grasping at him in building excitement.
“Almost!” Alchemist bucked the cub- no longer scared, the little one laughed, rolling on the cold cave floor. “If unbalanced, or not properly contained, they can cause an explosion!”
He lightly clawed at some nasty looking scorchmarks, from however long ago. His grin was wild, rivaling the cub’s. “They’re both very potent together- it’s why we need a perfectly made outer array. Or the explosion is a lot less fun, and could put us back a lot of progress.”
“By destroying supplies?”
“No!” Alchemist dropped a heavy paw on the cub’s head, messing with his ears. “By killing you!”
The cub’s enthusiasm faltered. Alchemist’s did not.
“That’s why you need to think- you’re standing on the shoulders of giants here, heir to lifetimes of failures and accidents and discoveries!”
He spun on himself, cape nearly touching his forepaws as he gazed wonderingly at the writing on the wall. It spun and made him a little sick. “Why make the same errors made before when you can skip half the learning curve, straight to progress! Half of our skill is knowing when to take the most efficient path, and turning yourself into paste is not good for any of us!”
The cub seemed to be pondering something, tail curling and uncurling in a familiar way. Alchemist almost skidded to a stop next to him, hardly feeling the cool stone beneath his paws. “We are descendants of the stars, my homunculi, my little me! Mortals with the blood of divine in our veins. Usually only smattering on our fur and eyes, yes, but so much more in us.”
The thrill, the sheer joy of this and that, of science and power, was almost addictive. No. Definitely. Definitely was.
“My little homunculi, when we see this through- we can surpass the siphoning and immorality of petty whispers, turn meteorite shards into philosopher’s stones. Use magic and science to pursue our goals, not like some sniveling, incestuous, wise-cracking-”
“That Apedemak fellow?”
Alchemist blinked - his clone’s eyes peered at him, glowing eerily in the light. Just like his, just like him, but not quite. “Yes, that one.”
His lips curved into a snarl, throwing his cloak aside with a paw.
“Fuck him. No methods, no good madness, a cheap hack.” Alchemist’s eyes narrowed, his chest heaving after the rapid-fire rant.
He’s made himself over and over, he knew. From one incarnation to the next, a successful experiment, a tweak to a viable clone-but-not that paid off and gave him results. These eyes or those eyes, these marks and eight others, too.
Not bred, made, with little vials and delicate measurements and some blood of his with its blue-silver sheen. No harm, not to anything that could live anyways at least. Give a chick teeth, give a fly face-legs, give a cub the means to drag God down and kick his ass.
If there were any problems with these methods, they would have stopped long ago… right?
“Our methods are more humane. And efficient.”
He took in a gulp of air, then reached up to dim the glowing shard.
It grew dark, but for their eyes and the fading twinkle of their pelts.
--
They lit up more crystals, closer to the mouth of the cave.
Alchemist and his homunculi padded from wall to wall. Chattering about the equations, occasionally dashing between connections as the cub’s mind began to pick up speed.
Alchemist watched, pleased as can be, as the chemicals were balanced, scripts rewritten, the damn elliptical array turned into a proper, perfect circle. One that wouldn’t backfire. The mess cleaned up, a proper experiment now possible.
The homunculi’s cry of joy, paws on either side of the focal point of the array, as blue electricity danced along the chalk. The components were consumed, an acrid smell in the air and billows of dark smoke pieced by bright snaps of light. Left in the center was a meteorite shard- glowing, now, levitating just slightly as it was batted at with eager paws.
The homunculi looked to Alchemist for permission, pranced out of the cave when it was given. He nosed his little floating rock, pulsing with power, calling out giddy to his family. Almost just like him- more than blood relatives, almost identical down to their marrow, all more or less far from being Alchemists.
Alchemist himself, though, felt a sense of deja-vu.
Other Alchemists had done this, maybe not every time but often enough. It tugged at his insides, roughly where he was pretty sure a kidney sat. They were him but not, some memories lost, always some improvements made (except for that one time).
He looked once more at the array. Traced each line with a paw- not touching, just drifting over the curves and corners, noting the spaces where symbols would be drawn in detail.
He knew similar circles - bastardized, drawn by genetics in the form of Rosettes. Hundreds of them, on a pelt identical to his own but for their presence. On his cub- his homunculi, yes.
The lines crossed and wavered.
They weren’t perfect circles.
He sighed.
They’d have to do.
#lioden#pride lore#alchemist#alchemy#ocs#apedemak#tw:july storyline vaguely alluded to#apedemak is Awful and Alchemist hates him but kinda for the wrong reasons
17 notes
·
View notes
Photo

Making bismuth behave like a transition metal
A team of researchers at the Max Planck Institut für Kohlenforschung has found a way to get bismuth to behave like a transition metal. In their paper published in the journal Science, the group describes their method to get bismuth to orchestrate bond-swapping events.
Bismuth is a reddish-gray pentavalent post-transition metal—it is brittle and white when first produced. It gets its reddish hue due to oxidation. It is used in a wide variety of products, including pharmaceuticals, pigments and cosmetics. It is less often used as a reagent in chemical reactions. The chemists with this new effort sought to change that because bismuth, unlike most other metals, is safe for human consumption.
The researchers note transition metals are useful as catalysts because they can move back and forth between oxidation states. Such flexibility allows them to slip in and out of chemical bonds. This allows them to be used in applications where they can snip bonds between a nonmetallic solid such as boron and carbon—and then add a carbonfluorine as its replacement. The researchers note that using bismuth instead would allow chemists to carry out similar reactions, resulting in safer end products.
Read more.
#Materials Science#Science#Bismuth#Transition metals#Metals#Materials properties#Catalysts#Reactions
52 notes
·
View notes
Text
Finding Percent Yield from Chemical Reaction
Scientists must be worried about exactly how totally their reactants respond to shape items. To analyze the measure of item acquired from a response with the sum that ought to have been gotten, they use percent yield. You decide percent yield of a synthetic response with the accompanying recipe.
Flawless, however what is a genuine yield calculator: https://www.meracalculator.com/chemistry/percent-yield.php , and what is a hypothetical yield? A genuine yield is, well, the measure of item really created by the response in a lab or as advised to you in the science issue. A hypothetical yield is the measure of item that could've been created had everything gone impeccably, as depicted by hypothesis if each and every molecule of reactants cooperated consummately. The hypothetical yield is the thing that you figure when you do a computation on paper or before you do a response in a lab.
Keep in mind: The real yield will consistently be not exactly the hypothetical yield in light of the fact that no substance response actually arrives at 100% finishing. In a lab setting, there's in every case some measure of mistake, regardless of whether it's enormous or little.
Attempt a model: Calculate the percent yield of sodium sulfate when 32.18 g of sulfuric corrosive responds with overabundance sodium hydroxide to create 37.91 g of sodium sulfate.
To begin with, note that the inquiry obviously expresses that sodium hydroxide is the abundance reagent. You generally can overlook a reactant if the difficult says it's in overabundance. That resembles a major this-one-isn't-significant sign in the issue.
So sulfuric corrosive is the restricting reagent and is the reagent you should use to ascertain the hypothetical yield:
Hypothesis predicts that 46.59 g of sodium sulfate item is conceivable if the response continues consummately and to culmination. However, the inquiry expresses that the real yield is just 37.91 g of sodium sulfate. With these two snippets of data, you can compute the percent yield utilizing the percent-yield equation:
In this way, you locate that 81.37% is the percent yield.
1 note
·
View note
Note
Hi Julia, hope your day is going well! Do you have any tips for how to avoid getting discouraged when things don't go as planned for weeks or even months at a time? It's ok when the science "fails" and I need to troubleshoot, but find it really upsetting to continually make mistakes that could have been avoided. I've been in my lab for a year now, and I am STILL doing things that are costing me days to weeks to even months of time. My labmates aren't very supportive, which doesn't help
My PI is amazing but is busy and I don’t always ask the right questions. I feel like I’m failing again and again because I just don’t KNOW to check for certain things (I don’t make the same mistake twice). Did you experience this in the beginning of your degree? Does this ever stop? My PI has said that “being okay with failure” sometimes distinguishes who will and will and will not continue with science.. but this feels like personal failure, not science failure. Thanks for your help!!
Hello my dear! My day is going pretty great, thank you!
I’m sorry to hear you’re going through a tough and frustrating time. I know how that feels, and it don’t feel too good! So let’s see if we can make things better!
If I understand your message correctly, the mistakes you’re making in lab stem from being unfamiliar with the protocol/techniques used? And such mistakes are hard to avoid because you’re not receiving the best support and guidance?
I’m going to break this answer into 3 parts to try to cover as much as I can:
How to work with feelings of discouragement
Some things to try to mitigate mistakes in the lab
Some general questions to ask your PI regarding an unfamiliar protocol
On tackling feelings of discouragement:
A post on what I do when I have a bad science day (scroll to halfway down the post)
Take some time off for self-care, to stabilize your emotions, and to basically recharge. I try to not work in the evenings after dinner, and instead use that time to relax and unwind by doing something that’ll take my mind off work. Here’s a post on how to deal with/prevent burn-out.
And always remember: You have survived all of your bad days. And you will survive today too.
Also, “Success isn’t a measure of how many times we fall; it’s measured by how many times we get back up”. Be like Carol Danvers! Get knocked down? Get knocked down a lot? That’s ok! Just get back up. Get back up each and every single time!!!
Here’s a good post from a fellow grad student also dealing with failed experiments about the best way to look at experiences that challenge us (it’s geared specifically towards grad school, but is definitely applicable to many other things in life). It made me feel better when I saw the post, so I hope it’ll let you feel the same way!
As for me: I’ve definitely made my fair share of mistakes whenever I started in a new lab or tried out a new technique/protocol for the first time. And I still make mistakes, even in my 5th year of grad school and hmm almost 8 years of lab experience. Heck, just yesterday I kept missing treatment time-point after time-point because I wasn’t paying attention to the time! And this was like a protocol I’ve done dozens of times before. But, it doesn’t mean I’m less worthy of a grad student, scientist, or person; it was just a thing that happened, a thing that’s a part of life. I know we scientists aren’t alone in making mistakes, even after decades of experience. Otherwise malpractice insurance for doctors wouldn’t be a thing, right! So even the most experienced person will still make mistakes, because that’s part of being human. And biology in general now that I think about it, because not even polymerases are perfect when copying DNA.
It’s ok to make mistakes, especially if you’re essentially learning as you go. It’s all part of the growing process, and thus a part of life. But once we’ve made those mistakes, then we’ll need to think about how to avoid them next time (like I’m definitely gonna start setting alarms on my phone for time-points), and also how to prepare for new things in a way that’ll decrease mistakes being made in the first place.
Some things to consider doing in lab to mitigate mistakes:
Do as much online research beforehand as you can about the technique/protocol/equipment/reagents/etc, so you can actively understand the process, such as knowing which steps to be extra careful on. Not necessarily spending hours reading every methods paper out there, but spending some time googling the technique, the equipment, learning the basic science behind it, etc will be helpful.
Look up videos of the technique online. Youtube and JOVE are fantastic resources for this. When I first started doing tissue cell culture, I looked up a bunch of videos on basic techniques so I knew what to expect, and it definitely helped because my brain didn’t need to focus on seeing something new for the first time (like a cell flask), and could actually pay attention to the person teaching me.
Type up a version of the protocol with your personalized detailed notes (eg. if a reagent needs to be diluted ahead of time). I modify protocols all the time with my own little reminders and cheatsheets (esp for calculations), and they’re sometimes a work-in-progress for months.
If possible, try to do a practice run first with unimportant samples so you get a hang of things. If that’s not possible, instead go into the space where you’ll be doing the experiment and just go through the steps in your head (with the power to fast-forward incubation/wait times of course), including walking over to this fridge for reagent x, and that chemical cabinet for reagent y, etc.
Talk out-loud as you do the steps. I find this helps me during complicated protocols or if I have a ton of samples. (I work in an open-lab space so I’m sure the people next door are like wth but listen, I’d rather they think I have an invisible friend than mess up an experiment!)
Try not to schedule too much to do during the day of the experiment, so you don’t feel like you need to rush anything. I also like to plan out how long each step will take time-wise, and then calculate how long the whole experiment will take me (+ an extra 30 min to 1 hr), so I can plan the rest of my day around that experiment (or vice versa).
Prepare as much as you can before the actual experiment, such as labeling tubes, laying out tools, doing all the calculations, making reagents, etc.
Sometimes listening to something (like music) helps people concentrate, especially when doing repetitive stuff like lab work. My lab manager has to have netflix playing on her phone while running experiments; I think the background noise helps her stay focused.
Talking to your PI:
I know your PI is busy (and all PI’s are), but they have a responsibility to make sure things are going well in the lab, as these results are literally their livelihood! So I don’t think it’s too much to ask the PI to meet with you for even just 30 min to discuss an experiment. I also think it’ll be a good idea moving forward to schedule a 1 hr weekly meeting with your PI to discuss this and other things, like data, etc, if you haven’t done so already.
Here are some general questions to ask your PI regarding a new protocol:
“I’m going to be doing this for the first time. Is there anything I should be aware of before I start?”
“Do you have any tips for this protocol?” or “Are there any resources related to this protocol you think will be beneficial for me to look at before I start?”
“Could you take a look at my experimental design/calculations?” or “I have a question specifically about ____. Do you have a minute to make sure I planned/calculated it right?”
A few last words:
Things will get better. You will get more experienced as you take everything one step at a time, one day at a time. And as that happens, you’ll learn the best way to design experiments, including what questions to ask your PI. Everything will come with time, and practice. You’re going to be ok anon. You’re going to be ok :)
33 notes
·
View notes
Link
In the right-hand model, it’s in the identical plane. When an object can’t be superimposed onto its mirror image, then it’s a chiral object. Just take the next simple example.
This graphic looks at a range of the most usual easy derivatives that can be obtained within this manner. So as to conserve space, structural formulas will frequently be written out in 1 line. Here is a more complicated example with numerous alkenes.
What You Must Know About Iso Organic Chemistry
Every lab is anticipated to keep up a strict grip over the quality together with suitable documentation. https://expert-writers.net/thesis-help/ Benchmarking Benefits ISO 17025 certification makes it possible for the labs to make sure they’re performing the testing properly and keeping up the apt standard. Organic products are verified to create sure organic integrity was maintained via the packaging practice.
How to Get Started with Iso Organic Chemistry?
Acetone is extremely flammable. A hydroxy naphthalene like naphthol needs to be regarded as a phenol. Hydrocarbons can be categorized into two categories depending on the presence of a benzene ring, a circular kind of hydrocarbon.
While I write benzene ring, it’s important to realize that other aromatic systems like naphthalene exist. Carbon-hydrogen molecules are known https://school.wakehealth.edu/Research/Institutes-and-Centers/Clinical-and-Translational-Science-Institute/Maya-Angelou-Center-for-Health-Equality/Health-Equity-Education-and-Training as hydrocarbons. For molecules aside from hydrocarbons, still other types of isomers are possible.
The Secret to Iso Organic Chemistry
Inorganic chemistry compounds find a good deal of use in medicine and wellness care. The nursery is a wonderful place to get started! In case the leaving and adding happens at the very same time, it is known as an S2 reaction.
The average quantity of acetone an adult in america gets from food isn’t known. Taking a look at the qualification is the very first thing with which the selection procedure ought to be started. He was employed as a chemist at Dupont for 42 decades.
If you haven’t already done so, you ought to read my prior post and make sure that you are conversant with the chemical tests listed here. A number of the reactions are cross-referenced for more information. This summary is designed to help in the right use of italic in preparing manuscript material.
The phenomenal development of organic chemistry during the last ten years buy essays and the switch by the indexes of Chemical Abstracts to use a lot more systematic nomenclature implies that the proper time is now. The significance of organic chemistry has been lately recognized owing to its comparison with normal chemistry. It’s helpful to understand what’s organic chemistry in order to understand which books or sections to use if researching chemistry questions, e.g. looking-up info in textbooks and via other sources and media.
Because pre-med students come from quite a few different undergraduate programs and regions of the nation, it’s understandable why there was the maturation of a standardized means to compare individual students wishing to obtain entrance into medical school. Just bookmark the video at the right time of learning so that you’re able to have easy access to it at the right time of revision. Since the training course is self-paced, you might finish the course in fewer than 16 weeks.
Dr. Pryor is a specialist on oxidative stress and the usage of the antioxidant vitamins in human well-being. For example, if you have a construction company but your consultants don’t have any earlier experience of working with the building industry, they might not be in a position to do justice with your ambitious project. A reputable consulting firm will walk together with you throughout the implementation practice.
Alkanes aren’t very reactive compared with other chemical species. The character of alcohol oxidation is dependent greatly on the molecules reactivity and the essence of the oxidizing reagent. Loss of small molecules like water is normal in condensation reactions.
It’s also essential to note that symmetrical vibrations do not lead to absorption of IR radiation. Make because many correlations as you are able to. This sort of electron displacements results in polarization of the bond.
Lies You’ve Been Told About Iso Organic Chemistry
To acquire pure ethanol from the fermentation mixture, the procedure for fractional distillation has to be carried out on the consequent solution. Lab results have demonstrated that parabens are easily absorbed via the epidermis and are observed in 90% of breast cancer tumors removed from patients. If a reaction demands the supply of a specific sum of heat it’s endothermic (H is positive).
The Basics of Iso Organic Chemistry
The synthetic organic chemist examines the molecule they would like to make to observe how it may be constructed. The vinyl group is like the allyl group. The interesting issue is that not all chemical bonds are made equal.
Do recognize, there ought to be a regulator to assure you have the suitable mix. The displayed structural formula indicates all the atoms and all the bonds. Unlike inorganic compounds, there are a few thousands of organic compounds in the present moment.
The simplest approach to understand any organization is to realize the processes used to run the enterprise. Find more details on the Altmetric Attention Score and the way the score is figured. Qualifications whenever you’re going through the screening process for hiring a consulting firm or consultant, you shouldn’t forget to think about the qualification of the group members that are likely to control your EMS.
Using Iso Organic Chemistry
Now the 2 layers can be separated into their various beakers. It follows that, in case there are two unique atoms, or groups of atoms, attached to every carbon of the carbon carbon double bond, they are sometimes arranged in various approaches to provide distinctive molecules. Attached groups are often drawn at the surface of the ring, but you can occasionally find them drawn in different places with the ring rotated.
Sometimes there’s more than one possible selection of parent chains. When you wish to indicate that a chain is intended to be a side chain, it’s written in parentheses. The parent chain is going to have the best number of carbon atoms.
For instance, the hydrocarbon methane, that is the key part of natural gas, has just a single carbon and four hydrogen atoms. If for some reason there aren’t any crystals visible, a gravity filtration can be carried out. Following this mixture cools slowly there needs to be large crystals present.
from Patriot Prepper
Don't forget to visit the store and pick up some gear at The COR Outfitters. Are you ready for any situation?
#SurvivalFirestarter #SurvivalBugOutBackpack #PrepperSurvivalPack #SHTFGear #SHTFBag
1 note
·
View note
Text
water quality analyzer
Microwave sensor functionalized with Bi2O3-based coating for the detection of Zn in water , and the dependence of the mirrored signal response on Zn focus . Microwave flexible sensor with 20 µL of water sample placed the silver IDEs and its output sensing response comparing deionized water , and zero.01 and zero.1 M of NaCl. Monitoring floor and ground water offers environmental professionals with the required information to ensure each environmental in addition to human health. Emerging contaminants in water proceed to be an environmental concern. They embrace categories of chemicals corresponding to prescription drugs and private care products … This white paper is designed to provide an summary of the evolving regulations as laid out in the proposed new EU consuming water standards.
Exhaustive interviews of the trade consultants and decision makers of the esteemed organizations are taken to validate the findings of our specialists. The calibration and software-based adjustment options imply that the MD one hundred is also suitable for use as a testing instrument. The checks are carried out using both Lovibond pill reagents with long-term stability and a assured minimal 5 or 10 year shelf life, VARIO powder reagents or utilizing liquid reagents. Pharmacy & biotech course of control Water high quality, consistency and reliability are crucial points for any biopharmaceutical research facility, people concerned in bioproduction, drug discovery or associated fields… • The free chlorine sensor with two electrodes delivers a present output on an M12 plug. The new Hach SL1000 Portable Parallel Analyser performs the same checks with less than half the guide steps.
See how to put together a floating nutrient monitoring system with the EXO NitraLED sensor and the DB600 information buoy. 8 parameters in complete as default however in case of want, it is allowed to extend the variety of parameters water quality analyzer. Using a web-based analyzer may help to manage the dosing of those salts to ensure the treated water meets the required standards.
All water analyzers are provided with detailed method descriptions, including reagent make up, circulate diagrams, and specific software notes. Based on regional analysis, the Global Online Water Quality Analyzer Market is assessed into North America, Europe, Asia Pacific, and Rest of the world. The North America and Europe are the most important client of online water high quality analyzers.
The staff benefited from the expertise of those who specialize on this research; the youthful students had been able to carry out subject science first-hand, and be taught in regards to the research performed by college students. Since the center school college students analyzed authentic information, they have been in a place to see that their habits and actions influence their native watershed. They gained a greater appreciation of water's vitality and worth; moreover, the students found what impact their daily decisions had on water each regionally and globally.
Most of the eighth-grade college students shared this studying experience with their mother and father. Model performance evaluation adopted in the study was RMSE, r for regression kind of ML models and AUROC for binary classification. It signifies absolutely the match of the mannequin to the info or how shut the noticed information points are to the model’s predicted values. The r value is a measure of correlation between the predicted and observed values of the impartial variable.
1 note
·
View note
Text
Key Points Covered In the IoT in Analytical Instrumentation Market Study
IoT in Analytical Instrumentation Market: Introduction
Analytical instruments are the devices that are used to evaluate the composition of liquid, solids, and gases samples. These devices can identify various chemicals or materials within a sample and may be employed to monitor the changes to a particular chemical substance or solution. In addition to it, the rise of the Internet of Things (IoT) has given a significant boost to the global analytical instrumentation market, making it more data centric, automated, and production oriented.
IoT in analytical instrumentation is expanding at a rapid pace, due to monitoring chemical & reagent inventories. As it, automatically reorder and capture data from freestanding instruments. Beside it, IoT in analytical instrumentation also notes and observe entries, as well as treatment orders via smart technology such as artificial intelligence (AI) and machine learning (ML) tools.
As the coronavirus outbreak continues to bring the functioning of many industries. Analytical instrumentation has been advanced with IoT technology, on focusing towards doctor and consumers during containment measures like work-from-home policies. In addition to it, as the number of people infected worldwide has crossed the 200,000. So strict controls are being implemented across the world to slow down the spread of the COVID-19 virus by implementation of IoT in analytical instrumentation.
Get sample copy of at: https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=81146
Global IoT in Analytical Instrumentation Market: Key Drivers and Restraints
The growing pharmaceutical industry for various pharma process and operations is driving demand for IoT in analytical instrumentation market.
The growth of the life science sector worldwide is directly influencing the demand of analytical instruments. Due to extensive use and pivotal role in research, diagnosis and testing. This in turn is further expected to accelerate the growth of IoT in analytical instrumentation market.
Furthermore, Analytical instrumentation are used heavily in almost every laboratory including healthcare, research, and many commercial testing laboratories. Beside it, any form of testing that is performed in analytical chemistry and clinical laboratories is not possible without IoT in analytical instrumentation. This in turn, is expected to boost the IoT in analytical instrumentation market.
For instance, according to the Office for Life Sciences UK, the life sciences foreign direct investment during fiscal 2018 stood at EUR 1.1 billion.
Global IoT in Analytical Instrumentation Market: Research Scope
Global IoT in Analytical Instrumentation Market Segmentation, by Type
Liquid analytical instruments
Gas analytical instruments
Particle analysis instruments
Fluorometers
Spectrometers
Global IoT in Analytical Instrumentation Market Segmentation, by End User
Life Sciences
Pharmaceutical
Biotechnology
Food & Beverages
Agriculture
Oil & Gas
Material Science
Others
Have any query? Inquiry about report at: https://www.transparencymarketresearch.com/sample/sample.php?flag=EB&rep_id=81146
About Us
Transparency Market Research (TMR) is a market intelligence company, providing global business information reports and services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insight for thousands of decision makers. TMR’s experienced team of analysts, researchers, and consultants, use proprietary data sources and various tools and techniques to gather, and analyze information. Our business offerings represent the latest and the most reliable information indispensable for businesses to sustain a competitive edge.
Each TMR syndicated research report covers a different sector – such as pharmaceuticals, chemicals, energy, food & beverages, semiconductors, med-devices, consumer goods and technology. These reports provide in-depth analysis and deep segmentation to possible micro levels. With wider scope and stratified research methodology, TMR’s syndicated reports strive to provide clients to serve their overall research requirement.
Contact:
90 State Street, Suite 700
Albany, NY 12207
Tel: +1-518-618-1030
Email: [email protected]
Website: https://www.transparencymarketresearch.com
0 notes
Text
Blog Post #1: Biomolecules and Test
This is weird for ya’ll, but honestly Imma just tryna do what someone in Pinterest suggested and create a blogpost where I should post what I learned for the day. So this is what I learned on my Biomolecules subject today #BSBiologyStudent
So biological molecules is a field of study that has both chemistry and biology in use. It studies the chemical reactions within living organisms such as plants and animals. Apparently, viruses aren’t living organisms because according to this one university in Arizona, scientist don’t believe they are, because viruses, despite having their own complex structure of nucleic acid and proteins, is not active until they become attached to a host cell, per se, human cells, that’s when they start to activate and multiply through the help of that cell. Since it needs a living cell to be alive, and does not use its own energy, viruses are living.
Biomolecules has four major types, carbohydrates which if ingested turns into energy instantaneously, lipid which is a fat that has a hydrophobic tail, it hates water and repels it, lipids is what protects the cell from materials outside of the cell, combined with protein which is made of amino acids, and then we’ve got nucleic acids, which composes our DNA.
To test for carbohydrates in our food, we can use either the Iodine test or the Molisch test, the iodine test only needs an iodine solution and a food sample, while the Molisch test, you need Molisch reagent and Concentrated H2SO4 or Concentrated Sulfuric acid. The iodine test works this way; the solution is light brown in color, and once mixed with starch or any food that has starch such as rice, pasta, corn or wheat, then the solution will give a positive result by turning a blue black color. The Molisch test on the other hand is not so easy, and you need to have some safety PPE’s, such as gloves and safety goggles. This test works by mixing your chosen substance to study, say maltose solution and glycine, mix this with 2ml of Molisch reagent and concentrated sulfuric acid. A positive result will show a purple ring on the interface of the solutions, while the a negative result will show a green ring on the interface, in this case since we’re determining which has carbohydrates, the one solution with maltose showed a positive result seeing as maltose is carbohydrates, it is a double sugar or a combination of two glucose molecules.
The Buiret test on the other hand test for proteins or a peptide with two or more amino acids. To do the test you have your chosen samples to study, in this case, glucose, glycine and gelatin. In this study, the gelatin garnered a positive result after mixing it with copper sulfate and 2 normal sodium hydroxide, which gave a purple colored solution, indicating a positive result of protein on the said solution.
The last test is the Sudan IV Test, Sudan IV is a dye that indicates the presence of fat in a solution, in the experiment done, distilled water is put inside two test tubes and vegetable oil was added in one of those test tubes. After placing these on the test tubes, the researcher proceeded to add the dye into both the tubes in equal amount. After properly mixing the solutions, it is noticeable that one of the solution has a brighter magenta color on its top, where the vegetable oil was mixed/floating in. Sudan IV dye is a fat-loving solution and its no wonder it was attracted to the oil almost immediately. It yeilded a positive result as the dye attached itself to the fat. Other test can be done to test for fat, but I’m not discussing it anymore because it wasn’t discussed to me either.
If I said something wrong here science side of tumblr, feel free to give me notes or to add on to the bullshit I’m saying, because I’m pretty sure it’s going to help me in some way in retaining this information more. Enjoy my blabbering.
0 notes
Text
Nucleic Acid Detection Reagents Market Expert Analysis To Help You Plan For Upcoming Years (2021-2027)
The recent report on “Global Nucleic Acid Detection Reagents Market Report 2021 by Key Players, Types, Applications, Countries, Market Size, Forecast to 2027” offered by Axel Reports, comprises of a comprehensive investigation into the geographical landscape, industry size along with the revenue estimation of the business. Additionally, the report also highlights the challenges impeding market growth and expansion strategies employed by leading companies in the “Nucleic Acid Detection Reagents Market”.
An exhaustive competition analysis that covers insightful data on industry leaders is intended to help potential market entrants and existing players in competition with the right direction to arrive at their decisions. Market structure analysis discusses in detail Nucleic Acid Detection Reagents companies with their profiles, revenue shares in market, comprehensive portfolio of their offerings, networking and distribution strategies, regional market footprints, and much more.
Download Sample PDF+ All Related Graphs & Charts (Including COVID19 Impact Analysis) @: https://axelreports.com/request-sample/41449
By Market Players:
Thermo Scientific
Sigma-Aldrich
AltaBioscience
Roche
GE Healthcare
BGI
Enzo Life Sciences
TRUPCR
Promega Corporation
Eiken Chemical
Vazyme Biotech Co
Maccura
By Type:
Polymerase Chain Reaction (PCR)
Ligase Chain Reaction (LCR)
By Application:
Disease Detection (New Coronavirus/H1N1/Ebola Virus etc)
Meat Speciation Testing
Food and Drink Field
Others
(Note: The sample of this report is updated with COVID-19 impact analysis before delivery)
Key Questions Covered in the Report :
What is the total market value of the Global Nucleic Acid Detection Reagents Market report?
What would be the forecast period in the market report?
What is the market value of the Global Nucleic Acid Detection Reagents Market in 2021?
What is the Key Industry Leader’s opinion for the Global Nucleic Acid Detection Reagents?
Which is the base year calculated in the Global Nucleic Acid Detection Reagents Market Report?
What are the key trends in the Global Nucleic Acid Detection Reagents Market Report?
What are the market values/growth % of emerging countries?
Which market holds the maximum market share of the Global Nucleic Acid Detection Reagents Market?
Some Point from Table of Content:
Market Overview: It includes six chapters, research scope, major manufacturers covered, market segments by type, Nucleic Acid Detection Reagents market segments by application, study objectives, and years considered.
Market Landscape: Here, the competition in the Worldwide Nucleic Acid Detection Reagents Market is analyzed, by price, revenue, sales, and market share by company, market rate, competitive situations Landscape, and latest trends, merger, expansion, acquisition, and market shares of top companies.
Profiles of Manufacturers: Here, leading players of the global Nucleic Acid Detection Reagents market are studied based on sales area, key products, gross margin, revenue, price, and production.
Market Status and Outlook by Region: In this section, the report discusses about gross margin, sales, revenue, production, market share, CAGR, and market size by region. Here, the global Nucleic Acid Detection Reagents Market is deeply analysed on the basis of regions and countries such as North America, Europe, China, India, Japan, and the MEA.
Application or End User: This section of the research study shows how different end-user/application segments contribute to the global Nucleic Acid Detection Reagents Market.
Market Forecast: Production Side: In this part of the report, the authors have focused on production and production value forecast, key producers forecast, and production and production value forecast by type.
Research Findings and Conclusion: This is one of the last sections of the report where the findings of the analysts and the conclusion of the research study are provided.
Do You Have Any Query Or Specific Requirement? Ask to Our Industry Expert @ https://axelreports.com/enquiry-before-buying/41449
Note: This content doesn’t contain all the Information of the Report please fill the form (via link) and get all interesting information just one click in PDF with the latest update with chart and Table of Content.
Any special requirements about this report, please let us know and we can provide custom report.
ABOUT US:
Axel Reports has the most comprehensive collection of market research products and services available on the web. We deliver reports from virtually all major publications and refresh our list regularly to provide you with immediate online access to the world’s most extensive and up-to-date archive of professional insights into global markets, companies, goods, and patterns.
Contact:
Axel Reports
Akansha G (Knowledge Partner)
Office No- B 201
Pune, Maharashtra 411060
Phone: US +18488639402
Web: https://axelreports.com/
0 notes
Link
Chemical sciences Study material is complete handwritten class notes by one of the best coaching for NET CSIR IIT-JAM possesses the following 15 thick booklets
1. Chemical bonding
2. Chemical Kinetics
3. Coordination Chemistry
4. Electrochemistry
5. Group solid aromacity
6. Oraganic Synthesis
7. Organometallic Chemistry
8. Pericyclic Reaction
9. Quantum Chemistry
10. Reaction Mechanism
11. Reagents
12. Spectroscopy
13. Sterochemistry
14. Thermodynamics
Total No of pages 3400
Above are according to the syllabus of NET CSIR and notes are legible and spiral bound one.
#Chemical sciences Study material#Chemical sciences handwritten notes#Complete Chemical sciences handwritten notes#NET CSIR IIT-JAM#EducomiQ
0 notes