#Clinical EDC
Explore tagged Tumblr posts
Text
Clinical Trial Technology and Complexity in the Real World – Why You Need a Flexible EDC System

The concept of EDC (electronic data capture) systems in clinical trials was first introduced over two decades ago. However, these archaic legacy systems are in no way equipped to deal with the sheer volume of data captured in a modern clinical trial. Over time, software firms offering an EDC system for clinical trials have established a significant market for their solutions, and yet innovative intuitive functionality is still lacking in most.
The idea of “if it isn’t broken, it doesn’t need fixing.” has held back innovation in the EDC tool system segment resulting in inherently flawed systems that could cost sponsors millions, not to mention years of wasted time.
As data management teams cope with the increasing complexity of trial design and protocol amendments, the fragility of legacy EDC software systems is becoming increasingly apparent. An inability to adapt to change and frequent downtimes are issues that can be worked around but they place an unnecessary burden on data management teams, resulting in fatigue, error, and most importantly, delays. In short, “It works well enough” just doesn’t cut it anymore. In short you need a highly efficient yet flexible EDC system.
This is where Octalsoft’s EDC system states its superiority as the best EDC software. Here are four ways Octalsoft's EDC solves these legacy challenges.
1. Capable of including Amendments with zero downtime
Existing EDC software systems are frequently cited as a source of customer dissatisfaction, given the frequency with which they crash anytime there is a change.
The fundamental database foundations of a traditional/legacy EDC are too rigid, which means that any time there is even the slightest change, the primary data structure needs to be reconstructed from the ground up.
In the fast-paced setting of a modern clinical trial, it is simply no longer acceptable to shut down the EDC software for hours at a time due to a large number of amendments and pivots that frequently take place in clinical investigations.
The contemporary and adaptable data structure of Octalsoft's regulatory compliant EDC system makes it possible for adjustments to be made with no downtime, removing the necessity to migrate data whenever an amendment is made and preventing end users from being kicked out of the system. This is more than just "no system downtime," it is in fact "no downtime for end users."
2. Maximizing Custom Functions
Every clinical trial is unique and hence requires an EDC that can adapt to the trial's specific requirements. Being forced to invest in additional systems just because the functionality of the EDC is sub-optimal is an expensive and wasteful approach. Octalsoft’s EDC tool includes every functionality that a modern clinical trial could need inclusive of a cloud-native platform and customization opportunities so that your EDC scales in tandem with your objectives.
Octalsoft's EDC software can handle complex edit checks directly from the system without requiring any external programming. Users can also organically add assessments, set derived values, and override targeted Source Data Verification in addition to emergency protocol deviations with minimal effort.
3. Intuitive and Effective Study Builds
Building out intuitive and effective studies is yet another essential component of efficient EDC tools. The integrated environment of Octalsoft’s EDC offers a simplified study builder functionality that allows users to do so in much shorter time frames without having to hand over control to development execs to convert protocols to code.
The Event Group functionality allows designers to work collaboratively within a single environment that ranges from individual treatment arms to a full sub-study of the master protocol. Subject Groups support named groups to facilitate seamless tracking and reporting.
The Octasoft EDC system validation difference report compares two distinct versions of a casebook. It identifies modifications for accurate User Acceptance Testing (UAT) by focusing on specific items, thus significantly reducing time and cost when it comes to including amendments.
Form Linking allows the user to establish a bi-directional connection between forms so as to easily capture relevant insights without switching between screens. e.g. An adverse event could be connected to its corresponding medication.
4. Enhanced UX
Handling the need gaps of legacy EDC software systems served as a strong foundation to build upon. Octalsoft identified the opportunity to make the entire user experience of our EDC a lot more streamlined and enjoyable for our users.
Octalsoft’s EDC user interface is designed to be both modern as well as deeply intuitive so that the user can navigate the system easily without spending hours in training sessions. There are many additional functionalities within our EDC system that make for a stellar UX.
The Autosave feature allows users to never lose any data, eliminating the stress and data loss) associated with systems timing out.
The EDC’s quick view feature offers CRAs and DMs an expansive yet cohesive view of task statuses. This is a massive upgrade from the form-by-form navigation of a legacy EDC. It does away with cluttered fields that are irrelevant to the task at hand.
This feature is designed to help users work in a way that is comfortable and yet does not compromise productivity or output quality. CRAS can now work incrementally without having to launch tiresome manual searches for every byte of data.
In Summation
Better features lead to an enhanced user experience and better UX leads to enhanced productivity and efficiency. This in turn results in better data and thus a better study. But our spirit of consistent innovation doesn’t stop with our EDC. As a core component of our eClinical suite Octalsoft's EDC is simply the starting point for revolutionizing clinical data management.
Interested in knowing how you can streamline capture, analyze, and report clinical trial data with the utmost precision? To find out, Book a Demo with us NOW!
0 notes
Text
Electronic Data Capture (EDC) systems are specialized software platforms designed to collect, manage, and store data in clinical trials. They replace traditional paper-based methods with secure, efficient, and highly accurate digital solutions. By automating data collection and streamlining management processes, EDC systems enhance data quality, minimize errors, and ensure compliance with regulatory standards. These systems are essential for modern clinical research, enabling real-time data access and integration with other clinical trial tools, thereby improving overall trial efficiency and decision-making.
1 note
·
View note
Text
Electronic data capture software | EDC software for clinical trials
Electronic Data Capture software has revolutionized clinical trials, enabling clinical research teams to streamline data management and enhance operational efficiency. However, the true potential of EDC is realized when coupled with advanced features that leverage AI and machine learning (ML). Among the sea of options, Clinion electronic data capture software stands out as a game-changer.
Discover how Clinion EDC’s advanced AI-enabled features like eProtocol Automation, Global Libraries, AI Medical Coding and Remote SDV Automation set it apart from the competition, making it the ideal choice for accelerating your trials.
0 notes
Text
How Can EDC Software Revolutionize Clinical Trials?
In the world of clinical trials, managing data accurately and efficiently is key to success. EDC software (Electronic Data Capture) plays a critical role in this process by streamlining data collection, minimizing errors, and ensuring timely access to real-time information. With its digital format, EDC software eliminates the need for paper-based forms, reducing the risk of data loss and inconsistencies.
This software enables clinical researchers to enter data directly into a system, ensuring faster, more accurate collection. It also allows for real-time monitoring, so any issues or discrepancies can be identified quickly. The best EDC systems offer features like data validation, customizable forms, and compliance with regulatory standards, making them indispensable tools for clinical trial teams.

Moreover, EDC software enhances collaboration among clinical teams by providing a centralized platform for data access, reducing delays and improving overall efficiency. With enhanced security measures such as encrypted data storage, patient information remains confidential and safe.
Ultimately, using the right EDC software can dramatically improve the accuracy, efficiency, and compliance of clinical trials, leading to faster and more reliable results.
Dacima Software provides powerful solutions designed to optimize clinical data management.
1 note
·
View note
Text

Plastics (EDCs) causing infertility Issues of Gender
And
Environmental exposure to plastics (xenoestrogens and oestrogen) related cancers: reproductive system, breast, lung, kidney, pancreas, and brain
13 notes
·
View notes
Text

Start Your Breakthrough Clinical Trials At AIIMS Hospital With BBMCT

Clinical trials are the backbone of medical advancements, helping researchers and healthcare professionals find new treatments, therapies, and solutions to pressing health issues. In India, AIIMS (All India Institute of Medical Sciences) has long been at the forefront of healthcare excellence, and when combined with **British Biomedicine Clinical Trials (BBMCT)**, it offers world-class research facilities and clinical expertise for cutting-edge breakthroughs. In this article, we’ll explore how BBMCT at AIIMS provides unmatched support for clinical research and trials.
— -
### **World-Class Research Facilities Available**
AIIMS Hospital is renowned globally for its state-of-the-art research infrastructure. The research facilities at AIIMS are designed to cater to diverse medical specialties, making it an ideal location for conducting advanced clinical trials. The hospital’s vast campus houses modern laboratories, research centers, and equipment, all of which are equipped with the latest technology to support clinical research.
British Biomedicine Clinical Trials (BBMCT) leverages these facilities to create an environment where studies can progress smoothly and efficiently. Whether it’s pharmacokinetics, bioequivalence studies, or clinical pharmacology trials, BBMCT at AIIMS offers a comprehensive setup for all kinds of advanced research. This enables researchers to gather precise data, ensuring faster and more accurate results.
— -
### **Expert Clinical Trials Management Team**
The success of clinical trials depends largely on the expertise and experience of the team managing them. BBMCT at AIIMS boasts an experienced team of medical professionals, researchers, and trial coordinators who specialize in clinical research and trial management. This team oversees every aspect of the clinical trial process, from initial planning to the final data analysis.
The clinical trial management team at BBMCT follows international best practices in Good Clinical Practice (GCP), ensuring that all trials meet rigorous scientific and ethical standards. Their profound knowledge in regulatory compliance, patient safety, and data integrity is a key asset for any study looking to achieve reliable and actionable results.
— -
### **Access to Diverse Patient Populations**
A unique advantage of conducting clinical trials at AIIMS is the access to a highly diverse patient population. AIIMS caters to patients from various socio-economic backgrounds, ethnicities, and regions. This diversity enhances the generalizability and relevance of clinical trial outcomes, ensuring that findings are applicable to a broad spectrum of people.
BBMCT at AIIMS takes full advantage of this diverse patient pool, allowing researchers to study the effects of treatments on a wide range of individuals. This helps researchers to detect varying responses to interventions, ensuring that the clinical trial results are robust, representative, and suitable for global healthcare applications.
— -
### **Cutting-Edge Technology Integration Offered**
To stay ahead in the fast-paced world of clinical trials, BBMCT integrates the latest technological advancements in clinical research at AIIMS. From electronic data capture (EDC) systems to cloud-based analytics platforms, AIIMS and BBMCT are fully equipped with cutting-edge technologies that streamline trial processes and improve accuracy.
These technologies enable real-time monitoring of trial data, faster recruitment and retention of participants, and more efficient management of study documentation. Furthermore, AIIMS is constantly upgrading its infrastructure to incorporate new innovations, ensuring that trials benefit from the most advanced tools available in medical research.
— -
### **Robust Ethical Oversight Ensured Here**
Clinical trials often involve testing new treatments on human participants, making ethical oversight a critical part of the research process. At AIIMS, ethical considerations are a top priority. BBMCT ensures that all clinical trials are conducted in accordance with the highest ethical standards, including patient informed consent, confidentiality, and protection from harm.
AIIMS has a dedicated ethics committee that reviews and monitors each clinical trial to ensure compliance with national and international ethical guidelines. This oversight gives patients confidence in participating and reassures researchers that their trials are conducted responsibly, ensuring the integrity and credibility of the results.
— -
### **Collaborate With Leading Medical Experts**
When conducting clinical research at AIIMS with BBMCT, you gain access to some of the leading medical experts in various fields. AIIMS is home to renowned specialists and researchers across disciplines, including oncology, cardiology, neurology, and infectious diseases.
Collaborating with these experts not only enhances the quality of research but also allows for interdisciplinary approaches to clinical trials. BBMCT fosters an environment of collaboration, where your study can benefit from the expertise and innovative solutions provided by these thought leaders in medicine and clinical research.
— -
### **Streamlined Processes for Quick Trials**
The faster clinical trials progress, the sooner medical breakthroughs can be made. At BBMCT, the processes involved in clinical trials are streamlined to reduce unnecessary delays and inefficiencies. From patient recruitment to data collection and analysis, BBMCT ensures that every phase of the trial is executed promptly.
AIIMS’ established infrastructure and resources facilitate quick approvals, recruitment, and trial management, meaning that studies can progress without the common bottlenecks seen in other settings. This streamlined approach is critical in accelerating the development of new treatments and improving patient outcomes.
— -
### **Enhance Your Study’s Success Rates**
Clinical trials require precision, efficiency, and an in-depth understanding of the research process. BBMCT at AIIMS is dedicated to enhancing the success rates of studies by offering the right resources, expertise, and infrastructure. With world-class facilities, expert teams, and cutting-edge technology, BBMCT ensures that trials are conducted optimally and that every potential challenge is mitigated.
The strategic support provided by BBMCT helps in better trial design, data accuracy, and recruitment strategies, which ultimately increase the likelihood of achieving positive study outcomes. Whether you are testing a new drug or a medical device, BBMCT maximizes your study’s chances of success.
/media/4ba4cf5bfd511c0ae4ae8e305b8459ec
— -
### **Frequently Asked Questions (FAQs)**
**1. What makes BBMCT at AIIMS different from other clinical trial organizations?**
BBMCT at AIIMS offers a combination of world-class research facilities, access to diverse patient populations, and expert clinical trial management. The integration of cutting-edge technologies and robust ethical oversight ensures that clinical trials are conducted efficiently and ethically. Additionally, AIIMS’ reputation and access to leading medical experts make BBMCT a standout choice for clinical research in India.
**2. How does AIIMS ensure ethical oversight during clinical trials?**
AIIMS has a dedicated ethics committee that reviews all clinical trials before they begin. The committee ensures that the trials adhere to national and international ethical standards, protecting the rights and well-being of participants. Informed consent, privacy, and safety protocols are central to their oversight, making sure trials are conducted responsibly.
**3. Can international researchers collaborate with BBMCT at AIIMS?**
Yes, BBMCT encourages international collaboration. Researchers from across the globe can partner with AIIMS and benefit from its vast resources, medical expertise, and advanced research facilities. International collaboration is particularly valuable in improving the scope and impact of clinical trials by incorporating diverse perspectives and expertise.
**4. What patient populations can BBMCT at AIIMS access for clinical trials?**
AIIMS serves a diverse patient population from various socio-economic backgrounds and regions. This diversity allows researchers to assess the effectiveness of treatments on a broad spectrum of individuals, increasing the relevance and applicability of study outcomes. BBMCT ensures that clinical trials can tap into this wide variety of patient groups for robust data collection.
**5. How do BBMCT’s cutting-edge technologies improve clinical trials?**
BBMCT integrates advanced technologies like electronic data capture, real-time monitoring systems, and cloud-based analytics to streamline trial processes. These technologies improve the accuracy of data, reduce trial delays, and enhance participant management. The use of such tools also accelerates the trial timeline, ensuring faster results and quicker access to new treatments.
— -
### **Conclusion**
AIIMS Hospital, in partnership with **British Biomedicine Clinical Trials (BBMCT)**, offers one of the most advanced environments for clinical research in the world. With world-class research facilities, an expert clinical trials management team, and a commitment to ethical oversight, BBMCT ensures that each clinical trial conducted at AIIMS is a step towards scientific breakthroughs and improved patient care. The integration of cutting-edge technology and access to diverse patient populations further enhances the success rates of trials, ensuring that medical innovations reach the people who need them most. If you are looking to advance your clinical research, starting your breakthrough trials with BBMCT at AIIMS is a choice you can trust.
Subscribe to BBMCLINICALTRIALS YouTube channel for Research Insights
Be sure to subscribe to the **BBMCLINICALTRIALS YouTube channel** for exclusive access to the latest updates and in-depth insights into British Biomedicine Clinical Trials (BBMCT). Stay informed on cutting-edge research, clinical trial advancements, patient safety protocols, and breakthrough therapies being tested at AIIMS Hospital. Our channel provides expert discussions, industry trends, and detailed videos on the clinical trial process across various therapeutic areas. Whether you’re a healthcare professional, researcher, or simply interested in biomedical innovation, subscribing will keep you at the forefront of clinical research developments. Don’t miss out — join our community today!
#anya mouthwashing#batman#artists on tumblr#agatha harkness#captain curly#cats of tumblr#bucktommy#agatha all along#911 abc
4 notes
·
View notes
Text
Top Skills You Need to Excel in Clinical Research
Clinical research is a dynamic and rewarding field that plays a vital role in advancing medical science and improving patient care. From testing new drugs to improving disease treatment strategies, clinical research professionals help shape the future of healthcare. But to truly excel in this field, there are certain key skills you must develop — both technical and interpersonal.
If you are considering stepping into this career path or planning to enroll in a clinical research course in Pune, it’s essential to understand what it takes to succeed. Let’s explore the top skills you need and how they align with various roles and responsibilities in clinical research.
Analytical Thinking and Attention to Detail
Clinical research involves working with data, protocols, and patient outcomes — all of which require careful analysis. Having strong analytical skills ensures that you can:
Interpret clinical data effectively
Spot inconsistencies in trial results
Follow protocols with accuracy
Detect even the smallest deviation that could affect the results
Whether you’re working on observational studies or interventional trials, a meticulous approach is crucial.
Strong Knowledge of Research Methodologies
Understanding the types of clinical research is foundational. The research you conduct could fall under several categories:
Interventional studies (e.g., clinical drug trials)
Observational studies
Diagnostic studies
Preventive or screening trials
Each type demands a different approach, and your skill set must match the research design to ensure success.
Regulatory and Ethical Awareness
One of the most essential skills in clinical research is being well-versed with regulatory guidelines and ethical standards. Since human lives are involved, there's no room for error. Professionals must:
Comply with GCP (Good Clinical Practice) guidelines
Understand ICH (International Council for Harmonisation) standards
Ensure patient safety and informed consent
Maintain proper documentation for audits and approvals
This is also where you understand the importance of clinical trials in contributing to ethical, safe, and effective medical advancements.
Communication and Interpersonal Skills
Clear communication is essential in every step of a clinical trial — whether it's coordinating with doctors, reporting to sponsors, or explaining procedures to participants. Good interpersonal skills allow you to:
Build trust with patients
Work effectively with a multidisciplinary team
Present data and updates to stakeholders
Resolve conflicts professionally
These soft skills are just as important as your technical knowledge.
Technical Proficiency and Data Management
Modern clinical research is powered by digital tools. Being familiar with data management systems and EDC (Electronic Data Capture) software gives you a significant edge. Skills in:
Data entry and cleaning
Statistical software like SPSS, SAS, or R
Using CTMS (Clinical Trial Management Systems)
Handling CRFs (Case Report Forms) are extremely valuable.
Project Management and Organization
Clinical trials are complex projects with multiple phases and deadlines. The ability to manage time and resources efficiently is key. You must:
Track timelines and milestones
Handle budgets and site coordination
Plan and prioritize tasks effectively
Ensure smooth trial operations
Learning this skill is often part of a structured curriculum offered by a top clinical research institute in India.
Problem-Solving and Adaptability
No clinical trial goes exactly as planned. Issues like patient dropouts, protocol deviations, or unexpected side effects can arise at any time. Quick thinking, creativity, and a flexible mindset are essential to:
Troubleshoot problems
Adapt trial procedures
Maintain compliance even under pressure
This kind of hands-on learning often comes from real-world exposure or an internship during a clinical research course in Pune.
Career-Specific Training and Certification
If you're wondering how to become a clinical research coordinator, the journey often starts with choosing the right academic and training program. Here’s a quick pathway:
Graduate in science, pharmacy, nursing, or medical fields
Enroll in a certified course in clinical research
Gain experience through internships or entry-level positions
Get certifications (e.g., ACRP, SOCRA) to boost your profile
The right training ensures you are job-ready and aware of both theoretical concepts and practical applications.
Bonus Insight: Future Scope of Clinical Research in India
The future scope of clinical research in India is incredibly promising. With increasing investment in pharmaceutical R&D, India is becoming a global hub for clinical trials. The demand for skilled professionals is higher than ever, especially in metropolitan areas and growing biotech hubs.
Some emerging trends include:
Personalized medicine trials
Artificial intelligence in data analysis
Remote or virtual clinical trials
Increased focus on rare diseases and vaccines
This makes it an excellent time to pursue a career in this field.
Final Thoughts
Excelling in clinical research isn't just about knowing scientific facts — it's about building a well-rounded skill set that includes technical, regulatory, and interpersonal capabilities. If you're ready to embark on this journey, consider learning from a top clinical research institute that offers hands-on training, real-world exposure, and industry-recognized certifications.
By mastering these top skills, you're not just preparing for a job — you're becoming a valuable contributor to the future of healthcare.
Digital marketing training curriculum
0 notes
Text
How EDC-Based SAE Reporting Reduces Time to Detection and Response in Clinical Trials
Serious adverse events (SAEs) are among the most critical data points collected during a clinical trial. The timeliness and accuracy of SAE reporting can mean the difference between a manageable issue and a significant safety crisis. As regulatory expectations increase and trial designs grow more complex, sponsors and CROs need tools that accelerate safety workflows without compromising data…
0 notes
Text
Risk Management in Late Phase Clinical Trials: Pharmacovigilance and Safety Monitoring

In the lifecycle of a pharmaceutical product, late-phase clinical trials—specifically Phase IIIb and Phase IV studies—represent a critical period for ensuring the continued safety, efficacy, and reliability of a treatment in real-world use. Unlike early-phase trials conducted in controlled environments with selective populations, late-phase studies involve broader, more diverse patient groups and reflect real-world usage patterns. This makes risk management, pharmacovigilance, and safety monitoring indispensable components of the process.
As regulators, healthcare providers, and patients demand higher standards for ongoing safety assurance, pharmaceutical companies must implement robust systems to identify, assess, and mitigate risks throughout late-stage development.
The Nature of Risk in Late Phase Clinical Trials
While early-phase trials focus on discovering therapeutic effects and optimal dosages, late-phase clinical trials are aimed at understanding how the drug performs in real-world settings, over longer durations, and in a more heterogeneous patient population.
Key sources of risk include:
Uncommon or long-term adverse drug reactions (ADRs)
Interactions with other medications or conditions
Off-label usage and patient misuse
Population-specific effects in pediatrics, geriatrics, or those with comorbidities
Since some of these risks may not manifest until the product is widely used, late phase trials provide the ideal framework for continuous safety evaluation.
Pharmacovigilance: The Cornerstone of Post-Market Safety
Pharmacovigilance (PV) refers to the science and activities related to the detection, assessment, understanding, and prevention of adverse effects or any other drug-related problem. In late-phase clinical trials, pharmacovigilance serves as the backbone of risk management.
Core components include:
Adverse Event Reporting: Capturing and classifying any negative health outcome experienced by participants, whether causally linked or not.
Signal Detection and Evaluation: Using statistical methods and data mining to identify patterns or clusters of adverse events that may indicate a safety concern.
Risk Evaluation: Determining the likelihood, severity, and relevance of potential risks in the context of the drug's benefits.
Risk Minimization Strategies: Implementing measures such as revised dosing instructions, boxed warnings, or restricted use programs to reduce patient exposure to known risks.
In many jurisdictions, pharmacovigilance in late phase trials is not just best practice—it’s a legal obligation.
Global Regulatory Requirements for Safety Monitoring
Various international regulatory authorities mandate structured safety reporting and risk management protocols during late-phase clinical trials:
FDA (USA): Requires sponsors to submit Periodic Adverse Drug Experience Reports (PADERs) and Risk Evaluation and Mitigation Strategies (REMS) when necessary. All serious and unexpected adverse events must be reported under 21 CFR 314.80.
EMA (Europe): Enforces Good Pharmacovigilance Practices (GVP) and mandates Periodic Safety Update Reports (PSURs) and Risk Management Plans (RMPs) under Directive 2001/83/EC.
MHRA (UK): Requires compliance with the Yellow Card Scheme, independent of EMA post-Brexit, with similar requirements for signal management and patient safety.
PMDA (Japan): Mandates Good Post-Marketing Study Practices (GPSP) and periodic re-evaluation of safety for up to 8 years post-approval.
Each of these agencies has specific timelines, formats, and criteria for safety data collection and reporting, necessitating a globally coordinated PV strategy.
Safety Monitoring Tools and Methodologies
To enhance safety monitoring in late-phase clinical trials, sponsors and CROs (Contract Research Organizations) utilize a mix of proactive and reactive tools:
1. Electronic Data Capture (EDC) and eCRFs
Digital systems enable near real-time adverse event documentation, streamlining communication between clinical sites and safety personnel.
2. Data Safety Monitoring Boards (DSMBs)
Independent committees that periodically review cumulative safety data and make recommendations on trial continuation, modification, or termination.
3. Signal Detection Algorithms
Automated tools apply disproportionality analysis (e.g., PRR, ROR) to detect unusual patterns in large safety databases such as FAERS or EudraVigilance.
4. Patient-Reported Outcomes (PROs)
Capture of subjective experiences such as fatigue, nausea, or quality-of-life impacts, offering deeper insight into treatment tolerability.
5. Real-World Evidence (RWE)
Supplemental safety data gathered from electronic health records, registries, and claims databases can complement traditional trial findings.
Risk Management Planning in Practice
An effective risk management plan (RMP) in late-phase trials should be customized based on the therapeutic area, patient population, and risk profile of the drug.
Key elements include:
Identification of known and potential risks
Summary of safety findings from pre-approval phases
Ongoing risk minimization activities (e.g., patient education)
Post-marketing study commitments and timelines
Continuous benefit-risk assessment and mitigation strategies
In some cases, post-marketing risk-sharing agreements with payers or REMS programs may also be developed based on safety data from late phase studies.
Case Example: Pharmacovigilance in Action
Consider a new oral anticoagulant approved for stroke prevention in atrial fibrillation. Phase III trials show strong efficacy, but concerns remain about bleeding risks in older adults. A post-marketing Phase IV trial includes:
A real-world patient cohort aged 65+
Adverse event tracking over 24 months
Integration with national health registries
Signal detection using RWE tools
The result? Identification of a higher-than-expected gastrointestinal bleeding rate in patients also taking NSAIDs—leading to updated labeling and targeted educational campaigns. This not only ensures patient safety but protects the brand from legal and reputational risks.
Conclusion
Late-phase clinical trials are a vital phase in the lifecycle of pharmaceutical products—not only for validating efficacy in diverse patient populations, but more importantly for monitoring and managing risk. Through rigorous pharmacovigilance and safety monitoring, sponsors can protect patient health, meet global regulatory obligations, and support a drug’s long-term commercial success.
As healthcare systems become increasingly reliant on real-world data and post-marketing evidence, the role of safety management in late-phase trials will continue to grow. Proactive investment in robust, global pharmacovigilance systems is no longer optional—it is a critical pillar of responsible, sustainable drug development.
1 note
·
View note
Text
Clinical Research Management Software — The Ultimate Guide for 2025 Your all-in-one blueprint to modernizing clinical operations with the latest CRMS innovations.
What you’ll discover: 🚀 The CRMS features CROs and sponsors can’t live without in 2025 🔐 Built-in compliance frameworks (think: GDPR, HIPAA, FDA) 🧠 How AI, automation & real-time analytics are changing the game ⚙️ Integration secrets: EDC, eTMF, CTMS, and beyond 📊 Proven tools that reduce manual errors & boost site performance
This isn’t just a guide — it’s your competitive edge in a high-stakes, high-speed industry.
👉 Get it now and future-proof your research:
#ClinicalTrials#ClinicalResearch#CRMS#LifeSciences#HealthTech#DigitalTransformation#CRO#PharmaInnovation#ResearchExcellence#AIinHealthcare
0 notes
Text
Bridging the Gap Between Investigators and Sponsors: TECCRO’s Coordinating Role as a Clinical Research Organization in Mumbai
Clinical research organizations play a vital role in the entire clinical trial process, from providing the sponsors a range of services to making things feasible for the investigators to just concentrate on research only. Synergy between investigators and sponsors is essential for a trial’s success. However, CROs often face the challenge of coordinating and managing the complex communication leading to misaligned expectations and operational inefficiencies. TECCRO, India’s niche Clinical Research Organization in Mumbai (CRO) specializing in dermatology, facial plastic surgery, cosmetic surgery, aesthetic medicine, and cosmetology, serves as the critical bridge that connects and aligns both ends of the research spectrum.
TECCRO provides a range of services, including project management, monitoring, protocol development, site selection, regulatory submissions, and data management over its expansive operational network across key metro hubs like Mumbai, Hyderabad, Delhi, Kolkata, Pune, Ahmedabad, Chennai, Bengaluru, Navi Mumbai, and Vadodara. TECCRO has established itself as a dependable partner for both sponsors and investigators – helping them work in tandem and thereby streamlining processes, reducing delays, and boosting overall trial delays.

Understanding the complexity of communication gaps in clinical trials
The most challenging task faced by CROs is to smoothen the communication between the involved stakeholders, including investigators and sponsors. Landscape of clinical trials in India has grown manifold in the last few years. Adaptive trial designs, decentralized and hybrid trials are the trends; regulatory changes have come into force and are constant and inevitable.
As the method of clinical trials has changed, relationships between top CROs in Mumbai , investigators, and sponsors are also evolving. Communications on staffing challenges, remote work, multiple vendors, use of EDCs, core imaging labs, etc. in real-time is the need of the hour.
What does a sponsor do?
Sponsors for clinical research are usually from pharmaceutical, biotech, or medical device companies, and their prime role is to fund and oversee the clinical trials.
What do investigators do?
Investigators are medical professionals who execute the trials on the ground.
The communication gap
Sponsors expect fast, accurate data and results from the investigators. These results should come after following protocol compliance and patient safety adherence rules. Investigators, on the other hand expect resources, clarity, and support.
However, many a time, even because of their shared goals, they often fall prey to a silos causing
Delays in site activation
Protocol amendments communications
Inconsistent documentation and reporting
Data quality issues
Delayed regulatory submissions and permissions
This is where the best CRO in India, TECCRO, steps in with its seamless operations and coordination models for all types of clinical trials.
TECCRO: The Link that holds the chain together
As a specialized CRO, a top one in India, TECCRO recognizes the clinical trials are only successful when there is transparency between all the stakeholders. TECCRO’s unique positioning allows it to:
Facilitate clear communications
By acting as a single point of contact between investigators and sponsors. The multifaceted nature of the CRO-let clinical trials leads to communication gaps. Additionally, time differences language barriers, and cultural differences further complicate the cause. TECCRO understands the sensitivity to language and cultural differences and eliminates confusion. Whether it is protocol clarification, site queries, or a logistical update, TECCRO streamlines communication and ensures alignment between the two sides.
Clear communication facilitates collaboration from the start of the trial by establishing preferred methods of communication and defining jobs and responsibilities. TECCRO uses video calls, emails, cloud-based platforms to keep the volume of communication under control.
Support Protocol Implementation
TECCRO includes hands-on training, SOP alignment, and periodic clarification to implement the final protocol effectively. Top clinical research organisation in Mumbai, TECCRO organizes periodic SIVs, workshops, etc. to ensure both investigators and sponsors understand the expectations and commitments towards the trial.
Monitor progress and milestones
Transparency when it comes to following the protocol is paramount. TECCRO has a reporting system in place providing real-time updates to sponsors on site selection, patient recruitment, adverse events, payments, delays, data entry, protocol submissions and such while simultaneously sharing transparent study updates, timelines, documentation processes and standards, which helps increase the efficiency of the trial.
Training and capacity building
TECCRO invests heavily in training both site and sponsor-facing staff for smooth coordination between the two parties. These capacity building exercises include GCP training, documentation for regulatory protocols, the latest EDC system usage, factual reporting of Adverse Event, and Serious Adverse Event reporting, and Trial Master File reporting. The dual training builds coordination between investigators and sponsors.
Regulatory support and ethics consideration
TECCRO’s standout feature is its in-house Institutional Ethics Committee (IEC). This expertise enables faster, compliant, and informed ethics approvals that fast-track clinical trials. For sponsors this means faster approvals, and for investigators it means clarity in ethical responsibilities. Reducing the gap between the stakeholders, TECCRO takes over CDSCO submissions, SAE follow-ups, audit readiness, and other site regulator packages. This backbone reduces the burden on investigators and enhances sponsor confidence.
Site management and patient recruitment
TECCRO includes SMO (Site Management Organization) services in its kitty and ensures every site is sponsor ready and, at the same time patient-friendly, meeting the criteria set by both sponsors and investigators. Both parties receive logistical and administrative support leading to fulfilled enrolment timelines, consistent data capture, and fewer protocol deviations, which are the key metrics of a trial’s success.
Why TECCRO’s Coordination Model Outshines others
Rapid Site Startup benefits both investigators and sponsors due to its documentation expertise and in-house IEC.
Real-time issue resolution with experts on ground for proactive responses.
Holistic oversight from start to end.
Personalized support with dedicated managers for each trial.
Specialized domain knowledge.
Conclusion: Paving the way to future-ready research
Best contract research organizations, TECCRO has emerged as a proactive, transparent, and agile coordination partner in the modern clinical trial landscape. It fills a critical void, making it easier for sponsors and investigators to work together without fretting over timelines and resources.
By bridging the gap between those who fund the trial and those who deliver the results, TECCRO is a catalyst in the development of safe, effective, and innovative solutions in the medicinal research space.
#best clinical research organizations in mumbai#cros in mumbai#contract research organization in mumbai
0 notes
Text
Clinical Trial Technology and Complexity in the Real World – Why You Need a Flexible EDC System

The concept of EDC (electronic data capture) systems in clinical trials was first introduced over two decades ago. However, these archaic legacy systems are in no way equipped to deal with the sheer volume of data captured in a modern clinical trial. Over time, software firms offering an EDC system for clinical trials have established a significant market for their solutions, and yet innovative intuitive functionality is still lacking in most.
The idea of “if it isn’t broken, it doesn’t need fixing.” has held back innovation in the EDC tool system segment resulting in inherently flawed systems that could cost sponsors millions, not to mention years of wasted time.
As data management teams cope with the increasing complexity of trial design and protocol amendments, the fragility of legacy EDC software systems is becoming increasingly apparent. An inability to adapt to change and frequent downtimes are issues that can be worked around but they place an unnecessary burden on data management teams, resulting in fatigue, error, and most importantly, delays. In short, “It works well enough” just doesn’t cut it anymore. In short you need a highly efficient yet flexible EDC system.
This is where Octalsoft’s EDC system states its superiority as the best EDC software. Here are four ways Octalsoft's EDC solves these legacy challenges.
1. Capable of including Amendments with zero downtime
Existing EDC software systems are frequently cited as a source of customer dissatisfaction, given the frequency with which they crash anytime there is a change.
The fundamental database foundations of a traditional/legacy EDC are too rigid, which means that any time there is even the slightest change, the primary data structure needs to be reconstructed from the ground up.
In the fast-paced setting of a modern clinical trial, it is simply no longer acceptable to shut down the EDC software for hours at a time due to a large number of amendments and pivots that frequently take place in clinical investigations.
The contemporary and adaptable data structure of Octalsoft's regulatory compliant EDC system makes it possible for adjustments to be made with no downtime, removing the necessity to migrate data whenever an amendment is made and preventing end users from being kicked out of the system. This is more than just "no system downtime," it is in fact "no downtime for end users."
2. Maximizing Custom Functions
Every clinical trial is unique and hence requires an EDC that can adapt to the trial's specific requirements. Being forced to invest in additional systems just because the functionality of the EDC is sub-optimal is an expensive and wasteful approach. Octalsoft’s EDC tool includes every functionality that a modern clinical trial could need inclusive of a cloud-native platform and customization opportunities so that your EDC scales in tandem with your objectives.
Octalsoft's EDC software can handle complex edit checks directly from the system without requiring any external programming. Users can also organically add assessments, set derived values, and override targeted Source Data Verification in addition to emergency protocol deviations with minimal effort.
3. Intuitive and Effective Study Builds
Building out intuitive and effective studies is yet another essential component of efficient EDC tools. The integrated environment of Octalsoft’s EDC offers a simplified study builder functionality that allows users to do so in much shorter time frames without having to hand over control to development execs to convert protocols to code.
The Event Group functionality allows designers to work collaboratively within a single environment that ranges from individual treatment arms to a full sub-study of the master protocol. Subject Groups support named groups to facilitate seamless tracking and reporting.
The Octasoft EDC system validation difference report compares two distinct versions of a casebook. It identifies modifications for accurate User Acceptance Testing (UAT) by focusing on specific items, thus significantly reducing time and cost when it comes to including amendments.
Form Linking allows the user to establish a bi-directional connection between forms so as to easily capture relevant insights without switching between screens. e.g. An adverse event could be connected to its corresponding medication.
4. Enhanced UX
Handling the need gaps of legacy EDC software systems served as a strong foundation to build upon. Octalsoft identified the opportunity to make the entire user experience of our EDC a lot more streamlined and enjoyable for our users.
Octalsoft’s EDC user interface is designed to be both modern as well as deeply intuitive so that the user can navigate the system easily without spending hours in training sessions. There are many additional functionalities within our EDC system that make for a stellar UX.
The Autosave feature allows users to never lose any data, eliminating the stress and data loss) associated with systems timing out.
The EDC’s quick view feature offers CRAs and DMs an expansive yet cohesive view of task statuses. This is a massive upgrade from the form-by-form navigation of a legacy EDC. It does away with cluttered fields that are irrelevant to the task at hand.
This feature is designed to help users work in a way that is comfortable and yet does not compromise productivity or output quality. CRAS can now work incrementally without having to launch tiresome manual searches for every byte of data.
In Summation
Better features lead to an enhanced user experience and better UX leads to enhanced productivity and efficiency. This in turn results in better data and thus a better study. But our spirit of consistent innovation doesn’t stop with our EDC. As a core component of our eClinical suite Octalsoft's EDC is simply the starting point for revolutionizing clinical data management. Interested in knowing how you can streamline capture, analyze, and report clinical trial data with the utmost precision? To find out, Book a Demo with us NOW!
0 notes
Text
North America Clinical Trials Market Drivers, Opportunities, Trends, and Forecasts by 2028

The North America clinical trials market is expected to grow from US$ 27,322.28 million in 2022 to US$ 36,818.15 million by 2028. It is estimated to grow at a CAGR of 5.1% from 2022 to 2028.
The US has emerged as a leading clinical research destination. Nearly half of the total clinical trials are conducted in the US. Additionally, most pharma research companies prefer to perform clinical trials in the US owing to established medical infrastructure, fast approval timelines, a favorable regulatory framework, and accepted clinical trial generated data globally. A World Health Organization (WHO) report states that the US registered the highest number of clinical trials (157,618) in 2021.
Exploring the Growth and Opportunities in the North America Clinical Trials Market
The North America Clinical Trials Market is witnessing significant growth, driven by advances in medical research, technological innovation, and increasing demand for novel therapeutics. As pharmaceutical companies continue to invest in R\&D, the region remains a global hub for clinical trials. The United States, in particular, accounts for a substantial share of the North America Clinical Trials Market, thanks to its robust healthcare infrastructure, presence of key industry players, and supportive regulatory framework.
📚 𝐃𝐨𝐰𝐧𝐥𝐨𝐚𝐝 𝐒𝐚𝐦𝐩𝐥𝐞 𝐏𝐃𝐅 𝐂𝐨𝐩𝐲@ https://www.businessmarketinsights.com/sample/BMIRE00029014
One of the primary drivers of the North America Clinical Trials Market is the rising prevalence of chronic diseases such as cancer, diabetes, and cardiovascular conditions. These health challenges have created a pressing need for innovative treatments, thereby boosting clinical trial activities. In addition, the aging population across North America has led to increased enrollment in trials focused on age-related illnesses, further fueling market expansion.
Technological advancements have also played a pivotal role in shaping the North America Clinical Trials Market. The integration of digital tools such as electronic data capture (EDC), remote monitoring, and decentralized clinical trials (DCTs) has streamlined the research process. These innovations enhance data accuracy, reduce costs, and improve patient participation—all of which contribute to the overall efficiency of the North America Clinical Trials Market.
The regulatory landscape in North America is another factor that supports market growth. Regulatory bodies such as the U.S. Food and Drug Administration (FDA) and Health Canada are committed to ensuring the safety and efficacy of clinical research. Their guidelines and expedited approval processes provide clarity and assurance to sponsors, making the North America Clinical Trials Market more attractive for investment.
Furthermore, partnerships between academic institutions, contract research organizations (CROs), and pharmaceutical companies are strengthening the clinical research ecosystem. These collaborations drive innovation and foster knowledge exchange, creating a more dynamic and competitive North America Clinical Trials Market. CROs, in particular, play a vital role by offering specialized services that enhance trial execution and data management.
📚𝐅𝐮𝐥𝐥 𝐑𝐞𝐩𝐨𝐫𝐭 𝐋𝐢𝐧𝐤 @ https://www.businessmarketinsights.com/reports/north-america-clinical-trials-market
The COVID-19 pandemic also brought the North America Clinical Trials Market into the spotlight. It highlighted the importance of rapid and flexible trial designs, particularly for vaccine and therapeutic development. The lessons learned during the pandemic have spurred greater adoption of virtual trials and hybrid models, setting new standards for the industry’s future.
Despite these opportunities, the North America Clinical Trials Market faces certain challenges, including high operational costs, patient recruitment issues, and complex regulatory requirements. However, ongoing efforts to address these barriers through innovation and policy reforms are expected to sustain the market’s upward trajectory.
In conclusion, the North America Clinical Trials Market is poised for continued growth, driven by a combination of technological advancements, regulatory support, and rising healthcare needs. As stakeholders focus on patient-centric approaches and data-driven solutions, the future of the North America Clinical Trials Market looks promising. With ongoing investments and strategic collaborations, the region is set to maintain its leadership position in global clinical research.
The List Of Companies
Charles River Laboratories InternationalInc
ICON Plc
IQVIA Holdings Inc
IXICO Plc
Laboratory Corp of America Holdings
Parexel International Corp
SGS SA
Syneos Health Inc
Thermo Fisher Scientific Inc
WuXi AppTec Co Ltd
Flourishing Pharmaceutical Industry and Increasing R&D Activities in Pharmaceutical Industry Fuels North America Clinical Trials Market
The pharmaceutical industry is one of the most R&D-intensive industries globally. The value of medicines is becoming increasingly important as pharmaceutical companies are keen to ensure that R&D achieves their intended goal. Over the last decade, the number of new drugs approved yearly has also increased. Efforts are being made to achieve greater effectiveness and efficiency in fulfilling patients' needs. The US is a leading country in R&D investments, producing over half of the world’s new molecules in the past decade. The US accounted for 62.3% of sales of new medicines launched during 2014–2019. R&D is a significant and essential part of the business of pharmaceutical companies as it enables them to come up with new molecules for various therapeutic applications with significant medical and commercial potential.
North America Clinical Trials Regional Insights
The geographic scope of the North America Clinical Trials refers to the specific areas in which a business operates and competes. Understanding local distinctions, such as diverse consumer preferences (e.g., demand for specific plug types or battery backup durations), varying economic conditions, and regulatory environments, is crucial for tailoring strategies to specific markets. Businesses can expand their reach by identifying underserved areas or adapting their offerings to meet local demands. A clear market focus allows for more effective resource allocation, targeted marketing campaigns, and better positioning against local competitors, ultimately driving growth in those targeted areas.
North America Clinical Trials Strategic Insights
Strategic insights for the North America Clinical Trials provides data-driven analysis of the industry landscape, including current trends, key players, and regional nuances. These insights offer actionable recommendations, enabling readers to differentiate themselves from competitors by identifying untapped segments or developing unique value propositions. Leveraging data analytics, these insights help industry players anticipate the market shifts, whether investors, manufacturers, or other stakeholders. A future-oriented perspective is essential, helping stakeholders anticipate market shifts and position themselves for long-term success in this dynamic region. Ultimately, effective strategic insights empower readers to make informed decisions that drive profitability and achieve their business objectives within the market.
Key Market Drivers
Several factors are fueling the growth of the clinical trials market in North America. Among the most significant is the increasing burden of chronic and complex diseases. Conditions such as cancer, cardiovascular disorders, and neurodegenerative diseases require ongoing research to develop new treatments. This medical need directly translates into a higher demand for clinical trials.
Technological advancements also contribute to the market’s growth. Innovations such as electronic data capture systems, remote monitoring tools, and AI-driven trial designs are enhancing the efficiency and accuracy of clinical research. These tools not only reduce the time and cost associated with trials but also improve patient recruitment and retention through personalized engagement strategies.
𝐀𝐛𝐨𝐮𝐭 𝐔𝐬:
Business Market Insights is a market research platform that provides subscription service for industry and company reports. Our research team has extensive professional expertise in domains such as Electronics & Semiconductor; Aerospace & Defense; Automotive & Transportation; Energy & Power; Healthcare; Manufacturing & Construction; Food & Beverages; Chemicals & Materials; and Technology, Media, & Telecommunications
You can see this-
North America Extruded Snacks Market- https://businessmarketresportsnews.blogspot.com/2025/04/north-america-market-research-share.html
North America Flexible Packaging Market- https://sites.google.com/view/bmi249/home
North America Hair Color Market- https://postyourarticle.com/north-america-hair-color-market-regional-analysis-key-players-future-projection-comprehensive-study-and-forecasts/
0 notes
Text
Simplifying Clinical Trial Protocol with Generative AI
AI-driven eProtocol generation represents a significant technological advancement in clinical trial protocol development. By leveraging machine learning, utilizing standardized templates, and automation, these tools are advancing the creation of high-quality protocols, enhancing efficiency, and reducing errors. As AI technology continues to evolve, its role in clinical research is expanding, offering new opportunities for improving the design and execution of clinical trials.
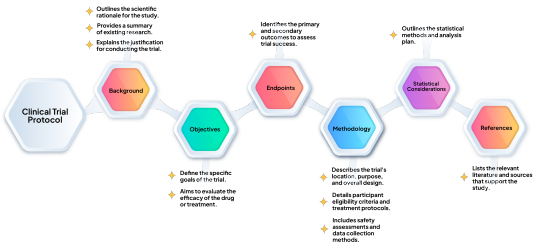
Read more: Simplifying Clinical Trial Protocol with Generative AI
0 notes
Text
Unlocking Efficiency: The Power of RTSM Software in Clinical Trials
Randomized Trial Supply Management (RTSM) software revolutionizes the management of clinical trial supplies, enhancing efficiency and ensuring the smooth progress of research endeavors. By automating inventory management, randomization, and drug dispensation processes, RTSM software minimizes errors and streamlines operations, saving valuable time and resources.

One of the key benefits of RTSM software is its ability to adapt to the dynamic nature of clinical trials. With features like real-time data tracking and forecasting, researchers can anticipate supply needs, mitigate risks of stockouts, and maintain adequate inventory levels throughout the trial duration. This proactive approach prevents disruptions, reduces costs, and accelerates trial timelines.
Moreover, RTSM software enables seamless integration with other clinical trial management systems, fostering collaboration among stakeholders and facilitating data exchange. This interoperability enhances visibility into trial operations, promotes transparency, and supports informed decision-making at every stage.
Furthermore, RTSM software enhances compliance with regulatory requirements by ensuring accurate documentation and audit trails. By maintaining comprehensive records of drug allocation and distribution, researchers can demonstrate protocol adherence and regulatory compliance, safeguarding the integrity and validity of trial results.
In essence, RTSM software represents a paradigm shift in clinical trial supply management, empowering researchers to navigate the complexities of trial logistics with precision and confidence. Its robust capabilities drive operational efficiencies, reduce risks, and ultimately contribute to the advancement of medical science.
0 notes
Text
𝐂𝐡𝐨𝐨𝐬𝐢𝐧𝐠 𝐭𝐡𝐞 𝐑𝐢𝐠𝐡𝐭 𝐌𝐞𝐝𝐢𝐜𝐚𝐥 𝐃𝐞𝐯𝐢𝐜𝐞 𝐂𝐑𝐎: 𝟕 𝐊𝐞𝐲 𝐅𝐚𝐜𝐭𝐨𝐫𝐬 𝐭𝐨 𝐂𝐨𝐧𝐬𝐢𝐝𝐞𝐫
Bringing a new medical device to market is an ambitious task. Along with innovation and engineering skill, you need airtight clinical data, an iron‑clad regulatory strategy, and expert project management. That’s why many companies, start‑ups, and global giants turn to a Medical Device CRO for help. A CRO can shorten timelines, control costs, and guide your product safely through the complexity of international regulations.
Still, not every contract research organization is right for every project. The wrong partnership can lead to delays, budget overruns, or missed regulatory milestones. To help you choose wisely, here are seven key factors you should weigh before signing any agreement.
Factors to Consider for the Right Medical Device CRO
1. Specialized Experience in Medical Devices
Clinical research for drugs and clinical research for devices are two different worlds. Devices often involve engineering changes mid‑study, unique usability endpoints, and combination products that challenge traditional trial designs. Ask potential partners:
How many device studies have you managed in the past five years?
Which therapeutic areas and device classes do you know best?
Can you provide case studies with successful regulatory approvals?
Look for a medical device CRO that understands device‑specific nuances, from design validation to post‑market surveillance.
2. Regulatory Expertise in Your Target Markets
A promising prototype is only half the battle; regulators decide when you can sell it. Each region has its own rules. Assess your CRO’s regulatory track record:
Up‑to‑date knowledge: Do they track evolving guidance documents and standards?
Submission success: What is their history?
Strategic planning: Can they map a step‑by‑step pathway, indicate the best predicate, and foresee data gaps?
A CRO with seasoned regulatory consultants can shave months off your approval timeline.
3. Global Site Network and Investigational Relationships
The best study design fails without capable investigators. Confirm that the CRO has:
Broad site reach: Established contacts in hospitals, specialty clinics, and academic centers across key regions.
Performance metrics: Data on each site’s enrollment speed, protocol compliance, and retention rates.
Backup options: A plan to activate alternate sites if the primary ones underperform.
Strong site management keeps enrollment on schedule and protects your budget.
4. Flexible Trial Design and Risk Management
Medical devices evolve quickly. You may tweak firmware or improve a catheter coating mid‑trial. Does the CRO have processes to:
Implement design changes: Rapidly changing protocols and communicating updates to sites and authorities.
Manage risk: Use real‑time data monitoring, adaptive designs, or Bayesian statistics to reduce sample sizes.
Mitigate supply chain issues: Handle recalls or production without disrupting the study.
Flexibility is vital when technology advances faster than paperwork.
5. Data Quality and Integrated Technology Platforms
Impressive data is your passport to the market. Investigate the CRO’s digital ecosystem:
Electronic data capture (EDC): Does it integrate with imaging, wearable sensors, or connected devices?
Real‑time dashboards: Can you track enrollment, adverse events, and data queries at a glance?
Cybersecurity: Compliant encryption and validated systems keep patient data safe.
A strong tech backbone accelerates cleaning and analysis, turning raw numbers into regulatory‑grade evidence.
6. Transparent Budgeting and Cost Control
Hidden fees drop many projects. Ask for a detailed budget model that covers:
Start‑up costs: Research submissions, site contracts, device shipping.
Variable costs: Per‑patient payments, monitoring visits, and data queries.
Change‑order policy: What happens if the timeline extends or the scope expands?
A trustworthy Medical Device CRO will walk you through each line item and offer contingency plans instead of surprises.
7. Cultural Fit and Communication Style
Even the most qualified CRO can disappoint if communication breaks down. During your selection process:
Meet the team: It is not just sales representatives; insist on speaking with the project manager and lead monitor.
Decision‑making process: Know who resolves issues and approves changes.
Projects run smoother when the CRO’s style meshes with yours—open, responsive, and proactive.
The Bottom Line
Selecting a CRO is one of the most critical choices you will make on the road to commercialization. A well‑matched partner keeps studies on track, controls budgets, and satisfies regulators. Use the seven factors above as your checklist, and you’ll dramatically improve your odds of success.
When you find a CRO that combines deep device know‑how, global reach, robust data systems, and clear communication, you’ve struck the research equivalent of gold. bioaccess® partnership won’t just move your device across the finish line—it will get it into the hands of clinicians and patients who need it most.
0 notes