#Clinical Trials Market Forecast
Text

#Clinical Trial Outsourcing Market#Clinical Trial Outsourcing Size#Clinical Trial Outsourcing Growth#Clinical Trial Outsourcing Trend#Clinical Trial Outsourcing segment#Clinical Trial Outsourcing Opportunity#Clinical Trial Outsourcing Analysis 2024#Clinical Trial Outsourcing Forecast
0 notes
Text
The clinical trial packaging and labelling market is expected to witness market growth at a rate of approximately 7.00% in the forecast period of 2021 to 2028. Data Bridge Market Research report on clinical trial packaging and labelling market provides analysis and insights regarding the various factors expected to be prevalent throughout the forecast period while providing their impacts on the market’s growth. The globalization of clinical trials and harmonization of regulations is escalating the growth of clinical trial packaging and labelling market.
The clinical trial refers to research study which assists in determining whether a medical strategy, treatment or device is safe, effective and useful for humans use. These studies find medical approach experiments are appropriate for certain diseases. These trials study strict scientific standards that protect patients and help in producing reliable study results. This is known to be the last stage in drug development that is carried out by scientists or researchers for a particular disease whether drug or medical device.
0 notes
Text
Clinical Trials Supplies Market: Examined in New Market Research
The research report provides detailed information on global market revenues, parent market trends, macroeconomic indicators and drivers, along with market attractiveness by market segment. The report provides an overview of the growth rate of clinical trial supplies during the forecast period, i.e. 2020-2027. More importantly, the report further identifies the qualitative impact of various market factors on market segments and geographies. The research segments the market based on product type, application, technology and region. To provide greater clarity regarding the industry, the report takes a closer look at the current state of various factors including, but not limited to, supply chain management, niche markets, distribution channel, trade, demand and supply and production capacity in different countries.
Request a sample copy of the clinical trial supplies at: https://www.theinsightpartners.com/sample/TIPRE00009672
Key vendors covered in this report:
1. Catalent, Inc.
2. Termo Fisher Scientific, Inc.
3. Almac Group
4.Parexel International Company
5. Biocair
6. UDG Healthcare plc (Sharp)
7. PCI health services
8. Owens & Minor Inc.
9. CLIFF
10. Rubicon Research Pvt. Limited.
The report outlines the key players in the industry, along with a detailed analysis of their individual positions in comparison to the global landscape. The study conducts a SWOT analysis to evaluate the strengths and weaknesses of key players in clinical trial supplies. The researcher provides in-depth analysis of the size, share, trends, overall earnings, gross revenue and profit margin of Clinical Trial Supplies to make accurate forecasts and provide expert insights to investors to keep them updated on the market trends.
Competitive scenario:
The study evaluates factors such as segmentation, description and applications of clinical trial supplies. It obtains accurate information to provide a holistic view of the dynamic characteristics of the business, including actions and profit generation, thus focusing on the critical aspects of the business.
Scope of the report
Clinical trial supplies research focuses on extracting valuable data on investment pockets, growth opportunities, and leading vendors in the market to help clients understand competitive methodologies. The research also segments clinical trial supplies by type and application for the 2020-2027 forecast period. Comprehensive analyses of critical aspects such as impact factors and competitive landscape are visualized with the help of vital resources such as graphs, charts and infographics.
The most important types of drugs supplied for clinical trials covered in this report are:
• Small molecule drugs
• Biological drugs
The main applications of clinical trial supplies covered in this report are:
• Oncology
• Cardiovascular disease
• Neurological disorders
• Respiratory disorders
Clinical Trial Supplies Segmented by Region/Country: North America, Europe, Asia Pacific, Middle East & Africa, Central & South America
sThank you for reading this article; You can also customize this report to get selected chapters or regional coverage with regions like Asia, North America, and Europe.
Who we are:
Insight Partners is a one-stop shop for market research reports and solutions for various companies across the globe. We assist our clients in their decision support system by helping them choose the most relevant and cost-effective research reports and solutions.
Contact us:
If you have any questions about this report or would like more information, please contact us:
The Insight Partners
Telephone: +1-646-491-9876
Email: [email protected]
#Clinical Trials Supplies Market#Clinical Trials Supplies Market Size#Clinical Trials Supplies Market Trends#Clinical Trials Supplies Market Forecast#Clinical Trials Supplies Market Growth#Clinical Trials Supplies Market Analysis
0 notes
Text
Global In Silico Clinical Trials Market Is Estimated To Witness High Growth Owing To Growing Demand For Virtual Clinical Trials

The global In Silico Clinical Trials Market is estimated to be valued at US$ 3,173.1 Mn in 2022 and is expected to exhibit a CAGR of 7.95% over the forecast period 2023-2030, as highlighted in a new report published by Coherent Market Insights.
A) Market Overview:
Silico Clinical Trials are virtual trials conducted using computer models and simulations to evaluate drug efficacy and safety. These trials provide several advantages over traditional clinical trials, such as reduced time and cost, ethical considerations, and the ability to collect large amounts of data.
Virtual trials eliminate the need for physical participation, making it more convenient for patients and reducing the burden on healthcare systems. They also allow for the exploration of various drug combinations and dosages, accelerating the development of personalized medicine.
B) Market Key Trends:
One key trend in the In Silico Clinical Trials Market is the growing demand for virtual clinical trials. With advancements in technology and the increasing need for efficient drug development processes, pharmaceutical companies are increasingly adopting virtual trials. These trials provide real-time data analysis, reduce patient recruitment time, and offer cost-effective solutions for drug development.
For example, Insilico Medicine Inc., a key player in the market, utilizes artificial intelligence (AI) algorithms to accelerate the drug discovery process. Their AI-based platform enables researchers to identify potential drug candidates and predict their efficacy using virtual models.
C) PEST Analysis:
Political: Regulatory frameworks play a crucial role in the adoption of virtual clinical trials. Governments need to establish guidelines and standards to ensure patient safety and data privacy.
Economic: Silico Clinical Trials offer cost-effective solutions compared to traditional clinical trials. They reduce the need for physical locations, extensive patient recruitment efforts, and travel expenses.
Social: Virtual trials provide opportunities for patients who may otherwise be unable to participate in traditional trials due to geographical constraints, physical limitations, or personal commitments.
Technological: Advancements in technology, such as AI, machine learning, and big data analytics, have facilitated the growth of virtual trials. These technologies enable researchers to analyze complex data sets and make predictions about drug efficacy and safety.
D) Key Takeaways:
Paragraph 1: The global In Silico Clinical Trials Market is expected to witness high growth, exhibiting a CAGR of 7.95% over the forecast period. This growth can be attributed to increasing demand for virtual trials due to their ability to reduce time and cost in drug development. For example, the use of virtual models and simulations enables researchers to predict drug efficacy and safety before conducting physical trials.
Paragraph 2: Regionally, North America is expected to dominate the In Silico Clinical Trials Market . The region has a well-established healthcare infrastructure, favorable regulatory environment, and a high adoption rate of advanced technologies. Additionally, collaborations between pharmaceutical companies, academic institutions, and research organizations in North America contribute to the region's growth in the market.
In conclusion, the In Silico Clinical Trials Market is witnessing significant growth due to the increasing demand for virtual trials. Advancements in technology, cost-effectiveness, and the ability to collect real-time data are driving the adoption of virtual trials in drug development. The market is dominated by key players who are continuously investing in research and development to stay ahead in the competitive landscape. As virtual trials become more widely accepted, they have the potential to revolutionize the drug development process and improve patient outcomes.
#In Silico Clinical Trials Market#Medical Devices#In Silico Clinical Trials Market Growth#In Silico Clinical Trials Market Analysis#In Silico Clinical Trials Market Forecast#In Silico Clinical Trials Market Future#In Silico Clinical Trials Market Overview
0 notes
Text
Clinical Trials Market Witnessed A Decline In 2020 Owing To COVID-19 Pandemic
The global clinical trials market size is expected to reach USD 78.3 billion by 2030, registering a CAGR of 5.8% during the forecast period, according to a new report by Grand View Research, Inc. An increase in the volume and complexity of clinical trials has been witnessed lately, which plays an important role in the R&D of new drugs and products. The market witnessed a decline of 6% in 2020 owing to the COVID-19 pandemic. However, the market is projected to recover from 2021 onwards. In addition, clinical trials have become increasingly costly, adding to the overall cost of developing a drug.
The increasing need for developing new drugs for chronic diseases, such as cancer, respiratory disorders, diabetes, cardiovascular diseases, and others, is creating immense pressure on the healthcare industry. The COVID-19 pandemic and the increasing demand for developing a suitable treatment are driving the market. The high number of people affected by the disease further depicts an increasing need for therapeutics & vaccines. Currently, there are 288 therapeutics and 106 vaccines under development, out of which, nearly 7.0% of therapeutics are in Phase IV, 21.0% in Phase III, and 43.0% & 13.0% in Phase II & Phase I, respectively.
Gain deeper insights on the market and receive your free copy with TOC now @: Clinical Trials Market Report
The pandemic has resulted in the global disruption of traditional onsite clinical trials. Hence, regulatory bodies worldwide have undertaken various initiatives for fast-tracking clinical trials for the development of innovative solutions. One such instance is Solidarity, an international clinical trial launched by the WHO to find effective treatment against COVID-19. Although the pandemic has forced many medical device & drug developers to revise the approach to such crises, integrating best practices within clinical trial procedures & adapting to virtual trials, which can support the continuous development of therapeutics.
#Clinical Trials Market Size & Share#Global Clinical Trials Market#Clinical Trials Market Latest Trends#Clinical Trials Market Growth Forecast#COVID-19 Impacts On Clinical Trials Market#Clinical Trials Market Revenue Value
0 notes
Text
Artificial Intelligence (AI)-based Clinical Trials Market by Product, Types, Procedure, Application, End-user Global Forecast to 2029
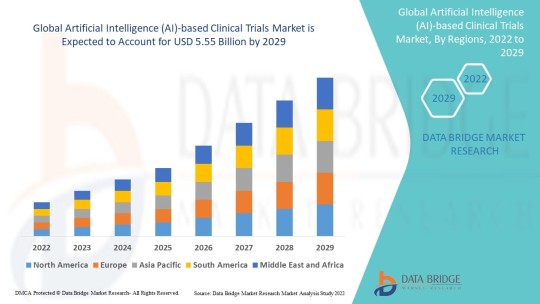
Industry Analysis
Data Bridge Market Research analyses that the artificial intelligence (AI)-based clinical trials market which was USD 1.3 billion in 2021, would rocket up to USD 5.55 billion by 2029, and is expected to undergo a CAGR of 19.90% during the forecast period 2022 to 2029.
Data Bridge market report covers an array of aspects of the market analysis which today’s businesses call for. This market document also defines a chapter on the global market and allied companies with their profiles, which provides important data pertaining to their insights in terms of finances, product portfolios, investment plans, and marketing and business strategies. This market research report is generated with a nice blend of industry insight, talent solutions, practical solutions and use of technology to advance user experience. An outstanding Data Bridge market report puts light on many aspects related to healthcare industry and market.
Market Insights and Scope
Artificial intelligence can be used to identify disease, provide healthcare services, and even develop new treatments, while improving clinical trials. The scale and speed of AI are significantly superior to any system that depends just on human activity. In many ways, this will be the biggest problem moving forward because we haven't yet reached an AI level that can run totally autonomously.
Artificial Intelligence (AI)-based Clinical Trials Market report helps the manufacturer in finding out the effectiveness of the existing channels of distribution, advertising programs, or media, selling methods and the best way of distributing the goods to the eventual consumers. Taking up such market research report is all the time beneficial for any company whether it is a small scale or large scale, for marketing of products or services. It makes effortless for healthcare industry to visualize what is already available in the market, what market anticipates, the competitive environment, and what should be done to surpass the competitor.
Industry Segmentation
The Artificial Intelligence (AI)-based clinical trials market is segmented on the basis of clinical trial phase, application and end-user. The growth amongst these segments will help you analyze meagre growth segments in the industries and provide the users with a valuable market overview and market insights to help them make strategic decisions for identifying core market applications.
Clinical Trial Phase
Phase-I
Phase-II
Phase-III
Application
Oncology
Cardiovascular Diseases
Neurological Diseases or Conditions
Infectious Diseases
End-user
Pharmaceutical Companies
Academia
Get a Free Sample of The Report: https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-artificial-intelligence-ai-based-clinical-trials-market
Market Country Level Analysis
The countries covered in the Artificial Intelligence (AI)-based clinical trials market report are
U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America.
Get full access to the report: https://www.databridgemarketresearch.com/reports/global-artificial-intelligence-ai-based-clinical-trials-market
Industry Share Analysis
Some of the major players operating in the Artificial Intelligence (AI)-based clinical trials market are:
Phesi (India)
CONSILX (Singapore)
DEEP LENS Inc. (U.S.)
Unlearn.AI, Inc. (U.S.)
Saama Technologies, LLC (U.S.)
Antidote Technologies, Inc. (U.K.)
Innoplexus (Germany)
Mendel.ai (U.S.)
Median Technologies (France)
Symphony AI (U.S.)
BioAge Labs, Inc. (U.S.)
AiCure (U.S.)
Halo Health Systems (U.S.)
An influential Artificial Intelligence (AI)-based Clinical Trials Market research report displays an absolute outline of the market that considers various aspects such as product definition, customary vendor landscape, and market segmentation. Currently, businesses are relying on the diverse segments covered in the market research report to a great extent which gives them better insights to drive the business on the right track. The competitive analysis brings into light a clear insight about the market share analysis and actions of the key industry players. With this info, businesses can successfully make decisions about business strategies to accomplish maximum return on investment (ROI).
Get TOC Details: https://www.databridgemarketresearch.com/toc/?dbmr=global-artificial-intelligence-ai-based-clinical-trials-market
Browse Related Reports@
Global 1, 4-Cyclohexanedimethanol Dibenzoate Market
Global Plant Hydrocolloids Market
U.S. Tahini Market
Asia-Pacific Hydroxyl-Terminated Polybutadiene (HTPB) market
West Africa Shisha Tobacco Market
Global Orthostatic Hypotension Drugs Market
Europe Customer Journey Analytics Market
About Us:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market
Contact:
Data Bridge Market Research
Tel: +1-888-387-2818
Email: [email protected]
#Artificial Intelligence (AI)-based Clinical Trials Market-by Product-Types-Procedure-Application-End User-Global-Forecast to-2029#Artificial Intelligence (AI)-based Clinical Trials Market-Global Opportunity-Analysis and-Industry-regional#Artificial Intelligence (AI)-based Clinical Trials Market-Growth-Competition-Scenario-Outlook#Artificial Intelligence (AI)-based Clinical Trials Market-Insights-Country-Share-Competitors-Research-Study#Artificial Intelligence (AI)-based Clinical Trials Market-Demands-Size-Share-Top Trends-Report-to-2029#Artificial Intelligence (AI)-based Clinical Trials Market Value-Segmentation-CAGR rate-Future Trends#Artificial Intelligence (AI)-based Clinical Trials Market-drivers-advantages-restraints-challenges#Artificial Intelligence (AI)-based Clinical Trials Market-Leading Brands-Business-Healthcare#Artificial Intelligence (AI)-based Clinical Trials Market-Growing Popularity-Traffic-DBMR
0 notes
Text
Clinical Trial Packaging Market Growth, Overview with Detailed Analysis 2022-2028
Clinical Trial Packaging Market Growth, Overview with Detailed Analysis 2022-2028
The Clinical Trial Packaging Market research report 2022-2030 provides an in-depth analysis of the changing trends, opportunities, and challenges influencing the growth over the next decade. The study includes a detailed summary of each market along with data related to demand, supply and distribution. The report examines Clinical Trial Packaging market growth strategies adopted by leading…
View On WordPress
#Clinical Trial Packaging#Clinical Trial Packaging forecast#Clinical Trial Packaging Industry#Clinical Trial Packaging Market#Clinical Trial Packaging price#Clinical Trial Packaging report#Clinical Trial Packaging research#Clinical Trial Packaging share#Clinical Trial Packaging trends#Covid-19 Impact Analysis
0 notes
Text
Transforming the Health Landscape: The Global Blockchain in Healthcare Market

The integration of blockchain technology into the healthcare sector is revolutionizing the way medical data is managed, shared, and secured. As the demand for transparent, efficient, and secure healthcare services grows, blockchain offers promising solutions to longstanding challenges.
Understanding Blockchain in Healthcare
Blockchain Technology is a decentralized digital ledger that records transactions across multiple computers in a way that ensures the security and transparency of data. In healthcare, blockchain can be used to manage patient records, track pharmaceuticals, ensure the integrity of clinical trials, and streamline administrative processes. The immutable nature of blockchain helps in preventing data breaches, ensuring data accuracy, and enhancing patient privacy.
According to BIS Research, the Global Blockchain in Healthcare Market was estimated to grow to a value of $5.61 billion by 2025, and still the market is showing a steep growth till 2030 witnessing a double-digit CAGR growth rate throughout the forecast period.
Key Market Dynamics
Several factors are driving the growth of the global blockchain in healthcare market:
Data Security and Privacy:
Need for robust data security and privacy solutions.
Healthcare data breaches are a growing concern.
Blockchain's secure, immutable nature protects sensitive patient information.
Interoperability and Data Sharing:
Facilitates seamless data sharing between healthcare providers and systems.
Overcomes current interoperability issues.
Leads to better patient outcomes by providing a comprehensive view of health history.
Supply Chain Transparency:
Tracks the entire lifecycle of drugs in the pharmaceutical industry.
Ensures the authenticity of medications.
Helps combat counterfeit drugs.
Efficient Administrative Processes:
Streamlines various administrative processes, such as billing and claims management.
Reduces fraud and administrative costs.
Support from Regulatory Bodies:
Increasing support from regulatory bodies and governments.
Initiatives by FDA and EMA to explore blockchain for drug traceability and clinical trials boost market growth.
Request for an updated Research Report on Global Blockchain in Healthcare Market Research.
Global Blockchain in Healthcare Industry Segmentation
Segmentation by Application:
Data Exchange and Interoperability
Supply Chain Management
Claims Adjudication and Billing Management
Clinical Trials and Research
Others
Segmentation by End-User:
Healthcare Providers
Pharmaceutical Companies
Payers
Others
Segmentation by Region:
North America
Europe
Asia-Pacific
Latin America and Middle East & Africa
Future Market Prospects
The future of the global blockchain in healthcare market looks promising, with several trends likely to shape its trajectory:
Integration with AI and IoT: The integration of blockchain with artificial intelligence (AI) and the Internet of Things (IoT) will enhance data analytics, predictive healthcare, and real-time monitoring.
Expansion of Use Cases: New use cases for blockchain in digital healthcare will emerge, including patient-centered care models, personalized medicine, and enhanced telemedicine services.
Focus on Patient-Centric Solutions: Blockchain will enable more patient-centric healthcare solutions, empowering patients with greater control over their health data and enhancing patient engagement.
Development of Regulatory Frameworks: The establishment of clear regulatory frameworks and industry standards will facilitate the widespread adoption of blockchain in healthcare.
Conclusion
The Global Blockchain in Healthcare Industry is poised for significant growth, driven by the need for enhanced data security, interoperability, supply chain transparency, and efficient administrative processes. By addressing challenges related to regulatory compliance, implementation costs, standardization, and scalability, and leveraging opportunities in technological advancements, investments, partnerships, and government initiatives, the potential of blockchain in healthcare can be fully realized. This technology promises to revolutionize healthcare delivery, enhancing efficiency, transparency, and patient outcomes, and setting new standards for the future of digital health.
#Blockchain in Healthcare Market#Blockchain in Healthcare Industry#Blockchain in Healthcare Market Report#Blockchain in Healthcare Market Research#Blockchain in Healthcare Market Forecast#Blockchain in Healthcare Market Analysis#Blockchain in Healthcare Market Growth#BIS Research#Healthcare
2 notes
·
View notes
Text
Know More About Pharma Database Service Provider Company | Chemxpert
At Chemxpert we have a comprehensive pharmaceutical company dataset prepared by these service providers, providing indispensable information for strategic decision making. From clinical trial data and patent information to competitive analysis and market forecasting, these datasets empower stakeholders to make informed decisions, minimize risks, and maximize opportunities.
2 notes
·
View notes
Text
"Unveiling the Future: How Data Science is Revolutionizing Upcoming Industries"
Data science continues to have a substantial impact on various industries, and its scope is expected to expand as new technologies emerge and businesses realize the potential of data-driven insights. Here are some upcoming industries where data science is likely to play a significant role:
Healthcare and Life Sciences: Data science can aid in personalized medicine, drug discovery, predictive analytics for patient outcomes, and healthcare operations optimization.
Financial Services: Financial institutions use data science for fraud detection, risk assessment, algorithmic trading, customer behavior analysis, and credit scoring.
Retail and E-Commerce: Data science helps optimize inventory management, pricing strategies, recommendation systems, and customer segmentation for targeted marketing.
Energy and Utilities: The energy sector benefits from data analytics for smart grid management, predictive maintenance of equipment, and energy consumption optimization.
Manufacturing: Data science improves manufacturing processes through predictive maintenance, quality control, supply chain optimization, and demand forecasting.
Agriculture: Precision agriculture utilizes data science to optimize crop yield, resource allocation, pest control, and environmental monitoring.
Transportation and Logistics: Data science plays a role in route optimization, fleet management, demand forecasting, and autonomous vehicles.
Telecommunications: Data science assists in customer churn prediction, network optimization, and personalized service offerings.
Media and Entertainment: Content recommendation, audience segmentation, and analyzing viewer engagement are areas where data science is making an impact.
Real Estate: Data science helps in property price prediction, market trend analysis, and investment decision-making.
Environmental Conservation: Data science aids in monitoring and analyzing environmental data, including climate patterns, pollution levels, and habitat preservation.
Education: Data science can personalize learning experiences, assess student performance, and optimize educational resources.
Government and Public Services: Data-driven decision-making is becoming increasingly important for optimizing public services, policy formulation, and resource allocation.
Insurance: Insurers use data science for risk assessment, claims processing, fraud detection, and customized pricing.
Travel and Tourism: Data science enhances traveler experiences through personalized recommendations, pricing optimization, and destination insights.
Pharmaceuticals: Data science plays a role in drug discovery, clinical trials optimization, and pharmacovigilance.
Smart Cities: The concept of smart cities involves integrating data science for efficient urban planning, traffic management, energy consumption, and public services.
Cybersecurity: Data science helps in identifying and responding to cyber threats by analyzing patterns and anomalies in network data.
As technology continues to advance and businesses recognize the value of data-driven insights, certybox is creating a difference in providing the top professional courses along with job assistance. It's essential for professionals in the field to stay updated with the latest developments and tools to make the most of these opportunities.

5 notes
·
View notes
Text
AI Transforming Drug Discovery: The Future of the Pharmaceutical Market
Artificial Intelligence in Pharmaceutical Market is projected to be valued at USD 3.05 billion in 2024 and is anticipated to reach USD 18.06 billion by 2029, with a CAGR of 42.68% during the forecast period (2024-2029).
Artificial Intelligence (AI) in Drug Discovery Market is gaining momentum as pharmaceutical companies increasingly adopt AI-driven solutions to streamline research and development (R&D) processes. According to Mordor Intelligence, the market is expected to grow significantly, driven by the rising need for faster, cost-effective drug discovery methods, combined with advances in AI technologies like machine learning, natural language processing (NLP), and deep learning.
AI’s Role in Transforming Drug Discovery
Accelerating Drug Discovery Process:
Traditionally, drug discovery is a time-consuming and costly process, often taking 10–15 years and billions of dollars to bring a new drug to market. AI is revolutionizing this by speeding up the identification of drug targets and optimizing lead compounds. Algorithms can rapidly analyze vast datasets, predicting drug behavior and outcomes more accurately than traditional methods.
Predictive Modeling and Simulation:
AI's ability to create predictive models is transforming how pharmaceutical companies approach drug discovery. AI can simulate how compounds will interact with biological targets, reducing the need for trial-and-error lab work. This not only accelerates the development process but also enhances the chances of finding successful drug candidates.
Data-Driven Research:
With the growing availability of biological and chemical data, AI can sift through and analyze enormous datasets, identifying patterns and insights that humans might miss. Machine learning models can analyze genomic data, protein structures, and chemical compounds, helping researchers understand complex biological mechanisms and predict potential therapeutic outcomes.
AI in Target Identification and Validation:
AI tools are being employed to identify novel biological targets for therapeutic intervention, as well as to validate their potential effectiveness. This is especially crucial in the development of drugs for complex diseases like cancer, neurodegenerative disorders, and infectious diseases.
AI for Drug Repurposing:
AI is also playing a pivotal role in drug repurposing, where existing drugs are investigated for new therapeutic uses. AI algorithms can identify new applications for existing compounds by analyzing data on drug interactions, mechanisms of action, and patient outcomes.
Reduction in Drug Development Costs:
By reducing the time and cost involved in early-stage drug discovery, AI is contributing to significant cost savings. Traditional methods often lead to high failure rates, especially in late-stage clinical trials. AI can help mitigate these risks by improving early predictions on a drug’s efficacy and safety profile.
Key Market Growth Drivers
Rising Demand for Personalized Medicine: AI’s ability to analyze genetic, environmental, and lifestyle factors is driving growth in personalized medicine. AI-powered platforms can predict how individuals will respond to treatments, leading to more targeted and effective therapies.
Increasing Partnerships between Pharma and AI Companies: Collaborations between pharmaceutical companies and AI technology firms are growing. These partnerships aim to combine pharma's clinical expertise with AI’s data-processing capabilities to revolutionize drug discovery and development.
AI in Clinical Trials: AI is optimizing the clinical trial process by identifying ideal patient cohorts, predicting trial outcomes, and improving trial design. This not only speeds up the trial process but also reduces costs, which is critical in the pharmaceutical market.
Challenges and Opportunities
Data Quality and Integration: While AI offers immense potential, one of the primary challenges remains the quality and integration of data. Pharmaceutical companies must ensure they have access to clean, structured data to fully leverage AI’s capabilities in drug discovery.
Regulatory Concerns: As AI becomes more integrated into drug discovery, regulatory agencies are working to establish frameworks to ensure the safety and efficacy of AI-driven drugs. Navigating these evolving regulatory landscapes will be crucial for pharmaceutical companies.
AI Talent Shortage: As the demand for AI in drug discovery grows, there is a shortage of skilled professionals who can build, implement, and manage these technologies. Addressing this talent gap is essential for sustained market growth.
Regional Insights
North America leads the market due to the presence of major pharmaceutical companies, strong healthcare infrastructure, and early adoption of AI technologies. The region’s advanced regulatory environment also supports the integration of AI in drug discovery.
Europe follows closely, driven by increased R&D funding and government support for AI initiatives in healthcare. Asia-Pacific is also expected to see rapid growth due to rising investments in AI and a growing pharmaceutical industry in countries like China and India.
Future Outlook
AI’s transformative impact on drug discovery is still in its early stages, but the potential is vast. As AI continues to evolve, it is expected to significantly reduce the cost and time associated with bringing new drugs to market, while also improving success rates in clinical trials. This will not only benefit pharmaceutical companies but also patients, who will gain faster access to innovative treatments.
In conclusion, AI is reshaping the future of the pharmaceutical industry by optimizing drug discovery processes, improving patient outcomes, and driving cost-efficiency. The next few years will be critical as AI’s role in drug discovery continues to expand, opening up new opportunities for innovation in the pharmaceutical market.
For a detailed overview and more insights, you can refer to the full market research report by Mordor Intelligence https://www.mordorintelligence.com/industry-reports/artificial-intelligence-in-pharmaceutical-market
#Artificial Intelligence (AI) In Pharmaceutical Market#Artificial Intelligence (AI) In Pharmaceutical Market Size#Artificial Intelligence (AI) In Pharmaceutical Market Share#Artificial Intelligence (AI) In Pharmaceutical Market Analysis#Artificial Intelligence (AI) In Pharmaceutical Market Trends#Artificial Intelligence (AI) In Pharmaceutical Market Report#Artificial Intelligence (AI) In Pharmaceutical Market Research#Artificial Intelligence (AI) In Pharmaceutical Industry#Artificial Intelligence (AI) In Pharmaceutical Industry Report
0 notes
Text

#Clinical Trial Outsourcing Market#Clinical Trial Outsourcing Size#Clinical Trial Outsourcing Growth#Clinical Trial Outsourcing Trend#Clinical Trial Outsourcing segment#Clinical Trial Outsourcing Opportunity#Clinical Trial Outsourcing Analysis 2024#Clinical Trial Outsourcing Forecast
0 notes
Text
Bone Cancer Drugs Market is driven by growing demand for targeted therapies
The global Bone Cancer Drugs market is primarily driven by the increasing prevalence of cancer including bone tumor or bone cancer. Bone cancer drugs help in treating cancer that starts in the bones, such as osteosarcoma or Ewing's sarcoma. Bone cancer drugs include molecular target drugs, chemotherapy and others that help inhibit or block the growth of cancer cells.
Bone cancer drugs are used for treating primary bone tumors and metastatic bone tumors. Primary bone tumors start within the bones whereas metastatic bone tumors spread to the bones from other parts of the body. Bone cancer drugs block the growth of cancer cells by interfering with cell division or inducing cell death. The increasing adoption of targeted therapies including monoclonal antibodies and small molecule inhibitors are helping treat various types of cancers more efficiently with lesser side effects. The growing prevalence of bone cancer coupled with availability of advanced targeted therapies are the major factors driving the growth of the global bone cancer drugs market.
The Global Bone Cancer Drugs Market is estimated to be valued at US$ 1423.67 Mn in 2024 and is expected to exhibit a CAGR of 5.2% over the forecast period 2024 to 2031.
Key Takeaways
Key players operating in the Bone Cancer Drugs market are Advaxis, Inc., Cellectar Biosciences, Inc., OPKO Health, Inc., Pfizer Inc., Amgen Inc., Novartis AG, Eli Lilly and Company, Debiopharm Group, Merck Co, Bayer AG, Bristol-Myers Squibb Company, Takeda Pharmaceutical, F. Hoffmann-La Roche Ltd and Teva Pharmaceutical. The demand for bone cancer drugs is increasing globally due to rising prevalence of primary bone tumors as well as bone metastasis. According to cancer research UK, around 2,300 new bone cancer cases are reported each year in the UK.
The increasing incidences of bone metastasis from cancers originating in breasts, lungs and prostate is a major factor driving the Bone Cancer Drugs Market Size. According to American Cancer Society, over 170,000 new cases of breast cancer are expected to be diagnosed in 2021 in the US, out of which approximately 40,000 will develop bone metastases. The global companies are expanding their presence into emerging markets of Asia Pacific, Middle East and Latin America to tap the growth opportunities in these regions.
Market key trends
The Bone Cancer Drugs market is witnessing trend of availability of personalized medicines. With advancement in understanding of genetics and specific mutations driving cancers, targeted therapies are being developed that can identify unique mutations in an individual patient's cancer and target them precisely. This enables delivering the right treatment to the right patient and optimizing treatment outcomes. Companies are investing heavily in development of novel targeted therapies and companion diagnostics that help deliver personalized cancer care. This trend is expected to significantly influence the bone cancer treatment landscape and support market growth over the forecast period.
Porter’s Analysis
Threat of new entrants: High research and development costs and regulatory barriers related to clinical trials make it difficult for new companies to enter the bone cancer drugs market.
Bargaining power of buyers: Patients have limited bargaining power due to serious nature of disease. However, availability of alternative treatment options provide some bargaining power.
Bargaining power of suppliers: Established drug manufacturers have significant bargaining power due to their large product portfolios and scarce manufacturing capabilities.
Threat of new substitutes: Alternatives like chemotherapy and radiation therapy impose a moderate threat as bone cancer drugs aim to target tumors specifically.
Competitive rivalry: Potential for high profits drives major pharmaceutical companies to intensely compete through intensive research and clinical trials.
Geographically, North America is currently dominating the bone cancer drugs market in terms of value owing to high prevalence, increasing healthcare expenditure and presence of major players. The market in Asia Pacific is projected to witness the fastest growth over the forecast period due to growing healthcare infrastructure, large patient pool and rising disposable income in developing countries.
Another major geographical region concentrated with bone cancer drugs market is Western Europe. Countries like Germany, United Kingdom and France collectively account for highest market share due to well-developed healthcare systems and availability of favorable reimbursement policies.
Get more insights on Bone Cancer Drugs Market
Discover the Report for More Insights, Tailored to Your Language
French
German
Italian
Russian
Japanese
Chinese
Korean
Portuguese
About Author:
Ravina Pandya, Content Writer, has a strong foothold in the market research industry. She specializes in writing well-researched articles from different industries, including food and beverages, information and technology, healthcare, chemical and materials, etc. (https://www.linkedin.com/in/ravina-pandya-1a3984191)

#Coherent Market Insights#Bone Cancer Drugs Market#Bone Cancer Drugs#Osteosarcoma Treatment#Bone Metastasis#Cancer Therapy#Chemotherapy#Targeted Therapy#Radiation Therapy#Bisphosphonates#Immunotherapy#Bone Tumors
0 notes
Text
Clinical Trial Imaging 2023 Industry Report Potential Growth, Share, Demand And Forecast to 2030
Clinical Trial Imaging Industry Overview
The global clinical trial imaging market size was estimated at USD 1.14 billion in 2023 and is projected to grow at a compound annual growth rate (CAGR) of 7.60% from 2024 to 2030. The market growth is anticipated to be fueled by the growing biotechnology and pharmaceutical sectors, coupled with rising investments in research and development for the creation of new drugs aimed at treating various diseases. Medical imaging plays a pivotal role in advancing the development of innovative life science products.
Gather more insights about the market drivers, restrains and growth of the Clinical Trial Imaging Market
Despite the ever-changing nature of the medical imaging industry, the biotechnology and pharmaceutical industries are showing sustained growth. This is primarily due to the increased investment in medical imaging companies, as well as the occurrence of mergers and acquisitions that involve the incorporation of cutting-edge imaging technologies to facilitate clinical trials for medical devices.
Advancements in technology are bringing substantial improvements to the collection, evaluation, and submission of clinical trial imaging data. Technology-enabled imaging, especially image analysis software, provides various benefits to clinical studies, such as consistency, data accuracy, adaptability, and compliance. For instance, image analysis software is used to direct and manage a reader by analyzing imaging time points. In addition, the increased use of imaging technology, along with the enhanced power of computing, is expected to drive the usage of imaging in clinical trials. The Quantitative Imaging Biomarkers Alliance (QIBA) protocol has come up with standardized methods and imaging procedures with uniform procedures to be implemented for attaining statistical and precise endpoints in clinical trials.
The Covid-19 pandemic has adversely impacted the healthcare system in most countries, leading to a disruption in medical studies, and research activities, and reduced sponsorship for research involving clinical trials. The pandemic hampered the clinical trial timeline as numerous ongoing studies were delayed and planned studies were cancelled. Unfavorable changes in regulations and guidelines, supply chain disruption, recruitment challenges for clinical trials, fear of viral spread, and shutting down of most manufacturers during lockdown have adversely impacted the market. However, introducing virtual imaging trials during the COVID-19 pandemic is expected to open new avenues for adopting these devices. The development of advanced computational models helps better assess CT and radiography images, which are expected to help in the early diagnosis of COVID-19 patients. The market has witnessed a bounce back by 2022 Q2 due to increased R&D activities and improvement in supply and distribution channels.
Many patents have been filed in the realm of enhancing image evaluation and capturing. In addition, imaging core lab provider’s offer patented technologies that are anticipated to assist pharmaceutical companies in reducing their development timelines. As an example, IXICO provides a diagnostic tool called Assessa, which enhances decision-making in clinical trials for conditions related to memory, including schizophrenia, Parkinson's, and Alzheimer's disease, as well as neurological disorders such as dementia and cognitive impairment.
However, the high cost of machinery and their installation, and the enormous cost of clinical trials may limit the market growth during the forecast periods. Advancements in technology are bringing substantial improvements to the collection, evaluation, and submit clinical trial imaging data. Technology-enabled imaging especially image analysis software provides various benefits to clinical studies such as consistency, data accuracy, adaptability as well as compliance. For instance, image analysis software is used to direct and manage a reader via analysis of imaging time points.
Browse through Grand View Research's Medical Devices Industry Research Reports.
• The global mobile stroke unit market size was valued at USD 35.80 million in 2023 and is projected to grow at a CAGR of 5.2% from 2024 to 2030. Rising incidence of strokes increased focus on early treatment of stroke patients are driving the demand for efficient and timely stroke care services.
• The global brain tumor diagnosis and therapeutics market size was valued at USD 3.11 billion in 2023 and is projected to grow at a CAGR of 7.1% from 2024 to 2030. The growing launches of brain tumor therapeutics products and the rise in cancer awareness for brain tumor medications drive the market over the forecast period.
Clinical Trial Imaging Market Segmentation
Grand View Research has segmented the clinical trial imaging market on the basis of on service, modality, application, end-use and region:
Clinical Trial Imaging Service Outlook (Revenue, USD Million, 2018 - 2030)
• Clinical Trial Design and Consultation Services
• Reading and Analytical Services
• Operational Imaging Services
• System and Technology Support Services
• Project and Data Management
Clinical Trial Imaging Modality Outlook (Revenue, USD Million, 2018 - 2030)
• Computed Tomography
• Magnetic Resonance Imaging
• X-Ray
• Ultrasound
• Optical Coherence Tomography (OCT)
• Others
Clinical Trial Imaging Application Outlook (Revenue, USD Million, 2018 - 2030)
• NASH
• CKD
• Diabetes
• Cardiovascular Diseases
• Ophthalmology
• Musculoskeletal
• Oncology
• Gastroenterology
• Pediatrics
• Others
Clinical Trial Imaging End-use Outlook (Revenue, USD Million, 2018 - 2030)
• Biotechnology and Pharmaceutical companies
• Medical Devices Manufacturers
• Academic and Government Research Institutes
• Contract Research Organizations (CROs)
• Others
Clinical Trial Imaging Regional Outlook (Revenue, USD Million, 2018 - 2030)
• North America
o U.S.
o Canada
• Europe
o UK
o Germany
o France
o Italy
o Spain
o Denmark
o Sweden
o Norway
• Asia Pacific
o India
o China
o Japan
o Australia
o Thailand
o South Korea
• Latin America
o Brazil
o Mexico
o Argentina
• Middle East & Africa
o South Africa
o Saudi Arabia
o UAE
o Kuwait
Order a free sample PDF of the Clinical Trial Imaging Market Intelligence Study, published by Grand View Research.
Key Companies profiled:
• IXICO plc
• Navitas Life Sciences
• Resonance Health
• ProScan Imaging
• Radiant Sage LLC
• Medpace
• Biomedical Systems Corp
• Cardiovascular Imaging Technologies
• Intrinsic Imaging
• BioTelemetry
Recent Developments
• In March 2023, Clario launched a cloud-based image viewer specifically for clinical trials. This innovation aims to streamline medical image analysis and improve its accessibility within the clinical research context
• In May 2023, Cleerly has partnered with ProScan Imaging to provide personalized solutions for cardiac health, which involve analyzing and devising treatment strategies for cardiovascular issues. The partnership is expected to leverage Cleerly's AI-powered platform to examine coronary CT angiography (CCTA) images
0 notes
Text
COVID-19 Pandemic Has Affected The Clinical Trial Equipment & Ancillary Solutions Market
The global clinical trial equipment & ancillary solutions market is expected to reach USD 5.09 billion by 2030, expanding at a CAGR of 8.1% over the forecast period according to a new report by Grand View Research, Inc. The growing pharmaceutical and medical sectors, globalization of clinical trials, and rising R&D expenditure are the factors driving the market. However, The COVID-19 pandemic has affected the global market, which resulted in slowing down the supply chain of the equipment & ancillary supplies. The COVID-19 pandemic has caused major changes in the clinical trial environment. Hundreds of experiments that were in progress before the pandemic was halted due to COVID-19 globally.
Gain deeper insights on the market and receive your free copy with TOC now @: Clinical Trial Equipment & Ancillary Solutions Market Report
The clinical trial procedure has evolved considerably in recent years. Complex clinical studies are creating new problems throughout the healthcare supply chain. Modern studies frequently involve huge numbers of patients and patient subgroups, as well as numerous nations and research sites. As a result, the number of challenges that supply chain managers confront while working with clinical trial equipment & ancillaries has increased. Renting medical equipment relieves the burden of storage, retrieval, and disposal. Another important decision-making reason is that renting equipment reduces significant upfront expenses and large investments required to furnish licensed clinical trial locations.
The pandemic has created a need for effective therapies and interventions. COVID-19 has changed the way clinical trials are conducted, encouraging the digitization of healthcare and the adoption of cutting-edge technologies and techniques. Such adoptions are likely to propel market growth. Moreover, the high R&D spending by the pharmaceutical company for developing innovative therapies for COVID-19 have further contributed to the market growth.
#Clinical Trial Equipment & Ancillary Solutions Market Size & Share#Clinical Trial Equipment & Ancillary Solutions Market Latest Trends#Clinical Trial Equipment & Ancillary Solutions Market Growth Forecast#COVID-19 Impacts On Clinical Trial Equipment & Ancillary Solutions Market#Global Clinical Trial Equipment & Ancillary Solutions Market
0 notes
Text
Seasonal Allergic Rhinitis Market Will Grow At Highest Pace Owing To Increasing Demand For Immunotherapy Treatment
The seasonal allergic rhinitis market comprises drugs, immunotherapy, and antihistamines used in the treatment of seasonal allergies, also known as hay fever. Allergic rhinitis is caused by an allergic reaction to airborne allergens such as pollen from trees, grass, flowers, and weeds. Common symptoms of seasonal allergic rhinitis include sneezing, nasal congestion, runny nose, and itchy, watery eyes. The demand for immunotherapy treatment, also known as allergy shots, is increasing as it provides long-term relief from allergies with minimal side effects as compared to medication.
The Seasonal Allergic Rhinitis Market is estimated to be valued at US$ 10.8 Bn in 2024 and is expected to exhibit a CAGR of 2.9% over the forecast period 2024-2031.
Key Takeaways
Key players operating in the seasonal allergic rhinitis market are Regeneron Pharmaceuticals, Revolo Biotherapeutics, Allergy Therapeutics, Emergo Therapeutics, ALKAbello. Regeneron Pharmaceuticals dominates the market with its successful drug Dupixent which treats both seasonal allergic rhinitis and other allergic conditions.
The seasonal allergic rhinitis market is witnessing high demand due to the growing prevalence of allergic rhinitis worldwide. According to a study by the American Academy of Allergy Asthma & Immunology, more than 50 million Americans suffer from allergies every year with seasonal allergic rhinitis affecting 20% of the global population.
Technological advancements are being made in allergy immunotherapy which involves exposing patients to gradually increasing doses of allergen extracts to boost immunity. New advanced therapies like sublingual and subcutaneous immunotherapy are gaining popularity due to easy mode of administration and higher efficacy.
Market Trends
The increased adoption of combination therapies using corticosteroids with antihistamines for treating moderate to severe symptoms is a key trend in the seasonal allergic rhinitis market. This provides better relief than single therapy. Another trend is the growing preference for generic drugs due to their lower cost compared to branded drugs.
Market Opportunities
Rising pollution levels have made seasonal allergies more severe creating opportunities for seasonal allergic rhinitis drugs. Over-the-counter remedies and nasal sprays are expected to witness high demand owing to convenience for self-treatment of mild symptoms.
Impact Of COVID-19 On Seasonal Allergic Rhinitis Market Growth
The COVID-19 pandemic has impacted the seasonal allergic rhinitis market in ways. The supply chain disruptions led to shortages in antihistamine nasal spray supplies causing patients difficulties. The lockdowns and social distancing measures reduced outdoor activities which provided temporary relief to seasonal allergy patients. However, it also delayed in-person doctor consultations and allergy testing limiting appropriate diagnoses and treatment plans. Telehealth emerged as an important tool for doctors to remotely monitor patients and adjust medications.
The pandemic shifted priorities of drug makers away from new product development and clinical trials towards vaccines and antiviral drugs. This slowed new seasonal allergy drug approvals. Post pandemic, the growth is expected to rebound faster in developing countries as healthcare budgets recover and access to treatment improves. Meanwhile, developed regions may see a moderate growth due to preference for online consultations, home-based allergy management devices and expectation of new innovations that provide lasting relief.
To sustain the market potential, companies need strategies addressing supply chain resilience, funding of new therapies and leveraging digital platforms. Collaborations with telehealth providers will help expand access while monitoring pandemics' long term impact on seasonal patterns is important for production planning.
Geography: Europe
Europe accounts for the largest share of the seasonal allergic rhinitis market, both in terms of value and volume. This is attributed to high per capita healthcare spending, advanced medical infrastructure and greater awareness about allergy diagnosis and management. Countries such as Germany, United Kingdom and France have a major market presence due to large patient pools and strong reimbursement structures supporting quality care.
The market is also rapidly growing in Central and Eastern European nations as healthcare reforms attract international pharmaceutical investments. Rising environmental pollution and allergen exposures in developing cities are contributing to higher disease incidence. Overall, Europe will continue dominating the seasonal allergic rhinitis space backed by strong research environments discovering novel therapeutic targets.
Get more insights on this topic: https://www.trendingwebwire.com/seasonal-allergic-rhinitis-market-is-estimated-to-witness-high-growth-owing-to-emerging-immunotherapies/
Author Bio:
Alice Mutum is a seasoned senior content editor at Coherent Market Insights, leveraging extensive expertise gained from her previous role as a content writer. With seven years in content development, Alice masterfully employs SEO best practices and cutting-edge digital marketing strategies to craft high-ranking, impactful content. As an editor, she meticulously ensures flawless grammar and punctuation, precise data accuracy, and perfect alignment with audience needs in every research report. Alice's dedication to excellence and her strategic approach to content make her an invaluable asset in the world of market insights. (LinkedIn: www.linkedin.com/in/alice-mutum-3b247b137 )
What Are The Key Data Covered In This Seasonal Allergic Rhinitis Market Report?
:- Market CAGR throughout the predicted period
:- Comprehensive information on the aspects that will drive the Seasonal Allergic Rhinitis Market's growth between 2024 and 2031.
:- Accurate calculation of the size of the Seasonal Allergic Rhinitis Market and its contribution to the market, with emphasis on the parent market
:- Realistic forecasts of future trends and changes in consumer behaviour
:- Seasonal Allergic Rhinitis Market Industry Growth in North America, APAC, Europe, South America, the Middle East, and Africa
:- A complete examination of the market's competitive landscape, as well as extensive information on vendors
:- Detailed examination of the factors that will impede the expansion of Seasonal Allergic Rhinitis Market vendors
FAQ’s
Q.1 What are the main factors influencing the Seasonal Allergic Rhinitis Market?
Q.2 Which companies are the major sources in this industry?
Q.3 What are the market’s opportunities, risks, and general structure?
Q.4 Which of the top Seasonal Allergic Rhinitis Market companies compare in terms of sales, revenue, and prices?
Q.5 Which businesses serve as the Seasonal Allergic Rhinitis Market’s distributors, traders, and dealers?
Q.6 How are market types and applications and deals, revenue, and value explored?
Q.7 What does a business area’s assessment of agreements, income, and value implicate?
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it
#Seasonal Allergic Rhinitis Market Trend#Seasonal Allergic Rhinitis Market Size#Seasonal Allergic Rhinitis Market Information#Seasonal Allergic Rhinitis Market Analysis#Seasonal Allergic Rhinitis Market Demand
0 notes