#Drug Delivery Technology Market
Text
The Controlled-Release Drug Delivery Technology Market in 2023 is US$ 54.81 billion, and is expected to reach US$ 124.69 billion by 2031 at a CAGR of 10.80%.
#Controlled-Release Drug Delivery Technology Market#Controlled-Release Drug Delivery Technology Market Growth#Controlled-Release Drug Delivery Technology Market Overview
0 notes
Text
Long Acting Drug Delivery Technologies and Services Market Report
Long Acting Drug Delivery technologies aim to prolong the duration of drug action within the body, allowing for less frequent dosing. This extension of therapeutic effect is particularly advantageous in the treatment of chronic conditions, where sustained release of medication can contribute to better disease management.
Traditional drug delivery methods often require patients to adhere to strict dosing schedules, leading to challenges in compliance. Long-acting formulations address this issue by reducing the frequency of administration, thereby improving patient adherence. This, in turn, has a positive impact on treatment efficacy and patient outcomes.
As of 2022, the global long-acting drug delivery technologies and services market held a value of $11.41 billion. The market is expected to grow at a CAGR of 11.31% during the forecast period 2023-2033 and attain a value of $36.82 billion by 2033.
Understanding Long Acting Drug Delivery Technologies:
Long Acting Drug Delivery Technologies refer to innovative methods of administering pharmaceuticals that extend the duration of drug action within the body, thereby reducing the frequency of dosing. These technologies offer several advantages, such as enhanced therapeutic efficacy, minimized side effects, and improved patient convenience.
Key Drivers of Market Growth:
Rising Chronic Diseases: The global burden of chronic diseases, such as diabetes, cardiovascular diseases, and neurodegenerative disorders, is on the rise. Long-acting drug delivery systems provide a convenient and effective way to manage these conditions, ensuring a sustained release of medication over an extended period.
Patient Compliance: Traditional drug delivery methods often require frequent dosing, leading to poor patient adherence. Long-acting formulations address this challenge by reducing the frequency of administration, promoting better adherence, and ultimately improving treatment outcomes.
Technological Innovations: Advances in drug delivery technologies, including biodegradable polymers, implantable devices, and nanotechnology, are driving the development of long-acting formulations. These innovations offer precise control over drug release kinetics, allowing for tailored therapeutic solutions.
Cost-effectiveness: While initial development costs may be higher for long-acting drug delivery systems, the potential cost savings in terms of reduced hospital visits, lower medication waste, and improved patient outcomes contribute to their economic viability in the long run.
Market Segmentation:
The Long Acting Drug Delivery Technologies and Services market can be segmented based on technology, application, and end-user.
Technology:
Implantable Devices
Polymeric Microparticles
Liposomal Drug Delivery
Nanoparticle-based Drug Delivery
Injectable Depot Formulations
Application:
Diabetes
Cardiovascular Diseases
Oncology
Neurological Disorders
Autoimmune Diseases
End-user:
Hospitals & Clinics
Ambulatory Surgical Centers
Research Institutes
Download the sample and understand better @ Long Acting Drug Delivery Technologies and Services Market Report
Challenges and Opportunities:
While the Long Acting Drug Delivery Technologies and Services market holds immense potential, it is not without challenges. Regulatory hurdles, complex formulation development, and concerns related to the safety and efficacy of long-acting formulations remain areas of focus. However, these challenges present opportunities for collaboration between pharmaceutical companies, research institutions, and regulatory bodies to address these concerns and propel the market forward.
Visit our precision medicine vertical page and grab more knowledge about the same
Future Outlook:
The future of the Long Acting Drug Delivery Technologies and Services market appears promising, with ongoing research and development efforts aimed at expanding the range of therapeutic areas and improving delivery mechanisms. As healthcare continues to evolve, the integration of these technologies into personalized medicine approaches is likely to reshape the treatment landscape.
Conclusion:
The Long Acting Drug Delivery Technologies and Services market is witnessing a transformative phase, offering innovative solutions to address the evolving needs of patients and healthcare providers. With a focus on improving treatment adherence, reducing healthcare costs, and enhancing therapeutic outcomes, these technologies are poised to play a pivotal role in the future of healthcare delivery. As the market continues to grow, collaboration and investment in research and development will be key drivers of success, ensuring that long-acting drug delivery technologies remain at the forefront of medical innovation.
#Long Acting Drug Delivery Technology and Services Market#Long Acting Drug Delivery Technology and Services Market Report#Long Acting Drug Delivery Technology and Services Industry .#Long Acting Drug Delivery Technology and Services Market Trends#Long Acting Drug Delivery Technologies and Services Key Players
0 notes
Text
0 notes
Text
The global Drug Delivery Technology market research gives a detailed and practical analysis of the products and services in this market which provides a competitive advantage to the existing and new businesses. In depth study and overview of the market has been collected by the overall insight of the industry and specifies the market segmentation, potential opportunities, growing market trends and events, current and future advancements, and other elements. This report will also showcase many possibilities of upscaling the global Drug Delivery Technology market share size.
0 notes
Text
A novel radiation treatment for cancer with a 100-percent success rate in its pilot trial is now in Phase 3 pivotal trials ahead of receiving Food and Drug Administration (FDA) approval.
Jerusalem-based startup Alpha TAU is expanding its trials of the treatment for skin and other cancer, after its first trial of 10 patients succeeded beyond the company’s expectations.

“Those patients got 100 percent CR [complete response],” Sofer says.
The pilot trial, conducted at multiple locations in the US last year, examined whether Alpha TAU’s DaRT (Diffusing Alpha-emitters Radiation Therapy) technology could successfully deliver targeted radiation therapy to patients with malignant skin and superficial soft tissue tumors that had returned or could not be removed surgically.
Alpha TAU had hoped that the treatment would be successful in at least seven of the 10 trial participants, but instead registered successful delivery to all 10. CT scans showed a 100 percent complete response rate at 12 weeks after the treatment and again at 24 weeks, with no evidence of the disease recurring in any of the subjects.

The results showed only mild or moderate side effects related to the device, and no systemic toxicity from it.
Radiation therapy for cancer normally uses beta and gamma particles. Alpha particles, while proving deadly for cancer cells in a tumor, are not traditionally used as they cannot travel far in solid masses.
Alpha DaRT, however, delivers the alpha particles directly into the tumor via a narrow device, inserted under local anesthetic, for a period of two to three weeks. The device is then removed and the patient monitored.
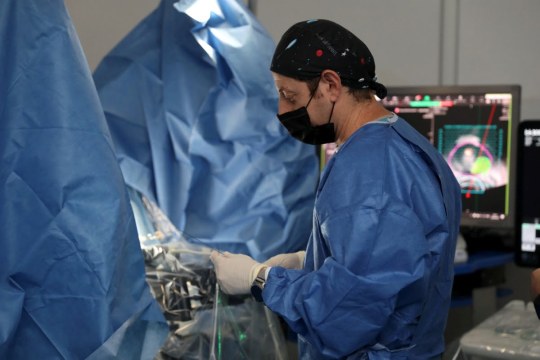
The findings of the pilot trial were published this month in the Journal of the American Medical Association (JAMA), months after submitting the results to the FDA.
The treatment is now undergoing its pivotal trial – the final one before the American agency gives it approval.
“We submitted the results that you see now to the FDA, and the FDA told us that we can submit the protocol for the last phase, the pivotal,” says Sofer.
A pivotal trial is required by the US and European Union drug agencies in order to receive approval to market a new form of medication; studies can involve thousands of subjects and test the efficacy and impact of a drug.
Sofer says that the successful findings of the trial has led to medical institutes around the world clamoring to work with Alpha TAU, but for now research is limited to just a handful of locations for the pivotal trial.
“Many, many, many centers all around the world want to participate,” he says. “We are working with 20 centers in the US, two or three centers in Canada and another four in Israel that are going to participate in this trial.”
youtube
Sofer says Alpha TAU will be ready to submit the findings of the pivotal trial in around a year and a half from now.
“We will have six months of follow up, then we will analyze the results and send it to the FDA,” he says of the current trial. “The submission to the FDA can be in about 18 months from now.”
The revolutionary treatment is also being tried on other cancers, according to Sofer, who clarifies that, “right now it’s only for solid tumors.”
“We’re working on pancreas and lung and breast [cancer],” he says, explaining that the company is currently at various stages of testing for these other forms of malignant tumors.

The device itself is easy to use and does not require specialized and often costly equipment in order to treat patients.
“When it is approved, it will be for any hospital, medical cancer center, all over the world,” Sofer explains.
“You don’t need any special equipment, and you don’t need the shielding,” he says, referring to the protective gear used in other forms of radiation therapy but are not needed for alpha particles.
“It will be very simple to implement. You don’t need special equipment or investment in capital expenditure or something like that, [just] regular tools.”
69 notes
·
View notes
Text

Government of Canada orders 4 new Airbus A330 MRTTs
Diego Alves By Diego Alves 07/26/2023 - 12:00m Military
In an effort to strengthen its continental defense capabilities, the government of Canada granted Airbus Defence and Space a contract for four newly built Airbus A330 Multi Role Tanker Transport (MRTT) aircraft and the conversion of five used A330-200s.
With an order value of approximately CAD $3 billion, this Strategic Air Transport and Refueling Capacity (STTC) initiative aims to replace the former CC-150 Polaris fleet (A310 MRTT) currently operated by the Royal Canadian Air Force (RCAF).
The A330 MRTT was considered the appropriate solution to Canada's requirements to protect its sovereignty and improve operations within the North American Aerospace Defense Command (NORAD) and NATO. “As the most advanced multi-mission tanker in the world, the A330 MRTT perfectly meets the needs of Canada,” said Mike Schoellhorn, executive director of Airbus Defence and Space. The aircraft's superior technological capabilities, combined with its interoperability with other allied client nations of the A330 MRTT, position it ahead of global competition.
The new A330-200 fleet will be set up in Toulouse, France, and will undergo a conversion at the A330 MRTT facility in Getafe, Spain, from 2025. The first MRTT is scheduled for delivery to the RCAF in 2027. The A330 MRTTs will be equipped with refueling options via hoses, drugs and booms, along with cybersecurity solutions and countermeasures. In addition, the aircraft will have the Airbus Medical Evacuation kit solution, with two Intensive Care Units and additional stretchers.

The contract includes advanced training services, featuring the Full Flight Simulator, to ensure crew readiness and modernize the air operational training infrastructure of the Canadian Armed Forces. Airbus was selected as the only qualified supplier for the replacement of the C-150 refuel after an acquisition process opened in April 2021.
With 76 requests from 15 customers and proven combat experience in theaters such as the Middle East and the Eastern Flank in Europe, the A330 MRTT has a market share of 90% outside the US and more than 270,000 flight hours. The history of interoperability, mission success and high availability rates of this mature platform highlights its remarkable performance and makes it the ideal choice for Canada's strategic defense needs.
Tags: A330 MRTTairbusMilitary AviationRCAF - Royal Canadian Air Force/Canada Air Force
Sharing
tweet
Diego Alves
Diego Alves
Related news
MILITARY
Concerns about the numbers of the RAF in the North Atlantic
26/07/2023 - 17:00
MILITARY
Poland buys Swedish early warning aircraft for $58 million
26/07/2023 - 13:00
COMMERCIAL
P&W says that "significant" number of engines of the A320neo family will have to be inspected "immediately"
25/07/2023 - 11:00
MILITARY
Delays in the delivery of the F-35 will cost Lockheed hundreds of millions in 2023
25/07/2023 - 09:00
MILITARY
Argentine Air Force awaits a "superior" proposal to choose F-16
24/07/2023 - 15:00
MILITARY
Indian Air Force considers A-400M, C-130J and C-390 to replace the average transport fleet
4 notes
·
View notes
Text

How Can Blockchain Revolutionize the Future of Healthcare and Pharmaceuticals?
Blockchain technology is changing many domains, and health care is no different. In this sector, the data security issues, inefficiencies, and skyrocketing costs bring a decentralized, transparent platform with blockchain. This blog shall focus on how blockchain technology is changing healthcare-expecially in pharmaceuticals-and companies like Justtry Technologies, which are helping unlock the potential of blockchain in this sector.
Why Blockchain in Health Care?
Blockchain makes use of distributed data and is, therefore, decentralized in that it has no central entity controlling the data. This can be invaluable in the healthcare industry, with sensitive patient information and, indeed, clinical trial data that must be kept secure. Blockchain does this by keeping healthcare data tamper-proof, thus instilling trust and security into the whole system.
Some major benefits of blockchain in health care are as follows:
More Security: Blockchain guards against cyberattacks and unauthorized access to the patient's information; therefore, health data remains private and secure.
Transparency in Data Sharing: Blockchain provides a hassle-free and reliable sharing of a patient's records with hospitals, doctors, and insurance companies.
It Prevents Fraud: Blockchain does not allow alterations in clinical trial data, and hence the integrity of research is kept intact.
Operational Cost Efficiency: It spares the healthcare providers from operational costs mainly because it saves healthcare providers from the hassle of going through intermediaries and automates administrative tasks.
At Justtry Technologies, we help healthcare organizations integrate blockchain solutions that provide data security, transparency, and operational efficiency.
How Blockchain is Applied in Healthcare?
Blockchain is being used in various critical areas of healthcare:
Patient Records: Blockchain allows patients who are choosing to share their medical histories securely to choose who can access the documents. This increases data privacy because the patients' personal information is kept anonymous. It also facilitates easy transfer of information among different healthcare providers.
One of the biggest hurdles in pharma is drug authenticity. Blockchain allows the validation of the entire journey of the medicine - its creation to its delivery - to restrict entrance of counterfeits in the market.
Applications in Clinical Trials: Blockchain makes sure the clinical trial data is held immutable with active transparency. Trust in the process is built because no one can manipulate or alter the data as part of clinical trials.
Blockchain in Medicine: Solving the Key Pharma Challenges
The pharmaceutical company is presently being faced by quite a few challenges, including fake drugs, strict regulatory compliance, and a long drug development process. Blockchain offers solutions to such problems, catering to safe data management and real-time insights.
A number of key applications related to blockchain within the pharmaceuticals include:
Drug Traceability: Using blockchain, a pharmaceutical company can trace their drugs all along the supply chain, meaning that with blockchain assurance, they know that their drugs are not tampered with, meaning that they, indeed, meet regulatory standards.
Personalized Medication: Blockchain sees to it that data is conveyed between parties, hence allowing for more accurate and bespoke treatments considering patient information.
Clinical Trial Data Integrity: Blockchain means that clinical trial data is secure and transparent. This helps in ensuring regulation compliance as well as safeguarding research integrity.
Justtry Technologies is integrating blockchain technology to help pharmaceutical companies improve security levels, efficiency, and speed in the entire development of drugs.
Blockchain Health Data: Security and Compliance
Healthcare organizations still face threats regarding security over health data; blockchain offers the best solution by giving decentralized, transparent, and immutable data storage. This not only enhances security but also assists in compliance to regulations on data privacy.
Blockchain allows healthcare organizations to ensure:
Secure Data Storage: Patient records are stored in an immutable format that cannot be altered to ensure data integrity.
Regulatory Compliance: Blockchain provides an easy, auditable trail of how the data accessed is utilized, thus significantly aiding such regulations as HIPAA in terms of compliance.
Trust and Transparency: Patients, healthcare providers, and regulators will trust that the data being put in blockchain is accurate and not tampered.
Conclusion: Future of Healthcare with Blockchain
Blockchain is unlocking the future of healthcare, some of the very tough challenges that the industry faces. From safeguarding patient data to improving the speed of the pharmaceutical supply chain, blockchain stands as the game changer. At its core lies the exciting innovative measure of stand-alone leader Justtry Technologies in revolutionizing the use of blockchain solutions tackling innovation and improvement in health care and pharmaceutical.
#blockchain in health sector#blockchain development services#blockchain development#blockchaintechnology#blockchain technologies#blockchain in medicine#blockchain in healthcare#blockchain development companies#blockchain development company in US
0 notes
Text
Polymer-Based Prefilled Syringe Market: Current Analysis and Forecast (2024-2032)
According to the UnivDatos Market Insights analysis, the increasing incidence of chronic conditions such as diabetes and rheumatoid arthritis, patient preference for self-administration, and reduced risk of contamination & needlestick injuries compared to traditional syringes. will drive the global scenario of the Polymer-Based Prefilled Syringe market. As per their “Polymer-Based Prefilled Syringe Market” report, the global market was valued at USD 2.87 Billion in 2023, growing at a CAGR of about 4.9% during the forecast period from 2024 – 2032.
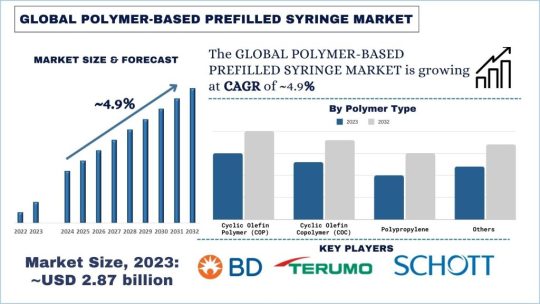
Over the years, there has been a shift of focus in the healthcare sector to develop new and advanced drug delivery systems, and prefilled syringes made of polymers have gained a lot of popularity among pharmaceutical organizations and healthcare centers. Such syringes produced from the highest quality polymers have several advantages over the traditional glass ones: safety, convenience, and a comparatively low price.
Demand
Several factors have led to the high demand for polymer-based prefilled syringes as explained below. Moreover, a trend toward the use of long-lasting drugs, which in turn requires frequent, accurate dosing due to such diseases as diabetes, rheumatoid arthritis, or cancer. Prefilled syringes provide a comfortable and safe way to administer injections to patients who may require multiple injections, hence enhancing the patient’s compliance with treatment and overall positive health impacts.
Applications
Polymer-based prefilled syringes are widely employed across different diseases such as immunology, oncology, and neurology among others. When integrated with the right technologies, they are ideal for dispensing biologic drugs, vaccines, and biosimilar medications, which require accurate dosing and maintaining drug integrity. These syringes are also being employed for administration and this helps the patients to administer their medication at home comfortably.
Access sample report (including graphs, charts, and figures): https://univdatos.com/get-a-free-sample-form-php/?product_id=61521
Factors Influencing the Cost of Syringe
The steps followed in making prefilled syringes from polymer materials include the selection of polymers, injection moulding, and final assembling. Polymeric materials, like cyclic olefin copolymer (COC), cyclic olefin polymer (COP), or their co-polymers, are preferable due to their high chemical and physical stability, and biocompatibility with most drugs. The syringes are then appropriately filled with the right medication and closed in a way that makes them ready for use; they are then sterilized using processes that have been through validation to ensure the product is safe and effective.
Manufacturing
Polymer selection, injection moulding, and subsequent assembly are typical phases of manufacturing pre-filled syringes from polymers. The materials are chosen for their good resistance to chemicals as well as compatibility with most of the drugs; COC or COP are preferred. The syringes are then prepped to contain the right dosage of medicine, closed, and autoclaved in a way that has been certified to be safe for patient use.
Conclusion
Consequently, the prefilled polymer syringes are a breakthrough in the polymer technology of drug delivery systems having the following advantages over glass syringes. Their safety, convenience, and relatively cheaper price make the device an attractive tool for pharma and healthcare providers as they seek better ways of attending to patients and cutting costs. With the increasing trend in technology later in the future, the features of polymer-based prefilled syringes can be developed to enhance the growth and use in the healthcare system.
Contact Us:
UnivDatos Market Insights
Email - [email protected]
Contact Number - +1 9782263411
Website -www.univdatos.com
0 notes
Text
Top 7 Growth Drivers of the Drug Delivery Devices Market By 2030

Introduction:
The drug delivery devices market is experiencing a significant surge, driven by various factors that are revolutionizing the way medications are administered and enhancing patient outcomes. By 2030, the market is expected to witness unprecedented growth, spurred by advancements in technology, increasing prevalence of chronic diseases, and a shift towards personalized medicine. Here, we delve into the top seven growth drivers that are shaping the future of the drug delivery devices market.
Download Free Sample: https://www.nextmsc.com/drug-delivery-devices-market/request-sample
1. Technological Innovations and Advancements
Technological innovation is at the forefront of the drug delivery devices market's growth. The development of advanced drug delivery systems such as micro-needles, smart pills, and implantable devices is transforming the landscape. These technologies offer improved precision in drug administration, reduce the frequency of doses, and enhance patient compliance. For instance, smart pills equipped with sensors can track medication adherence and provide real-time data to healthcare providers, ensuring effective treatment outcomes. Moreover, the integration of artificial intelligence (AI) and machine learning (ML) in drug delivery devices is enabling personalized treatment regimens, further driving market growth.
2. Rising Prevalence of Chronic Diseases
The increasing prevalence of chronic diseases such as diabetes, cancer, and cardiovascular diseases is a significant growth driver for the drug delivery devices market. Chronic diseases often require long-term treatment and management, necessitating the development of efficient drug delivery systems. For example, insulin pumps for diabetes management and transdermal patches for pain relief in cancer patients are gaining traction. The growing burden of chronic diseases is prompting healthcare providers to adopt innovative drug delivery solutions that enhance patient convenience and adherence to treatment protocols.
3. Growing Geriatric Population
The global population is aging, leading to a higher incidence of age-related diseases and conditions that require regular medication. The elderly population often faces challenges with traditional drug administration methods, such as difficulties in swallowing pills or remembering to take medications. Drug delivery devices that offer ease of use, such as inhalers, nasal sprays, and transdermal patches, are becoming increasingly popular among the geriatric population. This demographic shift is expected to significantly boost the demand for advanced drug delivery devices in the coming years.
Inquire Before Buying: https://www.nextmsc.com/drug-delivery-devices-market/inquire-before-buying
4. Shift Towards Home Healthcare
There is a growing trend towards home healthcare, driven by the need to reduce healthcare costs and the preference for personalized care. Drug delivery devices that can be used in home settings, such as auto-injectors, wearable infusion pumps, and self-administrable inhalers, are witnessing increased adoption. Home healthcare allows patients to manage their conditions more conveniently and reduces the burden on healthcare facilities. This shift is fostering the development and uptake of user-friendly drug delivery devices that empower patients to take control of their treatment.
5. Increased Focus on Biologics and Biosimilars
The pharmaceutical industry's focus is increasingly shifting towards biologics and biosimilars, which require specialized drug delivery systems. Biologics, derived from living organisms, are used to treat a wide range of conditions, including autoimmune diseases and cancers. These complex molecules necessitate innovative delivery methods to ensure stability and efficacy. Drug delivery devices such as pre-filled syringes, autoinjectors, and pen injectors are specifically designed to administer biologics effectively. The growing pipeline of biologics and biosimilars is expected to drive the demand for advanced drug delivery devices.
6. Regulatory Approvals and Reimbursements
Regulatory approvals and favorable reimbursement policies play a crucial role in the growth of the drug delivery devices market. Regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) are increasingly approving innovative drug delivery systems, recognizing their potential to improve patient outcomes. Additionally, reimbursement policies that cover the costs of advanced drug delivery devices encourage their adoption among healthcare providers and patients. These regulatory and financial incentives are critical drivers of market expansion.
7. Strategic Collaborations and Partnerships
Strategic collaborations and partnerships between pharmaceutical companies, technology providers, and healthcare institutions are accelerating the development and commercialization of advanced drug delivery devices. These collaborations enable the pooling of resources, expertise, and technologies, leading to the creation of innovative solutions that address unmet medical needs. For instance, partnerships between drug manufacturers and device developers can result in the co-development of combination products, such as drug-eluting stents and implantable infusion pumps. Such synergistic efforts are propelling the growth of the drug delivery devices market.
Conclusion
The drug delivery devices market is poised for significant growth by 2030, driven by technological advancements, the rising prevalence of chronic diseases, an aging population, the shift towards home healthcare, the focus on biologics and biosimilars, regulatory approvals, and strategic collaborations. As the market evolves, it will continue to revolutionize the administration of medications, enhancing patient outcomes and transforming the healthcare landscape. Stakeholders in the healthcare ecosystem, including pharmaceutical companies, device manufacturers, and healthcare providers, must stay abreast of these growth drivers to capitalize on the emerging opportunities in the drug delivery devices market.
0 notes
Text
https://rollbol.com/blogs/1831095/Controlled-Release-Drug-Delivery-Technology-Market-Size-Overview-Share-and
The Controlled-Release Drug Delivery Technology Market in 2023 is US$ 54.81 billion, and is expected to reach US$ 124.69 billion by 2031 at a CAGR of 10.80%.
#Controlled-Release Drug Delivery Technology Market#Controlled-Release Drug Delivery Technology Market Forecast#Controlled-Release Drug Delivery Technology Market Scope
0 notes
Text
Zirconia Nanoparticles Market Analysis: Size, Share, and Competitive Landscape 2024-2034
Zirconia nanoparticles, also known as zirconium dioxide nanoparticles (ZrO2), have gained widespread attention in various industries due to their exceptional mechanical, thermal, and chemical properties. These nanoparticles are highly resistant to heat and corrosion, which makes them useful in applications such as ceramics, coatings, biomedical materials, catalysts, and electronics. The growing demand for advanced materials in multiple sectors is driving the zirconia nanoparticles market. As industries increasingly look to enhance performance and durability, the role of zirconia nanoparticles has become crucial in meeting these objectives.
The global zirconia nanoparticles industry, valued at US$ 124.6 million in 2022, is projected to grow at a CAGR of 5.0% from 2023 to 2031, reaching US$ 193.3 million by the end of 2031.
Advancements in medical technology and surge in awareness about the potential benefits of zirconia nanoparticles are likely to offer lucrative opportunities to players in the global zirconia nanoparticles industry. Rise in government funding for R&D in zirconia nanoparticles is also anticipated to contribute to the zirconia nanoparticles market growth in the near future.
For More Details, Request for a Sample of this Research Report: https://www.transparencymarketresearch.com/zirconia-nanoparticles-market.html
Market Segmentation
The zirconia nanoparticles market can be segmented based on various parameters:
By Service Type: The market can be classified into customized nanoparticle solutions and standard nanoparticle solutions.
By Sourcing Type: This includes primary production and secondary sourcing. Primary production refers to companies that manufacture nanoparticles, while secondary sourcing includes companies that procure and use these materials.
By Application: The applications of zirconia nanoparticles are wide-ranging, including ceramics, electronics, biomedical applications (e.g., dental implants and drug delivery systems), coatings, and catalysts.
By Industry Vertical: The key industry verticals utilizing zirconia nanoparticles include healthcare, automotive, aerospace, electronics, and manufacturing.
By Region: The geographical segmentation includes North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa.
Regional Analysis
North America: The North American market is expected to dominate in terms of revenue, with a strong focus on healthcare and electronics applications. The presence of advanced industries and research institutions is fostering growth in this region.
Europe: Europe is another key region, driven by increasing demand for zirconia nanoparticles in automotive and biomedical applications. Germany, the UK, and France are leading contributors to market growth.
Asia Pacific: Asia Pacific is anticipated to witness the fastest growth, particularly in countries like China, Japan, and South Korea, where the electronics and manufacturing sectors are booming.
Latin America and Middle East & Africa: These regions are emerging markets with growing industrial applications for zirconia nanoparticles, particularly in healthcare and energy.
Market Drivers and Challenges
Drivers:
Increasing demand for advanced materials in healthcare and electronics.
Growth in the automotive and aerospace sectors, driving the need for high-performance materials.
Rising investment in nanotechnology research and development.
Challenges:
High production costs associated with zirconia nanoparticles.
Regulatory challenges concerning the environmental and health impacts of nanoparticles.
Competition from alternative materials in some applications.
Market Trends
The market is seeing increased focus on the miniaturization of electronic components, where zirconia nanoparticles offer superior performance in terms of durability and conductivity.
In the biomedical field, zirconia nanoparticles are gaining traction due to their biocompatibility and use in dental implants and drug delivery systems.
Green manufacturing practices and sustainability efforts are becoming increasingly important, with companies looking to reduce the environmental impact of nanoparticle production.
Future Outlook
The future of the zirconia nanoparticles market looks promising, with continued growth driven by innovation and expanding applications in emerging industries. As the demand for high-performance materials rises, especially in sectors like electronics and healthcare, zirconia nanoparticles will play a pivotal role. Furthermore, advancements in nanotechnology and sustainable production practices are likely to create new opportunities and market avenues.
Key Market Study Points
The growing application of zirconia nanoparticles in electronics, healthcare, and energy sectors.
R&D activities focused on enhancing the properties and applications of zirconia nanoparticles.
The role of regulations and standards in shaping the market landscape, particularly concerning environmental and safety issues.
Cost challenges associated with production and commercialization.
Buy this Premium Research Report: https://www.transparencymarketresearch.com/checkout.php?rep_id=85690<ype=S
Competitive Landscape
The zirconia nanoparticles market is highly competitive, with several key players operating globally. Some of the leading companies include:
Tosoh Corporation
Saint-Gobain
Showa Denko K.K.
Nanostructured & Amorphous Materials, Inc.
American Elements
Advanced Nano Products Co., Ltd.
These companies focus on continuous innovation, strategic partnerships, and expansion to maintain a competitive edge. They are investing heavily in research and development to improve nanoparticle properties and explore new applications.
Recent Developments
Tosoh Corporation has recently launched a new line of zirconia nanoparticles aimed at the dental and medical device industries.
Nanostructured & Amorphous Materials, Inc. announced plans to expand its production capabilities to meet the growing demand in the electronics sector.
Showa Denko K.K. is working on reducing the environmental impact of zirconia nanoparticle production by adopting green manufacturing practices.
About Transparency Market Research
Transparency Market Research, a global market research company registered at Wilmington, Delaware, United States, provides custom research and consulting services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insights for thousands of decision makers. Our experienced team of Analysts, Researchers, and Consultants use proprietary data sources and various tools & techniques to gather and analyses information.
Our data repository is continuously updated and revised by a team of research experts, so that it always reflects the latest trends and information. With a broad research and analysis capability, Transparency Market Research employs rigorous primary and secondary research techniques in developing distinctive data sets and research material for business reports.
Contact:
Transparency Market Research Inc.
CORPORATE HEADQUARTER DOWNTOWN,
1000 N. West Street,
Suite 1200, Wilmington, Delaware 19801 USA
Tel: +1-518-618-1030
USA – Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com
0 notes
Text
Viral Sensitizers Market Overview: Key Players and Strategies

Introduction to Viral Sensitizers MarketThe Viral Sensitizers Market is experiencing rapid growth due to the rising incidence of viral infections and the demand for effective treatments. Viral sensitizers enhance the efficacy of antiviral agents, making them crucial in managing diseases such as influenza and COVID-19. Key drivers include technological advancements in drug development, increased government funding for research, and heightened awareness of viral threats. However, challenges such as high R&D costs, regulatory hurdles, and intense market competition persist. Opportunities lie in emerging markets, combination therapies, and collaborations between biotech firms and research institutions. Overall, the market is poised for significant advancements and increased adoption in therapeutic applications.The Viral Sensitizers Market is Valued USD 4.8 Million by 2024 and projected to reach USD 17.28 billion by 2032, growing at a CAGR of 15.3% During the Forecast period of 2024-2032. These compounds enhance the efficacy of antiviral agents, making them crucial in the fight against emerging viral threats. With increasing research and development efforts, the market is witnessing significant advancements, particularly in drug formulations and delivery systems. As healthcare systems worldwide seek effective strategies to mitigate viral diseases, the demand for viral sensitizers is expected to rise. Key players in this market are focusing on collaborations and technological innovations to strengthen their positions and meet growing consumer needs.Access Full Report :https://www.marketdigits.com/checkout/3621?lic=sMajor Classifications are as follows:By Application:Antiviral Drug DevelopmentVaccine DevelopmentOncolytic Viral Therapies By End-users:Pharmaceutical CompaniesResearch InstitutesBiotechnology CompaniesKey Region/Countries are Classified as Follows:◘ North America (United States, Canada,)◘ Latin America (Brazil, Mexico, Argentina,)◘ Asia-Pacific (China, Japan, Korea, India, and Southeast Asia)◘ Europe (UK,Germany,France,Italy,Spain,Russia,)◘ The Middle East and Africa (Saudi Arabia, UAE, Egypt, Nigeria, and SouthKey Players of Viral Sensitizers MarketVirica Biotech.Market Drivers in Viral Sensitizers MarketRising Incidence of Viral Infections: The increasing prevalence of viral diseases, including influenza and COVID-19, drives demand for effective treatment options.Technological Advancements: Innovations in drug development and delivery systems enhance the efficacy of antiviral therapies.Government Initiatives: Increased funding for research on viral diseases fosters growth in the viral sensitizers market.Market Challenges in Viral Sensitizers MarketHigh R&D Costs: Developing viral sensitizers involves substantial financial investment, which can deter smaller firms.Regulatory Hurdles: Navigating complex regulatory pathways can delay product approvals and market entry.Market Competition: Intense competition from established antiviral therapies may hinder the adoption of new sensitizers.Market Opportunities of Viral Sensitizers MarketEmerging Markets: Untapped regions present significant growth opportunities for viral sensitizer applications.Combination Therapies: Developing combination therapies that incorporate viral sensitizers can enhance treatment effectiveness.Personalized Medicine: Tailoring treatments to individual patients’ needs can drive demand for specific sensitizers.ConclusionThe Viral Sensitizers Market is positioned for significant growth, driven by rising viral infection rates and advancements in technology. While challenges such as high R&D costs and regulatory complexities persist, the market offers substantial opportunities, particularly in emerging economies and through innovative therapeutic approaches. By addressing these challenges and leveraging market opportunities, stakeholders can contribute to a more effective response to viral infections, ultimately benefiting public health and patient outcomes.
0 notes
Text
gRNA Market 2024 Size, Application, Revenue, Types, Trends in Future, Scope to 2032
The global gRNA market, valued at USD 498.30 million in 2023, is projected to grow at an impressive compound annual growth rate (CAGR) of 19.61% over the forecast period from 2024 to 2032. By the end of 2032, the market is expected to reach a value of USD 2.30 billion. This robust growth underscores the increasing demand for advanced gene-editing technologies and their application in therapeutic and research settings.
gRNA, an essential component in CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats) technology, guides the Cas9 or other CRISPR-associated enzymes to specific locations in the genome, enabling precise gene editing. The expanding role of gRNA in gene-editing research, therapeutics, and agricultural applications is propelling the market forward.
Key Market Drivers
Increasing Application of CRISPR in Gene Therapy: The growing adoption of CRISPR technology in gene therapy, where it is used to correct genetic defects, has been a significant driver of gRNA demand. This technology allows scientists to target specific genetic sequences for deletion, insertion, or modification, paving the way for revolutionary treatments for genetic disorders such as cystic fibrosis, muscular dystrophy, and sickle cell anemia.
Advancements in Genomic Research: Advancements in genomics and the growing demand for personalized medicine have fueled the expansion of the gRNA market. gRNA-based tools are instrumental in understanding disease mechanisms, identifying drug targets, and developing precision medicines tailored to individuals’ genetic profiles. This trend is expected to accelerate in the coming years, given the ongoing focus on personalized therapies for complex diseases such as cancer and neurodegenerative disorders.
Agricultural Biotechnology Applications: Beyond human therapeutics, gRNA is being increasingly utilized in agricultural biotechnology for the development of genetically modified crops with enhanced traits such as disease resistance, improved yield, and tolerance to environmental stresses. As global food security becomes a pressing issue, the adoption of gene-editing technologies in agriculture will continue to drive the demand for gRNA.
Technological Innovations and Lower Costs: The continuous advancements in CRISPR-related technologies, including the improvement of gRNA design and delivery systems, have reduced the cost and complexity of gene-editing procedures. These innovations make gene-editing more accessible to a broader range of research institutions, biotechnology firms, and pharmaceutical companies, further driving market growth.
Access Free Sample Report: https://www.snsinsider.com/sample-request/4484
Challenges and Opportunities
While the gRNA market is poised for significant growth, several challenges remain. The ethical concerns surrounding gene-editing, particularly when it comes to human embryos and germline editing, continue to spark debates within scientific communities and regulatory bodies. Strict regulations and potential public backlash may pose barriers to the widespread adoption of CRISPR technology.
However, as regulatory frameworks evolve and more ethical guidelines are established, the gRNA market is expected to navigate these challenges successfully. Opportunities for growth will also arise from new developments in gene-editing therapies, especially as researchers work to address unmet medical needs in areas such as oncology, genetic disorders, and regenerative medicine.
Regional Insights
North America currently holds the largest share of the gRNA market, driven by significant investments in genomic research, a robust biotechnology sector, and increasing clinical trials focused on gene-editing therapies. The United States, in particular, leads the market due to its advanced healthcare infrastructure, funding for scientific research, and growing collaborations between academic institutions and biopharma companies.
Europe is also a prominent player, with considerable growth anticipated in countries such as Germany, the UK, and France, thanks to government funding for research and the expansion of biotechnology industries. Meanwhile, the Asia-Pacific region is expected to witness the highest growth rate during the forecast period. Rising investments in biotechnology, government initiatives to advance genomic research, and a growing pharmaceutical sector are driving market growth in countries like China, Japan, and India.
Future Outlook
The future of the gRNA market looks bright, with substantial growth expected across multiple industries, including biopharmaceuticals, agriculture, and academia. As gene-editing technologies become more advanced and accessible, the demand for precise and reliable gRNA tools will continue to rise. By 2032, the market is projected to exceed USD 2.30 billion, representing an era of unprecedented advancements in gene editing that will revolutionize medicine, agriculture, and scientific research.
In conclusion, the gRNA market is on a steep growth trajectory, driven by increasing demand for CRISPR-based therapies, advancements in gene-editing technologies, and expanding applications in biotechnology. With a forecasted CAGR of 19.61% from 2024 to 2032, the market is set to redefine the future of precision medicine and genomic research.
Other Trending Reports
Immunology Market Size
Medical Imaging Devices Market Size
Healthcare Mobility Solutions Market Size
Diabetes Devices Market Size
0 notes
Text
Ocular Inflammation Therapeutics Market Growth and Trends: Forecast to 2032
The Ocular Inflammation Therapeutics Market is expected to witness significant growth over the forecast period leading to 2032. Ocular inflammation refers to swelling, redness, and discomfort caused by various conditions affecting the eye, such as uveitis, keratitis, conjunctivitis, and scleritis. These conditions can arise from infections, autoimmune disorders, or trauma, potentially leading to vision loss if not treated promptly. The increasing prevalence of eye-related disorders, advancements in treatment options, and the growing elderly population are key factors propelling the market's expansion.
Ocular Inflammation Therapeutics Market Size was estimated at 14.16 (USD Billion) in 2023. The Ocular Inflammation Therapeutics Market Industry is expected to grow from 14.89(USD Billion) in 2024 to 22.2 (USD Billion) by 2032. The Ocular Inflammation Therapeutics Market CAGR (growth rate) is expected to be around 5.12% during the forecast period (2025 - 2032).
Market Growth Drivers
Rising Prevalence of Ocular Inflammatory Conditions
The increasing incidence of eye diseases, particularly among the aging population, is a major factor driving the demand for ocular inflammation therapeutics. Conditions such as uveitis, which affects the uveal tract of the eye, are increasingly diagnosed globally. According to the American Academy of Ophthalmology, uveitis is responsible for 10% to 15% of cases of total blindness in the U.S. alone. Other conditions such as scleritis and keratitis are also contributing to the rising demand for effective treatments.
Increasing Awareness and Early Diagnosis
Awareness campaigns promoting eye health and early diagnosis of ocular inflammatory diseases are contributing to market growth. With advancements in diagnostic technologies, such as Optical Coherence Tomography (OCT) and fundus imaging, clinicians are better equipped to identify inflammation in its early stages. This leads to more prompt and effective treatment, enhancing patient outcomes and driving the demand for therapeutic solutions.
Advancements in Therapeutics and Drug Development
Innovations in the field of ocular drug delivery and therapy are helping boost the market. The development of biologics, immunosuppressants, and corticosteroid formulations has transformed the treatment landscape for ocular inflammation. For example, corticosteroid implants and injectable therapies offer sustained drug release, providing long-term relief from chronic conditions like uveitis. Additionally, novel biologic therapies are emerging as targeted treatments that offer fewer side effects compared to traditional options, thus increasing adoption.
Market Segmentation
The Ocular Inflammation Therapeutics Market can be segmented based on drug class, indication, distribution channel, and geography.
By Drug Class: The market is segmented into corticosteroids, nonsteroidal anti-inflammatory drugs (NSAIDs), immunosuppressants, and biologics. Corticosteroids currently hold the largest share due to their widespread use in treating inflammation, although biologics are expected to see the highest growth owing to their targeted action and fewer side effects.
By Indication: Key conditions treated include uveitis, keratitis, conjunctivitis, and scleritis. Uveitis accounts for a significant portion of the market due to its frequency and potential severity.
By Distribution Channel: The primary distribution channels are hospital pharmacies, retail pharmacies, and online pharmacies. With the increasing prevalence of e-commerce and telemedicine, online pharmacies are expected to witness notable growth over the forecast period.
Key Market Trends
Biologics Gaining Traction
Biologic therapies are gaining ground in the ocular inflammation therapeutics market due to their precision in targeting specific pathways involved in the inflammatory response. Biologics like adalimumab have shown significant efficacy in treating severe uveitis, reducing the need for long-term corticosteroid use and minimizing associated side effects. As more biologic treatments receive regulatory approvals, their use is expected to expand, fueling market growth.
Rising Focus on Sustained Drug Delivery
The development of sustained drug delivery systems for ocular therapeutics is a growing trend. Implants and injectables that deliver medications over an extended period are becoming more popular, especially for chronic conditions like posterior uveitis. These systems improve patient compliance, reduce the frequency of dosing, and provide more consistent therapeutic outcomes.
Increasing Use of Immunosuppressants
Immunosuppressants, traditionally used in organ transplantation, are now being repurposed for treating ocular inflammatory conditions, particularly in patients unresponsive to corticosteroids. Drugs like cyclosporine and tacrolimus are gaining traction as they offer long-term control of inflammation without the side effects of prolonged steroid use.
Regional Analysis
North America holds the largest share of the ocular inflammation therapeutics market due to its advanced healthcare infrastructure, high prevalence of eye diseases, and growing geriatric population. The U.S. remains a major contributor to market growth, with high healthcare spending and an increasing number of clinical trials focused on novel therapies for ocular inflammation.
Europe follows closely, with countries like Germany, France, and the U.K. leading the way in adopting advanced ocular treatments. Awareness campaigns and government initiatives aimed at preventing vision impairment are driving market demand in this region.
Asia-Pacific is expected to witness the fastest growth during the forecast period, driven by improving healthcare infrastructure, increasing access to treatment, and a large, aging population. The rising incidence of diabetes, a key risk factor for ocular inflammation, further propels the demand for therapeutics in countries like India and China.
Competitive Landscape
Key players in the AbbVie ,Novartis ,Pfizer ,Roche ,Allergan ,Regeneron ,Alcon ,Takeda ,Iluvien ,Bausch & Lomb ,Biogen ,Santen ,Bayer ,Merck & Co ,Johnson & Johnson. These companies are focusing on product development, strategic collaborations, and mergers and acquisitions to strengthen their market presence. Ongoing research into new drug formulations, biologics, and drug delivery systems is expected to create new growth opportunities in the coming years.
Conclusion
The Ocular Inflammation Therapeutics Market is poised for substantial growth through 2032, driven by increasing awareness of eye health, advancements in drug development, and the rising prevalence of ocular inflammatory conditions. Innovations in biologics, sustained drug delivery systems, and early diagnosis technologies will continue to shape the market. As the demand for effective therapies grows, especially in emerging markets, key players in the industry are well-positioned to capitalize on these opportunities, driving future expansion.
0 notes