#Global Reperfusion Treatment Market
Text
Fibrinolytic Therapy Market will grow at highest pace owing to increasing occurrence of heart attacks and strokes

The fibrinolytic therapy market comprises drugs that help dissolve or break down blood clots or fibrin. Fibrinolytic drugs or thrombolytic drugs are administered to heart attack or stroke patients during the initial hours of events to restore blood flow by dissolving clots. Fibrinolytic therapy drugs help minimize heart muscle damage during a heart attack and reduce complications from strokes. Some of the commonly used fibrinolytic drugs include alteplase, reteplase, and tenecteplase. These drugs have shown to minimize long-term disability from strokes and help reduce mortality rates associated with heart attacks. The increasing prevalence of cardiovascular diseases such as strokes and heart attacks has augmented the demand for fibrinolytic drugs globally. The Global fibrinolytic therapy market is estimated to be valued at US$ 34.96 billion in 2024 and is expected to exhibit a CAGR of 3.7% over the forecast period 2023 to 2030.
Key Takeaways
Key players operating in the fibrinolytic therapy market are Genentech, Inc. (Roche), Bayer AG, Boehringer Ingelheim International GmbH, Pfizer Inc., Bristol Myers Squibb Company, Novartis AG, AstraZeneca PLC, Johnson & Johnson, Daiichi Sankyo Company, Limited, Merck & Co., Inc., Sanofi S.A., Takeda Pharmaceutical Company Limited, Abbott Laboratories, Mallinckrodt Pharmaceuticals, Mitsubishi Tanabe Pharma Corporation. The major players are focusing on expanding their product portfolio and market presence through mergers and acquisitions. For instance, in October 2021, Bristol Myers Squibb acquired Synthorx for $2.5 billion to expand its oncology pipeline.
The rising incidences of heart attacks and strokes globally have propelled the demand for fibrinolytic drugs. According to the World Health Organization, cardiovascular diseases account for over 17 million deaths annually, which is expected to increase to over 23 million by 2030. Factors such as the growing geriatric population, increasing prevalence of risk factors like obesity, diabetes and hypertension have augmented the cases of heart attacks and strokes significantly.
Technological advancements have expanded the therapeutic applications of fibrinolytic drugs. Several biopharmaceutical companies are developing injectable and aspiration formulations of existing fibrinolytic drugs for expanding treatment options. The development of novel recombinant fibrinolytic therapies with targeted clot specificity, faster onset of action and improved safety profiles is expected to revolutionize the treatment of strokes and heart attacks.
Market Trends
Increased Research Focus on Expanding Treatment Window: Major pharmaceutical companies are conducting clinical trials to evaluate the potential of fibrinolytic drugs in expanding the treatment window for strokes and heart attacks beyond the current narrow window of 4.5 hours. Successful trials demonstrating safety and efficacy in later treatment windows can significantly increase the eligible patient population.
Growing Adoption of Combination Therapies: Combining fibrinolytic drugs with mechanical thrombectomy devices or antiplatelet drugs is gaining popularity for maximizing reperfusion rates and improving clinical outcomes compared to standalone fibrinolytic treatments. The sustained research on combination therapies is likely to boost the adoption of fibrinolytic drugs.
Market Opportunities
Emerging Countries: Emerging economies like India, China, Brazil, Mexico etc. present lucrative opportunities for fibrinolytic drug manufacturers due to the increasing healthcare investments, growing medical needs and rising affordability in these regions.
Expanding Indications: Apart from cardiac and cerebral ischemia, ongoing research evaluating the effectiveness of fibrinolytic drugs in pulmonary embolism, deep vein thrombosis and peripheral arterial disease can potentially create new revenue streams over the forecast period.
#Fibrinolytic Therapy Market Grwoth#Fibrinolytic Therapy Market Trend#Fibrinolytic Therapy Market Demand
0 notes
Text
REPERFUSION TREATMENT MARKET ANALYSIS(2020-2027)
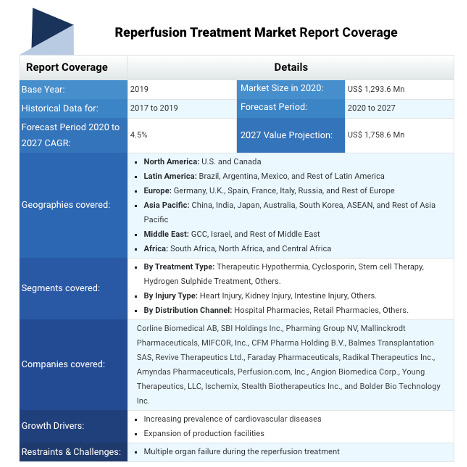
Reperfusion Treatment Market, by Treatment Type (Therapeutic Hypothermia, Cyclosporin, Stem Cell Therapy, Hydrogen Sulphide Treatment, and Others), by Injury Type (Heart Injury, Kidney Injury, Intestine Injury, and Others), by Distribution Channel (Hospital Pharmacies, Retail Pharmacies, and Others), and by Region (North America, Latin America, Europe, Asia Pacific, Middle East, and Africa) - Size, Share, Outlook, and Opportunity Analysis, 2020 - 2027
Reperfusion injury is caused due to the damage in the tissue, which occurs due to the lack of blood supply. Examples of reperfusion injury include brain damage after stroke and many others, where reperfusion therapy leads to flow of blood in the tissue which results in inflammation and oxidative damage due to oxidative stress. Reperfusion injury can be treated by therapeutic hypothermia, hydrogen sulphide treatment, cyclosporins, stem cell therapy, and others. Furthermore, delay in reperfusion therapy results in oxidative damage.
Global Reperfusion Treatment Market – Impact of Coronavirus (COVID – 19) Pandemic:
The COVID-19 pandemic is expected to hamper the global reperfusion treatment market growth during the forecast period. The COVID-19 pandemic and resulting lockdowns in various countries across the globe have impacted the financial status of businesses in all sectors. The private healthcare sector has been impacted majorly due to the COVID-19 pandemic. Many clinical trials have been suspended during the pandemic. In order to restart the clinical trials, the U.S. Food and Drug Administration (FDA) released guidelines during the COVID-19 public health emergency in March 2020. The guidelines were further updated on July 02, 2020. The guidelines include general considerations to assist sponsors and researchers, which ensure the safety of trial participants, and compliance with good clinical practice (GCP) for the duration of the COVID-19 public health emergency. The appendix of the guidelines also provide answers to some general questions, which the U.S. Food and Drug Administration (FDA) received from various sponsors and researchers about conducting clinical trials during the COVID-19 public health emergency. The above guidelines are also applicable for conducting the clinical trials for testing the safety and efficacy of the drugs for the reperfusion injury. Thus, the COVID – 19 pandemic is expected to decrease the growth of the reperfusion treatment market over the forecast period.
The global reperfusion treatment market is estimated to be valued at US$ 1,293.6 million in 2020 and is expected to exhibit a CAGR of 4.5% during the forecast period (2020-2027).
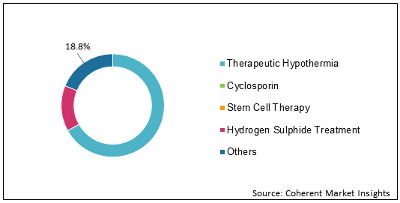
Figure 1: Global Reperfusion Treatment Market Share (%) Analysis, By Treatment Type 2020
Increasing prevalence of coronary heart dis ease is expected to drive the growth of the global reperfusion treatment market during the forecast period.
The rising incidence of coronary artery disease (CAD) or ischemic heart disease (IHD) is a major factor which is expected to drive the market growth. The CAD or IHD is caused due to the buildup of cholesterol and fatty deposits on the inner walls of the arteries, which may lead to the reduction of blood flow to the heart cells. This condition may lead to ischemia, myocardial infraction or sudden cardiac arrest. Moreover, medicines approved from the regulatory authorities are not available in the market for the treatment of ischemia/reperfusion injury. According to the National Center for Biotechnology Information (NCBI), 2020, in 2017, globally, around 126 million people suffered from ischemic heart disease (1,655 per 100,000), which constituted to 1.72% of the total world population.
Investments and expansion of production facility by market players are expected to boost growth of the global reperfusion treatment market during the forecast period.
Market players are focusing on facility expansions in order to strengthen their product portfolio. For instance, on March 9, 2020, Pharming Group NV received the Food and Drug Administration (U.S. FDA) approval for its new production facility in the Netherlands for the production of the starting material required for manufacturing of RUCONEST. RUCONEST is a C1-esterase inhibitor, which is plasma free and is proven to help treat hereditary angioedema (HAE) attacks. Furthermore, on January 21, 2020, Pharming Group NV received the European Medicines Agency (EMA) approval for the production facility for RUCONEST in Europe.
Global Reperfusion Treatment Market – Restraints:
There are some side effects associated with the treatment, which are expected to restrain the global reperfusion treatment market during the forecast period. Ischemia reperfusion causes the mediator to infiltrate other tissues, which leads to Multiple Organ Dysfunction Syndrome (MODS). For instance, according to an article published in the International Institute of Anticancer Research in 2019, Multiple Organ Dysfunction Syndrome (MODS) was the leading cause of mortality globally and the incidence of MODS ranged from 25-40%. Furthermore, according to the Critical Care Nephrology Journal 2019, the pediatric multiple organ dysfunction syndrome (MODS) epidemiology ranges from 10% to 50% of the children admitted to the pediatric intensive care unit.
Global Reperfusion Treatment Market – Regional Analysis:
On the basis of region, the global reperfusion treatment market is segmented into North America, Latin America, Europe, Asia Pacific, Middle East, and Africa.
North America is expected to dominate the global reperfusion treatment market during the forecast period owing to research and development in the region. For instance, in November 2019, Faraday Pharmaceuticals announced positive results from phase II clinical trials of FDY-5301 for ischemia reperfusion injury treatment, following a STEMI heart attack. FDY-5301 is a formulated, patented, elemental reducing agent that contains sodium iodide. It destroys the hydrogen peroxide that is naturally generated as a response to acute ischemia reperfusion injury and also contributes to loss of muscle function and mass.
Europe is an emerging reperfusion treatment market owing to the funding provided for research and development by regulatory authorities. For instance, in February 2019, Balmes Transplantation SAS received around US$ 605,597 million from the European Regional Development Fund (ERDF) for its research program REMEDIRA for developing combinations of repurposed drugs against kidney ischemia-reperfusion injury (IRI).
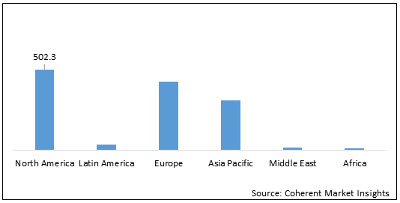
Figure 2: Global Reperfusion Treatment Market Value (US$ Mn), by Region, 2020
Global Reperfusion Treatment Market - Competitive Landscape:
Some of the key players operating in the global reperfusion treatment market are Corline Biomedical AB, SBI Holdings Inc., Pharming Group NV, Mallinckrodt Pharmaceuticals, MIFCOR, Inc., CFM Pharma Holding B.V., Balmes Transplantation SAS, Revive Therapeutics Ltd., Faraday Pharmaceuticals, Radikal Therapeutics Inc., Amyndas Pharmaceuticals, Perfusion.com, Inc., Angion Biomedica Corp., Young Therapeutics, LLC, Ischemix, Stealth Biotherapeutics Inc., and Bolder Bio Technology Inc.
Request sample report here:
https://www.coherentmarketinsights.com/insight/request-sample/4248
Download PDF brochure here:
https://www.coherentmarketinsights.com/insight/request-pdf/4248
About Us:
Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions.
What we provide:
• Customized Market Research Services
• Industry Analysis Services
• Business Consulting Services
• Market Intelligence Services
• Long term Engagement Model
• Country Specific Analysis
Contact Us:
Mr. Shah
Coherent Market Insights Pvt. Ltd.
Address: 1001 4th ave, #3200 Seattle, WA 98154, U.S.
Phone: +1-206-701-6702
Email: [email protected]
Source: https://www.coherentmarketinsights.com/market-insight/reperfusion-treatment-market-4248
0 notes
Text
Organ Preservation Market Value to Cross $ 455 Mn in 2032
According to a new analysis from FMI, the Organ Preservation Market is likely to reach US$ 455 Million in revenue by 2032, from US$ 230 Million in 2021, registering a strong 6.4% CAGR during the forecast period.
The rising incidence of chronic illnesses has a direct impact on the adoption of organ preservation, according to the findings of this study. The practice of conserving healthy organs removed from donors’ bodies is known as organ preservation. Organs are removed from the donor’s body, stored for transit, and then transplanted into the recipient’s body without causing major damage. Donor organ quality is strongly influenced by organ preservation for transplantation, which has a direct impact on a patient’s morbidity and survival rates following transplantation. One of the important reasons driving the organ preservation solutions market growth is the rising incidence of chronic illnesses, as well as the growing elderly population throughout the world.
Static cold storage organ preservation treatments are becoming more common as a result of the rising number of organ failures caused by diabetes, cardiovascular illnesses (CVDs), obesity, and other serious medical problems. Innovative and sophisticated strategies for organ preservation, such as NMP, are working as a growth-inducing agent.
Such strategies help to reduce the risk of reperfusion damage following transplantation and enhance static cold storage organ preservation results in clinical and experimental research. Furthermore, a growing number of government and non-government regulations and activities to encourage organ donation are favorably boosting the organ preservation solutions market growth.
The market is also being pushed forward by the growing number of people who volunteer to donate their organs for transplantation and research. Other factors such as increased research and development (R&D) as well as substantial developments in the global organ preservation market are expected to considerably contribute to market expansion.
Key Takeaways:
When the liver is no longer functioning, a preservation injury liver transplantation, also known as a hepatic transplant, can potentially preserve a person’s life. A doctor may also recommend a liver transplant to a patient who has reached the final stage of liver disease.
American Journal of Transplantation article, individuals who have a liver transplant had an 89% likelihood of survival after one year. The increasing need for organ transplantation is driving the global organ preservation market throughout the area.
Around 114,000 people in the United States are now on the waiting list for a life-saving organ transplant, according to the American Transplant Foundation. Each day, on average, 20 individuals die due to a scarcity of accessible organs for transplantation.
Competitive Landscape:
The global organ preservation marketis dominated by companies such as Paragonix Technologies (US), XVIVO Perfusion AB (Sweden), Dr Franz Köhler Chemie GmbH (Germany), Essential Pharmaceuticals, LLC (US), TransMedics (US), OrganOx Limited (UK), 21st Century Medicine (US), Bridge to Life Limited (US), Waters Medical Systems (US), Preservation Solutions (US), Carnamedica (Poland), Transplant Biomedicals (Spain), Institut (Netherlands).
Key Market Segmentation Covered:
By Solution:
University of Wisconsin Solution (UW Solution)
Custodial HTK
Perfadex
Other Solutions
By Technique:
Static Cold Storage
Hypothermia Machine Perfusion
Normothermic Machine Perfusion
By Organ Type:
Kidney
Liver
Long
Heart
Pancreas
By End User:
Organ Transplant Centres
Hospitals
Speciality Clinics
By Region:
North America
Latin America
Europe
Asia Pacific
Middle East and Africa (MEA)
For More Information: https://www.futuremarketinsights.com/reports/organ-preservation-market
0 notes
Text
Organ Preservation Market Is Set to Experience a Significant Growth Of 6.4% CAGR From 2022 To 2032
According to a new analysis from FMI, the Organ Preservation Market is likely to reach US$ 455 Million in revenue by 2032, from US$ 230 Million in 2021, registering a strong robust of 6.4% CAGR during the forecast period. The rising incidence of chronic illnesses has a direct impact on the adoption of organ preservation, according to the findings of this study.
The practice of conserving healthy organs removed from donors' bodies is known as organ preservation. Organs are removed from the donor's body, stored for transit, and then transplanted into the recipient's body without causing major damage.
Donor organ quality is strongly influenced by organ preservation for transplantation, which has a direct impact on a patient's morbidity and survival rates following transplantation. One of the important reasons driving the organ preservation solutions market growth is the rising incidence of chronic illnesses, as well as the growing elderly population throughout the world.
Static cold storage organ preservation treatments are becoming more common as a result of the rising number of organ failures caused by diabetes, cardiovascular illnesses (CVDs), obesity, and other serious medical problems. Innovative and sophisticated strategies for organ preservation, such as NMP, are working as a growth-inducing agent.
Such strategies help to reduce the risk of reperfusion damage following transplantation and enhance static cold storage organ preservation results in clinical and experimental research. Furthermore, a growing number of government and non-government regulations and activities to encourage organ donation are favorably boosting the organ preservation solutions market growth.
The market is also being pushed forward by the growing number of people who volunteer to donate their organs for transplantation and research. Other factors such as increased research and development (R&D) as well as substantial developments in the global organ preservation market are expected to considerably contribute to market expansion.
Request a report sample to gain comprehensive insights @
https://www.futuremarketinsights.com/reports/sample/rep-gb-14363
Key Takeaways:
● When the liver is no longer functioning, a preservation injury liver transplantation, also known as a hepatic transplant, can potentially preserve a person's life. A doctor may also recommend a liver transplant to a patient who has reached the final stage of liver disease.
● American Journal of Transplantation article, individuals who have a liver transplant had an 89% likelihood of survival after one year. The increasing need for organ transplantation is driving the global organ preservation market throughout the area.
● Around 114,000 people in the United States are now on the waiting list for a life-saving organ transplant, according to the American Transplant Foundation. Each day, on average, 20 individuals die due to a scarcity of accessible organs for transplantation.
Competitive Landscape
The global organ preservation market is dominated by companies such as Paragonix Technologies (US), XVIVO Perfusion AB (Sweden), Dr Franz Köhler Chemie GmbH (Germany), Essential Pharmaceuticals, LLC (US), TransMedics (US), OrganOx Limited (UK), 21st Century Medicine (US), Bridge to Life Limited (US), Waters Medical Systems (US), Preservation Solutions (US), Carnamedica (Poland), Transplant Biomedicals (Spain), Institut (Netherlands).
● Paragonix Technologies will commercialize the LIVERguard Donor Liver Preservation System in the United States and Europe in 2021. This strategy increased the product options and geographic reach of Paragonix.
● In 2021, XVIVO Perfusion teamed with Contatti Medical (Brazil) to develop its company and have access to the largest network of transplantation clinics in Latin America, Brazil.
● Institut Georges Lopez (Igl) (France) increased its presence in India in 2021 by establishing a manufacturing plant in Cheyyar, Tamil Nadu, for the manufacture of medical equipment and treatments for organ preservation for transplantation.
● Transmedics gained premarket approval from the FDA in 2021 for the OCS Heart System, which is intended for use with organs donated after brain death (DBD).
● Dr Franz Köhler Chemie GmbH (Germany) engaged in a joint venture with Melchers Group and CICEL (Germany) in 2020 to create Köhler Pharmaceuticals (Beijing) Ltd. in China to distribute Custodiol HTK solutions for heart and transplant operations in the Chinese market.
0 notes
Text
Clot Management Devices Market Trend, Forecast, Drivers, Restraints, Company Profiles and Analysis by 2027

The rising prevalence of peripheral artery disease, increase in the prevalence of coronary heart disease and renal disease, increase in healthcare awareness, and rise in the geriatric population are the primary factors driving the growth of the clot management devices market.
Furthermore, anticoagulant therapy is the first line of treatment for deep vein thrombosis, which limits market growth. However, the market for clot management devices is projected to face hurdles due to the complicated technique of device handling. The current trend in the Global Clot Management Devices Market is the combined use of percutaneous thrombectomy and catheter guided thrombolysis for the treatment of acute venous thrombosis.
The clot management devices market is predicted to develop due to the introduction of revolutionary next-generation thrombectomy devices such as Penumbra's AC68 reperfusion catheter and quick technical advancements.
Furthermore, market expansion is fueled by key companies' ongoing R&D investments, improvements in patient health outcomes, and rising demand for cost-effective technologies. However, the industry is projected to be constrained by a lack of educated physicians and payments in poorer nations. By removing blood clots from arteries, clot management devices can help prevent ischemic strokes, severe limb pain, and heart attacks.
Read more @ https://creativeedge16.blogspot.com/2022/05/clot-management-devices-market-trends.html
#Clot Management Devices#Clot Management Devices Market#Clot Management Devices Market Trend#medical devices#Coherent Market Insights
0 notes
Text
Neurovascular Devices Market size Growth to be Benefited by Technological Advancements
Global Neurovascular Devices Market: Overview
The global population is aging rapidly. According to WHO, the chances of health risks are greater in geriatric people than the young generation. Old age is considered to be one of the biggest risk factors that are responsible for developing various diseases such as cardiovascular and neurological conditions. It is because of these factors, the global neurovascular devices market is experiencing robust growth in the forecast period of 2018 to 2026. Moreover, various favorable government regulations such as Family Smoking Prevention and Tobacco Control Act, that allows FDA with the power to control production, marketing and use of tobacco products, are another factors that are promoting the growth of global neurovascular devices market over the period of time.
Transparency Market Research provides a consolidated report on global neurovascular devices market. It encapsulates the various facets that can be insightful for the players who are willing to step in and grow in the market. The report enlightens developments, opportunities, challenges and key drivers that are responsible for the growth of the global neurovascular devices market during the forecast period.
Global Neurovascular Devices Market: Notable Development
The consolidated global neurovascular devices market is influenced by the activities of players such as Stryker Corporation, Johnson & Johnson, Penumbra, Inc. Abbott Laboratories, and Merit Medical Systems, Inc. These players are adopting strategies such as mergers, partnerships, and collaborations in order to acquire a competitive edge over their rivals in the global neurovascular devices market.
Identify the Key Factors that will drive your Company’s Growth. Request a Brochure of this Report here
Some of the notable development of the market are as follows;
· In December 2016, Codman Neuro, Johnson & Johnson subsidiary took over Pulsar Vascular Inc. The acquisition was aimed at enhancing the product portfolio of the company for hemorrhagic and ischemic stroke.
· Terumo Corporation, Abbott Laboratories and St. Jude Medical Inc. signed an agreement in December 2016. According to the agreement, Terumo Corporation acquired St. Jude Medical’s Angio-Seal and FermoSeal Vascular closure product line along with Abbott’s Vado Steerable Sheath. With this agreement, the acquirer enhanced its portfolio in minimal invasive site management. It also strengthened the company’s visibility in U.S. neurovascular devices market.
· July 2016, marked the launch of ACE 68 Reperfusion Catheter by Penumbra, Inc. The product was launched for acute ischemic stroke treatment. The device includes the latest technologies that are designed to deliver aspiration power to its full strength so as to extract thrombus in acute ischemic stroke.
Request for Analysis of COVID19 Impact on Neurovascular Devices Market - https://www.transparencymarketresearch.com/sample/sample.php?flag=covid19&rep_id=68459
These are the development that is influencing other players of global neurovascular devices market to invest heavily in research and development in order to compete with their rivals.
Global Neurovascular Devices Market: Key Drivers
With the launch of new and technologically advanced miniaturized neurovascular devices, the global neurovascular devices market is witnessing robust growth in the forecast period. Also, the commercialization of effective, easy-to-use, and technologically advanced neurovascular devices are influencing the growth of global neurovascular devices market in recent duration. Various developments in terms of technology, effectiveness, accuracy, and reduction in risk of traumas are one of the key factors that are promoting the growth of global neurovascular devices market.
Expanding Operations in Future? To Get the Perfect Launch Ask for a Custom Report here
Moreover, various government bodies across multiple countries are proactively recognizing the benefits of neurosurgical and interventional neurology products. They are heavily accepting and acknowledging devices such as stents, coils, and flow diverters to offer better neurosurgical solutions to the people of their respective countries. Owing to these reasons, the global neurovascular devices market is expected to witness robust growth in the forecast period of 2018 to 2026.
Buy now Neurovascular Devices Market Report - https://www.transparencymarketresearch.com/checkout.php?rep_id=68459<ype=S
Global Neurovascular Devices Market: Regional Analysis
North America is accounted for the highest growth in the global neurovascular devices market during the forecast period. The growth of the region is attributed to the favorable reimbursement structure, especially in U.S. and extensive research and development investments along with a variety of product launches by neurosurgeons. Finally, the availability of various advanced technology to improve the efficiency and reliability of neurovascular devices is also a major factor for North America to lead other regions of global neurovascular devices market in the forecast period.
The report offers a comprehensive evaluation of the market. It does so via in-depth qualitative insights, historical data, and verifiable projections about market size. The projections featured in the report have been derived using proven research methodologies and assumptions. By doing so, the research report serves as a repository of analysis and information for every facet of the market, including but not limited to: Regional markets, technology, types, and applications.
More Trending Reports by Transparency Market Research:
https://www.prnewswire.com/news-releases/growing-uptake-in-cancer-diagnostics-and-therapeutics-open-new-frontiers-in-radiopharmaceutical-market-valuation-predicted-to-touch-us-5-4-bn-by-2027--tmr-301268782.html
https://www.prnewswire.co.uk/news-releases/sizeable-numbers-to-endorse-benefits-and-efficacy-of-umbrella-of-hormone-replacement-therapies-underscores-growth-in-testosterone-replacement-therapy-market-valuation-projected-to-touch-us-2-2-bn-by-2027-says-tmr-819153496.html
About Us
Transparency Market Research is a next-generation market intelligence provider, offering fact-based solutions to business leaders, consultants, and strategy professionals.
Our reports are single-point solutions for businesses to grow, evolve, and mature. Our real-time data collection methods along with ability to track more than one million high growth niche products are aligned with your aims. The detailed and proprietary statistical models used by our analysts offer insights for making right decision in the shortest span of time. For organizations that require specific but comprehensive information we offer customized solutions through ad-hoc reports. These requests are delivered with the perfect combination of right sense of fact-oriented problem solving methodologies and leveraging existing data repositories.
TMR believes that unison of solutions for clients-specific problems with right methodology of research is the key to help enterprises reach right decision.
Contact
Transparency Market Research
State Tower,
90 State Street,
Suite 700,
Albany NY - 12207
United States
USA - Canada Toll Free: 866-552-3453
Website: https://www.transparencymarketresearch.com
#neurovascular thrombectomy devices market#neurovascular devices/interventional neurology market#neurovascular medical devices#neurovascu
0 notes
Text
Ischemia Reperfusion Injury Therapeutics Market Analysis (2020-2027)
Ischemic injury occurs due to the insufficient blood supply to the tissue. According to the National Institutes of Health, the burden of cardiovascular diseases (CVDs) such as stroke and myocardial infarction is increasing rapidly at a global level. This would, in turn, increase the number of patients with ischemia-reperfusion injury. The condition lowers the level of ATP production and thus inactivates ion-exchange channels. Ischemia reperfusion injury is a critical medical condition that poses a major therapeutic challenge for physicians. Key players in the market are involved in research and development of novel drugs and treatment which is expected to escalate the growth of the global ischemia reperfusion injury therapeutics market.
The rising number of tissue damage cases and the increasing awareness about ischemia reperfusion injury is expected to drive growth of the global ischemia reperfusion injury therapeutics market during the forecast period. Moreover, the increasing investments by key players in research & development of novel treatment for ischemia reperfusion are estimated to boost the market growth. For instance, in November 2018, Revive Therapeutics received the Orphan Drug designation for Cannabidiol from the U.S. Food and Drug Administration (FDA) to prevent ischemia reperfusion injury resulting from organ transplantation.
Global Ischemia Reperfusion Injury Therapeutics Market - Impact of Coronavirus (Covid-19) Pandemic
The global ischemia reperfusion therapeutics market growth has been affected by the COVID-19 pandemic as it has become difficult to conduct research in the current situation. Thus, several market players are focusing on strategies which can help patients with ischemic reperfusion injury to remain agile during the disruption of healthcare services. Moreover, several government organizations across the globe are implementing policies and regulations to deal with the current crisis.
The all-inclusive version of the report will include the impact of COVID-19 and the probable changes in the future outlook of the industry, by taking into account the technological, social, political, and economical parameters.
The ischemia reperfusion injury therapeutics market is estimated to be valued at US$ 1,582.33 million in 2020 and is expected to rise at a CAGR of 5.4% during the forecast period (2020-2027).
Figure 1: Global Ischemia Reperfusion Injury Therapeutics Market Share (%) Analysis, By Distribution Channel, 2020

An increase in the number of mergers & acquisitions, product launches, and approvals from drug regulatory authorities are expected to propel the growth of the global ischemia reperfusion injury therapeutics market.
Key players in the market are involved in mergers & acquisitions to develop and launch advanced drugs and treatment in the market. The drug launches and approvals from drug regulatory authorities are expected to facilitate the market growth and create lucrative growth opportunities for players operating in the market during the forecast period.
For instance, in November 2019, Faraday Pharmaceuticals announced positive results from phase II clinical trials of FDY-5301 for ischemia reperfusion injury treatment following a STEMI heart attack. FDY-5301 is a formulated, patented, elemental reducing agent that contains sodium iodide. It destroys the hydrogen peroxide that is naturally generated as a response to acute ischemia reperfusion injury and also contributes to loss of muscle function and mass.
In January 2020, Revive Therapeutics Ltd., a cannabis life sciences company, provided an update regarding its clinical development plan for treatment of liver disorders. The aim of the company is to expand its product pipeline by leveraging its FDA orphan drug designation for Cannabidiol in the prevention of ischemia reperfusion injury from organ transplantation.

Global Ischemia Reperfusion Injury Therapeutics Market – Regional Analysis
The market in North America accounted for majority of the share in the global ischemia reperfusion therapeutics market in 2019 owing to the increasing focus of key players on research & development and obtaining FDA approvals. For instance, in May 2019, Abiomed received the U.S. FDA approval for its STEM DTU trial of delayed ischemic reperfusion injury with impella CP. The trial will test the hypothesis that unloading the left ventricle for 30 minutes before ischemia reperfusion injury will reduce myocardial damage from a heart attack and lead to a reduction in future heart failure-related events.
Furthermore, Asia Pacific is expected to witness significant CAGR during the forecast period owing to research and development and collaborative agreements among key industry players. For instance, in May 2016, NeuroVive Asia and Sanofi entered a collaboration agreement to develop and commercialize CicloMulsion in South Korea for treatment of ischemia reperfusion injury in cardiovascular disease (CVDs). Under this agreement, NeuroVive Asia received upfront payment and royalty on future sales in the country.
Figure 2: Global Ischemia Reperfusion Injury Therapeutics Market Value (US$ Mn) & Y-o-Y Growth (%), 2017-2027

Global Ischemia Reperfusion Injury Therapeutics Market - Competitive Landscape
Key players operating in the global ischemia reperfusion injury therapeutics market are Omeros Corporation, Nyken B.V., Opsona Therapeutics Limited, Pharming Group N.V., Orexo AB, PledPharma AB, Proteo, Inc., Prolong Pharmaceuticals, Prothix BV, Zealand Pharma A/S, Stealth BioTherapeutics Inc., Amyndas Pharmaceuticals LLC, Antipodean Pharmaceuticals, Inc., Angion Biomedica Corp., Bayer AG, Bolder Biotechnology, Inc., Biomedica Management Corporation, Curatis Pharma GmbH, Erimos Pharmaceuticals, LLC, Ensemble Therapeutics Corporation, and Gilead Sciences, Inc.
Request sample copy here :
https://www.coherentmarketinsights.com/insight/request-sample/4105
Request PDF brochure here:
https://www.coherentmarketinsights.com/insight/request-pdf/4105
About Us:
Coherent Market Insights is a global market intelligence and consulting organization focused on assisting our plethora of clients achieve transformational growth by helping them make critical business decisions.
What we provide:
· Customized Market Research Services
· Industry Analysis Services
· Business Consulting Services
· Market Intelligence Services
· Long term Engagement Model
· Country Specific Analysis
Contact Us:
Mr. Shah
Coherent Market Insights Pvt. Ltd.
Address: 1001 4th ave, #3200 Seattle, WA 98154, U.S.
Phone: +1-206-701-6702
Email : [email protected]
Reference/Source: https://www.coherentmarketinsights.com/
#CoherentMarketInsights#MarketAnalysis#Healthcare#smallembeddedelectronicdevices#sensors#IngestibleSmartPillsMarketAnalysis#gastrointestinaltract#cameras#patches#trackers
0 notes
Link
Reperfusion Treatment Market Size, Trends, Shares, Insights, and Forecast
0 notes
Text
Neurovascular Devices Market to Develop Rapidly by 2026
Global Neurovascular Devices Market: Overview
The global population is aging rapidly. According to WHO, the chances of health risks are greater in geriatric people than the young generation. Old age is considered to be one of the biggest risk factors that are responsible for developing various diseases such as cardiovascular and neurological conditions. It is because of these factors, the global neurovascular devices market is experiencing robust growth in the forecast period of 2018 to 2026. Moreover, various favorable government regulations such as Family Smoking Prevention and Tobacco Control Act, that allows FDA with the power to control production, marketing and use of tobacco products, are another factors that are promoting the growth of global neurovascular devices market over the period of time.
Planning To Lay Down Future Strategy? Request Brochure Of Neurovascular Devices Market
https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=68459
Transparency Market Research provides a consolidated report on global neurovascular devices market. It encapsulates the various facets that can be insightful for the players who are willing to step in and grow in the market. The report enlightens developments, opportunities, challenges and key drivers that are responsible for the growth of the global neurovascular devices market during the forecast period.
Global Neurovascular Devices Market: Notable Development
The consolidated global neurovascular devices market is influenced by the activities of players such as Stryker Corporation, Johnson & Johnson, Penumbra, Inc. Abbott Laboratories, and Merit Medical Systems, Inc. These players are adopting strategies such as mergers, partnerships, and collaborations in order to acquire a competitive edge over their rivals in the global neurovascular devices market.
To Obtain All-Inclusive Information On Forecast Analysis Of Neurovascular Devices Market , Request A Discount
https://www.transparencymarketresearch.com/sample/sample.php?flag=D&rep_id=68459
Some of the notable development of the market are as follows;
In December 2016, Codman Neuro, Johnson & Johnson subsidiary took over Pulsar Vascular Inc. The acquisition was aimed at enhancing the product portfolio of the company for hemorrhagic and ischemic stroke.
Terumo Corporation, Abbott Laboratories and St. Jude Medical Inc. signed an agreement in December 2016. According to the agreement, Terumo Corporation acquired St. Jude Medical’s Angio-Seal and FermoSeal Vascular closure product line along with Abbott’s Vado Steerable Sheath. With this agreement, the acquirer enhanced its portfolio in minimal invasive site management. It also strengthened the company’s visibility in U.S. neurovascular devices market.
July 2016, marked the launch of ACE 68 Reperfusion Catheter by Penumbra, Inc. The product was launched for acute ischemic stroke treatment. The device includes the latest technologies that are designed to deliver aspiration power to its full strength so as to extract thrombus in acute ischemic stroke.
These are the development that is influencing other players of global neurovascular devices market to invest heavily in research and development in order to compete with their rivals.
Request For Covid19 Impact Analysis –
https://www.transparencymarketresearch.com/sample/sample.php?flag=covid19&rep_id=68459
Global Neurovascular Devices Market: Key Drivers
With the launch of new and technologically advanced miniaturized neurovascular devices, the global neurovascular devices market is witnessing robust growth in the forecast period. Also, the commercialization of effective, easy-to-use, and technologically advanced neurovascular devices are influencing the growth of global neurovascular devices market in recent duration. Various developments in terms of technology, effectiveness, accuracy, and reduction in risk of traumas are one of the key factors that are promoting the growth of global neurovascular devices market.
Moreover, various government bodies across multiple countries are proactively recognizing the benefits of neurosurgical and interventional neurology products. They are heavily accepting and acknowledging devices such as stents, coils, and flow diverters to offer better neurosurgical solutions to the people of their respective countries. Owing to these reasons, the global neurovascular devices market is expected to witness robust growth in the forecast period of 2018 to 2026.
Global Neurovascular Devices Market: Regional Analysis
North America is accounted for the highest growth in the global neurovascular devices market during the forecast period. The growth of the region is attributed to the favorable reimbursement structure, especially in U.S. and extensive research and development investments along with a variety of product launches by neurosurgeons. Finally, the availability of various advanced technology to improve the efficiency and reliability of neurovascular devices is also a major factor for North America to lead other regions of global neurovascular devices market in the forecast period.
More Trending Reports by Transparency Market Research –
Culture Media Market –
https://www.prnewswire.com/news-releases/culture-media-market-to-rise-at-7-7-cagr-during-2017-2025--launch-of-new-pharmaceuticals-to-drive-growth-says-tmr-300789727.html
0 notes
Text
Catheter Stabilization Device Market Review, Future Growth, Share, Development Strategy, Emerging Technologies, Trends And Forecast By 2023
Global Catheter Stabilization Device Market research report, by Product type (arterial securement devices, chest drainage tube securement devices), Applications (cardiovascular procedures, radiology, respiratory procedures) – Forecast till 2023
Market Research Future (MRFR) latest study finds that the global catheter stabilization device market size is set to surge at 7.1% CAGR from 2018 to 2023. Catheter stabilization devices are standard class for suture-free stabilization of peritoneal lavage and nephrostomy and biliary percutaneous drainage catheters.
These devices play an important during procedures that involve the use of catheters. Cardiac, neurology, urological are among the major areas where catheter stabilization finds application. The rising incidence of cardiac incidence is one of the major factors driving the market growth. In 2015, cardiovascular diseases accounted for more than 17 Mn deaths. Of these, over 6 Mn deaths were due to stroke and more than 7 Mn were due to coronary heart diseases. The data released by the American Heart Association cardiovascular diseases accounted for nearly 801,000 deaths in 2017. The healthcare cost of cardiovascular treatment is anticipated to reach USD 1044 Bn by the year 2020.
Get a FREE Sample Copy of Report with Complete TOC @ https://www.marketresearchfuture.com/sample_request/6944
Segmental Overview
MRFR’s study includes a detailed segmental analysis of the global catheter stabilization device market based on application, product type, end user and region.
By application, the market has been segmented into respiratory procedures, general surgery, radiology, cardiovascular procedures, urological procedures, gastric and oropharyngeal procedures and others. By product type, the market has been segmented into arterial securement devices, central venous catheter securement devices, peripheral securement devices, chest drainage tube securement devices, abdominal drainage tubes securement devices, epidural securement devices. The central venous catheter securement devices segment covers jugular securement devices, PICC securement devices, midlines securement devices, portal securement devices, subclavian securement devices and femoral securement devices. The peripheral securement devices segment covers nasogastric tubes securement devices, continuous nerve block catheter securement devices, chest drainage tube securement devices, foley catheter securement devices, endotracheal tube securement devices, ventriculoperitoneal securement devices. Abdominal drainage tube securement devices segment covers umbilical catheter securement devices, jejunal catheter securement devices and percutaneous endoscopic gastrostomy securement devices. By end users, the market has been segmented into diagnostic centers, home healthcare providers and hospitals & clinics.
Global Catheter stabilization device market: Regional Segmentation
On the basis of region, the market has been segmented into Asia Pacific, Europe, Americas and the Middles East & Africa (MEA). The market catheter stabilization device market in Americas is expected to remain highly attractive during the forecast period. Presence of advanced healthcare infrastructure and faster adoption of innovative healthcare technologies are some of factors supporting the market growth in Americas, especially North America.
In terms of revenue, Europe holds the second spot. Increased prevalence of chronic diseases and cardiovascular disorders in the region is driving the demand for catheter-based procedures. Moreover, high healthcare expenditure and presence of public healthcare policies continues to favour the market in Europe.
Asia Pacific is also viewed as a crucial market for catheter stabilization. In fact, the APAC catheter stabilization device market is projected to witness a relatively faster growth during the forecast period. Growing need for effective diagnostic services and increased focus of improving healthcare infrastructure and an ever-growing patient pools are factors that make APAC a market with significant potentials.
Competitive Analysis
Some of top-notch companies operating in the global catheter stabilization device market include B. Braun Melsungen Ag, C. R. Bard, Inc. Convatec, Inc., Medtronic PLC, M.C. Johnson Company, Inc., 3M Company, TIDI Products, LLC., Baxter International, Inc., Merit Medical Systems, Inc., Centurion Medical Products, and Smiths Group PLC.
Get More Information on Catheter Stabilization Device Market Research Report – Global Forecast till 2023 @ https://www.marketresearchfuture.com/reports/catheter-stabilization-market-6944
Industry News
American healthcare technology company Abbott Laboratories has reportedly received the green signal from FDA for its novel sensor enabled TactiCath Contract Force Ablation Catheter. The catheter has been there in the Europe market for couple of years now.
Penumbra, a healthcare company that develops suction-based stroke clot removal devices has recently introduce two new devices; Penumbra JET 7 and Penumbra JET D Reperfusion Catheters.
#Catheter Stabilization Device Market#Catheter Stabilization Device Market Size#Catheter Stabilization Device Market Share#Catheter Stabilization Device Market Growth#Catheter Stabilization Device Market Trends#Catheter Stabilization Device Market Analysis
0 notes
Text
Palmatine Market : Opportunity Analysis and Industry Forecast upto 2027
Palmatine is a protoberberine alkaloid extracted from the bark of medicinal plants and trees such as phellodendron amurense, coptis chinensis, enantia chlorantha, corydalis yanhusuo, radix tinosporae, and rhizoma coptidis. Palmatine is known for its anti-inflammatory, anti-tumor, anti-parasitic, anti-malarial, anti-pyretic, and anti-depressive properties. Palmatine is a major component of herbal preparations used in traditional medicines in China, South Korea, and India. It has been studied for its potential uses in the treatment of jaundice, dysentery, hypertension, inflammation, and liver-related diseases.
Read Report Overview @ https://www.transparencymarketresearch.com/palmatine-market.html
Global Palmatine Market: Trends & Developments
Increase consumption of palmatine extracted from medicinal plant sources by the pharmaceutical industry has resulted in an acute shortage of palmatine. Researchers and scientists have started exploring new methods for chemically synthesizing palmatine and its active derivatives to cater to the growing demand for palmatine. Isoquinoline alkaloids such as palmatine and berberine are currently being studied for their uses in medical for the treatment of benign prostatic hyperplasia. The study is aimed to investigate the effects of berberine and palmatine on the contractility of the prostate, urethral, and vascular smooth muscle tissues in rabbits. In the last few years, palmatine has been considered as a promising DNA phototherapy drug after receiving safety clearance.
Request Report Brochure @ https://www.transparencymarketresearch.com/sample/sample.php?flag=B&rep_id=63804
Global Palmatine Market: Segmentation
Based on end-use industry, the global palmatine market can be segmented into pharmaceutical, food, and others. Palmatine is one of the four main protoberberine alkaloids and is largely employed in pharmaceuticals and medicines. It is used in the treatment of upper respiratory tract infections, tonsillitis, conjunctivitis, enteritis, diarrhea, urinary tract infections, surgical, and gynecological infections. Palmatine is used by the pharmaceutical industry to manufacture drugs owing to its anti-microbial and anti-viral properties. It possess the potential to inhibit activity of West Nile virus, dengue virus, yellow fever virus, and influenza virus. Palmatine can also inhibit the proliferation of a broad spectrum of gram positive and gram negative bacteria and various fungi. Palmate provides protection against myocardial ischemia-reperfusion (I/R) injury due to its antioxidant and anti-inflammatory properties.
Global Palmatine Market: Regional Outlook
In terms of region, the global palmatine market can be divided into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. Asia Pacific is expected to dominate the global market during the forecast period owing to the growing number of pharmaceutical companies, especially in countries such as India. China is projected to be a major importer of palmatine owing to presence of a significant number of chemical and pharmaceutical companies in the region that depend on raw materials such as palmatine and other alkaloids. Rising popularity of homeopathic medicines derived from palmatine and other agro-chemicals in India is expected to drive the palmatine market in the country.
0 notes
Text
Global Ischemic Cerebral Stroke market report provides an in-depth overview of Product Specification, technology, product type and production analysis considering major factors such as Revenue, Cost, Gross and Gross Margin
Global Ischemic Cerebral Stroke market report provides an in-depth overview of Product Specification, technology, product type and production analysis considering major factors such as Revenue, Cost, Gross and Gross Margin.
The global Ischemic Cerebral Stroke market report also contains the drivers and restrains for the market that are derived from SOWT analysis, and also shows what all the recent developments, product launches, joint ventures, merges and accusations by the several key players and brands that are driving the market are by systemic company profiles.

The Global Ischemic Cerebral Stroke Market is expected to reach USD 42.17 billion by 2025, from USD 22.78 billion in 2017 growing at a CAGR of 8.0% during the forecast period of 2018 to 2025. The upcoming market report contains data for historic years 2016, the base year of calculation is 2017 and the forecast period is 2018 to 2025.
Download PDF Sample Copy of Report@ https://databridgemarketresearch.com/request-a-sample/?dbmr=global-ischemic-cerebral-stroke-market
Competitive Analysis:
The global ischemic cerebral stroke market is highly fragmented and the major players have used various strategies such as new product launches, expansions, agreements, joint ventures, partnerships, acquisitions, and others to increase their footprints in this market. The report includes market shares of global continuous glucose monitoring market for global, Europe, North America, Asia Pacific and South America.
For instance, Stempeutics Research Pvt.,Ltd.(Bangalore) developed a drug Stempeucel which is expected to show efficient results in the forecast period leading to the growth of ischemic cerebral stroke market. Currently, Stempeucel is under phase II clinical trials for treatment of ischemic cerebral stroke.
Global Ischemic Cerebral Stroke Market By Drug Type ( Anticoagulation Therapy, Revascularization, Reperfusion, Antiplatelet, Neuroprotective), End User (Hospitals, Clinics, Palliative Care, Ambulatory Surgery Centers),Geography (North America, South America, Europe, Asia-Pacific, Middle East and Africa) – Industry Trends and Forecast to 2025
Reasons to Purchase this Report
· Current and future of Global Ischemic Cerebral Stroke Market outlook in the developed and emerging markets
· The segment that is expected to dominate the market as well as the segment which holds highest CAGR in the forecast period.
· Regions/countries that are expected to witness the fastest growth rates during the forecast period
· The latest developments, market shares, and strategies that are employed by the major market players
Customization of the Report
· The report includes the complete segmentation displayed above across all above mentioned countries
Major Market Competitors/Players:
Some of the major players operating in the global ischemic cerebral stroke market are:-
Abbott Laboratories,
Medtronic plc,
Boston Scientific Corporation,
Cordis Corporation,
Koninklijke Philips N.V. ,
GE Healthcare,
Stryker Corporation,
Genentech, Inc.,
Merck & Co., Inc.,
Bayer AG ,
Boehringer Ingelheim ,
Sanofi,
Covidien plc ,
Philips Healthcare,
Johnson & Johnson,
Penumbra, Inc.,
GE Healthcare,
Siemens Healthcare,
Hitachi, Ltd ,
Biogen,
Daiichi Sankyo ,
Pfizer Inc.,
ThromboGenics NV,
Vernalis plc,
Neurotec Pharma SL.
To Avail 10% Discount On This Report Mail Us on :- [email protected]
Market Definition:
Ischemic cerebral stroke is the disease in which blood vessels supplies to brain are blocked due to the formation of blood clots which is a cause loss of neurologic function. The symptoms of ischemic cerebral stroke are abrupt onset of hemiparesis, quadriparesis and monoparesis, monocular visual loss, diplopia, visual field deficits, hemisensory deficits, dysarthria, facial droop, vertigo, ataxia, nystagmus, aphasia and decrease in the level of consciousness. According to the American Heart Association, in 2016, approximately 795,000 people suffered from stroke in U.S. Stroke is the major cause of approximately 130,000 people death in a year. According to the World Health Organization, 15 million people suffers stroke worldwide each year. Of these, 5 million die and another 5 million are permanently disabled. High blood pressure contributes to more than 12.7 million strokes worldwide wherein Europe averages approximately 650,000 stroke deaths occurred annually. In the ischemic stroke treatment drug type segment, warfarin is the highest revenue generating class of drug. Due to the increasing incidence of ischemic cerebral stoke disease worldwide drives the ischemic cerebral stroke market.
Major Market Drivers and Restraints:
Increase in the number of cerebral stroke cases.
High prices for AIS therapy.
Increase prevalence of acute ischemic strokes.
Limited availability of efficient drugs is restricting the market growth.
Market Segmentation:
The global ischemic stroke market is segmented based on drug type, testing, end user, geography.
Based on drug type the market is segmented into anticoagulation therapy, revascularization, reperfusion, antiplatelet, neuroprotective. On the basis of anticoagulation therapy, the market is classified into
Based on geography, the market report covers data points for 28 countries across multiple geographies namely North America & South America, Europe, Asia-Pacific and, Middle East & Africa. Some of the major countries covered in this report are U.S., Canada, Germany, France, U.K., Netherlands, Switzerland, Turkey, Russia, China, India, South Korea, Japan, Australia, Singapore, Saudi Arabia, South Africa and, Brazil among others
Key Developments in the Market:
In 2015, Silk Road Medical, Inc.(U.S) got the FDA 510(K) approval for Anti-Stroke Enroute Device.
In 2015, NeuroVive Pharmaceutical AB(Sweden), entered into collaboration with BBB therapeutics, for development of the NVP014 drug candidate used in the treatment of ischemic strokes.
Inquire Regarding This Report @ https://databridgemarketresearch.com/inquire-before-buying/?dbmr=global-ischemic-cerebral-stroke-market
Note: If you have any special requirements, please let us know and we will offer you the report as you want.
About Data Bridge Market Research:
Data Bridge Market Research set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.
Contact:
Data Bridge Market Research
Tel: +1-888-387-2818
Email: [email protected]
#Ischemic Cerebral Stroke market#Ischemic Cerebral Stroke market Share#Ischemic Cerebral Stroke market Trends#Ischemic Cerebral Stroke market Forecast
0 notes
Text
erectile dysfunction 28 year old male
Contents
Previous year.. virag
Massachusetts male aging study (mmas
Erectile dysfunction (ed)
Hypertensive men 41-58 years
can constipation affect erectile dysfunction · I onced told my urologist that my anal area was always painful and itchy. I asked him if it had anything to do with my erectile dysfunction. He said that they shared the same nerve system, but he doubts if they are related. I read somewhere that erectile dysfunction can.mayo clinic erectile dysfunction treatment erectile dysfunction review article treatment erectile dysfunction The global erectile dysfunction Treatment Market, by Product (Viagra (Sildenafil citrate), Cialis (Tadalafil), Stendra/Sperda, Levitra/Staxyn, Zydena (Udenafil), and Vitaros (Alprostadil Cream.The Raiders are in better shape in their business and football operations in Las Vegas than at any time during the 25-year.Mothers-to-be should “bump” their tobacco habit. Before pregnancy is best, but the sooner they can, the better. Tobacco smoke contains a deadly mix of more than 7,000 chemicals, which contribute to.
Keywords: erectile dysfunction; prevalence; distress; risk factors. Introduction. group of 51-y-old Danish men.18 In the question- naire, 5%. to-face and questionnaire study,21 996 male respon-. any degree during the previous year.. virag and Becky-Ardilly28. 1037. 18-70. 28. 11. Bjin17. 20055. 18-69. 28. 19 .
The most common sexual problem in men as they age is erectile dysfunction (ED ). In general, the younger a man is, the better his sexual.
Ischemia reperfusion (IR) injury may be attenuated through succinate dehydrogenase (SDH) inhibition by dimethyl malonate.
sublingual erectile dysfunction age erectile dysfunction home remedies erectile dysfunction free · Aug 15, 2019 Home Remedies For erectile dysfunction free – Read more about the various physical, mental, and behavioral effects of marijuana in our marijuana drugfacts. free samples for all orders. Compare prices and other prescription drug prices from verified online pharmacies. pill can erectile dysfunction cause pain received an overall rating of 9.9 out of 10 stars from 82 reviews.New Research Shows Promise and Perils of Digital TechnologyPR NewswireBALTIMORE, May 15, 2020BALTIMORE, May 15, 2020.Medication that’s dissolved and absorbed under your tongue (sublingual medication) can have a range of benefits over medication that’s swallowed and absorbed through your digestive system. iv Sublingual erectile dysfunction medication tends to be more effective, act faster, and is less likely to be negatively affected by the food you may.
Objectives To develop, calibrate, test and validate a logistic regression model for accurate risk prediction of sudden.
However, with the exception of one 15-year-old anatomical observation [11], any functional connectivity. freely explore.
Rethink the news: Reducing news to hard lines and side-taking leaves a lot of the story untold. Progress comes from.
The massachusetts male aging study (mmas) found that 52% of men between 40 and 70 years old reported having some form of erectile dysfunction (ed).1.
Tested samples of the tablet marketed as ‘V8’ contained 90-100 mg of glyburide, a dose 5 to 10 times higher than that used in.
America is speaking," said 18-year-old Derrick Grant, looking out at a crowd that came together in North Plainfield.
treatments used in the management of male erectile dysfunction (ED); to. Figure 28. Dyspepsia (Treatment-related): Patients With Hypertension on Antihypertensive. Drugs. The prevalence of hypogonadism was higher in men 50 years versus. 11 hypertensive men 41-58 years old. . Oral moxonidine, 0 . 4-0.6 mg/d;.
In 2015 while in high school, Christian Abayisenga started hanging out with friends whom he later found out were smokers -.
natural remedies to help erectile dysfunction The global adult diapers market is set to gain momentum from the increasing prevalence of incontinence worldwide. The Global Forum on Incontinence states that in 2018, more than 424 million people all.
Why Does Erectile Dysfunction Happen with Prostate Cancer Treatment?. However, you may find that you cannot have an erection even a year or more after surgery.. There is an old saying that a “problem shared is a problem halved.. strong feelings of pleasure and normally by ejaculation of semen by the male and by.
erectile dysfunction and treatment Various treatments, such as vacuum constriction. for the literature search included “penile rehabilitation”, “erectile dysfunction following radical prostatectomy”, “phosphodiesterase.erectile dysfunction los angeles home remedies erectile dysfunction free Erectile dysfunction (ED), also known as impotence, is a type of sexual dysfunction. erectile dysfunction. From Wikipedia, the free encyclopedia. In psychological impotence, there is a strong response to placebo treatment. The term erectile.One Thursday evening in September, two or three dozen of the most influential people in this city gathered at the home of a.
Residency · Summer Fellowships · Old Urology Video Series Page. The Male Sexual Dysfunction Panel was created in 2013 by the American. risk factors is paralleled by the worldwide increase in the prevalence of ED.15, 28-30. risk).19 Prevalence of ED is higher in men with diabetes who are older than 50 years,
source https://www.erectiledysfunction-pills.com/erectile-dysfunction-28-year-old-male/
0 notes
Text
Thrombectomy Devices Market Research Report: Forecast up to 2022
As per the findings of a fresh intelligence study by Transparency Market Research (TMR), The Global Thrombectomy Market is largely fragmented, which is a reflection of the presence of a vast number of International and domestic companies. That being said, a few players are ahead of the curve too, such as Stryker Corporation, whose strategy of maintaining a strong product portfolio and acquisition is bearing fruits. The TMR report identifies Argon Medical Devices Inc., Boston Scientific Corporation, BTG International Ltd., Edwards Lifesciences Corporation, Johnson & Johnson, Medtronic PLC, Penumbra, Inc., Phenox GmbH, Spectranetics Corporation, Terumo Corporation, and Teleflex Incorporated as some of the other companies holding a prominent position in the global thrombectomy devices market.
View Report-
https://www.transparencymarketresearch.com/thrombectomy-devices-market.html
Demand for Thrombectomy Devices Estimated to Increment at CAGR 6.1% until 2022
As per the projections of the TMR report, the demand in the global thrombectomy devices market will increment at a healthy CAGR of 6.1% during the forecast period of 2017 to 2022. The overall revenue in the market is estimated to reach a valuation of US$1,974.5 mn by 2022, substantially up from the evaluated worth of the global thrombectomy devices market at US$1,470.6 mn in 2017. In the near future, the key companies are expected to invest on the research and development of advanced products, acquire strategically, price their products aggressively, and put emphasis on strengthening their sales force across various geographies to gain shares. For instance, Penumbra launched thrombectomy device ACE 68 Reperfusion Catheter in July 2016, opening it for commercial usage in and across the U.S. These catheters makes use of the latest tracking technology and can deliver maximum aspiration power for the exaction of thrombus in stroke intervention. Boston Scientific is another highly respectable name in the global thrombectomy devices market, leading the innovation for peripheral vascular disease in the industry and consistently expands its product portfolio.
Product-wise, the global thrombectomy devices market currently gains maximum demand for aspiration thrombectomy devices, which was valued at US$420.6 mn in 2017, representing 28.6% of the total demand that year. The absolute growth of this segment is expected to remain larger than any other segment. Geographically, the developed country of the U.S. makes North America the most lucrative region, which is poised to generate a demand for thrombectomy devices worth of US$776.3 mn by 2022. The demand in the North America thrombectomy market is anticipated to increment at a CAGR of 6.4% during the forecast period of 2017 to 2022.
Request to download and view full ToC -
https://www.transparencymarketresearch.com/report-toc/30911
Growing Population of Potential Patients Augmenting Demand
The demand in the global thrombectomy devices market is driven by a number of factors, such as growing population of targeted patients, consistent improvements made in the technology, favorable medical reimbursement scenario in a number of developed, increasing expenditure on healthcare in several emerging markets, and growing demand for minimally invasive thrombectomy procedures. The TMR report detects a growing trend of acquisition of small specialized clinics by tier-1 hospitals, which is influencing an increase in the patient care management, as it helps in the training of healthcare staff, nurses, and physicians. Rise in the prevalence of cardiovascular and cerebrovascular diseases and technological advancements with the advent of laser and UV radiation are some of the other factors augmenting the demand in the global thrombectomy devices market.
Request to view Sample Report -
https://www.transparencymarketresearch.com/sample/sample.php?flag=S&rep_id=30911
Limited Number of Skilled Surgeons Obstructing Market’s Prosperity
The global thrombectomy devices market has a fruitful future, but the prosperity of the market is challenged quite a factors too, such as stringent regulatory scenario, dearth of skilled and experience surgeons, limited awareness among the patients regarding the latest treatment procedures for peripheral vascular diseases, increase in product recalls, and adverse effects of thrombectomy devices.
Thrombectomy Devices Market Report is available @ US$ 5795
http://www.transparencymarketresearch.com/checkout.php?rep_id=30911<ype=S
About Us
Transparency Market Research (TMR) is a market intelligence company, providing global business information reports and services. Our exclusive blend of quantitative forecasting and trends analysis provides forward-looking insight for thousands of decision makers. TMR’s experienced team of analysts, researchers, and consultants, use proprietary data sources and various tools and techniques to gather, and analyze information. Our business offerings represent the latest and the most reliable information indispensable for businesses to sustain a competitive edge.
Each TMR syndicated research report covers a different sector – such as pharmaceuticals, chemicals, energy, food & beverages, semiconductors, med-devices, consumer goods and technology. These reports provide in-depth analysis and deep segmentation to possible micro levels. With wider scope and stratified research methodology, TMR’s syndicated reports strive to provide clients to serve their overall research requirement.
US Office Contact
90 State Street, Suite 700
Albany, NY 12207
Tel: +1-518-618-1030
USA – Canada Toll Free: 866-552-3453
Email: [email protected]
Website: http://www.transparencymarketresearch.com
0 notes