#Global supplier of API Intermediates
Explore tagged Tumblr posts
Text
Future Trends in API Intermediate Supply & Demand
The pharmaceutical industry is constantly evolving, and the supply and demand for API intermediates are undergoing significant changes. As the global healthcare landscape grows, the need for high-quality API intermediates continues to rise. A global supplier of API intermediates must adapt to emerging trends, including advancements in manufacturing, regulatory shifts, and increasing demand for specialized pharmaceutical raw materials. Companies like Jay Fine Chem are at the forefront of these transformations, ensuring that they meet the ever-changing needs of the industry.
With the increasing complexity of drug formulations, API intermediates manufacturers are facing new challenges and opportunities. From the rise of precision medicine to the impact of supply chain disruptions, understanding these trends is crucial for pharmaceutical intermediates suppliers and bulk drug intermediates exporters. By analyzing future developments in API intermediate production, manufacturers can prepare for the next wave of pharmaceutical advancements.
The Growing Demand for High-Quality API Intermediates
The pharmaceutical industry’s demand for API intermediates is expected to rise significantly in the coming years. The increasing prevalence of chronic diseases, expanding geriatric populations, and growing investments in drug development are key factors driving this demand. As a result, pharmaceutical intermediates suppliers must ensure a consistent supply of high-quality intermediates to meet the requirements of drug manufacturers worldwide.
A global supplier of API intermediates must also focus on improving manufacturing efficiency and regulatory compliance. With stricter quality standards set by organizations such as the FDA and EMA, API intermediates manufacturers need to adopt advanced production techniques that ensure the highest purity and efficacy of pharmaceutical raw materials. Companies that can maintain high standards while optimizing costs will remain competitive in the evolving market.
Technological Advancements in API Intermediate Production
Technological innovations are playing a major role in shaping the future of API intermediate production. Advanced chemical synthesis techniques, automation, and artificial intelligence (AI) are enhancing manufacturing efficiency, reducing waste, and improving overall product quality. Chemical synthesis services are incorporating AI-driven analytics to predict optimal reaction conditions, streamline production, and minimize human error.
Continuous manufacturing is another major shift in API intermediate production. Unlike traditional batch manufacturing, continuous processes allow for uninterrupted production, improving yield consistency and reducing production costs. A global supplier of API intermediates implementing this method can significantly improve supply chain efficiency while maintaining strict quality control standards. The adoption of automation and smart manufacturing technologies will continue to revolutionize how bulk drug intermediates exporters operate in the coming years.
Regulatory Changes and Their Impact on Supply
As regulatory requirements become more stringent, API intermediates manufacturers must stay updated with evolving compliance guidelines. Regulatory authorities worldwide are imposing stricter quality control measures to ensure drug safety and efficacy. A global supplier of API intermediates must invest in Good Manufacturing Practices (GMP), detailed documentation, and stringent quality assurance protocols to meet these evolving standards.
Pharmacopoeial standards such as the USP, EP, and JP are continuously being updated to ensure higher levels of product consistency and purity. Pharmaceutical intermediates suppliers must align their production processes with these standards to ensure seamless global distribution. Additionally, environmental regulations are becoming more prominent, requiring chemical synthesis services to adopt greener manufacturing practices and reduce their ecological footprint.
The Impact of Supply Chain Disruptions
Global supply chain disruptions have had a significant impact on the pharmaceutical industry, particularly in the sourcing and distribution of API intermediates. The COVID-19 pandemic highlighted vulnerabilities in pharmaceutical supply chains, leading to increased efforts in diversifying raw material sources and reducing dependency on single suppliers. A global supplier of API intermediates must now implement risk management strategies to ensure a steady supply of essential raw materials.
Geopolitical tensions, trade restrictions, and logistical challenges continue to affect bulk drug intermediates exporters. Many pharmaceutical companies are now looking for local or regional API intermediates manufacturers to mitigate risks associated with international supply chains. This shift is driving the growth of domestic manufacturing hubs and encouraging companies to invest in resilient supply chain strategies.
The Rise of Specialty API Intermediates
With advancements in precision medicine and biologics, there is an increasing demand for specialty API intermediates tailored for specific drug formulations. Pharmaceutical raw materials used in targeted therapies and biosimilars require advanced synthesis techniques and highly controlled production environments. A global supplier of API intermediates specializing in custom synthesis is better positioned to meet the needs of pharmaceutical companies developing next-generation medicines.
Biopharmaceuticals and complex generic drug formulations are also driving demand for high-purity API intermediates. Pharmaceutical intermediates suppliers must focus on enhancing their chemical synthesis capabilities to produce specialized intermediates for these advanced drug formulations. The ability to offer customized chemical synthesis services will be a key differentiator in the evolving pharmaceutical market.
Sustainability and Green Chemistry in API Manufacturing
Sustainability is becoming a priority for API intermediates manufacturers, with many companies adopting green chemistry principles to reduce environmental impact. Traditional chemical synthesis processes often involve hazardous reagents and generate waste that can harm the environment. The industry is now shifting towards eco-friendly alternatives that improve process efficiency while minimizing waste generation.
A global supplier of API intermediates investing in sustainable practices can enhance its reputation while ensuring compliance with stringent environmental regulations. Green chemistry approaches include solvent recovery, process intensification, and the use of biodegradable catalysts. Bulk drug intermediates exporters implementing these methods can improve cost efficiency and contribute to a more sustainable pharmaceutical industry.
The Future of API Intermediate Pricing and Cost Dynamics
The cost of API intermediates is influenced by multiple factors, including raw material availability, regulatory compliance costs, and manufacturing efficiency. As demand continues to grow, pharmaceutical intermediates suppliers must find ways to balance cost-effectiveness with high-quality production. A global supplier of API intermediates that invests in automation, process optimization, and supply chain resilience can maintain competitive pricing while ensuring consistent product quality.
Raw material shortages and increasing labor costs are also affecting pricing dynamics. Companies that diversify their sourcing strategies and invest in alternative raw materials will have a competitive advantage. Additionally, the use of digital supply chain management tools can help API intermediates manufacturers predict demand fluctuations and optimize inventory levels to reduce production costs.
The Role of Digital Transformation in API Supply Chain Management
Digital transformation is reshaping how bulk drug intermediates exporters manage their supply chains. Advanced data analytics, blockchain technology, and cloud-based management systems are improving traceability, security, and efficiency in API intermediate distribution. A global supplier of API intermediates utilizing digital supply chain solutions can enhance transparency and minimize disruptions.
Blockchain technology, in particular, is gaining traction as a tool for ensuring supply chain integrity. By providing an immutable record of transactions, blockchain enhances traceability and reduces the risk of counterfeit pharmaceutical raw materials entering the market. Pharmaceutical intermediates suppliers adopting digital solutions can improve regulatory compliance, streamline logistics, and build stronger relationships with pharmaceutical manufacturers.
Conclusion
The future of API intermediate supply and demand is being shaped by technological advancements, regulatory changes, and evolving market needs. A global supplier of API intermediates must stay ahead of these trends to ensure consistent quality, regulatory compliance, and competitive pricing. As the demand for high-purity pharmaceutical raw materials continues to rise, API intermediates manufacturers must embrace innovation to enhance production efficiency and sustainability.Companies like Jay Fine Chem are leading the way in adapting to these emerging trends. By investing in advanced chemical synthesis services, digital transformation, and environmentally friendly manufacturing processes, the industry can meet the growing demands of pharmaceutical companies worldwide. As the pharmaceutical landscape evolves, pharmaceutical intermediates suppliers that prioritize innovation and regulatory excellence will remain at the forefront of API intermediate production.
0 notes
Text
Leading Pharma Chemical Suppliers in India – Amizara Chemicals
Amizara Chemicals is the leading pharma chemicals suppliers in India. India’s pharmaceutical industry has witnessed exponential growth, becoming one of the largest global producers of generic medicines and active pharmaceutical ingredients (APIs). At the heart of this growth lies a strong network of reliable chemical suppliers who ensure consistent availability of high-quality raw materials. One such prominent name in this sector is Amizara Chemicals, a trusted supplier of pharmaceutical-grade chemicals across India.

Who is Amizara Chemicals?
Amizara Chemicals is a leading supplier of industrial and pharmaceutical chemicals, serving a wide range of industries including pharmaceuticals, biotech, agrochemicals, and specialty chemicals. Headquartered in Mumbai, the company has built a reputation for offering superior-quality chemicals, timely delivery, competitive pricing, and customer-focused service.
Our commitment to quality, safety, and regulatory compliance has made us a preferred partner for pharmaceutical manufacturers and research laboratories across India. We offer a broad portfolio of pharma-grade chemicals essential for synthesis, formulation, and various drug development processes.
Our Key Pharmaceutical Chemicals
1. Acetone Chemicals
Acetone is one of the most commonly used solvents in the pharmaceutical industry. It plays a critical role in the extraction, purification, and crystallization of various drug compounds. At Amizara Chemicals, we provide high-purity acetone suitable for lab and industrial use. Our acetone complies with industry standards and is ideal for drug formulation and cleaning purposes in GMP environments.
2. Acetonitrile Chemicals
Acetonitrile is widely used in high-performance liquid chromatography (HPLC) and as a solvent in organic synthesis. It’s essential in pharmaceutical analysis and the production of vitamins and antibiotics. We supply high-quality acetonitrile with consistent purity, ensuring accuracy and reproducibility in critical applications.
3. Cyclohexanone Chemicals
Cyclohexanone serves as a key intermediate in the production of active pharmaceutical ingredients. It’s used in the manufacturing of antibiotics, anti-inflammatory drugs, and other pharmaceutical formulations. Amizara Chemicals offers pharma-grade cyclohexanone with strict adherence to safety and purity norms.
4. Diisopropyl Ether
Diisopropyl Ether is a low-polarity solvent used in various pharmaceutical extractions and crystallization processes. It is especially useful in the purification of APIs. Our Diisopropyl Ether is supplied in leak-proof packaging and conforms to pharmaceutical-grade specifications, ensuring both safety and effectiveness.
5. Dimethyl Sulphoxide (DMSO)
DMSO is known for its exceptional solvent properties and ability to penetrate biological membranes. It is widely used in drug delivery research and pharmaceutical formulations. Amizara Chemicals provides ultra-pure DMSO that supports both R&D and commercial scale manufacturing, maintaining consistency across all batches.
6. Methane Sulfonyl Chloride
Methane Sulfonyl Chloride is an important reagent in organic synthesis and pharmaceutical intermediate production. It is used in sulfonation reactions that lead to the development of several therapeutic compounds. At Amizara Chemicals, we supply highly stabilized Methane Sulfonyl Chloride, ensuring safe storage and reliable results in sensitive processes.
Why Choose Amizara Chemicals?
Unmatched Quality: We follow rigorous quality assurance protocols to deliver chemicals that meet international standards.
Wide Inventory: From solvents to intermediates, we stock a comprehensive range of chemicals ready for immediate dispatch.
Customer-Centric Approach: We offer customized solutions, including bulk packaging, timely deliveries, and technical support.
Regulatory Compliance: All our products are sourced and handled as per regulatory guidelines to support your pharma compliance needs.
Industries We Serve
While we specialize in pharmaceutical-grade chemicals, our offerings also support biotech firms, research laboratories, contract manufacturing organizations (CMOs), and chemical research institutes across India.
Conclusion
In a sector where quality and consistency are non-negotiable, Amizara Chemicals stands as a dependable partner for pharmaceutical chemical needs. Whether it’s high-purity solvents like Acetone and Acetonitrile or key intermediates like Methane Sulfonyl Chloride and DMSO, we ensure that you get the right chemical for the right application — delivered safely, swiftly, and sustainably.
Frequently Asked Questions (FAQs)
Q1: What industries does Amizara Chemicals serve? A: While our primary focus is the pharmaceutical sector, we also serve industries such as biotechnology, agrochemicals, cosmetics, and specialty chemicals. Our diverse portfolio ensures we can cater to various sectors with tailored chemical solutions.
Q2: Do you supply pharma-grade chemicals in bulk quantities? A: Yes, we specialize in bulk supply of pharmaceutical-grade chemicals. Whether you need small lab quantities or large industrial-scale volumes, we can accommodate your requirements with reliable logistics and timely deliveries.
Q3: What quality standards do your products comply with? A: All our chemicals meet stringent quality benchmarks and regulatory guidelines. We provide high-purity products with proper COA (Certificate of Analysis), MSDS (Material Safety Data Sheet), and batch testing reports as needed.
Q4: Can you supply chemicals like Acetone, Acetonitrile, and DMSO for research purposes? A: Absolutely. We supply these solvents and chemicals in both lab-grade and pharma-grade variants, suitable for research, analysis, and production.
Q5: How do you ensure safe packaging and transportation of chemicals? A: We follow industry best practices for chemical packaging, labeling, and transport. Leak-proof, corrosion-resistant containers are used to ensure safety during transit, along with proper hazard documentation.
Q6: Do you offer technical support or product consultation? A: Yes, our expert team is available to provide technical support, suggest alternatives, and assist with product compatibility or process integration queries.
Q7: How can I place an order with Amizara Chemicals? A: You can contact us via our website, email ([email protected]), or call us at +91–9221044646. Our team will guide you through the ordering process, provide quotations, and arrange delivery as per your needs.
#pharma chemicals#pharmabusiness#chemical dealers#chemical suppliers#bulkchemicalsindia#amizarachemicals#chemicalcompanyindia#chemicalsmanufacturers#chemicalsolutions#chemicaldealers#chemicalmanufacturersindia#pharmaindustry
0 notes
Text

Vidgastech – Ruxolitinib Intermediates Manufacturer
Vidgastech is a leading manufacturer and global supplier of Ruxolitinib Intermediates. We specialize in high-purity pharmaceutical intermediates for API production, supporting the global pharma industry with quality, consistency, and innovation.
#Ruxolitinib Intermediates#Pharmaceutical Intermediates#Ruxolitinib Raw Material#API Supplier#Vidgastech#GMP Manufacturer#Bulk Drug Intermediates#Custom Synthesis
0 notes
Text
Best 2-DIETHYLAMINOETHYL CHLORIDE HCL (DEC) Manufacturer in Philippines

Introduction
The pharmaceutical industry depends heavily on high-quality chemical intermediates for the production of life-saving medicines. One such essential compound is 2-Diethylaminoethyl Chloride Hydrochloride. It is widely used in the synthesis of antihistamines, local anesthetics, and other pharmaceutical drugs. With rising global demand, choosing the best 2-DIETHYLAMINOETHYL CHLORIDE HCL (DEC) Manufacturer in Philippines ensures access to a consistent supply of this critical ingredient.
A trusted pharmaceutical drug manufacturer in Philippines focuses on providing superior-grade intermediates that meet global regulatory standards, ensuring safety and performance in drug development.
What is 2-Diethylaminoethyl Chloride Hydrochloride?
2-Diethylaminoethyl Chloride Hydrochloride is a chemical compound that plays a key role in the production of several pharmaceutical agents. It is mainly used as an intermediate in the synthesis of:
Antihistamines
Local anesthetics
Antispasmodic agents
Neurological medications
This compound is highly reactive and requires precise handling and manufacturing controls to maintain its purity and effectiveness.
Applications in the Pharmaceutical Industry
The primary use of 2-Diethylaminoethyl Chloride Hydrochloride is in drug synthesis. Pharmaceutical companies rely on its chemical properties to produce active pharmaceutical ingredients (APIs). Some important applications include:
Development of pain-relief and nerve-related medications
Production of drugs that control allergic reactions
Preparation of therapeutic compounds for gastrointestinal treatment
Using this compound in medicine manufacturing helps improve drug efficacy and patient outcomes.
Why Choose a Manufacturer from the Philippines?
1. High-Quality Standards
Manufacturers in the Philippines follow strict Good Manufacturing Practices (GMP) and adhere to global quality standards. Their production systems are audited regularly to maintain consistency and safety.
2. Advanced Technology
Facilities in the Philippines use advanced chemical synthesis and quality testing techniques. This ensures every batch of 2-Diethylaminoethyl Chloride Hydrochloride is reliable and meets global requirements.
3. Skilled Workforce
The Philippines has a skilled scientific workforce trained in chemical engineering, pharmaceutical manufacturing, and research. This helps ensure accurate production and quality control.
4. Affordable Manufacturing
The country offers cost-effective solutions without compromising product quality. This makes it ideal for companies seeking affordable but high-standard pharmaceutical intermediates.
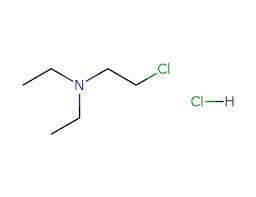
5. Global Supply Chain Experience
A leading pharmaceutical drug manufacturer in Philippines is familiar with international logistics and export documentation. They can support clients worldwide with timely delivery and proper regulatory paperwork.
How to Select the Right Manufacturer?
When choosing a 2-Diethylaminoethyl Chloride Hydrochloride manufacturer in Philippines, look for the following:
Proven track record in pharmaceutical intermediate production
Certification in GMP, ISO, and other global standards
Modern facilities with in-house R&D and quality testing labs
Ability to supply custom quantities — from small to bulk orders
Transparent communication and customer support
Sustainability and Safety
Manufacturers in the Philippines also emphasize eco-friendly practices and workplace safety. They implement waste management systems, safe handling protocols, and energy-efficient production methods.
These initiatives make them reliable and responsible manufacturing partners.
Conclusion
If you're looking for a dependable supplier of 2-Diethylaminoethyl Chloride Hydrochloride, the Philippines stands out as a competitive and trustworthy location. With a focus on quality, affordability, and global supply capabilities, the country is home to some of the best pharmaceutical drug manufacturers in Philippines. These manufacturers not only offer top-grade products but also ensure you receive regulatory-compliant, timely deliveries worldwide.
#2-DIETHYLAMINOETHYL CHLORIDE HCL (DEC) Manufacturer in Philippines#2-DIETHYLAMINOETHYL CHLORIDE HCL (DEC) supplier in Philippines#2-DIETHYLAMINOETHYL CHLORIDE HCL (DEC) exporter in Philippines
0 notes
Text
The Role of 4-Desdimethylamine 4-Oxo Omadacycline in Antibiotic Development
The pharmaceutical industry continues to innovate in the fight against antibiotic resistance, and 4-Desdimethylamine 4-Oxo Omadacycline stands out as a pivotal intermediate in this mission. As a structural precursor in the synthesis of omadacycline, this compound plays a central role in enabling the production of next-generation antibiotics with improved efficacy and resistance profiles.
What is 4-Desdimethylamine 4-Oxo Omadacycline?
4-Desdimethylamine 4-Oxo Omadacycline is a chemically engineered intermediate designed for use in the synthesis of omadacycline, a novel aminomethylcycline antibiotic. Its molecular structure is critical in allowing modifications that enhance antibacterial activity while maintaining a broad spectrum of action, even against drug-resistant bacteria.
This compound is frequently used in:
Pharmaceutical R&D pipelines
Structure-activity relationship (SAR) studies
Intermediate stages of GMP-grade active pharmaceutical ingredient (API) manufacturing
Explore this product: 4-Desdimethylamine 4-Oxo Omadacycline — Aquigen Bio
Related Omadacycline Intermediates & Impurities
In addition to 4-Desdimethylamine 4-Oxo Omadacycline, other related compounds serve important functions in synthesis, impurity profiling, and pharmacological testing. These include:
1. Dihydro Omadacycline Impurity
This impurity is commonly encountered in omadacycline synthesis and is crucial for analytical method development and stability testing. It plays a vital role in ensuring regulatory compliance through impurity profiling.
2. N-(Hydroxymethyl) Omadacycline
Often used in the synthesis of modified tetracycline derivatives, this compound helps scientists optimize structural analogues and improve pharmacokinetic profiles.
3. N-Methyl Omadacycline
This methylated analogue is studied for its structural influence on omadacycline’s antibacterial spectrum and resistance stability. It can also be useful in toxicity and metabolic pathway studies.
Why These Compounds Matter
Each of these compounds, including 4-Desdimethylamine 4-Oxo Omadacycline, contributes uniquely to the research and development of new antibiotics:
Enhancing efficacy against resistant pathogens
Enabling structural modifications for drug optimization
Supporting analytical research through impurities and metabolites
Together, they form a toolkit for scientists working to stay ahead in the global battle against antimicrobial resistance.
Sourcing from a Trusted Supplier
For researchers and manufacturers seeking reliable, high-purity antibiotic intermediates and impurities, Aquigen Bio offers:
GMP-compliant sourcing
Consistent batch quality
Responsive technical support
Whether you’re scaling synthesis or conducting early-stage research, Aquigen Bio ensures access to compounds that meet both scientific and regulatory demands.
Final Thoughts
As antibiotic innovation accelerates, key intermediates like 4-Desdimethylamine 4-Oxo Omadacycline, along with related compounds like Dihydro Omadacycline Impurity, N-(Hydroxymethyl) Omadacycline, and N-Methyl Omadacycline, will continue to empower breakthroughs in drug discovery and development.
Looking for a quote or COA? Visit Aquigen Bio or explore their Contact Page to connect with a specialist.
0 notes
Text
Anisole Prices Index: Trend, Chart, News, Graph, Demand, Forecast
In the first quarter of 2025, the global anisole market experienced a downward price trajectory across key regions including North America, Asia-Pacific, and Europe, primarily influenced by fluctuating feedstock availability, evolving demand patterns, and global economic uncertainties. Anisole, a key intermediate chemical used in the production of fragrances, pharmaceuticals, and agrochemicals, saw its market behavior shaped by both upstream and downstream factors. In North America, particularly in the United States, anisole prices consistently declined throughout the quarter. January marked the beginning of this bearish phase, driven by weakened demand from personal care and industrial sectors. Reduced procurement activities coupled with steady domestic production created a supply-demand imbalance, pushing prices downward. By February, this trend intensified as lower ocean freight rates reduced import costs and geopolitical tensions disrupted the supply of phenol, a major feedstock for anisole synthesis. These disruptions, while initially expected to support prices due to potential shortages, instead led to market uncertainty and conservative purchasing behavior.
As March unfolded, anisole prices continued to decrease in the U.S. market. Even though Chinese production levels remained largely stable, persistent supply chain bottlenecks such as port congestion and fluctuating freight conditions made the timely movement of goods more challenging. Despite these logistical complications, inventory levels remained high due to sluggish demand recovery, particularly in the mass-market beauty segment, which reported a decline in consumer spending. However, the premium personal care segment demonstrated marginal growth, reflecting a consumer shift toward sustainability and higher-quality products. This shift, while notable, was not sufficient to offset the broader reduction in demand. Meanwhile, the pharmaceutical sector offered a cushion to market stability as it maintained steady consumption of anisole for the synthesis of active pharmaceutical ingredients (APIs). This application segment remained a cornerstone of demand throughout the quarter, helping prevent a sharper price collapse despite broader market weakness.
Get Real time Prices for Anisole: https://www.chemanalyst.com/Pricing-data/anisole-1123
In the Asia-Pacific region, the anisole price trend presented a more dynamic pattern during the first quarter. January began on a strong note with a notable price increase, especially in China, where robust demand from the pharmaceutical sector and steady consumption in the personal care industry supported market sentiment. Rising feedstock costs further contributed to bullish pricing, as manufacturers faced increased input expenses. However, this positive momentum was short-lived. February brought a shift in market dynamics as overstocking from the previous month led to a surplus in supply, while economic uncertainties caused buyers to adopt a more cautious stance. As a result, anisole prices softened, and transactional activity slowed. By March, the bearish outlook intensified due to persistent inventory overhang and insufficient demand revival. Even though personal care consumption showed resilience, it could not offset the excess material in the market. Consequently, producers began offering discounts to move stock, further depressing prices.
In Europe, anisole prices also followed a declining path throughout Q1 2025. Germany, as a leading market, witnessed consistent price drops from January through March. At the start of the quarter, the pharmaceutical industry experienced a downturn, significantly reducing demand for anisole. Elevated stock levels from late 2024 weighed heavily on suppliers, who had to implement price cuts to stimulate sales. February brought little relief as freight rates declined and domestic production operated at moderate but steady levels, contributing to persistent oversupply. Although geopolitical tensions affected feedstock imports, their impact was diluted by the broader lack of demand. On the brighter side, the cosmetics sector showed steady performance, supported by consumer demand and regulatory initiatives such as the Critical Medicines Act, which bolstered local pharmaceutical production. However, this support was not robust enough to reverse the overall downward pricing pressure.
By March, logistical efficiencies returned to European markets with fewer disruptions related to port congestion and trade tariffs, yet the price decline persisted due to unchanged demand fundamentals. While the cosmetics industry in the region continued to show modest growth, producers remained cautious with their forecasts, mindful of the lingering geopolitical uncertainties and the pace of recovery in pharmaceutical applications. Across all regions, a key theme emerged: the anisole market in Q1 2025 was predominantly shaped by external macroeconomic pressures, sector-specific demand variation, and supply chain adjustments rather than any major shifts in production technology or regulatory environment. The persistent oversupply in most markets, coupled with conservative buyer sentiment and selective end-use growth, created an environment of price erosion.
Looking ahead, market participants are expected to monitor feedstock phenol availability, freight cost fluctuations, and policy shifts in pharmaceutical production to assess potential price recovery in the coming quarters. The rising interest in premium and sustainable personal care products could support long-term demand for anisole, particularly if economic conditions improve and consumer confidence rebounds. Additionally, the pharmaceutical industry’s continued reliance on anisole as a precursor may provide a degree of price stability despite broader volatility. In conclusion, the anisole market in Q1 2025 exhibited a generally bearish tone driven by supply-demand imbalances, shifting sectoral demand, and complex logistical and geopolitical factors, with cautious optimism prevailing regarding future recovery.
Get Real time Prices for Anisole: https://www.chemanalyst.com/Pricing-data/anisole-1123
Contact Us:
ChemAnalyst
GmbH - S-01, 2.floor, Subbelrather Straße,
15a Cologne, 50823, Germany
Call: +49-221-6505-8833
Email: [email protected]
Website: https://www.chemanalyst.com
0 notes
Text
Chemox Pharma Powers Global TB & Diabetes Drug Supply
The global medicinal force chain plays a critical part in icing the vacuity of life- saving specifics for conditions like tuberculosis( TB) and diabetes. As a leading manufacturer of high- quality intermediates, Chemox Pharma serves as a strategic mate in this force chain, enabling the product of essential medicines similar as Bedaquiline for TB and SGLT2 impediments for diabetes. By supplying crucial intermediates with perfection, trustability, and scalability, Chemox Pharma helps pharmaceutical companies meet global demand, ameliorate treatment availability, and eventually save lives.

The Global Burden of TB and Diabetes Tuberculosis and diabetes are two of the world's most burning health challenges. TB remains one of the deadliest contagious conditions, with millions of new cases reported annually. medicine- resistant TB strains, similar as multidrug- resistant TB( MDR- TB), bear advanced treatments like Bedaquiline, a advance medicine that has significantly bettered issues for cases. On the other hand, diabetes, particularly Type 2 diabetes, has reached epidemic proportions, affecting over 500 million people worldwide. SGLT2 impediments, a class of antidiabetic medicines, have surfaced as a vital treatment option due to their efficacity in managing blood glucose situations and reducing cardiovascular pitfalls. The product of these life- saving specifics depends on a steady force of high- quality intermediates the structure blocks used in their conflation. This is where Chemox Pharma plays a vital part. Chemox Pharma's part inAnti-TB Drug Production Bedaquiline Intermediates Bedaquiline, retailed under the brand name Sirturo, is a foundation in the treatment of MDR- TB. Its complex molecular structure requires technical intermediates for effective and cost-effective manufacturing. Chemox Pharma supplies critical intermediates that insure the flawless product of Bedaquiline, addressing crucial challenges similar as Consistency & Purity: High- chastity intermediates are essential to maintain the medicine's efficacity and safety. Chemox Pharma adheres to strict quality control measures, icing that every batch meets global nonsupervisory norms. Scalability: With TB affecting millions, the demand for Bedaquiline is ever- adding . Chemox Pharma's scalable product capabilities help manufacturers ramp up force without compromising quality. Cost- Effectiveness: By optimizing conflation routes and reducing product costs, Chemox Pharma enables more affordable access to Bedaquiline, particularly in low- and middle- income countries where TB is most current. Through these benefactions, Chemox Pharma supports global health enterprise like the WHO's End TB Strategy, icing that cases admit timely and effective treatment. Supporting Diabetes Treatment SGLT2 Asset Intermediates SGLT2 impediments, including medicines like Empagliflozin, Dapagliflozin, and Canagliflozin, have revolutionized diabetes operation. These medicines work by precluding glucose reabsorption in the feathers, leading to better glycemic control and fresh benefits like weight loss and heart protection. The conflation of SGLT2 impediments relies on advanced intermediates, and Chemox Pharma serves as a trusted supplier by furnishing
High- Quality structure Blocks: The efficacity of SGLT2 impediments depends on the perfection of their chemical conflation. Chemox Pharma's intermediates insure that the final API( Active Pharmaceutical component) meets strict pharmacopeial norms.
Regulatory Compliance: With diabetes being a habitual condition taking long- term drug, nonsupervisory compliance isnon-negotiable. Chemox Pharma's intermediates are manufactured in compliance with FDA, EMA, and other global nonsupervisory conditions.
Supply Chain Reliability: Diabetes is a lifelong condition, and any dislocation in medicine force can have severe consequences. Chemox Pharma's robust force chain ensures continued vacuity of intermediates, precluding product detainments.
By easing the effective product of SGLT2 impediments, Chemox Pharma helps ameliorate the quality of life for millions of diabetic cases worldwide. Why Chemox Pharma Stands Out as a Strategic Partner Technical Expertise With deep knowledge of complex chemistry, Chemox Pharma develops intermediates that streamline API conflation, reducing time and cost for medicine manufacturers.
Custom Synthesis Solutions: Beyond standard intermediates, Chemox Pharma offers acclimatized results to meet specific client conditions, accelerating medicine development timelines.
Sustainability & Innovation: The company invests in green chemistry and sustainable manufacturing practices, minimizing environmental impact while maintaining high effectiveness.
Global Reach: Serving pharmaceutical companies across North America, Europe, and Asia, Chemox Pharma ensures that critical intermediates are available wherever they're demanded.
Conclusion Chemox Pharma's part in the global medicinal force chain is necessary. By supplying high- quality intermediates for medicines like Bedaquiline and SGLT2 impediments, the company helps combat two of the world's most grueling conditions — TB and diabetes. With a commitment to quality, scalability, and invention, Chemox Pharma strengthens the capability of medicine manufacturers to deliver life- saving curatives to cases worldwide. As the demand for effective TB and diabetes treatments continues to rise, Chemox Pharma remains a trusted mate, icing that the trip from intermediate to finished drug is flawless, dependable, and poignant. Through strategic collaboration and nonstop enhancement, Chemox Pharma contributes to a healthier future for all.
0 notes
Text
B.J. Madan & Co.: Pioneering Excellence in Peptide Manufacturing
In the dynamic field of pharmaceuticals and biotechnology, peptides manufacturers have become indispensable. Their role in therapeutic drug development, diagnostics, and cutting-edge research continues to expand rapidly. In this high-precision industry, one name that has consistently delivered quality and trust is B.J. Madan & Co., a leading manufacturer and supplier of pharmaceutical-grade peptides in India and abroad.
Why Peptides Are the Future of Therapeutics
Peptides, short chains of amino acids, are vital to many biological functions. They are increasingly being used in:
Therapeutic Drugs: For treating cancers, diabetes, cardiovascular diseases, and more.
Cosmeceuticals: As active agents in anti-aging and skin-repair products.
Nutraceuticals: Supporting athletic performance and general wellness.
Biotech Research: As molecular tools in genomics, proteomics, and drug discovery.
Precision, purity, and scalability are key requirements in peptide production—areas where B.J. Madan & Co. has built a legacy.
B.J. Madan & Co.: A Legacy of Pharmaceutical Excellence
Founded in 1939, B.J. Madan & Co. has grown from a respected pharmaceutical company into a trusted peptide manufacturer, offering high-quality solutions tailored to research and commercial needs. With decades of industry knowledge and a strong regulatory framework, the company is recognized for its reliable supply of niche and hard-to-source APIs, intermediates, and now, peptides.
What Sets B.J. Madan Apart in Peptide Manufacturing:
Deep Technical Expertise: A team of skilled chemists and process engineers develop and optimize peptide synthesis routes.
Quality Assurance: Rigorous testing and validation protocols ensure every batch meets global standards (IP, BP, USP, Ph. Eur.).
Custom Synthesis Services: Offering tailored peptides for R&D, pilot studies, and commercial supply.
Global Distribution Network: Serving pharmaceutical companies, CROs, and research institutions worldwide.
Focus on Quality and Innovation
With an emphasis on quality, compliance, and innovation, B.J. Madan ensures that all peptides undergo extensive analytical testing—including HPLC, mass spectrometry, and purity analysis. Their facilities operate with strict adherence to Good Manufacturing Practices (GMP) and are continually upgraded to match evolving regulatory requirements.
Your Trusted Partner in Peptide Sourcing
Whether you are a pharmaceutical developer looking for clinical-grade peptides or a research lab in need of custom synthesis, B.J. Madan & Co. provides dependable, scalable solutions. Their reputation for integrity, competitive pricing, and consistent quality makes them a preferred partner in the global life sciences industry.
Conclusion
As peptide-based therapies become more central to modern medicine, having a reliable manufacturer is essential. With its rich history, technical capabilities, and commitment to excellence, B.J. Madan & Co. continues to shape the future of peptide manufacturing—delivering innovation one molecule at a time.
Visit:- https://www.bjmadan.com/peptides.html
0 notes
Text
Active Pharmaceutical Ingredients Manufacturers in India
The Backbone of Global Pharma: Active Pharmaceutical Ingredient (API) Manufacturers in India
India, often dubbed the "Pharmacy of the World," holds a prominent position in the global pharmaceutical landscape. A critical part of this identity stems from its robust and expansive ecosystem of Active Pharmaceutical Ingredient (API) manufacturing. APIs are the core components of any drug formulation—responsible for producing the intended therapeutic effect. Over the decades, India has emerged as a leading supplier of these crucial drug substances, catering to domestic demands and supplying to markets across the globe.
What Are Active Pharmaceutical Ingredients?
An Active Pharmaceutical Ingredient (API) is the biologically active component in a pharmaceutical drug. While most medications contain multiple ingredients—including stabilizers, preservatives, and fillers—the API is the element that produces the desired pharmacological activity. For example, in a pain relief tablet, the compound that actually alleviates the pain is the API, while other substances help in delivering it effectively within the body.
APIs can be synthesized through chemical processes or derived from natural sources, and more recently, biotechnological methods have become increasingly prevalent. Their production is subject to stringent regulatory oversight, given the direct impact on drug safety and efficacy.
The Rise of India’s API Manufacturing Sector
India’s rise as an API powerhouse didn’t happen overnight. It is the result of decades of policy decisions, infrastructure investments, skilled workforce development, and an entrepreneurial mindset. In the 1970s and 1980s, India implemented a series of patent laws that favored the local production of drugs, especially APIs, without infringing on international patents. This provided a unique environment where domestic companies could innovate and scale.
Additionally, India’s vast base of chemical manufacturing experience and relatively lower production costs compared to Western countries gave it a competitive edge. As Western pharmaceutical companies increasingly outsourced their manufacturing to reduce costs, India became a natural partner.
Strategic Advantages of Indian API Manufacturers
Several factors contribute to India’s continued leadership in API manufacturing:
Cost Efficiency: Lower labor and operational costs allow Indian manufacturers to offer competitive pricing without compromising on quality.
Skilled Workforce: A strong base of chemists, pharmacists, and engineers drives innovation and quality in manufacturing processes.
Regulatory Compliance: Many Indian facilities are approved by major regulatory agencies such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and the World Health Organization (WHO). This global compliance enables Indian APIs to be used in drug formulations worldwide.
Vertical Integration: Many manufacturers in India are vertically integrated, producing both APIs and finished dosage formulations. This integration streamlines production and quality control, ensuring a more reliable supply chain.
Government Support: Recognizing the strategic importance of the API sector, the Indian government has launched various initiatives, such as Production Linked Incentive (PLI) schemes and bulk drug parks, to encourage local API manufacturing and reduce import dependency.
Challenges Facing the Indian API Sector
Despite its many strengths, the Indian API manufacturing sector is not without challenges:
Dependency on Imports for Key Starting Materials (KSMs): A significant proportion of raw materials and intermediates used in API production are imported, particularly from China. This dependency poses a risk to supply chain stability.
Environmental Compliance: API production can involve hazardous chemicals and generate waste. Adhering to increasingly stringent environmental regulations requires continuous investment in clean technologies and waste management systems.
Price Pressures: Global competition and buyer expectations for low costs can erode profit margins, especially for commoditized APIs.
Innovation Gaps: While India excels in manufacturing generics and well-established APIs, innovation in new drug molecules and high-potency APIs (HPAPIs) is still developing.
The Road Ahead
India’s API manufacturing landscape is evolving. With the COVID-19 pandemic highlighting the risks of global supply chain disruptions, there is renewed focus on domestic self-sufficiency and resilience. Initiatives aimed at backward integration—producing more raw materials and intermediates domestically—are gaining traction.
Moreover, advances in green chemistry, continuous manufacturing, and digital process control are being explored to make API production more sustainable and efficient. The growing focus on biosimilars and biopharmaceuticals also opens new avenues for API development beyond traditional small-molecule drugs.
Conclusion
India’s API manufacturers play a critical role not only in the national pharmaceutical ecosystem but also in ensuring global access to affordable, quality medicines. As healthcare needs continue to evolve, and demand for generic drugs and complex therapeutics rises, India is well-positioned to expand its footprint.
To sustain and enhance its leadership, the industry must continue to innovate, invest in sustainability, and reduce its dependency on external sources for key inputs. With coordinated efforts from industry stakeholders and government, India’s API sector will remain a cornerstone of the global pharmaceutical supply chain for years to come.
URL: For more information, visit Bhasya International: Active Pharmaceutical Ingredients Manufacturers in India
0 notes
Text
Bromos Organics – Trusted Bromine Derivatives Manufacturers in India
When it comes to reliable and high-purity brominated compounds, Bromos Organics stands out as one of the top Bromine Derivatives Manufacturers India. With a focus on consistent quality, technical precision, and customer satisfaction, Bromos Organics has carved a niche in the chemical manufacturing sector. Our expertise spans various applications, including pharmaceuticals, agrochemicals, and specialty chemicals, offering clients a dependable source for custom and bulk brominated products.
The Role of Bromine Derivatives in Industrial Applications
Bromine and its derivatives are vital components across multiple industries due to their reactivity and versatility. These compounds play a significant role in:
Pharmaceutical Intermediates: Used in the synthesis of APIs and drug precursors.
Agrochemicals: Essential in producing pesticides, fumigants, and herbicides.
Dyes and Pigments: Aid in color development for textiles and inks.
Flame Retardants: Enhance fire resistance in plastics, textiles, and electronics.
Oil & Gas Drilling: Applied in drilling fluids for better borehole stability.
Given their wide utility, it’s essential to source these compounds from a manufacturer that guarantees high purity, proper handling, and consistent delivery—this is where Bromos Organics delivers unmatched value.
Why Choose Bromos Organics?
As one of the most respected Bromine Derivatives Manufacturers India has to offer, Bromos Organics focuses on delivering quality without compromise. Here's what sets us apart:
1. High-Quality Production Standards
Our manufacturing process is designed to ensure the highest standards of purity and performance. By combining modern techniques with rigorous quality control, we ensure each batch meets industry and regulatory standards.
2. Advanced R&D and Customization
Our in-house R&D team works closely with clients to develop customized brominated solutions tailored to specific industrial applications. Whether you need a niche intermediate or large-scale supply, we adapt to your project needs.
3. Environmentally Responsible Manufacturing
Sustainability is at the core of our operations. Our production methods focus on minimizing waste, reducing emissions, and ensuring safe disposal practices. Our commitment to green chemistry helps support clients seeking sustainable supply chain partners.
4. Comprehensive Product Range
From alkyl bromides and aryl bromides to specialty compounds, Bromos Organics offers a broad spectrum of bromine derivatives. This allows businesses from different sectors to rely on one trusted supplier for all their bromine-based requirements.
Industries We Serve
Our brominated compounds are widely used by industries such as:
Pharmaceutical & Life Sciences
Agrochemical Formulations
Flavors and Fragrances
Chemical Research and Development
Electronics and Plastics Manufacturing
Bromos Organics supports small-scale labs as well as large manufacturing units by delivering consistent quality and scalable production capabilities.
Commitment to Regulatory Compliance
In a highly regulated chemical industry, compliance is critical. Bromos Organics adheres strictly to both Indian and international safety, quality, and environmental guidelines. Our facilities are regularly audited, and we maintain transparent documentation for traceability and confidence.
Nationwide and Global Reach
With a strategically located production facility in Northern India, we efficiently supply clients across India and export to international markets. Our logistics and packaging processes are designed for safe transport and timely delivery, no matter the destination.
Partner with Bromos Organics
Choosing the right chemical manufacturer can significantly influence your product quality, safety, and market success. By partnering with Bromos Organics, you gain access to:
Over a decade of expertise in bromine chemistry
Flexible order quantities and competitive pricing
Custom synthesis and toll manufacturing options
Transparent communication and responsive support
Whether you're a pharmaceutical formulator, chemical distributor, or R&D lab, our bromine derivatives can help enhance your process and product outcomes.
Final Thoughts
In the evolving landscape of chemical manufacturing, businesses need partners who bring more than just supply—they need reliability, innovation, and sustainability. As a leading Bromine Derivatives Manufacturers India recognized, Bromos Organics continues to raise the bar by combining scientific rigor with ethical production practices.
Reach out to our team today to explore how we can support your bromine derivative needs with precision, performance, and partnership.
#BromineDerivativesManufacturersIndia#BromineDerivativesManufacturersNorthernIndia#HYDROBROMICACID48%
0 notes
Text
(R)-(-)-N-Boc-3-Pyrrolidinol Market, Report: Trends, Opportunities, and Forecast 2025-2032
(R)-(-)-N-Boc-3-Pyrrolidinol Market, Global Outlook and Forecast 2025-2031
The global (R)-(-)-N-Boc-3-Pyrrolidinol market is experiencing steady expansion, currently valued at USD 5.4 million in 2024 with projections indicating growth to USD 9.7 million by 2031, representing a CAGR of 9.1%. This chiral building block has become indispensable in pharmaceutical synthesis, particularly for developing complex drug molecules requiring specific stereochemistry.
(R)-(-)-N-Boc-3-Pyrrolidinol serves as a critical intermediate in producing active pharmaceutical ingredients (APIs) for neurological and cardiovascular therapies. Its Boc-protected pyrrolidine structure offers both stability during synthesis and easy deprotection when needed, making it increasingly valuable as drug development pipelines grow more complex.
Download FREE Sample Report: https://www.24chemicalresearch.com/admin24cr/download-sample/290603/global-nbocpyrrolidinol-forecast-market-2025-2031-719
Market Overview & Regional Analysis
China dominates production with approximately 80% market share, leveraging its established pharmaceutical chemical infrastructure and cost advantages. North America follows with 10% share, driven by stringent quality requirements for drug development, while Europe accounts for 5% with its focus on innovative therapies.
The Asia-Pacific region demonstrates the strongest growth momentum, particularly in India and South Korea where generic drug manufacturers are expanding their capabilities. Meanwhile, North America maintains premium pricing for high-purity grades used in clinical-stage compounds, creating a two-tier market dynamic.
Key Market Drivers and Opportunities
Three primary factors are propelling market expansion: the surge in neurological drug development (where pyrrolidine structures are particularly valuable), increasing outsourcing of intermediate production by Western pharma companies, and advancements in asymmetric synthesis technologies that improve production efficiency.
Emerging opportunities include the compound's growing use in PROTAC drug development and as a building block for next-generation RNA therapeutics. Furthermore, the push for continuous manufacturing in pharma production is creating demand for reliable, consistent supplies of key intermediates like (R)-(-)-N-Boc-3-Pyrrolidinol.
Challenges & Restraints
The market faces several headwinds, including regulatory scrutiny of Chinese API facilities, intellectual property disputes around synthetic routes, and the technical complexity of maintaining stereochemical purity at scale. Additionally, the trend toward simpler drug molecules in some therapeutic areas could limit growth potential.
Supply chain vulnerabilities have emerged as a significant concern, particularly for Western pharmaceutical companies dependent on Asian suppliers. This has led to increased inventory holding and dual-sourcing strategies that are reshaping procurement patterns across the industry.
Market Segmentation by Type
95% Purity
98% Purity
Others
Download FREE Sample Report: https://www.24chemicalresearch.com/admin24cr/download-sample/290603/global-nbocpyrrolidinol-forecast-market-2025-2031-719
Market Segmentation by Application
Pharmaceutical Intermediates
Organic Synthesis
Others
Market Segmentation and Key Players
SiChuan Tongsheng Biopharmaceutical
Sichuan Hengkang Technology Development
Changzhou Anxuan Chemical
BTC Pharmaceuticals Technology
Zhengzhou Alfa Chemical
Chemspon Bio-Tech
Shanghai Longsheng Chemical
Report Scope
This comprehensive analysis covers the global (R)-(-)-N-Boc-3-Pyrrolidinol market from 2024 to 2031, providing detailed insights into:
Market size estimations and growth projections
Detailed segmentation by purity level and application
Regional demand patterns and growth hotspots
The report also includes in-depth profiles of major market participants, examining:
Production capacities and expansion plans
Quality control systems and regulatory compliance
Pricing strategies and customer relationships
Research and development initiatives
Our research methodology combined extensive primary interviews with industry experts and comprehensive analysis of secondary sources, including:
Trade data and customs records
Company financial disclosures
Patent filings and scientific literature
Regulatory documents and industry databases
Get Full Report Here: https://www.24chemicalresearch.com/admin24cr/reports/290603/global-nbocpyrrolidinol-forecast-market-2025-2031-719
About 24chemicalresearch
Founded in 2015, 24chemicalresearch has rapidly established itself as a leader in chemical market intelligence, serving clients including over 30 Fortune 500 companies. We provide data-driven insights through rigorous research methodologies, addressing key industry factors such as government policy, emerging technologies, and competitive landscapes.
Plant-level capacity tracking
Real-time price monitoring
Techno-economic feasibility studies
With a dedicated team of researchers possessing over a decade of experience, we focus on delivering actionable, timely, and high-quality reports to help clients achieve their strategic goals. Our mission is to be the most trusted resource for market insights in the chemical and materials industries.
International: +1(332) 2424 294 | Asia: +91 9169162030
Website: https://www.24chemicalresearch.com/
Follow us on LinkedIn: https://www.linkedin.com/company/24chemicalresearch
0 notes
Text
Jay Fine Chem – Your Trusted Global Supplier of API Intermediates & Fine Chemicals
Jay Fine Chem – Your Trusted Global Supplier of API Intermediates & Fine Chemicals
In the dynamic world of pharmaceuticals and specialty chemicals, sourcing high-quality materials from a reliable partner is crucial. Jay Fine Chem stands as a global supplier of API intermediates and a global supplier of fine chemicals, delivering high-purity substances to meet the evolving demands of various industries. Our commitment to Good Manufacturing Practice (GMP) compliance and stringent quality standards ensures that we provide superior pharmaceutical raw materials and specialty chemicals worldwide.
Leading the Way in API Intermediates Supply
As a renowned global supplier of API intermediates, Jay Fine Chem specializes in providing active pharmaceutical ingredients (APIs) and their precursors for drug manufacturing. Our expertise in custom synthesis allows us to offer tailored solutions for pharmaceutical companies, contract manufacturers, and research institutions. With a focus on innovation and quality, we ensure that our high-purity substances meet international standards and regulatory requirements.
We work closely with contract manufacturing organizations (CMOs) and pharmaceutical firms to streamline the supply chain, ensuring timely delivery of pharmaceutical raw materials. Our API intermediates are essential for developing life-saving drugs and therapeutic solutions across multiple healthcare sectors.
Global Supplier of Fine Chemicals – Quality & Innovation
Jay Fine Chem is also a leading global supplier of fine chemicals, catering to industries such as pharmaceuticals, biotechnology, agrochemicals, and cosmetics. Our portfolio includes a diverse range of organic compounds and chemical synthesis products that meet the highest quality benchmarks.
With state-of-the-art manufacturing facilities and a commitment to custom chemical manufacturing, we produce specialty chemicals that serve a wide range of applications. Whether you need high-performance materials for industrial processes or precise chemical formulations for research and development, Jay Fine Chem is your trusted partner.
Why Choose Jay Fine Chem?
Unmatched Quality Assurance – Our adherence to GMP compliance and rigorous testing protocols ensures that every batch meets stringent industry standards.
Global Reach & Supply Chain Efficiency – As a global supplier of API intermediates and fine chemicals, we maintain a robust logistics network for seamless international deliveries.
Customized Solutions – With our expertise in custom synthesis, we provide tailor-made solutions to meet unique client requirements.
Commitment to Innovation – We continuously invest in research and development to stay ahead in the rapidly evolving chemical industry.
Reliability & Compliance – Our operations align with international regulatory guidelines, ensuring consistent quality and reliability.
Partner with Jay Fine Chem for Your API & Fine Chemical Needs
If you are looking for a global supplier of API intermediates or a global supplier of fine chemicals, Jay Fine Chem is your trusted partner. Our dedication to excellence, combined with our extensive product portfolio, makes us the preferred choice for pharmaceutical and chemical companies worldwide.
Get in touch with Jay Fine Chem today to explore how our high-purity substances, specialty chemicals, and custom chemical manufacturing capabilities can meet your industry needs. Together, we can drive innovation and success in the world of pharmaceuticals and fine chemicals.

0 notes
Text
Overcoming the “Choke Points” in Semaglutide Side Chain Synthesis with Core Technologies to Enable Efficient GLP-1 Drug Manufacturing Semaglutide, a groundbreaking product in the GLP-1 drug class, owes its extended half-life and enhanced receptor affinity largely to its unique side chain, Ste-Glu-AEEA-AEEA-OSU (CAS: 1169630-40-3). This side chain covalently modifies the peptide backbone, significantly improving pharmacokinetics and therapeutic performance. However, its complex structure presents two critical synthetic challenges: - Precise Assembly of Repetitive AEEA Units:The side chain features consecutive AEEA (aminoethoxyethoxyacetic acid) units, which require stepwise coupling via highly activated intermediates (e.g., AEEA-AEEA). Any impurities or deviations compromise downstream reaction efficiency and may trigger irreversible byproducts. - Stereochemistry and Stability of Glutamic Acid (Glu):The glutamic acid component must maintain strict L-configuration, and its carboxyl groups require directional protection (e.g., OtBu) to preserve biological activity. Leveraging deep expertise in peptide chemistry and industrial-scale manufacturing, Watson has successfully broken through the synthetic bottlenecks of these two key intermediates—AEEA series and glutamic acid derivatives—emerging as an “invisible champion” in global semaglutide API production. I. AEEA Series Intermediates: From Molecular Design to Industrial Scale-Up As the hydrophilic spacer in the side chain, the quality of AEEA units directly impacts drug solubility and metabolic behavior. Watson’s innovations have set new industry standards through: 1. Dual Activation Strategies for Flexible Supply To address the diverse process requirements in semaglutide side chain synthesis, Watson offers two intermediate options: - Fmoc-AEEA (CAS: 166108-71-0):Featuring Fmoc-protected amino and pre-activated carboxyl groups, this option allows direct use in solid-phase synthesis, eliminating 2-3 activation steps and shortening production cycles by over 30%. - AEEA-AEEA (CAS: 1143516-05-5):Retains a non-activated hydroxyl end for custom activation approaches. Both approaches leverage precision molecular engineering to ensure superior product consistency and performance. 2. Ultra-High Purity and Batch Consistency Given the hygroscopic and oxidation-sensitive nature of AEEA intermediates, Watson employs “inert gas protection + low-temperature crystallization” purification processes to achieve purity >99.0% and limit single impurities to 99.0% Chemical Purity: The Foundation of Compliance and Cost Reduction Semaglutide side chain synthesis demands >99.0% chemical purity, as residual trace impurities (e.g., oxidative byproducts) from lower-grade materials can amplify exponentially in downstream steps, jeopardizing entire batches. Watson employs gradient crystallization and supercritical chromatographic purification combined with a 200+ impurity database and end-to-end monitoring, ensuring purity exceeds 99.0%—enhancing coupling yields by 8%-12% and reducing purification costs. 2. 99.5% Optical Purity: Uncompromising Chiral Control Watson’s Fmoc-Glu-OtBu (CAS: 84793-07-7) and Glu-OtBu (CAS: 45120-30-7) products achieve >99.5% optical purity through asymmetric synthesis and dynamic kinetic resolution. In-line process analytical technology (PAT) ensures batch-to-batch consistency in stereochemistry, completely eliminating risks of drug inactivation from isomer contamination. 3. Tight Control of Single Impurities: Watson’s “Dual-Standard” Approach While many suppliers focus solely on overall purity, neglecting the toxicological risks of specific impurities, Watson sets a new industry benchmark by limiting maximum single impurity to Read the full article
0 notes
Text
Unlocking the Power of 6-Aminouracil Intermediates in Drug Development – A Vidgastech Perspective
In the rapidly advancing world of pharmaceuticals, high-performance intermediates are the foundation of innovative drug formulations. Among these, 6-Aminouracil stands out as a vital compound used in the synthesis of multiple therapeutic agents. Renowned chemical and pharma supplier Vidgastech has positioned itself as a leader in the production and distribution of 6-Aminouracil intermediates, supporting pharmaceutical companies worldwide in delivering effective treatments.
What is 6-Aminouracil?
6-Aminouracil is a pyrimidine derivative used as an intermediate in the development of active pharmaceutical ingredients (APIs). Its chemical structure makes it a versatile compound that supports the synthesis of:
Antiviral medications
Anticancer drugs
Immunosuppressive therapies
Nucleoside analogs
With its broad applicability and stable characteristics, 6-Aminouracil plays a critical role in modern drug discovery and formulation.
Applications of 6-Aminouracil Intermediates
The importance of 6-Aminouracil intermediates spans across pharmaceutical and biochemical research sectors. These intermediates are widely used for:
Preclinical drug development
Research formulations
API production
Synthesis of high-performance molecules
Their chemical stability and adaptability make them ideal for laboratory research as well as large-scale manufacturing.
Why Choose Vidgastech for 6-Aminouracil Intermediates?
Vidgastech is a trusted global supplier of pharmaceutical intermediates with a strong focus on quality, consistency, and compliance. Here's why companies prefer Vidgastech for their intermediate needs:
GMP-compliant manufacturing processes
High-purity chemical compounds
In-house R&D and quality assurance teams
Customizable supply volumes for all scales
Timely global logistics support
Vidgastech ensures that each batch of 6-Aminouracil meets international regulatory and safety standards, making it suitable for critical pharmaceutical applications.
Sustainability & Innovation at Vidgastech
Vidgastech is also deeply committed to environmental sustainability and responsible manufacturing. With state-of-the-art production facilities and eco-friendly processes, the company balances industrial output with environmental consciousness—helping clients meet ESG goals without compromising product quality.
Conclusion
For pharmaceutical companies and research institutions looking to optimize their drug development processes, 6-Aminouracil intermediates are essential. With Vidgastech’s expertise, quality assurance, and global reach, you can trust that you're working with one of the most reliable names in the industry.
🔗 Learn more about 6-Aminouracil intermediates or request a quote at: 👉 https://www.vidgastech.com
#6-Aminouracil#pharmaceutical intermediates#drug intermediates#API intermediates#Vidgastech#chemical intermediates#pharma chemicals#active pharmaceutical ingredients#bulk drug intermediates#GMP manufacturer
0 notes
Text
Best Bromoethane or ethyl bromide Manufacturer in South Africa

Bromoethane, also known as ethyl bromide, is a vital chemical used in pharmaceutical synthesis, agrochemical production, and research applications. It plays a key role as an alkylating agent in organic chemistry. If you're looking for the best Bromoethane manufacturer in South Africa, Dolphin Pharmaceutical is a name you can trust.
We are a recognized pharmaceutical drug manufacturer in South Africa, known for quality, reliability, and compliance with global standards.
What is Bromoethane?
Bromoethane is a colorless, flammable liquid with a faint sweet odor. Its chemical formula is C₂H₅Br. It is used to introduce ethyl groups into compounds and is important in:
API manufacturing
Laboratory synthesis
Industrial chemical reactions
Agrochemical production
Because of its reactive nature, it must be manufactured and handled under strict safety and quality protocols.
Why Choose Dolphin Pharmaceutical?
Dolphin Pharmaceutical is one of the leading producers of Bromoethane in South Africa. We use advanced technology, safe processing methods, and strict quality checks to ensure you receive the best product.
High Purity and Stability
We offer high-purity Bromoethane with low impurities and excellent stability for various industrial applications.
GMP-Compliant Manufacturing
Our production follows Good Manufacturing Practices, ensuring safety, quality, and traceability at every stage.
Regulatory Documentation
We provide essential documents like COA, MSDS, and export papers to help you meet local and international compliance standards.
Bulk Supply & Fast Delivery
Whether you need small quantities or bulk supply, we offer flexible volumes and reliable shipping across South Africa and abroad.
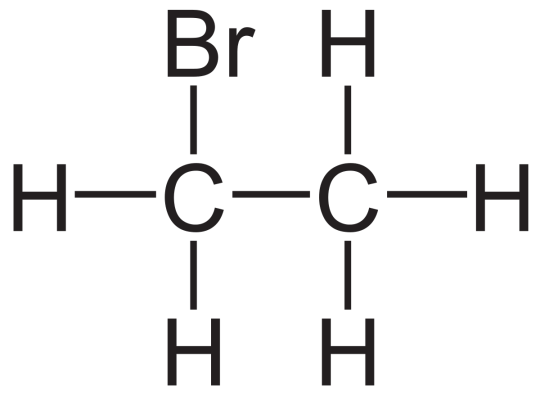
Applications of Bromoethane
Pharmaceutical Intermediates – for synthesis of active ingredients
Agrochemical Formulations – as a building block for pesticides
Industrial Use – in rubber, textiles, and dye manufacturing
R&D Labs – for organic synthesis research
Dolphin as a Pharmaceutical Drug Manufacturer in South Africa
Dolphin Pharmaceutical is not just a chemical supplier—we are a trusted pharmaceutical drug manufacturer in South Africa. We support our clients with custom synthesis, consistent batch quality, and long-term supply partnerships.
Our manufacturing plant follows strict safety measures, and our expert team ensures that every product meets both local and global standards.
Conclusion
If you need a dependable partner for high-quality ethyl bromide, Dolphin Pharmaceutical is your answer. As the best Bromoethane manufacturer in South Africa, we are committed to safe manufacturing, superior quality, and trusted service.
Choose Dolphin—your reliable pharmaceutical drug manufacturer in South Africa, ready to meet your chemical and API needs with integrity and care.
#Bromoethane or ethyl bromide Manufacturer in South Africa#Bromoethane or ethyl bromide supplier in South Africa#Bromoethane or ethyl bromide exporter in South Africa
0 notes
Text
High-Purity METHYLAMINE 2M IN THF Supplier | Nikava Pharmaceuticals Industries

Nikava Pharmaceuticals Industries is a premier manufacturer and supplier of METHYLAMINE 2M IN THF, delivering high-purity solutions to pharmaceutical companies, research labs, and chemical industries across the globe. Our reputation is built on precision, purity, and performance — making us a trusted name in the field of specialty chemicals.
About METHYLAMINE 2M IN THF
METHYLAMINE 2M IN THF is a solution of methylamine gas (CH₃NH₂) in tetrahydrofuran (THF), with a 2 molar concentration. This reagent is widely used in organic synthesis, particularly in the pharmaceutical and agrochemical sectors. THF serves as an excellent solvent, offering good solubility and reaction stability, while the 2M concentration ensures optimal reactivity and control in a range of chemical processes.
This compound is valued for its role in:
Amination reactions
Formation of pharmaceutical intermediates
Heterocyclic compound synthesis
Research and development in medicinal chemistry
Why Choose Nikava Pharmaceuticals?
At Nikava Pharmaceuticals Industries, we are committed to delivering consistent and compliant chemical products. Our METHYLAMINE 2M IN THF is manufactured under strict quality control protocols in ISO-certified facilities, ensuring high levels of purity, stability, and performance in every batch.
Our strengths include:
High-purity formulations: Guaranteed purity for research and industrial applications
Custom packaging options: Available in a variety of volumes to suit small-scale labs or large-scale manufacturing
Global logistics support: Safe and timely delivery across domestic and international markets
Regulatory compliance: Comprehensive documentation including COA, MSDS, and export certifications
Technical assistance: Dedicated team to support application queries and custom requirements
Applications of METHYLAMINE 2M IN THF
Nikava’s METHYLAMINE 2M IN THF is used in several key chemical sectors:
Pharmaceuticals: For the synthesis of APIs and intermediates
Agrochemicals: In the development of herbicides and pesticides
Fine Chemicals: As a building block in custom synthesis
Academic Research: For specialized organic synthesis projects
Contact Us
Whether you are a researcher looking for reliable lab-grade reagents or a manufacturer seeking large-volume supply, Nikava Pharmaceuticals Industries in Ghatkopar, Mumbai has the capabilities to fulfill your requirements.
Contact us today to request a quotation, product sample, or to discuss custom packaging for METHYLAMINE 2M IN THF. For more information call at +91 9653317212 or visit Nikava Pharmaceutical Industries.
0 notes