#Protein Crystallization and Crystallography review
Text
Figuring out structure
Proteins are like molecular machines. Unlike actual machines, proteins can fold into intricate 3-D structures on their own. Wouldn't it be convenient if your bookshelf from IKEA could do that? If we want to understand how proteins work, we need a blueprint. Some parts of the protein are essential for stability; other parts are crucial for getting things done through binding or catalysis. We need to understand how different parts of the protein fit together; this is where Structural Biology comes in. Getting the 3-D structure is one of the main steps in characterising new anemone toxins. I will cover some of the techniques we use to investigate the toxin structure.
The hierarchy of protein structure goes like this: primary structure-->secondary structure-->tertiary structure-->quaternary structure. For anemone toxins, we don't have to worry about the quaternary structure. Usually, anemone toxins have one polypeptide, not multiple polypeptides.
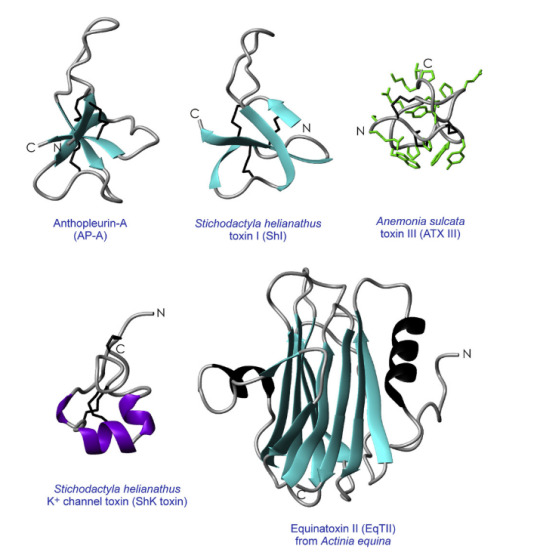
Figure 1. Structures of anemone toxins (Source: Norton, R. S, 2009)
Primary structure:
It's the amino acid sequence or 'code' for the protein. You can get the amino acid sequence from DNA, mRNA or protein sequencing. Protein sequencing just gives you the sequence for your protein. However, if we use DNA or mRNA sequencing, then we have to figure out the 'mature' peptide sequence. DNA and mRNA code for the 'first draft' of the peptide. After translating mRNA to protein, the signal peptide and pro-peptide are attached to the 'mature peptide.' Those two parts of the protein get cut out before our mature peptide can go out in the world and decimate prey.
Secondary structure:
Our flat polypeptide chain will fold into different secondary structures like alpha-helices, beta-sheets, beta turns, etc. Circular dichroism and Fourier transform infrared radiation (FT-IR) can tell us about the secondary structure of the toxin. CD and FT-IR are commonly used to predict the amount of each secondary structure in proteins. For CD, we measure how much of circularly polarised light in the far-UV region is absorbed by the sample. We use curve fitting programs to determine which combination of secondary structures fits the spectra the best (e.g. 50% beta-sheet, 10% alpha-helix, 20 % random coil and 20 % beta-turn). FT-IR works similarly, but we use infrared radiation instead of polarised light. FT-IR is a better option for investigating the interactions between pore-forming toxins and lipid membranes. The lipid vesicle or 'sacs' scatter polarised light, which it makes challenging to use CD for the same purpose.
One of the best things about spectroscopic techniques is that they are not as time-consuming as, X-ray crystallography. You can have multiple CD, and FT-IR runs in 1-2 hours and get the processed data before lunch. However, CD and FT-IR are low-resolution techniques. They don't tell you the exact position and orientation of tryptophan 32. We need atomic resolution structures to get an in-depth understanding of how anemone toxins work.
Tertiary structure:
The full 3-D structure of proteins will have secondary structure plus the disulphide linkages, salt bridges, hydrophobic interactions. There are two main ways of getting atomic resolution structures: X-ray crystallography and NMR. X-ray crystallography can give high-resolution 3-D structures—if you manage to please the crystal gods. If you don't have the ideal crystallisation conditions—no crystal for you. I know PhD students who spent 12-18 months on crystallising their protein. However, once you get past the crystallisation bottleneck, the diffraction experiments and data processing doesn't take as much time. In theory, you can get better resolution with X-ray crystallography than NMR. Let's assume you don't have time for crystallisation and you go with NMR. NMR is excellent for finding the structure of smaller proteins, but for larger proteins, you have to use isotopic labelling. Some proteins that are too big for NMR. In X-ray crystallography, the protein is a 'frozen' state. However, NMR also lets you study proteins in solution to understand the dynamics of the protein. You can observe how the protein changes when it binds to something.
We can also predict the 3-D structure of the toxin-based on the sequence alone.
How?
Computer magic !
With the protein data bank and structure prediction tools like SWISS-MODEL, you can predict the 3-D structure of a new toxin. If there is an anemone toxin in the database with an experimentally determined 3-D structure and it has high sequence similarity with your new toxin, you can use homolog modelling. If you can't use homology modelling, you can use de novo structure prediction like I-TASSER. In de novo modelling, the program predicts the structure for smaller sections of the protein and uses those bits to build the full predicted model. Model prediction isn't 100% accurate, and we still have to determine the 3-D structure experimentally. However, predicted 3-D structures act as a guide.
Every technique has its strengths and weaknesses. A good researcher tries to use the most suitable method for their project to its full potential. You have to realise the power that's inside.
So, what can you do once you have the 3-D structure of a toxin?
I am going to use APETx1 from the clonal anemone as an example. APETx1 is a potassium channel toxin, which targets the Ether-a-gogo channels in heart cells. Since APETx1 is a small potassium channel toxin, Chagot and company could use NMR. They found that APETx1 belong to the Defensin family. A variety of anti-bacterial proteins and ion channel toxins belong to this family. Proteins with Defensin-4 domain, have double or triple-stranded antiparallel b-sheets linked to a short a-helix by two disulphide bridges. Since NMR can reveal disulphide linkages, Chagot found APETx1 has an inhibitor cysteine knot (ICK). The inhibitor cysteine is a very popular protein fold because it's so stable that it makes proteins resilient against heat stress and proteolysis. Getting the structure of a toxin allows us to classify the toxin and compare it with structurally similar toxins. Since APETx1 has structurally similarity with two other toxins BeKm1 and CnErg1, you can infer it would have a similar mechanism for blocking potassium channels. Chagot suggested that five key amino acids on APETx1 (i.e. Tyr 5, Tyr 32, Phe33, Lys8 and Lys 18) 'block' the Ether-a-gogo channels through electrostatic interactions.
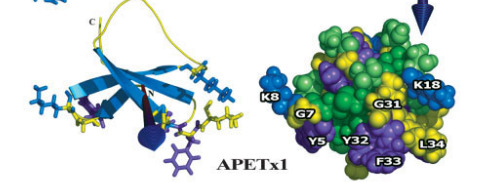
Figure 2. 3-D structure for APETx1 (Source: Chagot et al, 2005)
In short, we can use a variety of biophysical techniques to get structural information for new toxins and the structure provides insight into the function. Protein structure and function are different sides of the same coin. Next time, I will flip the coin by covering how we figure out function.
Citations:
Chagot, B., Diochot, S., Pimentel, C., Lazdunski, M. & Darbon, H. Solution structure of APETx1 from the sea anemone Anthopleura elegantissima: A new fold for an HERG toxin. Proteins Struct. Funct. Genet. 59, 380–386 (2005).
Jouiaei, M. et al. Ancient venom systems: A review on cnidaria toxins. Toxins (Basel). 7, 2251–2271 (2015).
Norton, R. S. Structures of sea anemone toxins. Toxicon 54, 1075–1088 (2009).
Norton, R. S. & Pallaghy, P. K. The cystine knot structure of ion channel toxins and related polypeptides. Toxicon 36, 1573–1583 (1998).
Dauplais, M. et al. On the Convergent Evolution of Animal Toxins. J. Biol. Chem. 272, 4302–4309 (1997).
Belmonte, G. et al. Primary and secondary structure of a pore-forming toxin from the sea anemone, Actinia equina L., and its association with lipid vesicles. BBA - Biomembr. 1192, 197–204 (1994).
Honma, T. & Shiomi, K. Review Article Peptide Toxins in Sea Anemones: Structural and Functional Aspects. Mar. Biotechnol. 8, 1–10 (2006).
Bernard Gilquin, Judith Racape, Anja Wrisch, Violeta Visan, Alain Lecoq, Stephan Grissmer, Andre´Menez, and S. G. Structure of the BgK-Kv1.1 Complex based on distance restraints identified by double mutant cycles: Molecular basis for convergent evolution of Kv1 channel blockers. J. Biol. Chem. 277, 37406–37413 (2002).
3 notes
·
View notes
Text
Global Protein Crystallization and Crystallography Market at A Highest CAGR of 11.90% With Leading Players Trends and share
The protein crystallization and crystallography market research report is a stunning aide for an imperative thought, improved fundamental authority and better business frameworks. The report joins estimations of the ongoing state of the market, CAGR values, market size and overall industry share, income age and significant changes required later on items. The protein crystallization and crystallography market report has information and data as graphs, tables and outlines that can be adequately understood by the associations. The market thinks about, bits of learning and investigation joined into this overall protein crystallization and crystallography market report keeps business focus indisputably into the concentration with which you can reach to the business objectives.
Market Analysis:
Global protein crystallization and crystallography market is registering a healthy CAGR of 11.90% in the forecast period of 2019-2026. The report contains data from the base year of 2018 and the historic year of 2017. The rise in the market value can be attributed due to surging need for high resolution facts on protein structures and expansion of new technologies contributing to the growth of the market.
Influencing players of this market are:
Few of the major market competitors currently working in the global protein crystallization and crystallography market are GENERAL ELECTRIC, Thermo Fisher Scientific Inc., QIAGEN, HAMPTON RESEARCH CORP., Anton Paar GmbH, Danaher, PerkinElmer Inc., Tecan Trading AG, Jena Bioscience GmbH, Agilent Technologies Inc., MīTeGen LLC, Rigaku Corporation, Bruker, Molecular Dimensions Ltd, Formulatrix, Art Robbins Instruments, LLC, Lonza, Creative Biostructure, Fluidigm Corporation and Dynamic Devices among others.
Get Exclusive Sample Copy of this Report@
https://www.databridgemarketresearch.com/request-a-sample/?dbmr=global-protein-crystallization-crystallography-market
This protein crystallization and crystallography market report consolidates comprehensive industry examination with exact data and conjectures that offers total research arrangements and brings the most extreme industry clarity for decision making.
Analysis based on various segments-:
This analysis gives an examination of various segments that are relied upon to witness the quickest development amid the estimated forecast frame.
And this is done on the basis of-:
By Technology
Protein Purification Systems
Chromatography
Electrophoresis
Protein Crystallization
Affinity Chromatography
Ion-Exchange Chromatography
Gel-Filtration Chromatography
High Pressure Liquid Chromatography (HPLC)
Gel Electrophoresis
Capillary Electrophoresis
Others
Instruments
Reagents/Consumables
Protein Crystal Mounting
Protein Crystallography
Liquid-Handling Systems & Robots
Crystallization Plates/Microplates
Dialysis Buttons
Incubators
Cooling Crystallizers
Other Instruments
Crystallization Screens
Salts
Alcohols
Buffers & Additives
By Technology
By Product
X-Ray Crystallography
NMR Spectroscopy
Cryo-Electron Microscopy
Others (SAXS)
Instruments
Services & Software
By Product
Instruments
Reagents/Consumables
Services & Software
By End Users
Pharmaceutical Companies
Biotechnology Companies
Government Institutes
Academic Institutions
By Geography
North America
South America
Europe
Asia-Pacific
Middle East & Africa
S.
Canada
Mexico
Brazil
Rest of South America
Germany
France
United Kingdom
Italy
Spain
Russia
Turkey
Belgium
Netherlands
Switzerland
Rest of Europe
Japan
China
South Korea
India
Australia
Singapore
Malaysia
Indonesia
Thailand
Philippines
Rest of Asia-Pacific
South Africa
Rest of Middle East & Africa
Regional Insights-
Regional analysis helps the market players to take an exhaustive assessment of the protein crystallization and crystallography market region wise so that it becomes easy for them to distinguish and investigate the developing pattern and hidden opportunities all over the world.
The protein crystallization and crystallography market covers regions such as- South America, North America, Europe, Asia-Pacific, Middle East, and Africa.
Speak To Analyst @
https://www.databridgemarketresearch.com/speak-to-analyst/?dbmr=global-protein-crystallization-crystallography-market
Analysis based on Competition-:
The protein crystallization and crystallography market report presents profiles of key market players and information about different techniques they have utilized, for example, new product dispatches, extensions, understandings, joint endeavors, associations, acquisitions, and others to expand their impressions in this market so as to continue in long run.
Key queries addressed in this report-:
What will be the market size and market share in the upcoming future?
What are the new and hidden opportunities in the market?
Who are the top players in market?
How the challenge goes later on?
Which are the main regions impacting the market growth?
What are the difficulties in future?
Key Issues addressed here-:
Uncertainty of upcoming revenue pockets and growth areas.
Understanding business sector sentiments.
Understanding the most dependable venture focuses.
Competitiveness of Top industry players
Trending factors which are impacting the market growth.
Challenges and threats faced by the leading players
Research strategies and tools used-:
This protein crystallization and crystallography market research report helps the readers to know about the overall market scenario, strategy to further decide on this market project. It utilizes SWOT analysis, Porter’s Five Forces Analysis and PEST analysis.
Contact:
Data Bridge Market Research
US: +1 888 387 2818
UK: +44 208 089 1725
Hong Kong: +852 8192 7475
Email: [email protected]
Report Related :
global practice management systems market
global professional diagnostics market
global protein crystallization crystallography market
#Protein Crystallization and Crystallography#Protein Crystallization and Crystallography markets#Protein Crystallization and Crystallography share#Protein Crystallization and Crystallography size#Protein Crystallization and Crystallography trends#Protein Crystallization and Crystallography analysis#Protein Crystallization and Crystallography overview#Protein Crystallization and Crystallography report#Protein Crystallization and Crystallography research#Protein Crystallization and Crystallography insight#Protein Crystallization and Crystallography application#Protein Crystallization and Crystallography forecast#Protein Crystallization and Crystallography review
0 notes
Text
RCB Project Scientist & Associate Vacancies – Biotech & Life Science Job
New Post has been published on https://biotechtimes.org/2020/08/19/rcb-project-scientist-associate-vacancies-biotech-life-science-job/
RCB Project Scientist & Associate Vacancies – Biotech & Life Science Job

RCB Project Scientist & Associate Vacancies
RCB Project Scientist & Associate Vacancies – Biotech & Life Science Job. Excellent opportunity to join Regional Centre for Biotechnology as a Project Scientist or Project Associate. RCB is currently recruiting candidates from Life Sciences and Biotechnology field. Interested candidates may check out the details below:
Interview for Project Scientist II, Project Associate II & Animal / Laboratory Attendant Positions
Advt. No. 18/Project/2020/HR
Title of Project: “Development of small molecule antivirals against Chikungunya and Japanese Encephalitis Viruses”.
Principal Investigator: Dr. Deepak T. Nair, Professor.
Duration: Initially for 12 months and maybe extended after review
Post-I
Name of Position: Project Scientist II
Age Limit: 40 Years
No. of Positions: Four
Emoluments: Rs. 83,080/- (consolidated).
Essential Qualifications: (i) Doctoral Degree in Life Science/ Biotechnology; and (ii) Three years experience in Research and Development in Academic Institutions with peer-reviewed publications in international journals. Research experience in cell, molecular biology and virology with practical knowledge of high throughput screening in cultured cells (2 positions) OR Research experience and practical knowledge of protein production, purification, biochemical assays and protein crystallography (1 position) OR Research experience and practical knowledge of computational drug discovery and biochemical assays (1 position).
Desirable Qualifications: Knowledge and Research Experience in virology and experience in screening for antiviral compounds (2 positions) OR Knowledge and Research Experience in the area of viral proteins plus experience in structural biology of protein-inhibitor complexes (2 positions).
Post-II
Name of Position: Project Associate II
Age Limit: 35 Years
No. of Positions: Four
Emoluments: As per DST guidelines issued vide OM No. SR/S9/Z-05/2019 dated 10.07.2020.
Essential Qualifications: (i) Master’s degree in Life Sciences/Chemistry/ Biotechnology/Veterinary sciences with minimum 60 % and (ii) two years research experience in Research and Development in Academic Institutions. Research experience in virology and drug discovery (2 positions) OR protein chemistry, protein production and crystallization (1 position) OR protein chemistry and biochemical assays (1 position) is essential.
Post-III
Name of Position: Animal / Laboratory Attendant
Age Limit: 30 Years
No. of Positions: Three
Emoluments: Rs. 22,320/- (consolidated)
Essential Qualifications: Graduate in Science, minimum 60 % marks in B. Sc or equivalent degree. Knowledge and experience of handling animals for scientific research (2 positions) OR handling supporting activities in a research laboratory (1 position) is essential.
Desirable Qualifications: Research experience in handling of mice and guinea pigs for virology projects is desirable.
How to Apply
Interested candidates should apply online by 27th August 2020, 5:00 PM
Please upload copies of the following documents:
MSc or any other equivalent course in Life Sciences.
National Level JRF eligibility test certificate
Certificate of experience as mentioned.
The shortlisted candidates will be informed by 28th August 2020, 5.00 PM through email to appear in the online interview to be held at 10.00 AM on 1st September 2020.
For any query, you may contact PI by email: [email protected]
Download Notification
Apply Online
0 notes
Text
Protein Crystallization Market: Review, Research, Statistics and Growth to 2020

Protein crystallography is a technique used to visualize protein structure and enhance understanding about protein function. The process of protein crystallography includes protein purification, protein crystallization, protein crystal mounting and protein crystallography. Protein crystallization is a process of formation of protein crystals. Protein crystallization technique helps to identify three dimensional structure of protein. Protein crystallization is used by researcher during drug discovery and development. In addition, the technique is also used in protein engineering, drug designing and other applications. Various techniques used during protein crystallization technology are ion-exchange chromatography, high-performance liquid chromatography (HPLC), gel-electrophoresis, x-ray crystallography and nuclear magnetic resonance (NMR). On the basis of methods of crystallization, protein crystallization technique includes vapor diffusion method and high through-put method. Protein crystallization technique is used in various sectors such as biotechnology, pharmaceutical, chemical industries and others.
North America has the largest market for protein crystallization followed by Europe, due to rising investments from government and private sectors, technological advancements and increasing research activities in the region. Asia is expected to show high growth rates in the next five years due to rising investment by key market players and increasing R&D activities in this region.
For detailed insights on enhancing your product footprint, request for a Sample here @ https://www.persistencemarketresearch.com/samples/3056
Increasing technological advancement, rising government and private sector funding, new and innovative product launches, growing demand for high-resolution information on protein structure and increasing research and development activities in pharmaceutical and biotechnological areas are some of the key factors driving the growth for global protein crystallization market. In addition, increasing spending in research and developments is expected to drive the protein crystallization market. However, lack of qualified and experienced researchers, high cost involved and time consuming process and economic downturn are some of the major factors restraining the growth for global protein crystallization market.
Emerging markets in the developing countries such as India and China would lead to growth in protein crystallization market in Asia. In addition, growing need to identify new ligands for the drug discovery process would develop opportunity for global protein crystallization market. Lab automation and x-ray free electron lasers in protein crystallization are some of the latest trends that have been observed in protein crystallization market.
To receive extensive list of important regions, ask for TOC here @ https://www.persistencemarketresearch.com/toc/3056
Some of the major companies operating in the global protein crystallization market are:
Rigaku Corporation
Jena Bioscience GmbH
Molecular Dimensions Limited
HAMPTON RESEARCH CORP.
Agilent Technologies.
Other companies which have significant presence in protein crystallization market are Bruker Corporation, GE Healthcare, Thermo Fisher Scientific, Anton Paar GmbH, MiTeGen, LLC and QIAGEN.
#protein crystallization market#protein crystallization market size#protein crystallization market sahre
0 notes
Text
Proteins out of bounds
Gerhard Wagner
Harvard Medical School
As a physics undergraduate student in Munich, Gerhard Wagner worked on an esoteric atomic measurement of iron in a protein molecule. Then he heard from his supervisor, who was on sabbatical at Bell Labs in New Jersey. There, the same molecule, hemoglobin, the iron-rich protein that carries oxygen in red blood cells, was being probed by nuclear magnetic resonance (NMR) spectroscopy.
The technology caught Wagner’s attention. Why measure a single parameter, as he was doing, when you could measure many aspects at once with NMR and learn so much more, he asked himself. Little did he know, but that thought launched a lifetime of NMR research.
Wagner hadn’t planned on a career in academia. He was born in Czechoslovakia just as World War II ended. His German-speaking family became refugees, fleeing to nearby Bavaria and starting again with nothing. In science, Wagner also helped develop a new field from scratch.
When he graduated from Technical University in 1972, he found a sweet spot in Switzerland—home of his girlfriend, skiing, and a PhD training position with Kurt Wüthrich, one of the early scientists to use NMR on proteins and who later won a Nobel Prize. He spent most of the next 14 years in Zurich.
At ETH Zurich, Wagner launched a family and a career in NMR that soon brought him to the attention of the world’s top structural biologists. At the time, protein structures could only be determined by X-ray crystallography. Wagner helped develop suites of techniques for the fledgling NMR analyses of proteins that made it possible to assign resonances and solve their structures.
Early on, he observed one of the NMR advantages: protein dynamics. “I found a lot of mobility in proteins,” he says. These days, NMR is well known for its unique ability to reveal thermodynamic and kinetic aspects of proteins. More than 40 years ago, those protein wiggles were nearly impossible for some to imagine.
Wagner was testing the new techniques on a cow protein with stable ring-shaped parts (called aromatic side chains in chemistry parlance). Analyzed by NMR, the rings flipped when the temperature went up or down, opening the protein structures by several Ångströms. The unexpected results were controversial.
Wüthrich planned to talk about the new data at a physics conference in Germany. Behind the stage, conference organizer and Nobel Laureate Max Perutz didn’t believe the results and objected to the presentation. Perutz famously had solved the structure of hemoglobin, which also sported aromatic side chains. Another prominent scientist, Robert Huber, who later also was awarded a Nobel Prize convinced Perutz to let the talk proceed as planned, Wagner says. (Later, the same data provided a test bed for the development of molecular dynamic simulations by Martin Karplus.)
After a one-year postdoctoral position at the Massachusetts Institute of Technology in Cambridge, Wagner continued his work in Wüthrich’s lab in a postgraduate teaching and research position called privatdozent. Soon it came time to present Wagner’s latest work, the NMR structure of cadmium-bound metallothionein, a small protein that binds zinc or other metals in plants and animals. Wagner got a worried call in the middle of the night from for Wüthrich. It seems the NMR structure was very different from a crystal structure of the same molecule being presented at the same meeting. Wagner and his coworkers had applied a new technique, proton cadmium correlations, to determine the topology and structure. He scrambled to review the data. It all checked out correct.
“It was also an early time in crystallography, so people could still make mistakes,” Wagner says. “It gave me some visibility. It showed NMR could do a structure and get it right.” Later, the two NMR and crystal teams published a joint paper showing agreement between their final metallothionein structures.
NMR eventually became an accepted method for solving protein structures, especially of smaller proteins in lower concentrations. Wagner took a faculty position at University of Michigan in 1987. There he just had time to develop the first triple resonance experiments, a strategy that is now the basis for most protein NMR, before he was recruited to Harvard Medical School in Boston in 1990.
“When I came to Harvard 25 years ago, I came as an expert in NMR,” Wagner says. “I thought I should also have an important biological project.” He looked for areas with little research and landed on translation initiations, or how a transcribed gene is turned into a protein.
After a gene is transcribed into RNA, a large protein complex grabs hold of the messenger RNA tail to prepare to make a protein. In a 2003 Cell paper, Wagner’s lab reported the structure of the first two proteins in this complex (eIF4E and eIF4G) and how they enable the ribosome to bind to the 5’ end of mRNA and start making protein.
Above: In this NMR image, a complex of two proteins (eIF4E in red and yellow and eIF4GI in blue) attach to the 5’ cap structure of mRNA, and eIF4G forms a “molecular bracelet” around the N-terminus of eIF4E, prompting the initial event of ribosome recruitment to start making a protein. Courtesy G.Wagner
“Then the idea came that oncoproteins have long 5’ UTRs,” or untranslated tails, Wagner said. “Our hypothesis is that nature developed long tails to make it more difficult to translate dangerous proteins.” Other labs have shown that the long tail effectively down regulates some proteins. In cancer, such controls on the oncoproteins may be broken, he says. Restoring them may be a new way to treat cancer.
Wagner and his collaborators screened for small molecules to inhibit some of the translation initiation proteins his lab had characterized. Now they have a major program to improve the compounds they found with anti-cancer activity.
These days, NMR remains well suited for characterizing smaller proteins and to catch the gymnastic moves of molecules in their active biological roles. In particular, Wagner believes NMR has potential to illuminate RNA in complex with other proteins. “Another area where NMR may have some impact is proteins that are only partially folded,” he says.
For example, the nuclear factor of resting T cells has a long unstructured tail hanging in the cytoplasm of cells. The tail has many phosphorylation sites, keeping it in a resting state. Working with another DFCI collaborator, Wagner’s group developed a new NMR technique (known as direct 15N detection) to elucidate a key internal step in activating T cells, which is important to fight infections but also needs to be regulated to prevent rejections of organ transplants. In a collaboration with Haribabu Arthanari at Dana-Farber Cancer Institute, the team identified a key molecular interaction in activated T cells. The Wagner lab is developing small molecules that inhibit the interaction, ideally targeting a protein-protein interaction with fewer side effects than current anti-rejection drugs.
In another project, Wagner’s group has found a better way to study membrane proteins in a more natural environment. A team led by postdoctoral fellow Mahmoud Nasr improved the design of tiny nanodiscs, a popular model of cell membranes used to study proteins entering cells.
The Wagner lab’s modification of the design made the nanodiscs more stable using some fancy chemistry bonding membrane scaffolding proteins. The resulting covalently circularized nanodiscs can be precisely sized from 9 to 50 nanometers and used to study viruses and other membrane interactions by adding a receptor. In their first published demonstration in 2017 in Nature Methods, the team and its collaborators observed polioviruses opening a putative pore and injecting genetic material through the nanodiscs, as the virus might do when it infects a cell.

Left: A cartoon illustrates the poliovirus (ball) attaching to its receptor CD155 in the membrane interior of a DNA-corralled nanodisc. Right: The EM micrograph series shows the sequence of the poliovirus engaging with the membrane, ejecting RNA through the nanodisc. Then the empty virus leaves behind a putative ejection pore.
When Wagner moved on to study HIV and the process by which it fuses with a cell membrane, the nanodiscs became too big for NMR. The ongoing work requires cryo-EM analysis. Collaborating with William Shih’s lab at the Wyss Institute, Wagner’s group developed a larger nanodisc design with the lipid bilayer inside DNA scaffolding. He sees many possibilities ahead with the new technology, including nanodisc complexes.
- Carol Cruzan Morton
0 notes