#Western blot protocol
Text
Western blot protocol

Place the membrane in blocking buffer (5X DiluObuffer ) for 1 hour on shaker at room temperature or overnight at 4 o C.Stain with Ponceaus to assess the quality of transfer and to mark the MW markers on membrane.For high MW/large glycosylated proteins, reduced the concentration of methanol form 20% to up to 15% and transfer for longer times (plate electrodes: Limit 250mA at 50 volts for 7 hours) the conditions are different for wire electrodes, overnight transfers will be preferred for HMW proteins at low methanol concentrations). Note: Immobilon membranes must be wetted in 100% methanol before using for transfer.Transfer the gel onto nitrocellulose/ immobilon (PVDF) membrane in Towbins buffer.Load the samples (20-65ug protein in 10-25ul volume) into their respective wells run the gel until dye reaches the bottom.Prepare SDS gel to the desired percentages (8% for antibodies >50kDa 10% for antibodies 30kDa 12% gel for antibodies Note: target proteins that are highly glycosylated/large in size should be avoided freeze thaw cycles or they form oligomers that will not enter in to the gel.The cells extracts were heated to 90 o C for 3 minutes before applying on SDS-PAGE.The samples were mixed in SDS-PAGE Laemmli’s buffer and reduced by adding 2.5% BME.Tissues can be grinded and then homogenized at 10% w/v in SolObuffer with protease inhibitors. Cells (106 cells are homogenized/ polytron /sonicate) in 1ml of SolObuffer with protease inhibitor A and B or with phosphatase inhibitors (depending upon experiment).The membrane exposure time to the film/imager will depend on the abundance of the protein and the detection system.Note: If using a commercial kit, follow the manufacturer’s instructions. Proceed to detection using an enhanced chemiluminescence (ECL) system.Wash the membrane with Washing Buffer for 3 x 10 minutes at room temperature.Incubate the secondary antibody in PBS with 1% BSA and 0.1% Tween-20 for 1 hour at room temperature with gentle agitation.Note: If you are using a secondary antibody conjugated to HRP, do not use solutions containing NaN 3 from this point on. Wash the membrane with Washing Buffer (PBS and 0.1% Tween-20) for 3 x 10 minutes at room temperature.Incubate for 2–3 hours at room temperature or overnight at 4☌ with gentle agitation.Note: If you use a blocking peptide as a negative control, refer to our Peptide Blocking Protocol for WB. Add the primary antibody diluted in PBS 1% BSA, 0.1% Tween-20, and 0.05% NaN 3.Block the membrane with Blocking Solution (PBS with 3% BSA and 0.05% NaN 3) for 2–5 hours at room temperature with gentle agitation.Note: High molecular weight proteins may need a longer transfer time (see Western Blot Protocol for High Molecular Weight (HMW) Proteins ). For dry transfer, follow the manufacturer’s instructions. Transfer to a nitrocellulose membrane at 200 mA for 2.5 hours at 4☌ for wet transfer.Run the gel according to the manufacturer’s instructions.*Load 80–100 µg tissue lysate/lane or lysate from 2–5 x 10 5 cells/lane. Heat the samples in Laemmli buffer at 70–100 ☌ for 10 minutes.If you have any problems, please see our extensive troubleshooting guides. An important and widely used tool in biology, western blotting involves separating proteins according to their size via gel electrophoresis and then transferring them to a membrane to detect with specific antibodies. Subscribe – Newsletters and Email UpdatesĪ western blot (WB) lets you detect and evaluate the size of specific proteins in cell or tissue extracts.

0 notes
Text
The Tower of London
The Romans, Anglo-Saxons, Vikings, French, Germans and all the “nationalities” that make up the people soup of London have been joined by the civilizations that soup colonized. It is a wondrous, multi-cultural city. Smack dab in the middle is a fortress that started as a medieval palace and became infamous for executions. The central building was erected by William the Conqueror who is responsible for making the English at least partly French even if they will not admit it as England and France became the Cain and Abel of Western Europe for centuries.
Power, monarchy and human weakness fed war and cruelty. Edward the first taxed the Jewish population higher than anyone else to pay for the construction of towers. Then he kicked them all out of England. The one room dedicated to devices of torture has boards glibly stating that there was not nearly as much torture as you would think. Oh no. There were only 81 cases of state-sanctioned torture. Mmmm hmmm. Who are you trying to kid? That statement should not be allowed to assuage any guilt felt by the largest purveyor of medieval hijinks and abject colonization. There is a quaint little pub across the street from the Tower called the Hung, Drawn. And Quartered. Own it England.
There are some things that have not evolved well. In the 50 or so years since my last visit, the ravens of the tower are now kept locked up. When I was a child, they free-roamed the grounds when tourists were there. Men just cannot be trusted.
Also, not one of us avoids death. Life is for living.
Haman
“So they hanged Haman on the gallows that he had prepared for Mordecai.”
Esther 7:10
“The loveliest lynchee was our Lord.”
Gwendolyn Brooks
Haman, good provider, brought his own rope.
Arranged with care his own unique reward.
He was risen higher in public death
Than he dared hope to rise in public life,
High as the best carpenters of the realm
Could build, high as the best gallows makers
He could afford to hire could lofty reach.
He twists slowly, slowly, at his rope’s end,
Turning slowly, his gaze could see for miles
Around now if still his eyes could see,
Turning slowly, could scan the capital,
The ways and and avenues that lead to power,
Turning slowly, South, East, North, West, search for
The junction where it all went somehow wrong.
Always and only he had expected
Simple justice: just what he had coming,
Had served his king, had shirked no drudging task,
Kept his desk clean, filed reports on time,
Learned decorum proper to high command—
Whose wife to flirt with and whom to avoid,
How to carve the roast, when to chill the wine,
How to serve up what the king wants to hear
At conference, and serve it up sincere.
Order, protocol, rank, degree, respect—
He knew his place and merely asked that those
Below know theirs; he wasn’t asking much:
The easy bow, the bending of the knee
To rank, acknowledging the earned degree.
His wife at first had thought his ravings odd,
A petty agnostic fret; his friends
Had humored him and failed to understand
His point that so much more than wounded pride
Was on the line, that the whole nation reeled
When one small wretched Jew refused to kneel.
If order, rank, and rule were not for all,
None would have them—the gutted state would fall.
The king, poor blind mindless amorous fool,
Must be saved from himself like it or not,
The state pushed back from the brink of chaos:
Blot out a people to save a nation,
Encourage a race for civilization.
The sentimental sops might call it cruel,
But realists would cautiously applaud:
And see him clear: a man doing the job
That years of public life had trained him for.
He liked to think that the years had prepared
Him precisely to meet this Jewish threat:
A moment to shine high in the klieg lights
Of all the focusing historians.
The man who knew his job and got it done.
Let the klieg lights of time affix him now
Twisting, slowly, slowly, at his rope’s end.
See him now in the bright harsh light of time
As man the butt of all ironic jokes,
Prickled on his own barbed wire, blown to hell
By his own bombs, gassed in the seclusion
Of his own chambers, and asking always
Only for what he has coming to him
And always, always, always getting it.
Man twists, slowly, slowly, at his rope’s end.
Turning slowly, scanning North, East, South, West:
History’s avenues all lead to death.
The light winks, the bands play, the boots march on.
Man dances absurd at the end of his rope.
For life is gala lynching party
Where every swinger brings his own rope:
It’s bring your own rope and reap your reward.
Except once: that grim party crashed by Him,
Intruding, who brought no rope of His own,
But borrowing man’s He stole the scene
And died, took what wasn’t coming to Him.
Look on Him, scene stealer, on His hilltop,
Changing the rules, muddling simple justice
With mercy, redemption, something called grace,
And cheating man of his hard earned reward:
Man’s antic rope’s end dance eclipsed at last
By the still shadow high on Golgotha.
E.W. Oldenburg 1936 - 1974
2 notes
·
View notes
Text
What You Need To Know About The Conjugated Antibodies For Flow Cytometry
Choosing the right antibody reagents for flow cytometry is crucial. A poor choice can cause a loss of sample material, money, and time, resulting in incorrect and unreliable results. Researchers need to consider plenty of factors, including antibody format and the antigenic target’s nature, for the success of flow cytometry. Another crucial factor they need to focus on when selecting an antibody is whether to use an unconjugated or directly conjugated product for flow cytometry.
Compared to unconjugated antibodies, directly conjugated antibodies are preferred by researchers for flow as it allows simultaneous use of multiple antibodies in the same species. It also simplifies the assay protocol and reduces the risk of potential mistakes during sample preparation. Read on to know about the conjugated antibodies for flow cytometry.
Conjugated antibodies are monoclonal or polyclonal antibodies with an attached molecule, used for creating a detectable signal by fluorescence, color-generation, or other signals. Besides flow cytometry, conjugated antibodies are used in various applications, like immunocytochemistry, and immunohistochemistry, to mention a few.
Direct and indirect are two types of antibody detection methods. The direct detection method uses a primary antibody conjugated directly to a label. Researchers can directly detect the target by conjugating primary antibodies, eliminating the need for secondary antibodies. It makes the use of multicolor staining easier and eliminates the problems related to the non-specific binding of secondary antibodies.
On the other hand, the indirect detection method uses an unconjugated primary antibody or conjugated secondary antibody, specifically for the primary antibody’s host species. Researchers prefer this method when the target of interest has low expression levels, and it allows signal amplification as secondary antibodies carrying multiple labels bind to primary antibodies. The indirect detection method has higher sensitivity levels and generates more intense signals.
Using fluorescently conjugated antibodies, researchers can detect multiple targets simultaneously. Fluorochromes, used in fluorescent dyes, have absorption and emission wavelengths. The emission of a photon at one wavelength excites another in the presence of light. Since overlapping of emission is common when performing co-staining, taking note of excitation and emission spectra is crucial when using multiple dyes. Fluorescently conjugated antibodies are used in varied techniques, including flow cytometry.
When antibodies are conjugated to enzymes such as alkaline phosphatase and horseradish peroxidase, they react to substrates and localize an insoluble colored product to antigen expression sites. Chromogenic labeled antibodies are majorly used for immunohistochemistry and Western Blot. Biotin conjugated antibodies are used if the target expression is low.
While researchers widely use primary conjugated antibodies, secondary antibodies are preferred as they make it easier to choose different conjugates irrespective of the primary antibodies. Using secondary antibodies reduces the need for chemically labeled primary antibodies, which are a bit costlier.
Author’s Bio – The author is an online blogger. This article is about conjugated antibodies
3 notes
·
View notes
Text
Researchers adopt approach for more ethical plant research
Researchers adopt approach for more ethical plant research
https://ift.tt/fs2AelW
Elizabete Carmo-Silva stays up to date on innovative plant science research methods, but one aspect of her work always concerned her – the process used to make the antibodies she needs for her research.
Traditionally the process of making antibodies for research involves the use of a small number of animals – typically rabbits – which are killed for their blood products to be used. Animal-sourced antibodies are even used in plant sciences, for example, to characterize the abundance of photosynthetic proteins in crop plants. Antibodies that selectively bind to proteins of interest are labeled with fluorescent dyes or isotopes selectively bind to enable detection and quantification.
In recent years, advances in technology using cell cultures have begun to enable recombinant antibody production. Recombinant antibodies are produced by synthetic cell culture – removing the need to use animals.
Antibody generation methods from Gray et al. 2020
“This technology has existed for years, but it has not been used much in plant research either because scientists don’t know that it’s an option or because it’s cost-prohibitive,” said Duncan Bloemers, former doctoral researcher in the Carmo-Silva lab at Lancaster University and now a data analyst for LiNaEnergy.
A new book chapter by Bloemers and Carmo-Silva outlines how plant scientists can apply this method to their work. The chapter, “Antibody Design for the Quantification of Photosynthetic Proteins and Their Isoforms” appears in Photosynthesis: Methods and Protocols, a book sharing methods, protocols, and best practices with the photosynthesis research community. The chapter includes how the duo went about designing and developing the antibodies specific to the different isoforms of their protein of interest (Rubisco activase), what was involved in the design, and how to validate that the proteins are being properly recognized by the antibodies. Importantly, researchers can use these detailed methods to create antibodies for virtually any protein and continue to use their current detection methods like immunoblotting and Western blotting.
Elizabete Carmo–Silva, professor in crop physiology at Lancaster University.
“The ethics of our research are very important,” said Carmo-Silva, Professor of plant physiology and principal investigator for Realizing Increased Photosynthetic Efficiency (RIPE). “We are very thankful to those who have made it possible to adopt this strategy and use it in our lab. We hope others will use it as well.”
Read the chapter:
Bloemer, D., Carmo-Silva, E. A. (2024). Antibody Design for the Quantification of Photosynthetic Proteins and Their Isoforms. In: Covshoff, S. (eds) Photosynthesis. Methods in Molecular Biology, vol 2790. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-3790-6_21
The post Researchers adopt approach for more ethical plant research appeared first on Botany One.
via Botany One https://botany.one/
May 20, 2024 at 09:47PM
0 notes
Text
The main reason behind my burning dislike for biomaterials (especially hydrogels and 'synthetic cells') is the fact that this subject is built upon a basis so unsteady that often data massaging (quoting a certain person here...) is necessary to make it appear workable.
Regarding how rampant data massaging is, I have four anecdotes:
Friend of mine, when she was starting out, the postdoc she was assigned with told her to replicate a set of experiments. She spent a month following the exact same protocol used but all data that came out were obviously not the same.
Said postdoc above once remarked to my friend: the error bars on your data are too large. You need to massage your data.
Same postdoc has a biobattery published on Nature in 2022. The idea and protocol used were exactly the same as a Nature paper in 2019. Obvious problems with innovation aside, he openly claimed on multiple occasions that the biobattery can last 30 minutes to 2 hours with an output of 1.9 V, but when he tried to replicate his own experiments in another lab, he failed hard and blamed the collaborator's microscope incubator for being faulty. Quoting the collaborator: "He says it can last for 30 minutes to 2 hours? More like 30 seconds."
Another person in said lab has a project where her assumptions were built on an earlier paper from the group. That paper deliberately didn't include lots of data that should've been there, and acknowledged "we have lots of unpublished data that supports our findings" in the text. Both me and my friend thinks this paper has been significantly massaged, and this unfortunate person spent 4 years trying to make her project work but it never did.
In practice there's indeed no way to figure out if the data has or hasn't been massaged just by looking the current-voltage figures they've put on the paper. One can easily take a system that has previously been established to be working, then adjust the input manually so that the output looks like what you want. One can also splice multiple different current-voltage traces together, something often done in dishonest Western blotting.
And I'm fucking glad I've left this field.
0 notes
Text
Welcome to Kwality Pharma, a hub of innovation and excellence. We're urgently seeking passionate individuals for Executive / Senior Executive positions in Quality Control. Dive into the details below to discover an incredible opportunity to contribute to the field of vaccines, biosimilars, and Mab.
Introduction: Embark on a rewarding journey with Kwality Pharma, a pioneering name in the pharmaceutical industry. Our founders' vision and a dynamic startup culture make Kwality Pharma the ideal workplace for those aspiring to make a difference. Currently, we have urgent openings for talented individuals to join our Quality Control team.
About the Company (Kwality Pharma): Kwality Pharma, with a rich history and a commitment to quality, stands as a symbol of excellence in the pharmaceutical sector. Founded on the principles of innovation and integrity, Kwality Pharma has evolved into a trusted name in the industry.
Company Vacancies List:
Position Title: Executive / Senior Executive - Quality Control
Experience: 3 to 5 Years
Qualifications: MSc Biochemistry / Biotechnology / Microbiology / & any Life science background / B Tech Biotechnology / Biochemistry
Job Description:
About the Department & Responsibilities:
Department: Quality Control - Microbiology/ Molecular Biology/ Analytical Science / Chromatographic / Bio assay
Roles and Responsibilities:
Preparation of SOP's & Method Validation protocols
Analytical Method validation & verification
Good Lab Techniques and Good Documentation
RT PCR techniques and operation
ELISA techniques and operation
SDS PAGE, Western Blotting Techniques and Gel Doc operation
Biosafety cabinet/LAFU operations
CO2 Incubator operations
Stability chamber operation and Stability study execution
Lab Chemicals management
Inverted Microscope operation
Plating techniques and Environmental Monitoring
HPLC operation
UV Spectroscopy operation
Thermomixer operation
Protein estimation by LOWRY method
TOC analysis
Method Validation/Cleaning Validation
OOT/OOS Investigations
How to Apply: Ready to be a part of our dynamic team? Email your CV at [email protected] and take the first step towards a fulfilling career in the pharmaceutical industry
0 notes
Text
In Vitro Budding and Floatation based enrichment of Mitochondria-derived Vesicles for Proteomics from Rat Heart
Mitochondria-derived vesicles (MDVs) are a novel class of vesicles whose biogenesis and trafficking are poorly characterized. Here, we describe the protocol to in vitro generate MDVs from mitochondria isolated from animal heart tissues. The protocol can be coupled with Transmission Electron Microscopy (TEM), proteomics or western blotting as final readouts. The protocol is optimized to improve the yield and purity of vesicles for downstream proteomics and facilitates selection of key regulators to be validated within cells. http://dlvr.it/T3lrFZ
0 notes
Text
Understanding Market Dynamics in the Autoimmune Disease Diagnostics Market Industry
Market Overview –
The size of the market for autoimmune disease diagnostics was estimated at USD 4.9 billion in 2022 and is expected to increase at a compound yearly growth rate (CAGR) of 6.7% from USD 5.23 billion in 2023 to USD 8.78 billion by 2032.
The autoimmune disease diagnostics market is witnessing growth driven by advancements in the diagnosis of lupus and other autoimmune conditions. With increasing awareness and understanding of these diseases, there's a rising demand for accurate and efficient diagnostic tools. Innovations in biomarker testing and imaging techniques are enhancing early detection and personalized treatment strategies, driving market expansion.
The autoimmune disease diagnostics market focuses on providing tools and tests for diagnosing autoimmune diseases, a group of conditions where the immune system attacks healthy cells and tissues. These diseases can affect various organs and systems in the body, leading to chronic inflammation, tissue damage, and functional impairment.
Market growth is driven by the increasing prevalence of autoimmune diseases worldwide, attributed to factors such as genetic predisposition, environmental triggers, and lifestyle factors. With millions of people affected by autoimmune diseases, there is a growing demand for accurate and timely diagnostic tests to facilitate early intervention and improve patient outcomes.
Technological advancements and innovations in autoimmune disease diagnostics are shaping the market, offering new biomarkers, imaging techniques, and molecular assays to enhance diagnostic accuracy and precision. From serological tests and genetic testing to advanced imaging modalities and point-of-care devices, these advancements enable healthcare providers to diagnose autoimmune diseases more effectively and tailor treatment strategies to individual patient needs.
Moreover, the growing recognition of the importance of early detection and personalized medicine is driving market growth, as healthcare systems prioritize preventive healthcare and precision diagnostics to improve patient care and reduce healthcare costs.
However, challenges such as variability in disease presentation, overlapping symptoms, and limited awareness among healthcare providers pose obstacles to market growth. Addressing these challenges requires greater education and training for healthcare professionals, improved access to diagnostic tools and resources, and collaboration between industry stakeholders to develop standardized diagnostic protocols and guidelines.
Segmentation –
The global autoimmune disease diagnostics market has been segmented on the basis of disease type, test type, and end-user.
The market, by disease type, has been classified as systemic autoimmune disease, and localized autoimmune disease. The systemic autoimmune disease segment has been further classified into psoriasis, multiple sclerosis, rheumatoid arthritis, systemic lupus erythematosus (SLE), and others. Localized autoimmune disease segment has been divided into inflammatory bowel disease, thyroid, type 1 diabetes, and others.
On the basis of test type, the market has been segregated into western blotting, enzyme-linked immunosorbent assay (ELISA), double immune diffusion, dot blot, counter immune-electrophoresis, line blot, immunofluorescence assays (IFA), multiplex immunoassay, agglutination, and others.
The end-user segment has been divided into, has been segmented into hospitals, diagnostics centers, research laboratories, and others
Regional Analysis –
The autoimmune disease diagnostics market varies regionally due to differences in disease prevalence, diagnostic infrastructure, and healthcare expenditure. Developed regions like North America and Europe have a higher prevalence of autoimmune diseases and well-established diagnostic capabilities, leading to a robust market for autoimmune disease diagnostics. In contrast, developing regions face challenges such as limited access to specialized testing facilities and lower awareness of autoimmune diseases, resulting in underdiagnosis and undertreatment.
Moreover, variations in regulatory frameworks and reimbursement policies influence market dynamics across different regions. As the burden of autoimmune diseases continues to rise globally, there is a growing need for affordable and accessible diagnostic solutions, particularly in underserved regions. Collaborative efforts between healthcare stakeholders and diagnostic companies are essential to address regional disparities and improve patient outcomes in the autoimmune disease diagnostics market.
Key Players –
Autoimmune disease diagnostics companies include Siemens Healthcare Private Limited, bioMérieux SA, Bio-rad Laboratories, Beckman Coulter, AstraZeneca, EUROIMMUN AG, Hoffmann-la Roche, Inova Diagnostics, Myriad Genetics, Thermo Fisher Scientific, Trinity Biotech Plc., and Hemagen Diagnostics.
Related Reports –
Urinalysis Test
Celiac Disease Treatment
Healthcare Enterprise Software
Amniotic Membrane
For more information visit at MarketResearchFuture
#Autoimmune Disease Diagnostics Market#Autoimmune Disease Diagnostics Market Size#Autoimmune Disease Diagnostics Market Share#Autoimmune Disease Diagnostics Market Growth#Autoimmune Disease Diagnostics Market Trends
0 notes
Text
How Advancements in Rabbit Polyclonal Production are Shaping Research
In the dynamic realm of scientific research, the quest for innovative tools and methodologies is ceaseless. One such pivotal advancement is seen in the field of Rabbit Polyclonal Antibody Production, where custom antibody development has become a linchpin in shaping the landscape of modern research methodologies. As scientists delve deeper into the intricacies of biological systems, the evolution of rabbit polyclonal antibodies emerges as a game-changer, offering unparalleled specificity and versatility.
Custom Antibody Development Tailoring Precision for Research Excellence: At the heart of this paradigm shift lies the custom antibody development process. Unlike monoclonal antibodies derived from a single cell line, rabbit polyclonal antibodies are generated by immunizing rabbits with the target antigen. This approach results in a heterogeneous mix of antibodies, each recognizing different epitopes on the antigen. The diversity achieved through this method grants researchers a potent tool for a wide array of applications, from Western blotting to immunohistochemistry.
The Versatility of Rabbit Polyclonal Antibodies: A Crucial Asset in Research, Rabbit polyclonal antibodies production, due to their unique process, exhibit high specificity and sensitivity. This characteristic makes them ideal for detecting multiple protein isoforms and post-translational modifications. The ability to recognize various epitopes provides a more comprehensive understanding of protein interactions, facilitating nuanced insights into complex biological processes.
Rapid Advancements in Techniques: Enhancing Custom Antibody Development, Recent strides in molecular biology techniques have catalyzed the refinement of rabbit polyclonal antibody production. Advanced purification methods and state-of-the-art immunization protocols have significantly improved the yield and specificity of antibodies. These developments not only streamline the production process but also contribute to the production of high-affinity antibodies, ensuring reliable and reproducible results in diverse experimental settings.
Final Thoughts: In the realm of scientific discovery, the role of rabbit polyclonal antibody production in shaping research is indisputable. As scientists strive for precision and versatility in their experiments, the custom antibody development process offers a bespoke solution. The advancements in techniques witnessed in recent years have not only bolstered the efficiency of production but have also elevated the quality and reliability of rabbit polyclonal antibodies. Researchers worldwide now have at their disposal a powerful tool that is reshaping the boundaries of what can be achieved in the laboratory.
The journey from antigen immunization to the production of rabbit polyclonal antibodies has traversed remarkable milestones, opening new avenues for exploration and understanding in the scientific community. As we witness the transformative impact of these antibodies on research methodologies, it becomes evident that the advancements in rabbit polyclonal antibody production are not just a scientific evolution; they are a catalyst for breakthroughs that redefine the possibilities within the realm of life sciences.
0 notes
Text
HIV Testing: Ensuring Your Health and Well-being with DrSafeHands
In today’s world, where health concerns are on the rise, it’s important to stay informed and take proactive steps to protect ourselves. One such crucial aspect of healthcare is HIV testing. HIV (Human Immunodeficiency Virus) is a serious condition that can lead to AIDS if left untreated. DrSafeHands, a trusted name in healthcare, offers reliable and confidential HIV testing services that prioritize your well-being and peace of mind. In this article, we will explore the importance of HIV testing, the testing options available, and how DrSafeHands can assist you in this journey.
Understanding the Importance of HIV Testing:
HIV is primarily transmitted through unprotected sexual intercourse, sharing needles, or from an infected mother to her child during childbirth or breastfeeding. While HIV can affect anyone, certain groups, such as sexually active individuals, intravenous drug users, and those with multiple partners, are at higher risk. Timely HIV testing plays a crucial role in preventing the spread of the virus and ensuring early detection for effective treatment and care.
Types of HIV Tests :
DrSafeHands offers a range of HIV testing options tailored to your specific needs:
Rapid HIV Tests: Rapid tests provide quick results, usually within 20 minutes. These tests are conducted using blood, oral fluid, or urine samples and can be performed in a clinical setting or in the privacy of your home. Rapid tests are highly accurate and are an excellent choice for individuals seeking immediate results.
ELISA/Western Blot Tests: These tests are commonly used in diagnostic laboratories and healthcare facilities. ELISA (Enzyme-Linked Immunosorbent Assay) detects the presence of HIV antibodies in the blood, while Western Blot confirms the diagnosis. These tests are highly accurate and recommended for those who require a definitive diagnosis.
Self-Testing Kits: DrSafeHands offers self-testing kits, allowing you to discreetly test yourself for HIV in the comfort of your home. These kits include step-by-step instructions and provide accurate results, empowering you to take control of your health.
HIV RNA Tests: HIV RNA tests detect the presence of the virus itself by analyzing the genetic material (RNA) in the blood. These tests are highly sensitive and can detect HIV infection as early as 10 days after exposure. They are particularly useful for individuals who suspect recent exposure to HIV.
Confidentiality and Support: At DrSafeHands, we understand the importance of privacy and confidentiality when it comes to HIV testing. We maintain strict protocols to ensure your personal information remains secure and confidential. Our trained counselors are also available to provide support and guidance throughout the testing process, addressing any concerns or questions you may have.
Why Choose DrSafeHands?
Expertise and Experience: DrSafeHands is a leading healthcare provider with years of experience in HIV testing and counseling. Our team of medical professionals and counselors are highly trained to offer the best care possible.
Convenience and Accessibility: We understand that your time is valuable. DrSafeHands provides a hassle-free experience with online appointment scheduling, at-home testing options, and quick turnaround times for results.
Quality and Accuracy: Our commitment to quality and accuracy ensures that you receive reliable and trustworthy results. We utilize state-of-the-art testing methods and follow stringent quality control measures to maintain the highest standards.
Comprehensive Care: DrSafeHands goes beyond testing. We offer comprehensive care and support services for those living with HIV, including treatment options, counseling, and educational resources to help you lead a healthy and fulfilling life.
Conclusion: Taking charge of your health includes regular HIV testing. With DrSafeHands, you can access accurate, confidential, and convenient testing options that prioritize your well-being. Don’t delay — get
0 notes
Text
METHODS FOR PURIFYING AND ANALYSING PROTEINS VIA WESTERN BLOTTING
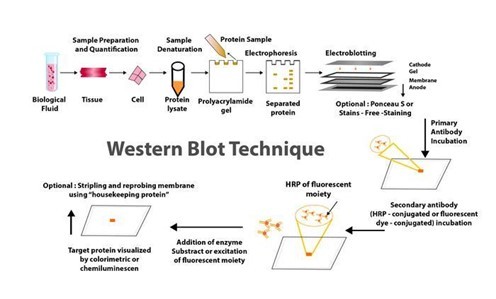
For many years, western blotting was a primary method in molecular biology and techniques such as proteomics (the large-scale study of proteomes: a set of proteins produced in an organism, system, or biological context). Given that western blot is a multistep process that frequently necessitates specialised interpretation, potential errors and variations at any step can jeopardise the reliability and reproducibility of its results.
Given this situation, many advanced and sophisticated approaches to improving the western blot protocol have been developed over the last two decades. These enhancements are more automated and sensitive, with high potential for reducing potential issues with the traditional western blot method. Western blotting's improved sensitivity and innovative equipment advancements have the potential to broaden the field of clinical applications for this fundamental method.
Helvetica Health Care explains the western blot technique as a method of purifying and analysing proteins, as well as new developments in western blotting methodology, in this article.
WHAT EXACTLY IS THE WESTERN BLOT TECHNIQUE?
Traditionally, the western blotting method involves seven steps, as below.
Sample preparation from cells or tissue lysates
Separation of proteins by gel electrophoresis
Protein transfer in a nitrocellulose or polyvinylidene fluoride (PVDF) membrane,
Blocking of non-specific proteins on membrane
Primary Antibody incubation
Secondary Antibody incubation
Protein detection & analysis
WHAT ARE THE NEW DEVELOPMENTS IN THE WESTERN BLOT DOMAIN AND WHAT CHARACTERISTICS DO THEY HAVE?
Below are some innovations that have enhanced the western blot protocol and addressed the potential problems in its application in protein purification and analysis.
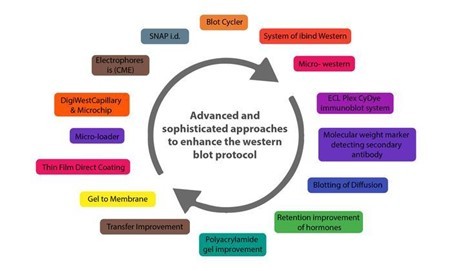
CAPILLARY AND MICROCHIP ELECTROPHORESIS (MCE)
MCE has higher sensitivity and resolution than traditional techniques, allowing for the detection of multiple target proteins from a single cell lysate sample. The method not only allows for parallel multiplexed tests of a set of proteins using a small sample amount, but it also eliminates blocking stages and has faster analysis times (8 min for electrophoretic resolution). MCE, while still in development, has the potential to be significantly improved with additional work.
AUTOMATED MICROFLUIDIC PROTEIN IMMUNOBLOTTING
Automated protein immunoblotting is a programmable and controllable technology that combines PAGE (polyacrylamide gel electrophoresis) and blotting in a single device. It saves time, avoids multiple test stages, and reduces the amount of equipment and reagents required. Because it can detect free prostate-specific antigens in a sample of human seminal fluid in less than 5 minutes, this technology is both cost-effective and reagent-efficient. The method is constantly being improved to improve sensitivity and enable protein quantification.
SINGLE CELL-RESOLUTION WESTERN BLOTTING
This sensitive method has also been used to investigate protein variations in stem cell signalling and differentiation. It can detect specific cell-to-cell changes in protein expression. MiloTM (ProteinSimple), the world's first Single-Cell Western platform, can measure protein expression (up to 4 proteins per cell simultaneously) in 1000 single cells in about 4 hours.
DIGIWEST
DigiWest, a simplified version of the standard procedure, improves western blotting throughput by requiring less lysate, target detection, and antibody. However, this method requires biotinylation of the target proteins, requires specialised reagents and equipment, incurs initial startup costs, has issues with translating the digital DigiWest results to western blot mimics, and has specifics about some procedures, such as the complex and difficult elution of proteins from PVDF membranes.
SIMPLE WESTERN
Simple Western is a method developed by ProteinSimple (San Jose, California) that is based on CE-SDS (Capillary electrophoresis sodium dodecyl sulphate), in which separated proteins are linked to the capillary wall by a patented photo- (ultraviolet) induced chemical crosslink. The method can use up to 90 different antibodies at the same time, is relatively quick, and requires small amounts of sample. It can also perform standard curve quantification, similar to high-pressure liquid chromatography (HPLC), and improve reproducibility. However, it necessitates the use of specialised reagents.
MICRO-LOADER
The Micro-loader technology has improved the sensitivity of the western blot assay and PAGE. To load samples, it employs a sample micro-loader device with a funnel-like design. This technique is highly effective for measuring protein expression and phosphorylation in samples that are few, difficult to find, and limited in quantity because it only requires a small number of loading samples for signal detection.
THIN-FILM DIRECT COATING WITH SUCTION-WESTERN BLOTTING (TDCS)
Western blotting has significant limitations due to high antibody consumption and lengthy operating times. TDCS, a capillary-tube-based technique, aims to reduce antibody and time consumption in western blotting. This quick and sensitive detection method allows for the quantitative study of multiple antigen-antibody interactions, as well as multiple protein interactions.
BLOTTING OF DIFFUSION
SDS-PAGE Single-prefabricated gels on plastic support are a quick and easy way to make multiple blots using diffusion blotting. Diffusion blotting allows for the comparison of several proteins on blots from the same gel. Protein transmission rates by diffusion blotting are 10% in 3 minutes, 20% in 20 minutes, and 40% to 60% in 3 hours. When compared to electroblotting, the technique may increase the amount of proteins efficiently delivered to the membrane surface. As a result, when quantitative protein exchange is not required, diffusion blotting is the preferred method.
MICRO WESTERN
Soon, micro western will be a proper western blot technique for confirming the diagnosis of purified HIV p24 and gp120 proteins in blood plasma. The Micro-Western may be vital for detecting infectious diseases and cancer, despite not being widely available.
SYSTEM OF IBIND WESTERN
Thermo Fisher Scientific's iBind Western procedure makes use of a low-cost semi-automated western blotting structure that uses sequential flow technology to distribute chemicals and antibodies to different compartments. Cleaner western blots will result from using the iBind Western Systems and highly specialised primary and secondary antibodies.
BLOT CYCLER
Every step of membrane processing is automated with the BlotCycler (Precision Biosystems, USA), and up to 12 membranes can be processed at once. The BlotCyclerTM is an Automated Western Blot Development system that improves repeatability and sensitivity by completing all blot cleaning, trapping, and incubation steps. It is also simple to programme all of the actions for specific operations.
SYSTEM OF SNAP I.D.
In a low-cost system of SNAP i.d. 2.0, solvents are uniformly distributed via the tissue using a technique based on a vacuum and a flow distribution system (Merck, USA). Its main advantage is that washing, antibody incubation, and blocking all take about 30 minutes. The SNAP i.d.® 2.0 gadget actively forces reagents through the membrane, unlike conventional Western blotting, which depends on diffusion to transfer reagents.
Washes, antibody recollection, and antibody-antigen binding have been improved. The SNAP i.d.® 2.0 delivers new, cutting-edge western blotting characteristics with an immunodetection capability in 30 minutes without using additional reagents (e.g., antigen, antibody, or detection reagents).
RETENTION IMPROVEMENT OF HORMONES OF THE PEPTIDE ON THE MEMBRANE OF BLOTTING
Western blotting is significantly improved by treating the PVDF membrane used in this method with glutaraldehyde (0.2%) for fifteen minutes in a saline solution containing tween-80. In western blotting, the addition of glutaraldehyde to the membrane prevented or reduced the quantity of insulin loss.
Apart from these, two more techniques that have improved the conventional method are
Analysis of western blot utilising molecular weight marker detecting secondary antibody
Total and target protein co-detection by immunoblotting of fluorescent ECL and labelling of Cydye
Many innovations in western blotting techniques to transfer proteins from gel to membrane have also been observed, such as vacuum blotting, centrifuge blotting, multiple tissue blotting, and so on. The Trans-Blot Turbo, the iBlot® dry blotting system, and the PierceTM Power Blot Cassette are all improvements in protein transfer methods from gels to membranes. The transfer time is reduced from an hour or overnight to at most 10 minutes when these new techniques are used.
HHC offers the ZeptoMetrix WESTERN BLOT for in vitro detection of antibodies to SIV in serum or plasma and is available in 10 or 30-strip kit formats. We also provide a bench top, a western blot processor, and the AUTOBLOT 3000. It controls incubation times, dispensing volumes and washing programs. Ten user-defined protocols can be programmed easily. Contact us now to know how we can help you get accurate and high-quality output with our products when performing the western blotting procedure.
0 notes
Text
Western Blot Troubleshooting Tips - Technology Networks
Western Blot Troubleshooting Tips – Technology Networks
We’ve updated our Privacy Policy to make it clearer how we use your personal data.We use cookies to provide you with a better experience. You can read our Cookie Policy here.You’ve read the protocol a hundred times, you’ve tried your lab mate’s suggestions and left various offerings to the western blotting Gods – but it’s just no good. Your western blot still looks a mess, the bands don’t appear…

View On WordPress
0 notes
Text
Lzip pgc1a

Membrane Blocking and Antibody Incubations Electrotransfer to nitrocellulose membrane ( #12369).Ĭ.NOTE: Loading of prestained molecular weight markers ( #59329, 10 µl/lane) to verify electrotransfer and biotinylated protein ladder ( #7727, 10 µl/lane) to determine molecular weights are recommended. Load 20 µl onto SDS-PAGE gel (10 cm x 10 cm). Heat a 20 µl sample to 95–100☌ for 5 min cool on ice.Sonicate for 10–15 sec to complete cell lysis and shear DNA (to reduce sample viscosity).Immediately scrape the cells off the plate and transfer the extract to a microcentrifuge tube. Lyse cells by adding 1X SDS sample buffer (100 µl per well of 6-well plate or 500 µl for a 10 cm diameter plate).Aspirate media from cultures wash cells with 1X PBS aspirate.Treat cells by adding fresh media containing regulator for desired time.Protein Blotting A general protocol for sample preparation. Detection Reagent: SignalFire™ ECL Reagent ( #6883).ī.Secondary Antibody Conjugated to HRP: Anti-rabbit IgG, HRP-linked Antibody ( #7074).Pore size 0.2 µm is generally recommended. Blotting Membrane and Paper: ( #12369) This protocol has been optimized for nitrocellulose membranes.Blue Prestained Protein Marker, Broad Range (11-250 kDa): ( #59329).Biotinylated Protein Ladder Detection Pack: ( #7727).Primary Antibody Dilution Buffer: 1X TBST with 5% BSA for 20 ml, add 1.0 g BSA to 20 ml 1X TBST and mix well.Blocking Buffer: 1X TBST with 5% w/v nonfat dry milk for 150 ml, add 7.5 g nonfat dry milk to 150 ml 1X TBST and mix well.10X Tris Buffered Saline with Tween ® 20 (TBST): ( #9997) To prepare 1 L 1X TBST: add 100 ml 10X TBST to 900 ml dH 2O, mix.10X Tris-Glycine Transfer Buffer: ( #12539) To prepare 1 L 1X Transfer Buffer: add 100 ml 10X Transfer Buffer to 200 ml methanol + 700 ml dH 2O, mix.10X Tris-Glycine SDS Running Buffer: ( #4050) To prepare 1 L 1X running buffer: add 100 ml 10X running buffer to 900 ml dH 2O, mix.1X SDS Sample Buffer: Blue Loading Pack ( #7722) or Red Loading Pack ( #7723) Prepare fresh 3X reducing loading buffer by adding 1/10 volume 30X DTT to 1 volume of 3X SDS loading buffer.10X Tris Buffered Saline (TBS): ( #12498) To prepare 1 L 1X TBS: add 100 ml 10X to 900 ml dH 2O, mix.20X Phosphate Buffered Saline (PBS): ( #9808) To prepare 1 L 1X PBS: add 50 ml 20X PBS to 950 ml dH 2O, mix.NOTE: Prepare solutions with reverse osmosis deionized (RODI) or equivalent grade water. Solutions and Reagentsįrom sample preparation to detection, the reagents you need for your Western Blot are now in one convenient kit: #12957 Western Blotting Application Solutions Kit NOTE: Please refer to primary antibody product webpage for recommended antibody dilution. For western blots, incubate membrane with diluted primary antibody in 5% w/v BSA, 1X TBS, 0.1% Tween ® 20 at 4☌ with gentle shaking, overnight.

0 notes
Text
Primary antibody incubation time
As a universal standard protocol, western blot antibody incubation should be placed for one hour. However, from interactions with primary antibody incubation time and secondary antibodies, the process is not always fast and easy. This is because there is a detection limit that can not be achieved by incubation even after 48 hours of incubation and a 16-hour incubation of primary and secondary antibodies. With longer incubations, the possibility of background does not increase.
0 notes
Text
Metabolomics unveiled the impact of alterations in nucleotide glucose metabolism on palmitic acid-induced TLR4 activation in THP-1 macrophages
Objective: The association of palmitic acid with macrophage inflammation and its promotion of the expression of inflammatory factors through TLR4 have been demonstrated. It has been observed that immature TLR4 localizes to the endoplasmic reticulum and Golgi apparatus, necessitating glycosylation for migration to the cell membrane. The objective of this study was to identify potential biomarkers associated with N-glycosylation subsequent to the activation of TLR4 inflammatory signaling in human macrophages by palmitic acid. Approach and Results: The co-cultivation of palmitic acid with THP-1 macrophages was conducted for a duration of 24 hours, followed by the collection of cell extracts for subsequent metabolomic and lipidomic analyses using high performance liquid chromatography-tandem mass spectrometry. Multivariate and univariate statistical analyses were conducted to identify potential biomarkers, in accordance with established scientific protocols. The impact of palmitic acid on the TLR4 signaling pathway and macrophage N-glycosylation was assessed at various time points using Western blot analysis, immunofluorescence staining, Elisa assays, and chemical labeling techniques. The TLR4 inflammatory signaling pathway was examined in macrophages at different time points, revealing that PA induced the upregulation of MyD88 and TRAF6 expression as well as NF-{kappa}B phosphorylation, indicating the activation of classical NF-{kappa}B signaling. After 24 hours, TLR4 translocated from the cell membrane to the cytoplasm and initiated internalization, accompanied by significant colocalization with GalAz in the cytosol. In addition, the metabolites in the cell extract were found to be altered in both the control and model groups. Significantly alterations in two N-glycosylation related metabolites were observed in the model group, including guanosine diphosphate-L-fucose and uridine diphosphate-N-acetylglucosamine/uridine diphosphate-N-acetylgalactosamine. Conclusion: THP-1 macrophages incubated with palmitic acid exhibited a distinct metabolomic profile compared to the control group. Our findings suggest that metabolomics analysis holds promise in identifying disease-specific biomarkers for diagnosing fatty acid-induced inflammatory responses in macrophages. http://dlvr.it/T0wF97
0 notes
Text
man, I’ve been trying to write out making a Western blot for Do A Science, but I haven’t had to do one myself in a couple years and I forgot. I forgot that they’re too many fucking steps
#western blots: separate proteins from a sample out by size and then stain with marked antibodies to see if#a protein of interest is there#my lab's protocol made this take TWO FUCKING DAYS#fic complaints
3 notes
·
View notes