#western blot protein
Text
0 notes
Text
A Step-by-Step Guide to Performing Western Blotting Protein

Western blotting is a powerful and widely used technique in molecular biology and protein analysis. In this comprehensive guide provided by Kendrick Labs Inc., we will walk you through the step-by-step process of performing Western blotting with precision and accuracy. From sample preparation and protein extraction to antibody incubation and data interpretation, each key stage of the Western blotting workflow will be detailed to help researchers and scientists achieve reliable results in protein detection and analysis. Follow along to master the fundamentals of Western blotting and enhance your experimental skills in the laboratory.
What is Western Blotting?
If science had a dating app, Western blotting would be the swiping right participant. It's a technique used to detect specific proteins in a sample, kind of like finding Waldo in a sea of stripes.
Applications of Western Blotting
Western blotting is the undercover agent of the lab world, utilized in various fields like molecular biology, immunology, and biochemistry. It helps researchers identify proteins, study protein expression levels, and even solve mysteries like a true detective.
Overview of Western Blotting Process
Key Steps in Western Blotting
Think of Western blotting as a protein talent show, where the key steps include protein separation, transfer to a membrane, blocking, primary and secondary antibody incubation, and finally, detection.
Importance of Protein Detection
Proteins are the Beyoncé of cells - they run the show. Detecting and quantifying proteins through Western blotting helps scientists understand cellular processes, biomarker discovery, and even evaluate treatment responses.
Sample Preparation and Protein Extraction
Tissue or Cell Lysis
It's like breaking into a safe, but a scientific one. Tissue or cell lysis is the process of breaking open cells to release proteins, akin to cracking open a delicious protein-filled piñata.
Protein Quantification Methods
Quantifying proteins is like counting the number of cookies in a jar – it's crucial for accurate results. Various methods like Bradford assay or BCA protein assay help determine protein concentrations for the Western blotting magic show.
Gel Electrophoresis and Protein Separation
Preparation of SDS-PAGE Gel
Creating an SDS-PAGE gel is like laying down the red carpet for proteins. It provides a platform for protein separation based on size, ensuring each protein gets its moment in the spotlight.
Running the Gel and Protein Separation
Running the gel is the protein marathon – proteins move through the gel at different speeds based on size, creating distinct bands like a molecular fingerprint. This step sets the stage for the Western blotting revelation to come.
Protein Transfer Techniques
Overview of Protein Transfer Methods
When it comes to transferring proteins from a gel to a membrane, Western blotting offers a variety of methods to suit your needs. From traditional wet transfers to speedy semi-dry transfers, there's a method for every protein aficionado.
Optimizing Transfer Efficiency
For a successful protein transfer, ensure your transfer buffer is fresh, your voltage is just right (not too high, not too low), and your proteins are evenly transferred. Remember, even proteins deserve a smooth journey from gel to membrane!
Antibody Incubation and Detection
Primary and Secondary Antibody Incubation
Like a perfectly crafted sandwich, the primary antibody is the flavorful filling that binds specifically to your protein, while the secondary antibody adds the visual flair for detection. Don't forget to incubate with care and wash away any unbound antibodies.
Visualization of Protein Bands
Once your antibodies have worked their magic, it's time to uncover those elusive protein bands. Whether you opt for chemiluminescence, fluorescence, or colorimetric detection, make sure your proteins are ready for their close-up moment.
Data Analysis and Interpretation
Image Acquisition and Analysis Software
Now that your protein bands have posed for the camera, it's time for some data analysis fun! Choose your favorite image analysis software and let it work its magic to quantify those bands and normalize your data. Numbers never looked so good!
Quantification and Normalization of Protein Bands
When it comes to interpreting Western blot data, remember the golden rule: quantify, quantify, quantify! Normalize your protein bands to housekeeping genes or total protein levels for an accurate representation of your results. It's all about that protein balance!
Troubleshooting and Common Pitfalls
Addressing High Background Noise
Ah, the pesky background noise that loves to photobomb your protein bands. Combat this common culprit by tweaking your blocking buffer, optimizing antibody concentrations, or just giving that membrane a good wash. Say goodbye to noisy neighbors!
Strategies for Weak Signal Detection
When your protein bands are feeling a bit camera-shy, fear not! Boost your signal with a longer exposure time, optimize your antibody dilutions, or try out signal amplification techniques. A little patience and creativity can go a long way in revealing those shy proteins.
In conclusion, mastering the art of Western blotting is essential for researchers and scientists looking to delve deeper into the realm of protein analysis. By following the detailed steps and guidelines outlined in this guide provided by Kendrick Labs Inc., you can enhance your understanding and proficiency in Western blotting techniques. With practice and attention to detail, you can harness the power of Western blotting to unlock valuable insights into protein expression and function, furthering your research endeavors and contributing to advancements in the field of molecular biology.
Original Sources: https://kendricklabs.blogspot.com/2024/05/a-step-by-step-guide-to-performing.html
0 notes
Text
A small funny tidbit from the realm of Biology/Chemistry:
In 1975, the scientist Edwin Southern developed a method to separate DNA fragments in a gel, then transfer it to a membrane, which makes it possible to label it according to their sequence. The method was named 'Southern blot' to honour its creator.
Later, similar methods were made for RNA and Protein. And these were aptly named 'Northern blot' and 'Western blot'
And I find that beautiful.
29 notes
·
View notes
Text
Heyy,
Today I'm taking the day off from now on (it is already almost 6pm, but better that than nothing)
I had a car class, and on monday i have the practic exam!!!!!! AAAAAAAAAAKDKDKDKDKDKD SO NERVOUS
Today at the lab a molecular weight's tube (like to make a guide line in western blot, a gel to separate proteins based on their weight) got lost. I left early yesterday, so i didn't see who put it away or anything, and my parter doesn't remember taking it and my teacher neither. I'll keep you updated on the mystery if it appears. We even looked in every trash can in the lab

Pic: Today waiting in the only shadow around: the one of a signal post
#leian rambles#realistic studyblr#neuroscience studies#study blog#studyblr#student#realistic study life
4 notes
·
View notes
Note
Cheadle with the prompt late night studying/working? (if that’s prompty enough)
Cheadle fought back a yawn as she analyzed the smudgy results of her western blot, trying to discern concentrations of a protein she was studying.
It was late, as everyone else in her lab had already gone to bed, yet she wanted to finish everything.
Sleeping always felt a lot better when it was a reward for completion.
6 notes
·
View notes
Text
DYNE-251 for the treatment of Duchenne Muscular Dystrophy Received FDA Fast Track Designation
HK-Magicure -- On October 31, Dyne Therapeutics announced that the U.S. FDA has granted Fast Track designation for DYNE-251 for the treatment of Duchenne muscular dystrophy (DMD) mutations amenable to exon 51 skipping. DYNE-251 is being evaluated in the Phase 1/2 DELIVER global clinical trial.

DYNE-251 is Dyne’s product candidate being developed for people living with Duchenne muscular dystrophy (DMD) who are amenable to exon 51 skipping. DYNE-251 consists of a phosphorodiamidate morpholino oligomer (PMO) conjugated to a fragment antibody (Fab) that binds to the transferrin receptor 1 (TfR1) which is highly expressed on muscle. It is designed to enable targeted muscle tissue delivery and promote exon skipping in the nucleus, allowing muscle cells to create a truncated, functional dystrophin protein, with the goal of stopping or reversing disease progression.
In preclinical studies, robust and durable exon skipping and dystrophin expression were observed in the mdx mouse model in skeletal and cardiac muscle as well as reduced muscle damage and increased muscle function. In non-human primates, DYNE-251 demonstrated a favorable safety profile.
DELIVER is a Phase 1/2 global clinical trial evaluating DYNE-251, consisting of a 24-week multiple ascending dose (MAD) randomized placebo-controlled period, a 24-week open-label extension and a 96-week long-term extension. The trial, which is designed to be registrational, is expected to enroll approximately 46 ambulant and non-ambulant males with DMD who are ages 4 to 16 and have mutations amenable to exon 51 skipping therapy. The primary endpoints are safety, tolerability and change from baseline in dystrophin levels as measured by Western blot. Secondary endpoints include measures of muscle function, exon skipping and pharmacokinetics. Dyne anticipates reporting data from the MAD placebo-controlled portion of the DELIVER trial on safety, tolerability and dystrophin in the second half of 2023.

About Duchenne Muscular Dystrophy (DMD)
Duchenne Muscular Dystrophy (DMD) is a rare disease caused by mutations in the gene that encodes for dystrophin, a protein critical for the normal function of muscle cells. These mutations, the majority of which are deletions, result in the lack of dystrophin protein and progressive loss of muscle function.
DMD occurs primarily in males and affects an estimated 12,000 to 15,000 individuals in the U.S. and 25,000 in Europe. Loss of strength and function typically first appears in pre-school age boys and worsens as they age. As the disease progresses, the severity of damage to skeletal and cardiac muscle often results in patients experiencing total loss of ambulation by their early teenage years and includes worsening cardiac and respiratory symptoms and loss of upper body function by the later teens. There is no cure for DMD and currently approved therapies provide limited benefit.
For more articles on medicines, click here: hkmagicure
Hong Kong Magicure Medical Center has long been focusing on the import and export of new drugs, special drugs and rare disease drugs in the field of oncology. Welcome to inquiry: [email protected].
3 notes
·
View notes
Text
Botulinum Toxin Market Segmentation, Parameters and Prospects 2024 to 2030 Industry Research Report
Botulinum Toxin Industry Overview
The global botulinum toxin market size was valued at USD 11.1 billion in 2023 and is expected to grow at a compound annual growth rate (CAGR) of 9.8% from 2024 to 2030. Botulinum toxin is a protein and neurotoxin produced by the bacterium clostridium botulinum. The toxin is a selective blocker of acetylcholine released from nerves, which blocks neural transmission from the nerves when injected into muscle. Botulinum toxin is one of the most exceptional substances encountered in medicine and science. The adoption of botulinum toxin in cosmetology has grown rapidly over the decade, and at present it is one of the most common and widely performed aesthetic procedures in the world. The increasing concern for aesthetic features in developed and developing regions has led to an increase in the number of cosmetic procedures.
Gather more insights about the market drivers, restrains and growth of the Botulinum Toxin Market
The availability of a variety of botulinum toxin products such as botox, xeomin, dysport, and others will accelerate the market growth. The increasing usage of botulinum toxin injection in several aesthetic procedures including the treatment of glabellar lines, chemical browlift, forehead lines, and others is expected to boost the botulinum toxin industry growth over the coming years. Presently, a few products of botulinum toxin - Type-A and only one product of Type-B (Myobloc) are commercially available in the market.
However, rising investment in R&D programs by major manufactures to explore the therapeutic use of botulinum toxin is building opportunities for the expansion of its therapeutic application area in the near future. A shift from invasive to minimally invasive procedures is expected to fuel the market growth over the forecast period. For instance, according to ISAPS, 17,598,888 noninvasive procedures were performed globally in 2021, out of which 5,355,604 were performed in the U.S. A large number of people are opting for permanent procedures, such as fillers, fat grafting, and lip advancement.
Minimally invasive procedures offer advantages such as smaller incisions, shorter hospital stays, quick outpatient services, rapid wound healing, lesser pain, and lower risk of complications than invasive surgeries. Thus, the demand for botulinum toxin is expected to increase over the forecast period. Moreover, many dermatologists believe that COVID-19 may act as a springboard that will drastically increase patient footfall post-pandemic.
According to a survey conducted on 1,000 American women in 2020 by American Society of Plastic Surgeons, 11% of participants indicated that they are more inclined to opt for minimally invasive procedures post-pandemic than pre-COVID-19. Even during the pandemic, Botox and soft tissue fillers remained the most popular minimally invasive procedures in the U.S.
Browse through Grand View Research's Medical Devices Industry Research Reports.
• The global bronchoscopes market size was valued at USD 1.14 billion in 2023 and is projected to grow at a CAGR of 11.4% from 2024 to 2030. The rising incidences of respiratory disorders and growing awareness about early diagnosis are driving demand for bronchoscopes.
• The global western blotting market size was valued at USD 986.2 million in 2023 and is projected to grow at a CAGR of 6.1% from 2024 to 2030. The rising rate of brain disorders, research initiatives in the biotech and pharma sectors, and funding by the pharmaceutical and biotechnology agencies drive market growth.
Botulinum Toxin Market Segmentation
Grand View Research has segmented the global botulinum toxin market report based on product type, application, end-use, and region:
Botulinum Toxin Product Type Outlook (Revenue, USD Billion, 2018 - 2030)
• Type A
o Botox
o Dysport
o Xeomin
o Others
• Type B
Botulinum Toxin Application Outlook (Revenue, USD Billion, 2018 - 2030)
• Therapeutic
o Chronic Migraine
o Overactive Bladder
o Cervical Dystonia
o Spasticity
o Others
• Aesthetic
o Glabellar Lines
o Crow’s Feet
o Forehead Lines
o Others
Botulinum Toxin End-use Outlook (Revenue, USD Billion, 2018 - 2030)
• Hospitals
• Dermatology Clinics
• Cosmetic Centers and Medspas
Botulinum Toxin Regional Outlook (Revenue, USD Billion, 2018 - 2030)
• North America
o US
o Canada
• Europe
o UK
o Germany
o France
o Italy
o Spain
o Denmark
o Sweden
o Norway
• Asia Pacific
o India
o Japan
o China
o South Korea
o Australia
o Thailand
• Latin America
o Brazil
o Mexico
o Argentina
• Middle East & Africa
o South Africa
o Saudi Arabia
o UAE
o Kuwait
Order a free sample PDF of the Botulinum Toxin Market Intelligence Study, published by Grand View Research.
Key Companies profiled:
• Ipsen Group
• Allergen, Inc.
• Metabiologics
• Merz Pharma
• US Worldmeds
• Evolus
• Galderma
• Lanzhou Institute of Biological Products
0 notes
Text
Unlocking the Potential of Recombinant Proteins: A Comprehensive Guide for Researchers
Recombinant proteins are essential tools in modern biological research and biotechnology. Produced using recombinant DNA technology, these proteins play a crucial role in a wide range of applications, from basic research to therapeutic development. This article provides an overview of recombinant proteins, their applications, and key considerations for choosing the right product for your research needs.
1. What Are Recombinant Proteins?
Recombinant proteins are proteins that are produced by inserting a gene of interest into a host cell, such as bacteria, yeast, or mammalian cells. These host cells then express the protein, which is subsequently purified and used for various research and therapeutic purposes. Recombinant proteins are valuable because they can be produced in large quantities and with high purity, allowing for consistent and reproducible results in experiments.
2. Applications of Recombinant Proteins
Recombinant proteins have a wide range of applications across different fields:
Basic Research: In fundamental research, recombinant proteins are used to study protein function, interactions, and structure. By providing specific proteins in a controlled environment, researchers can investigate their roles in cellular processes and disease mechanisms.
Drug Development: Recombinant proteins are pivotal in drug development and therapeutic research. They are used to create biologics, such as monoclonal antibodies and hormone therapies, which target specific diseases or conditions. For example, recombinant insulin is a widely used therapeutic protein for managing diabetes.
Diagnostic Tools: Recombinant proteins are employed in diagnostic assays and tests, including ELISA and Western blotting. They help in detecting specific antibodies or antigens, facilitating accurate disease diagnosis and monitoring.
Vaccine Development: In vaccine research, recombinant proteins are used to produce antigens that stimulate an immune response. These proteins can be used to develop vaccines against various infectious diseases, improving public health and disease prevention.
Protein Engineering: Recombinant protein technology allows for the modification and optimization of proteins for specific applications. Researchers can engineer proteins with altered properties, such as enhanced stability or binding affinity, to meet the needs of their studies.
3. Choosing the Right Recombinant Protein
Selecting the appropriate recombinant protein involves considering several factors:
Purity and Quality: Ensure that the recombinant protein is of high purity and quality. Look for products that are rigorously tested and verified to meet quality standards, as impurities can affect experimental results.
Expression System: Different host cells (e.g., bacteria, yeast, mammalian cells) can be used to produce recombinant proteins, each with its advantages and limitations. Choose a product based on the expression system that best suits your research needs.
Functional Activity: Verify that the recombinant protein retains its biological activity and functionality. Check for product information on activity assays and functional validation to ensure the protein performs as expected.
Documentation and Support: Look for suppliers that provide comprehensive product documentation, including detailed protocols, handling instructions, and technical support. This information is essential for optimizing the use of recombinant proteins in your experiments.
4. Best Practices for Handling Recombinant Proteins
To achieve the best results with recombinant proteins, follow these best practices:
Storage and Stability: Properly store recombinant proteins according to the manufacturer’s recommendations. Pay attention to storage conditions, such as temperature and buffer composition, to maintain protein stability.
Avoid Contamination: Use aseptic techniques and clean equipment to prevent contamination. Contaminants can interfere with protein function and lead to inaccurate results.
Optimize Experimental Conditions: Adjust experimental conditions, such as concentration and buffer composition, to suit the specific recombinant protein. Perform preliminary tests to optimize conditions for your particular application.
5. Innovations and Future Directions
The field of recombinant protein technology continues to advance, with ongoing innovations enhancing protein production and applications. Recent developments include improved expression systems, advanced purification techniques, and novel protein engineering approaches. These innovations are expanding the potential of recombinant proteins in research, diagnostics, and therapeutics.
Conclusion
Recombinant proteins are versatile and valuable tools in biological research and biotechnology. By understanding their applications, selecting high-quality products, and following best practices, researchers can effectively utilize recombinant proteins to advance their studies and develop new therapeutic solutions. As technology evolves, recombinant proteins will continue to play a key role in driving scientific discoveries and innovations.
0 notes
Text

How to Elute Proteins from Protein G Magnetic Beads Effectively
Protein G Magnetic Beads are widely used for isolating antibodies and their complexes in various biological studies, particularly in immunoprecipitation and co-immunoprecipitation experiments. These beads are coated with Protein G, a bacterial protein that exhibits a strong affinity for the Fc region of immunoglobulins (IgG) from multiple species. The magnetic nature of these beads allows for quick separation and easy handling during protein isolation procedures.
An essential step in immunoprecipitation is the elution of the target protein or protein complex from the beads. In this article, we will explore the best practices and methods for efficiently eluting proteins from Protein G Magnetic Beads to ensure high yield and purity.
What Are Protein G Magnetic Beads?
Protein G Magnetic Beads are small magnetic particles coated with Protein G, a bacterial protein that binds strongly to antibodies, particularly IgG. Unlike Protein A, which has a more selective affinity for certain species and subclasses of IgG, Protein G binds to a broader range of IgG subclasses across different species. This makes Protein G Magnetic Beads a more versatile option for antibody capture and immunoprecipitation.
These beads are often used in studies involving protein-protein interactions, signaling pathways, and post-translational modifications, where isolating specific antibodies and their bound antigens is essential.
Why Is Elution Critical in Protein G Magnetic Beads Protocols?
The elution step is a crucial part of the Protein G Magnetic Beads protocol, as it allows the captured proteins (antibodies and their bound antigens) to be released from the beads for downstream applications, such as Western blotting, mass spectrometry, or enzyme-linked immunosorbent assays (ELISA). Efficient elution is essential to ensure that the target protein is fully recovered while maintaining its functionality and structural integrity.
Several factors can influence the success of protein elution, including buffer composition, pH, and elution conditions. Therefore, optimizing the elution process is key to achieving high yields and minimizing protein degradation or loss.
Methods for Eluting Proteins from Protein G Magnetic Beads
There are several elution strategies that researchers can use to recover proteins bound to Protein G Magnetic Beads. Each method has its advantages and considerations based on the nature of the target protein, downstream analysis, and experimental conditions.
Low pH Elution
One of the most commonly used methods for eluting proteins from Protein G Magnetic Beads is using a low pH buffer, typically in the range of pH 2.5-3.5. At this pH, the interaction between Protein G and the antibody is disrupted, allowing the antibody and its bound antigen to be released from the beads.
Protocol:
Prepare an elution buffer, such as 0.1 M glycine-HCl, pH 2.5-3.0.
Add the elution buffer to the Protein G Magnetic Beads after the final wash step.
Incubate the beads with the elution buffer for 5-10 minutes at room temperature with gentle mixing.
Place the tube on a magnetic separator to pellet the beads and carefully transfer the supernatant containing the eluted protein to a clean tube.
Immediately neutralize the eluted fraction by adding 1 M Tris-HCl, pH 8.0, to prevent protein denaturation.
Considerations:
Advantages: Low pH elution is highly effective in releasing proteins from the beads, and it is a simple and cost-effective method.
Limitations: Some proteins may denature at low pH, particularly if they are sensitive to acidic conditions. Therefore, immediate neutralization is necessary to maintain protein integrity.
SDS-Based Elution
For some applications, such as SDS-PAGE or Western blotting, an SDS-containing buffer can be used for elution. SDS (sodium dodecyl sulfate) is a detergent that disrupts protein-protein interactions and denatures proteins, making it a useful method for fully recovering bound proteins from Protein G Magnetic Beads.
Protocol:
Prepare an elution buffer, such as 1X SDS sample buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 0.01% bromophenol blue, and 5% β-mercaptoethanol).
Add the SDS sample buffer to the beads and incubate at 95°C for 5-10 minutes to denature and release the proteins.
Use a magnetic separator to pellet the beads, and carefully transfer the supernatant to a clean tube for SDS-PAGE analysis.
Considerations:
Advantages: SDS-based elution is ideal for preparing samples for SDS-PAGE or Western blotting, as the detergent denatures the proteins and ensures complete release.
Limitations: SDS denatures the proteins, so this method is not suitable for applications where native protein conformation or activity is required.
High Salt Elution
Another approach for eluting proteins from Protein G Magnetic Beads is using a high-salt buffer to disrupt the ionic interactions between Protein G and the antibody. This method is gentler than low pH or SDS-based elution, making it suitable for applications that require functional or structurally intact proteins.
Protocol:
Prepare a high-salt elution buffer, such as 3 M sodium chloride (NaCl) or 2-3 M MgCl2 in PBS.
Incubate the beads with the high-salt buffer for 10-15 minutes at room temperature with gentle mixing.
Use a magnetic separator to pellet the beads and carefully transfer the eluted protein into a clean tube.
Considerations:
Advantages: High-salt elution is gentler than low pH and SDS-based methods, making it suitable for preserving protein activity and structure.
Limitations: The efficiency of high-salt elution can be lower than low pH methods, and additional optimization may be required to achieve complete protein recovery.
Competitive Elution with Free Antigen
For some applications, particularly when working with antigen-antibody complexes, it may be possible to elute the target protein by using a free antigen that competes with the bound antigen for the antibody's binding site. This method preserves both the antibody and antigen's native structure, making it useful for functional assays.
Protocol:
Prepare a solution of free antigen at a concentration that is 5-10 times higher than the concentration of the bound antigen.
Add the free antigen solution to the beads and incubate at room temperature for 30-60 minutes with gentle mixing.
Use a magnetic separator to pellet the beads and transfer the eluted protein into a clean tube.
Considerations:
Advantages: Competitive elution preserves both the antibody and antigen in their native forms, which is beneficial for downstream functional studies.
Limitations: This method requires a high concentration of free antigen, which may not always be feasible or cost-effective.
Tips for Effective Elution from Protein G Magnetic Beads
Optimize Elution Conditions: The best elution method depends on your specific protein and downstream applications. If one method doesn’t provide satisfactory results, try adjusting buffer composition, pH, or incubation times.
Handle Proteins Gently: When using low pH or high salt buffers, ensure that proteins are neutralized or dialyzed promptly to maintain their activity and prevent degradation.
Avoid Protein Loss: Perform multiple elutions if needed to recover all of the bound protein from the beads. Sometimes, the first elution may not capture the entire protein yield.
Test for Compatibility: Different antibodies and antigens may require different elution strategies, so testing several elution buffers in pilot studies can help identify the optimal method for your system.
Conclusion
Eluting proteins from Protein G Magnetic Beads is a critical step in immunoprecipitation and protein purification protocols. By selecting the right elution method—whether low pH, SDS-based, high salt, or competitive elution—you can achieve efficient recovery of your target proteins while preserving their functionality and structural integrity for downstream applications. Experimentation and optimization of elution conditions are key to maximizing the yield and purity of proteins isolated using Protein G Magnetic Beads.
Original Source: https://lyticsolutions.blogspot.com/2024/09/how-to-elute-proteins-from-protein-g.html
0 notes
Text
METHODS FOR PURIFYING AND ANALYSING PROTEINS VIA WESTERN BLOTTING
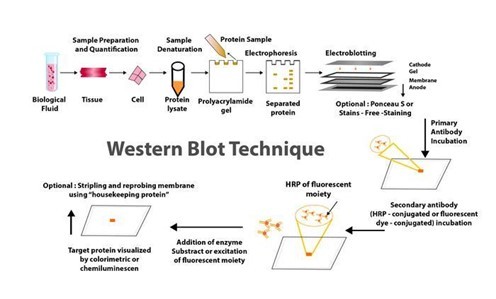
For many years, western blotting was a primary method in molecular biology and techniques such as proteomics (the large-scale study of proteomes: a set of proteins produced in an organism, system, or biological context). Given that western blot is a multistep process that frequently necessitates specialised interpretation, potential errors and variations at any step can jeopardise the reliability and reproducibility of its results.
Given this situation, many advanced and sophisticated approaches to improving the western blot protocol have been developed over the last two decades. These enhancements are more automated and sensitive, with high potential for reducing potential issues with the traditional western blot method. Western blotting's improved sensitivity and innovative equipment advancements have the potential to broaden the field of clinical applications for this fundamental method.
Helvetica Health Care explains the western blot technique as a method of purifying and analysing proteins, as well as new developments in western blotting methodology, in this article.
WHAT EXACTLY IS THE WESTERN BLOT TECHNIQUE?
Traditionally, the western blotting method involves seven steps, as below.
Sample preparation from cells or tissue lysates
Separation of proteins by gel electrophoresis
Protein transfer in a nitrocellulose or polyvinylidene fluoride (PVDF) membrane,
Blocking of non-specific proteins on membrane
Primary Antibody incubation
Secondary Antibody incubation
Protein detection & analysis
WHAT ARE THE NEW DEVELOPMENTS IN THE WESTERN BLOT DOMAIN AND WHAT CHARACTERISTICS DO THEY HAVE?
Below are some innovations that have enhanced the western blot protocol and addressed the potential problems in its application in protein purification and analysis.
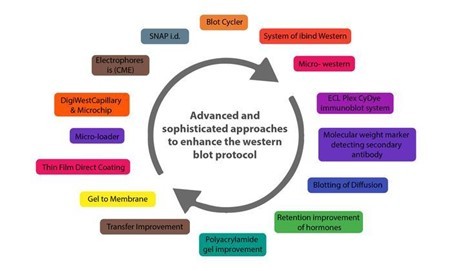
CAPILLARY AND MICROCHIP ELECTROPHORESIS (MCE)
MCE has higher sensitivity and resolution than traditional techniques, allowing for the detection of multiple target proteins from a single cell lysate sample. The method not only allows for parallel multiplexed tests of a set of proteins using a small sample amount, but it also eliminates blocking stages and has faster analysis times (8 min for electrophoretic resolution). MCE, while still in development, has the potential to be significantly improved with additional work.
AUTOMATED MICROFLUIDIC PROTEIN IMMUNOBLOTTING
Automated protein immunoblotting is a programmable and controllable technology that combines PAGE (polyacrylamide gel electrophoresis) and blotting in a single device. It saves time, avoids multiple test stages, and reduces the amount of equipment and reagents required. Because it can detect free prostate-specific antigens in a sample of human seminal fluid in less than 5 minutes, this technology is both cost-effective and reagent-efficient. The method is constantly being improved to improve sensitivity and enable protein quantification.
SINGLE CELL-RESOLUTION WESTERN BLOTTING
This sensitive method has also been used to investigate protein variations in stem cell signalling and differentiation. It can detect specific cell-to-cell changes in protein expression. MiloTM (ProteinSimple), the world's first Single-Cell Western platform, can measure protein expression (up to 4 proteins per cell simultaneously) in 1000 single cells in about 4 hours.
DIGIWEST
DigiWest, a simplified version of the standard procedure, improves western blotting throughput by requiring less lysate, target detection, and antibody. However, this method requires biotinylation of the target proteins, requires specialised reagents and equipment, incurs initial startup costs, has issues with translating the digital DigiWest results to western blot mimics, and has specifics about some procedures, such as the complex and difficult elution of proteins from PVDF membranes.
SIMPLE WESTERN
Simple Western is a method developed by ProteinSimple (San Jose, California) that is based on CE-SDS (Capillary electrophoresis sodium dodecyl sulphate), in which separated proteins are linked to the capillary wall by a patented photo- (ultraviolet) induced chemical crosslink. The method can use up to 90 different antibodies at the same time, is relatively quick, and requires small amounts of sample. It can also perform standard curve quantification, similar to high-pressure liquid chromatography (HPLC), and improve reproducibility. However, it necessitates the use of specialised reagents.
MICRO-LOADER
The Micro-loader technology has improved the sensitivity of the western blot assay and PAGE. To load samples, it employs a sample micro-loader device with a funnel-like design. This technique is highly effective for measuring protein expression and phosphorylation in samples that are few, difficult to find, and limited in quantity because it only requires a small number of loading samples for signal detection.
THIN-FILM DIRECT COATING WITH SUCTION-WESTERN BLOTTING (TDCS)
Western blotting has significant limitations due to high antibody consumption and lengthy operating times. TDCS, a capillary-tube-based technique, aims to reduce antibody and time consumption in western blotting. This quick and sensitive detection method allows for the quantitative study of multiple antigen-antibody interactions, as well as multiple protein interactions.
BLOTTING OF DIFFUSION
SDS-PAGE Single-prefabricated gels on plastic support are a quick and easy way to make multiple blots using diffusion blotting. Diffusion blotting allows for the comparison of several proteins on blots from the same gel. Protein transmission rates by diffusion blotting are 10% in 3 minutes, 20% in 20 minutes, and 40% to 60% in 3 hours. When compared to electroblotting, the technique may increase the amount of proteins efficiently delivered to the membrane surface. As a result, when quantitative protein exchange is not required, diffusion blotting is the preferred method.
MICRO WESTERN
Soon, micro western will be a proper western blot technique for confirming the diagnosis of purified HIV p24 and gp120 proteins in blood plasma. The Micro-Western may be vital for detecting infectious diseases and cancer, despite not being widely available.
SYSTEM OF IBIND WESTERN
Thermo Fisher Scientific's iBind Western procedure makes use of a low-cost semi-automated western blotting structure that uses sequential flow technology to distribute chemicals and antibodies to different compartments. Cleaner western blots will result from using the iBind Western Systems and highly specialised primary and secondary antibodies.
BLOT CYCLER
Every step of membrane processing is automated with the BlotCycler (Precision Biosystems, USA), and up to 12 membranes can be processed at once. The BlotCyclerTM is an Automated Western Blot Development system that improves repeatability and sensitivity by completing all blot cleaning, trapping, and incubation steps. It is also simple to programme all of the actions for specific operations.
SYSTEM OF SNAP I.D.
In a low-cost system of SNAP i.d. 2.0, solvents are uniformly distributed via the tissue using a technique based on a vacuum and a flow distribution system (Merck, USA). Its main advantage is that washing, antibody incubation, and blocking all take about 30 minutes. The SNAP i.d.® 2.0 gadget actively forces reagents through the membrane, unlike conventional Western blotting, which depends on diffusion to transfer reagents.
Washes, antibody recollection, and antibody-antigen binding have been improved. The SNAP i.d.® 2.0 delivers new, cutting-edge western blotting characteristics with an immunodetection capability in 30 minutes without using additional reagents (e.g., antigen, antibody, or detection reagents).
RETENTION IMPROVEMENT OF HORMONES OF THE PEPTIDE ON THE MEMBRANE OF BLOTTING
Western blotting is significantly improved by treating the PVDF membrane used in this method with glutaraldehyde (0.2%) for fifteen minutes in a saline solution containing tween-80. In western blotting, the addition of glutaraldehyde to the membrane prevented or reduced the quantity of insulin loss.
Apart from these, two more techniques that have improved the conventional method are
Analysis of western blot utilising molecular weight marker detecting secondary antibody
Total and target protein co-detection by immunoblotting of fluorescent ECL and labelling of Cydye
Many innovations in western blotting techniques to transfer proteins from gel to membrane have also been observed, such as vacuum blotting, centrifuge blotting, multiple tissue blotting, and so on. The Trans-Blot Turbo, the iBlot® dry blotting system, and the PierceTM Power Blot Cassette are all improvements in protein transfer methods from gels to membranes. The transfer time is reduced from an hour or overnight to at most 10 minutes when these new techniques are used.
HHC offers the ZeptoMetrix WESTERN BLOT for in vitro detection of antibodies to SIV in serum or plasma and is available in 10 or 30-strip kit formats. We also provide a bench top, a western blot processor, and the AUTOBLOT 3000. It controls incubation times, dispensing volumes and washing programs. Ten user-defined protocols can be programmed easily. Contact us now to know how we can help you get accurate and high-quality output with our products when performing the western blotting procedure.
0 notes
Text
Advances in Immunoblot Western Blot Technology
If you've ever wondered how scientists play detective with proteins, enter Immunoblot Western Blotting - a technique that helps researchers identify specific proteins in a sample. It's like finding Waldo in a sea of stripes, only way cooler and much more scientifically sound.
Evolution of Immunoblot Technology in Biomedical Research
From its humble beginnings to a pivotal role in modern research, Immunoblot technology has come a long way. It's like the superhero origin story of the science world - once an underdog, now a powerhouse in the fight against protein mysteries.
Overview of Kendrick Labs Inc
Company Background and Mission
Kendrick Labs Inc isn't just your average lab - it's a hub of innovation, committed to advancing scientific discovery. Think of them as the cool nerds of the science world, rocking lab coats and breaking boundaries like it's nobody's business.
Key Products and Services Offered by Kendrick Labs
From cutting-edge technology to top-notch services, Kendrick Labs has got it all. They're like the one-stop shop for all your Immunoblot needs, making protein research feel like a walk in the park.
Key Features and Benefits of Kendrick Labs' Immunoblot Technology
High Sensitivity and Specificity
When it comes to detecting proteins, Kendrick Labs doesn't mess around. Their technology is so sensitive, it can spot a protein molecule in a haystack. Say goodbye to blurry results and hello to crystal-clear insights.
Automation and Workflow Efficiency
Who has time for tedious manual processes? Not Kendrick Labs. With their automation wizardry, researchers can breeze through experiments like never before. It's like having a protein detective assistant that never sleeps.
Customization and Flexibility for Researchers
One size doesn't fit all in the world of protein research, and Kendrick Labs, Inc gets that. They offer customization options that make researchers' hearts sing. It's like having your own protein detective kit tailored just for you.
Applications of Immunoblot Western Blot Technology in Research
Protein Detection and Quantification
Need to find out how much of a certain protein is hanging out in your sample? Immunoblot technology is here to save the day. It's like having x-ray vision for proteins, allowing researchers to see what's going on at a molecular level.
Biomarker Discovery and Validation
Looking for that one-in-a-million protein that could change the game in disease research? Immunoblot technology is your trusty sidekick. It's like having a protein bloodhound sniffing out potential biomarkers with precision and speed.
Drug Development and Pharmacological Studies
When it comes to developing life-saving drugs, every protein clue counts. Immunoblot technology plays a key role in drug research by helping researchers understand how drugs interact with proteins. It's like having a secret weapon in the battle against diseases.
Comparison with Traditional Western Blot Methods
When it comes to comparing Immunoblot technology with traditional methods, it's like pitting a modern superhero against a classic comic book character. Immunoblot technology swoops in with its precision and sensitivity, leaving traditional methods feeling a bit outdated. With Immunoblot, researchers can detect even the faintest traces of proteins with ease, making the process quicker and more reliable.
Advantages of Immunoblot Technology Over Traditional Methods
Immunoblot technology brings a host of advantages to the table. Say goodbye to the days of struggling to detect low-abundance proteins or dealing with nonspecific binding issues. With Immunoblot, researchers can achieve higher sensitivity and specificity, leading to more accurate and reproducible results. Plus, the ability to multiplex and analyze multiple proteins simultaneously is a game-changer in the world of protein analysis.
Technical Considerations and Limitations
While Immunoblot technology is a superhero in its own right, it's not without its kryptonite. Like any technology, there are technical considerations and limitations to keep in mind. Factors such as sample preparation, antibody selection, and data interpretation can impact the success of Immunoblot experiments. Researchers must also be mindful of potential sources of variability and ensure proper controls are in place to mitigate any issues that may arise.
Case Studies Highlighting the Success of Kendrick Labs' Technology
In the world of research, success stories speak louder than words. Kendrick Labs' Immunoblot technology has been the secret weapon behind numerous research breakthroughs, propelling scientists towards new discoveries and insights. Real-world case studies showcase the power of Immunoblot in unraveling complex biological mysteries and opening doors to novel therapeutic interventions.
Real-World Examples of Research Breakthroughs Using Kendrick Labs' Immunoblot Technology
From uncovering biomarkers for early disease detection to elucidating signaling pathways critical for cancer progression, Kendrick Labs' Immunoblot technology has left an indelible mark on the landscape of scientific discovery. These real-world examples serve as a testament to the impact and significance of Immunoblot in advancing our understanding of various biological processes.
Future Developments and Innovations in Immunoblot Western Blot Technology
As technology continues to evolve at lightning speed, the future of Immunoblot technology holds exciting possibilities. With emerging trends and innovations on the horizon, researchers can look forward to even greater advancements in protein analysis and detection methods.
Emerging Trends and Technologies in the Field
From enhanced multiplexing capabilities to improved automation and data analysis tools, the field of Immunoblot technology is poised for a revolution. Researchers can expect to see a shift towards more integrated and user-friendly platforms that streamline experimental workflows and provide deeper insights into protein biology.
Potential Impact on Biomedical Research and Clinical Diagnostics
The potential impact of future developments in Immunoblot technology extends far beyond the laboratory walls. With enhanced sensitivity and specificity, researchers can uncover novel biomarkers for early disease detection and personalized medicine applications. In the realm of clinical diagnostics, Immunoblot technology holds the promise of revolutionizing patient care by enabling faster and more accurate protein analysis for diagnostic purposes.
Original Source: https://kendricklabs.blogspot.com/2024/03/advances-in-immunoblot-western-blot.html
0 notes
Text
Biomedicines, Vol. 12, Pages 1981: Abnormally High Expression of DNAJB6 Accelerates Malignant Progression of Lung Adenocarcinoma
DNAJB6, a major member of the DNAJ/HSP40 family, plays an important role in tumor development. We explored the effect of DNAJB6 expression on the prognosis of patients and its biological role in lung adenocarcinoma (LUAD). #mRNA and clinical data were obtained from The #cancer Genome Atlas (TCGA). Enriched pathways were determined by the Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses. A nomogram incorporating DNAJB6 and three clinical features was constructed to predict the survival rate. DNAJB6 expression and function in LUAD were explored using immunohistochemistry, Western blotting, proliferation, cell cycle analysis, #RNA sequencing, and xenograft tumor assays. DNAJB6 #mRNA levels were elevated in the LUAD-TCGA dataset. DNAJB6 protein levels were higher in LUAD tumor tissues than in normal tissues. A high DNAJB6 level was an independent risk factor for poor prognosis in patients with LUAD. The proportion of tumor-infiltrating immune cells significantly differed between high and low DNAJB6 expression. DNAJB6 was associated with cell cycle pathways; therefore, its knockdown induced G2/M cell cycle arrest and inhibited LUAD cell proliferation. This is the first report of the DNAJB6 requirement for LUAD cell proliferation and its potentially crucial role in LUAD prognosis. https://www.mdpi.com/2227-9059/12/9/1981?utm_source=dlvr.it&utm_medium=tumblr
0 notes
Text
My vision of the molecular biology of the nearest future
My vision of the molecular biology of the future is a single letter, sent through regular mail, that would allow a person with no previous molecular biology experience to start doing it without any purchases of specialized equipment or reagents.
The person who receives this letter should have in their hands the full toolkit to do molecular cloning and assembly, delivery and editing systems that support wide variety of biological organisms to be studied, quality control of genetic constructions, de novo DNA synthesis and massive parallel DNA sequencing.
This will require and therefore facilitate the adherence to and development of standards of molecular biology allowing for tighter collaboration of people from different institutions: part domestication, multiplication of standard primers in-house, better quality control of in house reagents, standartized protein scaffolds for affinity experiments, highly reproducibile reporters for a variety of tasks from Western blotting to ELISA to in vivo microscopy.
This will increase lucrativeness of various open source hardware assembly protocols, because now people will see direct results from spending time to build it.
As more people will be engaged with it, molecular biology will stop being an Ivory Tower science - it will go out the the homeless shelters, factories and fields. Small self-funded genomic surveillance outposts, if they will be as ubiquitous as home radiotelescopy once was, will collect data to build a resilient shield of humanity against infectuous diseases.
That's within grasp. And I don't imagine what is next. But I will not make the mistakes I once made. I will become strong enough to protect what I hold dear. I will be able to honestly and proudly answer "What have I done today for the sake of tomorrow?".
1 note
·
View note
Text

Imagine having the power to pinpoint and analyze the presence of specific proteins in a complex sample with unparalleled precision – that's the magic of Western blotting, and today, we'll explore its incredible potential.
Learn more about its applications and troubleshooting by reading the full article on the PraxiLabs blog.
0 notes
Text
Research grade Proteins: Production and Scale-Up Challenges

Research-grade proteins are essential tools in the field of scientific discovery, serving as foundational elements in a variety of biological and medical research applications. These proteins, characterized by their high purity and consistency, enable researchers to conduct experiments with a high degree of reliability and reproducibility. Their use spans numerous disciplines, including biochemistry, molecular biology, pharmacology, and biotechnology.
The production of research-grade proteins involves several sophisticated techniques to ensure their purity and functionality. These techniques often include recombinant DNA technology, where genes encoding the desired proteins are inserted into expression systems such as bacteria, yeast, or mammalian cells. Once expressed, the proteins are purified using methods like affinity chromatography, ion exchange chromatography, and gel filtration. The goal is to obtain proteins free from contaminants and with the correct folding and post-translational modifications necessary for their activity.
Research-grade proteins play a crucial role in drug development and screening processes. They are used to study the binding affinities and specificities of potential therapeutic compounds, aiding in the identification of promising drug candidates. For instance, proteins such as enzymes, receptors, and ion channels are targeted by pharmaceutical companies to develop new medications for a variety of diseases. The high quality of research-grade proteins ensures that the data generated from these studies are accurate and reproducible, which is critical for the success of drug discovery programs.
In addition to drug development, research-grade proteins are vital for structural and functional studies. Techniques like X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy, and cryo-electron microscopy rely on high-quality proteins to determine their three-dimensional structures. Understanding protein structures at the atomic level provides insights into their mechanisms of action and interactions with other molecules, which is essential for advancing our knowledge of biological processes and developing targeted therapies.
The application of research-grade proteins in diagnostic assays is another area of significant impact. Proteins such as antibodies and antigens are used in various diagnostic tests, including enzyme-linked immunosorbent assays (ELISAs) and western blotting, to detect and quantify biomolecules in clinical samples. The accuracy and sensitivity of these tests depend on the quality of the proteins used.
Despite their importance, producing research-grade proteins presents challenges, including ensuring stability and functionality over time, and maintaining ethical and regulatory standards in their production. Nonetheless, ongoing advancements in protein engineering and purification technologies continue to enhance the availability and quality of research-grade proteins, driving forward scientific innovation and discovery.
0 notes
Text
Board Certified Radiologist Germany
Dr. Samuel Kobba embarked on his illustrious medical journey at the University of Bonn Medical School in 2001, where he laid the foundation for a career that would span various aspects of the medical field, especially in research and radiology. His academic pursuit in medicine was rigorous and comprehensive, culminating in 2006 when he was on the cusp of completing his medical degree, pending his final exams. However, his passion for research led him to the University of California, Davis, where he undertook a doctorate thesis at the Genome and Biomedical Sciences Facility under the expert guidance of Professor Knowlton. During this period, Dr. Kobba delved into the intricate process of isolating cardiac myocytes—a procedure that demanded nearly six hours of meticulous work, followed by a series of experiments utilizing heat shock factor and TNF. These experiments employed cutting-edge techniques such as Western blot and ELISA (enzyme-linked immunosorbent assay), both of which are paramount in the separation, identification, and detection of proteins and antigens in biological samples, through highly specific antibody-antigen interactions. His groundbreaking work in this field not only showcased his commitment to medical research but also earned him the title of Medical Research Scholar of the Year. Board Certified Radiologist Germany
Dr. Kobba’s doctoral thesis, “Heat Shock Paradox: Role of Heat Shock Factor,” was a testament to his innovative research and was subsequently published in a renowned medical journal, marking a significant milestone in his career. Furthermore, during his time at the University of California Davis, he immersed himself in clinical rotations at the University of California Davis Medical Center in Sacramento, working under the tutelage of Dr. Matthew Bobinski, Dr. Arthur Brooks Dublin, Dr. Rosalie Hagge, and Dr. Adam Greenspan, where he completed his courses with honors.
Upon his return to Germany, Dr. Kobba successfully completed the State exams in 2009, which allowed him to begin to practice. He commenced his professional journey as a resident physician at the University Clinic in Bonn, where he continued to engage in research. Throughout his journey, he was mentored by luminaries such as Professor Baumgarten, Professor Knüffeman, and PD Dr. Se-Chan Kim, who played pivotal roles in shaping his career. He extends his heartfelt gratitude to these mentors, especially the late Professor H. Shimassek, his personal mentor, and Dr. Seitz, who supported him through challenging times. His brother, Dr. Joseph Kobba, his cousin and wife, Mr. and Mrs. Strasser-King, in Sacramento, Carlifornia, also deserve special mention for their unwavering support throughout his academic and professional journey.
Dr. Kobbas career path led him to Berlin in 2004, where he continued his training at institutions, like Vivantes Klinikum and Charite. After completing his residency he worked as a fellow at Vivantes Klinikum until 2008. Then transitioned to a role as an attending physician at Saint Augustinus Krankenhaus in Düren. During this time his expertise in radiology flourished, eventually leading him to a consultancy position in 2019. In collaboration with Professor Brassel and Professor Sommer he contributed to a chapter titled “Vene of Galen Aneurysmal Malformations” in the book “Pineal Region Lesions; Management Strategies and Controversial Issues.”
His career took a turn when he joined Radio-log, a network of practices in Bavaria. However the outbreak of the COVID 19 pandemic prompted him to move to blikk Radiologie (now known as evidia) which has than 75 locations throughout Germany. Additionally he made contributions to Klinikum Aschaffenburg and Klinikum Darmstadt as a consultant. Dr. Kobbas broad experience in radiology spans areas such as neuroradiology, musculoskeletal radiology, cardiovascular radiology( cardiac computed tomograms and cardiac magnetic resonance imaging) , ultrasound examinations, Ultrasound and CT guided procedures and particularly MRI diagnostics. His work in Doppler ultrasound technology and his emphasis on diagnosing and treating strokes demonstrate his commitment, to advancing knowledge and improving patient care. Consultant Radiologist Frankfurt
The Radiology Department, at Klinikum Darmstadt has provided me with an opportunity to enhance my skills in radiology significantly. Although the workload was initially daunting, I saw it as a chance to grow than shy away from it. Stepping out of your comfort zone is essential for development. I had the chance to perform procedures ranging from computed tomography guided draining of abdomenal abscesses to intricate lung, liver and bone biopsies from different parts of the body with a focus on the back (vertebral column), particularly in cases like spondylodiscitis. My proficiency in conducting and interpreting cardiac computed tomograms (cardiac CTs) and cardiac magnetic resonance imaging (cardiac MRIs) has improved tremendously. Being part of Praxis Radiolog, in Bavaria based in Passau and Klinikum Aschaffenburg made me feel like I belonged to an caring community.
0 notes