#biocompatibilitytesting
Explore tagged Tumblr posts
Text
Medical Device Testing Outsourcing: $3.5B in 2023 to $7.2B by 2033 (7.5% CAGR)
Medical Device Analytical Testing Outsourcing Market offers specialized services provided by third-party organizations to rigorously test and analyze medical devices. These services ensure compliance with regulatory standards, enhance product safety and efficacy, and support manufacturers in accelerating time-to-market while reducing operational costs.
To Request Sample Report: https://www.globalinsightservices.com/request-sample/?id=GIS25883 &utm_source=SnehaPatil&utm_medium=Article
Key Market Drivers
The market is experiencing robust growth, driven by:
Increasing Regulatory Scrutiny: Rising emphasis on product safety and efficacy has amplified the need for comprehensive testing.
Cost-Efficiency: Outsourcing helps manufacturers optimize resources and focus on core competencies.
Technological Advancements: The growing complexity of medical devices necessitates specialized and advanced testing capabilities.
Market Insights
The chemical characterization segment leads the market, accounting for 38% of the share in 2023, driven by stringent safety standards and the necessity for thorough material analysis.
Microbiology and sterility testing follow closely with a 32% share, underscoring the importance of ensuring device sterility and patient safety.
Biocompatibility testing holds a 30% share, reflecting the demand for evaluating device compatibility with biological systems.
In 2023, the global market recorded approximately 1.2 billion tests conducted, showcasing the growing reliance on outsourced testing services.
Regional Highlights
North America dominates the market due to advanced healthcare infrastructure and the high concentration of medical device manufacturers.
Europe follows, supported by a robust regulatory framework and a rising demand for innovative medical technologies.
The United States and Germany stand out as leading contributors, reflecting significant demand for outsourced testing services in these regions.
Market Segmentation
By Type: Chemical Testing, Physical Testing, Microbiological Testing, Biocompatibility Testing, Stability Testing, Electromagnetic Compatibility Testing, Packaging Testing By Product: Instruments, Reagents, Consumables By Services: Method Development, Method Validation, Extractables and Leachables Testing, Material Characterization, Batch Release Testing, Product Life Cycle Testing By Technology: Chromatography, Spectroscopy, Polymerase Chain Reaction (PCR), Flow Cytometry, Mass Spectrometry, Electrophoresis, Microscopy By Component: Sensors, Transducers, Microfluidics By Application: Cardiology Devices, Orthopedic Devices, In Vitro Diagnostic Devices, Ophthalmic Devices, Dental Devices, Drug Delivery Devices By Device: Wearable Devices, Portable Devices, Stationary Devices By Process: Preclinical Testing, Clinical Testing, Post-Market Surveillance By End User: Medical Device Manufacturers, Research Laboratories, Academic Institutions
Leading Market Players
Key players such as Eurofins Scientific, SGS SA, and Charles River Laboratories drive the market with their advanced technologies and robust testing capabilities, maintaining a competitive edge in this dynamic industry.
#MedicalDevices #AnalyticalTesting #OutsourcingServices #MedicalInnovation #HealthcareSafety #RegulatoryCompliance #BiocompatibilityTesting #ChemicalCharacterization #SterilityTesting #AdvancedTechnologies #GlobalHealthcare #PatientSafety #DeviceTesting #ResearchAndDevelopment #QualityAssurance
The Medical Device Analytical Testing Outsourcing Market is set to expand further as advancements in medical device technologies and stringent regulatory requirements fuel the demand for precise, specialized testing services.
0 notes
Text


SAFE OHNE #AMALGAM
Viele Patienten fragen immer noch nach #Amalgamfüllungen. Zum Wohle der Patienten gibt es die bei uns seit 20 Jahren nicht mehr - als eine der ersten #Zahnarztpraxen damals in #Berlin.

Klick auf den Link für mehr Infos über unsere professionelle und safe #Amalgamentfernung unter #Kofferdam:
#amalgam#zahnarzt#dentist#dentistry#ferrari#kyosho#dental#endodontics#mercury#denture#prosthodontics#filling#silverfilling#endo#holisticdentistry#silverfillings#holisticdentist#dentalmaterials#art#periodontics#teeth#rubberdam#biocompatibilitytesting#biocompatibility #blombe #füllung #zahnfüllung #ku64 #diezahnarztwoche
0 notes
Text

It is our immense pleasure to announce our participation in India's No.1 & largest Medical Equipment Exhibition, Medicall, Chennai. Meet us at our stall 4B6 - Hall 4. For your queries, please write to [email protected].
0 notes
Photo

Identify the Cytotoxic potential of your medical device with Eurofins Advinus. ISO 10993-5 Cytotoxicity testing Biological evaluation of Medical devices. To know more please email us at [email protected].
0 notes
Text

It is our immense pleasure to announce our participation in India's No.1 & largest Medical Equipment Exhibition, Medicall, Chennai. Meet us at our stall 4B6 - Hall 4. For your queries, please write to [email protected].
0 notes
Text

It is our immense pleasure to announce our participation in India's No.1 & Largest Medical Equipment Exhibition, Medicall Chennai. Visit us at our stall: 4B6 - Hall 4. For your queries, please write to [email protected].
#medicallchennai#visitourstall#liveonbiolabs#preclinicalcro#biocompatibilitytesting#medicaldevicetesting#CRO
0 notes
Text

#Liveonbiolabs#preclinicalcro#biocompatibilitytesting#genotoxcity . Please drop a mail to [email protected]#Log into www.liveonbiolabs.com
0 notes
Text

0 notes
Text

0 notes
Text

0 notes
Text

0 notes
Photo

Looking to test your Personal Protective Equipment (PPE)? Eurofins Advinus is accredited for all biocompatibility testing for medical/ surgical masks and PPE as per GLP and ISO 17025:2017 compliance. Connect with our team at [email protected] for more details.
0 notes
Link
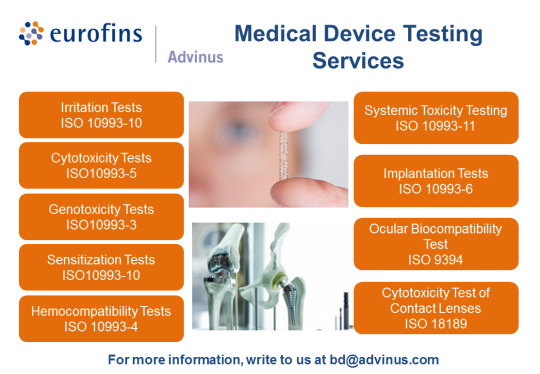
0 notes
Text

#Liveonbiolabs#preclinicalcro#medicaldevice#ISO10993#biocompatibilitytesting Login to www.liveonbiolabs.com#Drop a mail to [email protected]
0 notes
Text

It is our immense pleasure to announce our participation in ARAB HEALTH 2023
For your queries, please write to
#arabhealth2023 #liveonbiolabs #preclinicalcro #biocompatibilitytesting
0 notes
Text

#Arabhealth2023 #weareparticipating #liveonbiolabs #biocompatibilitytesting #medicaldevices
0 notes