#endothermicreaction
Text
What are Endothermic Reactions and Exothermic Reactions?
Endothermic reactions are chemical reactions that absorb energy from their surroundings in heat. The term “endothermic” is derived from the Greek words “endo,” meaning inside, and “therme,” meaning heat. In an endothermic reaction, the energy needed to break the bonds of the reactants exceeds the energy released when new bonds are formed in the products. Exothermic reactions are chemical reactions that release energy into their surroundings, typically in the form of heat. The word “exothermic” originates from the Greek words “exo,” meaning “outside,” and “therme,” meaning “heat.” In an exothermic reaction, the energy released during the formation of bonds in the products is greater than the energy required to break the bonds in the reactants. Enroll now at Tutoroot.
0 notes
Photo
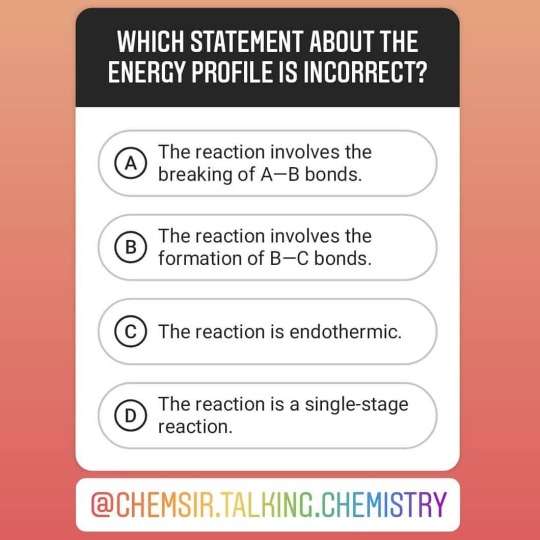
[M.C. Challenge] Which of the following statements regarding the above energy profile is INCORRECT? A. The reaction involves the breaking of A—B bonds. B. The reaction involves the formation of B—C bonds. C. The reaction is endothermic. D. The reaction is a single-stage reaction. 🤔 ------------------------------------------------------------------------ 👍Please like, comment and share @chemsir.talking.chemistry and tag your friends. ------------------------------------------------------------------------ 👉Follow @chemsir.talking.chemistry and @chemsir_lee_wai_hon for more chemistry materials. ------------------------------------------------------------------------ 🙏🏻Thank you for your support. Wish you enjoy the page! ------------------------------------------------------------------------ #ChemsirTalkingChemistry #chem #chemistry #science #ChemsirMCChallenge #EnergyProfile #energy #PotentialEnergy #ReactionCoordinate #EnthalpyChange #EnthalpyLevelDiagram #EnthalpyLevel #enthalpy #ActivatedComplex #TransitionState #ActivationEnergy #ExothermicReaction #EndothermicReaction #exothermic #endothermic #2021dse #hkdse #dse #dsefighter #dse2021 #HongKong #Chemsir同你講化學 #化學 #科學 #Chemsir https://www.instagram.com/p/CB7mpEFBV_U/?igshid=6qdx4x01xzdj
#chemsirtalkingchemistry#chem#chemistry#science#chemsirmcchallenge#energyprofile#energy#potentialenergy#reactioncoordinate#enthalpychange#enthalpyleveldiagram#enthalpylevel#enthalpy#activatedcomplex#transitionstate#activationenergy#exothermicreaction#endothermicreaction#exothermic#endothermic#2021dse#hkdse#dse#dsefighter#dse2021#hongkong#chemsir同你講化學#化學#科學#chemsir
0 notes
Text
youtube
Endothermic and Exothermic Reaction - Atech Classes
Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Similarly, the formation of chemical bonds requires an input of energy.
Example-:
Melting ice cubes.
Melting solid salts.
Evaporating liquid water
What is an endothermic and exothermic reaction?
An exothermic process is one that gives off heat. This heat is transferred to the surroundings. An endothermic process is one in which heat has to be supplied to the system from the surroundings. A thermoneutral process is one that neither requires heat from the surroundings nor gives off energy to the surroundings
ATECH ACADEMY📷
Achieving Excellence Together
With specialization in -
+1 |+2 | IITJEE | NEET | MEDICAL | NDA | COMPETITIONS
Coaching Notes | Doubt Clearing | Revision | Exam Tips | CBSE | ICSE | NCERT
📷Get a different experience of education with us
Contact for Online, Classroom & Home Tutions
+91 9041152777
+91 9041062077
https://AtechEdu.com
ATECH ACADEMY is a fast, simple, and fun app that I use for learning and growing every day.
Get it at
https://play.google.com/store/apps/details?id=com.mohit.ingenium.tca504
https://youtu.be/wRUy9vQ0ikw
#Endothermicreactions#exothermicreactions#chemicalreactions#science#Class10#AtechAcademy#Coaching#CBSE#ICSE#NCERT#Education#Learning#Study#Exams#Topprs#iit#Jee#Competitions#Scholar#classesonline#Youtube
2 notes
·
View notes
Video
youtube
Chemical Reactions and Equation Class -10 (Part 1)
Chapter -1 Chemical reactions & Equations, Part-1,Class-10,CBSE, The videos we have made of this chapter are aimed to develop the better understanding of different concepts related to the chemical changes taking place around ourselves. Chemical changes involve chemical reactions and different types of chemical reactions have been explained in the videos of this chapter as per the updated syllabus of class 10.In order to fulfill the law of conservation of mass these reactions have to be balanced so balancing the chemical reactions is also an important part in this chapter. I have also prepared the video covering important questions and questions asked in previous year papers with answers so that you will be aware that how to write answers of the questions and reactions as part of answers. ************************************************** The links of the videos of this chapter are given below- Part -1 https://youtu.be/K5YG3oCKG_U Part 2 https://youtu.be/Is_L1JaTWvU Part 3 https://youtu.be/h_1IHAAmAUM Part 4 https://youtu.be/knuu7fbOJ_M (having important solved questions from last years papers) ************************************************** In these videos I have explained these topics from this chapter – • Importance of knowing about chemical reactions • Differences between physical and chemical changes • What are chemical reactions? • What are chemical equations? • Steps in writing a chemical equations / How to write a balanced chemical reaction? • Balancing the chemical equations with example • Points to be taken care while writing chemical reaction and balancing chemical equations • Different types of chemical reactions / what are the different types of chemical reactions? • Combination reactions & examples of Combination reactions • Decomposition reaction • Common examples of Decomposition types of reactions • Single displacement reactions • Double displacement reactions • Oxidation reduction reactions /redox reactions • Applications of oxidation-reduction reactions • applications of displacement reactions • Common examples of displacement and oxidation-reduction reactions • Cations-anions • Rancidity • How to prevent rancidity? • Corrosion • Factors causing corrosion • Rusting • Ways to prevent rusting I have used 12 years of my teaching experience for making these videos and have tried to explain the content in an easy to understand way. Please support us by subscribing to this channel on which we make such videos for the easy understanding of concepts. These videos are available free for you. Thanks for watching EduAchievers. You can follow us on following platforms- Facebook-https://www.facebook.com/EduAchievers... Instagram-https://www.instagram.com/eduachievers/ Twitter-https://twitter.com/EduAchievers Youtube-https://www.youtube.com/channel/UCfM4... ************************************************** #Combinationreactionso #decompositionreactions #commonexamplesofcombinationreactions #commonexamplesofdecompositionreactions #class10 #cbseclass10 #updatedsyllabus #chemistry #typesofreactionschemicalreaction #typesofreactions #Displacementreactions #Oxidationreductionreactions #applicationofoxidationreductionreactions #redoxreactions #applicationsofdisplacementreactions #chemicalequation #Combinationreactions #decompositionreactions #commonexamplesofcombinationreactions #commonexamplesofdecompositionreactions #class10 #cbseclass10 #updatedsyllabus #importanceofknowingaboutchemicalreactions #chemistry #differencesbetweenphysicalandchemicalchanges #Whatarechemicalreactions #Whatarechemicalequations #stepsinwritingachemicalequations #balancingthechemicalequationswithexample #pointstobetakencarewhilewritingchemicalreactionandbalancingchemicalequations #Differenttypesofchemicalreactions #asperpdatedsciecesllabs2020 #Applicationsofoxidationreductionreactions #rancidity #corossion #rusting #spoilageofoilyfoodpacket #exothermicreactions #endothermicreactions #decompositionofsilverchlorideinsunlight #whathappenswhencopperpowderisheated #oxidisingagent #reducingagent #activityofcoppersulphateandiron #chemical #chemistry #reactions #laboratory #colour #industry #kimya #analysis #skeletalchemicalreaction #engineering #lab #chemist #sodiumchloride #sodiumhydroxide #burningofmagnesiumribbon #decompositionofsilverchloride #blackandwhitephotography #useofnitrogeninpackagingfooditems #useofnitrogeninpackagingchipspackets #khimikaty #chimique #chemisch #kimi #kemija #chemie #kemi #chemie #chemija #chemia #cemeg #reaksion #reakcija #reaktion #reactie #kemiskreaktion #chemical #chemistry #science #laboratory #chemist #lab #reaction #analysis #tokorenda #laboratuvary # #chemicals #kimyager #colourSHOW LESS
1 note
·
View note
Text
A no prep, 20-point self-grading quiz on the difference between endothermic and exothermic reactions.
Simply share the quiz with your students and once they have completed the answers they can access the automatic feedback to see where they have made their mistakes. The teacher will also have access to all the answered quizzes and no changes can be made by the students once they have submitted their answers.
This quiz makes for an excellent revision exercise or assessment and can be used to test the students' knowledge and understanding of the basic differences between enthermic and exothermic reactions.
#atarchemistry #year12chem #year12chemistry #endothermicandexothermicreactions
#selfgradingactivities
#selfgradingassessments #selfgradingquiz #chemistryteachers #chemistryteacherreource #chemteach #chemistryactivity #atarche #atar #atarchemterm1 #australianteachers #australianchemistry #australianchemistryteacher #austeachers #digitalchemistryactivity #noprepresources #wateachers #wachem #waatarche #endothermicreaction #exothermicreaction #chemistryeducation #chemistrytest #chemistryquiz #digitalteachingandlearning #digitalresources

0 notes
Text
What are Endothermic Reactions Exothermic Reactions?
In chemistry, reactions can either absorb or release energy. Understanding the difference between endothermic and exothermic reactions is crucial for grasping how energy changes affect chemical processes. These reactions are fundamental concepts in thermodynamics, playing a key role in various natural and industrial processes. This article will explore the definitions, examples, and differences between endothermic and exothermic reactions, making it clear and understandable for students.
What are Endothermic Reactions?
Definition and Explanation
Endothermic reactions are chemical reactions that absorb energy from their surroundings in heat. The term “endothermic” is derived from the Greek words “endo,” meaning inside, and “therme,” meaning heat. In an endothermic reaction, the energy needed to break the bonds of the reactants exceeds the energy released when new bonds are formed in the products. As a result, there is a net intake of energy, causing the surrounding environment to cool down.
These reactions are characterized by a positive enthalpy change (ΔH > 0), indicating that the energy of the products is higher than that of the reactants. Endothermic reactions are essential in various natural processes, such as photosynthesis in plants, where sunlight is absorbed to convert carbon dioxide and water into glucose and oxygen.
In a laboratory setting, endothermic reactions often require continuous heating to proceed, as they rely on an external energy source to overcome the activation energy barrier. These reactions are not spontaneous and require careful control of temperature and energy input.
What are Exothermic Reactions?
Definition and Explanation
Exothermic reactions are chemical reactions that release energy into their surroundings, typically in the form of heat. The word “exothermic” originates from the Greek words “exo,” meaning “outside,” and “therme,” meaning “heat.” In an exothermic reaction, the energy released during the formation of bonds in the products is greater than the energy required to break the bonds in the reactants. This results in a net release of energy, causing the surrounding environment to heat up.
Exothermic reactions have a negative enthalpy change (ΔH < 0), indicating that the energy of the products is lower than that of the reactants. These reactions are often spontaneous and can occur rapidly, sometimes producing light, heat, or sound as by-products.
Exothermic reactions are common in everyday life and are essential in various industrial processes, such as combustion in engines and the setting of concrete. They play a vital role in biological systems as well, including cellular respiration, where glucose is broken down to release energy for cellular activities.
Difference Between Endothermic Reactions and Exothermic Reactions
Endothermic and exothermic reactions differ primarily in how they handle energy. Endothermic reactions draw energy from their surroundings, causing a reduction in the temperature near the reaction site. In contrast, exothermic reactions release energy, causing an increase in the surrounding temperature. These reactions can be identified by measuring the temperature change or by examining the enthalpy change (ΔH) associated with the reaction.
Understanding these differences is crucial in predicting how a reaction will behave under different conditions. For example, in industrial applications, controlling the temperature of an exothermic reaction is essential to prevent overheating, while ensuring a constant energy supply is vital for maintaining an endothermic reaction.
Understanding the difference between endothermic and exothermic reactions is fundamental to grasping how chemical processes interact with energy. These reactions are not just theoretical concepts but are integral to everyday life and industrial processes. By recognizing whether a reaction absorbs or releases energy, scientists and engineers can control and optimize these reactions for various applications.
If you’re looking to deepen your understanding of subjects just like this one, the Tutoroot Blog offers more simplified and insightful explanations. For a personalised learning experience, consider exploring Tutoroot’s Chemistry Online Tuitions. Our expert tutors are here to help you master complex topics with ease. Start your journey with Tutoroot today by booking a FREE DEMO session and take the next step in your academic success.
0 notes
Photo

The energy profile of a chemical reaction is the energy change of the species involved during the course of the reaction. What else do you know about the energy profile of a chemical reaction?🤔 🤔 ------------------------------------------------------------------------ 👍Please like, comment and share @chemsir.talking.chemistry and tag your friends. ------------------------------------------------------------------------ 👉Follow @chemsir.talking.chemistry and @chemsir_lee_wai_hon for more chemistry materials. ------------------------------------------------------------------------ 🙏Thank you for your support. Wish you enjoy the page! ------------------------------------------------------------------------ Source: Internet ------------------------------------------------------------------------ #ChemsirTalkingChemistry #chem #chemistry #science #EnergyProfile #energy #PotentialEnergy #ReactionCoordinate #EnthalpyChange #EnthalpyLevelDiagram #EnthalpyLevel #enthalpy #ActivatedComplex #TransitionState #ActivationEnergy #ExothermicReaction #EndothermicReaction #exothermic #endothermic #PhysicalChemistry #chemistryisfun #chemistryfun #chemistrylover #chemistrylovers #chemistryaddict #chemistryaddicts #Chemsir同你講化學 #化學 #科學 #Chemsir https://www.instagram.com/p/CB5SDiXBCLt/?igshid=1azs17g0a0bft
#chemsirtalkingchemistry#chem#chemistry#science#energyprofile#energy#potentialenergy#reactioncoordinate#enthalpychange#enthalpyleveldiagram#enthalpylevel#enthalpy#activatedcomplex#transitionstate#activationenergy#exothermicreaction#endothermicreaction#exothermic#endothermic#physicalchemistry#chemistryisfun#chemistryfun#chemistrylover#chemistrylovers#chemistryaddict#chemistryaddicts#chemsir同你講化學#化學#科學#chemsir
0 notes