#hwsolutions
Explore tagged Tumblr posts
Text
cbe 142 hw 3
Problem 2 (15 points):
Liquid A decomposes with n th-order kinetics in a batch reactor: A→B, r=kC_A ^n
The conversion of A reaches 50% in a five-minute run. What is the order (n) of the reaction if it takes...
(a) (9 points) 10 minutes to reach 75% conversion?
(b) (3 points) 20 minutes to reach 75% conversion?
(c) (3 points) 30 minutes to reach 75% conversion?
15/15 soln:

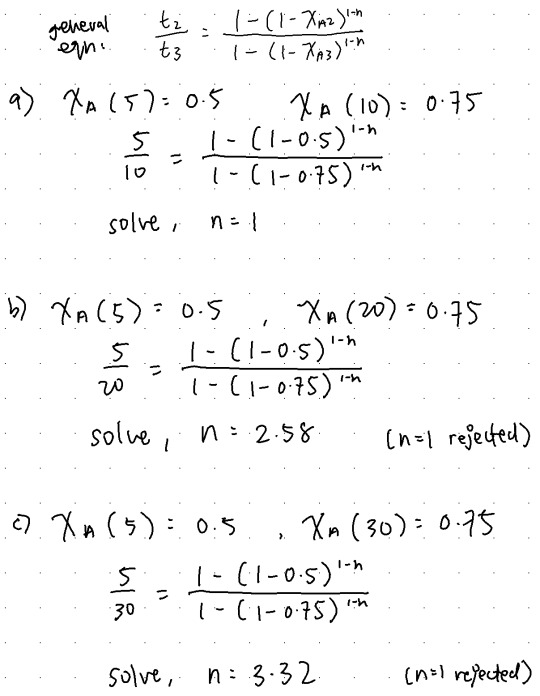
1 note
·
View note
Text
HW Solutions
https://www.studocu.com/en/document/orange-coast-college/calculus-based-physelecmag/book-solutions/hwsolutions-chapter-21zemansky/2003763/view
2 notes
·
View notes
Photo


Monev - Monitoring dan Evaluasi System : adalah aplikasi bantu yang dapat digunakan untuk monitoring dan evaluasi pelayanan pasien JKN bersumber data dari txt INACBG , dengan software ini didapatkan informasi : 1. Monitoring jumlah kasus dan klaim BPJS Kesehatan 2. Melihat 10 penyakit terbanyak 3. Pasien dengan LOS terlama 4. Tren ALOS Dan masih banyak lagi Aplikasi ini khusus digunakan untuk Rumah Sakit. Info lebih lanjut : Email : [email protected] Telp : 08112956608
1 note
·
View note
Text
cbe 142 hw 2
Problem 4 (5 points): Variable volumetric flowrate
A chemical reactor consists of a tube with gaseous inflow and outflow. Within the chem- ical reactor, a gas-phase chemical reaction occurs. Determine and state if the volumetric flowrate at the entrance is greater or less than the volumetric flowrate at the reactor exit for the following three situations. Please provide an explanation only in words. Assume that the gas phase is ideal.
(a) The reactor is at constant temperature and pressure. The gas phase reaction is A −−→ B+C.
(b) The reaction is A −−→ B and at constant pressure. The temperature increases within the reactor.
(c) The reaction is A −−→ B and at constant temperature. The pressure decreases within the reactor.
5/5 soln

1 note
·
View note
Text
cbe 142 hw 2
Problem 3 (20 points): Graphical Reactor Sizing
In companies such as Shell, Dow, and BASF, polymers are a standard product. To cre- ate a polymer, we feed in monomers (A) to the system and we have an initiator acting as a catalyst to start the polymerization process. We can treat the system as a reactor in which the reaction occurs throughout the reaction volume. For a fixed initiator concentration, and a volumetric flow rate of 0.5 m3/min, the following results from an ideal isothermal liquid-phase CSTR were tabulated:
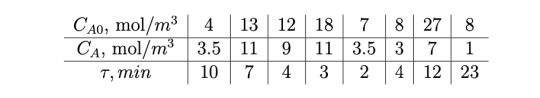
(a) (8 points) Plot the Levenspiel graph (FA0/−rA vs. XA) for the reaction above.(At XA=0 (FA0/−rA =40)
(b) (2 points) For 35% conversion, calculate the reactor volume if an ideal CSTR is used to conduct the reaction.
(c) (4 points) For 35% conversion, calculate the reactor volume if an ideal PFR is used to conduct the reaction. Feel free to either use a numerical integrator or implement by hand a scheme like Simpson’s rule or the trapezoidal rule.
(d) (2 points) Compare parts (b) and (c) and decide which reactor would occupy a lower volume in order to achieve 35% conversion.
(e) (4 points) What is the optimal reactor configuration (single reactor, reactor in series) to reach 75% conversion? What are the suggested reactor volume(s) for the system?






0 notes
Text
cbe 142 hw2
Problem 2 (10 points): Mole balance and stoichiometry
The reaction
2A + 3B → C (1)
takes place in an isothermal, unsteady CSTR. The feed consists of only A and B in stoichio- metric proportions. Which of the following set of equations gives the correct mole balances on A, B, and C? Species A and B are disappearing and Species C is being formed. Circle the correct answer where all mole balances are correct.

10/10 soln

0 notes
Text
cbe 142 hw2
Problem 1 (15 points): Isothermal reactions, conversion
(a) (7 points) Given a first-order isothermal reaction, A −−→ P, rA = −kcA , simplify the general mole balance equation to get the design equations for the following ideal liquid phase reactors operating under isothermal conditions : i) batch reactor, ii) CSTR and iii) PFR. Your final answer should be in terms of CA0,k,cA and the residence time τ. τ gives us the average time any volume element spends in the reactor for any flow reactor, given as V/q0. For a batch reactor it refers to how long the reaction has taken place in the reactor, which is the time t.
(b) (8 points) Let the conversion of the reactant A be XA. Express the design equations from part a) in terms of XA, τ and k. Let k=1s−1. Plot XA versus τ for all the three reactor types. XA should vary from 0 to 0.9.Why does the plot for PFR and batch reactor look similar? Why is the conversion for the CSTR lower than the PFR?
15/15
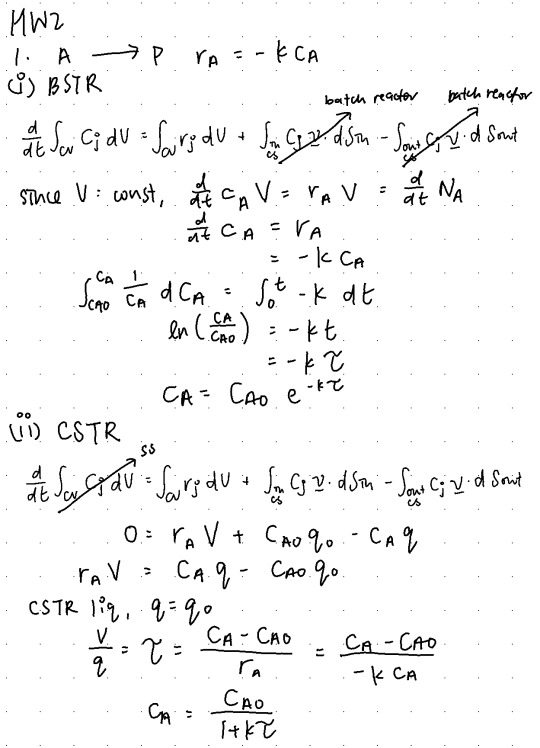



0 notes
Text
cbe 142 hw1
Problem 3 (25 points): ODE solving in MATLAB
Systems with multiple reactions can often display complex behavior depending on the prop- erties of the system. Reactors can exhibit stable or unstable behavior depending on the specifics of the reactions and it is thus pivotal to understand these dynamics when designing reactors. Use MATLAB to solve the following system of differential equations and visualize the dynamical behavior of a system of reacting chemical species.
Consider a reaction system for the conversion of A to B via the intermediates X and Y:
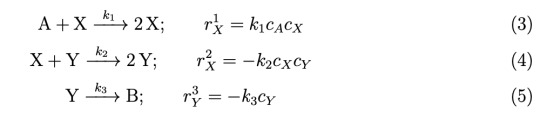
Assume that a controller is present to keep the concentration of A constant at cA0 and k1 = 0.1 (M s)−1, k2 = 0.3 (M s)−1, and k3 = 0.5 (s)−1. Here M [=] moles/Liter
(a) (6 points) Derive a set of ordinary differential rate equations to describe each species present in this system. Are these equations linear or nonlinear? Explain why.
(b) (2 points) What are the independent and dependent variables of the system of equations you derived in Part (a)?
(c) (2 points) Solve for the steady-state values of cX and cY in this system.
(d) (7 points) We are interested in the intermediate values of cX and cY . Using MATLAB, plot cX (t) and cY (t) over an appropriate time range to visualize the system’s dynamics. AssumecA0 =5M,cX0 =3M,cY0 =4M,andcB0 =0M.Pleaseattachascreenshot of your code.
(e) (4 points) Does your result in Part (d) agree with what you expected from your result in Part (c)? If not, explain the apparent discrepancy between these two results.
(f) (4 points) Generate a phase portrait of the intermediate species by plotting them against each other where the independent variable is implicit. What does this plot represent?
24/25 soln




0 notes
Text
cbe 142 hw1
Problem 2 (10 points): Pseudo-first order kinetics
Recall from class the homogeneous bimolecular reaction A + B −−→ P, which is first or- der in each of the two reactants A and B, and therefore second order overall. We derived in class the expression for the concentrations of A and B as a function of time,
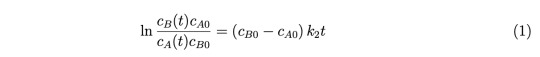
Here, cA0 and cB0 are the starting concentrations of A and B at t = 0.
(a) (4 points) Consider the instance that cA0 = cB0, i.e. the reactants are in stoichiometric ratio. Convince yourself that in this situation cA(t) = cB(t) for all times. By integrating differential form of the rate law, derive an equation for cA(t) in terms of cA0, t and k2.
(b) (2 points) For the situation in (a), suppose you are given data for cA(t) vs. t. Can you think of a nice way to plot it to extract the value of the rate constant k2?
(c) (4 points) Let’s return to the case in (a) where cA0 and cB0 are not equal. It often occurs for second-order reactions that one reactant can be maintained at a much larger concentration than the other, so that no matter how much the limiting reactant is consumed, the concentration of the excess reactant will be affected little. In this vein, assume that cB0 >> cA0, and that cB(t) ≈ cB0. In this scenario, convince yourself that equation 1 reduces to the first order form,
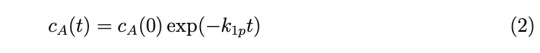
where k1p is the pseudo-first order rate constant. How is k1p related to k2?
10/10 soln
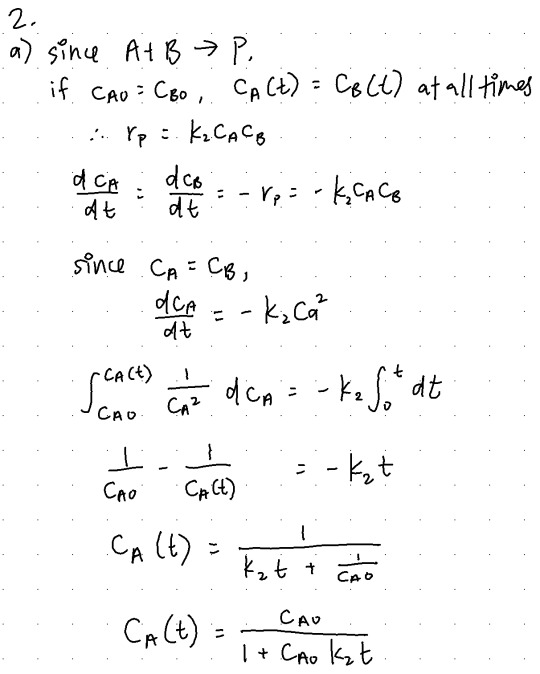
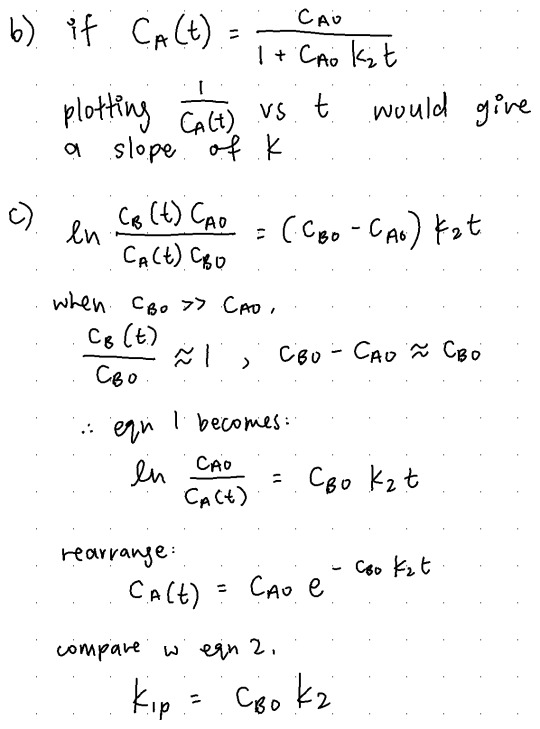
0 notes
Text
cbe 142 hw1
Problem 1 (15 points): Rate constants and units
For units, use centimeters for length, pascals (Pa) for pressure, moles for species amount, and seconds for time. cX denotes the concentration of species X and PX denotes the partial pressure of species X. The generation rate rA is defined as the rate of generation of A per unit time per unit volume. rA′ is defined as the rate of generation of A per unit time per unit mass of catalyst. r′′ is defined as the rate of generation of A per unit time per unit surface area of catalyst. rA,r′ and r′′ have the following units (following the convention in Appendix F of Fogler’s book),
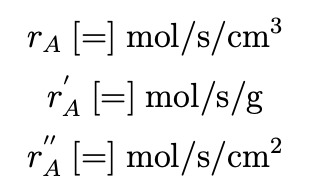
Given these definitions, determine the units of the rate constants (k’s) and the equilibrium constants (K’s) in the following rate laws. Use the principle of dimensional homogeneity that you should be familiar with from 150A.
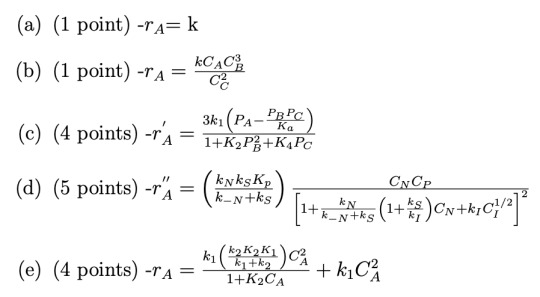
Hint: In a sum of terms a + b + c, all terms must have the same units according to the principle of dimensional homogeneity. In an equation A = B, the quantities A and B must have the same units
15/15 soln




0 notes
Text
mse 45 hw10
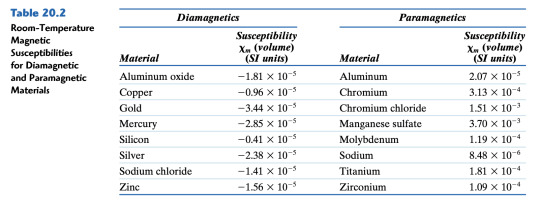
Q7. (12 pts.) A coil of wire 0.5 m long and having 20 turns carries a current of 1.0 A. Answer the following: (4 pts.) Compute the flux density if the coil is within a vacuum. (4 pts.) A bar of an iron–silicon alloy, the B-H behavior for which is shown in Figure 20.29, is positioned within the coil. What is the flux density within this bar? (4 pts.) Suppose that a bar of molybdenum is now situated within the coil. What current must be used to produce the same B field in the Mo as was produced in the iron–silicon alloy (part b) using 1.0 A?
Figure 20.29

Table 20.2
12/12
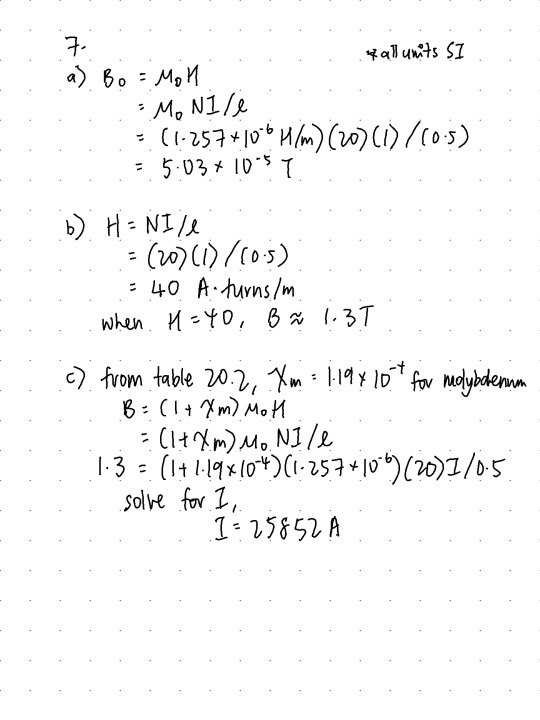
i have no idea how the units work but it does work soo
0 notes
Text
mse 45 hw10
Q6. (14 pts.) Assume there exists some hypothetical metal that exhibits ferromagnetic behavior and that has (1) a simple cubic crystal structure, (2) an atomic radius of 0.125 nm, and (3) a saturation flux density of 0.85 T. Determine the number of Bohr magnetons per atom for this material.
14/14
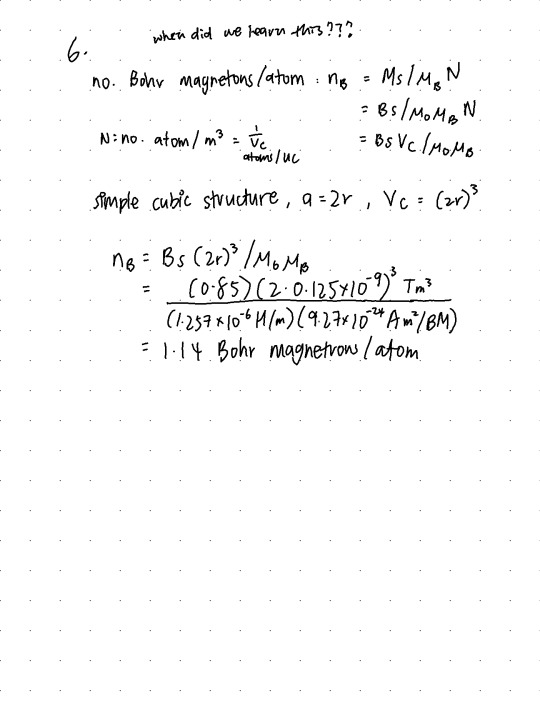
always, make sure your units cancel out nicely ! also we weren't taught this in lecture so,,, thank u google (and hence that complain at the top of my hw)
0 notes
Text
mse 45 hw10
Q5. (12 pts) The magnetic flux density within a bar of some material is 0.630 T at an H field of 5 × 10^5 A/m. Compute the following for this material: (4 pts.) The magnetic permeability. (4 pts.) The magnetic susceptibility. (4 pts.) What type(s) of magnetism would you suggest is (are) being displayed by this material? Why?
12/12
*for (c), its better to say paramagnetic since Xm is small and positive

the units for magnetism are confusing as heck
0 notes
Text
mse 45 hw10
Q4. (20 pts.) A charge of 2.0 × 10-10 C is to be stored on each plate of a parallel-plate capacitor having an area of 650 mm2 (1.0 in2) and a plate separation of 4.0 mm (0.16 in). Answer the following: (4 pts.) What voltage is required if a material having a dielectric constant of 3.5 is positionedwithin the plates? (4 pts.) What voltage would be required if a vacuum were used? (4 pts.) What are the capacitances for parts (a) and (b)? (4 pts.) Compute the dielectric displacement for part (a). (4 pts.) Compute the polarization for part (a).
20/20

0 notes
Text
mse 45 hw10
Q3. (12 pts.) For CaO, the ionic radii for Ca2+ and O2- ions are 0.100 and 0.140 nm, respectively. If an externally applied electric field produces a 5% expansion of the lattice, compute the dipole moment for each Ca2+-O2- pair. Assume that this material is completely unpolarized in the absence of an electric field.
10/12 *charge is ±2 so Q should be 2*e

0 notes
Text
mse 45 hw10
Q2. (14 pts.) a. (6 pts.) A parallel-plate capacitor using a dielectric material having an εr of 2.2 has a plate spacing of 2 mm (0.08 in.). If another material having a dielectric constant of 3.7 is used and the capacitance is to be unchanged, what must be the new spacing between the plates? b. (8 pts.) A parallel-plate capacitor with dimensions of 38 mm by 65 mm and a plate separation of 1.3 mm must have a minimum capacitance of 70 pF (7 × 10-11 F) when an ac potential of 1000 V is applied at a frequency of 1 MHz. Which of the materials listed in Table 18.5 are possible candidates? Why?
Table 18.5
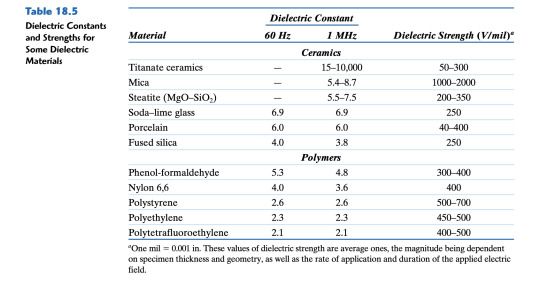
14/14

0 notes