#mercuric oxide
Text

1 note
·
View note
Text
How to Use Mercurochrome Safely for Minor Cuts and Scrapes
Mercurochrome and Red Mercuric Oxide are well-known antiseptic agents used in various medical applications. Mercurochrome is commonly applied to minor cuts and wounds to prevent infection, while Red Mercuric Oxide is used in ophthalmic treatments for specific eye infections. Both substances are essential in the healthcare industry due to their antimicrobial properties. At Spa Corporation, we provide high-quality Mercurochrome and Red Mercuric Oxide to meet the needs of medical professionals and researchers. Explore our product offerings for top-notch chemical solutions.
0 notes
Text
#pediculosis treatment market#pediculosis treatment market size#pediculosis treatment market shape#pediculosis treatment market forecast#pediculosis treatment market analysis#pediculosis treatment market report#pediculosis treatment market research#pediculosis treatment market price#Isopropyl Alcohol#Mercuric Oxide Ointment#Dimeticone Lotion
0 notes
Text

Best Greetings
Mars Original Orbit Was Between Mercury And Venus
Physics Nobel Prize For Imaginary Ideas!
or
Mars Original Orbit Was Between Mercury And Venus with orbital distance 84 million km- Later Mars had migrated to its current position– Mars had collided with Venus and with the Earth in its migration- indeed – Mars Is the planet caused the Earth Moon creation (Giant-Impact hypothesis is wrong)
THE DETAILS
Mars was the next planet after Mercury with original orbital distance =84 mkm and Mars had migrated into Mars current orbital distance (227.9 mkm)
Through Mars Displacement from (84 mkm) to (227.9 mkm), Mars had collided with Venus (at first) and then with Earth (at second) and then reach to its current orbital distance point (227.9 mkm).
Mars had moved from (84 mkm) to (227.9 mkm) in a direct displacement – and had pushed with it all collisions debris in its direction
From these collisions debris the Earth could create its own moon and also Mars moons are created from them and the rest debris be attracted by Jupiter Gravity and Created The Asteroid Belt.
Means,
Mars Migration Theory is in full harmony with (Giant- Impact Hypothesis), but
Mars itself had collided with Earth and caused the moon creation and not the ancient planet (theia) as supposed by the Giant-impact hypothesis.
The point is that, the planets order (Mercury – Venus –Earth) shows that Mars was the second planet after Mercury – and Mars had migrated from (84 mkm) to (227.9 mkm) –through this displacement – Mars has collided with Venus and Earth–
Mars collisions with Earth and Venus left many proves behind which prove that Mars did these collisions and not the planet (theia).
Mars Migration theory answers many of the Giant-impact hypothesis questions.
Let's do that in following
(1st Question)
Why has Venus no a moon? spite it suffered from a similar collision as Earth?
Mars had moved its displacement from (84 mkm) to (227.9 mkm), and through this displacement Mars pushed all debris with it in its motion direction and because of that, Venus had found no debris around, And couldn't create its own moon because of that.
But the debris lost some of their motion momentum at Earth position and Earth gravity is more strong than Venus, by that Earth could attract some debris by which Earth created its moon.
(2nd Question)
What's The Lunar Magma Ocean (LMO) Origin?– It's Venus
Because
The Earth moon rocks are created from the 3 planets debris (Mars- Venus – Earth) and for that the moon has a Magma Ocean (LMO).
(3rd Question)
Why The Iron Oxide (Feo) of the Moon= (13%)?
Because The rate (13%) is a middle rate between Mars rate (18%) and the terrestrial mantle (8%).
Notice no.(1)
Mars Moons Creation is a good proof for Mars Migration Theory.
The collisions debris lost the great part of their momentum when reach to Mars orbital distance point (227.9 mkm), because of that, Mars with its small mass could attracted 2 moons, because the debris is too much around it and can attract them easily.
But Venus great gravity couldn't create even one moon because no debris be found around it
Notice no.(2)
The asteroid belt is one more proof for Mars Migration
The collisions debris which were pushed with Mars motion to its current orbital distance point (227.9 mkm) couldn't all be attracted by Mars gravity because of its small mass, Mars had attracted only its 2 small moons
But
Jupiter great gravity attracted the rest debris and they created the asteroid belt.
The whole trajectory is filled with references of the event and shows that it's a fact.
Notice no.(3)
Mars diameter before Migration was 7070 km and be 6792 mkm after migration as a result for the collisions Venus and Earth
We know that because
The equation (7070 km x 1092 = 84 mkm), where 84 mkm=Mars original orbital distance.
The equation (d = r x 1092) is the equation controls planet orbital distance based on its diameter. The equation be used by Mercury, Earth and Saturn but the planets migration effects negatively on the other planets using of it.
From this analysis we conclude, Mars Diameter be decreased with 4.1%
Additional Data
Mercury Orbital Distance 57.9 mkm = Mercury Diameter 4879 km x 1092
Earth orbital distance 149.6 mkm = Earth diameter 12756 km x 1092
Saturn orbital distance 1433.5 mkm =Saturn diameter 120536 km x 1092
Gerges Francis Tawdrous +201022532292
Physics Department- Physics & Mathematics Faculty
Peoples' Friendship university of Russia – Moscow (2010-2013)
Curriculum Vitae https://www.academia.edu/s/b88b0ecb7c
E-mail [email protected], [email protected]
ORCID https://orcid.org/0000-0002-1041-7147
Facebook https://www.facebook.com/gergis.tawadrous
VK https://vk.com/id696655587
Tumblr https://www.tumblr.com/blog/itsgerges
Researcherid https://publons.com/researcher/3510834/gerges-tawadrous/
Google https://scholar.google.com/citations?user=2Y4ZdTUAAAAJ&hl=en
Livejournal https://gerges2022.livejournal.com/profile
Pocket https://getpocket.com/@646g8dZ0p3aX5Ad1bsTr4d9THjA5p6a5b2fX99zd54g221E4bs76eBdtf6aJw5d0?src=navbar
PUBLICATIONS
box https://app.box.com/s/47fwd0gshir636xt0i3wpso8lvvl8vnv
Academia https://rudn.academia.edu/GergesTawadrous
List of publications http://vixra.org/author/gerges_francis_tawdrous
Slideshare https://www.slideshare.net/Gergesfrancis
#русский tumblr#quantum physics#physique#astronomy#астрофизика#квантовая физика#наш физик легенда#физика#астрономи космос наука#physics#astrophysics#учеба#ошибки#scientists#science#10 класс#mathematics#maths#школа#русская школа#русский тамблер#решение задач#задачи#механика#quantum mechanics#science side of tumblr#философия#philosophy#электричество#electromagnetism
17 notes
·
View notes
Text
What is Sydney experimenting with?
I had in front of me some of the finest destructive agents you could wish to light upon—carbon-monoxide, chlorine-trioxide, mercuric-oxide, conine, potassamide, potassium-carboxide, cyanogen
Carbon monoxide - a colourless, odourless, tasteless, poisonous gas. It binds to haemoglobin and prevents the blood from carrying oxygen and expelling carbon dioxide.
Chlorine trioxide - a gas that kills bacteria, viruses and fungi. In large doses it damages red blood cells and the lining of the GI tract.
Mercuric oxide - a highly toxic substance that irritates the eyes, skin and respiratory tract, and can damage the kidneys. It's banned as a pesticide in the EU.
Conine - a compound that can be isolated from poisonous hemlock. It disrupts the central nervous system and causes death by respiratory paralysis.
Potassamide - this one... seems to be fine, unless Google is failing me. It's flammable and ignites on contact with moisture.
Potassium carboxide - again, this is relatively harmless compared with the rest of this list. It's caustic, and so can cause tissue damage on contact. But in small amounts people sometimes use it in baking.
[Edited: @finite-fractal points out this is probably not potassium carbonate, which was my assumption, but an explosive made with potassium and carbon monoxide. Which would be much more in line with the rest of the list.]
Cyanogen - this is a colourless and highly toxic gas. Inhaling 100-150 milligrams is fatal.
#the beetle weekly#sydney wasn't lying#these agents can destructive#this is all from googling i am not good at chemistry
32 notes
·
View notes
Text
Joseph Priestley

A portrait of Priestley commissioned by his publisher and close friend Joseph Johnson from Henry Fuseli (c. 1783)
an English chemist, natural philosopher, separatist theologian, grammarian, multi-subject educator, and liberal political theorist. He published over 150 works, and conducted experiments in electricity and other areas of science. He was a close friend of, and worked in close association with Benjamin Franklin involving electricity experiments

Priestley, painted late in life by Rembrandt Peale(c. 1800); Americans knew Priestley less as a man of science and more as a defender of the freedom of the colonies and of Dissenters.
Priestley is credited with his independent discovery of oxygen by the thermal decomposition of mercuric oxide, having isolated it in 1774. During his lifetime, Priestley's considerable scientific reputation rested on his invention of carbonated water, his writings on electricity, and his discovery of several "airs" (gases), the most famous being what Priestley dubbed "dephlogisticated air" (oxygen). Priestley's determination to defend phlogiston theory and to reject what would become the chemical revolution eventually left him isolated within the scientific community.
Priestley's science was integral to his theology, and he consistently tried to fuse Enlightenment rationalism with Christian theism. In his metaphysical texts, Priestley attempted to combine theism, materialism, and determinism, a project that has been called "audacious and original". He believed that a proper understanding of the natural world would promote human progress and eventually bring about the Christian millennium. Priestley, who strongly believed in the free and open exchange of ideas, advocated toleration and equal rights for religious Dissenters, which also led him to help found Unitarianism in England. The controversial nature of Priestley's publications, combined with his outspoken support of the American Revolution and later the French Revolution, aroused public and governmental contempt; eventually forcing him to flee in 1791, first to London and then to the United States, after a mob burned down his Birmingham home and church. He spent his last ten years in Northumberland County, Pennsylvania.
A scholar and teacher throughout his life, Priestley made significant contributions to pedagogy, including the publication of a seminal work on English grammar and books on history; he prepared some of the most influential early timelines. The educational writings were among Priestley's most popular works. Arguably his metaphysical works, however, had the most lasting influence, as now considered primary sources for utilitarianism by philosophers such as Jeremy Bentham, John Stuart Mill, and Herbert Spencer.

Priestley by Ellen Sharples
The intellectually stimulating atmosphere of Warrington, often called the "Athens of the North" (of England) during the 18th century, encouraged Priestley's growing interest in natural philosophy. He gave lectures on anatomy and performed experiments regarding temperature with another tutor at Warrington, his friend John Seddon. Despite Priestley's busy teaching schedule, he decided to write a history of electricity. Friends introduced him to the major experimenters in the field in Britain—John Canton, William Watson, Timothy Lane, and the visiting Benjamin Franklin who encouraged Priestley to perform the experiments he wanted to include in his history. Priestly also consulted with Franklin during the latter's kite experiments. In the process of replicating others' experiments, Priestley became intrigued by unanswered questions and was prompted to undertake experiments of his own design. (Impressed with his Charts and the manuscript of his history of electricity, Canton, Franklin, Watson, and Richard Price nominated Priestley for a fellowship in the Royal Society; he was accepted in 1766.)
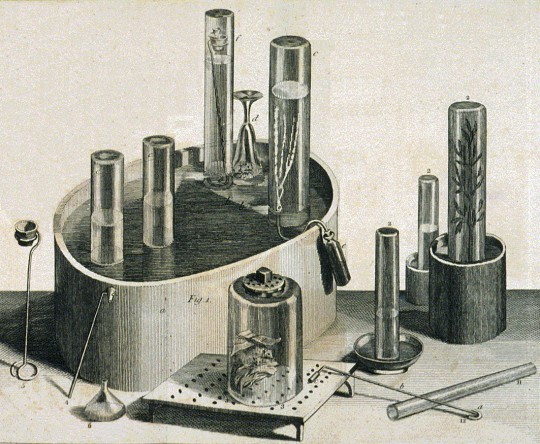
Equipment used by Priestley in his experiments on gases
Priestley's years in Calne were the only ones in his life dominated by scientific investigations; they were also the most scientifically fruitful. His experiments were almost entirely confined to "airs", and out of this work emerged his most important scientific texts: the six volumes of Experiments and Observations on Different Kinds of Air (1774–86). These experiments helped repudiate the last vestiges of the theory of four elements, which Priestley attempted to replace with his own variation of phlogiston theory. According to that 18th-century theory, the combustion or oxidation of a substance corresponded to the release of a material substance, phlogiston.
Priestley's work on "airs" is not easily classified. As historian of science Simon Schaffer writes, it "has been seen as a branch of physics, or chemistry, or natural philosophy, or some highly idiosyncratic version of Priestley's own invention". Furthermore, the volumes were both a scientific and a political enterprise for Priestley, in which he argues that science could destroy "undue and usurped authority" and that government has "reason to tremble even at an air pump or an electrical machine".
Volume I of Experiments and Observations on Different Kinds of Air outlined several discoveries: "nitrous air" (nitric oxide, NO); "vapor of spirit of salt", later called "acid air" or "marine acid air" (anhydrous hydrochloric acid, HCl); "alkaline air" (ammonia, NH3); "diminished" or "dephlogisticated nitrous air" (nitrous oxide, N2O); and, most famously, "dephlogisticated air" (oxygen, O2) as well as experimental findings that showed plants revitalised enclosed volumes of air, a discovery that would eventually lead to the discovery of photosynthesis. Priestley also developed a "nitrous air test" to determine the "goodness of air". Using a pneumatic trough, he would mix nitrous air with a test sample, over water or mercury, and measure the decrease in volume—the principle of eudiometry. After a small history of the study of airs, he explained his own experiments in an open and sincere style. As an early biographer writes, "whatever he knows or thinks he tells: doubts, perplexities, blunders are set down with the most refreshing candour." Priestley also described his cheap and easy-to-assemble experimental apparatus; his colleagues therefore believed that they could easily reproduce his experiments. Faced with inconsistent experimental results, Priestley employed phlogiston theory. This led him to conclude that there were only three types of "air": "fixed", "alkaline", and "acid". Priestley dismissed the burgeoning chemistry of his day. Instead, he focused on gases and "changes in their sensible properties", as had natural philosophers before him. He isolated carbon monoxide (CO), but apparently did not realise that it was a separate "air".
In August 1774 he isolated an "air" that appeared to be completely new, but he did not have an opportunity to pursue the matter because he was about to tour Europe with Shelburne. While in Paris, Priestley replicated the experiment for others, including French chemist Antoine Lavoisier. After returning to Britain in January 1775, he continued his experiments and discovered "vitriolic acid air" (sulphur dioxide, SO2).
In March he wrote to several people regarding the new "air" that he had discovered in August. One of these letters was read aloud to the Royal Society, and a paper outlining the discovery, titled "An Account of further Discoveries in Air", was published in the Society's journal Philosophical Transactions. Priestley called the new substance "dephlogisticated air", which he made in the famous experiment by focusing the sun's rays on a sample of mercuric oxide. He first tested it on mice, who surprised him by surviving quite a while entrapped with the air, and then on himself, writing that it was "five or six times better than common air for the purpose of respiration, inflammation, and, I believe, every other use of common atmospherical air". He had discovered oxygen gas (O2).
Priestley assembled his oxygen paper and several others into a second volume of Experiments and Observations on Air, published in 1776. He did not emphasise his discovery of "dephlogisticated air" (leaving it to Part III of the volume) but instead argued in the preface how important such discoveries were to rational religion. His paper narrated the discovery chronologically, relating the long delays between experiments and his initial puzzlements; thus, it is difficult to determine when exactly Priestley "discovered" oxygen. Such dating is significant as both Lavoisier and Swedish pharmacist Carl Wilhelm Scheele have strong claims to the discovery of oxygen as well, Scheele having been the first to isolate the gas (although he published after Priestley) and Lavoisier having been the first to describe it as purified "air itself entire without alteration" (that is, the first to explain oxygen without phlogiston theory).
In his paper "Observations on Respiration and the Use of the Blood", Priestley was the first to suggest a connection between blood and air, although he did so using phlogiston theory. In typical Priestley fashion, he prefaced the paper with a history of the study of respiration. A year later, clearly influenced by Priestley, Lavoisier was also discussing respiration at the Académie des sciences. Lavoisier's work began the long train of discovery that produced papers on oxygen respiration and culminated in the overthrow of phlogiston theory and the establishment of modern chemistry.
Around 1779 Priestley and Shelburne – soon to be the 1st Marquess of Landsdowne – had a rupture, the precise reasons for which remain unclear. Shelburne blamed Priestley's health, while Priestley claimed Shelburne had no further use for him. Some contemporaries speculated that Priestley's outspokenness had hurt Shelburne's political career. Schofield argues that the most likely reason was Shelburne's recent marriage to Louisa Fitzpatrick—apparently, she did not like the Priestleys. Although Priestley considered moving to America, he eventually accepted BirminghamNew Meeting's offer to be their minister.
Both Priestley and Shelburne's families upheld their Unitarian faith for generations. In December 2013, it was reported that Sir Christopher Bullock– a direct descendant of Shelburne's brother, Thomas Fitzmaurice (MP) – had married his wife, Lady Bullock, née Barbara May Lupton, at London's Unitarian Essex Church in 1917. Barbara Lupton was the second cousin of Olive Middleton, née Lupton, the great-grandmother of Catherine, Duchess of Cambridge. In 1914, Olive and Noel Middleton had married at Leeds' Mill Hill Chapel, which Priestley, as its minister, had once guided towards Unitarianism.
Many of the friends that Priestley made in Birmingham were members of the Lunar Society, a group of manufacturers, inventors, and natural philosophers who assembled monthly to discuss their work. The core of the group included men such as the manufacturer Matthew Boulton, the chemist and geologist James Keir, the inventor and engineer James Watt, and the botanist, chemist, and geologist William Withering. Priestley was asked to join this unique society and contributed much to the work of its members. As a result of this stimulating intellectual environment, he published several important scientific papers, including "Experiments relating to Phlogiston, and the seeming Conversion of Water into Air" (1783). The first part attempts to refute Lavoisier's challenges to his work on oxygen; the second part describes how steam is "converted" into air. After several variations of the experiment, with different substances as fuel and several different collecting apparatuses (which produced different results), he concluded that air could travel through more substances than previously surmised, a conclusion "contrary to all the known principles of hydrostatics". This discovery, along with his earlier work on what would later be recognised as gaseous diffusion, would eventually lead John Dalton and Thomas Graham to formulate the kinetic theory of gases.
In 1777, Antoine Lavoisier had written Mémoire sur la combustion en général, the first of what proved to be a series of attacks on phlogiston theory; it was against these attacks that Priestley responded in 1783. While Priestley accepted parts of Lavoisier's theory, he was unprepared to assent to the major revolutions Lavoisier proposed: the overthrow of phlogiston, a chemistry based conceptually on elements and compounds, and a new chemical nomenclature. Priestley's original experiments on "dephlogisticated air" (oxygen), combustion, and water provided Lavoisier with the data he needed to construct much of his system; yet Priestley never accepted Lavoisier's new theories and continued to defend phlogiston theory for the rest of his life. Lavoisier's system was based largely on the quantitative concept that mass is neither created nor destroyed in chemical reactions (i.e., the conservation of mass). By contrast, Priestley preferred to observe qualitative changes in heat, color, and particularly volume. His experiments tested "airs" for "their solubility in water, their power of supporting or extinguishing flame, whether they were respirable, how they behaved with acid and alkaline air, and with nitric oxide and inflammable air, and lastly how they were affected by the electric spark."
By 1789, when Lavoisier published his Traité Élémentaire de Chimie and founded the Annales de Chimie, the new chemistry had come into its own. Priestley published several more scientific papers in Birmingham, the majority attempting to refute Lavoisier. Priestley and other Lunar Society members argued that the new French system was too expensive, too difficult to test, and unnecessarily complex. Priestley in particular rejected its "establishment" aura. In the end, Lavoisier's view prevailed: his new chemistry introduced many of the principles on which modern chemistry is founded.
Priestley's refusal to accept Lavoisier's "new chemistry"—such as the conservation of mass—and his determination to adhere to a less satisfactory theory has perplexed many scholars. Schofield explains it thus: "Priestley was never a chemist; in a modern, and even a Lavoisierian, sense, he was never a scientist. He was a natural philosopher, concerned with the economy of nature and obsessed with an idea of unity, in theology and in nature." Historian of science John McEvoy largely agrees, writing that Priestley's view of nature as coextensive with God and thus infinite, which encouraged him to focus on facts over hypotheses and theories, prompted him to reject Lavoisier's system. McEvoy argues that "Priestley's isolated and lonely opposition to the oxygen theory was a measure of his passionate concern for the principles of intellectual freedom, epistemic equality and critical inquiry." Priestley himself claimed in the last volume of Experiments and Observations that his most valuable works were his theological ones because they were "superior [in] dignity and importance".
2 notes
·
View notes
Text
Who discovered oxygen?
The discovery of oxygen is attributed to the combined efforts of several scientists in the 18th century, each contributing uniquely to our understanding of this vital element.
Joseph Priestley, an English clergyman and chemist, is often credited with the discovery of oxygen on August 1, 1774.
Priestley conducted experiments by focusing sunlight on mercuric oxide (HgO), which released a gas he termed "dephlogisticated air."
He observed that this gas allowed a candle to burn brighter and longer and supported animal respiration better than normal air.

Carl Wilhelm Scheele, a Swedish pharmacist, also discovered oxygen independently in the early 1770s.
Scheele produced oxygen by heating compounds like mercuric oxide and potassium nitrate but published his findings later in 1777.
Antoine Lavoisier, a French chemist, named the element "oxygen" and clarified its nature through experiments in the 1770s and 1780s.
Lavoisier debunked the phlogiston theory of combustion and respiration, demonstrating that oxygen is a distinct element.
He showed that oxygen is a crucial component of air and essential for combustion and respiration.
Lavoisier's work laid the foundation for modern chemistry, providing a clearer understanding of oxygen's role in chemical reactions.
The discovery of oxygen is a collective achievement attributed to Joseph Priestley, Carl Wilhelm Scheele, and Antoine Lavoisier.
0 notes
Text
Diferenta dintre acumulatorii NiMH si NiCad?
Popularitatea absoluta a acestor baterii reincarcabile ridică adeseori întrebarea „Care este diferența dintre bateriile NiMH și NiCad?”
Pe scurt, principalele diferente dintre acumulatorii NiMH si NiCad tin de capacitatea energetica marita cu 40%, dezvoltarea mai greoaie a efectului de memorie si compatibilitatea mai ridicata cu mediul. iluminat impotriva panicii
Acumulatorul nichel-metal hybrid are o capacitate energetica mai mare decat acumulatorul nichel-cadmiu, ceea ce inseamna ca poate genera o energie mai mare pentru consumatorul sau, fie ca o putere mai mare furnizata, fie ca un timp mai mare de lucru. orp iluminat antipanica
Un alt avantaj al acumulatorilor Ni-MH este ca nu sufera de efectul de memorie, adica “nu vor uita” abilitatea de a se incarca total de-a lungul timpului. corp de iluminat impotriva panicii
Si in final, acumulatorii NiMH sunt mult mai prietenosi cu mediul decat variantele NiCad.
Cu toate acestea acumulatorii NiCad au un avantaj major fata de NiMH, cum ar fi performanta la temperaturile extreme (uzual reci).
Acumulatorii nichel-metal hibrid (NiMH)
Acumulatorii NiMH au aparut mai tarziu decat NiCad, cercetarea si dezvoltarea lor a inceput in 1967 si a devenit satisfacatoare dupa mai bine de 20 ani in 1987. schema electrica iluminat de siguranta
Compozitia chimica a unui acumulator nichel-metal hibrid arata in felul urmator:
- un electrod pozitiv de hidroxid de nichel;
- un electrod negativ cu ioni de hidrogen;
- un separator;
- si un electrolit alcalin precum hidroxidul de potasiu.
Acumulatorii NiMH se utilizeaza frecvent in bateriile auto, instrumentele medicale, telefoane mobile, camera video, periute de dinti electrice si alte produse de larg consum.
Iata cateva dintre avantajele utilizarii unui acumulator NiMH:
· capacitate energetica mare in comparatie cu alte baterii reincarcabile;
· rezistent la ciclurile de supraincarcare si subdescarcare;
· usor ca si greutate;
· prietenos cu mediul in sensul ca nu exista chimicale periculoase precum cadmiu, mercur sau acid.
Iata si cateva dintre dezavantajele unui acumulator NiMH:
· mai scump decat alti acumulatori;
· descarcare rapida pe raft in stoc;
· terminarea brusca a energiei inmagazinate fata de o scurgere lenta;
· anumiti acumulatori se incarca doar cu incarcatorul producatorului.
Atentie! Acumulatorii NIMH sunt mai sensibili la temperaturile mari decat alti acumulatori.
Asadar e important sa fie tinuti la temperatura camerei si e de evitat sa fie expusi la temperaturi ridicate. Mai mult decat atat acumulatorii NiMH nu se compoarta asa bine la temperaturi joase, asadar e important sa fie mentinuti la o temperatura calduta atunci cand sunt folositi in medii cu temperatura joasa. iluminat de securitate pentru evacuare
Acumulatorii nichel-cadmiu (NiCd)
Acumulatorii NiCad au fost produsi in Suedia in 1899, dar de atunci au suferit imbunatatiri frecvente pana azi.
Bateriile nichel-cadmiu sunt reincarcabile, si sunt unele dintre cele mai vechi si cele mai utilizate baterii reincarcabile din piata.
Atentie, cadmiu este un metal toxic!
Un acumulator NiCad este compus din:
- un electrod oxid-hidroxid de nichel;
- un electrod negativ de cadmiu;
- un separator;
- si un electrolit alcalin precum hidroxidul de potasiu.
Acumulatorii NiCad se utilizeaza frecvent in jucarii, lampi iluminat siguranta, echipamente medicale, produse comericale si industriale, masini de ras, radio-uri, scule de mana electrice.
Iata cateva dintre avantajele utilizarii unui acumulator NiCad:
· relativ ieftine;
· incarcare simpla si rapida;
· transport si depozitare usoara;
· numar mare de cicluri de incarcare;
· functioneaza la temperaturi joase.
Unul dintre avantajele bateriilor NiCd este capacitatea lor de a fi reîncarcate de mai multe ori.
Au un ciclu de viata mare, ceea ce înseamna ca pot fi încarcate și descarcate în mod repetat, fara a-si pierde capacitatea de a stoca energie. lampa led cu kit emergenta
De fapt, bateriile NiCd pot fi reincarcate de sute, daca nu de mii, de ori.
Acest lucru le face o alegere excelentă pentru aplicatiile in care bateriile sunt utilizate frecvent și trebuie reîncarcate des.
Un alt avantaj al bateriilor NiCd este rata lor mare de descarcare.
Ele pot furniza o ieșire de curent mare, facandu-le potrivite pentru utilizarea în dispozitive care necesită multa putere, cum ar fi sculele electrice și masinile radiocomandate.
Bateriile NiCd au, de asemenea, o rata scazuta de auto-descarcare, ceea ce înseamna ca isi pot mentine încarcarea pentru o perioada lunga de timp, facandu-le ideale pentru aplicatii în care bateria nu poate fi utilizata pentru perioade lungi de timp.
Iata si cateva dintre dezavantajele unui acumulator NiCad:
· nu atat de puternic precum alti acumulatori;
· dezvolta efectul de memorie sau acumulator lenes (pierde treptat din capacitatea de stocare si necesita cicluri COMPLETE pentru descarcare);
· contine metale periculoase pentru mediu.
Cum sa incarcam acumulatorii Ni-Cd?
Incarcarea acumulatorilor nicehl-cadmiu (Ni-Cd) implica respectarea catorva pasi pentru a avea o incarcare sigura si eficienta.
Iata o regula generala pentru incarcarea acumulatorilor Ni-Cd.
Asigurati-va ca acumulatorul este complet descarcat inainte de incarcare.
Acest verificare va fereste de o supraincarcare, ce poate defecta acumulatorul.
Conectati incarcatorul la o sursa de energie si verificati tensiunea setata.
Ar trebui sa corespunda cu tensiunea nominala a acumulatorului Ni-Cd pe care doriti sa il incarcati.
Atasati incarcatorul la bornele acumulatorului, asigurandu-va de polaritatea corecta a terminalelor.
Porniti incarcatorul si monitorizati temperatura acumulatorului in timpul procesului de incarcare.
Acumulatorii Ni-Cd se pot incazli foarte mult in procesul de incarcare, asadar e important sa aruncati un ochi pe temperatura acumulatorului si sa evitati sa il incarcati prea mult.
Cand acumulatorul e pe deplin incarcat, deconectati incarcatorul si sursa de energie.
Pentru a evita efectul de memorie al acumulatorilor NiCd este recomandat ca acumulatorii sa fie complet descarcati si apoi complet reincarcati la intervale de timp regulate, in loc sa le reincarcati partial de mai multe ori.
0 notes
Text

Benefits of Mercury Batteries
Mercury batteries, also known as mercuric oxide batteries, were once commonly used in various applications due to their unique characteristics. However, they have largely been phased out and replaced with other battery technologies due to environmental concerns associated with mercury. Still, it's essential to understand the benefits and drawbacks of mercury batteries.
For more info<
https://dreamworksdirect.com/
0 notes
Text
How to Red Mercuric Oxide Online Buy and Find Reliable Acriflavine Suppliers in India: A Guide by Spa Corporation
Spa Corporation is a trusted name in the industry, known for providing high-quality chemicals and compounds to customers worldwide. With their extensive network and experience, they can help you source red mercuric oxide and reliable acriflavine suppliers in India efficiently and cost-effectively.
0 notes
Text
Cette journée fut placée sous le signe de l'eau ... Fort heureusement pour nous, pas trop sur le bout de notre nez, on passe entre les gouttes !

Nous sommes allées nous balader à la surface de la lune ce matin !

(j'y peux rien si c'est le nom officiel de la zone moi). On est sur une plaine avec un peu beaucoup d'activité géothermique, des geysers (maintenant éteints), des explosions partout, ça a donc fait des jolis trous ... La légende maori raconte que la zone est devenue volcanique suite à une légère sur-reaction de deux déesses quand leur frère leur a dit qu'il avait froid.


Petit rappel sur la création des volcans : en simplifié, la couche océanique passe sous une autre. En plongeant, la croûte fond, ça fait du magma, et sous la pression ça remonte et finit par faire un joli volcan !

Ici, on est dans une zone avec beaucoup d'eau prisonnière sous la croûte : elle monte à plus de 300°C (différence de pression oblige, c'est l'inverse d'essayer de faire cuire des patates en haute montagne), crée de la vapeur, et finit par trouver une faille et s'y engouffrer, faisant des grosses fumerolles ! Si elle ne trouve pas d'échappatoire, la pression monte monte monte, et ça finit par exploser!

La zone avait plein de très belles couleurs : en fonction du type de minéraux/éléments présents dans les fumerolles, les différents cratères n'avaient pas les mêmes teintes. Ça allait du violet (mercure) au saumon (sulfate de mercure) en passant par le jaune orange (soufre ou soufre + arsenic ), ou le rouge (oxide de fer)! En plus, si les températures ne sont pas trop élevées, il y a des végétaux qui poussent là dedans (lol), ça faisait donc de jolis contrastes avec le vert !

Bref, une petite matinée fort sympathique! On est ensuite tombées sur un magasin de souffleur de verre, aux couleurs juste incroyables. Bon, les prix aussi étaient un peu incroyables (32000$ pour un gros vase incroyable), donc on s'est contentées de baver. On nous a fort gentiment offert l'accès gratuitement aux jardins, où il y avait plein d'oeuvres d'exposées !

Après un petit échec d'accès à une autre balade en zone geothermale (le typhon de début d'année a laissé des séquelles conséquentes partout, ici 150 arbres au milieu du chemin), mais qui s'est soldée par la rencontre d'un lapin-singe (Sisi, les yeux de Clochette sont excellents), d'un papi farceur (qui m'a mis un paquet entre les doigts en me faisant croire que c'était des oeufs de tarentule géante), de poules suicidaires se cachant sous les roues, de lamas déguisés en moutons et de dindes visiblement fort intéressées par la potentialité d'un goûter, nous avons pu confirmer que mes six mois de vadrouille ont fortement amélioré mon système digestif. Clochette a moins apprécié le poulet bizarre du déjeuner, ce qui ne nous a finalement pas empêché de profiter du reste de la journée !

Nous voici donc aux chutes d'eau d'Aratiatia, où nous sommes arrivées juste à temps pour l'ouverture du barrage, où nous sommes donc passés d'un ruisseau à sec à un énorme torrent entraînant tout sur son passage ... Très très impressionnant ! Et toujours cette couleur bleue absolument magnifique

Et là, on est posées tranquilles au bord d'un lac, bien au chaud (en doudoune/polaire/écharpe dans le sac de couchage et sous la couette) dans le van alors qu'il pleut dehors ! Pas de mission toilettes ce soir ><
Bizouuuuux
1 note
·
View note
Text
Are Skin Brightening Products Effective?
A shopping point that was launched not long agone
in Saudi Arabia, the United Arab Emirates and Egypt. It's a veritably strong store that offers products at the stylish prices in the request, and you may indeed find prices for some products that are better and cheaper than the same product in the store under your house.
Assuming that you wish to buck up or ease up your skin hue, spots, age spots, skin inflammation marks, dull underarms, melasma, or your general skin tone, also, at that point, this will be the main composition you will at any point read.
Skin réclame, skin easing up, and skin fading are the acts of exercising conflation or regular substances to ease up complexion or negotiate an indeed skin coloring by lessening the melanin preoccupation in the skin. Melanin is the shade that gives mortal skin, hair, and eyes their variety. Melanin is delivered by cells called melanocytes. It gives some assurance against skin detriment from the sun, and the melanocytes gain their creation of melanin in light of sun openness.
By exercising skin easing- up strategies, unambiguous zones of unexpectedly high achromatism, for illustration, spots, intelligencers, and hue might be depigmented to match the encompassing skin. likewise, in cases of vitiligo, innocent skin might be eased up to negotiate a further steady appearance.
Utmost skin- easing up medicines, which can reduce or hinder some measure of melanin creation, is directed toward repressing tyrosinase. Tyrosinase is an oxidizing protein, passing in the plant and creature towels, that faves up the oxidation of tyrosine into melanin and different tones. Hindering tyrosinase action decreases the union of melanin so exceptionally that skin cells generally slip keratinocytes with lower melanin at the end brought to the face, giving the skin a lighter, all further indeed conditioned composition.
There are numerous conversations and negative good impacts skin cheering particulars. There's substantiation to propose that numerous kinds of skin- cheering particulars use dynamic seasoning( like mercurous chloride) and hydroquinone which can be hurtful; however, 2012 deals of skin easing up creams in India alone added up to 258 tons and the worldwide request for skin lighteners is projected to communicateUS$19.8 billion by 2018 given deals development constitutionally in the US, Asia, Africa, and the Center East. In this way, there is an immense request for skin- cheering particulars.
numerous regular hotspots for skin easing up are voluntary lighteners got from normal wellsprings of hydroquinone. unadulterated types of arbutin are viewed as further strong for impacting skin easing up. Arbutin is gotten from the leaves of bearberry, cranberry, mulberry, or blueberry backwoods, and likewise is available in multitudinous feathers of pears. It can have melanin- hindering parcels. Arbutin and other plant removes are viewed as defended options in distinction to regularly employed depigmenting specialists to make the skin more affable. Clinical examinations have shown the proficiency of arbutin for skin easing up.
Make certain to explore any item you endlessly use particularly regular particulars that are 100 percent assured!
For more info :-
how to make ugly feet pretty
strongest skin bleach
Source Url :- https://www.diigo.com/item/note/999mt/0m5o?k=b97fb9f3f9e15b9d89aee9469adccd81
0 notes
Text
Here are 10 fascinating facts about mercury chemical
Mercury is the main metal that is fluid at standard temperature and tension. The main fluid component under standard circumstances is bromine (a halogen), albeit the metals rubidium, cesium, and gallium liquefy at a temperature simply above room temperature. Mercury has an extremely high surface pressure, so it structures adjusted dots of fluid.
Despite the fact that mercury and every one of its mixtures is known to be profoundly harmful, it was viewed as helpful all through quite a bit of history.
The advanced component image for mercury is Hg, which is the image for one more name for mercury: hydrargyrum. Hydrargyrum comes from Greek words for "water-silver" (hydr-implies water, Argyros implies silver).
Mercury is an extremely uncommon component in the World's outside layer. It represents just around 0.08 parts per million (ppm) and is chiefly viewed as in the mineral cinnabar, which is mercuric sulfide. Mercuric sulfide is the wellspring of the red color called vermilion.
Mercury, for the most part, isn't permitted on airplanes since it consolidates so promptly with aluminum, a metal that is normal on airplanes. At the point when mercury shapes a mixture with aluminum, the oxide layer that safeguards aluminum from oxidizing is disturbed. This makes aluminum consume similarly to iron rusts.

Mercury doesn't respond with most acids.
Mercury is a moderately unfortunate guide of intensity. Most metals are magnificent warm conduits. It is a gentle electrical transmitter. The edge of freezing over (- 38.8 C) and limit (356 C) of mercury are nearer together than any remaining metals.
In spite of the fact that mercury generally shows a +1 or +2 oxidation state, once in a while it has a +4 oxidation state. The electron design makes mercury act fairly like an honorable gas. Like honorable gases, mercury shapes somewhat feeble synthetic bonds with different components. Its structures blends with a wide range of various metals aside from iron. This settles on iron as a decent decision to fabricate compartments for holding and shipping mercury.
The component mercury is named for the Roman god Mercury. Mercury is the main component to hold its catalytic name as its advanced normal name. The component was known to old human advancements, tracing all the way back to something like 2000 BCE. Vials of unadulterated mercury have been tracked down in Egyptian burial places from the 1500s BCE.
Mercury is utilized in fluorescent lights, thermometers, float valves, dental mixtures, and medication, for the creation of different synthetics, and to make fluid mirrors. Mercury(II) explode is an unstable utilized as groundwork in guns. The sanitizer mercury compound thimerosal is an organomercury compound found in immunizations, tattoo inks, contact focal point arrangements, and beauty care products.
For more info:-
Mercury Element For Sale Usa
Mercury Chemical Element For Sale
1 note
·
View note
Text
Alcohol functional group

Other nucle-ophiles such as alcohols, acids, and hydrogen peroxide Gives higher yields and cleaner product mixtures because rearrangements are The advantage is that the mercuric electrophileįorms a bridged or partially bridged intermediate that is stabilized and hence Serves as a surrogate for the proton during the addition step and is replacedīy hydrogen in the reduction. Reductive removal of mercury with NaBH 4. For example, the preferred way to produce the Markovnikov alcoholįrom an olefin is to use hydroxymercuration for the addition step followed by Instead methods are used which permit much greater regiochemical control andĪre milder. However, simpleĪcid-catalyzed addition of water is often the least desirable alternative. Thus a common method for the preparation of alcohols. Addition of water across an olefinic dou-ble bond is Aldehydes give primary alcohols while ketonesĪnd olefins are at the same oxidation level and are interconvertible without aĬhange in oxidation level. Milder than LAH and does not require aprotic conditions (an alcohol is often As such it provides a selective way to reduce acidsĪnd produce alcohols in the presence of most other functional groups.Īnd ketones are conveniently reduced by sodium borohydride, which is much Increases the reactivity of the boron hydride and delivers the hydride by an Unique reactivity comes from the fact that borane first forms a Lewis acid –īase complex with the acid and then a boron – carboxylate intermediate which With carboxylic acids than with esters or other acid derivatives. Reduced easily by borane, which is the only reducing agent that reacts faster Carboxylic acids, but not esters, are also Reductive methods have been reported for the preparation of alcohols.Ĭarboxylic acids and esters react vigorously with lithium aluminum hydride Groups and consequently are readily prepared by reduction. Methods for the preparation of alcohols are still among the most usefulĪre at a fairly low oxidation level compared to other oxygen-containing functional The alcohol group, but the alcohol group can be prepared from many other groups and converted to many functional groups. Not only do many important compounds and pharmaceuticals contain Not only do many important compounds and pharmaceuticals contain the alcohol group, but the alcohol group can be prepared from many other groups and converted to many functional groups.Īlcohol functional group is a very important functional group in organicĬhemistry. The alcohol functional group is a very important functional group in organic chemistry. Naming phenols can be done in many ways.Ĭontinue reading about the other functional groups.Chapter: Organic Chemistry : Functional Group Synthesis When the hydroxyl group is bonded directly to a benzene ring, the compound is classified as a phenol. If it is bonded to three other carbons, it is a tertiary (3 o) alcohol. If this carbon is bonded to two other carbons, it is a secondary (2 o) alcohol. If this carbon is bonded to one other carbon atom, it is a primary (1 o) alcohol. Just as with alkenes, alkynes, and ketones, the location of the hydroxyl group is made by numbering the molecule such that the hydroxyl group has the lowest number possible.Īlcohols are subdivided by examining the carbon to which the hydroxyl group is bonded. Structure Example Compound Official Name of ExampleĪ hydroxyl group is a hydrogen bonded to an oxygen that is covalently bonded to the rest of the molecule.

0 notes