#payload linker
Explore tagged Tumblr posts
Text

How to Choose the Right Linker Payload for Your Research Project – Precise PEG Learn how to select the best linker payload for stability, targeting, and efficacy in drug development, ADCs, and bioconjugation. Optimize your research results today!
0 notes
Text
Global Antibody Drug Conjugates Market Expected to Achieve Consistent 9% CAGR Expansion by 2030
The antibody drug conjugates (ADCs) market is projected to grow at a CAGR of ~9% over the forecast period. Major factors driving growth include the growing prevalence of cancer, increasing demand for targeted cancer therapies, advancement in ADC technology, growing investment in R&D for introducing new antibody-drug conjugates, and increasing approvals by regulatory bodies such as the FDA and EMA for ADCs. However, the market encounters certain challenges, including the high cost of development, stringent regulatory requirements that make the approval process lengthy and expensive, and increasing competition from emerging therapies.
Antibody-drug conjugates or ADCs are a novel class of highly potent biopharmaceuticals consisting of a monoclonal antibody chemically linked to a biologically active drug or cytotoxic compound. These targeted therapies harness the precise targeting capabilities of antibodies, enabling accurate differentiation between healthy and cancerous tissues, while delivering the cell-killing effects of cytotoxic agents. An ideal ADC has:
A highly selective monoclonal antibody (mAb) for a tumor-associated antigen that has restricted or no expression on normal (healthy) cells
A potent cytotoxic agent (generally a small molecule drug with high systemic toxicity) designed to induce target cell death after being internalized in the tumor cell and released
A linker that is stable in circulation, but releases the cytotoxic agent in target cells
Discover the more details-Download the PDF brochure:
Growing prevalence of cancer is expected to drive market growth
Cancer remains one of the leading causes of death globally. According to GLOBOCAN 2024, in 2022, nearly 20 million new cancer cases were reported, alongside 9.7 million cancer-related deaths worldwide. Therapeutic approaches for treating cancer or tumors include chemotherapy, immunotherapy, radiation therapy, stem cell therapy, laser treatment, hyperthermia, surgery, and photodynamic therapy, among others. Among these, chemotherapy remains the primary treatment method.
However, antibody-drug conjugates (ADCs) are rapidly gaining attention due to their unique capability to combine targeted therapy with potent cytotoxic agents while leaving healthy cells unharmed. This innovative approach provides a promising solution for treating various types of cancers, including those resistant to conventional therapies. To date, 14 ADCs have received market approval for the treatment of different cancers, with over 200 currently undergoing clinical development. Further, ADCs are revolutionizing cancer treatment by offering a highly specific mechanism of action, delivering targeted and effective therapy, and bringing new hope to cancer patients worldwide.
Technological advancements in antibody-drug conjugate (ADC) technology to propel market growth
Continuous advancements in the design and development of antibody-drug conjugates (ADCs) have significantly improved their efficacy and safety, making them increasingly attractive as treatment options. These innovations span several critical areas, including antibody engineering, linker technology, cytotoxic payloads, and manufacturing processes.
One breakthrough is the adoption of site-specific conjugation techniques, which provide precise control over the drug-to-antibody ratio, enhancing the consistency and therapeutic performance of ADCs. Another key innovation is the development of novel linkers that respond to specific conditions, such as the low pH in the tumor microenvironment, enabling targeted drug release and reducing off-target toxicity. Additionally, advances in antibody engineering and selection have led to the creation of antibodies with enhanced binding specificity, affinity, and pharmacokinetics, further boosting the potency and effectiveness of ADCs. Improvements in manufacturing technologies, such as the integration of single-use systems, have addressed some of the production challenges, increasing efficiency and scalability. As research in ADCs continues to progress, even more sophisticated and effective technologies are expected to emerge, broadening the scope of their applications.
Competitive Landscape Analysis
The global antibody drug conjugates (ADCs) market is marked by the presence of established and emerging market players such as Takeda Pharmaceutical Company Ltd.; AstraZeneca PLC; F. Hoffmann-La Roche Ltd.; Pfizer, Inc.; AbbVie’s; Gilead Sciences, Inc.; Merck & Co.; ADC Therapeutics SA; Bolt Biotherapeutics; Mersana Therapeutics; and Daiichi Sankyo Company Ltd.; among others. Some of the key strategies adopted by market players include new product development, strategic partnerships and collaborations, and investments.
Unlock key findings! Fill out a quick inquiry to access a sample report
Global Antibody Drug Conjugates (ADCs) Market Segmentation
This report by Medi-Tech Insights provides the size of the global antibody drug conjugates (ADCs) market at the regional- and country-level from 2023 to 2030. The report further segments the market based on product, application, target, and technology.
Market Size & Forecast (2023-2030), By Product, USD Million
Kadcyla
Enhertu
Adcetris
Padcev
Trodelvy
Polivy
Others
Market Size & Forecast (2023-2030), By Application, USD Million
Breast Cancer
Blood Cancer
Other Cancers
Market Size & Forecast (2023-2030), By Target, USD Million
HER2
CD22
CD30
Others
Market Size & Forecast (2023-2030), By Technology USD Million
Linker Type
Cleavable Linker
Non-cleavable Linker
Linker less
Payload Technology
MMAE/ Auristatin
Maytansinoids
Camptothecin
Others
Market Size & Forecast (2023-2030), By Region, USD Million
North America
US
Canada
Europe
UK
Germany
Italy
Spain
Rest of Europe
Asia Pacific
China
India
Japan
Rest of Asia Pacific
Latin America
Middle East & Africa
About Medi-Tech Insights
Medi-Tech Insights is a healthcare-focused business research & insights firm. Our clients include Fortune 500 companies, blue-chip investors & hyper-growth start-ups. We have completed 100+ projects in Digital Health, Healthcare IT, Medical Technology, Medical Devices & Pharma Services in the areas of market assessments, due diligence, competitive intelligence, market sizing and forecasting, pricing analysis & go-to-market strategy. Our methodology includes rigorous secondary research combined with deep-dive interviews with industry-leading CXO, VPs, and key demand/supply side decision-makers.
Contact:
Ruta Halde Associate, Medi-Tech Insights +32 498 86 80 79 [email protected]
0 notes
Text
🌟 𝐓𝐫𝐚𝐧𝐬𝐟𝐨𝐫𝐦𝐢𝐧𝐠 𝐂𝐚𝐧𝐜𝐞𝐫 𝐓𝐫𝐞𝐚𝐭𝐦𝐞𝐧𝐭: 𝐓𝐡𝐞 𝐑𝐢𝐬𝐞 𝐨𝐟 𝐀𝐧𝐭𝐢𝐛𝐨𝐝𝐲 𝐃𝐫𝐮𝐠 𝐂𝐨𝐧𝐣𝐮𝐠𝐚𝐭𝐞𝐬 (𝐀𝐃𝐂𝐬) 🌟-IndustryARC™
The Antibody Drug Conjugate Market size is estimated to reach $15275 million by 2030, growing at a CAGR of 14.20% during the forecast period 2024-2030.
👉 𝐃𝐨𝐰𝐧𝐥𝐨𝐚𝐝 𝐒𝐚𝐦𝐩𝐥𝐞
𝐇𝐞𝐫𝐞 𝐚𝐫𝐞 𝐬𝐨𝐦𝐞 𝐤𝐞𝐲 𝐟𝐢𝐧𝐝𝐢𝐧𝐠𝐬 𝐟𝐫𝐨𝐦 𝐭𝐡𝐞 𝐫𝐞𝐩𝐨𝐫𝐭
𝐀𝐝𝐯𝐚𝐧𝐜𝐞𝐦𝐞𝐧𝐭𝐬 𝐢𝐧 𝐓𝐚𝐫𝐠𝐞𝐭𝐞𝐝 𝐓𝐡𝐞𝐫𝐚𝐩𝐲: ADCs represent a targeted approach to cancer therapy, allowing for selective delivery of cytotoxic drugs to cancer cells, reducing damage to healthy cells and enhancing treatment effectiveness. This shift towards targeted therapies is a major trend in oncology.
𝐆𝐫𝐨𝐰𝐢𝐧𝐠 𝐏𝐢𝐩𝐞𝐥𝐢𝐧𝐞 𝐨𝐟 𝐀𝐃𝐂𝐬: Pharmaceutical companies are actively developing ADCs, with a growing number of ADC candidates in clinical trials. Increased R&D investments and strategic collaborations are fueling the expansion of ADC pipelines, particularly for solid tumors and hematologic cancers.
𝐈𝐧𝐜𝐫𝐞𝐚𝐬𝐢𝐧𝐠 𝐅𝐃𝐀 𝐀𝐩𝐩𝐫𝐨𝐯𝐚𝐥𝐬 𝐚𝐧𝐝 𝐑𝐞𝐠𝐮𝐥𝐚𝐭𝐨𝐫𝐲 𝐒𝐮𝐩𝐩𝐨𝐫𝐭: Regulatory bodies are accelerating approvals for ADCs due to their effectiveness and safety profile in cancer treatment. Recent approvals of ADCs, such as those targeting breast and bladder cancers, have driven further interest and investment.
𝐓𝐞𝐜𝐡𝐧𝐨𝐥𝐨𝐠𝐢𝐜𝐚𝐥 𝐈𝐦𝐩𝐫𝐨𝐯𝐞𝐦𝐞𝐧𝐭𝐬 𝐢𝐧 𝐋𝐢𝐧𝐤𝐞𝐫 𝐓𝐞𝐜𝐡𝐧𝐨𝐥𝐨𝐠𝐲: Innovations in linker technology, which attaches the antibody to the drug payload, are enhancing ADC stability and precision. Advanced linkers improve the therapeutic index, enabling more controlled drug release and minimizing off-target effects.
𝐅𝐨𝐜𝐮𝐬 𝐨𝐧 𝐍𝐨𝐯𝐞𝐥 𝐏𝐚𝐲𝐥𝐨𝐚𝐝𝐬: ADCs are moving beyond traditional cytotoxic agents, incorporating novel payloads such as immune modulators and DNA-damaging agents. These novel payloads expand ADC applications and offer enhanced potency against resistant cancer cells.
𝐄𝐱𝐩𝐚𝐧𝐬𝐢𝐨𝐧 𝐢𝐧𝐭𝐨 𝐍𝐨𝐧-𝐎𝐧𝐜𝐨𝐥𝐨𝐠𝐲 𝐀𝐩𝐩𝐥𝐢𝐜𝐚𝐭𝐢𝐨𝐧𝐬: While oncology remains the primary focus, ADCs are increasingly being explored for autoimmune and infectious diseases. This diversification presents new opportunities and broadens the market’s scope beyond cancer.
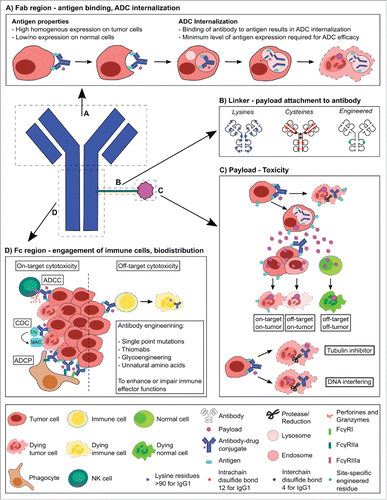
#antibodydrugconjugates#oncology#cancertherapy#targetedtherapy#precisionmedicine#cancerresearch#biotechnology#drugdevelopment#pharma#cancerimmunotherapy#biopharma#noveltherapeutics#tumortargeting#personalizedmedicine
0 notes
Text
Explore the growing Antibody Drug Conjugates (ADC) market, including industry trends, key players, growth factors, and future projections. Discover the role of ADCs in cancer therapy and other medical applications.
Key Trends in the ADC Market
Increased Cancer Prevalence The rising incidence of cancers worldwide is a major factor driving the demand for innovative treatments. As the number of cancer patients grows, the need for more effective and less invasive therapies, such as ADCs, becomes crucial.
Technological Advancements in ADC Development Recent innovations in linker technology, drug payloads, and antibody engineering are enhancing the effectiveness and safety of ADCs. Improved stability and the ability to target more cancer cell types are key factors fueling market growth.
Focus on Targeted Therapies The shift towards personalized medicine is propelling the growth of targeted therapies like ADCs. By targeting specific cancer cell markers, ADCs can offer better precision, fewer side effects, and improved patient outcomes.
Partnerships and Collaborations Major pharmaceutical companies and biotech firms are increasingly collaborating to develop next-generation ADCs. Strategic partnerships are helping to overcome the complex challenges involved in ADC development, accelerating the time-to-market for new therapies.
Regulatory Approvals In recent years, several ADCs have gained approval from regulatory bodies like the FDA and EMA. This trend has led to greater market confidence and expansion, as more ADC-based treatments become available for patients.
Key Drivers of Growth
Rising Cancer Cases The global cancer burden is growing due to factors such as aging populations, lifestyle changes, and environmental factors. ADCs provide an effective solution for treating a variety of cancers, including breast cancer, lung cancer, and blood cancers like leukemia and lymphoma.
R&D Investment Increased investment in research and development (R&D) by pharmaceutical companies is fostering the creation of more advanced ADCs. The exploration of new targets, payloads, and drug delivery systems is likely to expand the market significantly in the coming years.
Emerging Markets As healthcare infrastructure improves in emerging markets, there is growing access to novel therapies like ADCs. The increasing availability of cutting-edge treatments in regions such as Asia-Pacific and Latin America is expected to drive the global market.
0 notes
Text
o2h is currently seeking an experienced Sr. Research Associate - Anti Drug Conjugates (ADC) for their Ahmedabad, Gujarat site. This role focuses on the synthesis, purification, and characterization of complex ADC molecules, making it an ideal opportunity for professionals with a background in organic chemistry and ADC chemistry. Job Overview Company: o2h Location: Ahmedabad, Gujarat, India Position: Sr. Research Associate – Anti Drug Conjugates (ADC) Experience: 2-5 years in ADC chemistry Qualifications: MSc or M.Pharm in Organic Chemistry If you have a passion for synthesizing and purifying antibody drug conjugates (ADC) and experience in contract research organizations (CROs), this position could be your next career move. Key Responsibilities As a Sr. Research Associate in the ADC department, your role will focus on: Synthesis and Purification of complex antibody drug conjugate (ADCs) molecules. Synthesis of different type of linkers - using natural and un-natural amino acids, PEGs Synthesis of high potent drug molecules – payloads Purification of complex and large molecules by flash column and HPLC. Handling various type chemistry with different payloads Interpretation and Characterization of higher Molecular Weight Compounds [caption id="attachment_66270" align="aligncenter" width="930"] o2h Group Recruitment Alert[/caption] Qualifications and Skills To qualify for this role, candidates should meet the following requirements: Educational Qualification: MSc or M.Pharm in Organic Chemistry or a related field. Experience Technical Expertise: 2-5 years of experience in ADC chemistry from the CRO industry Adherence to safe practices and procedures. Contribution to the development of safety protocols and systems. Completion of mandatory training related to data integrity, health, and safety. Must have good manual dexterity and demonstrate careful attention to detail. Interpersonal skills to interact effectively with people in other departments. Must be able to prioritize work responsibilities to meet deadlines. Must possess a great deal of integrity to maintain confidentiality. How to Apply Interested candidates are encouraged to apply by submitting their resumes through the o2h careers portal. To explore more details about the role and the company, click here.
0 notes
Text
The Global ADC Contract Manufacturing Market To Grow Significantly Due To Increasing Demand For Personalized Medicine

The Global ADC Contract Manufacturing market involves developing antibody drug conjugates (ADC) for pharmaceutical and biotechnology companies. ADCs are monoclonal antibodies that are attached to cytotoxic compounds or drugs to selectively target and kill cancer cells. The market is driven by the need for personalized medicine in the treatment of chronic diseases like cancer.
Global ADC Contract Manufacturing Market is estimated to be valued at US$ 1.79 Billion in 2024 and is expected to exhibit a CAGR of 13% over the forecast period 2024-2031.
Key players operating in the Global ADC Contract Manufacturing market include AbbVie Contract Manufacturing, Abzena, CARBOGEN AMCIS, Catalent Pharma Solutions, and Cerbios-Pharma. These players specialize in developing ADCs using various linker-drug combinations and conjugation technologies to maximize the potency and efficacy of the antibody drug conjugate. The Global ADC Contract Manufacturing Market is driven by the increasing demand for ADCs in cancer treatment due to their ability to target specific cancer antigens with high precision. Technological advancements in bioconjugation techniques have also improved the therapeutic index of ADCs.
Key Takeaways
Key players: Key players operating in the Global ADC Contract Manufacturing market are AbbVie Contract Manufacturing, Abzena, CARBOGEN AMCIS, Catalent Pharma Solutions, and Cerbios-Pharma.
Growing demand: The increasing demand for personalized medicine in cancer treatment is a major driver of the Global ADC Contract Manufacturing market. ADCs allow for targeted delivery of cytotoxic drugs to tumor cells while minimizing adverse effects on non-cancerous cells.
Technological advancement: Technological innovations in areas such as site-specific conjugation, optimal linker-payload combinations, and conjugation processes have improved the selectivity and efficacy of ADCs. These advancements are expected to increase the clinical potential of ADCs and fuel demand for contract manufacturing.
Market Trends
Consolidation of players: Large players are acquiring smaller players to expand their capabilities across various antibody engineering and ADC technologies. This allows for a one-stop-shop for end-to-end ADC development and manufacturing services.
Outsourcing of manufacturing: Pharma companies prefer outsourcing ADC manufacturing to contract manufacturers to avoid investments in facility setup and specialized expertise. This is a major trend driving the growth of contract manufacturing.
Market Opportunities
Development of solid tumor ADCs: Most approved ADCs target hematological cancers. Further development of ADCs for solid tumors like lung, breast and prostate cancer presents key opportunities.
Manufacturing of next-gen ADCs: Innovation in linker technologies, conjugation methods, and novel cytotoxic payloads can yield so-called "next-generation" ADCs with improved stability, tolerability and efficacy. This provides opportunities for contract manufacturers.
Impact Of COVID-19 On Global ADC Contract Manufacturing Market:
The COVID-19 pandemic had a significant impact on the global ADC contract manufacturing market. During the initial outbreak in early 2020, production and manufacturing facilities were shut down due to strict lockdown measures imposed globally. This led to major disruptions in supply chains and logistics networks. The demand for ADC therapeutics also declined initially due to the diversion of resources towards managing the crisis. However, as the pandemic intensified, emphasis on developing effective treatments grew substantially. This stimulated research activities into antibody-drug conjugates for COVID-19. Several pharma companies partnered with contract manufacturers to expedite their development.
As lockdowns eased from mid-2020, contract manufacturers steadily scaled up operations while implementing stringent safety protocols. The demand regained momentum as clinical trials entered late-stage testing. By late 2020, contract manufacturers worked round the clock to meet surge in demand. Their production capacities were under immense pressure to deliver on timely commitments. Ensuring workforce safety and minimizing disruptions remained key priorities. Looking ahead, partnerships are expected to increase further to enhance manufacturing throughput. Advanced manufacturing technologies will help optimize processes and flexibility. Efforts to diversify supplier networks can strengthen resilience against future public health emergencies.
Geographical Regions With Highest ADC Contract Manufacturing Value:
North America remains the dominant regional market for ADC contract manufacturing in terms of value. This is primarily due to strong presence of leading pharma companies and contract service providers in the US. Countries like the US have witnessed approvals and launch of several ADC drugs in cancer therapy in recent years. This has propelled demand for their large-scale production via reliable contract routes. Growing pipeline of ADC candidates entering clinical trials also offers lucrative opportunities. Meanwhile, Asia Pacific has emerged as the fastest growing regional market driven by expanding biotech industries in China, India, South Korea and others. Improving regulatory environment and lower production costs have encouraged companies to outsource to Asia Pacific contract manufacturers.
Fastest Growing Regional Market:
Asia Pacific is poised to be the fastest growing regional market for ADC contract manufacturing during the forecast period. This is attributed to increasing investments by international drug makers into the biologics manufacturing infrastructure of Asia Pacific nations. Countries like China and India offer skilled workforce, well-developed supply chains and strong government support for biopharma sector. Additionally, Asia Pacific contract manufacturers have enhanced their technological expertise to meet stringent quality standards. Their expanding service portfolios covering complex conjugation and analytical testing attract more collaborations. These factors are facilitating Asia Pacific's rise as a valuable outsourcing destination, thus driving the fastest growth of its regional ADC contract manufacturing market.
Get more insights on this topic: https://www.pressreleasebulletin.com/global-adc-contract-manufacturing-market-is-estimated-to-witness-high-growth-owing-to-advancement-in-drug-development-technologies/
Author Bio:
Money Singh is a seasoned content writer with over four years of experience in the market research sector. Her expertise spans various industries, including food and beverages, biotechnology, chemical and materials, defense and aerospace, consumer goods, etc. (https://www.linkedin.com/in/money-singh-590844163 )
What Are The Key Data Covered In This Global ADC Contract Manufacturing Market Report?
:- Market CAGR throughout the predicted period
:- Comprehensive information on the aspects that will drive the Global ADC Contract Manufacturing Market's growth between 2024 and 2031.
:- Accurate calculation of the size of the Global ADC Contract Manufacturing Market and its contribution to the market, with emphasis on the parent market
:- Realistic forecasts of future trends and changes in consumer behaviour
:- Global ADC Contract Manufacturing Market Industry Growth in North America, APAC, Europe, South America, the Middle East, and Africa
:- A complete examination of the market's competitive landscape, as well as extensive information on vendors
:- Detailed examination of the factors that will impede the expansion of Global ADC Contract Manufacturing Market vendors
FAQ’s
Q.1 What are the main factors influencing the Global ADC Contract Manufacturing Market?
Q.2 Which companies are the major sources in this industry?
Q.3 What are the market’s opportunities, risks, and general structure?
Q.4 Which of the top Global ADC Contract Manufacturing Market companies compare in terms of sales, revenue, and prices?
Q.5 Which businesses serve as the Global ADC Contract Manufacturing Market’s distributors, traders, and dealers?
Q.6 How are market types and applications and deals, revenue, and value explored?
Q.7 What does a business area’s assessment of agreements, income, and value implicate?
*Note: 1. Source: Coherent Market Insights, Public sources, Desk research 2. We have leveraged AI tools to mine information and compile it
#Global ADC Contract Manufacturing Market Trend#Global ADC Contract Manufacturing Market Size#Global ADC Contract Manufacturing Market Information#Global ADC Contract Manufacturing Market Analysis#Global ADC Contract Manufacturing Market Demand
0 notes
Text
Growing Antibody-Drug Conjugates Market Owing to Rising Demand for Targeted Cancer Therapy.

Antibody-drug conjugates (ADCs) are a type of bioconjugate consisting of monoclonal antibodies that are attached by chemical linkers to highly potent anti-cancer payloads. ADCs selectively target antigens that are highly expressed on tumor cells while sparing normal tissues through the use of antibodies. Linkers attached between the antibody and cytotoxic drug allow for the drug to be delivered unchanged until it reaches the intended tumor site, minimizing harm to healthy cells. ADCs have demonstrated clinical efficacy in treating various cancers including lymphoid malignancies, breast cancer, and solid tumors.
The Global Antibody-Drug Conjugates Market is estimated to be valued at US$ 5.38 Bn in 2024 and is expected to exhibit a CAGR of 14% over the forecast period 2023 to 2030. Key Takeaways: Key players operating in the Antibody-Drug Conjugates are AstraZeneca PLC, Daiichi Sankyo Company, Limited, Novasep, ADC Therapeutics SA, Alentis Therapeutics AG, F. Hoffmann-La Roche, Gilead Sciences, Inc., AbbVie Inc., Biosion USA, Inc., Astellas Pharma Inc., Duality Biologics (Suzhou) Co. Ltd., BioNTech SE, LaNova Medicines Ltd., Bliss Biopharmaceutical, Eisai Co., Ltd., ProfoundBio, Pfizer, Inc., ImmunoGen Inc., Mersana Therapeutics Inc., Sorrento Therapeutics Inc., Oxford BioTherapeutics Ltd, and Takeda Pharmaceutical Company Ltd. Growing demand for targeted cancer therapy with minimal side effects is expected to drive significant growth of the ADC market over the forecast period. Additionally, ongoing technological advancements in linker chemistry, increasing pipeline products and approvals are further fueling the market growth. Market Trends: The ADC market is witnessing increasing adoption of cleavable linkers that are stable in circulation but rapidly release the drug payload intracellularly upon internalization into target tumor cells. Additionally, the development of novel conjugation technologies such as DBCO-azide click chemistry is allowing for site-specific conjugation without effect on bioactivity and efficacy of ADCs. Market Opportunities: The significant opportunities in the ADC market are in developing ADCs for liquid and solid tumor indications with unmet medical needs. Additionally, optimization of physiochemical properties of molecules to improve pharmacokinetics is another key area that ADC developers are increasingly focusing on to enhance therapeutic index and efficacy of ADCs.
#Antibody Drug Conjugates Market Share#Antibody Drug Conjugates Market Growth#Antibody Drug Conjugates Market Analysis
0 notes
Text
The demand for antibody drug conjugate was valued at USD 9984.20 million in 2023 and is expected to reach USD 26594.21 million in 2032, growing at a CAGR of 11.50% between 2024 and 2032.The Antibody-Drug Conjugate (ADC) market represents a groundbreaking intersection of biotechnology and pharmacology, offering a novel approach to cancer treatment. ADCs are sophisticated biopharmaceuticals designed to deliver cytotoxic drugs directly to cancer cells, minimizing damage to healthy tissues. This targeted therapy approach has gained substantial attention in recent years due to its potential to enhance the efficacy and safety profiles of cancer treatments. As the ADC market continues to expand, it is poised to revolutionize oncology and drive significant advancements in personalized medicine.
Browse the full report at https://www.credenceresearch.com/report/antibody-drug-conjugate-market
Market Overview
The global ADC market has witnessed rapid growth, driven by increasing cancer prevalence, technological advancements in drug development, and rising demand for targeted therapies. According to recent market research, the ADC market was valued at approximately USD 4.8 billion in 2022 and is expected to reach over USD 12 billion by 2028, growing at a compound annual growth rate (CAGR) of 16.7% during the forecast period.
This growth is fueled by the rising incidence of cancer worldwide, coupled with the limitations of conventional cancer therapies, such as chemotherapy and radiation, which often result in severe side effects. ADCs, by contrast, offer a more precise mechanism of action, delivering cytotoxic agents directly to cancer cells while sparing healthy tissues. This specificity reduces adverse effects and improves patient outcomes, making ADCs a promising option in the oncology landscape.
Technological Advancements
Advancements in ADC technology have been pivotal in driving market growth. Early-generation ADCs faced challenges such as low therapeutic indices, off-target toxicities, and limited efficacy. However, recent innovations have addressed these issues, leading to the development of more stable linkers, improved antibody engineering, and the use of highly potent cytotoxic agents.
Modern ADCs utilize cleavable linkers that release the drug payload only in the presence of specific enzymes or conditions within the cancer cell, ensuring targeted drug delivery. Additionally, advancements in monoclonal antibody engineering have enhanced the ability of ADCs to bind selectively to tumor-specific antigens, further improving their therapeutic potential.
Key Market Players
Several pharmaceutical companies are at the forefront of the ADC market, investing heavily in research and development to bring new ADCs to market. Notable players include:
1. Seagen Inc.: Known for its pioneering work in ADCs, Seagen’s ADC technology has led to the successful development of drugs like Adcetris, used in the treatment of Hodgkin lymphoma and other CD30-expressing lymphomas.
2. Roche: A global leader in oncology, Roche has developed Kadcyla, an ADC used in the treatment of HER2-positive breast cancer. Kadcyla combines the HER2-targeting properties of trastuzumab with a cytotoxic agent, offering a targeted approach to treating this aggressive form of breast cancer.
3. AstraZeneca: With the development of Enhertu, an ADC for HER2-positive breast cancer, AstraZeneca has further solidified its position in the ADC market. Enhertu’s unique design allows it to deliver a higher drug-to-antibody ratio, enhancing its therapeutic efficacy.
Challenges and Opportunities
Despite the promising outlook, the ADC market faces several challenges. The complexity of ADC development, high production costs, and stringent regulatory requirements can hinder market growth. Additionally, issues related to drug resistance and the need for personalized approaches to treatment pose ongoing challenges.
However, these challenges also present opportunities for innovation. Companies are exploring new ADC technologies, such as bispecific ADCs that target multiple antigens, and the use of alternative payloads to overcome drug resistance. Furthermore, ongoing research into biomarker-driven patient selection is expected to enhance the precision of ADC therapies, aligning them with the principles of personalized medicine.
Future Outlook
The future of the ADC market looks promising, with continued advancements in technology and a growing pipeline of ADC candidates in clinical trials. As more ADCs receive regulatory approval and enter the market, the adoption of this targeted therapy is expected to increase, offering new hope to cancer patients worldwide.
Moreover, the expanding application of ADCs beyond oncology, such as in the treatment of autoimmune diseases, presents additional growth opportunities. As research in this area progresses, ADCs may become a cornerstone of targeted therapy across various therapeutic areas.
Key Players
Seagen Inc.
Takeda Pharmaceutical Company Ltd.
AstraZeneca Plc.
F. Hoffmann-La Roche Ltd.
Pfizer Inc.
ImmunoGen Inc.
Gilead Sciences Inc.
Daiichi Sankyo Company Ltd.
Segmentation
By Product
Kadcyla
Enhertu
Adcetris
Padcev
Trodelvy
Polivy
Others
By Disease Type
Breast Cancer
Blood Cancer
Others
By Linker Type
Non-Cleavable
Cleavable
By Target
HER2
CD22
CD30
Others
By Payload Type
MMAE/Auristatin
Calicheamicin
Maytansinoids
Others
By Region
North America
The U.S
Canada
Mexico
Europe
Germany
France
The U.K.
Italy
Spain
Rest of Europe
Asia Pacific
China
Japan
India
South Korea
South-east Asia
Rest of Asia Pacific
Latin America
Brazil
Argentina
Rest of Latin America
Middle East & Africa
GCC Countries
South Africa
Rest of the Middle East and Africa
Browse the full report at https://www.credenceresearch.com/report/antibody-drug-conjugate-market
About Us:
Credence Research is committed to employee well-being and productivity. Following the COVID-19 pandemic, we have implemented a permanent work-from-home policy for all employees.
Contact:
Credence Research
Please contact us at +91 6232 49 3207
Email: [email protected]
Website: www.credenceresearch.com
0 notes
Text
Antibody-Drug Conjugates Market: A Targeted Approach to Cancer Treatment
The fight against cancer is relentless, and researchers are constantly innovating to develop more effective therapies. Antibody-drug conjugates (ADCs) have emerged as a promising class of drugs with the potential to revolutionize cancer treatment. This article delves into the ADC market, exploring its current landscape, future prospects, and key driving forces.
Understanding ADCs: A Marriage of Precision and Power
ADCs are ingenious biopharmaceutical weapons. They combine the targeting ability of monoclonal antibodies with the cytotoxic power of potent chemotherapy drugs. The monoclonal antibody acts like a smart missile, recognizing and binding to specific antigens on cancer cells. A chemical linker then connects the antibody to the cytotoxic payload. Once inside the cancer cell, the linker releases the payload, triggering cell death with minimal harm to healthy tissues.
Market on the Move: A Booming Landscape
The ADC market is experiencing significant growth. Estimates suggest the global market reached a value of $9.7 billion in 2023 and is projected to reach $19.8 billion by 2028, with a healthy CAGR (Compound Annual Growth Rate) of 15.2%. This surge is attributed to several factors, including:
Rising Cancer Rates: The global burden of cancer is undeniably increasing. This fuels the demand for effective treatment options, propelling the ADC market.
Enhanced Efficacy: ADCs offer a targeted approach, potentially leading to improved treatment outcomes and reduced side effects compared to traditional chemotherapy.
Expanding Pipeline: Pharmaceutical Antibody-Drug Conjugates market companies are actively developing new and improved ADCs, with a robust pipeline of candidates in various stages of clinical trials.
Favorable Regulatory Landscape: Regulatory bodies are increasingly recognizing the potential of ADCs, leading to faster approvals and market access.
Market Segmentation: A Multifaceted Landscape
The ADC market can be segmented based on various factors, including:
Target: ADCs can target a wide range of cancer types, with HER2-positive breast cancer and B-cell malignancies being some of the initial targets. Research is ongoing to explore their efficacy against other cancers.
Payload: The cytotoxic payload is a crucial component of ADCs. Different payloads offer varying mechanisms of action and potency.
Linker Technology: The linker plays a vital role in ensuring the stability of the ADC during circulation and the efficient release of the payload within the target cell. Advancements in linker technology are critical for developing next-generation ADCs.
Region: The ADC market is geographically diverse, with North America currently holding the largest share. However, the Asia Pacific region is expected to witness the fastest growth due to factors like rising disposable income and increasing healthcare awareness.
Challenges and Considerations: A Path Forward
Despite the promising outlook, the ADC market faces certain challenges. Manufacturing ADCs is a complex process, and ensuring their efficacy and safety requires rigorous testing. Additionally, the high cost of development and treatment can be a limiting factor.
Buy the Full Report for More Insights on the Key Marketed Drugs in the ADC Market
Download a Free Sample Report
0 notes
Text
Performed felled
Durability/performance/ safety/Se
Related infections
Result
transmission rates
• prove the case
• Me bought is posible in what store Instrumentive 90% in the intuiticion Patient injury
I Endoscopy design
small convention sgap expected contamination In 0%
Sign and sinthomy
Risk of contamination
For not cime constipation new scope 10%
now one solution what is 5the future hold
Señas de proves
Novel washing modalitié Table top
& Some remove biofil
other modalities Capaple o esterilazation long lumens, in develoments
*pinzas *electrolisis
*
sponge
*Refrigator
*Pale Cleaner
China Karten
*Desinfection termica- baño de maria
*alcohol
*Miltrex Instrument
*Surgery cleaner
*desinfection for methods chemystre
*evaporation
*Germicide[ Glutaraldehido; peroxido de hidrogeno y acido peritico
Cloruro mg
Sales de sodio
Silicato ppm
Valor ph
Acido citrico
Fosforicos
Orto-phalardehido
Peroxido de hidrogeno
Acido peracético
Miltex instrument
Surgery Jeaner
Destinacion por metodo
Circulo de Sinner
resistence corrosive presencia el agua
potable
Desalinizacion unsesfully del ag
Sal regenerator dragged ion exchangers
Comprobar con Norma Iso 10993 -3 Evaluacion Biologica de productos sanitarios
Level Antidrug concentracion high Concentration can result increase toxicity Low result in efficary biologics immunogenicity non- I ENF
Commonly performen other situation in
ransomized of considered dosie an adition
of therapy drug monitorizing
Ciclosporine an tacroliti-mas user organ
trasplation
Dancomycin angentamycin in sepsia
The presentation regarder show the monotherapy and study How is needly the cortecosteroide and others transition
considerations in the treatment inclusive options treatment
Conmeeting have the wormen virgin period its premenospause
Maintenance drops, for induction and derivates of quimiotherapy of that list of medicament is necesary for the study and benefict of patient pharmaeconomic benefict.
Potencial TDM and STRC
Redused in drug concentration
Monitorizing Drug with inmunohilatores to antibodys of immunogenocity..
Certolizumab pegol >20
Golimumab post induction > 10
Vedolizumab > 3
Ustekinomab > 5
Cledolizumab week >2,4 10 Cledoligimen. week 2,8 post induction, 8, 3, 5
of week 14 levels prediting duabiling
Sustained
PIFX Dose Dashboard
For Symphony of reflux electrodo-up the next quipement
One consicered
GERD = Infectious System To prevent rejection of organ transplant recipients
Hermozone: Cream with metamethazone conicoids: gel fusicor
Gel fusico GERD = Infectious System To prevent rejection of organ transplant recipients
What are antibodys - Drug congugates monoclonal antibodys Linked to a cytotoxic drug des to -n the therapeulic window by focusing a delivery to specifics cells.
*Tumor antigen *binding site
*monoclonal antibody
* selective for antigeno
with high copy numbers in target tumor celk with minimal inmyngenic response.
Linkers Can be cleavable (a temo Cells, Linkers Can be cleabank via tumor-
associated factors) or non-cleavable (via tumor associated factor of monel cavable (Lysosomalal degration
Payload:enhances cytoxicity all Thoughes and septicity although antibody raty afecct and clearance
Antibody bind target antigeno. Internalized → → payload releas +
bystander gel
ADCS may circulate as dynamic mixture of contact conjugate, naked antibodys,and
free payload
ACDS Reach tumors via capiliares releasing somepayload in micro
enviroment ass diffuse toward target
Any's independing tacles
Antibody engagement payload -
independent antitumor activity
Disruption o receptor dimerization and
funtion leads to disruption ofdownstream signaling
Antibody Drug congugates: Mechanism of action
• what are antibodys - Drug Congutgates + Monoclonal antidays Linked To a
CYTOTOXIC drog designes to ovide the therapevtic window by focusing a delivery To specific cells
Turmor antigen the hy
•Antibody binds target antigen Internalized 7 Payload release ADCS may circulate as dynamic mixture of context congujate, naked antibodys,
Conjugate, naked antibody, and free payload
ADCS reach tumors via capiliares releasing some payload in tumor micro enviroment aa diffuse toward target
ADCS: More than the Sum of their parts
Efective in heavily pretreated patient of those who have resistance to treatment antibody or chemotherapy components may be due to a superior therapeutic index Components vary, eg, FDM1 vs I-Dxd! vs Additive benefit from best. bystander Effect
Identifying the mechanist of effacey is challenging given multiple contributing part
ACDS toxicityies
Grade 3/4 Neutropenia by ADC on targeted ADC pivatuzumab off target an off tumor more difusse payload release inadverted
cellular uptake.
Anemia, neutropenia seen with many ADCS unknow pulmonaty toxicitys with many ADCS unknow pulmonaty with t-Dxd
THC imunohistochemistry
predictive biomarkers for ADCs hard to establish
•Where will ADCs for within the current
treatment Porading for lung cancer?
• Consideration of ADCs in combination with agent thar increase APC delivery modulate target antigen any or for internalization Promove antitumor immunity,
in comon, up Be complex bypass infysion guideline Opideline (ACD proteins)
by an antibody drug conjugate 's wely expressed IGFR mutation with con monosiclive tumor with adquired resistance To EGFR Targeted is Antigen is the ADC ressistention to antibodies
monociclive from tumor cell via endoson recicly prior to payload release Minimum issues chemotherapy or quimiotherapy
Halganior promore antllemor
is Antigen is the ADC. ressistention to Antibodies monactive From Tumor cell vier end es gon feciding, prior to praylord Teles
Lover is the thored meal for cance
example Urothelia Camer
Whal I describe of Her 3 itsel
Lmmunohibidor
HER3 DX d
operacion (Surgery 2 operasion (Surgery)
By pas asi minth
The atmen
I operacion (Sunger) operasion (Surgery,
By pas asiaminib
fuimia her afte -reatmen is overall 3 Mon
inimum issues
Insugnificance issues
Chemotherapy or Quimiotherapy Mechanism of resistance, to osimetris
Toaclina
Water
Witch GI is no have phrase
Hiz incidence of lineage plasticity including both small cell and squamous transformation by Frequent adquired gene alteration (eg, gene fusion)
Anticipate a role for non biomarker select in therapies in setting of osirmertinib resistance
Progresion Oh Osimertinib subpectos
• obtein brain MRI and chest/abdomen/ pelvis Ct
•Radiographic progressing lesion and submit for biomarker testing consider Liquid biopsy if tissue biopsy not feasible
but less sensitive for fusion/
transformations.
• Oligome ometastatic continue TX with addition of log ablative
•Slows and/or asymptomatic. Continue to beyond progression
•
CNS only. If Limited, continue tx with addition
if diffuse transition to CNS-penetrant Systemic
Progression on Osimerinibs. Supped to
Obtein brain MRI and Chess/aboomen, peluk- Pabgraphy of progresing confirmed! Consider whether special circumstances are obtein tissue present
obiopsy of progresing lesion and Submit for biomarker testing: consider liquid biopsy of tissue biopsy nor feaside, but
less sensitive for fusion/ transformation
bypass pathways
⚫met amplification
⚫clinical trial of Combined EGFR/MET
Inhibition
• Osimetinib + Met inhibitor (eg, crizotinibtinib, capmatinib
Fusion clinical trial
RET: OsimerTinibt
Ret Inhibitor (eg, Pralsetinib selpercatinib
ARK: Osimertive +ALK TIKE
Ros1 osimertinibt ROSTRI
Anexo.
maintenance Therapy observation
Osimetinib is almost in clinical
Considerated
is the standar of care Chemo Therapy (Histology, driven, platinum-based
Long Cancer
Anemia >
EGFR
PHASE III
Pembrolizumab - Carboplatin /
permetraxes.co alone in Advanced NSCLC Vs Chemotherapy add EGFR mutations with L858R or del19 EFRG mutations after EGFR IKI Therapy
mechanism or fusion of therapy
transformation of histology teatretment, follow up with, X Ray, abdomen of confirmed Progress
1First chemotherapy
2 on therapy I will aproximing
3 hemoestasis
4 Metastasis
trombotic Nervious sistem
Adjuvant treatmen in deumatic diseases o Spookify your armets, Juvenile reumatoid arthritis, post-traumatic Osteoarthritis, etc.) or in exacerbation of rheumatoid psoriatic arthrit.
Peace dose gradually avoid disease High doses decrease song every 1-2 days Weeks up to 18mg gay 19 Average dose 20-60mg day decreasdz by 5mg by 5.g
Starting at 10 ming day, decrease by 2,5 mg every 1-2 weeks.
mild dermal and Join Outbreaks Can be enough Short Courses of Cortecosteroids evidence how to be the construction in future of organ trasplnt in benefic of surge
What Phase no Have metatasis.
Ciclofosfamida
Classic Indications
Vasculitis Sistemicas con afectation and organs remopulmonary syndrome
Nefropatia Lupica Tipo III, IV Alveolitis fibrosane of multiple sclerosis sistemica
In monarche patient with hormone receptor (HR)-positive/HER2-mode- positive high risk EBC and previous
surgery and radiotherapy an indicated and adjuvant chemotherapy were randomize to receive adjuvant endocrine Therapy with or without adjuvant abemaceclir for two year. After a median follow of 19.1 month
treatmen with aberacidib up plus endocrine therapy significantly reduced the risk of an invasive desease-free
survival (IDFS) event hazan ratio 0,71, 95% Cl: 0,58-082; P=01) The benefit was even greater in patient with a Ki-67=20% (Harzard ratio 0.69, 95% CI: 052-092
Lon biopsy confirms invasive ductal carcinome estrogen receptor (ER) Positive, progesterone keeptor PR positive and HER2 negative Ki-67- Scome was 60% Germline mutation testing confirms no patogeny Vananita She plans to underjo Meoadjuvant Chemotherapy followed by
surgery
Guideline Recommended Biomaker testing For Breast Cancer
[on biopsy confirms invasive ductal carcinome estrogen receptor (ER) positive, progesterone keeptor positive and HER2 negative K1-67- Scone was 60% Germline noutation testing confins no patogeny Varianita She plans to undergo
Medadjuvant Chemotherapy followed by
surgery
Guideline Recommended Biomarker testing For Breast Cancer
Breast cancer receptor status Should be determinated as part of the diagnostic Workup for patient worth newly aliagnosed or current breast cancer, including Here statue by inmonohistochemistry with or witho Fluorescence in situ hybridization if needed and HR Status including ER and PR F3. several gene expres assays are avalaible that can help predict risk of recurrence and response to systemic therapy More recently germine mutation resting has been incorporated"
manregements of £BC, especif booking for BREA mutation To quide at uvant Herapy Select For patient with Her 2 negative breast cancer. "Las
me asurement of the Ki-67 antigen can be helpful when Considery specific targeted the rapies Such as aberma why the begen of breast tumlr like drive to cancer
Among pation with metas
breast cancer approximately 50% will desselop brain To F
metastaser managing brain metastases is a critical Before the last year
part of caring the population - There were no agent that Where no feven to be to treat central nervous metastases and result improved outcome me
Optimising systemic therapy for patient with Herz Pos. The early breast cancer
based den deseas charac-
terist and risk
0 notes
Text
Peptide linkers for Antibody-drug conjugates (ADCs)
Antibody-drug conjugates (ADCs) are a type of targeted cancer therapy that combines the specificity of monoclonal antibodies with the cytotoxic effects of drugs. Peptide linkers play a crucial role in ADC design, as they connect the antibody and the cytotoxic payload, facilitating controlled release of the drug within the target cells. The choice of linker can impact stability, drug release kinetics, and overall efficacy of the ADC. The choice of a specific linker depends on factors such as the pharmacokinetics of the ADC, the desired release mechanism, and the characteristics of the drug payload. It's crucial to balance stability in circulation with efficient drug release at the target site to maximize the therapeutic effect of the ADC. Additionally, advancements in linker technology continue to contribute to the development of novel and improved ADCs for cancer therapy.
The advantage Of Peptide Linker
1. Biological Compatibility
Peptide linkers are composed of natural amino acids, which are biocompatible and less likely to induce an immune response. This can contribute to the overall safety profile of the ADC.
2. Specificity and Selectivity
Peptide linkers can be designed to incorporate specific cleavage sites for proteases that are overexpressed in the target cells. This allows for selective drug release within the tumor microenvironment, enhancing the therapeutic window.
3. Stability in Circulation
Peptide linkers can be engineered for stability in the bloodstream, minimizing premature drug release during circulation. This stability is crucial for maintaining the integrity of the ADC and preventing off-target effects.
4. Tunable Pharmacokinetics
The properties of peptide linkers, such as their size and hydrophilicity, can be fine-tuned to influence the pharmacokinetics of the ADC. This tunability allows for optimization of drug delivery and distribution in vivo.
5. Ease of Synthesis
Peptide synthesis techniques are well-established, making it relatively straightforward to design and produce peptide linkers. This ease of synthesis contributes to the scalability and cost-effectiveness of ADC manufacturing.
6. pH Sensitivity
Some peptide linkers can be designed to be pH-sensitive, allowing for drug release in the acidic environment of endosomes or lysosomes within target cells. This pH responsiveness enhances the specificity of drug delivery to cancer cells.
7. Multifunctionality
Peptide linkers can be engineered to have multiple functions, such as facilitating site-specific conjugation, improving solubility, or enhancing overall stability. This versatility contributes to the design of ADCs with optimized properties.
8. Well-Characterized Cleavage Mechanisms:
Proteolytic cleavage of peptide linkers by cellular proteases is a well-characterized biological process. This predictability allows for a better understanding of the drug release mechanism and facilitates rational design of ADCs.
0 notes
Text
Global Antibody Drug Conjugates Market Expected to Achieve Consistent 9% CAGR Expansion by 2030
The antibody drug conjugates (ADCs) market is projected to grow at a CAGR of ~9% over the forecast period. Major factors driving growth include the growing prevalence of cancer, increasing demand for targeted cancer therapies, advancement in ADC technology, growing investment in R&D for introducing new antibody-drug conjugates, and increasing approvals by regulatory bodies such as the FDA and EMA for ADCs. However, the market encounters certain challenges, including the high cost of development, stringent regulatory requirements that make the approval process lengthy and expensive, and increasing competition from emerging therapies.
Antibody-drug conjugates or ADCs are a novel class of highly potent biopharmaceuticals consisting of a monoclonal antibody chemically linked to a biologically active drug or cytotoxic compound. These targeted therapies harness the precise targeting capabilities of antibodies, enabling accurate differentiation between healthy and cancerous tissues, while delivering the cell-killing effects of cytotoxic agents. An ideal ADC has:
A highly selective monoclonal antibody (mAb) for a tumor-associated antigen that has restricted or no expression on normal (healthy) cells
A potent cytotoxic agent (generally a small molecule drug with high systemic toxicity) designed to induce target cell death after being internalized in the tumor cell and released
A linker that is stable in circulation, but releases the cytotoxic agent in target cells
Discover the more details-Download the PDF brochure:
Growing prevalence of cancer is expected to drive market growth
Cancer remains one of the leading causes of death globally. According to GLOBOCAN 2024, in 2022, nearly 20 million new cancer cases were reported, alongside 9.7 million cancer-related deaths worldwide. Therapeutic approaches for treating cancer or tumors include chemotherapy, immunotherapy, radiation therapy, stem cell therapy, laser treatment, hyperthermia, surgery, and photodynamic therapy, among others. Among these, chemotherapy remains the primary treatment method.
However, antibody-drug conjugates (ADCs) are rapidly gaining attention due to their unique capability to combine targeted therapy with potent cytotoxic agents while leaving healthy cells unharmed. This innovative approach provides a promising solution for treating various types of cancers, including those resistant to conventional therapies. To date, 14 ADCs have received market approval for the treatment of different cancers, with over 200 currently undergoing clinical development. Further, ADCs are revolutionizing cancer treatment by offering a highly specific mechanism of action, delivering targeted and effective therapy, and bringing new hope to cancer patients worldwide.
Technological advancements in antibody-drug conjugate (ADC) technology to propel market growth
Continuous advancements in the design and development of antibody-drug conjugates (ADCs) have significantly improved their efficacy and safety, making them increasingly attractive as treatment options. These innovations span several critical areas, including antibody engineering, linker technology, cytotoxic payloads, and manufacturing processes.
One breakthrough is the adoption of site-specific conjugation techniques, which provide precise control over the drug-to-antibody ratio, enhancing the consistency and therapeutic performance of ADCs. Another key innovation is the development of novel linkers that respond to specific conditions, such as the low pH in the tumor microenvironment, enabling targeted drug release and reducing off-target toxicity. Additionally, advances in antibody engineering and selection have led to the creation of antibodies with enhanced binding specificity, affinity, and pharmacokinetics, further boosting the potency and effectiveness of ADCs. Improvements in manufacturing technologies, such as the integration of single-use systems, have addressed some of the production challenges, increasing efficiency and scalability. As research in ADCs continues to progress, even more sophisticated and effective technologies are expected to emerge, broadening the scope of their applications.
Competitive Landscape Analysis
The global antibody drug conjugates (ADCs) market is marked by the presence of established and emerging market players such as Takeda Pharmaceutical Company Ltd.; AstraZeneca PLC; F. Hoffmann-La Roche Ltd.; Pfizer, Inc.; AbbVie’s; Gilead Sciences, Inc.; Merck & Co.; ADC Therapeutics SA; Bolt Biotherapeutics; Mersana Therapeutics; and Daiichi Sankyo Company Ltd.; among others. Some of the key strategies adopted by market players include new product development, strategic partnerships and collaborations, and investments.
Unlock key findings! Fill out a quick inquiry to access a sample report
Global Antibody Drug Conjugates (ADCs) Market Segmentation
This report by Medi-Tech Insights provides the size of the global antibody drug conjugates (ADCs) market at the regional- and country-level from 2023 to 2030. The report further segments the market based on product, application, target, and technology.
Market Size & Forecast (2023-2030), By Product, USD Million
Kadcyla
Enhertu
Adcetris
Padcev
Trodelvy
Polivy
Others
Market Size & Forecast (2023-2030), By Application, USD Million
Breast Cancer
Blood Cancer
Other Cancers
Market Size & Forecast (2023-2030), By Target, USD Million
HER2
CD22
CD30
Others
Market Size & Forecast (2023-2030), By Technology USD Million
Linker Type
Cleavable Linker
Non-cleavable Linker
Linker less
Payload Technology
MMAE/ Auristatin
Maytansinoids
Camptothecin
Others
Market Size & Forecast (2023-2030), By Region, USD Million
North America
US
Canada
Europe
UK
Germany
Italy
Spain
Rest of Europe
Asia Pacific
China
India
Japan
Rest of Asia Pacific
Latin America
Middle East & Africa
About Medi-Tech Insights
Medi-Tech Insights is a healthcare-focused business research & insights firm. Our clients include Fortune 500 companies, blue-chip investors & hyper-growth start-ups. We have completed 100+ projects in Digital Health, Healthcare IT, Medical Technology, Medical Devices & Pharma Services in the areas of market assessments, due diligence, competitive intelligence, market sizing and forecasting, pricing analysis & go-to-market strategy. Our methodology includes rigorous secondary research combined with deep-dive interviews with industry-leading CXO, VPs, and key demand/supply side decision-makers.
Contact:
Ruta Halde Associate, Medi-Tech Insights +32 498 86 80 79 [email protected]
0 notes
Text
Bioconjugation Service
Bioconjugation Service at AxisPharm
Bioconjugation is the process of linking molecules/polymers or biomolecules/biopolymers together by covalent bonds. Conjugation can enhance drug efficacy by improving pharmacokinetic and pharmacodynamic properties and by reducing plasma protein binding. For instance, introducing polyethylene glycol (PEG) or polysaccharide reduces toxicity and improves solubility and stability.
AxisPharm offer our clients with affordable and high quality custom bioconjugation service that covers:
Early de-risking and optimization
Process development and optimization
Formulation development
Analytical method development
Drug substance manufacture
Drug product manufacture
Payload and linker manufacturing
Our bio conjugation service delivers affordable and high-quality custom bioconjugation service, using combinations of payloads, linkers, and conjugation methods.
Antibody-Drug Conjugates (ADCs)
Antibody-Oligonucleotide Conjugates (AOCs)
Protein-drug Conjugation
Fluorophore Conjugation (products: fluorescent dyes)
Biotin conjugation (products: biotin peg)
Polymer–drug conjugation (products: polymer peg)
Polymer-drug-target ligand conjugation
PEGylation and cross-linking
Cysteine-based conjugation
Lysine-based conjugation
Thio-engineered antibody
Carbohydrate-based conjugation
Unnatural amino acids-based conjugation (product: amino peg)
Analytical – AxisPharm has the analytical tools and experience that are critical for this field. We are equipped with sophisticated mass spectrometers for small and large molecules to analyze and quantify protein, antibody and their conjugates. We routinely use high resolution mass spectrometer on real time analysis of drug-antibody ratio for bioconjugation synthesis, process optimization and quality control. Please click for real time analysis case study.
Synthetic Chemistry and Biology Expertise – AxisPharm has a large collection of ADC linker, such as PEG linker, Peptide Linker, Sugar Liner, Dye Probe in stock and has extensive experience in synthesis, purification and characterization of both small and large molecules to support bioconjugation R&D.

1 note
·
View note
Text
Shaping the Future of Cancer Care: Antibody Drug Conjugates Market Dynamics and Trends
Introduction
The Antibody Drug Conjugates (ADCs) market is a dynamic and rapidly growing sector in the pharmaceutical and biotechnology industries. ADCs represent a novel approach to cancer therapy, combining the specificity of monoclonal antibodies with the potency of cytotoxic drugs. This synergy has led to significant advancements in the treatment of various cancers, and the market is witnessing a surge in research, development, and investment. In this article, we will delve into the Antibody Drug Conjugates market, exploring its current state, key players, challenges, and future prospects.
Understanding Antibody Drug Conjugates
Antibody Drug Conjugates are a class of targeted therapies designed to deliver cytotoxic drugs directly to cancer cells while sparing healthy tissues. The concept is elegantly simple: a monoclonal antibody is engineered to recognize and bind to a specific antigen expressed on the surface of cancer cells. This antibody is then conjugated to a potent cytotoxic drug, creating a highly selective and effective therapeutic agent.
Current Market Landscape
The ADC market has been steadily growing over the past decade, and it is projected to continue its upward trajectory. One of the primary drivers of this growth is the increasing prevalence of cancer worldwide. As the global cancer burden rises, there is an urgent need for more effective and less toxic treatments, making ADCs an attractive option.
Key Players and Developments
Several pharmaceutical and biotechnology companies have made significant investments in ADC research and development. Key players in the ADC market include Roche, Seattle Genetics, Immunomedics (now part of Gilead Sciences), and Daiichi Sankyo. These companies have brought several ADCs to market and have a robust pipeline of candidates in various stages of development.
One of the groundbreaking developments in the ADC field is the approval of Trodelvy (sacituzumab govitecan-hziy) by the U.S. Food and Drug Administration (FDA) in 2020. Trodelvy, developed by Immunomedics, has shown remarkable efficacy in treating triple-negative breast cancer and metastatic urothelial cancer, marking a significant milestone in ADC therapy.
Challenges and Opportunities
Despite the promising potential of ADCs, there are several challenges that the market faces. One of the primary challenges is the complexity of ADC development. Designing and manufacturing these molecules requires a deep understanding of antibody engineering, linker chemistry, and drug payload selection. Additionally, the regulatory landscape for ADCs is evolving, which can pose hurdles in the approval process.
Another challenge is the cost of ADC therapy. These drugs are often expensive, and there is a need for greater accessibility to ensure that patients can benefit from this advanced treatment. As more ADCs gain approval and competition increases, there is hope that pricing may become more competitive.
The future of the ADC market holds immense promise. Advancements in antibody engineering, linker technology, and drug payloads are expected to improve the safety and efficacy of ADCs. Moreover, ongoing research is exploring the potential of ADCs in treating a broader range of cancer types and even other diseases beyond oncology.
Conclusion
The Antibody Drug Conjugates market is a dynamic and evolving sector in the field of cancer therapy. With a growing number of ADCs gaining approval and a robust pipeline of candidates, there is a sense of optimism about the future of cancer treatment. As researchers and pharmaceutical companies continue to innovate, the potential for ADCs to revolutionize the way we approach cancer therapy remains high. While challenges persist, the progress made in this field is a testament to the dedication of scientists and the hope they bring to cancer patients worldwide. As we look ahead, the Antibody Drug Conjugates market is poised to play a pivotal role in the fight against cancer, offering new possibilities for improved patient outcomes and quality of life.
0 notes