#solvothermal process
Text
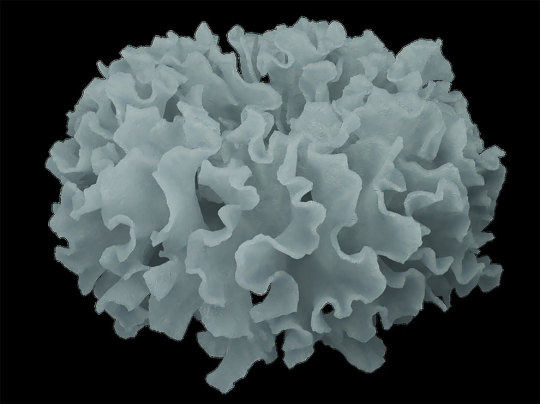
These cabbage-like nickel phosphate particles have been synthesised by a solvothermal process. Not suitable for Chiko Roll filler.
Photograph: Valentino Kaneti/AIBN
2023 Australian Institute For Bioengineering And Nanotechnology Image Contest
#valentino kaneti#photographer#australian institute for bioengineering and nanotechnology image contest#micro photography#cabbage-like nickel phosphate particles#solvothermal process#nature
10 notes
·
View notes
Photo

Nickel nanowires enhance microwave absorption, study finds
In a study published in Advanced Materials Interfaces, a research team led by Prof. Wang Hui and associate Prof. Sheng Zhigao from the Hefei Institutes of Physical Science (HFIPS) of the Chinese Academy of Sciences reported the synthesis of polyvinylpyrrolidone-directed nickel nanowires (PNNWs) via solvothermal method assisted by a high magnetic field, and applied them to enhance microwave absorption.
Among many microwave absorbers that have been studied, one-dimensional magnetic nanowires have attracted much attention because of their excellent mechanical properties, large aspect ratio and excellent electronic transmission performance. However, the smooth surface and insufficient magnetism of nickel nanowires synthesized by traditional methods inhibit the process of electromagnetic wave (EW) passing through the absorber and dissipating as heat energy. Therefore, it is urgent to find a new method to increase the surface roughness and magnetic properties of nickel nanowires and to enhance their microwave absorption performance.
In this study, the researchers used solvothermal method induced by external magnetic field to synthesize PNNWs, and the morphology and properties of PNNWs showed a magnetic field strength-dependent relationship.
Read more.
16 notes
·
View notes
Text
Activated Carbon’s Use in Gold Recovery
With the use of magnetic activated carbon produced via a solvothermal synthesis, gold may be recovered from cyanide solutions easily and sustainably.
The removal of organic impurities with activated carbon is noted as successful.
A type of carbon that has been treated to contain low-volume holes that enhance the surface area accessible for adsorption or chemical reactions is known as activated carbon.

How does the gold cyanidation process work with the activated carbon?
1. C02 in Pulp
When the ore is finely ground, the gold is dissolved using a cyanide leach inside the tank, and activated carbon is added to the leached slurry. The gold will be extracted from the slurry solution by the activated carbon, and the slurry will then be separated from the slurry by coarse screening.
2. Leach with Carbon
When activated carbon is introduced to a leach solution, a process known as carbon in leach (CIL) occurs. Gold is then adsorbed while being dissolved in a cyanide solution. Before any leaching takes place, activated carbon is applied.
3. C02 in Column
In comparison to the first two approaches, carbon in a column, or CIC, uses a distinct procedure. placing enormous columns filled with the gold cyanide solution
There are several kinds of activated carbon with special properties for specific purposes.
4. Adsorptive Power (known as K-value)
This is the maximum amount that activated carbon can take in.
ACTIVATED CARBON FOR GOLD RECOVERY: ITS CHARACTERISTICS
Rate of Adsorption (known as R-value):
The physical and chemical characteristics of the activated carbon have an impact on the rate of adsorption.
Mechanical sturdiness and hardness/wear resistance:
Utilizing mechanical strength to prevent attrition losses that can result in carbon loss and loss of recoverable gold
Size Distribution of Particles:
Another important consideration while selecting activated carbon for gold recovery is the adsorption rate.
We manufacture and supply activated carbon products in India.
For additional information about our activated carbons, PAC (powdered activated carbon), GAC (granular activated carbons), and EAC (extruded activated carbons/pellets), get in touch with us.
Get in touch with us.
Contact no: +91 7383224411
#activated carbon#activated carbon in India#activated carbon manufacturer in India manufacturer#activated carbon manufacturer
1 note
·
View note
Text
New Post has been published on Vivan Life Sciences
New Post has been published on https://blog.vivanls.com/1694-2/

According to researchers from Purdue University in the US, polyethylene in plastic bags could be an inexpensive source of energy-storing carbon.
Scientists have created a way to convert plastic bags into carbon chips that could be used in batteries powering our smartphones and other devices. Plastic bag pollution has become a huge environmental problem, prompting some cities and countries to heavily tax or ban the sacks.
Many plastic bags are used only once and then disposed, ending up in landfills, oceans and elsewhere in the environment, where they can take hundreds of years to decompose. According to researchers from Purdue University in the US, polyethylene in plastic bags could be an inexpensive source of energy-storing carbon.
However, previous methods to upcycle polyethylene into pure carbon have been inefficient or required expensive, complex processes. The team wanted to develop a simpler yet efficient approach to convert plastic waste into useful carbon-containing materials.
The researchers immersed polyethylene plastic bags in sulphuric acid and sealed them inside a solvothermal reactor, which heated the sample to just below polyethylene’s melting temperature.
This treatment caused sulfonic acid groups to be added to the polyethylene carbon-carbon backbone so that the plastic could be heated to a much higher temperature without vapourising into hazardous gases.
Then, they removed the sulphonated polyethylene from the reactor and heated it in a furnace in an inert atmosphere to produce pure carbon. The team ground the carbon into a black powder and used it to make anodes for lithium-ion batteries. The resulting batteries performed comparably to commercial batteries.
Reference: Indian Express
0 notes
Photo

Jun Jiao and Kavita Meduri, Department of Mechanical and Materials Engineering faculty, co-authored an article titled, “Unique Structural Characteristics of Catalytic Palladium/Gold Nanoparticles on Graphene”, published in Microscopy and Microanalysis, January 2019.
Adding Au to Pd nanoparticles (NPs) can impart high catalytic activity with respect to hydrogenation of a wide range of substances. These materials are often synthesized by reducing metallic precursors; hence, sonochemical and solvothermal processes are commonly used to anchor these bimetals onto thin supports, including graphene. Although similar NPs have been studied reasonably well, a clear understanding of structural characteristics relative to their synthesis parameters is lacking, due to limitations in characterization techniques, which may prevent optimization of this very promising catalyst. In this report, a strategic approach has been used to identify this structural and material synthesis correlation, starting with controlled sample preparation and followed by detailed characterization. This includes advanced scanning transmission electron microscopy and electron energy loss spectroscopy; the latter using a state-of-the-art instrumentation to map the distribution of Pd and Au, and to identify chemical state of the Pd NPs, which has not been previously reported. Results show that catalytic bimetal NP clusters were made of small zero-valent Pd NPs aggregating to form a shell around an Au core. Not only can the described characterization approach be applied to similar material systems, but the results can guide the optimization of the synthesis procedures.
0 notes