#tumorsuppressor
Text
youtube
#TumorSuppressor#CancerResearch#Genetics#DNARepair#CellGrowth#Apoptosis#CancerTherapy#TP53#BRCA1#RB1#GeneMutation#Oncology#cancer#youtube#Youtube
0 notes
Text

Hochreines CBD und positive Wirkung auf bestimmten Tumortypen
Cannabidiol gegen Krebszellen
Es gab schon eine Vermutung dasCannabidiol gegen Krebs helfen kann, jetzt könne diversen wissenschaftlichen Studien zahlreiche positive Effekte von CBD Produkten auf den Menschen nachweisen. Die Forscher des Klinikums der Universität München zeigten wie die Natur bei der Bekämpfung von Krankheiten helfen kann. Die hochreinem CBD nicht nur Nebenwirkungen von Krebstehrapien linder kann, sondern auch die Krebszellen in den Zelltod treiben könne. Die Wissenschaftler betonen jedoch, dass dies nur mit bestimmten Tumortypen wie Glioblastome und auch nur mit hochreinem CBD funktioniert. Um herauszufinden, welchen Effekt CBD auf diesen Hirntumor haben, führten Glaß und sein Team Zellkulturversuche mit Glioblastomzellen von Menschen und Mäusen durch.
.
Dazu verwendeten Sie das hochreines CBD in Form Epilepsiepräparats Epidiolex und analysierten, wie die Krebszellen darauf reagierten. Sie nutzten dazu biochemische sowie zellbiologische Analysen, führten pharmakologische Assays durch und untersuchten die sogenannte Genexpression der Zellen.
Die meisten Krebszellen starben bereits nach zwei bis drei Tagen ab. Die spätere Auswertung aller Daten zeigte, dassCBD den Signalweg, welcher für das Wachstum der Tumorzellen verantwortlich ist, effektiv blockiert. Dabei wird der NFKB Transkriptionsfaktor in der Anwesenheit von CBD in einen Tumorsuppressor umgewandelt. Als direkte Folge sterben die Tumorzellen ab. Damit liegt den Wissenschaftler der eindeutige Beweis vor, dass hochreines CBD einen tumorbekämpfenden Effekt beim Menschen besitzt – zumindest bei diesem speziellen Gehirntumor.
Auch früher in 2015 gab es erste Berichte von US-Krebsforschungszentrum National Cancer Institute, in dem beschrieben wurde das der Konsum von Cannabis positiv auf die Heilung von Krebs auswirken könnte.
Die Cannabinoide die grüne wunder Pflanze soll demnach in der Lage sein, Tumorzellen abzutöten. „Labor- und Tierstudien haben gezeigt, dass Cannabioide möglicherweise in der Lage sind, Krebszellen abzutöten, während sie normale Zellen schützen”, heißt es auf der Seite des National Cancer Institute.
Das ist eine Hoffnung auf die neuen Möglichkeiten, die durch die Cannabinoide langsam sich öffnen.
Quelle: Neuro-Oncology; doi: 10.1093/neuonc/noab095 und
Cannabidiol gegen Hirntumore
0 notes
Text
Triple negative "opportunity": is soy effectively useful against this breast cancer?

Breast cancer is the most common cancer and the leading cause of cancer death in women worldwide. It is not considered a single disease but a heterogeneous group of several diseases of distinct molecular subtypes. Breast cancer can be classified into four subtypes, luminal A, luminal B, basal and HER2-positive. Although the overall mortality rate for breast cancer patients has decreased in developed countries, patients diagnosed with the baseline subtype have a poorer short-term prognosis than those diagnosed with other breast cancer subtypes. About one sixth of breast cancer cases are classified as triple negative breast cancer (TNBC), named after the absence of estrogen receptor (ER), progesterone receptor (PRa) and human epidermal growth factore receptor (EGRF or HER2) expression.
Most cases of basal-type breast cancer are also referred to as TNBC because it is characterized by the lack of expression of these three biomarkers. Soy products have long been suggested to be useful in the prevention of cancer development. Epidemiological studies have shown the preventive effect of soy intake for breast cancer. In particular, Asian women who consume a diet rich in soy products have a lower incidence of breast cancer and a lower risk of breast cancer recurrence than women in western counties. Genistein, a phytoestrogen, is the main isoflavonoid contained in soybeans and is considered the active micronutrient responsible for its chemopreventive effect. Things are a little different in the case of the intake of soy or soy-based foods in the context of patients who have breast cancer.
Genistein is an isoflavone that was originally found to be an estrogen receptor agonist (ER-alpha), although later and more recent studies have shown that it urges preference for the beta isoform (ER-beta), which is not directly implicated in the proliferation of normal or malignant breast cells. Indeed, the results of in vitro mechanistic studies show that the antitumor activity of genistein in breast cancer cells is largely attributable to the preferential induction of ERβ, which suppresses ERα signaling. Given the possibility, however, that genistein behaves as an aromatic compound capable of activating the ER-alpha receptor, oncologists and endocrinologists advise against taking soy and its derivatives in case of classic breast cancer that expresses receptors for estradiol and / or progesterone.
This may be different in the case of triple-negative tumors. This isoflavone has a broad spectrum of anticancer properties in triple negative breast cancer cells. Genistein can inhibit cell growth, induce G2/M phase arrest and/or apoptosis, and decrease cell invasiveness in TNBC cells. This happens as genistein can enhance the expression of stop proteins (p53, p21WAF) while repressing cell death protectors such as Bcl-2 and the transcription factor NF-kB. Overall, it is known that genistein can inhibit some tyrosine kinases such as c-Src and the EGF receptor and can interfere with nuclear enzymes, such as topoisomerase I (Top-1) and DNA-methyltransferase (DNMT-1). There is some evidence that it might interfere as well with MEK-1, a protein kinase component of the MAP kinase signaling pathway.
More recent analyzes based on phospho-proteomics and bioinformatics have shown that genistein can modulate phosphorylation on proteins involved in cell cycle regulation and DNA damage response. They include critical components of the DNA replication fork, the cohesion complex, kinetochores and the BRCA1 complex. A particular regulation in triple-negative tumor cells was recorded in proteins such as histone H2.1, cyclin B1, thymidine kinase and few others. The vast majority of cellular proteins, therefore, do not appear to be conditioned in their expression in the context of triple-negative malignant cells. What seems to change, however, is their secondary modification state or phosphorylative state: nearly a hundred proteins undergo this variation when cultured MDA-MB-231 (triple-negative) cells are treated with genistein.
Of these, more than 20 are involved in the cellular response to radiation-induced genomic damage (such as ATR, BACH-1, ATRIP, TOPBP1, RAP80 and also the famous tumor suppressor BRCA-1). These responses are not late (24h or more), but occur after just 3 hours, indicating that genistein affects transduction pathways made of protein kinase. Other cellular responses, on the other hand, could depend on the involvement of longer genetic responses. The tumor suppressor BRCA-1 seems to be present in triple-negative malignant cells, but the genetic and epigenetic variations seem to keep it in a silent state that prevents it from acting on cellular malignancy. Genistein seems to restore its expression through the activation of a pathway sensitive to aromatic compounds, that of the hydrocarbon receptor or AhR, a transcription factor that can be activated or inhibited depending on what type of aromatic compound it manages to bind.
It is generally recognized that plant polyphenols and flavonoids block it, which leads to a tumor growth suppression effect and therefore to a chemopreventive effect. The genetic activity of BRCA-1 is also essential for the ER-alpha receptor to be expressed, which gives cancer cells sensitivity to estrogen or, even better, to anti-estrogens used in breast cancer chemotherapy. In fact, a very recent study has shown that TNBC cells treated with genistein show alterations of the epigenome (methylation state) in the BRCA-1 gene. In this case, from hyper-methylated the gene becomes hypomethylated and resumes its expression.It is curious that when the AhR receptor is bound to aromatic compounds, it also drags with it the DNMT1 enzyme for DNA methylation on the promoter of the BRCA-1 gene, which coincidentally is also inhibited by genistein itself.
This process allows the function of BRCA-1 and the subsequent expression of the ER-alpha receptor. In this way, the triple-negative cells become sensitive again to tamoxifen, one of the historical antiestrogens used against breast cancer. The study showed that the effect of genistein appears not only in cultured cell models, but also in laboratory mice to which genistein has been added to the diet. Scientists deem that in the case of triple-negative breast cancers, genistein supplementation or introduction of soy in the diet, might help manage cancer status, enhancing the possibility to restore its sensibility to drugs of the antiestrogens group.
Edited by Dr. Gianfrancesco Cormaci, PhD, specialist in Clinical Biochemistry.
Scientific references
Donovan MG, Selmin O et al. Nutrients 2019; 11:2559.
Jiang H et al. Onco Targets Ther. 2018; 11:8153.
Fang Y, Zhang Q et al. Int J Oncol. 2016: 48:1016.
Xie et al. Genes Chromos Cancer 2014; 53:422-31.
Liu X, Sun C et al. Molecules 2013; 18:13200-13217.
Yang Z et al. Antican Ag Med Chem 2012; 12:1264.
Pan H, Zhou W et al. Int J Mol Med 2012; 30:337-343.
Li Z, Li J et al. Toxicol In Vitro 2008; 22:1749-1753.
Vauzour D et al. Arch Biochem Biophys 2007; 468:159.
Cappelletti V et al. J Cell Biochem 2000; 79: 594-600.
Read the full article
#anticancer#antiestrogens#breastcancer#cellproliferation#DNAdamage#DNAmethylation#epigenetics#genistein#metastasis#nuclearreceptor#oncogene#phytoestrogens#soyfoods#tamoxifen#transcriptionfactor#triple-negative#tumorsuppressor
0 notes
Text
Tumor-suppressor protein dynamics determine if tissues survive radiation
Tumor-suppressor protein dynamics determine if tissues survive radiation
Credit: CC0 Public Domain
Exposure to radiation can wreak indiscriminate havoc on cells, tissues, and organs. Curiously, however, some tissues are more vulnerable to radiation damage than others.
Scientists have known these differences involve the protein p53, a well-studied tumor-suppressor protein that initiates a cell’s auto-destruct programs. Yet, levels of this sentinel protein are often…

View On WordPress
0 notes
Text
Why elephants are resistant to cancer and what they teach us about cancer prevention
By Swati Mishra, Ph.D.
5 minutes read
Last week I have attended the 2018 Nanomedicine and Drug Delivery Symposium (NanoDDS), hosted here in Portland, Oregon. This three days scientific meeting brought together some of the world’s eminent scientists and clinical researchers from diverse array of fields including nanotechnology, materials science, imaging, cell biology, tissue engineering, gene editing, drug and gene delivery, who are trying to solve range of clinical problems with a common goal of improving the quality of life. The aim of this meeting was to highlight new groundbreaking discoveries and developments in nanomedicine and drug delivery. One of the talks that particularly enticed my attention was on developing “Personalized Cancer Nanomedicines”. The talk was delivered by Dr. Avi Schroeder from Technion-Israel Institute of Technology. Dr. Schroeder’s work is inspired by the cancer-resistance mechanism in elephants and is an attempt to apply this knowledge to humans in the hopes of developing new cancer treatments for children and families at high risk for developing cancer.
To summarize it for those who are not familiar with the pathogenesis of cancer, in healthy individuals, on an average a cell will divide for 50-70 times before it grows old and dies. Cancers develop when some of the cells in ours body accumulate mutations in their DNA that cause them to divide and proliferate uncontrollably and eventually start spreading into surrounding tissues. Mutations in DNA, leading to cancer, can start in any one of the 30 trillion cells that make our body, which means all the cells are equally susceptible to cancer causing mutations. Therefore, the risk of cancer causing DNA mutation in any species theoretically increases with the total number of cells, which is directly proportional to the body-size and species life span, which is correlated to the total number of cell divisions. Though statistically this correlation holds true with in the species for eg, taller and over-sized humans are more likely to develop malignant tumors and bigger dogs are more susceptible to get cancers, this is not necessarily true across species. Which means it is not true that larger and longer-lived animals – like elephants and whales – would have a higher risk of cancer than smaller and shorter-lived animals – like mice and Chihuahuas.
Peto’s Paradox, published some 35 years ago by British epidemiologist Richard Peto of the University of Oxford, suggests that larger and longer-lived mammals develop less cancer than expected.
Though scientists have come up with multiple hypothesis while trying to solve Peto’s paradox over the years, the exact reason behind this mismatch of species size and cancer rate was mostly unknown until 2015, when two independent scientific discoveries by Dr. Joshua Schiffman and his team as well as by an evolutionary biologist at University of Chicago Dr. Vincent Lynch and his team, reported an important cellular mechanism for this phenomenon of cancer resistance. Before diving into the details reported in these papers, I would like to draw your attention to our body’s natural defense mechanism against cancer and to the role of a tumor suppressor gene that provides instructions for making tumor suppressor protein p53 (one of the most important players in cancer biology), which is often referred as “guardian of the genome”. Protein p53 is a crucial tumor protein that helps control cell growth and fight against cancer by either repairing the broken or damaged strands of DNA (repairing mutations), or kills off cells containing defective DNA. With p53 working enthusiastically against cancer, the probability of getting cancer causing mutation is one in billion: which means if exposed to massive DNA damaging carcinogenic environment like high doses of nuclear radiation, toxic chemicals, ultraviolet (UV) rays from sunlight etc. p53 will kill all the damaged cells and only about one in a billion damaged cells will contain a mutation likely to cause cancer. But, what if you get a mutation in the gene that encodes “guardian of the genome”. It has been seen that greater than half of all the tumors exhibit mutation in this gene and normal p53 function is compromised in most of human tumors. It is now known that the “mutant p53 proteins” (produced after mutation in the “guardian of the genome”) surprisingly gain tumor-promoting functions, which means inactivation of normal p53 protein leads to three cancer specific characteristics in the mutated cells - (1) cells with mutant p53 stimulate their own growth (cells will grow forever); (2) they resist inhibitory signals that might otherwise stop their growth (their DNA will be full of mutations); (3) they resist their programmed cell death even after infinite divisions (cells will never die).
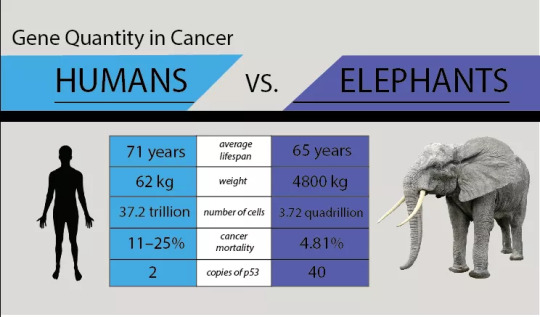
Picture Courtesy
Each and every cell in our body contains two functional copies of this p53 gene. Here it is important to note that – with two functional p53 gene copies, the odds of getting cancer in your lifetime without ever being exposed into carcinogenic environment are; 1 in 2 for men and 1 in 3 for women. Now let’s discuss the findings of the above two papers, Lynch and Schiffman hypothesized that elephants held some biological insight that could explain their resistance to develop cancer for an animal of their size and wondered whether that insight could help fight against cancer in humans. In two independent studies they subsequently found out that this large long-lived mammal indeed have 40 functional copies of p53 gene. It was later found that the african-elephant’s genome holds 40 copies of p53 where as asian-elephant’s genome holds p53 copies ranging from 30 to 40. These extra copies make elephant cells more sensitive to DNA mutations, and instead of repairing DNA the elephant cells prefer to launch the self-suicide program at levels of damage that human cells would tolerate. So under conditions that trigger DNA damage, elephant cells would just die to kill the tumor in the bud before it becomes malignant and that explains why elephants are so resistant to cancer for an animal of their size.
This is not the end of the story, just so you know that it's not only elephants - there are some smaller species too that are highly cancer-resistant, for example naked mole rats, microbats and grey squirrels. Extensive molecular level investigation on these cancer resistance animals would allow scientists to explore – genetic differences, underlying mechanisms against cancer and cellular responses to DNA damage. And I am hopeful that the findings would soon help us putting the learnt lessons into a perspective that could strategically be applied in humans to prevent, to diagnose and to develop better treatment modalities for – cancer.
Connect with me on LinkedIn, Facebook and Follow me on Twitter
0 notes
Text
Warum mir persönlich die Coronapolitik momentan
LePenseur:"wesentlich wichtiger ist als die massiv gestiegene Zuwanderung Zu einem Problem-Ablenkungs-Artikel von Unterberger, in welchem er sich – freilich nicht ohne Berechtigung! – über abgefackelte Polizeiautos erregt, folgender
Gastkommentarvon elfenzauberin
Man weiß mittlerweile, dass dasSpike-Protein auf das p53-gen und das BRACA1- und BRACA2-Gen einwirkt in dem Sinne, dass deren Wirkungen eingeschränkt werden.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7324311/
Was bedeutet das? Das p53-Gen und die Braca-Gene sind Tumorsupressor-Gene. Insbesondere das p53-Protein ist ein Wächter der DNA, das geschädigte Gene wieder reparieren kann. Ist der Genabschnitt, der das p53-Protein codiert, selbst beschädigt, dann entsteht ein funktionslosen p53, das die DNA nicht mehr zu schützen in der Lage ist. Geschädigte Gene werden so nicht mehr repariert und es entstehen bösartige Tumore.
Es gibt eine seltene und scheußliche Erbkrankheit, in der das p53-Protein funktionslos ist, das Li-Fraumeni-Syndrom. Die Betroffenen erkranken praktisch alle an ganz verschiedene bösartige Tumore, wobei nicht nur Erwachsene, sondern auch Jugendliche und Kinder betroffen sind. Diese Krankheit illustriert sehr klar die Bedeutung des Reparaturgenes für p53.
Die BRACA-Gene sind ebenfalls Tumorsuppressor-Gene, die Doppelstrangbrüche der DNA reparieren können. Sind diese Gene defekt, dann entsteht sehr häufig Brustkrebs.
Die Spike-Proteine interagieren genau mit diesen Genen und schlimmer noch - die mRNA-Impfstoffe codieren die S2-Untereinheit des Spike-Proteins, also genau den Eiweißkörper, der die p53-Gene und Braca-Gene behindert oder sogar inaktiviert.
Mir ist schon wiederholt aufgefallen, dass der Krebs bei diversen Krebspatienten nach der Covid19-Impfung buchstäblich explodiert ist. Solche Phänomene gab es zwar schon immer, nur nicht in der Ausprägung.
Es ist wichtig zu verstehen, dass Schädigungen der Tumorsupressor-Gene Phänomene sind, die mit-unter mit großem zeitlichen Abstand erfolgen. Sogar Patienten mit Li-Fraumeni-Syndrom, die den Defekt schon von Geburt an haben, erreichen mitunter das Erwachsenenalter, bevor sich der Krebs mani-festiert; es können also Jahrzehnte vergehen, bevor der Gendefekt wirksam wird.
Wenn einmal eine Impfung die Tumorsuppressor-Gene zusammenschießt, dann ist jedenfalls Feuer am Dach, insbesondere wenn weite Teile der Bevölkerung mit so einem (Nicht-)Impfstoff geimpft wurden.
Falls jetzt irgendwer glaubt, dass die Impf-Spike-Proteine genauso schnell verschwinden, wie die Impf-RNA, dann ist derjenige auf dem Holzweg. Die Impf-Spikeproteine wurde noch viele Monate nach Impfstoffgabe nachgewiesen - das schädliche Agens ist also lange vorhanden, insbesondere wenn man ständig boostert. Demgegenüber sind ein paar abgebrannte Polizeiautos in der Tat Nebensache. ----- P.S.: Für Vektorimpfstoffe gilt oben Geschriebenes in gleicher Weise, da die Endstrecke, nämlich der unkontrollierte Einbau von Spikeproteinen in die Zelloberfläche, völlig identisch ist.
Für Totimpfstoffe gilt Geschriebenes, wenn überhaupt, in viel geringeren Ausmaß, da es zu keiner un-kontrollierten Vermehrung von Spikeproteinen kommt. Hier wird eine streng definierte Menge an in-aktivierten Viren verabreicht, die zwar das Spikeprotein an ihrer Oberfläche tragen, jedoch nicht ver-mehrungsfähig sind.
Ein Totimpfstoff löst eine immunologische Reaktion aus und er wird — soweit ich weiß — vom Körper recht schnell abgebaut. Das schädigende Agens wirkt also nur kurze Zeit.
Das ist ein fundamentaler Unterschied zu Vektor- und mRNA-Impfstoffen. http://dlvr.it/SJT57N "
1 note
·
View note
Link
Forscher der University of California San Diego School of Medicine berichten, dass der Nachweis von "Copy Editing" durch ein Stammzellenenzym namens ADAR1, das in mehr als 20 Tumorarten aktiv ist, eine Art molekulares Radar zur Früherkennung von Malignomen bereitstellen kann und ein neues therapeuti...
0 notes
Text
RT @letlifehappen: Tumor-suppressing protein... http://bit.ly/2mpwNi5 #anti-cancertarget #cancer #P53 #proteinPHLDB3 #TulaneUniversity #tumorsuppressor
Tumor-suppressing protein... http://bit.ly/2mpwNi5
#anti-cancertarget #cancer #P53 #proteinPHLDB3 #TulaneUniversity #tumorsuppressor
— Let Life Happen (@letlifehappen) March 14, 2017
via Twitter https://twitter.com/NiteStar
March 14, 2017 at 04:46PM
0 notes
Text
youtube
#WarburgEffect#Glycolysis#CancerMetabolism#GlutamineAddiction#Mitochondria#Oncogenes#TumorSuppressors#MetabolicReprogramming#AerobicGlycolysis#MetabolicFlexibility#oncology#cancer#Youtube
0 notes
Text
Tumorsuppressor
Synonym: Tumorsuppressorprotein, Antionkogen
Englisch: tumor suppressor
1 Definition
Als Tumorsuppressoren werden Proteine verstanden, welche durch ihre Aktivität denn Zellzyklus und damit die Proliferation einer Zelle kontrollieren. Zusätzlich können sie, bei Vorliegen ireparabler DNA-Schäden, die Zelle in die Apoptose schicken.
2 Hintergrund
Bei normalen Zellen führen Beschädigungen der DNA,…
View On WordPress
0 notes
Link
Wie entsteht Brustkrebs und warum sind manche Patientinnen resistent gegen etablierte Therapien? Forschende der Universität Basel haben neue Erkenntnisse zu den molekularen Prozessen im Brustgewebe gewonnen. Sie identifizierten den Tumorsuppressor LATS als zentralen Akteur in der Entwicklung und Beh...
0 notes
Text
Tumorsuppressorgen
1 Definition
Als Tumorsupressorgene werden Gene bezeichnet, deren Produkte die unkontrollierte Teilung genomisch geschädigter Zellen unterdrücken, und dadurch die Entstehung von Tumoren verhindern können.
2 Beispiele
Genprodukte der Tumorsupressorgene sind unter anderem:
2.1 Protein 53 (p53)
Protein 53 ist ein Transkriptionsfaktor, der HDM2-gebunden inhibiert im Zellkern vorliegt. Bei Schädigung…
View On WordPress
0 notes