#zeroDefectPackaging
Explore tagged Tumblr posts
Text
The Hidden Risks of Ignoring Flip-Off Cap Inspection in Pharma Packaging

In pharmaceutical manufacturing, every detail counts — from sterile filling to labeling. But one component often overlooked is the flip-off cap that seals injectable vials. It’s small, yes — but the consequences of not inspecting it are anything but.
Let’s talk about why ignoring flip-off seal inspection is a risk no modern pharma brand should take, and how Optomech’s FOSIS system brings precision, automation, and peace of mind to the process.
🧨 The Problem: Small Defects, Big Consequences
You may think a cap is just a cap. But even minor defects in flip-off seals can cause:
Regulatory failures during batch audits
Sterility breaches in transit or storage
Patient safety risks due to contaminated vials
Brand reputation damage from recalls or customer complaints
Typical issues include:
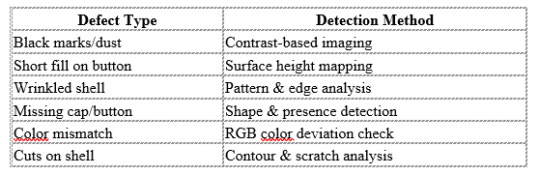
Black spots
Short filling of the PP button
Cut or dented aluminum shells
Color mismatches
Improper or missing plastic caps
Now imagine these defects slipping through manual or random inspections. That’s a compliance risk pharma companies can no longer afford.
✅ The Solution: Meet FOSIS – Flip-Off Seal Inspection System
FOSIS is Optomech’s high-speed, camera-based vision inspection system — purpose-built to scan, detect, and reject defective flip-off caps with unmatched accuracy.
🎯 Key Features at a Glance:
Speed: Inspects up to 600 caps/min
Vision Accuracy: Detects 20+ types of defects
Automation: No manual checking, no guesswork
Rejection System: Faulty caps are auto-ejected instantly
Compliance: Fully 21 CFR Part 11 compliant
🧠 How It Works:
Caps are fed into the system via a vibratory bowl.
High-speed cameras capture images from multiple angles.
Advanced algorithms compare each cap to a defect-free reference.
Defective caps are rejected, and logs are generated.
Reports can be exported via Excel/network sharing.
📊 Defects Detected by FOSIS:
🏭 Who Needs FOSIS?
FOSIS is ideal for:
Pharmaceutical manufacturers of injectable drugs
CMOs (Contract Manufacturing Organizations)
Flip-off cap producers
Companies targeting zero-defect packaging
Whether you're building a new packaging line or upgrading your existing setup, FOSIS integrates seamlessly and boosts inspection reliability from day one.
🔒 Why Flip-Off Cap Inspection Can’t Be Optional Anymore
In today’s regulated pharma landscape:
Quality audits are stricter
Sterility concerns are growing
Brand protection is non-negotiable
FOSIS isn't just a machine — it's your frontline quality gate. It helps you catch defects before regulators or customers do.
🏁 Final Takeaway: Inspection Is No Longer Optional — It's Strategic
Flip-off seal inspection may be a “last step” in the process, but with FOSIS, it becomes a first-class safeguard for your production integrity. Speed, accuracy, compliance — all in one automated system.
📞 Want to see FOSIS in action or schedule a demo? ���� [email protected] 🌐 www.optomech.in/flipoff-inspection-system-2
#flipoffsealinspection#pharmapackaging#capdefectdetection#machinevisionqc#21cfrcompliant#injectablepackaging#optomechinspection#capinspectionmachine#visualinspectionpharma#zeroDefectPackaging
0 notes